Abstract
The human visual system prioritizes processing of novel information, leading to faster detection of novel stimuli. Novelty facilitates conflict resolution through the enhanced early perceptual processing. However, the role of novel information processing during the conflict-related response selection and inhibition remains unclear. Here, we used a face-gender classification version of the Simon task and manipulated task-difficulty and novelty of task-relevant information. The novel quality of stimuli was made task-irrelevant, and an in-group bias was tightly controlled by manipulation of a gender of picture stimuli. We found that the in-group bias modulated the role of novelty in executive control. Novel opposite-sex stimuli facilitated response inhibition only when the task was not demanding. By contrast, novelty enhanced response selection irrespective of the in-group factor when task-difficulty was increased. These findings support the in-group bias mechanism of visual processing, in cases when attentional resources are not limited by a demanding task. The results are further discussed along the lines of the attentional load theory and neural mechanisms of response-inhibition and locomotor activity. In conclusion, our data showed that processing of novel information may enhance executive control through facilitated response selection and inhibition.
Introduction
Flexible behavioral control is an ability to track and respond to salient changes in a dynamic environment. Indeed, humans rapidly detect and evaluate novel stimuli, irrespective of its task-relevance [1–4]. Processing of novelty seems to have a certain priority in the brain; novel information attracts attention and elicits an orienting reflex [1, 2]. In line with this hypothesis, evidence suggests that novelty facilitates early visual processing and cognitive control [3, 4]. For instance, in a modified Stroop task, Krebs and colleagues [3] showed that novel information attenuates semantic interference, speeds up conflict processing, and enhances visual perception [4].
By contrasts, the role of novel information in response selection and inhibition is less clear. Recent evidence suggests that cognitive system employs partially dissociable control mechanisms to resolve conflicts at the early visual processing and response selection stages [5, 6]. In the Stroop task, conflicts are mainly resolved by enhanced processing of the task-relevant information during the early visual processing [7, 8]. Alternatively, in the Simon task, conflicts are resolved by inhibition of the task-irrelevant information during the later response selection stage [9, 10]. Previous studies reported motor slowing after observing an unexpected novel event [11]. Participants were instructed to verbally report one of the two target letters presented on the screen. Prior to the target, a short sound was delivered to participants via the headphones. The results of the study showed greater motor slowing after novel sounds (20% of all trials) relative to familiar sounds (80%). However, the question remains whether novel task-irrelevant component of the target stimulus would influence response inhibition when presented within trials, relative to when presented prior to the target [11]. Additionally, it is not clear whether the influence of novelty on motor inhibition comes from the perceptual saliency of novel objects relative to the surprise value of a stimulus, as novel stimuli usually compose 1/5 of all trials.
The current study investigated the influence of novelty on the response selection and inhibition during a Simon task. Participants were presented with a male or female picture (face) displayed either to the left or the right of the fixation cross. Participants were asked to make a decision whether they saw a female or male picture by pressing either the right or left-hand button. In the congruent condition, the picture occurred in the same relative location as the response, while in the incongruent condition the picture and response were in conflict. Also, half of the pictures were familiarized prior to the experiment yielding novel and familiar pictures (picture novelty), which however was task-irrelevant. Previous studies showed that participants automatically code the direction of perceived gaze, although completely irrelevant to the task [12]. Therefore, all the face stimuli in the current study looked straight ahead to avoid the bias.
Furthermore, there is some evidence for the existence of an in-group bias effect when participants tend to pay more attention to and remember the faces of their gender [13–15]. Therefore, half of the stimuli were pictures of males and the other half were female images. This resulted in a 2 (congruent, incongruent) by 2 (novel, familiar) by 2 (male pictures, female pictures) factorial design with a gender of participants as a between-subject factor. Importantly, in line with previous studies [3], novel and familiar pictures occurred with equal probability to ensure that the effect of stimulus novelty is driven by perceptual saliency itself [16, 17] rather than by the mere surprise value of an event [18].
We hypothesized that novel stimuli would facilitate response selection, similar to facilitation of semantic interference [3]. Alternatively, salient task-irrelevant novel stimuli could have a detrimental effect by drawing attention away from the task-relevant information, similar to other task-irrelevant salient stimuli; for example, negative emotions [19]. Additionally, we hypothesized that the in-group pictures would elicit stronger effects in either facilitating or inhibiting response-selection, due to their high saliency and biological relevance [13].
Experiment 1
Materials and Methods
Participants and experimental procedure
Forty healthy right-handed individuals (mean age: 21.4 years; range: 19–24 years; 20 males) completed a Simon task. All participants were recruited from the student population of the North South University, Dhaka, Bangladesh. Written informed consent was obtained prior to experiment from all of the participants. The experimental protocol was approved by the ethical committee of the School of Health and Life Sciences, North South University.
The stimuli were composed of pictures taken from the database of adult facial stimuli [20] as well as pictures of Bangladeshi males and females (the database was manually collected at North South University). Prior to the testing session, participants completed a familiarization task, in which half of the picture stimuli (80 pictures) were repeatedly presented in random order (four times each) intermixed with 40 non-repeat pictures that were not used in the Simon task [3]. Each picture was presented for 1500 ms with a variable stimulus-onset asynchrony between 1000 and 2000 ms. To ensure that participants looked at all pictures during the familiarization session, we asked to indicate for each picture presentation whether they had seen the current picture before or not by pressing a button (>95% of pictures were reported as familiar after the fourth presentation). All pictures were presented in the center of a grey screen (visual angle 9 × 6°).
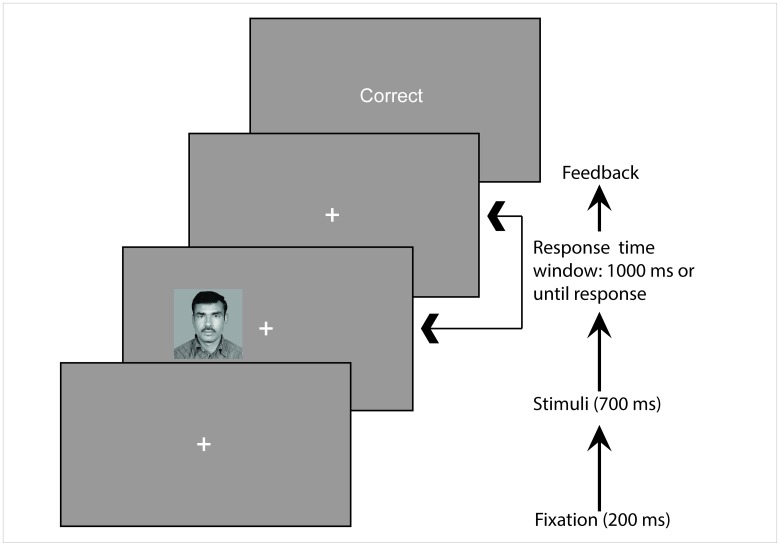
During the Simon task (Fig 1), the 80 familiarized (50% male) and 80 completely novel (50% male) male and female pictures were displayed once in random order for 700 ms each on either the left (x = 0.02°, y = 0.36°) or the right (x = 0.82°, y = 0.36°) side of the fixation cross (Fig 1). For the stimulus presentation, we used PychoPy stimulus delivery software [21]. In each trial, the side of the stimulus could be either congruent (50%) or incongruent (50%) with the response hand. This resulted in a 2 (congruent, incongruent) by 2 (novel, familiar) by 2 (female, male pictures) factorial design. Gender of participants was a between-subject factor. Participants were instructed to attend to the picture and to decide as quickly as possible whether they saw a male or a female picture by responding with either left or right index finger (counterbalanced across participants). Importantly, the familiarity/novelty manipulation of the pictures was entirely irrelevant to the task. Throughout the experiment, a small fixation cross was visible in the center of the screen and participants were instructed to maintain accurate fixation.
Fig 1. Example of a trial sequence.
Each trial started with a fixation cross that was present throughout the whole trial. Afterwards, the target display was briefly presented and participants decided via button press if they saw a male or a female picture. Each trial ended with a visually presented performance feedback.
Data Analysis
The reaction time and error rates were split according to conditions and submitted to the repeated-measures ANOVA using the R software [22]. The RT below and over the 2.5 standard deviations (SD) from the mean were excluded from further analysis, which resulted in < 2% of data excluded from analysis. Importantly, the excluded data did not influence the results of statistical analysis. Finally, only statistically significant main effects and interactions that involve the critical factors of novelty and congruence are reported in the results section.
Results
RT
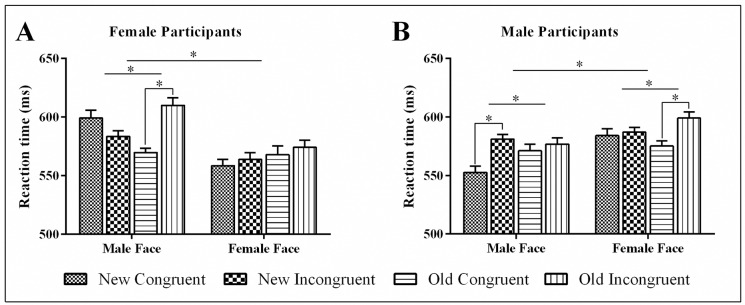
We observed a four-way interaction of novelty by congruence by gender of the picture by gender of participants (F(1, 38) = 26.91, p < 0.001, = 0.415) and resolved it by participants’ gender (Fig 2). In female participants, novelty influenced cognitive control in the pictures of male (F(1, 19) = 23.14, p < 0.001, = 0.549) but not female stimuli (F(1, 19) = 0.005, p > 0.9, = 0.000). We found that novel male stimuli facilitated cognitive control by reducing the interference effect (F(1, 19) = 2.59, p > 0.1, = 0.120) relative to the familiar male pictures (F(1, 19) = 27.67, p < 0.001, = 0.593).
Fig 2. RT data.
The figure represents RT data to congruent and incongruent stimuli as a function of novelty, gender of stimuli and gender of participants. The conflict effect is smaller for familiar compared to novel pictures of an opposite gender. For the same-gender pictures, novelty either impeded conflict processing (male participants) or had no effect on conflict processing (female participants).
In male participants, novelty influenced cognitive control in both male pictures (F(1, 19) = 7.80, p < 0.02, = 0.291) and female pictures (F(1, 19) = 6.98, p < 0.02, = 0.269). Novel male pictures inhibited cognitive control by increasing the RT conflict effect (F(1, 19) = 15.72, p < 0.001, = 0.453) relative to the familiar male pictures (F(1, 19) = 0.378, p > 0.5, = 0.019). On the other hand, female novel pictures facilitated cognitive control by reducing the RT conflict effect (F(1, 19) = 0.148, p > 0.7, = 0.008) relative to the familiar female pictures (F(1, 19) = 9.54, p < 0.01, = 0.334).
Error rate
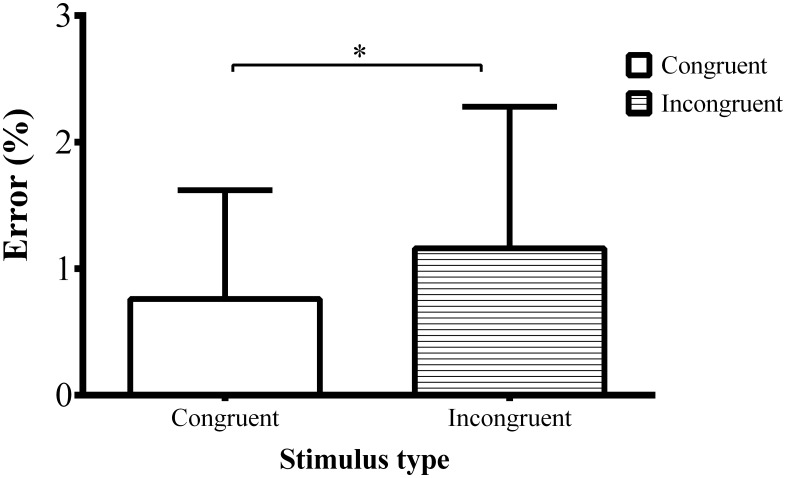
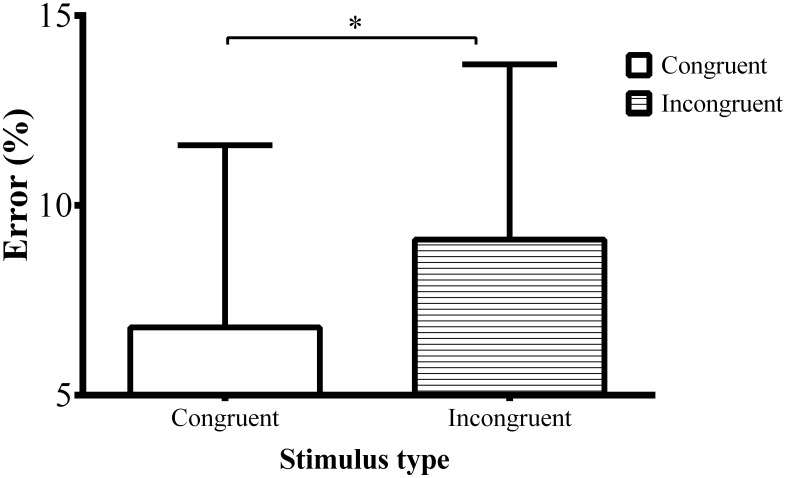
Incongruent stimuli produced more errors relative to congruent stimuli (Fig 3; F(1, 38) = 14.47, p < 0.01, = 0.276).
Fig 3. Error rate.
The figure represents error rate to congruent and incongruent stimuli. The conflict effect is larger for the own-race stimuli.
The results showed that novelty facilitated response inhibition in the opposite gender stimuli. However, as the task was easy (error rate ~ 2%), the performance was already at ceiling and the effect of novelty might have not been as pronounced. In other words, due to the ease of the task used in the current set of studies, the cognitive task may not have been demanding enough, and the influence of novelty could also be minimized. Therefore, we conducted Experiment 2 and made the task more demanding to ensure higher error rates.
Experiment 2
Participants and experimental procedure
Twenty healthy right-handed individuals (mean age: 22.6 years; range: 19–25 years; 10 males) naïve to the purpose of the study participated in Experiment 2. All participants were recruited from the student population of the North South University, Dhaka, Bangladesh. Written informed consent was obtained before the experiment from all of the participants. The experimental protocol was approved by the ethical committee of the School of Health and Life Sciences, North South University.
The familiarization and experimental procedure were identical to Experiment 1. Additionally, we reduced and randomly varied the presentation duration of stimuli (i.e., 200, 250 and 300 ms).
Data Analysis
The data analysis procedure was identical to Experiment 1. Only statistically significant main effects and interactions that involve the critical factors of novelty and congruence are reported in the results section.
Results
RT
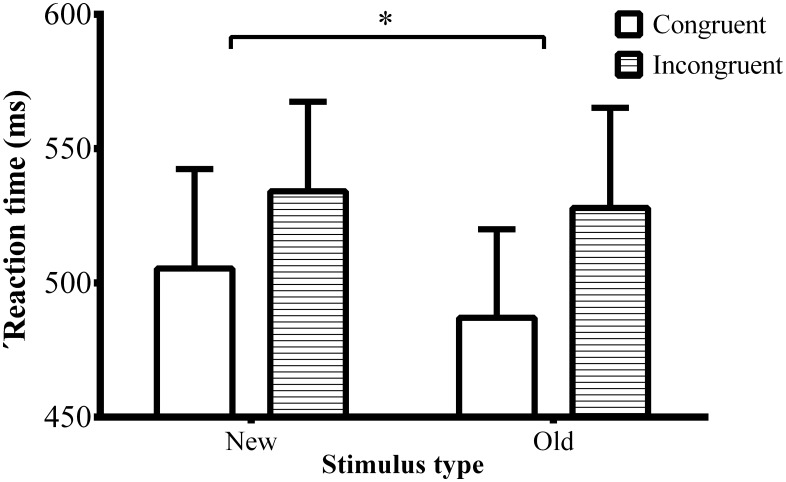
Novel stimuli elicited speeded responses relative to familiar stimuli (Fig 4; F(1, 18) = 13.734, p < 0.002, = 0.433). Additionally, incongruent stimuli took longer to be processed relative to congruent stimuli (F(1, 18) = 42.309, p < 0.001, = 0.702). Finally, we observed an interaction of novelty by congruence (F(1, 18) = 5.03, p < 0.04, = 0.218). Participants were faster to process the incongruence in novel stimuli (F(1, 18) = 17.067, p < 0.001, = 0.487) relative to familiar stimuli (F(1, 18) = 70.799, p < 0.001, = 0.797). However, the four-way interaction of novelty by congruence by gender of the picture by gender of participants was not significant anymore (F(1, 18) = 3.51, p > 0.05, = 0.163).
Fig 4. RT data.
The figure represents RT data to congruent and incongruent stimuli as a function of novelty. The conflict effect is smaller for familiar compared to novel pictures.
Error rate
Incongruent stimuli resulted in larger number of erroneous responses relative to congruent stimuli (Fig 5; F(1, 18) = 15.999, p < 0.001, = 0.471).
Fig 5. Error rate.
The figure represents error rate to congruent and incongruent stimuli. The conflict effect is larger for incongruent stimuli.
In summary, Experiment 2 showed that novelty facilitated response selection and inhibition. Importantly, this facilitation seems to be independent of an in-group bias when the task is demanding.
Discussion
We studied the role of novelty in response selection and inhibition during the cognitive conflict processing. We also determined the role of an in-group bias (gender) in response inhibition and conflict processing as a function of task difficulty. Three main findings emerged: First, participants responded slower and made more errors in incongruent compared to congruent trials. Second, participants responded faster and produced fewer errors when processing pictures of their gender. Third, novel stimuli facilitated response inhibition in the opposite gender stimuli, while novel pictures of the own gender either impeded response inhibition (male participants) or had no influence on response inhibition (female participants). By contrasts, novelty facilitated response inhibition independently of the in-group factor when the task difficulty level was increased.
We replicated previous findings: participants took longer and made more errors to process incongruent than congruent trials [23]. Conflict processing is characterized by increased competition for attentional resources during response selection and inhibition, which prolongs reaction times and increases error rates [24].
We found an own-gender bias effect. We observed that participants were faster and more efficient to process pictures of their gender. Current results are consistent with the previous findings that showed female participants to have biased processing of female than male faces [14]. Notably, previous studies only observed the own-gender bias effect in female but not male participants. For example, female participants can remember more female faces than male faces [25]. In this context, our findings extend the knowledge of the previously observed own-gender bias to male participants (see also [26]). One of the possible explanations for this finding is that evolutionary it makes sense to recognize one’s competitors rather than possible mates [26, 27]. Further, studies have also shown that the same-gender compared to the opposite-gender role models are the most effective in advertisements [28], and the majority of photographs in the fashion magazines are of people of the same gender as the target audience [26]. In contrast to the previous studies, we tested the in-group bias using the cognitive control task, rather than a memory task. Further research may explain the role of the task in the own-gender bias effect.
Additionally, novel compared to familiar stimuli facilitated response inhibition and conflict processing in pictures of an opposite gender and either inhibited (male participants) or had no influence (female participants) on conflict processing in the same-gender pictures. Facilitated attentional allocation for novel compared to familiar pictures during early perceptual processes has been reported in the past [3, 4]. However, the present study has further elucidated the role of novelty in response selection and inhibition, as well as an in-group bias.
Importantly, the in-group bias effect was absent when task-difficulty was increased. As the task in Experiment 1 was not demanding (error rate ~ 2%), attentional resources could be additionally allocated elsewhere and, thus, bias the role of novelty in response selection and inhibition. For instance, studies reported enhanced neural responses to salient emotional compared to neutral stimuli [29–31]. However, such biased processing of emotions was diminished when the corresponding task difficulty was increased. In other words, scarce attentional resources may be preferentially allocated to salient, although task-irrelevant, information when the task is not demanding. However, as the task-difficulty is increased (error rate ~ 10%), attentional resources are redistributed to focus on the task, reducing attention interference by other factors (e.g., stimulus gender).
The present findings are consistent with the attentional load theory [32]. The theory postulates that distracter interference is reduced under the condition of high perceptual load. However, unlike in the attentional load theory, gender of stimuli was not a distractor, but rather a task-irrelevant component of the target. Furthermore, Wessel and Aron [11] showed that unexpected novel events presented before target stimuli may induce motor slowing via a brain mechanism for action-stopping. This motor slowing becomes beneficial when the spatial target location and the corresponding response are in conflict, as people can easily overcome and inhibit prepotent response tendencies. Importantly, unlike the in-group factor in the current study and emotional information in previous studies [31], the effect of novelty remained when the task-difficulty was increased. This finding might suggest that emotion and novelty have a differential mechanism of impact on executive control.
Furthermore, it was also shown that the neural response to reward is not modulated by task-difficulty [33]. There seems to be a link between processing of novelty and reward; reward was shown to facilitate conflict processing in different versions of the Stroop task [3, 34]. For instance, Krebs and colleagues (37) showed that color-naming performance in the classical Stroop task was enhanced on trials with potential-reward versus those without, whereas incongruent reward-related information in a task-irrelevant dimension can impede task performance. The neural basis of the reward-related enhancement of cognitive control may rely on dopaminergic pathways that are known to be involved in both reward processing [35–38] and conflict processing [39, 40]. Interestingly, processing of novel information was also shown to activate dopaminergic pathways [41, 42]. Therefore, manipulating stimulus’ novelty may support response selection and inhibition through the dopaminergic pathways [3, 4].
Dopaminergic neurons have projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc). Both VTA and NAc play an important role in the regulation of locomotor activity [43]. Animal studies suggest that the integrity of the VTA and NAc circuits is required for the manifestation of novelty-induced motor activity [44]. There seems to exist a connection between novelty-induces dopamine circuits, VTA and NAc [45]. Consistent with these findings, our results show that novelty can modulate executive control by facilitating response selection and inhibition.
Simon task requires shielding of the response selection from the task-irrelevant information. It is possible that since same-gender pictures have processing advantage [26], novel same-gender pictures might attract attention [3], but away from the task-relevant information, and, thus impede conflict processing. On the contrary, this effect is reversed in the opposite-gender stimuli. Studies reported distinct underlying mechanisms or even different neural populations that code male and female faces [46]. Furthermore, infants categorize and process male and female faces differently with an advantage for the female faces [47]. The observed disadvantage with male faces implies a weaker representation of the male category, at least in babies whose primary caregiver is a female and especially during the first year of life [47, 48]. Possibly, this early exposure-related difference in gender processing may result in the gender-specific processing mechanism later in adulthood. However, this early exposure does not explain the opposite-gender pattern observed in the female group. It is also possible, that processing of novel opposite-gender pictures facilitates disengagement rather than engagement of attention from the task-irrelevant components, thus speeding up conflict processing. However, this question is beyond the scope of the current study and further studies are necessary to elucidate this point.
Several points may have potentially impacted our results. Previous studies showed that culture and state anxiety might shape the way people process facial and novel information [49–51]. However, we have only tested students in Bangladesh, which may limit interpretation of our results. Therefore, future studies should control for culture differences and state anxiety to investigate the role of the in-group bias, task-difficulty and novelty in response selection and inhibition.
Conclusion
Novel information results in a prioritized perceptual processing in the brain: it attracts attention and enhances visual perception. As a consequence, the novelty was shown to increase motivation, elicit exploratory behavior, and promote learning. However, the role of novelty in response selection and inhibition was less clear. Our results show that novelty can either facilitate or inhibit response inhibition, depending on the in-group bias effect. However, when the task is demanding, the influence of the in-group bias is neutralized.
Supporting Information
(XLSX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Sokolov E. N. (1963). Higher Nervous Functions—Orienting Reflex. Annual Review of Physiology, 25, 545–&. 10.1146/annurev.ph.25.030163.002553 [DOI] [PubMed] [Google Scholar]
- 2.Huang S, Belliveau JW, Tengshe C, Ahveninen J. Brain Networks of Novelty-Driven Involuntary and Cued Voluntary Auditory Attention Shifting. Plos One. 2012;7(8). ARTN e44062 10.1371/journal.pone.0044062. WOS:000308213600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs RM, Fias W, Achten E, Boehler CN. Picture novelty attenuates semantic interference and modulates concomitant neural activity in the anterior cingulate cortex and the locus coeruleus. Neuroimage. 2013;74:179–87. 10.1016/j.neuroimage.2013.02.027. WOS:000317441300019. [DOI] [PubMed] [Google Scholar]
- 4.Schomaker J, Meeter M. Novelty enhances visual perception. Plos One. 2012;7(12):e50599 10.1371/journal.pone.0050599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18(1):42–57. 10.1006/nimg.2002.1319. WOS:000180015200004. [DOI] [PubMed] [Google Scholar]
- 6.Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, et al. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res. 2002;13(3):427–40. . [DOI] [PubMed] [Google Scholar]
- 7.Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18(6):1475–84. 10.1093/cercor/bhm179 . [DOI] [PubMed] [Google Scholar]
- 8.Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8(12):1784–90. 10.1038/nn1594 . [DOI] [PubMed] [Google Scholar]
- 9.Sturmer B, Leuthold H. Control over response priming in visuomotor processing: a lateralized event-related potential study. Exp Brain Res. 2003;153(1):35–44. 10.1007/s00221-003-1579-1 . [DOI] [PubMed] [Google Scholar]
- 10.Sturmer B, Leuthold H, Soetens E, Schroter H, Sommer W. Control over location-based response activation in the Simon task: behavioral and electrophysiological evidence. J Exp Psychol Hum Percept Perform. 2002;28(6):1345–63. . [DOI] [PubMed] [Google Scholar]
- 11.Wessel JR, Aron AR. Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. J Neurosci. 2013;33(47):18481–91. Epub 2013/11/22. 10.1523/jneurosci.3456-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zorzi M, Mapelli D, Rusconi E, Umilta C. Automatic spatial coding of perceived gaze direction is revealed by the Simon effect. Psychon Bull Rev. 2003;10(2):423–9. . [DOI] [PubMed] [Google Scholar]
- 13.Dickter CL, Bartholow BD. Racial ingroup and outgroup attention biases revealed by event-related brain potentials. Soc Cogn Affect Neurosci. 2007;2(3):189–98. 10.1093/scan/nsm012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loven J, Herlitz A, Rehnman J. Women's own-gender bias in face recognition memory. Exp Psychol. 2011;58(4):333–40. 10.1027/1618-3169/a000100 . [DOI] [PubMed] [Google Scholar]
- 15.Herzmann G, Curran T. Neural Correlates of the In-Group Memory Advantage on the Encoding and Recognition of Faces. Plos One. 2013;8(12). ARTN e82797 10.1371/journal.pone.0082797. WOS:000328737700032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krebs RM, Schott BH, Schutze H, Duzel E. The novelty exploration bonus and its attentional modulation. Neuropsychologia. 2009;47(11):2272–81. 10.1016/j.neuropsychologia.2009.01.015. WOS:000267839900014. [DOI] [PubMed] [Google Scholar]
- 17.Nyberg L. Any novelty in hippocampal formation and memory? Curr Opin Neurol. 2005;18(4):424–8. . [DOI] [PubMed] [Google Scholar]
- 18.Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neurosci Biobehav Rev. 2001;25(4):355–73. . [DOI] [PubMed] [Google Scholar]
- 19.Hart SJ, Green SR, Casp M, Belger A. Emotional priming effects during Stroop task performance. Neuroimage. 2010;49(3):2662–70. 10.1016/j.neuroimage.2009.10.076. WOS:000273626400069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minear M, Park DC. A lifespan database of adult facial stimuli. Behav Res Methods Instrum Comput. 2004;36(4):630–3. . [DOI] [PubMed] [Google Scholar]
- 21.Peirce JW. Generating Stimuli for Neuroscience Using PsychoPy. Front Neuroinform. 2008;2:10 10.3389/neuro.11.010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Team RDC. R Development Core Team. R Foundation for Statistical Computing, Vienna, Austria: 2008;ISBN 3-900051-07-0. [Google Scholar]
- 23.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62. 10.1037/0096-3445.121.1.15. WOS:000188522600055. [DOI] [Google Scholar]
- 24.Simon JR, Rudell AP. Auditory S-R compatibility: the effect of an irrelevant cue on information processing. J Appl Psychol. 1967;51(3):300–4. . [DOI] [PubMed] [Google Scholar]
- 25.Herlitz A, Reuterskiold L, Loven J, Thilers PP, Rehnman J. Cognitive sex differences are not magnified as a function of age, sex hormones, or puberty development during early adolescence. Dev Neuropsychol. 2013;38(3):167–79. 10.1080/87565641.2012.759580 . [DOI] [PubMed] [Google Scholar]
- 26.Wright DB, Sladden B. An own gender bias and the importance of hair in face recognition. Acta Psychol (Amst). 2003;114(1):101–14. . [DOI] [PubMed] [Google Scholar]
- 27.Schott BH, Seidenbecher CI, Richter S, Wustenberg T, Debska-Vielhaber G, Schubert H, et al. Genetic Variation of the Serotonin 2a Receptor Affects Hippocampal Novelty Processing in Humans. Plos One. 2011;6(1). ARTN e15984 10.1371/journal.pone.0015984. WOS:000286519500034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bochner S. The effectiveness of same-sex versus opposite-sex role models in advertisements to reduce alcohol consumption in teenagers. Addict Behav. 1994;19(1):69–82. . [DOI] [PubMed] [Google Scholar]
- 29.O'Toole LJ, DeCicco JM, Hong M, Dennis TA. The impact of task-irrelevant emotional stimuli on attention in three domains. Emotion. 2011;11(6):1322–30. Epub 2011/06/29. 10.1037/a0024369 . [DOI] [PubMed] [Google Scholar]
- 30.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11458–63. Epub 2002/08/15. 10.1073/pnas.172403899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28(1):249–55. Epub 2005/07/05. 10.1016/j.neuroimage.2005.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of experimental psychology General. 2004;133(3):339–54. Epub 2004/09/10. 10.1037/0096-3445.133.3.339 . [DOI] [PubMed] [Google Scholar]
- 33.Jasinska AJ, Yasuda M, Rhodes RE, Wang C, Polk TA. Task difficulty modulates the impact of emotional stimuli on neural response in cognitive-control regions. Frontiers in psychology. 2012;3:345 Epub 2012/10/13. 10.3389/fpsyg.2012.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krebs RM, Boehler CN, Woldorff MG. The influence of reward associations on conflict processing in the Stroop task. Cognition. 2010;117(3):341–7. 10.1016/j.cognition.2010.08.018. WOS:000284746300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28(52):14311–9. 10.1523/JNEUROSCI.2058-08.2008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7(2):191–7. . [DOI] [PubMed] [Google Scholar]
- 37.Wise RA. Dopamine and food reward: back to the elements. Am J Physiol Regul Integr Comp Physiol. 2004;286(1):R13 10.1152/ajpregu.00590.2003 . [DOI] [PubMed] [Google Scholar]
- 38.Zellner MR, Ranaldi R. How conditioned stimuli acquire the ability to activate VTA dopamine cells: a proposed neurobiological component of reward-related learning. Neurosci Biobehav Rev. 2010;34(5):769–80. 10.1016/j.neubiorev.2009.11.011 . [DOI] [PubMed] [Google Scholar]
- 39.Holroyd CB, Coles MG, Nieuwenhuis S. Medial prefrontal cortex and error potentials. Science. 2002;296(5573):1610–1 author reply -1. . [DOI] [PubMed] [Google Scholar]
- 40.Jocham G, Ullsperger M. Neuropharmacology of performance monitoring. Neurosci Biobehav Rev. 2009;33(1):48–60. 10.1016/j.neubiorev.2008.08.011 . [DOI] [PubMed] [Google Scholar]
- 41.Costa VD, Tran VL, Turchi J, Averbeck BB. Dopamine modulates novelty seeking behavior during decision making. Behav Neurosci. 2014;128(5):556–66. 10.1037/a0037128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rangel-Gomez M, Hickey C, van Amelsvoort T, Bet P, Meeter M. The detection of novelty relies on dopaminergic signaling: evidence from apomorphine's impact on the novelty N2. PLoS One. 2013;8(6):e66469 10.1371/journal.pone.0066469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swanson CJ, Kalivas PW. Regulation of locomotor activity by metabotropic glutamate receptors in the nucleus accumbens and ventral tegmental area. J Pharmacol Exp Ther. 2000;292(1):406–14. . [PubMed] [Google Scholar]
- 44.Hooks MS, Kalivas PW. The role of mesoaccumbens—pallidal circuitry in novelty-induced behavioral activation. Neuroscience. 1995;64(3):587–97. . [DOI] [PubMed] [Google Scholar]
- 45.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14(9):609–25. 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Little AC, DeBruine LM, Jones BC. Sex-contingent face after-effects suggest distinct neural populations code male and female faces. Proc Biol Sci. 2005;272(1578):2283–7. 10.1098/rspb.2005.3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramsey JL, Langlois JH, Marti NC. Infant categorization of faces: Ladies first. Dev Rev. 2005;25(2):212–46. 10.1016/j.dr.2005.01.001. WOS:000229498400004. [DOI] [Google Scholar]
- 48.Rennels JL, Davis RE. Facial experience during the first year. Infant Behav Dev. 2008;31(4):665–78. 10.1016/j.infbeh.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masuda T, Ellsworth PC, Mesquita B, Leu J, Tanida S, Van de Veerdonk E. Placing the face in context: cultural differences in the perception of facial emotion. J Pers Soc Psychol. 2008;94(3):365–81. 10.1037/0022-3514.94.3.365 . [DOI] [PubMed] [Google Scholar]
- 50.Goh JO, Tan JC, Park DC. Culture Modulates Eye-Movements to Visual Novelty. Plos One. 2009;4(12). ARTN e8238 10.1371/journal.pone.0008238. WOS:000272834000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ousdal OT, Andreassen OA, Server A, Jensen J. Increased Amygdala and Visual Cortex Activity and Functional Connectivity towards Stimulus Novelty Is Associated with State Anxiety. Plos One. 2014;9(4). ARTN e96146 10.1371/journal.pone.0096146. WOS:000335240300141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.