Summary
Background
Chronic obstructive pulmonary disease (COPD) is associated with eosinophilic airway inflammation in 10–20% of patients. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, depletes blood and sputum eosinophils. We aimed to establish whether benralizumab reduces acute exacerbations of COPD in patients with eosinophilia and COPD.
Methods
We did this randomised, double-blind, placebo-controlled, phase 2a study between Nov 18, 2010, and July 13, 2013, at 26 sites in the UK, Poland, Germany, Canada, the USA, Denmark, and Spain. Adults aged 40–85 years, with moderate-to-severe COPD, at least one acute exacerbation of COPD, and a sputum eosinophil count of 3·0% or more within the previous year, were randomly assigned (1:1) via computer-generated permuted block randomisation (block size of four), with an interactive voice or web-response system, to receive placebo or 100 mg benralizumab subcutaneously, every 4 weeks (three doses), then every 8 weeks (five doses) over 48 weeks. Study site personnel included in study assessments, participants, and data analysts, were masked to treatment allocation. The primary endpoint was the annualised rate of acute exacerbations of COPD at week 56, defined as the number of acute exacerbations divided by total duration of person-year follow-up. Secondary and exploratory endpoints included COPD-specific Saint George’s Respiratory Questionnaire (SGRQ-C), Chronic Respiratory Questionnaire self-administered standardised format (CRQ-SAS), pre-bronchodilator forced expiratory volume in 1 second (FEV1), and safety. We did a prespecified subgroup analysis by baseline blood eosinophil count. Analyses were by intention to treat and per-protocol. This trial is registered with ClinicalTrials.gov, number NCT01227278.
Findings
We randomly assigned 101 patients to receive placebo (n=50) or benralizumab (n=51), of whom 88 (87%) patients completed the study. Six patients who completed the study were excluded from the per-protocol population because of major protocol violations; the per-protocol population thus included 82 patients. Benralizumab did not reduce the annualised rate of acute exacerbations of COPD compared with placebo in the per-protocol population, with rates of 0·95 (0·68–1·29; n=40) versus 0·92 (0·67–1·25; n=42). Mean pre-bronchodilator FEV1 change from baseline to week 56 was −0·06 L (SD 0·24) with placebo, and 0·13 L (0·41) with benralizumab (p=0·014). Numerical, albeit non-significant, improvement in acute exacerbations of COPD, SGRQ-C, CRQ-SAS, and FEV1 were greater in benralizumab-treated patients with baseline blood eosinophil concentrations of 200 cells per µL or more or 300 cells per µL or more. Incidence of treatment-emergent adverse events was similar between the two groups, with the most common events being respiratory disorders (31 [62%] of 50 patients given placebo vs 32 [63%] of 51 given benralizumab) and infections (28 [56%] vs 27 [53%]). A higher incidence of serious treatment-emergent adverse events were recorded in patients in the benralizumab group than in those in the placebo group (14 vs nine patients), although none of these events were considered by the investigator to be benralizumab related.
Interpretation
Compared with placebo, benralizumab did not reduce the rate of acute exacerbations of COPD. However, the results of prespecified subgroup analysis support further investigation of benralizumab in patients with COPD and eosinophilia.
Funding
MedImmune.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by partly irreversible airflow obstruction through a combination of emphysema and destruction of small airways.1,2 Acute exacerbations of COPD are associated with airflow obstruction, a substantial reduction in health-related quality of life, and high levels of mortality and morbidity.3 Inflammation, predominantly neutrophilic, contributes to the narrowing of small airways in patients with COPD1 and is increased during acute exacerbations.4 However, in 10–20% of patients with COPD, evidence has been reported of eosinophilic airway inflammation both during stable periods and during acute exacerbations;4–8 titration of corticosteroid treatment to reduce concentrations of airway eosinophils attenuates the frequency of severe acute exacerbations of COPD6.
Because of the potential role of eosinophil-mediated inflammation in patients with COPD, development of treatment options that reduce eosinophil concentrations is of great interest. Interleukin-5 regulates the differentiation, proliferation, survival, and activation of eosinophils via the interleukin-5 receptor.9 Antibodies to interleukin-5 greatly reduce severe asthma exacerbations in patients with evidence of eosinophilic inflammation.10–12
Benralizumab, a humanised, afucosylated monoclonal antibody to interleukin-5 receptor α, reduces sputum and blood eosinophil counts by enhancement of antibody-dependent cell-mediated cytotoxic effects.13,14 We did this study to assess the efficacy and safety of benralizumab in patients with eosinophilic COPD.
Methods
Study design and participants
We did this phase 2a, randomised, double-blind, placebo-controlled study between November, 2010 (the first patient was enrolled on Nov 18, 2010, and the first dose of study drug was given on Feb 25, 2011), and July 13, 2013, at 26 sites in the UK, Poland, Germany, Canada, the USA, Denmark, and Spain. Patients had to be exacerbation free in the 4-week run-in period, hence the delay between screening and delivery of the first dose.
We enrolled adults aged 40–85 years, with moderate-to-severe COPD2 and at least one acute exacerbation of COPD requiring oral corticosteroids, antibiotics, or hospital admission in the past year. Participants were present or past smokers with a tobacco history of ten or more pack-years, a post-bronchodilator forced expiratory volume in 1 second (FEV1) of less than 80% predicted at screening, airflow obstruction with a post-bronchodilator FEV1-to-forced vital capacity ratio of less than 70%, and a sputum eosinophil count of 3·0% or more 12 months before, or at, screening. We excluded patients who had no historical data showing a sputum eosinophil count of at least 3·0% and could not produce sputum at the screening visit. Furthermore, we excluded patients with a clinically significant cardiovascular history; additional clinically significant pulmonary disease or asthma; parasitic or chronic mycobacterial or viral infection; or those who had used oral immunosuppressive drugs, not including oral corticosteroids within the past 28 days. The appendix provides full inclusion and exclusion criteria.
The study protocol was developed by the chief investigator (CEB) and MedImmune and was approved by the institutional review board at each study site. Participants provided written informed consent, and the study was done in accordance with the Declaration of Helsinki.
Randomisation and masking
Participants were randomly assigned (1:1), via computer-generated permuted block randomisation (block size of four) with a central telephone and web-based system, to receive 100 mg benralizumab or matched placebo, subcutaneously. Benralizumab and placebo were prepared by a site investigational product manager who was unmasked to treatment allocation and an independent site investigational product monitor, also unmasked, did investigational product accountability. Both investigational product manager and investigational product monitor were not included in study assessments. Treatments were given to patients by the site staff after reconstitution. All other study site personnel, participants, and sponsors, including data analysts, were masked to treatment allocation.
Procedures
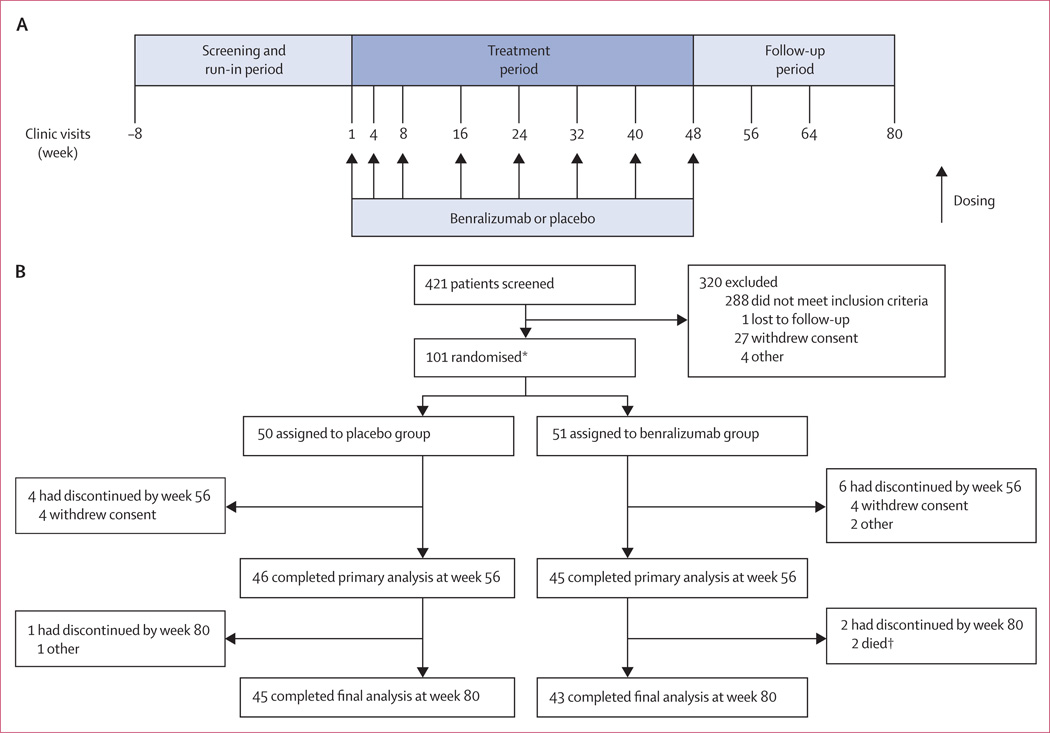
During screening (from week −8 to −4; figure 1), each patient’s FEV1 was measured to identify their maintenance treatment. For the duration of the study from week −4, patients whose FEV1 was at least 50% but less than 80% received tiotropium (18 ug) once daily, and those with less than 50% FEV1 received two inhalations of budesonide–formoterol (200 ug and 6 ug, respectively) twice daily. If patients with an FEV1 less than 50% predicted were stable (ie, no change in drug for 3 months before screening) with triple treatment (tiotropium with any inhaled corticosteroid and longacting β agonist) before screening, they could remain on this treatment. Patients could continue use of previous COPD drugs. Study drug was given as two subcutaneous 50 mg injections in an outpatient setting. The first three doses were given at weeks 1, 4, and 8, followed by doses at weeks 16, 24, 32, 40, and 48 (figure 1). Vital signs were obtained before study drug was given and patients were monitored for 2 h after.
Figure 1. Study design (A) and trial profile (B).
*These patients comprised the intention-to-treat and safety population; six patients were excluded from the per-protocol population for major protocol violations: eligibility criteria not checked appropriately (n=4), use of restricted drugs (n=1), and non-compliance with maintenance treatment (n=1).†Neither death was considered related to treatment.
We defined an acute exacerbation of COPD as a worsening of at least two major symptoms (dyspnoea, sputum volume, and sputum purulence) or any major symptom together with one of several minor symptoms (sore throat, cold, fever without other cause, and increased cough or wheeze for more than 2 consecutive days).15 Acute exacerbations were categorised by severity on the basis of the treatment given: mild required an increased use of short-acting bronchodilators, moderate required treatment with systemic corticosteroids or antibiotics, and severe required hospital admission. Other assessments undertaken were disease-specific health status with the Saint George’s Respiratory Questionnaire-COPD (SGRQ-C);16,17 reductions in hospital admissions for acute exacerbations; bodymass index (BMI), the airflow Obstruction, Dyspnoea, and Exercise capacity (BOD E) index;18 and health-related quality of life with the Chronic Respiratory Questionnaire self-administered standardised format (CRQ-SAS);19,20 time to first acute exacerbation; FEV1; and counts of peripheral blood and sputum eosinophil cells. The sputum induction and processing procedures were standardised and sputum cell counts were done by a central laboratory. Induction was contraindicated in patients with an FEV1 of less than 1·0 L. We monitored safety and tolerability by recording treatment-emergent adverse events. Assessments were continued to week 56, with safety monitoring continuing for a further 24 weeks. The appendix provides full details of study assessments.
Outcomes
The primary endpoint was annualised rate of moderate and severe acute exacerbations of COPD at week 56, defined as the number of acute exacerbations divided by total duration of person-year follow-up. Secondary endpoints were change from baseline at week 56 in SGRQ-C and CRQ-SAS scores, reduction in hospital admissions for acute exacerbations, BODE index, and safety. Exploratory endpoints were rate of acute exacerbations by maintenance treatment, time to first acute exacerbation, change from baseline in FEV1 at week 56, number of exacerbation-free days, as measured by the Exacerbations of Chronic Pulmonary Disease Tool–Patient Reported Outcome (EXACT-PRO), symptom scores, all-cause mortality, and blood and sputum eosinophil counts.
Statistical analysis
The intention-to-treat population included all randomised participants. Efficacy analyses were done with the per-protocol population, which included all patients with no major protocol violations who received at least six doses of study drug (including at both weeks 1 and 4) and completed the study to at least week 56. The per-protocol population was identified before database lock. Sample size and power calculations were based on simulation with the van Elteren test (two sided). With the assumption of a drop-out rate of 20%, 90 participants were needed for 77% power to detect a 40% reduction in rate of moderate and severe acute exacerbations of COPD compared with placebo, assuming an annual rate of two acute exacerbations to week 56 in the placebo group, with a two-sided α of 0·05. We did a van Elteren test, including no other stratification factors, on the per-protocol population to compare differences in the primary endpoint between treatment groups. These results were also assessed with a Poisson regression model adjusted for overdispersion and a negative binomial model (appendix). We chose an annual exacerbation rate of two on the basis of published literature.21–24 A 40% reduction in COPD exacerbations was thought to be commercially relevant and clinically important for biological treatments. Thus, the study was powered to detect a 40% reduction. Because the study was a small phase 2a study rather than a pivotal phase 3 study, the slightly higher rate of type-2 errors was deemed to be acceptable and therefore 90% power was not used. We compared the other endpoints between treatment groups with ANCOVA. A prespecified subgroup analysis by baseline blood eosinophil count was added to the statistical analysis before database lock, on the basis of published literature.12 Post-hoc analyses of sputum eosinophil count cutoff points were done for all endpoints. Subpopulation treatment effect pattern plots (STEPP), which are designed to present estimates of treatment effect derived from different but overlapping subsets of data, were generated to explore the patterns of relative treatment effectiveness across patient subpopulations. The subpopulations were defined with both sputum and blood eosinophil counts within a range of cutoff values corresponding to the 0–40th, 10–50th, 20–60th, 30–70th, 40–80th, 50–90th, and 60–100th percentile. The safety population, defined as all participants who received at least one dose of study drug, was used to summarise safety and tolerability. Analyses were done with SAS (version 9.3).
This trial is registered with ClinicalTrials.gov, number NCT01227278.
Role of the funding source
The study protocol was developed by the study sponsor and CEB (principal investigator and lead author). The investigators obtained all study data, which were analysed by the sponsor. The report was written by the authors with a medical writer funded by the study sponsor. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Figure 1 shows the trial profile. We randomly assigned 101 patients to receive benralizumab (n=51) or placebo (n=50), of whom 88 (87%) completed the study. The entry criterion for sputum eosinophil count (≥3·0%) was met historically in 32 (32%) patients, and in 69 (68%) patients at screening. However, of 95 participants with assessable baseline sputum, only 62 (65%) had a sputum eosinophil count of 3·0% or more, showing variability. No participants withdrew because of subcutaneous injections. Six (7%) patients of the 88 who completed the study (three per treatment group) were excluded from the per-protocol population because of major protocol violations; the per-protocol population thus included 82 patients (42 in the placebo group and 40 in the benralizumab group). Demographic and baseline clinical characteristics were similar between treatment groups (table 1).
Table 1.
Baseline demographics and clinical characteristics (intention-to-treat population)
| Placebo group (n=50) | Benralizumab group (n=51) | |
|---|---|---|
| Demographics | ||
| Age (years) | 64·6 (7·5) | 62·9 (8·2) |
| Female | 21 (42%) | 16 (31%) |
| White | 50 (100%) | 50 (98%) |
| BMI (kg/m2) | 26·5 (4·8) | 26·6 (5·6) |
| COPD exacerbations in the previous 12 months resulting in*: | ||
| Hospital admission | 0·2 (0·4) | 0·3 (0·6) |
| Visit to emergency department | 0·1 (0·4) | 0·1 (0·5) |
| Visit to physician’s office | 1·7 (1·4) | 1·3 (1·0) |
| Phone call to physician’s office | 0·4 (0·9) | 0·3 (0·6) |
| COPD exacerbations in previous 12 months† | 1·6 (1·0) | 1·6 (1·0) |
| Present smoker | 21 (42%) | 17 (33%) |
| Ex-smoker | 29 (58%) | 34 (67%) |
| Number of pack-years smoked‡ | 49·4 (22·0) | 49.3 (28·1) |
| COPD characteristics | ||
| GOLD 2001 status§ | ||
| I and II | 31 (62%) | 24 (48%) |
| III and IV | 19 (38%) | 26 (52%) |
| SGRQ-C score¶ | 48·1 (19·9) | 52·7 (19·3) |
| Symptoms | 63·7 (23·9) | 67·7 (20·9) |
| Activity | 58·7 (23·7) | 64·0 (24·7) |
| Impact | 36·6 (20·0) | 40·8 (21·8) |
| Airway function | ||
| Pre-bronchodilator FEV1 (L) | 1·4 (0·6) | 1·3 (0·5) |
| Post-bronchodilator FEV1 (L) | 1·5 (0·6) | 1·5 (0·5) |
| FEV1 (% predicted; L) | 49·9 (17·9) | 44·2 (16·1) |
| FEV1 to FVC ratio | 48·4 (13·5) | 47·1 (13·7) |
| Baseline concentrations of blood and sputum eosinophils | ||
| Blood eosinophils (cells per µL) | 229·2 (164·5) | 248·8 (193·4) |
| Sputum eosinophils (%)‖ | 8·7% (14·1) | 12·0% (14·4) |
| Background treatment at baseline | ||
| LAMA | 24 (49%) | 20 (40%) |
| ICS and LABA | 5 (10%) | 5 (10%) |
| ICS and LABA, and LAMA | 20 (41%) | 25 (50%) |
Data are mean (SD) or n (%), unless otherwise indicated.
BMI=body-mass index. COPD=chronic obstructive pulmonary disease. GOLD=Global Initiative for Chronic Obstructive Pulmonary Disease. SGRQ-C=St George’s Respiratory Questionnaire–COPD. FEV1=forced expiratory volume in 1 second. FVC=forced vital capacity. LAMA=long-acting muscarinic antagonists. ICS=inhaled corticosteroids. LABA=long-acting β agonists.
These values are not mutually exclusive.
We did not capture the exact number of previous exacerbations; we captured only the data as shown for hospital admission, emergency department admission, physician visit, and physician phone call. Thus, if a patient had two physician visits and one hospital admission, we took a conservative approach in calculation of the total exacerbation count, whereby one physician visit and one hospital admission were the same exacerbation and were calculated as two exacerbations (ie, two physician visits) not three (ie, two physician visits and one hospital visit).
One pack year is 20 cigarettes smoked every day for 1 year.
GOLD status shows severity of disease as established by spirometry results, with stage I being the mildest.25
Scores on the SGRQ-C range from 0 to 100, whereby a lower score shows better health status.
Baseline sputum data were available for 80 patients with day 1 data and for 15 patients with induced sputum at screening; only 62 (65%) of these patients had a sputum eosinophil count of 3·0% or more at baseline.
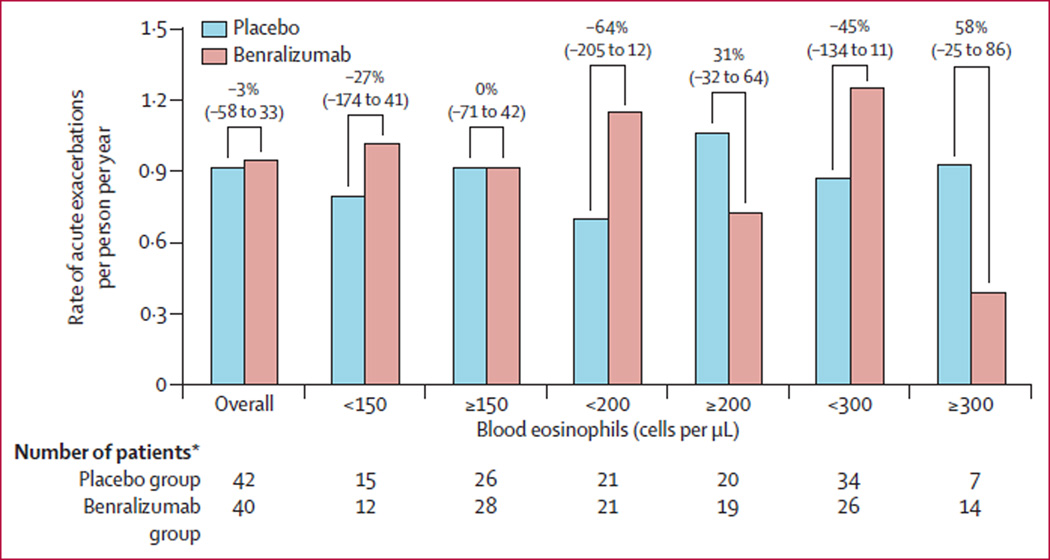
The primary endpoint was not met, with patients in the placebo group showing annualised rates of acute exacerbations of COPD at week 56 of 0·92 (95% CI 0·67–1·25), and those in the benralizumab group showing annualised rates of 0·95 (0·68–1·29). The rate reduction after benralizumab versus placebo was −3% (95% CI −58 to 33; p=0·94; figure 2). Analysis of the primary endpoint by Poisson regression and negative binomial calculations supported the results from the primary van Elteren test (appendix). In the per-protocol population, 25 of 42 (60%) patients in the placebo group and 22 of 40 (55%) of those in the benralizumab group needed oral corticosteroids or intravenous antibiotics for exacerbations, or both; corresponding numbers in the intention-to-treat population were 28 of 50 (56%) and 27 of 51 (53%), respectively. There was no difference in rate of hospital admissions for acute exacerbations between the placebo and benralizumab groups (appendix).
Figure 2. Rate of annualised moderate and severe acute exacerbations of COPD at week 56 (per-protocol population).
Values above the bars show rate reduction (95% CI) with benralizumab. One patient in the benralizumab group was not included in the subgroup analyses because no baseline eosinophil count was available. One patient in the placebo group did not have baseline blood eosinophil count, resulting in one patient less for subgroup analyses in this group. COPD=chronic obstructive pulmonary disease. *Number of patients in each subgroup.
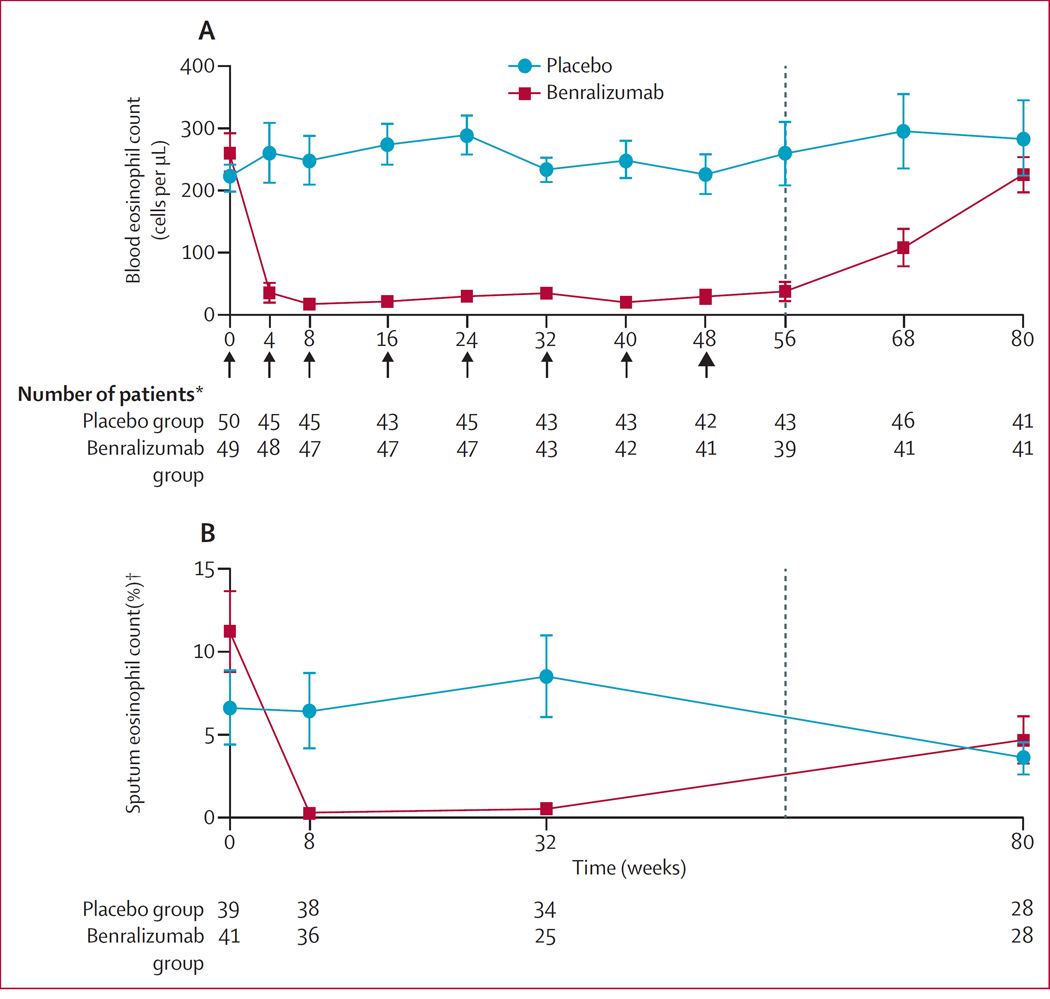
Benralizumab depleted both blood and sputum eosinophils by the first timepoint analysed (weeks 4 and 8, respectively; figure 3). These counts remained reduced throughout the treatment period (figure 3). Compared with placebo, we recorded greater mean percentage reductions from baseline in sputum eosinophil percentages in the benralizumab group at week 8 (11·2% [SD 1·2] vs 97·0% [1·4] reduction) and week 32 (−11·2% [1·4] increase vs 96·3% [1·2] reduction).
Figure 3. Mean (SE) eosinophil count over time in peripheral blood (A) and induced sputum (B) in the safety population.
Arrows represent when doses were given; bold arrow shows time of final dose. *Number of patients for each timepoint. †Out of 400 cells.
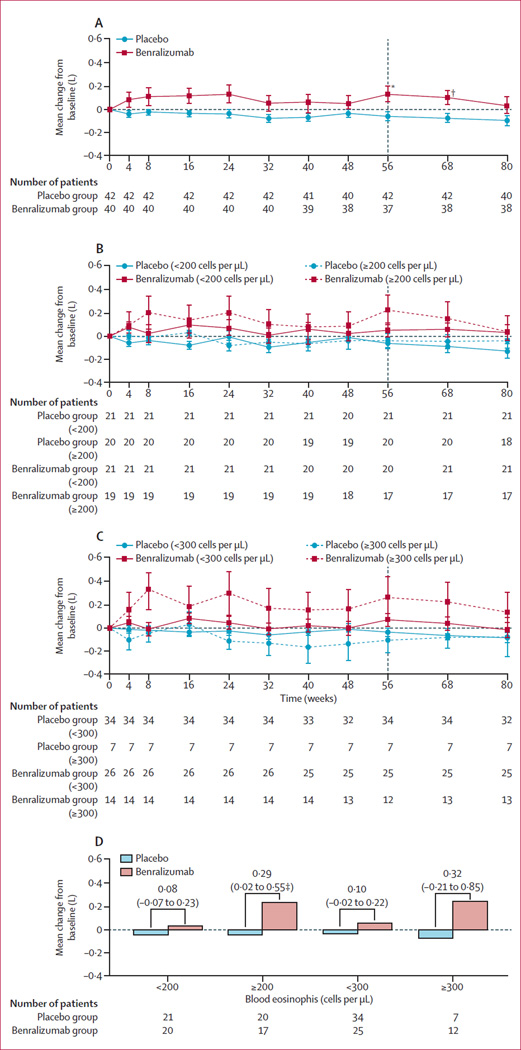
Mean change from baseline to week 56 for pre-bronchodilator FEV1 was −0·06 L (SD 0·24) for patients given placebo and 0·13 L (0·41) for those given benralizumab (p=0·014). Compared with patients in the placebo group, we noted a significant improvement (p=0·014) in post-bronchodilator FEV1 in those in the benralizumab group (−0·08 L [SD 0·21] vs 0·09 L [0·39]). Improvements in pre-bronchodilator and post-bronchodilator FEV1 in the benralizumab group happened as early as week 4 (the first timepoint analysed) and were sustained until week 80, 32 weeks after the last dose (figure 4). No significant differences between treatment groups were noted in change from baseline at week 56 for mean SGRQ-C, CRQ-SAS, or BODE scores (appendix). The mean change from baseline to week 56 in SGRQ-C total score was −4·43 in the placebo group and −5·51 in the benralizumab, group (table 2). The appendix provides results for the exploratory endpoints (6-min walk test, visual analogue scale, BMI, airflow obstruction, dyspnoea, exercise capacity, and number of exacerbation-free days, as measured by EXACT-PRO).
Figure 4.
Mean (SE) change from baseline in pre-bronchodilator forced expiratory volume in 1 second for the overall study population (A) and over time for the 200 cells per µL cutoff subgroup (B), the 300 cells per µL cutoff subgroup (C), and to week 56 by eosinophil subgroup (D; per-protocol population)
Values above the bars in panel D show the difference (95% CI) between treatment groups. Tables show number of patients for each timepoint or in each subgroup. One patient in the benralizumab group was not included in the subgroup analyses because no baseline eosinophil count was available. *p=0·014. †p=0·03. ‡p=0·035.
Table 2.
Change from baseline in the St George’s Respiratory Questionnaire−COPD score to week 56 (per-protocol population)*,†
| Overall | <200 cells per µL | ≥200 cells per µL | <300 cells per µL | ≥300 cells per µL | |
|---|---|---|---|---|---|
| Total | |||||
| Placebo | −4·43 (11·71; n=42) | −4·26 (10·17; n=21) | −4·79 (13·64; n=20) | −4·40 (11·04; n=34) | −5·11 (16·24; n=7) |
| Benralizumab | −5·51 (16·64; n=37) | −2·10 (16·47; n=20) | −9·52 (16·42; n=17) | −1·90 (14·97; n=25) | −13·03 (18·08; n=12) |
| p value | 0·739 | 0·614 | 0·345 | 0·463 | 0·354 |
| Symptom | |||||
| Placebo | −3·19 (17·44; n=42) | −5·56 (18·34; n=21) | −1·40 (16·79; n=20) | −3·88 (17·40; n=34) | −1·87 (19·35; n=7) |
| Benralizumab | −9·02 (21·27; n=37) | −3·22 (14·16; n=20) | −15·84 (26·24; n=17) | −3·40 (12·84; n=25) | −20·72 (30·00; n=12) |
| p value | 0·185 | 0·651 | 0·051 | 0·909 | 0·156 |
| Activity | |||||
| Placebo | −5·65 (14·85; n=42) | −5·09 (12·66; n=21) | −6·51 (17·44; n=20) | −6·06 (13·65; n=34) | −4·47 (21·78; n=7) |
| Benralizumab | −4·37 (24·89; n=37) | −3·96 (28·35; n=20) | −4·86 (20·94; n=17) | −1·91 (26·03; n=25) | −9·49 (22·52; n=12) |
| p value | 0·780 | 0·868 | 0·795 | 0·431 | 0·641 |
| Impacts | |||||
| Placebo | −4·16 (14·62; n=42) | −3·33 (14·95; n=21) | −4·99 (14·98; n=20) | −3·63 (14·77; n=34) | −6·61 (15·87; n=7) |
| Benralizumab | −4·95 (16·97; n=37) | −0·61 (17·04; n=20) | −10·06 (15·88; n=17) | −1·38 (16·57; n=25) | −12·40 (15·94; n=12) |
| p value | 0·826 | 0·589 | 0·324 | 0·585 | 0·455 |
Data are expressed as mean (SD; n). p values were calculated by ANCOVA. One patient in the placebo group did not have baseline blood eosinophil count, resulting in one patient less for subgroup analyses in this group. Not all patients in the per-protocol population had a change from baseline in St George’s Respiratory Questionnaire score across different timepoints.
In the per-protocol population, 54 (66%) patients (26 in the placebo group and 28 in the benralizumab group) had baseline blood eosinophil counts of 150 cells per µL or more, 39 (48%) patients (20 vs 19 patients) had counts of 200 cells per µL or more, and 21 (26%) patients (seven vs 14 patients) had counts of 300 cells per µL or more.
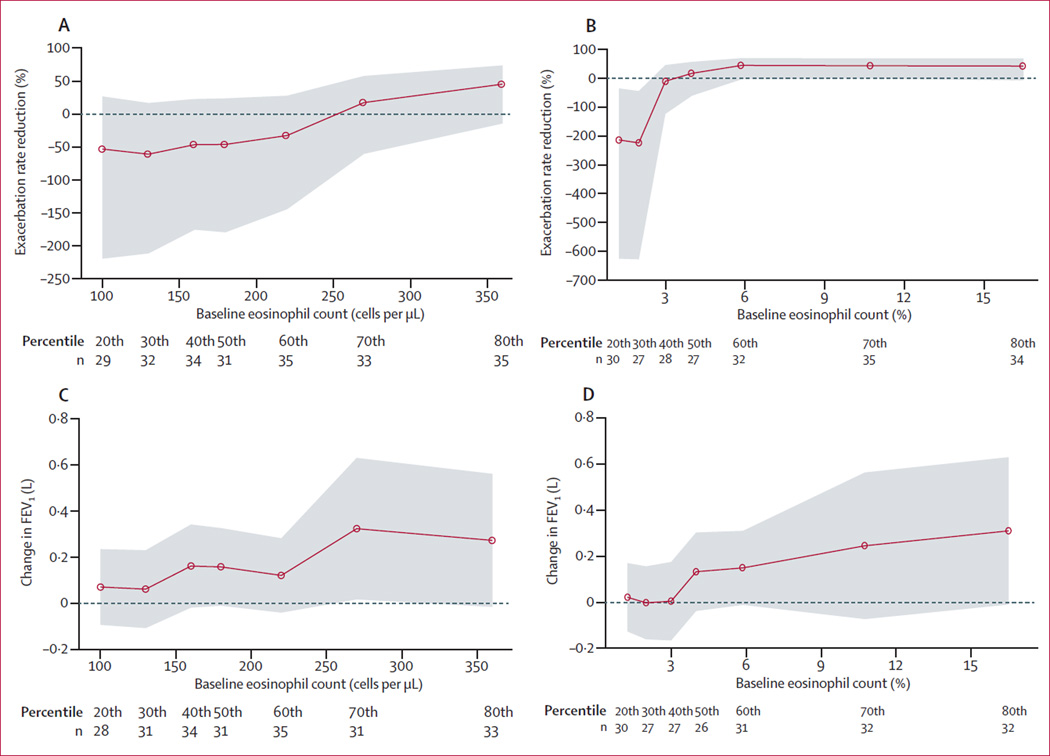
Reductions in rates of acute exacerbations with benralizumab were non-significant compared with placebo in patients with baseline eosinophil counts of 150 cells per uL or more (p=0·84), 200 cells per uL or more (p=0·26), or 300 cells per uL or more (p=0·28; figure 2). By contrast, patients with blood eosinophil counts of less than 150 cells per µL, 200 cells per µL or 300 cells per µL had a greater, non-significant, frequency of exacerbations with benralizumab than with placebo (exacerbation rate 1·02 vs 0·80, p=0·70 for <150 cells per µL; 1·15 vs 0·70, p=0·27 for <200 cells per µL; and 1·25 vs 0·87, p=0·28 for <300 cells per µL). Improvements from baseline in pre-bronchodilator FEV1 at week 56 with benralizumab compared with placebo were significant in patients with blood eosinophil counts of 150 cells per µL or more (p=0·031; appendix) or 200 cells per µL or more (p=0·035), and non-significant in those with counts of 300 cells per µL or more (p=0·22; figure 4). These findings were confirmed by STEPP analysis, which showed a greater numerical reduction in rate of acute exacerbations and FEV1 improvements with benralizumab in patients with higher baseline blood eosinophil counts (figure 5).
Figure 5. Subpopulation treatment effect pattern plots by blood and sputum eosinophil cutoffs.
Rate reduction of acute exacerbations of chronic obstructive pulmonary disease by blood (A) and sputum eosinophils (B), and FEV1 by blood (C) and sputum eosinophils (D). Each subpopulation contains roughly 40% of the patients with eosinophil counts around the selected cutoff; cutoffs correspond to the 20th, 30th, 40th, 50th, 60th, 70th, and 80th percentiles. Data below panels show number of patients in each percentile. Y axes show the difference in measure between between benralizumab and placebo. Shaded areas represent 95% CIs. FEV1=forced expiratory volume in 1 second.
We recorded numerical, although not statistically significant, improvements from baseline in both mean SGRQ-C and CRQ-SAS scores in the benralizumab group compared with the placebo group in patients with blood eosinophil counts of 200 cells per µL or more and 300 cells per µL or more (table 2, appendix). Effects were less evident in patients with blood eosinophil counts of 150 cells per µL or more (appendix). We did not analyse BODE index by peripheral blood eosinophil subgroups.
Post-hoc efficacy analyses by baseline sputum eosinophil cutoffs of 3·0% and 10·0% showed a positive association between changes in acute exacerbations, FEV1, SGRQ-C, and intensity of sputum eosinophilia (appendix). STEPP analysis showed greater reductions in rates of acute exacerbations and FEV1 improvements in patients in the benralizumab group than in those in the placebo group, with higher concentrations of sputum eosinophils (figure 5).
Little difference between groups was noted in the total number of treatment-emergent adverse events, the proportion of patients who reported at least one treatment-emergent adverse event, and events with an incidence of 5% or greater (table 3). Although we recorded a higher incidence of serious treatment-emergent adverse events in patients in the benralizumab group than in those in the the placebo group, none of these events were considered by the investigator to be benralizumab related. Two (4%) patients in the benralizumab group died during the study (one myocardial infarction and one sudden death of unknown cause); however, neither death was considered to be related to benralizumab. One (2%) patient in the placebo group died from bronchitis after study completion. No hypersensitivity reactions or immune complex diseases related to treatment were reported.
Table 3.
Summary of treatment-emergent adverse events (safety population)
| Placebo group (n=50) |
Benralizumab group (n=51) |
|
|---|---|---|
| Number of treatment-emergent adverse events | 240 | 282 |
| Patients reporting one or more treatment-emergent adverse event | 41 (82%) | 45 (88%) |
| Number of treatment-emergent serious adverse events | 16 | 25 |
| Patients reporting one or more treatment-emergent serious adverse event |
9 (18%) | 14 (27%) |
| Patients with treatment-emergent adverse events leading to discontinuation |
2 (4%) | 3 (6%) |
| Treatment-emergent adverse events by system organ class recorded in 5% or more patients in the benralizumab group* | ||
| Respiratory, thoracic, and mediastinal disorders | 31 (62%) | 32 (63%) |
| Infections and infestations† | 28 (56%) | 27 (53%) |
| Musculoskeletal and connective tissue disorders | 10 (20%) | 16 (31%) |
| General disorders and administration-site disorders | 4 (8%) | 13 (25%) |
| Nervous-system disorders | 6 (12%) | 10 (20%) |
| Investigations‡ | 4 (8%) | 10 (20%) |
| Gastrointestinal disorders | 14 (28%) | 9 (18%) |
| Skin and subcutaneous-tissue disorders | 3 (6%) | 9 (18%) |
| Injury, poisoning, and procedural complications | 6 (12%) | 7 (14%) |
| Cardiac disorders | 2 (4%) | 7 (14%) |
| Vascular disorders | 6 (12%) | 5 (10%) |
| Metabolism and nutrition disorders | 5 (10%) | 3 (6%) |
| Renal and urinary disorders | 2 (4%) | 3 (6%) |
| Reproductive system and breast disorders | 1 (2%) | 3 (6%) |
Data are n or n (%).
Patients were counted only once for each system organ class, irrespective of how many events they had.
Includes infections and infestations of any organ system.
Covers changes to clinical laboratory results, including in C-reactive protein, rates of erythrocyte sedimentation, and weight.
Discussion
To our knowledge, this study is the first of a biological treatment for eosinophilic COPD. Consistent with a previous study in the asthma setting,14 benralizumab rapidly depleted both sputum and blood eosinophils in patients with COPD to a much greater extent than did inhaled or oral corticosteroids in other studies,5,26 and was reversible after treatment washout. The primary endpoint in this study was not met because benralizumab did not reduce the rate of acute exacerbations of COPD compared with placebo in the per-protocol population, nor did it attenuate symptoms or health-related quality of life. However, benralizumab did provide clinically significant (≥100 mL) improvements in lung function, determined by pre-bronchodilator and post-bronchodilator FEV1, with improvements sustained up to week 80, 32 weeks after the last dose. In the pre-specified and post-hoc analyses, non-significant improvements in acute exacerbations of COPD, FEV1, and SGRQ-C were greater in benralizumab-treated patients with raised concentrations of peripheral blood and sputum eosinophils. Because improvements of 100 mL or more in FEV1, or of 4 points or more in SGRQ-C, are considered clinically significant,27 the statistically non-significant improvements recorded for these measures might represent real-world beneficial effects in these populations. Thus, to our knowledge, benralizumab is the first anti-interleukin-5 biological drug to show relevant reduction in eosinophilic inflammation and potentially beneficial clinical effects in subgroups of COPD patients.
Previous studies in patients with COPD have shown that eosinophilic airway inflammation is associated with acute exacerbations of COPD4,6 and can predict a beneficial response to treatment with corticosteroids.5,26,28,29 Additionally, treatment with inhaled and oral corticosteroids to reduce the proportion of sputum eosinophils in COPD has been associated with a reduction in the frequency of severe exacerbations (panel).6 Therefore, we postulated that patients with a sputum eosinophil count of 3·0% or more would respond to benralizumab.
To enrich our study, we recruited patients with evidence of sputum eosinophilia either at, or in the 12 months before, screening. However, by contrast with studies of patients with severe asthma,12,30 our findings do not show reductions in acute exacerbations of COPD. This difference might be explained by our small sample size, a less important role of eosinophils in exacerbations of COPD than in asthma, or that our study design failed to adequately identify patients with persistent sputum eosinophilic inflammation. The challenge of obtaining adequate and reliable sputum samples in a clinical setting emphasises the need for alternative biomarkers of eosinophilic inflammation. Furthermore, despite our use of historic or screening evidence of a sputum eosinophil count of 3·0% or more, only about two-thirds of patients had sputum eosinophilia at this concentration at baseline. The reproducibility of testing for sputum eosinophils in the asthma setting has been questioned in the past,31 and our calculations showed that testing for sputum eosinophilia between historical and day 1 samples was repeatable in almost 70% of patients. Our protocol-standardised inhaled corticosteroid treatment might have affected the repeatability of the sputum eosinophil count. We recorded the greatest improvements in response to benralizumab in patients with evidence of eosinophilic inflammation at baseline; therefore, the inclusion of some individuals on the basis of historic sputum eosinophilia alone probably diminished the likelihood of significant benefits being shown.
Although the most sensitive and specific measure of sputum eosinophilia at exacerbation is proportion of peripheral blood eosinophil cells,4 the association between sputum and blood eosinophils in patients with stable COPD has not been well described. Evidence has shown that baseline blood eosinophil counts predict response to anti-interleukin-5 monoclonal antibody treatments in patients with severe asthma12,30,32 and can be used to effectively direct corticosteroid treatment in patients with COPD.33,34 Although early studies were unable to show upregulation of interleukin-5 in the bronchial mucosa in patients with COPD,35,36 with use of sensitive platforms, investigators have shown that interleukin-5 can be detected both in sera and sputum of patients with COPD.4,33,37 On the basis of these data and the mechanism of action of benralizumab, with effective reduction in peripheral blood eosinophils, we also prespecified and assessed an alternative hypothesis that benralizumab would be effective in patients with COPD with baseline blood eosinophil counts of 150 or more, 200 or more, and 300 or more cells per µL. The aim of this prespecified analysis was to establish whether blood, rather than sputum, eosinophils were a better predictive biomarker of benralizumab response in patients with COPD. These results should be interpreted with caution because the numbers of patients in the subgroups were small. Nonetheless, our findings suggested that benralizumab could have had an effect on exacerbations, health status, symptoms, and lung function in patients with higher baseline peripheral blood eosinophil counts of 200 or more and 300 or more cells per µL. Similarly, post-hoc analyses showed outcomes favouring benralizumab versus placebo in patients with higher counts of sputum eosinophil cells. These findings might be the result of sustained eosinophilic airway inflammation over time, or an increased inflammatory state amenable to benralizumab intervention. By contrast, exacerbations seemed to be increased in patients with lower blood or sputum eosinophil counts. This finding might be due to several patients having repeated exacerbations, or because reducing eosinophil concentrations in patients with already low baseline eosinophil counts might result in increased susceptibility to infectious exacerbations. Inhaled corticosteroids, which also modulate the immune system, increase the risk of pneumonia,38 and COPD exacerbations associated with a low peripheral blood eosinophil count have a poorer outcome after treatment with oral corticosteroids than with placebo.33 Thus, blood eosinophil counts might identify a subpopulation for which benralizumab could have potential deleterious effects, and a subpopulation with persistent or present blood eosinophilia that benefits from benralizumab.
By contrast with our finding suggesting that benralizumab might have a clinically important effect on lung function in patients with COPD, studies with the anti-interleukin-5 monoclonal antibody mepolizumab have been unable to show a benefit in lung function in people with asthma, although this strategy reduced asthma exacerbations.10,12 In people with asthma, both benralizumab30 and reslizumab32 improved FEV1. Thus, the effects noted for COPD in our study are consistent with findings reported for asthma. Whether the scarcity of response in lung function to mepolizumab in patients with severe asthma shows differences in the pharmacology between these biological treatments or is a consequence of different patient populations is unclear; additionally, whether mepolizumab and benralizumab have different effects in COPD needs to be addressed.
Our study has several limitations. First, the study was underpowered because the exacerbation rate assessed was lower than two per year. The study was also not powered for the subgroup analyses done. Furthermore, recruited patients were selected for inclusion by sputum eosinophilia, but prespecified analyses were based on peripheral blood eosinophils. Although what the treatment effect would be in patients with discordant concentrations of sputum and blood eosinophils is unclear, our post-hoc analyses by sputum eosinophils seemed to confirm the results of the prespecified analyses. Despite this finding and the marked reductions in sputum and blood eosinophils, patients in the benralizumab group still had exacerbations, suggesting that not all acute exacerbations of COPD are related to eosinophilia. However, we did not investigate possible pathogenic causes of exacerbation events. Moreover, not all patients were taking inhaled corticosteroids, which are well known to reduce concentrations of sputum eosinophils in COPD5 and asthma.39 This diversity in patient population could have made the clinical effect of benralizumab seem greater than it actually is.
The safety profile was similar to that noted in previous studies of subcutaneous benralizumab in patients with asthma.14,30 However, serious adverse events were reported by a slightly higher proportion of patients in the benralizumab group than in the placebo group, although none were considered to be related to treatment. These findings need to be extended to larger studies.
To our knowledge, this study is the first to provide evidence suggesting effectiveness of treatment with a biological compound that affects eosinophils in reducing exacerbations and improving other functional abnormalities in specific groups of patients with COPD. These findings support further clinical development of benralizumab in patients with eosinophilic COPD, and phase 3 studies are underway (ClinicalTrials.gov, numbers NCT02155660 and NCT02138916) to assess the effect of benralizumab on exacerbation reduction and concentrations of blood eosinophils.
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed with the terms “COPD”, “chronic obstructive pulmonary disease”, “interleukin 5” and “eosinophil” for articles published up to Aug 7, 2014, with no restrictions. We did not identify any studies of anti-interleukin-5 monoclonal antibody treatment in patients with chronic obstructive pulmonary disease (COPD). CEB was an investigator in Siva and colleagues’ 2007 study,6 which showed that a treatment strategy to titrate corticosteroid treatment, with an aim to normalise the sputum eosinophil count, reduced acute exacerbations of COPD. Furthermore, similar findings in patients with eosinophilic asthma prescribed anti-interleukin-5 monoclonal therapy also showed positive findings with respect to reductions in exacerbations. We therefore postulated that reduction of sputum eosinophils with an anti-interleukin-5 antibody would reduce exacerbations in patients with COPD and eosinophilia. We now know that eosinophils play a large part in the pathogenesis of COPD, because up to 30% of patients with COPD have sputum eosinophilia; however, at the time the study commenced, little data were available in this area. In the intervening period, more data have been published, and are referenced in this manuscript.
Interpretation
Our findings confirm that in a subgroup of patients with COPD with raised concentrations of eosinophils, a proportion seem to respond to treatments that reduce the numbers of these cells—ie, treatments that target interleukin-5. Benralizumab might improve lung function and reduce exacerbations in this patient population; however, improvements recorded in our trial were not significant. On the basis of our findings, phase 3 studies have been started to investigate further and establish whether benralizumab can significantly improve lung function and reduce exacerbations in these patients.
Acknowledgments
Declaration of interests
CEB received a grant for this study from MedImmune, and grants outside the submitted work from GlaxoSmithKline, Roche, Novartis, and Chiesi. ERB has received grants outside the submitted work from SARP, AsthmaNet, Spiromics, and Pharmacogenetics; has been involved in clinical trials through his institution for Amgen, MedImmune–AstraZeneca, Boehringer Ingelheim/Pfizer, Forest, Genentech–Roche, GlaxoSmithKline, Janssen–Johnson & Johnson, Novartis, Sanofi, and Teva; and has received personal consultancy fees from Amgen, MedImmune–AstraZeneca, Boehringer Ingelheim–Pfizer, Forest, Genentech–Roche, GlaxoSmithKline, Merck, Novartis, and Regeneron–Sanofi. RAP has received grants outside the submitted work from AstraZeneca, Gilead, Johnson & Johnson, Merck, Roche, Sanofi, and the National Institutes of Health; and personal fees from AstraZeneca, Gilead, Johnson & Johnson, Merck, and Teva. MB has received travel reimbursement outside the submitted work from Almirall, Boehringer Ingelheim, and GlaxoSmithKline. XX is an employee of AstraZeneca. DS, CKW, CB, and RvdM are employees of MedImmune and hold AstraZeneca shares. CKW has a patent pending to MedImmune.
This study was funded by MedImmune. CEB was funded by the Wellcome Trust (senior clinical fellowship). CEB and MB were funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, or the Department of Health. We thank the investigators and staff at the study sites; the patients who participated in this study; Diana Swanson (AstraZeneca), Sherahe Fitzpatrick (MedImmune), and Sophie Walton (QXV Communications, Macclesfield, UK) for assistance with manuscript preparation; Sonia Matadial (AstraZeneca) and Jacqueline Mazza (MedImmune) for management of study delivery; and Janice Chen, Deling Niu (both MedImmune), and the rest of the statistical programming team for data analysis.
Footnotes
See Online for podcast interview with Leonardo M Fabbri
See Online for appendix For the study protocol see http://www.astrazenecaclinicaltrials.com/Submission/View?id=44
Contributors
CEB contributed to study design; data collection, analysis, and interpretation; and manuscript preparation and review. ERB contributed to data analysis and interpretation, and manuscript preparation and review. RAPJr contributed to protocol assessment, data analysis and interpretation, and manuscript preparation and review. MB contributed to study design; data collection, analysis, and interpretation; and manuscript preparation and review. XX contributed to study design, data analysis and interpretation, and manuscript review. DS and CKW contributed to study design, data analysis and interpretation, and manuscript preparation and review. CeB contributed to data cleaning and analysis, and manuscript review. RvdM contributed to study design and set up; data cleaning, analysis, and interpretation; and manuscript preparation and review.
References
- 1.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of COPD. [accessed March 19, 2014];2014 Jan; http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. [Google Scholar]
- 3.Seemungal TA, Hurst JR, Wedzicha JA. Exacerbation rate, health status and mortality in COPD–a review of potential interventions. Int J Chron Obstruct Pulmon Dis. 2009;4:203–223. doi: 10.2147/copd.s3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 5.Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356:1480–1485. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 6.Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29:906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 7.Barker BL, Brightling CE. Phenotyping the heterogeneity of chronic obstructive pulmonary disease. Clin Sci. 2013;124:371–387. doi: 10.1042/CS20120340. [DOI] [PubMed] [Google Scholar]
- 8.Kolsum U, Brightling CE, Agusti A, Locantore N, Tal-Singer R, Singh D. The prevalence and clinical characteristics associated with eosinophilic inflammation in COPD patients from The ECLIPSE (Evaluation Of COPD Longitudinally To Identify Predictive Surrogate Endpoints) cohort. Am J Respir Crit Care Med. 2014;189:A2745. [Google Scholar]
- 9.Takatsu K, Takaki S, Hitoshi Y. Interleukin-5 and its receptor system: implications in the immune system and inflammation. Adv Immunol. 1994;57:145–190. doi: 10.1016/s0065-2776(08)60673-2. [DOI] [PubMed] [Google Scholar]
- 10.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 12.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 13.Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125:1237–1244. doi: 10.1016/j.jaci.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132:1086–1096. doi: 10.1016/j.jaci.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 16.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 17.Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George Respiratory Questionnaire. Chest. 2007;132:456–463. doi: 10.1378/chest.06-0702. [DOI] [PubMed] [Google Scholar]
- 18.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42:773–778. doi: 10.1136/thx.42.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams JE, Singh SJ, Sewell L, Guyatt GH, Morgan MD. Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR) Thorax. 2001;56:954–959. doi: 10.1136/thorax.56.12.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast--an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 22.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respir Med. 2008;102:1099–1108. doi: 10.1016/j.rmed.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6:320–329. doi: 10.1080/15412550903140881. [DOI] [PubMed] [Google Scholar]
- 25.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHBLI/WHO workshop report. [accessed March 19, 2014];2001 Mar; http://www.goldcopd.org/uploads/users/files/GOLDWkshp2001.pdf. [Google Scholar]
- 26.Brightling CE, McKenna S, Hargadon B, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60:193–198. doi: 10.1136/thx.2004.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189:250–255. doi: 10.1164/rccm.201310-1863PP. [DOI] [PubMed] [Google Scholar]
- 28.Pizzichini E, Pizzichini MM, Gibson P, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1998;158:1511–1517. doi: 10.1164/ajrccm.158.5.9804028. [DOI] [PubMed] [Google Scholar]
- 29.Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27:964–971. doi: 10.1183/09031936.06.00072105. [DOI] [PubMed] [Google Scholar]
- 30.Castro M, Gossage DL, Ward CK, et al. Benralizumab reduces exacerbations and improves lung function in adults with uncontrolled eosinophilic asthma. Am J Respir Crit Care Med. 2014;189:A3699. [Google Scholar]
- 31.Al-Samri MT, Benedetti A, Prefontaine D, et al. Variability of sputum inflammatory cells in asthmatic patients receiving corticosteroid therapy: A prospective study using multiple samples. J Allergy Clin Immunol. 2010;125:1161–1163. doi: 10.1016/j.jaci.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 33.Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bafadhel M, Davies L, Calverley PM, Aaron SD, Brightling CE, Pavord ID. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J. 2014 doi: 10.1183/09031936.00062614. published online June 12. [DOI] [PubMed] [Google Scholar]
- 35.Saetta M, Di SA, Maestrelli P, et al. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;150:1646–1652. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- 36.Saetta M, Di SA, Maestrelli P, et al. Airway eosinophilia and expression of interleukin-5 protein in asthma and in exacerbations of chronic bronchitis. Clin Exp Allergy. 1996;26:766–774. [PubMed] [Google Scholar]
- 37.Bafadhel M, Saha S, Siva R, et al. Sputum IL-5 concentration is associated with a sputum eosinophilia and attenuated by corticosteroid therapy in COPD. Respiration. 2009;78:256–262. doi: 10.1159/000221902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68:1029–1036. doi: 10.1136/thoraxjnl-2012-202872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djukanovic R, Wilson JW, Britten KM, et al. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. Am Rev Respir Dis. 1992;145:669–674. doi: 10.1164/ajrccm/145.3.669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.