Abstract
In female mammals, activation of Xist (X-inactive specific transcript) is essential for establishment of X chromosome inactivation. During early embryonic development in mice, paternal Xist is preferentially expressed whereas maternal Xist (Xm-Xist) is silenced. Unlike autosomal imprinted genes, Xist imprinting for Xm-Xist silencing was erased in cloned or parthenogenetic but not fertilized embryos. However, the molecular mechanism underlying the variable nature of Xm-Xist imprinting is poorly understood. Here, we revealed that Xm-Xist silencing depends on chromatin condensation states at the Xist/Tsix genomic region and on Rnf12 expression levels. In early preimplantation, chromatin decondensation via H3K9me3 loss and histone acetylation gain caused Xm-Xist derepression irrespective of embryo type. Although the presence of the paternal genome during pronuclear formation impeded Xm-Xist derepression, Xm-Xist was robustly derepressed when the maternal genome was decondensed before fertilization. Once Xm-Xist was derepressed by chromatin alterations, the derepression was stably maintained and rescued XmXpΔ lethality, indicating that loss of Xm-Xist imprinting was irreversible. In late preimplantation, Oct4 served as a chromatin opener to create transcriptional permissive states at Xm-Xist/Tsix genomic loci. In parthenogenetic embryos, Rnf12 overdose caused Xm-Xist derepression via Xm-Tsix repression; physiological Rnf12 levels were essential for Xm-Xist silencing maintenance in fertilized embryos. Thus, chromatin condensation and fine-tuning of Rnf12 dosage were crucial for Xist imprint maintenance by silencing Xm-Xist.
Author Summary
X-inactive specific transcript (Xist) is essential a large non-coding RNA for establishment of X chromosome inactivation in female mammals. The aberrant X chromosome inactivation critically affects cellular viability. Therefore, spatiotemporal regulation of Xist expression is required for proper development. In mice, Xist expression is imprinted in early embryonic development and maternal Xist is never expressed during preimplantation phases irrespective of the presence of Xist activator, maternal Rnf12. Generally, parental origin-specific expression pattern of autosomal imprinted genes is maintained in various types of embryos. However, Xist imprinting for transcriptional silencing of maternal Xist was erased in cloned or parthenogenetic but not fertilized embryos. Here, we dissect the molecular mechanism underlying the variable nature of Xist imprinting. We show that in fertilized embryos, chromatin condensation states are essential maternal Xist repression in early preimplantation phases, whereas at late preimplantation stages, pluripotency factor Oct4 serves as a chromatin opener and the maintenance of Xist silencing depends on Rnf12 expression dosage. Although the Oct4 mediated chromatin decondensation also occurs in parthenogetic embryos, Rnf12 overdose causes maternal Xist derepression at late preimplantation phases. Thus these findings reveal that the chromatin regulation by pluripotency factor and Xist activator dose define Xist imprinting state.
Introduction
In mice, the expression of Xist, an essential non-coding RNA for the initiation of X-chromosome inactivation (XCI) [1–3], commences around early preimplantation phases [4,5]. The expression pattern during preimplantation phases is imprinted; paternal Xist (Xp-Xist) is activated and maternal Xist (Xm-Xist) is never expressed although the Xist activator, Rnf12/Rlim [6,7], is abundantly deposited in oocytes [8]. The predominant expression of Xp-Xist is reprogrammed in embryonic-tissues after implantation and maintained in extra-embryonic tissues [5,9]. Therefore, Xp-Xist mutation causes embryonic lethality owing to an over-dose of X-linked genes in extra-embryonic tissues [2,3,10]. Notably, at late preimplantation phases, the imprinted Xm-Xist silencing (Xm-Xist imprinting) is partially disrupted in parthenogenetic embryos, which have two maternal X-chromosomes (XmXm) [4,11]. A previous study showed that histone 3 lysine 9 trimethylation (H3K9me3) and/or histone acetylation was involved in Xm-Xist derepression from early preimplantation phases [4]. However, little is known about the molecular mechanism underlying Xm-Xist imprint maintenance or loss in fertilized (male: XmY or female: XmXp) or XmXm embryos (parthenogenotes), respectively. Furthermore, the question of whether transient alteration of histone modifications from early preimplantation phases could lead to stable Xm-Xist derepression in postimplantation stages has not previously been addressed.
In this study, we demonstrated that chromatin condensation at Xm-Xist/Tsix genomic loci was essential for Xm-Xist silencing in early preimplantation phases. This condensation was involved in H3K9me3 and histone acetylation. Once the chromatin was decondensed in early preimplantation phase, Xm-Xist was stably derepressed and resulted in the rescue of female lethality by Xist paternal deletion (XmXpΔ), indicating that loss of Xm-Xist imprinting was irreversible and genetic lethality could be overcome without direct gene manipulation. At late preimplantation phases, Rnf12 dosage played an important role in Xm-Xist silencing in fertilized and parthenogenetic embryos. Furthermore, we found Oct4 served as a chromatin opener to create transcriptional permissive states at Xm-Xist/Tsix genomic loci in fertilized and parthenogenetic embryos. Together, we propose that Xist imprinting maintenance depends on chromatin condensation states and the regulators differ at developmental stage.
Chromatin decondensation at Xm-Xist/Tsix loci through loss of H3K9me3 and gain of histone acetylation in early preimplantation phases
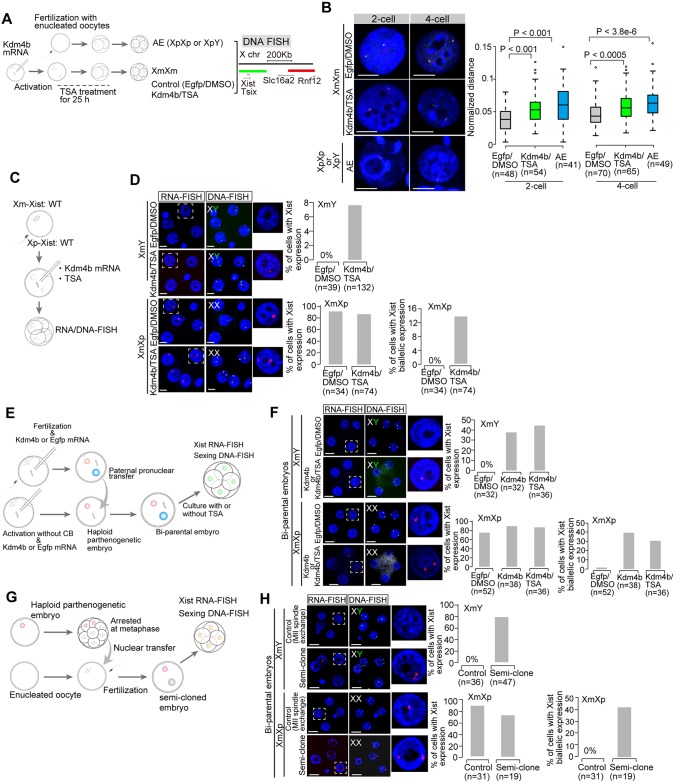
A previous study using XmXm embryos (parthenogenotes) showed that loss of H3K9me3 via Kdm4b, which is a H3K9me3 demethylase [12], or gain of histone acetylation by trichostatin A (TSA) treatment, induced Xm-Xist derepression [4] (S1 Fig). More recently, chromatin decondensation was shown to be associated with Xist expression [13]. Thus, we first investigated whether the chromatin condensation states of Xm-Xist/Tsix regions at the 2- and 4-cell stages could be altered by Kdm4b overexpression and TSA treatment using XmXm embryos (Kdm4b/TSA-XmXm) (Fig 1a). We also examined the chromatin states of androgenetic embryos with Xist RNA positive alleles [14]. DNA fluorescence in situ hybridization (DNA-FISH) analysis around Xist/Tsix genomic regions revealed that Kdm4b/TSA-XmXm embryos showed significantly relaxed chromatin states in both stages compared with Egfp/DMSO (control)-XmXm embryos, although the chromatin was the most relaxed in Xp at both stages (Fig 1b).
Fig 1. Chromatin decondensation induced by loss of H3K9me3 and gain of histone acetylation.
(a) Experimental scheme for chromatin condensation assay using DNA-FISH. Each colour corresponds to the FISH image. Androgenetic embryos (XpXp or XpY) were produced by in vitro fertilization with enucleated oocytes. For the production of Kdm4b/TSA- or Egfp/DMSO-XmXm embryos, mRNA was injected into MII oocytes and then the oocytes were activated by SrCl2. (b) DNA analysis in control (Egfp mRNA with DMSO treatment: Egfp/DMSO)-XmXm, Kdm4b/TSA-XmXm, and androgenetic embryos at 2-cell and 4-cell stages. Representative images and the values of the normalized distance between two signals are shown as pictures and graphs, respectively. The P-values were calculated by the Mann-Whitney U test. (c) Experimental scheme of bi-parental embryos production with Kdm4b and TSA treatment. Oocytes were subjected to in vitro fertilization and Kdm4b mRNA was injected at 1.5 hours after insemination. The Kdm4b/TSA-treated fertilized embryos were cultured and analyzed at 4-cell stage by RNA/DNA-FISH. The sexing probe for X chromosome detection targets the XqF4 regions. (d) RNA/DNA-FISH analysis in Egfp/DMSO- or Kdm4b/TSA-XmY and -XmXp embryos at the 4-cell stage. The graph shows quantification of RNA-FISH signals. n, the number of cells analysed. Scale bars show 20 μm. (e) Experimental scheme of bi-parental embryo production. Oocytes were subjected to in vitro fertilization and Kdm4b mRNA was injected at 1.5 hours after insemination. Haploid parthenogenetic embryos (hPE) injected with Kdm4b mRNA were produced by SrCl2-mediated activation without cytochalasin B (CB). Egfp mRNA was used as an injection control. At 6 hours after insemination or activation, a paternal pronucleus that was larger than the maternal pronucleus in the fertilized embryo was transferred into hPE to produce a bi-parental embryo. The bi-parental embryos were subjected to Xist RNA-FISH and sexing by DNA-FISH at the 4-cell stage. (f) RNA/DNA-FISH analysis of bi-parental embryos. Representative images of bi-parental embryos with Kdm4b/TSA or Kdm4b. The graph shows quantification of RNA-FISH signals. n, the number of cells analysed. Scale bars show 20 μm. (g) Experimental scheme of semi-cloned embryo production. hPE were cultured at the morula stage and the nuclei were arrested by nocodazol treatment. The metaphase-arrested nuclei were transferred into enucleated oocytes and the constructed oocytes were subjected to fertilization, resulting in semi-cloned embryos. For control embryos, spindles were exchanged between oocytes. (h) RNA/DNA-FISH analysis of semi-cloned embryos. Representative images of semi-cloned and control embryos. The graph shows quantification of RNA-FISH signals. n, the number of cells analysed. Scale bars show 20 μm.
We next examined whether Kdm4b/TSA treatment could induce Xm-Xist derepression in XmXp and XmY embryos (fertilized embryos), respectively, at the 4-cell stage (Fig 1c). RNA combined with DNA-FISH (RNA/DNA-FISH) analysis showed that Xm-Xist derepression was observed in 7.6% of Kdm4b/TSA-XmY cells and that 13.5% of Kdm4b/TSA-XmXp cells showed biallelic expression (Fig 1d). Thus, these results indicated that the loss of H3K9me3 and gain of histone acetylation induced chromatin decondensation at Xm-Xist/Tsix genomic regions, resulting in Xm-Xist derepression.
Although these results indicated that the chromatin alterations induced Xm-Xist derepression in XmY and XmXp embryos, the induction efficiency was low compared with XmXm embryos (Fig 1d and S1b Fig). In comparison, a previous study showed that the sole induction of Kdm4b mRNA sufficiently induced Xm-Xist derepression in XmXm 4-cell embryos [4]. Notably, it has been shown that the transcriptional capacity of maternal pronuclei in haploid parthenogenetic embryos (hPE) was higher than that of paternal and maternal pronuclei in zygotes [15]. Furthermore, although histone H4 acetylation was predominantly imposed on the paternal pronuclei in zygotes, maternal pronuclei in parthenogenetic embryos exhibited an H4 acetylated state [16]. These results suggested that the absence of the paternal genome during pronuclear formation might provide a transcriptionally permissive state within the maternal genome. Consistent with this notion, very few XmXm embryos showed Xm-Xist derepression at the 4-cell stage, although Xm-Xist was never expressed in XmY and XmXp embryos [4] (S1b Fig).
In light of these findings, we speculated that the presence of paternal genome during pronuclear formation would impede Xm-Xist derepression. In order to inspect the possibility, we constructed Kdm4b overexpressing bi-parental embryos wherein the parental pronuclei were of different derivation: the maternal pronucleus was formed by SrCl2 activation whereas the paternal pronucleus was formed by in vitro fertilization. Then, to produce bi-parental embryos, paternal pronuclei were transferred into haploid maternal embryos derived from SrCl2 activation (Fig 1e). At the 4-cell stage, Xist RNA-FISH analysis revealed that the constructed bi-parental embryos with Kdm4b overexpression showed marked increase of the cells with Xm-Xist derepression in XmY embryos (Fig 1f: 37.5%; 4.9 fold compared with Kdm4b/TSA-XmY in Fig 1d) and with bialleleic Xist expression in XmXp embryos (Fig 1f: 39.5%; 2.9 fold compared with Kdm4b/TSA-XmXp in Fig 1d), whereas control embryos showed no Xm-Xist derepression in XmY embryos and only a cell was biallelic expression in XmXp embryos (2%) (Fig 1f). The combination of TSA with Kdm4b mRNA injection was also able to induce Xm-Xist derepression (Fig 1f). Thus, these results indicated that the presence of the paternal genome during pronuclear formation impeded Xm-Xist derepression by chromatin alterations.
However, the question remained whether Xm-Xist could be derepressed irrespective of the presence of the paternal genome during pronuclear formation when the maternal chromatin was sufficiently decondensed before fertilization. To test this, we constructed oocytes with decondensed maternal chromatin derived from hPE at the morula stage (Fig 1g), because a previous study had indicated that Xm-Xist/Tsix genomic regions in XmXm embryos became gradually relaxed during preimplantation phases [13]. The constructed oocytes were subjected to fertilization and resulted in semi-cloned embryos (Fig 1g). At the 4-cell stage, Xist RNA-FISH analysis revealed that 78.7% of the cells in XmY semi-cloned embryos exhibited Xm-Xist derepression (Fig 1h: 10.3-fold increase compared with Kdm4b/TSA-XmY in Fig 1c) and 36.8% of cells in XmXp semi-cloned embryos showed biallelic expression (Fig 1h: 2.7-fold increae compared with Kdm4b/TSA-XmXp in Fig 1c). In contrast, in control embryos (spindle-exchanged oocytes), we did not observed Xm-Xist derepression in XmY embryos or biallelic expression in XmXp embryos (Fig 1h).
Taken together, these results demonstrated that Xm-Xist could be derepressed if the chromatin was decondensed even when the paternal genome was present during pronuclear formation, indicating that chromatin condensation of the maternal genome represents the primary factor for imprinting maintenance to silence Xm-Xist. The condensation could be relaxed by loss of H3K9me3 and gain of histone acetylation.
Xm-Xist derepression causes global silencing of X-linked genes in XmXpΔ embryos
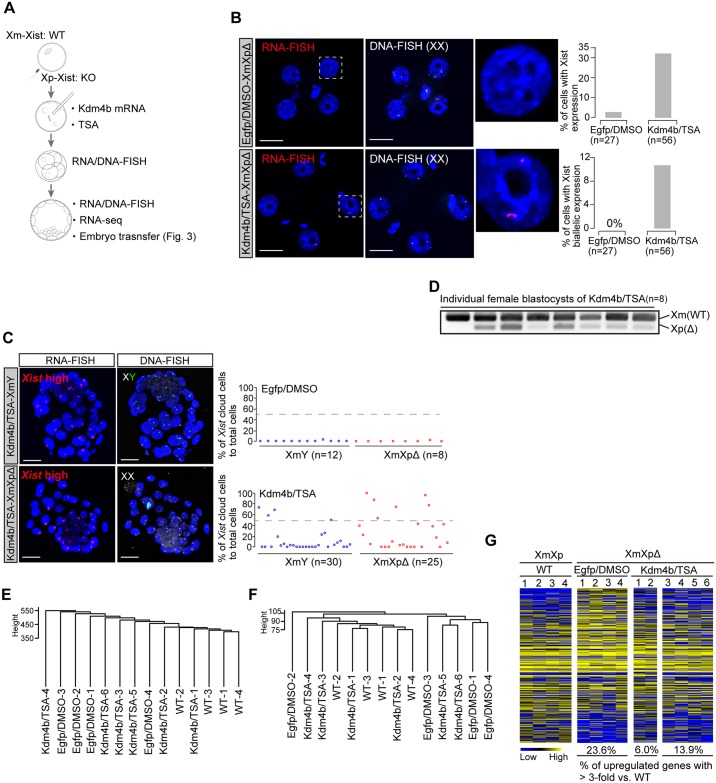
Next, we asked whether the derepression of Xm-Xist during the early preimplantation stages could be stably maintained. To facilitate the analysis of Xm-Xist derepression state in female embryos, we used female embryos devoid of Xp-Xist expression because of a paternal deletion in the repeat-A region [10] (Fig 2a). We first checked Xist expression states by RNA/DNA-FISH analysis at the 4-cell stage, demonstrating that 32.1% of Kdm4b/TSA-XmXpΔ cells but only one Egfp/DMSO-XmXpΔ cell (3.7%) exhibited an Xist signal (Xist+) (Fig 2b). Furthermore, 10.7% of Kdm4b/TSA-XmXpΔ cells showed biallelic expression (Fig 2b). Given that Egfp/DMSO-XmXpΔ embryos showed no Xist cloud and biallelic expression, the results indicated that histone modification alteration induced Xist expression on not only Xm but also XpΔ alleles.
Fig 2. Xm-Xist induces global X-linked gene silencing in XmXpΔ embryos.
(a) Experimental scheme to construct Kdm4b/TSA-XmXpΔ embryos. (b) RNA/DNA-FISH analysis in Kdm4b/TSA- or Egfp/DMSO-XmXpΔ embryos at the 4-cell stage. The graph shows quantification of RNA-FISH signals. The upper and lower graphs show the percentages of Xist-expressing and biallelic cells, respectively. n, the number of cells analysed. Scale bars show 20 μm. (c) RNA/DNA-FISH analysis in Kdm4b/TSA or control blastocysts. Each circle shows individual embryos. n, the number of embryos analysed. Scale bars show 20 μm. (d) Strand-specific RT-PCR analysis for Xist detection. Individual Kdm4b/TSA-XmXpΔ blastocysts were used for the assay. (e and f) Hierarchical clustering analysis by RNA-Seq from individual blastocysts of WT-XmXp, Egfp/DMSO-XmXpΔ, and Kdm4b/TSA-XmXpΔ. The genes expressed in at least one sample (TMM > 10) were used for analysis. All genes expressed (e) and X-linked genes (f). (g) Heat map showing X-linked genes expression. Colours show expression levels; blue: low, black: middle, and yellow: high. The percentages are the genes with > 3-fold upregulated compared with WT.
We further examined whether Xm-Xist derepression could be stably maintained through late preimplantation stages. In blastocysts, RNA/DNA-FISH showed that 13.3% of Kdm4b/TSA-XmY and 24% of Kdm4b+TSA-XmXpΔ embryos exhibited robust Xist expression states (>50% of cells), whereas no Xist expression was found in Egfp/DMSO treated embryos (Fig 2c). We also examined the effect of Kdm4b induction alone on Xist expression. Although Xist expression was induced in XmXpΔ embryos, no embryos of either gender exhibited >50% of Xist positive cells (S2 Fig), indicating that both H3K9me3 loss and the absence of histone deacetylases were required for strong Xist induction.
The antisense RNA for Xist, commences around the blastocysts stage [17,18]. Therefore, to investigate allele-specific Xist expression, we performed strand-specific reverse transcription polymerase chain reaction (RT-PCR) analysis. This demonstrated that Xm-Xist expression was clearly induced in Kdm4b/TSA-XmXpΔ embryos (Fig 2d). Thus, Xm-Xist derepression at early preimplantation phases could last until the late phases of preimplantation.
To further examine Tsix expression states, we performed RNA/DNA-FISH analysis using Tsix-specific detection probes (S3a Fig). Kdm4b/TSA treatment resulted in an increase of cells with Xist but not Tsix in XmY and XmXp embryos (S3b and S3c Fig). Quantitative PCR (qPCR) analysis also confirmed the lack of Tsix upregulation although some X-linked genes, i.e., Pgk1 and Plac1, were downregulated in Kdm4b/TSA-XmY and -XmXpΔ embryos compared with Egfp/DMSO treated embryos (S3d and S3e Fig). We also confirmed H3K27me3 enrichment in some cells in Kdm4b/TSA-XmY or -XmXpΔ blastocysts [19], indicating that the normal XCI process occur in Kdm4b/TSA treated embryos (S3f Fig).
To gain further insights into transcriptome states, we performed RNA deep sequencing (RNA-Seq) analysis using an individual XmXpΔ embryo with Kdm4b/TSA, Egfp/DMSO, and wild-type (WT). Notably, hierarchical clustering analysis indicated that the transcriptome states of two Kdm4b/TSA-XmXpΔ (Kdm4b/TSA-1/2) embryos resembled those of WT (Fig 2e), indicative of normal X-linked genes expression in the Kdm4b/TSA-1/2 embryo. Consistent with this, hierarchical clustering based on X-linked genes showed that Kdm4b/TSA-1/2 clustered with WT embryos (Fig 2f). Out of 331 X-linked genes expressed, 23.6% and 13.9% were upregulated in Egfp/DMSO and Kdm4b/TSA-3/4/5/6 embryos, respectively (Fig 2g and S1 Table). However, only, 6.0% were upregulated in Kdm4b/TSA-1/2 embryos (Fig 2g and S1 Table). Although group specific differentially-expressed genes were also identified (S4 Fig), these results indicated that Kdm4b/TSA treatment induced Xm-Xist derepression and reduced the number of upregulated X-linked genes in XmXpΔ embryos.
Xm-Xist compensates for imprinted XCI
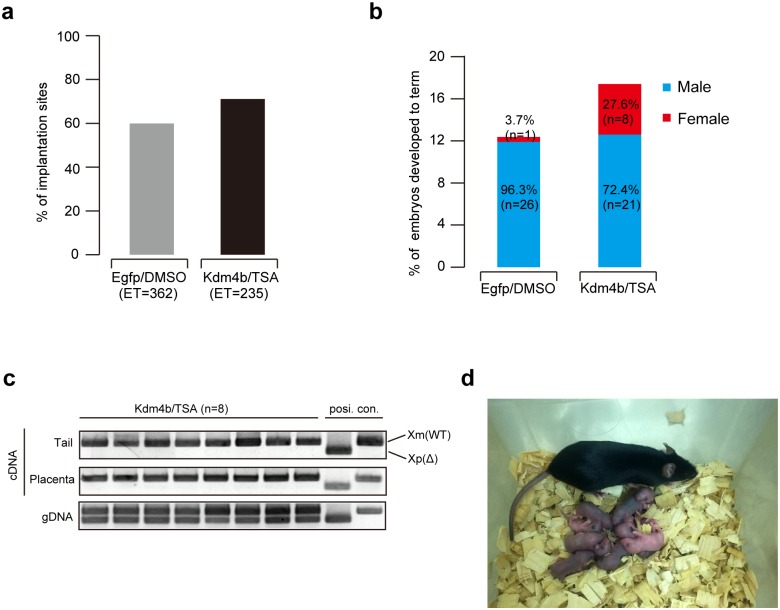
To test whether transient histone alterations in preimplantation phases could lead to stable Xm-Xist derepression in embryonic- and extraembryonic tissues and rescue the lethal phenotype of XmXpΔ embryos without gene manipulation, we conducted embryo transfer experiments and assessed the developmental ability of XmXpΔ embryos. We transferred 362 Egfp/DMSO- and 235 Kdm4b/TSA-blastocysts that were recovered at embryonic day 19.5, identifying 60% and 71% of implantation sites in Egfp/DMSO- and Kdm4b/TSA-groups, respectively (Fig 3a). We obtained 26 XmY pups (96.3% of total pups) from the Egfp/DMSO treatment. Unexpectedly, one XmXpΔ pup was also born in the group (Fig 3b). However, we have not yet replicated this result. In contrast, 8 XmXpΔ (27.6%) and 21 XmY (72.4%) pups were born in the Kdm4b/TSA group (Fig 3b).
Fig 3. Developmental competency of Kdm4b/TSA-XmXpΔ embryos.
(a) % of implantation sites at E19.5. n, the number of transferred embryos. (b) % of embryos developed to term at E19.5. n, the number of recovered embryos. (c) PCR analysis using cDNA and genomic DNA (gDNA) in rescued XmXpΔ females. cDNA from the tails and placentas and gDNA from placentas were used for the assay, respectively. (d) The rescued females developed to adults with normal reproductive ability.
RT-PCR analysis of embryonic- and extra-embryonic tissue from rescued XmXpΔ embryos exhibited predominant Xm-Xist expression (Fig 3c). The rescued females displayed normal reproduction and gave birth to viable offspring (Fig 3d). Taken together, these results clearly demonstrated that the Xm-Xist compensated for imprinted XCI and exhibited the functional equivalency of Xm-Xist to Xp-Xist in both embryonic- and extraembryonic tissues.
Decrease of Xm-Tsix expression in XmXm embryos compared with that in XmXp and XmY embryos
One of the remaining questions is the molecular mechanisms involved in loss of the Xm-Xist imprint (Xm-Xist silencing) in XmXm morula embryos despite the imprint maintenance in XmY and XmXp embryos [4,11]. As the chromatin at Xist/Tsix genomic loci was gradually relaxed during preimplantation development [13] and Xm-Tsix began to be expressed around the morula stage [17], we investigated the Tsix expression state. RNA-FISH analysis for Xist and Tsix revealed that Tsix was also not detected until the 16-cell stage in XmXp, XmY, and XmXm embryos. In XmXp embryos, the cells showing a Tsix signal or an Xist cloud averaged 23% and 94%, respectively (S5 Fig). XmY embryos contained 34% of cells with Tsix expression and no Xist expression (S5 Fig). In XmXm embryos at the 16-cell stage, an Xist cloud was observed in 34% of nuclei. Notably, the ratio of Tsix expressing cells in XmXm embryos was less than that in XmXp and XmY embryos (S5 Fig, XmXm: 18%). Considering that Xm-Tsix was present in two copies in XmXm embryos, these results implied that Xm-Xist derepression in XmXm embryos might be associated with the repression of Tsix.
Rnf12 is overexpressed and induces Xm-Xist activation via Tsix repression in XmXm embryos
Previous studies indicated that the dose-dependent Xist activator, RNF12 [6,7], was highly expressed in early preimplantation phases [4]. RNF12 activates Xist via REX1 protein degradation and REX1 plays a role in Tsix elongation [20,21]. Thus, we performed single cell qPCR assays against Rnf12 mRNA using XmY, XmXp, and XmXm preimplantation embryos. To identify the sex of the cells in fertilized embryos at the 2-cell stage, DNA-FISH analysis was conducted in the remaining cell in each embryo not used for qPCR analysis. From the 4-cell stage onward, Xist expressing cells were defined as female. The analysis exhibited that Rnf12 expression levels were markedly higher in oocytes and at the 1-cell stage (Fig 4a). Although Rnf12 expression levels gradually decreased in most of the embryos as the embryos developed, the levels of XmXm (Xist−) cells tended to be high compared with those of XmXp from the 2-cell stage onward (Fig 4b). At 8-cell and morula stages, the Rnf12 levels of XmXm (Xist+) cells were downregulated compared with XmXm (Xist−) and XmXp (≥ 2 fold on average) (Fig 4b). These results suggested that excess Rnf12 might facilitate Xm-Xist derepression via Xm-Tsix repression in XmXm embryos.
Fig 4. Rnf12 over-dosage induces Xm-Xist derepression in XmXm, XmY and XmXp embryos.
(a) Average Rnf12 expression levels in single XmY, XmXp, and XmXm cells from oocytes to morula embryos. n, the number of cells analysed. The average expression levels of MII oocytes were set as one. (b) Rnf12 expression profiles in individual cells. The average expression levels of XmXp embryos were set as one in each stage. (c) Xist/Tsix RNA-FISH analysis in Rnf12KD-XmXm and Rex1/Rnf12 double KD XmXm morulae. Representative images from scramble (siRNA control), Rnf12KD, and Rex1/Rnf12 double KD embryos. The graphs show quantification of FISH signal patterns. The P-values were calculated by the Fisher’s exact test. n, the number of cells analysed. Scale bars show 20 μm. (d) Experimental scheme of construction of RNF12 overexpressing fertilized embryos by Rnf12 mRNA injection. Egfp mRNA was used for the control. (e and f) Immunofluorescence combined with RNA/DNA-FISH analysis of Rnf12 overexpressing XmY (Rnf12-XmY) and control XmY (Egfp-XmY) (e), Rnf12-XmXp, and Egfp-XmXp (f). Representative images from Rnf12 or Egfp overexpressing embryos. The graph shows % of Xist cloud cells in XmY and XmXp embryos or of Xist biallelic cells. n, the number of cells expressing RNF12 or EGFP. Scale bars show 20 μm.
To test this possibility, we constructed Rnf12-depleted XmXm embryos by siRNA injection (Rnf12KD-XmXm) (S6a and S6b Fig). The RNA-FISH analysis for Xm-Xist/Tsix revealed that Rnf12 repression caused a remarkable increase of Tsix+ (scramble-XmXm: 14% vs. Rnf12KD-XmXm: 64%, Fig 4c), and the proportion of Xist+ cells significantly declined (scramble-XmXm: 59% vs. Rnf12KD-XmXm: 28%, Fig 4c). Next, we tested the previous notion in differentiating ES cells, which indicated that RNF12-mediated Xist upregulation was involved in the Rex1 expression state [20], as determined by Rnf12/Rex1 double knockdown (KD) experiments in XmXm embryos (S6c Fig). As expected, the Xm-Xist repression with Tsix upregulation seen in Rnf12KD-XmXm embryos was rescued in XmXm embryos with Rnf12/Rex1 double depletion, although there was no marked effect on Xm-Xist/Tsix expression in Rex1 single KD embryos (Fig 4d). Thus, we concluded that the Rnf12 overdose in XmXm embryos caused Xm-Tsix repression and resulted in Xm-Xist activation.
Additional RNF12 expression induces Xm-Xist activation in XmY and XmXp embryos
Given the above results from XmXm embryos, we inferred that the decrease of Rnf12 in XmY and XmXp embryos around morula stages might be essential for Xm-Tsix activation to repress Xm-Xist since the chromatin was decondensed. To test this possibility, we constructed Rnf12-overexpressing fertilized embryos. As RNF12 turnover was implied to be quick [4], we injected Rnf12 mRNA into 2-cell blastomeres (Fig 4d). At the blastocyst stage, we carried out immunofluorescence against RNF12 combined with RNA/DNA-FISH (IF-RNA/DNA-FISH). Of the RNF12 overexpressing cells in XmY, 11.1% exhibited Xist cloud states (Fig 4e), whereas embryos with Egfp mRNA never showed Xm-Xist derepression (Fig 4e). In XmXp embryos, biallelic expression was significantly induced in Rnf12 overexpressing cells (Egfp: 1.9% vs. Rnf12 overexpression: 11.2%, Fig 4f). These results indicated that proper Rnf12 expression levels were required for Xm-Xist silencing in fertilized embryos.
Oct4 positively controls Xm-Xist/Tsix in XmXm embryos at late preimplantation phases
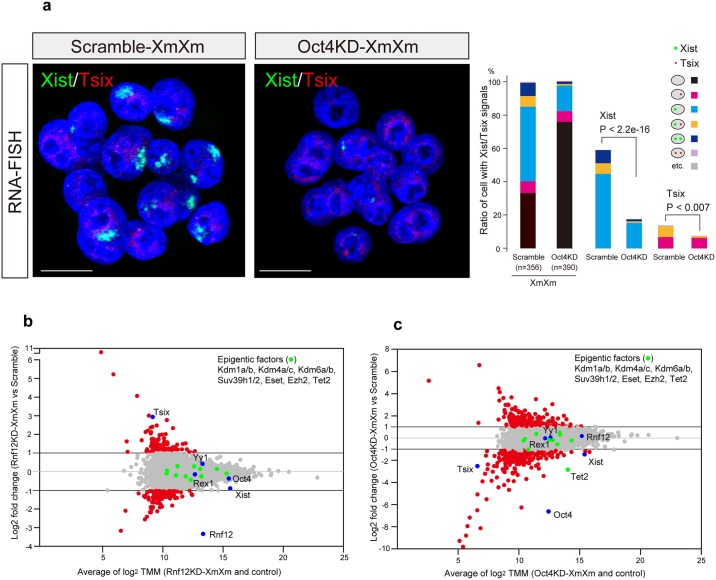
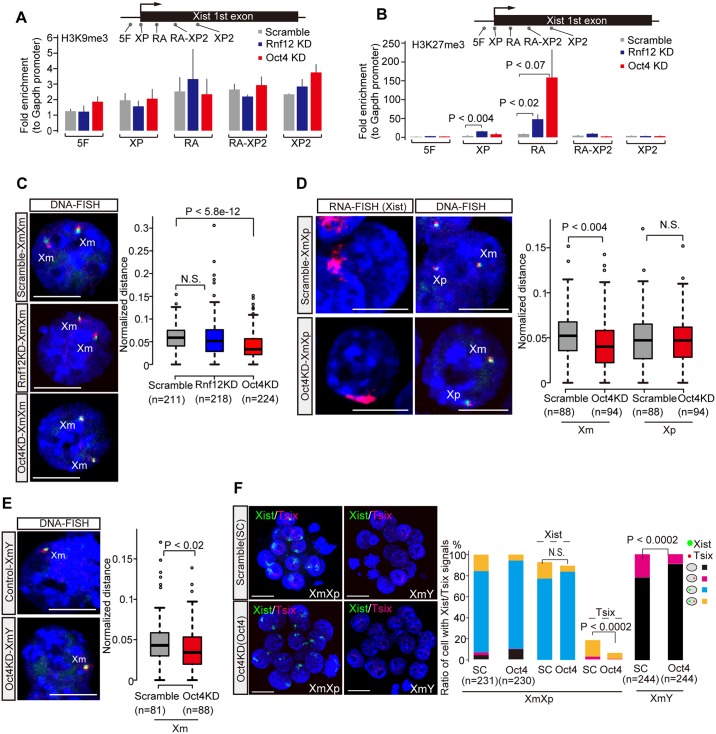
Around the morula stage, many pluripotency-factors that were shown to regulate Xist begin to be expressed [21–23]. Therefore, using XmXm morulae, we investigated the involvement of pluripotency-factors in Xm-Xist/Tsix regulation. YY1 and Oct4 were selected for candidate pluripotency-factors based on previous reports [23–25] and we conducted siRNA-mediated KD experiments. qPCR and Xist RNA-FISH analysis revealed that Oct4 depletion induced significant reduction of Xm-Xist (S7 Fig), implying that Oct4 is an important factor for regulating Xm-Xist imprint erasure.
Further, Xist/Tsix RNA-FISH analysis in Oct4KD-XmXm embryos revealed that the proportions of cells with Xist and Tsix signals were extremely reduced (Oct4KD-XmXm: Xist 18% and Tsix 8%, Fig 5a and scramble: Xist 59% and Tsix 14%, shown in Fig 4c), indicating that opposed to RNF12, Oct4 controls not only Xm-Xist derepression but also Xm-Tsix activation.
Fig 5. Oct4 depletion disrupts Xm-Xist/Tsix expression in XmXm embryos.
(a) Xist/Tsix RNA-FISH analysis in Oct4KD-XmXm and scramble-XmXm morulae. Scramble data is the same as in Fig 4c. The graphs show quantification of FISH signal patterns. The P-values were calculated by the Fisher’s exact test. n, the number of cells analysed. Scale bars show 20 μm. (b and c) MA plot of RNA-Seq analysis in Rnf12KD-XmXm embryos (b) and Oct4KD-XmXm embryos (c). Red circles show fold change over 2-fold. Representative epigenetic markers and known Xist/Tsix regulators are depicted as green and blue, respectively.
To gain further insights into the effect of Oct4 and Rnf12 depletion in XmXm morulae, we performed RNA-Seq analysis. Out of 7898 genes with > 10 trimmed mean of M values (TMM) [26] in at least one group, 280 and 613 genes were differentially expressed (more than 2-fold) in Rnf12KD-XmXm and Oct4KD-XmXm embryos, respectively (Fig 5b and 5c and S2 Table), indicating the high impact of Oct4 depletion on the transcriptome. Notably, the differentially expressed genes following Oct4 or Rnf12 depletion were randomly distributed across the chromosomes (S8 Fig).
Consistent with the FISH results, Xist and Tsix were down- and upregulated in Rnf12KD-XmXm embryos, respectively (Fig 5b and S2 Table), whereas both were repressed in Oct4KD-XmXm embryos (Fig 5c and S2 Table). Oct4 and Rnf12 expression levels were comparable to those of scramble-XmXm embryos in Rnf12KD-XmXm and Oct4KD-XmXm embryos, respectively (Fig 5b and 5c and S2 Table). The expression states of major histone modifiers including members of the H3K9me3 demethylase Kdm4-family were not dramatically affected (Fig 5b and 5c and S2 Table). However, we found that Tet2, which is associated with DNA methylcytosine dioxygenase [27], was markedly downregulated (14% of scramble) only in Oct4KD-XmXm morulae (Fig 5c and S2 Table). To examine the impact of Tet2 depletion on Xm-Xist derepression, we constructed Tet2KD-XmXm embryos and evaluated their Xm-Xist expression states. RNA-FISH analysis indicated that the extent to which Tet2 mediated Xm-Xist repression was modest compared with Oct4 depletion (S9 and S7c Figs). However, as we could not exclude the possibility that the Tet2 KD efficiency might be insufficient, given that DNA methylation was not responsible for Xm-Xist expression [28], these results suggested that dysregulation of epigenomic factors were not likely to be the primary cause for Xm-Xist repression following Oct4 depletion.
The known Xist activators on the X chromosome (Jpx and Ftx) [29,30] were not detectable in either group by qPCR analysis. Taken together, these results indicated that the mechanism by which Oct4 mediated Xist regulation was different from that underlying Rnf12-mediated regulation.
Oct4 directs chromatin decondensation at Xm-Xist/Tsix loci in XmY, XmXp, and XmXm embryos
Previous studies demonstrated that H3K9me3 was involved in Xm-Xist silencing and that Tsix transcripts altered H3K27me3 states at Xist promoter regions [31,32]. As such, we investigated the two histone modifications at Xm-Xist promoter regions in Oct4KD-XmXm and Rnf12KD-XmXm embryos using embryo-chromatin immunoprecipitation combined qPCR [4,13]. Notably, we found nosignificant changes of H3K9me3 modifications in Oct4KD- and Rnf12KD-XmXm embryos compared with scramble-XmXm embryos in the regions examined (Fig 6a) whereas, as expected, in Rnf12KD-XmXm embryos, significant hypermethylation of H3K27me3 at the promoter regions compared with scramble-XmXm embryos was observed (4.7-fold increase, Fig 6b). Oct4KD-XmXm embryos also showed this effect, albeit more modest, (2.4-fold increase compared to scramble-XmXm, Fig 6b). The repeat-A regions were also markedly hypermethylated in Rnf12 or Oct4 depleted XmXm embryos (Rnf12KD: 5.5 fold and Oct4: 18.4 fold increase compared to scramble-XmXm, respectively, Fig 6b). Thus, these results indicate that Oct4 and Rnf12 were involved in the alteration of histone modifications leading to transcriptional active states around Xist regulatory regions.
Fig 6. Oct4 directs chromatin decondensation at Xm-Xist/Tsix regions in XmY, XmXp, and XmXm embryos.
(a) H3K9me3 and H3K27me3 states around Xist regulatory regions in XmXm morulae. Examined regions for eChIP-qPCR in XmXm morula embryos were shown above the graph. The XP region is major promoter. H3K9me3 (a) and H3K27me3 (b) states. In all cases, more than three biological replicates were tested. The error bars show standard error. The P-values were calculated using the Student’s t-test. (c) DNA-FISH analysis in Oct4KD- and Rnf12KD-XmXm morulae. (d and e) RNA/DNA-FISH analysis in Oct4KD-XmY and -XmXp morulae. Xist cloud cells by RNA-FISH were identified as Xp alleles. BAC DNA probes shown in Fig 1a were used for the DNA-FISH assay (c-e). Representative images and the values of normalized distance between two signals are shown as pictures and graphs, respectively. The P-values were calculated by the Mann-Whitney U test. Scale bars show 10 μm. (f) Xist/Tsix RNA-FISH analysis in Oct4KD-XmY and -XmXp morulae. The graphs show quantification of FISH signal patterns. The P-values were calculated by the Fisher’s exact test. Scale bars show 20 μm. n, the number of cells analysed.
Since Oct4 repression caused not only Xm-Xist but also Xm-Tsix silencing in XmXm embryos, we inferred that Oct4 might also regulate chromatin condensation states at Xm-Xist/Tsix genomic regions. To test this possibility, we conducted DNA-FISH analysis in XmXm morulae. Chromatin condensation at the loci was significantly induced by Oct4 depletion but was not observed upon Rnf12 repression (Fig 6c). These results indicated that Oct4 mediated chromatin relaxation facilitated transcription around Xist/Tsix regions and resulted in Xm-Xist/Tsix activation in XmXm embryos.
Next, we investigated whether Oct4 served as chromatin opener in XmXp and XmY embryos. To distinguish Xp and Xm alleles, we conducted RNA/DNA-FISH at the morula stage. Notably, Oct4 depletion significantly induced chromatin contraction in both XmY and XmXp embryos at Xm-Xist/Tsix (Fig 6d and 6e). Given this, we sought to investigate whether Oct4 might affect Xm-Tsix expression states, by Xist/Tsix RNA-FISH analysis in Oct4KD-XmY and -XmXp embryos. Notably, Xist expression states in XmXp embryos were comparable between scramble and Oct4KD embryos (Fig 6f), indicating that Oct4 did not affect Xp-Xist expression. However, as expected, Oct4KD embryos exhibited a significant reduction of cells with Tsix+ in both XmY (9.0%) and XmXp (6.1%) compared to scramble-XmY (21.7%) and -XmXp (18.2%) cells counterparts (Fig 6f). Taken together, these results revealed the novel role of Oct4 as a chromatin opener to induce the activation of Xm-Xist/Tsix in XmXm and of Xm-Tsix in XmY and XmXp embryos.
Discussion
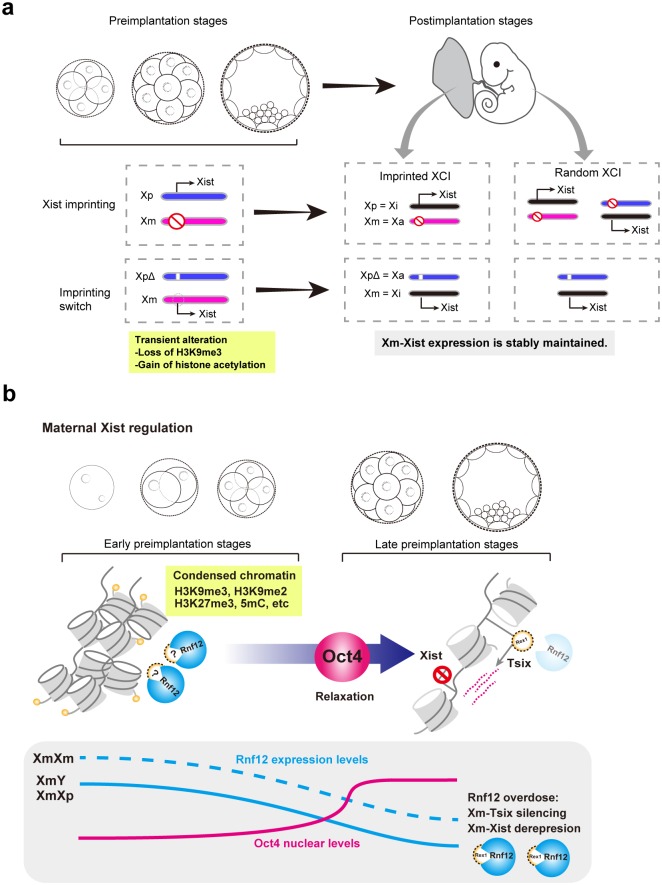
The establishment of XCI is crucial for faithful development [2,3]. The present study addressed two unresolved questions about imprinted XCI in mice, the first being the irreversibility of Xm-Xist imprinting. Once Xm-Xist was derepressed at early preimplantation phases by transient alteration of histone modifications, it could be stably maintained and genetic lethality of XmXpΔ embryos could be rescued without gene manipulation (Fig 7a). The other is the molecular mechanism of imprinted XCI maintenance and erasure. The maintenance mechanism of Xm-Xist imprinting differed by developmental phase: in early preimplantation phases, chromatin condensation states determined the Xm-Xist silencing, whereas in the late preimplantation phase, as chromatin was relaxed by Oct4, the occurrence of maintenance or erasure of the Xist imprinting depended on Rnf12 dosage state (Fig 7b).
Fig 7. Proposed model.
(a) Irreversible Xm-Xist imprinting. The transient alteration of histone modifications through loss of H3K9me3 and gain of histone acetylation induces stable Xm-Xist derepression and results in the rescue of lethality in XmXpΔ without gene manipulation. The imprinting switch in XCI does not affect cellular integrity. (b) Maintenance and erasure of the Xm-Xist imprint. In early preimplantation phases, chromatin at Xm-Xist/Tsix regions is condensed by various epigenetic modifications. At late stage, Oct4 localizes to nucleus (see discussion) and serves as a chromatin opener at Xm-Xist/Tsix regions, creating transcriptional permissive states around Xist/Tsix regions. In XmXm embryos, Rnf12 expression levels represent a double dose compared with those of XmY or XmXp embryos, leading to Xm-Xist activation by Tsix silencing, which depends on REX1 state. In XmY and XmXp embryos, on the other hand, physiological expression levels of Rnf12 are essential for Xm-Xist silencing by Xm-Tsix activation.
Chromatin condensation and imprinted XCI
Species-specific imprinted XCI has been observed and one study indicated that human embryos showed no imprinted XCI [33]. Previously, Sado and Sakaguchi proposed that chromatin condensation states in parental genomes differed in each specie and that this might define imprinted XCI [34]. In the current study, we showed that the asymmetric chromatin condensation states of parental Xist/Tsix genomic regions are crucial for the initiation of Xist expression in mice (Fig 7b). In mice, the Xm-Xist imprint is established during oogenesis [13,35]. During the phases, a maternal genome state is imposed on many transcriptionally repressive marks such as 5mC of DNA, H3K27me3, and H3K9me2/3 [4,36–38]. Furthermore, HDAC2, which mediates induced histone deacetylation, is not highly expressed until the full grown oocyte stage [39]. Thus, maternal chromatin becomes condensed during oocyte growth [13]. In mice, the maternal pronucleus is smaller than its paternal counterpart after fertilization, reflective of the maternal genome condensation. As a reflection of the chromatin condensation states in the maternal genome immediately after fertilization, the maternal pronuclear size is smaller than paternal size in mice [4], whereas in humans, the parental pronuclei size was equal and non-imprinted XIST expression was observed [40].
Oct4-mediated chromatin decondensation
Our findings disclosed a novel role of Oct4 in Xist regulation in vivo. Maternal Oct4 has been shown to be dispensable for embryonic development [41]. Recently, we found that the Oct4 protein was not localized to the nucleus until the 8–16-cell stage in mice [42]. Moreover, Oct4 overexpression altered chromatin conformation during early preimplantation phases[42]. More recently, Oct4 has been shown to relax chromatin in 8-cell embryos [43]. These findings supported the conclusion in the present study that Oct4 is a functional chromatin remodeler around Xist/Tsix genomic loci. However, the mechanism by which Oct4 induces chromatin decondensation remains unknown. RNA-Seq analysis showed that the expression levels of major chromatin remodelling factor genes such as Caf1, Brg1, Ring1b, and Ezh2 [44–46] were not dramatically altered by Oct4 depletion (S2 Table).
One of the other possibilities for controlling chromatin remodeling is direct binding of Oct4 around Xist/Tsix regions. In ES cells, Oct4 could bind to XqD regions including Xist/Tsix loci [47] (S10 Fig). Moreover, recent study revealed that Nanog was necessary for an open heterochromatin organization in ES cells by direct binding to major satellite regions [48]. Thus, the direct bindings of Oct4 around Xist/Tsix loci might recruit transcriptional activators or evict transcriptional repressors.
RNF12-mediated Xist activation
RNF12 is an essential factor for imprinted XCI [8]. The role of RNF12 as a dose-dependent Xist activator [7,49] is supported by the present study (Fig 7b). At late preimplantation embryos, as shown in previous studies using differentiating ES cells [20,21], Rnf12 controls Xm-Xist expression by silencing Tsix, which was induced by Rex1 in XmXm embryos (Fig 7b). Thus, the primary role of Rnf12 at late preimplantation phases is the silencing of Rex1 leading to Tsix repression. However, the Rnf12 expression levels of XmY and XmXp embryos markedly declined compared with those of XmXm embryos (Fig 4b). Therefore, under the physiological conditions of XmY and XmXp embryos, Rnf12 double dosage never occurs and Tsix can be expressed from the Xm allele to induce chromatin alteration at Xist promoter regions.
In contrast, the role of RNF12 in Xp-Xist activation at early preimplantation phases remains a large question. Makhlouf et al. demonstrated that YY1 binds to Xist exon1 loci and can activate Xist in somatic cells [24]. These YY1 binding sites are CpG regions and DNA methylation inhibited this YY1 binding [24]. In support of the importance of YY1 binding sites for Xist activation, a DNA methylome study revealed that a part of the exon1 regions in the sperm genome were hypomethylated [50], implying YY1 binding in Xp-Xist. Furthermore, Gontan et al. showed the interaction of RNF12 with YY1 [20]. Therefore, the examination of the role of YY1 for Xp-Xist activation will aid in determining the mechanism of RNF12-mediated Xp-Xist activation.
Materials and Methods
Oocyte and sperm collection
Female B6D2F1 and male C57BL/J mice were purchased from CLEA and Sankyo Labo service (Japan) and oocytes and sperm were collected according to standard methods [4]. Repeat-A deletion mice were obtained from RIKEN BRC (B6.Cg-Xist<tm5Sado>). All animals were maintained and used in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Japanese Association for Laboratory Animal Science and the National Research Institute for Child Health and Development (NRICHD) of Japan. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the NRICHD (Permit Number: 05–006).
Embryo manipulations
The production of parthenogenetic and androgenetic embryos was previously described [4]. In brief, oocytes were incubated in Ca-free M16 medium containing 8 mM SrCl2 and 5 μg/mL cytochalasin B (Sigma-Aldrich) for 5–6 hours. For production of haploid parthenogenetic embryos (hPE), the cytochalasin B was removed in the activation medium. All embryos were cultured in KSOM medium (EMD Millipore) in an atmosphere containing 5% CO2 at 37°C. In the TSA experiment, the embryos were cultured for 25 h in activation and culture media containing 50 nM TSA (Sigma-Aldrich). siRNAs were purchased from Life Technologies; siRNA sequences are described in S3 Table. siRNA injection into ovulated oocytes was conducted using a Piezo drive (Sutter Instrument Company). For expression or FISH experiments, the embryos were collected at 24–26 (2-cell), 48–50 (4-cell), 57–59 (8-cell), and 72–74 (morula) h after activation or insemination, respectively.
For nuclear transfer, HVJ-E (Ishihara Sangyo, Japan)-mediated fusion methods were used for all nuclear transfer experiments. Prior to nuclear transfer, zona pellucida was silted by a grass knife and the 1st polar body was removed to prevent fusion with oocytes by HVJ-E. In male pronuclear transfer experiments, large pronucleus was selected and transferred into hPE. For the preparation of metaphase nuclei of hPE, hPE at the morula stage were incubated with M2 containing 1 μg/mL Nocodazole (Sigma-Aldrich) for 4–5 hours and used as donor cells. The reconstructed oocytes were subjected to intracytoplasmic sperm injection.
For embryo transfer, pseudopregnant ICR mice (Clea Japan) were used as embryo recipients. At E19.5, the embryos were recovered from the uterus.
In vitro mRNA synthesis
The preparation of in vitro synthesized Kdm4b and Egfp mRNA was described previously [4]. For Rnf12 mRNA synthesis, the full length coding sequence (CDS) was amplified by PCR using KOD-Plus-Neo DNA polymerase (Toyobo, Osaka, Japan) from 1-cell embryos. The amplified DNA was used as a template for the generation of PCR products with Poly-A tail and a T7 promoter and the products were subjected to in vitro transcription. The primers used for Rnf12 CDS amplification are shown in S3 Table.
qPCR analysis of morula embryos
The qPCR analysis was conducted using TaqMan probes (Life Technologies) as described previously [4]. Total RNA from morula embryos (72 h after activation) was extracted using an RNeasy micro kit (Qiagen) according manufacturer instructions. Gapdh (Mm99999915_g1) was used as an internal control for normalization of target genes (Oct4: Mm00658129_gH, Yy1: Mm00456392_m1, and Rex1: Mm01194090_g1)
Single cell qPCR analysis of preimplantation embryos
The zona pellucida was removed by treatment with acid Tyrode’s solution (Sigma) and single cells from each preimplantation stage were collected using a micromanipulator. Total RNA isolation and cDNA synthesis were performed using a Single Cell-to-CT™ qRT-PCR Kit (Thermo Fisher) with slight modifications. In brief, half volumes of all reagents were used in this study. The qPCR analysis using TaqMan probes (Rnf12: Mm00488044_m1 Xist: Mm01232884_m1) was conducted without a cDNA preamplification step. A total of 4 or more embryos were randomly selected from which to collect single cells used in the assay. The remaining cells at the 2-cell stage in fertilized embryos were subjected to DNA-FISH analysis as described below.
Immunofluorescence
Embryos were fixed and permeabilised as previously described [13]. In brief, zona pellucida embryos were fixed with 2% PFA in PBS containing 0.1% PVA (Sigma) for 15 min at room temperature and then permeabilised with 0.25% Triton-X in PBS-PVA for 10 min at room temperature. After blocking with 1% BSA, the samples were incubated with the primary antibody RNF12 (1:500 diluted by blocking buffer, Abnova, H00051132-M01), H3K27me3 (1:200, Millipore, 07–449), or Oct4 (1:200, Santa Cruz Biotechnology, C-10), respectively. For H3K9me3 (1:500, Abcam, ab8898) and H3K9Ac (1:500, Abcam, ab12179) detection, fixation and permeabilisation treatments were simultaneously conducted and the primary antibodies were simultaneously incubated. The images were observed using a LSM510 laser scanning confocal microscope (Carl Zeiss). For quantification of the signal intensity, the same laser intensity was applied to each sample and the signals were calculated using U.S. National Institutes of Health (NIH) ImageJ software (http://rsb.info.nih.gov/ij/).
eChIP-qPCR
Embryo-ChIP (eChIP) analysis for preimplantation embryos was based on previous reports. At least 15 XmXm morulae were used per assay. The primer/probe sequences used were described previously [13]. In addition, antibodies for H3K9me3 (Abcam, ab8898) and H3K27me3 (Millipore, 07–449) were used.
RNA-FISH
The samples for RNA-FISH were prepared as previously described [4]. In brief, for Xist detection, the pXist12.9 plasmid containing the majority of the Xist cDNA was used (kindly gifted by T. Sado). For Tsix detection, the region (around 7 kb) of the Tsix locus from chr X: 103,448,873 to 103,455,853 was amplified by PCR and the products were subjected to nick translation (Abbott Laboratories). The region from chr X: 103,459,241 to 103,460,958 was amplified by PCR and cloned in the PUC118 vector (Takara), resulting in PCU118-Tsix1.7. The plasmid was also subjected to nick translation along with the PCR products of the 7 kb region. The FISH images were observed using a LSM510 laser scanning confocal microscope using C-.Apochromat 40x/1.2 W (Carl Zeiss).
DNA-FISH
The DNA-FISH procedures were based on a previous study [13]. The fixed and permeabilised embryos were treated with RNaseA and then incubated with 0.2N HCl containing 0.05% tween-20 solution on ice for 10 min. The samples were incubated at 85°C for 10 min and then for overnight at 37°C. BAC DNA probes (RP23-311P7 and RP23-36C20) were prepared by nick translation. For evaluation of chromosome pairing, the probe derived from RP23-311P7 was used. Both probes were used for the chromatin condensation assay. For embryo sexing, the probes of X-chromosome (XqF4 regions) and Y-chromosome were purchased from Chromosome Science Labo (Sapporo, Japan). Distance measurements were based on previous reports [13]. Briefly, the signal centroid was calculated by NIH ImageJ software. Each nuclear radius used for distance normalization was calculated using the DAPI-stained area measurement. For image capture of all DNA FISH analyses, LSM510 laser scanning confocal microscopy using a Plan-Apochromat 100×/1.46 Oil DIC objective (Carl Zeiss) was used.
RNA/DNA-FISH
Morula stage embryos were used for RNA/DNA-FISH analysis. The RNA-FISH procedure and image capture were carried out as in the above method and after image capture, the samples were washed with PBS and incubated with RNaseA for 1.5 h. After washing, the samples were treated with a solution including 0.01N HCl, 0.1% Tween20, and 100 μg/ml Pepsin (Sigma) for 7 min at 37°C. After washing, the samples were hybridized with probes at 85°C for 10 min and then overnight at 37°C. The image capture and distance calculations were performed as described above.
Immunofluorescence combined with RNA/DNA-FISH
The IF-RNA/DNA-FISH procedures were based on a previous report [51]. In brief, the embryos were fixed with 2% PFA-PVA for 15 min at RT and then permeabilised with 0.25% Triton X-100 in PBS-PVA for 10 min. After washing with PBS-PVA, the samples were blocked in 1% BSA-PBS-PVA containing 1.3 U ml−1 RNaseOUT (Life Technologies) for 40 min. After washing, the embryos were incubated with primary antibodies (anti-RNF12, Abnova, diluted 1:200 in blocking buffer containing RNaseOUT) for 1 h. After incubation with the secondary antibody, the samples were subjected to RNA/DNA-FISH as described above except that the pepsin treatment in blastocysts was for 4 min.
Transcriptome analysis
The Hiseq system (Illumina, Inc.) was used for RNA-sequencing. In brief, total RNA from each sample (30 pooled embryos) or single blastocysts were extracted using a Qiagen RNeasy Micro Kit (Qiagen), and the remaining DNA was degraded by DNase treatment. In blastocyst samples, a fraction of the total RNA was used for qPCR analysis to screen female samples. For Kdm4b/TSA-XmXpΔ samples, we selected samples with high Xist expression. For construction of sequencing libraries, we used an Ovation Single Cell RNA-Seq System (NuGEN) according the manufacturer’s instruction. BAM format data yielded by Tohat 2.0.11 were subjected to successive analyses using AvadisNGS 1.6 (Agilent Technologies). The counts of raw reads allocated for each gene/transcript, which link to UCSC transcripts, were normalized using the TMM method (AvadisNGS 1.6). Normalized values were described as log2 values. For clustering analysis, the R function “hclust” (https://www.r-project.org/) was used to produce unsupervised clustering. The raw data was deposited in SRA (http://www.ncbi.nlm.nih.gov/sra) under accession I.D.: PRJNA312739 and PRJNA305455.
Oct4 binding regions in ES cells
The published data of Oct4 ChIP-seq [52] (GSM566277) in ES cells was visualized via the UCSC genome browser (https://genome.ucsc.edu/) using custom tracks.
Supporting Information
(a) IF analysis of H3K9me3 and H3K9ac in Kdm4b/TSA-XmXm 2-cell embryos. For control embryos, Egfp mRNA was injected and cultured with DMSO (Egfp/DMSO). The same leaser intensity was applied to all samples. Blue, red, and green show DAPI, H3K9ac, and H3K9me3, respectively. (b) RNA-FISH analysis in Kdm4b/TSA-XmXm embryos at the 4-cell stage. n, the number of cells analysed. The P-values were calculated by the Fisher’s exact test. Scale bars show 20 μm.
(TIF)
RNA/DNA-FISH analysis in Kdm4b overexpressing blastocysts. Representative images of XmY and XmXpΔ embryos are shown. Circles represent individual embryos in lower graph. n, number of embryos analyzed. Scale bars, 20 μm.
(TIF)
(a) Schematic view of RNA-FISH probes. Xist/Tsix and Tsix signals are shown in green and red, respectively. (b and c) RNA-FIHS analysis of Xist/Tsix in Kdm4b/TSA-XmY (b) and -XmXpΔ (c). The sexing of embryos was determined by DNA-FISH (see methods). (d and e) qPCR analysis in individual blastocysts in XmY of WT, Egfp/DMSO, and Kdm4b/TSA treated embryos (d) and XmXp (WT), XmXpΔ of control and Kdm4b/TSA treated embryos (e). The sexing of embryos was based on the presence of Eif2s3y mapped on the Y-chromosome. (f) Immunofluorescence analysis of H3K27me3 in Kdm4b/TSA treated embryos (Kdm4b/TSA-XmY or -XmXpΔ).
(TIF)
Venn diagram shows differentially expressed genes (DEGs) in each group. Upregulated (a) and downregulated (b). The average expression levels of each group were used for analysis and > 3-fold genes compared with WT were identified as DEGs.
(TIF)
(a) RNA-FISH analysis in XmXp, XmY, and XmXm embryos during preimplantation stages. Xist/Tsix and Tsix signals are shown in green and red, respectively. Representative images (b). Quantification of FISH signal patterns. n, the number of cells analysed.
(TIF)
(a) qPCR analysis of Rnf12KD-XmXm morulae. (b) Immunofluorescence analysis of RNF12 in Rnf12KD-XmXm morulae. Representative images were shown in picture and the graph showed signal intensities. The P-values were calculated by the Mann–Whitney U test. (c) qPCR analysis of Rex1KD-XmXm morulae. For qPCR analysis, pooled XmXm morulae were analyzed with two to three biological replicates. It was noted that we could not obtain antibody reacted to mouse REX1. The error bars show standard errors.
(TIF)
(a) The expression of Xist was examined in XmXm morula embryos treated with siRNA injection (Oct4 or Yy1). Two to three independent experiments were conducted for each target gene. The error bars show standard errors. Expression levels of scramble controls were set to one. (b) IF analysis to examine the knockdown efficiency of OCT4 protein at the morula stage. n, the number of cells. The scale bars show 20 μm. The P-values were calculated using a student’s t-test. It was noted that we could not obtain an antibody reacted to mouse YY1. (c) RNA-FISH analysis in Oct4KD- and Yy1KD-XmXm morulae. The probes used for FISH detected Xist/Tsix signals.
(TIF)
The genes with over 2-fold changes compared with controls were identified as differentially expressed genes in Rnf12KD-XmXm (a) and Oct4KD-XmXm (b) embryos.
(TIF)
(a) qPCR analysis of Tet2 and Xist expression states. (b) Representative image of RNA-FISH using a Xist/Tsix detection probe. The graph showed quantification of Xist RNA-FISH results. The P-value was calculated by a Fisher’s exact test. n, the number of analysed cells.
(TIF)
ChIP-seq data of Oct4 in undifferentiated ES cells is shown using a UCSC custom track. The BAC probe regions used in this study are shown.
(TIF)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Dr. S. Kikugawa (DNA Chip Research Inc.) and Y. Takahashi (Mitsui Knowledge Industry Co., Ltd.) for assistance with bioinformatics analysis and T. Takigashira for microscopic observation.
Data Availability
The raw data are available from SRA (http://www.ncbi.nlm.nih.gov/sra) under accession I.D.: SRP068485 and SRP071762.
Funding Statement
This work was supported by: Ministry of Education, Culture, Sports, Science, and Technology http://www.mext.go.jp/english/a06.htm JSPS KAKENHI Grant-in-Aid for Young Scientists(26861350); a Grant-in-Aid for Scientific Research (21390456) https://www.jsps.go.jp/english/e-grants/; Research Institute for Child Health and Development grant to AF (26-39) http://www.ncchd.go.jp/; and JSP-CREST http://www.jst.go.jp/kisoken/crest/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brockdorff N, Ashworth A, Kay GF, McCabe VM, Norris DP, et al. (1992) The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell 71: 515–526. [DOI] [PubMed] [Google Scholar]
- 2.Marahrens Y, Panning B, Dausman J, Strauss W, Jaenisch R (1997) Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev 11: 156–166. [DOI] [PubMed] [Google Scholar]
- 3.Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N (1996) Requirement for Xist in X chromosome inactivation. Nature 379: 131–137. 10.1038/379131a0 [DOI] [PubMed] [Google Scholar]
- 4.Fukuda A, Tomikawa J, Miura T, Hata K, Nakabayashi K, et al. (2014) The role of maternal-specific H3K9me3 modification in establishing imprinted X-chromosome inactivation and embryogenesis in mice. Nat Commun 5: 5464 10.1038/ncomms6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augui S, Nora EP, Heard E (2011) Regulation of X-chromosome inactivation by the X-inactivation centre. Nat Rev Genet 12: 429–442. 10.1038/nrg2987 [DOI] [PubMed] [Google Scholar]
- 6.Barakat TS, Gunhanlar N, Pardo CG, Achame EM, Ghazvini M, et al. (2011) RNF12 activates Xist and is essential for X chromosome inactivation. PLoS Genet 7: e1002001 10.1371/journal.pgen.1002001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonkers I, Barakat TS, Achame EM, Monkhorst K, Kenter A, et al. (2009) RNF12 is an X-Encoded dose-dependent activator of X chromosome inactivation. Cell 139: 999–1011. 10.1016/j.cell.2009.10.034 [DOI] [PubMed] [Google Scholar]
- 8.Shin J, Bossenz M, Chung Y, Ma H, Byron M, et al. (2010) Maternal Rnf12/RLIM is required for imprinted X-chromosome inactivation in mice. Nature 467: 977–981. 10.1038/nature09457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takagi N, Sasaki M (1975) Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 256: 640–642. [DOI] [PubMed] [Google Scholar]
- 10.Hoki Y, Kimura N, Kanbayashi M, Amakawa Y, Ohhata T, et al. (2009) A proximal conserved repeat in the Xist gene is essential as a genomic element for X-inactivation in mouse. Development 136: 139–146. 10.1242/dev.026427 [DOI] [PubMed] [Google Scholar]
- 11.Nesterova TB, Barton SC, Surani MA, Brockdorff N (2001) Loss of Xist imprinting in diploid parthenogenetic preimplantation embryos. Dev Biol 235: 343–350. 10.1006/dbio.2001.0295 [DOI] [PubMed] [Google Scholar]
- 12.Fodor BD, Kubicek S, Yonezawa M, O'Sullivan RJ, Sengupta R, et al. (2006) Jmjd2b antagonizes H3K9 trimethylation at pericentric heterochromatin in mammalian cells. Genes Dev 20: 1557–1562. 10.1101/gad.388206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda A, Mitani A, Miyashita T, Umezawa A, Akutsu H (2015) Chromatin condensation of Xist genomic loci during oogenesis in mice. Development 142: 4049–4055. 10.1242/dev.127308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto I, Tan S, Takagi N (2000) X-chromosome inactivation in XX androgenetic mouse embryos surviving implantation. Development 127: 4137–4145. [DOI] [PubMed] [Google Scholar]
- 15.Aoki F, Worrad DM, Schultz RM (1997) Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev Biol 181: 296–307. 10.1006/dbio.1996.8466 [DOI] [PubMed] [Google Scholar]
- 16.Adenot PG, Mercier Y, Renard JP, Thompson EM (1997) Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential transcriptional activity in pronuclei of 1-cell mouse embryos. Development 124: 4615–4625. [DOI] [PubMed] [Google Scholar]
- 17.Sado T, Wang Z, Sasaki H, Li E (2001) Regulation of imprinted X-chromosome inactivation in mice by Tsix. Development 128: 1275–1286. [DOI] [PubMed] [Google Scholar]
- 18.Lee JT, Davidow LS, Warshawsky D (1999) Tsix, a gene antisense to Xist at the X-inactivation centre. Nat Genet 21: 400–404. 10.1038/7734 [DOI] [PubMed] [Google Scholar]
- 19.Plath K, Fang J, Mlynarczyk-Evans SK, Cao R, Worringer KA, et al. (2003) Role of histone H3 lysine 27 methylation in X inactivation. Science 300: 131–135. 10.1126/science.1084274 [DOI] [PubMed] [Google Scholar]
- 20.Gontan C, Achame EM, Demmers J, Barakat TS, Rentmeester E, et al. (2012) RNF12 initiates X-chromosome inactivation by targeting REX1 for degradation. Nature 485: 386–390. 10.1038/nature11070 [DOI] [PubMed] [Google Scholar]
- 21.Navarro P, Oldfield A, Legoupi J, Festuccia N, Dubois A, et al. (2010) Molecular coupling of Tsix regulation and pluripotency. Nature 468: 457–460. 10.1038/nature09496 [DOI] [PubMed] [Google Scholar]
- 22.Hamatani T, Carter MG, Sharov AA, Ko MS (2004) Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell 6: 117–131. [DOI] [PubMed] [Google Scholar]
- 23.Navarro P, Chambers I, Karwacki-Neisius V, Chureau C, Morey C, et al. (2008) Molecular coupling of Xist regulation and pluripotency. Science 321: 1693–1695. 10.1126/science.1160952 [DOI] [PubMed] [Google Scholar]
- 24.Makhlouf M, Ouimette JF, Oldfield A, Navarro P, Neuillet D, et al. (2014) A prominent and conserved role for YY1 in Xist transcriptional activation. Nat Commun 5: 4878 10.1038/ncomms5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donohoe ME, Silva SS, Pinter SF, Xu N, Lee JT (2009) The pluripotency factor Oct4 interacts with Ctcf and also controls X-chromosome pairing and counting. Nature 460: 128–132. 10.1038/nature08098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson MD, Oshlack A (2010) A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11: R25 10.1186/gb-2010-11-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, et al. (2010) Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466: 1129–1133. 10.1038/nature09303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiba H, Hirasawa R, Kaneda M, Amakawa Y, Li E, et al. (2008) De novo DNA methylation independent establishment of maternal imprint on X chromosome in mouse oocytes. Genesis 46: 768–774. 10.1002/dvg.20438 [DOI] [PubMed] [Google Scholar]
- 29.Sun S, Del Rosario BC, Szanto A, Ogawa Y, Jeon Y, et al. (2013) Jpx RNA activates Xist by evicting CTCF. Cell 153: 1537–1551. 10.1016/j.cell.2013.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chureau C, Chantalat S, Romito A, Galvani A, Duret L, et al. (2011) Ftx is a non-coding RNA which affects Xist expression and chromatin structure within the X-inactivation center region. Hum Mol Genet 20: 705–718. 10.1093/hmg/ddq516 [DOI] [PubMed] [Google Scholar]
- 31.Sado T, Hoki Y, Sasaki H (2005) Tsix silences Xist through modification of chromatin structure. Dev Cell 9: 159–165. 10.1016/j.devcel.2005.05.015 [DOI] [PubMed] [Google Scholar]
- 32.Navarro P, Pichard S, Ciaudo C, Avner P, Rougeulle C (2005) Tsix transcription across the Xist gene alters chromatin conformation without affecting Xist transcription: implications for X-chromosome inactivation. Genes Dev 19: 1474–1484. 10.1101/gad.341105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto I, Patrat C, Thepot D, Peynot N, Fauque P, et al. (2011) Eutherian mammals use diverse strategies to initiate X-chromosome inactivation during development. Nature 472: 370–374. 10.1038/nature09872 [DOI] [PubMed] [Google Scholar]
- 34.Sado T, Sakaguchi T (2013) Species-specific differences in X chromosome inactivation in mammals. Reproduction 146: R131–139. 10.1530/REP-13-0173 [DOI] [PubMed] [Google Scholar]
- 35.Tada T, Obata Y, Tada M, Goto Y, Nakatsuji N, et al. (2000) Imprint switching for non-random X-chromosome inactivation during mouse oocyte growth. Development 127: 3101–3105. [DOI] [PubMed] [Google Scholar]
- 36.Santos F, Peters AH, Otte AP, Reik W, Dean W (2005) Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Dev Biol 280: 225–236. 10.1016/j.ydbio.2005.01.025 [DOI] [PubMed] [Google Scholar]
- 37.Puschendorf M, Terranova R, Boutsma E, Mao X, Isono K, et al. (2008) PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat Genet 40: 411–420. 10.1038/ng.99 [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Liu YJ, Nakashima H, Umehara H, Inoue K, et al. (2012) PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature 486: 415–419. 10.1038/nature11093 [DOI] [PubMed] [Google Scholar]
- 39.Ma P, Pan H, Montgomery RL, Olson EN, Schultz RM (2012) Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development. Proc Natl Acad Sci U S A 109: E481–489. 10.1073/pnas.1118403109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beuchat A, Thevenaz P, Unser M, Ebner T, Senn A, et al. (2008) Quantitative morphometrical characterization of human pronuclear zygotes. Hum Reprod 23: 1983–1992. 10.1093/humrep/den206 [DOI] [PubMed] [Google Scholar]
- 41.Wu G, Han D, Gong Y, Sebastiano V, Gentile L, et al. (2013) Establishment of totipotency does not depend on Oct4A. Nat Cell Biol 15: 1089–1097. 10.1038/ncb2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda A, Mitani A, Miyashita T, Kobayashi H, Umezawa A, et al. (2016) Spatiotemporal dynamics of Oct4 protein localization during preimplantation development in mice. Reproduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu F, Liu Y, Inoue A, Suzuki T, Zhao K, et al. (2016) Establishing Chromatin Regulatory Landscape during Mouse Preimplantation Development. Cell 165: 1375–1388. 10.1016/j.cell.2016.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terranova R, Yokobayashi S, Stadler MB, Otte AP, van Lohuizen M, et al. (2008) Polycomb group proteins Ezh2 and Rnf2 direct genomic contraction and imprinted repression in early mouse embryos. Dev Cell 15: 668–679. 10.1016/j.devcel.2008.08.015 [DOI] [PubMed] [Google Scholar]
- 45.Cheloufi S, Elling U, Hopfgartner B, Jung YL, Murn J, et al. (2015) The histone chaperone CAF-1 safeguards somatic cell identity. Nature 528: 218–224. 10.1038/nature15749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Dieuleveult M, Yen K, Hmitou I, Depaux A, Boussouar F, et al. (2016) Genome-wide nucleosome specificity and function of chromatin remodellers in ES cells. Nature 530: 113–116. 10.1038/nature16505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Xu H, Yuan P, Fang F, Huss M, et al. (2008) Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133: 1106–1117. 10.1016/j.cell.2008.04.043 [DOI] [PubMed] [Google Scholar]
- 48.Novo CL, Tang C, Ahmed K, Djuric U, Fussner E, et al. (2016) The pluripotency factor Nanog regulates pericentromeric heterochromatin organization in mouse embryonic stem cells. Genes Dev 30: 1101–1115. 10.1101/gad.275685.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barakat TS, Loos F, van Staveren S, Myronova E, Ghazvini M, et al. (2014) The trans-activator RNF12 and cis-acting elements effectuate X chromosome inactivation independent of X-pairing. Mol Cell 53: 965–978. 10.1016/j.molcel.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi H, Sakurai T, Imai M, Takahashi N, Fukuda A, et al. (2012) Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks. PLoS Genet 8: e1002440 10.1371/journal.pgen.1002440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Namekawa SH, Lee JT (2011) Detection of nascent RNA, single-copy DNA and protein localization by immunoFISH in mouse germ cells and preimplantation embryos. Nat Protoc 6: 270–284. 10.1038/nprot.2010.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ang YS, Tsai SY, Lee DF, Monk J, Su J, et al. (2011) Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell 145: 183–197. 10.1016/j.cell.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) IF analysis of H3K9me3 and H3K9ac in Kdm4b/TSA-XmXm 2-cell embryos. For control embryos, Egfp mRNA was injected and cultured with DMSO (Egfp/DMSO). The same leaser intensity was applied to all samples. Blue, red, and green show DAPI, H3K9ac, and H3K9me3, respectively. (b) RNA-FISH analysis in Kdm4b/TSA-XmXm embryos at the 4-cell stage. n, the number of cells analysed. The P-values were calculated by the Fisher’s exact test. Scale bars show 20 μm.
(TIF)
RNA/DNA-FISH analysis in Kdm4b overexpressing blastocysts. Representative images of XmY and XmXpΔ embryos are shown. Circles represent individual embryos in lower graph. n, number of embryos analyzed. Scale bars, 20 μm.
(TIF)
(a) Schematic view of RNA-FISH probes. Xist/Tsix and Tsix signals are shown in green and red, respectively. (b and c) RNA-FIHS analysis of Xist/Tsix in Kdm4b/TSA-XmY (b) and -XmXpΔ (c). The sexing of embryos was determined by DNA-FISH (see methods). (d and e) qPCR analysis in individual blastocysts in XmY of WT, Egfp/DMSO, and Kdm4b/TSA treated embryos (d) and XmXp (WT), XmXpΔ of control and Kdm4b/TSA treated embryos (e). The sexing of embryos was based on the presence of Eif2s3y mapped on the Y-chromosome. (f) Immunofluorescence analysis of H3K27me3 in Kdm4b/TSA treated embryos (Kdm4b/TSA-XmY or -XmXpΔ).
(TIF)
Venn diagram shows differentially expressed genes (DEGs) in each group. Upregulated (a) and downregulated (b). The average expression levels of each group were used for analysis and > 3-fold genes compared with WT were identified as DEGs.
(TIF)
(a) RNA-FISH analysis in XmXp, XmY, and XmXm embryos during preimplantation stages. Xist/Tsix and Tsix signals are shown in green and red, respectively. Representative images (b). Quantification of FISH signal patterns. n, the number of cells analysed.
(TIF)
(a) qPCR analysis of Rnf12KD-XmXm morulae. (b) Immunofluorescence analysis of RNF12 in Rnf12KD-XmXm morulae. Representative images were shown in picture and the graph showed signal intensities. The P-values were calculated by the Mann–Whitney U test. (c) qPCR analysis of Rex1KD-XmXm morulae. For qPCR analysis, pooled XmXm morulae were analyzed with two to three biological replicates. It was noted that we could not obtain antibody reacted to mouse REX1. The error bars show standard errors.
(TIF)
(a) The expression of Xist was examined in XmXm morula embryos treated with siRNA injection (Oct4 or Yy1). Two to three independent experiments were conducted for each target gene. The error bars show standard errors. Expression levels of scramble controls were set to one. (b) IF analysis to examine the knockdown efficiency of OCT4 protein at the morula stage. n, the number of cells. The scale bars show 20 μm. The P-values were calculated using a student’s t-test. It was noted that we could not obtain an antibody reacted to mouse YY1. (c) RNA-FISH analysis in Oct4KD- and Yy1KD-XmXm morulae. The probes used for FISH detected Xist/Tsix signals.
(TIF)
The genes with over 2-fold changes compared with controls were identified as differentially expressed genes in Rnf12KD-XmXm (a) and Oct4KD-XmXm (b) embryos.
(TIF)
(a) qPCR analysis of Tet2 and Xist expression states. (b) Representative image of RNA-FISH using a Xist/Tsix detection probe. The graph showed quantification of Xist RNA-FISH results. The P-value was calculated by a Fisher’s exact test. n, the number of analysed cells.
(TIF)
ChIP-seq data of Oct4 in undifferentiated ES cells is shown using a UCSC custom track. The BAC probe regions used in this study are shown.
(TIF)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The raw data are available from SRA (http://www.ncbi.nlm.nih.gov/sra) under accession I.D.: SRP068485 and SRP071762.