Highlights
-
•
Simplified cardiomyocyte electrophysiology model optimised for patient specific modelling.
-
•
Modified Mitchell and Schaeffer model.
-
•
No spurious pacemaker behaviour.
-
•
Suitable for parameter fitting.
-
•
Consistent leading order APD approximation.
Keywords: Mitchell–Schaeffer model, Pacemaker behaviour, Parameter fitting
Abstract
Ionic models with two state variables are routinely used in patient specific electro-physiology simulations due to the small number of parameters to be constrained and their computational tractability. Among these models, the Mitchell and Schaeffer (MS) action potential model is often used in ventricle electro-physiology due to its ability to reproduce the shape of the action potential and its restitution properties. However, for some choices of parameters characterising this ionic model, unwanted pacemaker behaviour is present. The absence of any a priori criterion to exclude unstable parameter combinations affects parameter fitting algorithms, as unphysiological solutions can only be discarded a posteriori. In this paper we propose an adaptation of the MS model that does not exhibit pacemaker behaviour for any combination of the parameters. The robustness to pacemaker behaviour makes this model suitable for inverse problem applications.
1. Introduction
Cardiac ionic models are mathematical models describing the electrical response of a cardiac myocyte following an applied electrical stimulus.
When an electrical stimulus is applied to a cardiac myocyte, an action potential is generated by the flux of ionic species across the cell membrane. Complex mathematical models [2], [15], [24] describe the ionic current generated by each ionic species. Though physiologically accurate, these models are expensive to solve numerically due to their large number of state variables and their non-linear formulation; moreover, when personalising such models, an additional challenge arises from the large number of parameters to constrain.
In contrast, phenomenological ionic models aim to describe the collective effects of the ionic currents by a smaller number of state variables (usually 2–3) and parameters, and are obtained either by simplifying a complex ionic model [7], [16], or by trying to reproduce the shape of the action potential [1], [8]. In the field of personalised models, particular emphasis has been given to the models with two state variables, due to their numerical tractability and the reduced number of parameters to be constrained [4], [10], [20], [23], [27].
Among the available two state-variable ionic models, the one introduced by Mitchell and Schaeffer (MS) [16] is often employed in ventricular electro-physiology inverse problems [4], [21], [23]. This model is capable of reproducing the shape of the action potential and the restitution properties of the action potential duration (APD), [12], [14]. The MS model is characterised by 5 parameters. It is obtained by simplifying the model proposed by Fenton and Karma, [7] (3 state variables, 13 parameters), which in turn is obtained by simplifying the more complex Luo Rudy I [15] biophysical ionic model (8 state variables, 63 parameters).
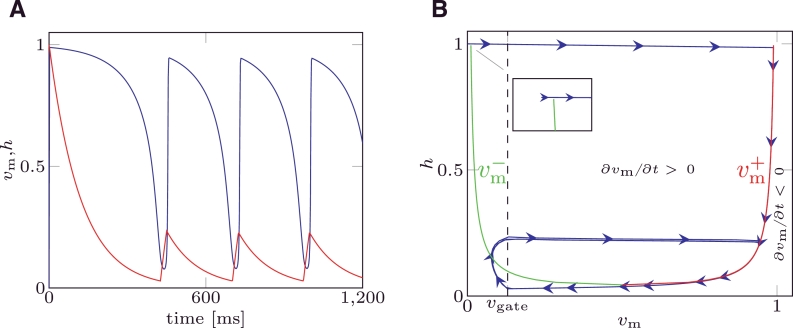
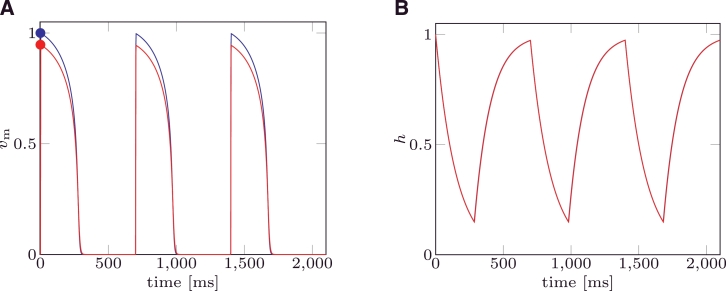
For some choices of the parameters, the MS model suffers from the so called “pacemaker cell behaviour”. That is, the trans-membrane potential cyclically depolarises and repolarises in the absence of any applied external stimulus, as depicted in Fig. 1A. In Fig. 1B (blue line) the solution of the MS model under pacemaker behaviour is depicted in the phase space. From an analysis of the MS model equations, it is possible to analytically define the relation between the gate variable and the trans-membrane potential delimiting the values of the state variables producing a depolarisation. This curve is called a nullcline, and it is split into a left branch, () and a right branch, () depicted in Fig. 1B. Once activated when the system moves towards the initial (at rest) condition, if the phase portrait crosses the nullcline branch the system “falls” into a condition where a depolarisation occurs, and a new action potential will be produced even though no external stimuli were applied. The sets of parameters that yield pacemaker cell behaviour do not delimit a closed region of the parameter space. This phenomenon has been observed and reported both in 0D and tissue models, [5], [22]. However, to the best of our knowledge there are no criteria for determining a priori which combination of parameters will produce pacemaker activity.
Fig. 1.
(A) State variable for MS model affected by pacemaker behaviour. (B) Nullclines (green: left branch, ; red: right branch, ) and phase portrait for MS model affected by pacemaker behaviour. Parameter values are reported in the Table 2 of example 2. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For patient specific modelling, this unwanted behaviour represents a problem for parameter estimation, since it necessitates a stability test for each estimated parameter set, [5].
Particularly affected by this phenomenon are the sequential data assimilation techniques [3], [9], [11]: since the values of the parameters are sequentially updated to minimise the discrepancy between the output of the model and the measurements of the system under study, if a combination of parameters yielding pacemaker behaviour is produced, the algorithm adopted could become unstable and diverge.
To overcome these limitations, in this paper we derive and introduce a two state-variable ionic model that describes the action potential phases by 5 parameters, has the same benefits as the MS model, and is robust to pacemaker behaviour. These characteristics make it suitable for generating personalised electrophysiology models for clinical applications, in particular when a sequential data assimilation technique is employed. This paper is organised as follows: in Section 2 we introduce the mathematical formulation of the new ionic model; in Section 3 we prove the absence of pacemaker behaviour in the phase plane; in Section 4 we derive an asymptotic derivation of the restitution curves and compare it to the one described in [16] for the standard MS model; and in Section 5 we compare the solutions and the restitution properties of the new model with the MS model for some numerical examples.
2. The modified Mitchell–Schaeffer ionic model
The standard MS ionic model [16] describes the ionic currents that flow across the cell membrane with a gated-inward ionic current, representing the current produced by the flux of the sodium ions, and an ungated outward ionic current, representing the current produced by the flux of the potassium ions. The two state variables characterising the MS model represent the electric potential of the cell membrane and the gate dynamics of the sodium ion channels. The model can be written in the generalised form proposed by [6]:
| (1) |
| (2) |
where Jstim is an externally applied electrical stimulus, vm is the trans-membrane potential, h is the gate variable of the inward current, vgate is the activation threshold potential and τin, τout, τopen, τclose are the 4 time constants affecting the 4 characteristic phases of the trans-membrane potential. The standard MS model described in [16] is obtained by imposing ; the parameters a and λ were introduced by [6] and used to control the excitability of the system.
The modified Mitchell–Schaeffer (mMS) ionic model presented in this paper is obtained by first replacing and in (1): consequently, if vm < vgate, then ∂vm/∂t < 0 and the system naturally evolves towards the rest condition instead of producing an action potential, in contrast to the original MS model where there is a range of values yielding ∂vm/∂t > 0.
The effect of the potassium ion current is negligible when vm ≤ vgate and the cell is returning to a quiescent state. It is possible to introduce gating effects to the outward ionic current with the complement of the gate variable, (1h), adopting an expression similar to the one introduced in [25]. This leads to the following system of ODEs:
| (3) |
| (4) |
Remark
The robustness to pacemaker behaviour is obtained by modifying the cubic polynomial on the right hand side of Eq. (1). The addition of gating on the outward current has three advantages: first, when the threshold value of the transmembrane voltage above which an action potential is triggered is equal to vgate; second, for the transmembrane potential at the end of the upstroke will be ; third, as will be discussed in Section 4, the analytical solution of the mMS model coincides to the analytical solution of the MS model for the same set of ionic parameters for a particular choice of vgate.
3. Robustness to pacemaker behaviour
The study of the robustness of the mMS model to pacemaker behaviour consists in determining if the condition ∂vm/∂t > 0 could occur without an external stimulus being applied. In the phase plane, the nullclines of Eq. (1) for the MS model and Eq. (3) for the mMS model define the boundary between the region where the depolarisation occurs and the region where the system naturally evolves towards the rest condition; when h recovers towards its rest state if the phase portrait crosses the nullcline branch as depicted in Fig. 1B, then the system depolarises again and a new action potential is produced without an external stimulus having been applied.
The robustness of the mMS model to pacemaker behaviour is shown by proving that its phase portrait cannot intersect the nullcline branch unless an external stimulus is applied. To this aim, we first derive the nullcline of Eq. (3), we then determine the three characteristic points corresponding to the minimum and maximum potentials delimiting the region where depolarisation occurs, and the point where the and branches merge; we also compare these points with the corresponding points from the original MS model. Once the nullcline and characteristic points are known, we show that the phase portrait of mMS cannot cross the nullcline branch during the recovery of h.
3.1. Nullclines
Denoting the minimum value of h on the nullclines in the MS model by
| (5) |
the nullclines of MS are defined in [16] as follows:
| (6) |
denoting by
| (7) |
the minimum value of h on the nullcline of the mMS model, the nullclines of the new mMS model described in Eq. (3) are defined as follows:
| (8) |
Eqs. (6) for MS and (8) for mMS define two regions in the phase plane: the first one defined by the interval where ∂vm/∂t > 0; the second one defined by and where ∂vm/∂t < 0. Fig. 2A depicts the nullclines of MS ( ) and mMS (
) and mMS ( ). In the same figure, the black dashed line represents the threshold potential vgate where h switches between closing and opening (recovery); the region vm < vgate of the phase plane is critical in the study of the robustness to pacemaker behaviour.
). In the same figure, the black dashed line represents the threshold potential vgate where h switches between closing and opening (recovery); the region vm < vgate of the phase plane is critical in the study of the robustness to pacemaker behaviour.
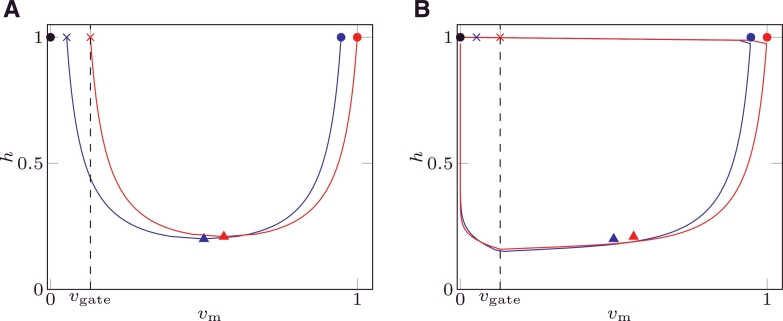
Fig. 2.
Nullclines (A) and phase portrait (B) of the mMS ( ) and MS (
) and MS ( ) models. The black dashed line represents ; the points (
) models. The black dashed line represents ; the points ( ) and (
) and ( ) represent the points 1,2 and 3 for the mMS and MS models, respectively. The zeroth point (•) represents the rest (initial) state and coincides for both models. The values of the parameters were taken from [16].
) represent the points 1,2 and 3 for the mMS and MS models, respectively. The zeroth point (•) represents the rest (initial) state and coincides for both models. The values of the parameters were taken from [16].
The following 4 characteristic points are defined for Eqs. (6) and (8) and are depicted in Fig. 2:
-
•
Point 0: rest (initial) state. In both models, this point (•) is characterised by in the phase plane. This point does not belong to nullclines (6) and (8).
-
•
Point 1: end of depolarisation from a rest state, . This point characterises the value of vm at the end of the depolarisation upstroke and it is equal to (1, 1) for mMS (
 ) and to (
) and to ( ) for MS.
) for MS. -
•
Point 2: merging point of the and branches. This point defines the minimum value of h on the nullcline: it approximates the state point where the phase portrait “falls off the nullcline”, and is equal to (
 ) for mMS and to (
) for mMS and to ( ) for MS.
) for MS. -
•
Point 3: minimum potential on branch . This point represents the minimum value an external stimulus has to rise vm, to produce an action potential when the system is fully recovered. It is equal to (vgate, 1) (
 ) for mMS and to (
) for mMS and to (  ) for MS.
) for MS.
In the phase plane, the phase portrait is defined as the curve described by the values (vm(t), h(t)) which constitute the solution of the ionic model. The phase portraits of MS ( ) and mMS (
) and mMS ( ) are depicted in the right panel of Fig. 2 for a non-pacemaker solution; the phase portrait for a pacemaker solution for the MS model is depicted in Fig. 2B.
) are depicted in the right panel of Fig. 2 for a non-pacemaker solution; the phase portrait for a pacemaker solution for the MS model is depicted in Fig. 2B.
3.2. Robustness
The nullcline and characteristic points were defined above. From these points we show that the phase portrait of mMS cannot cross the nullcline branch during the recovery of h unless an external stimulus is applied. To simplify the notation, we denote the value of the trans-membrane potential of Point 2 in mMS by
We then split the phase portrait into two parts: the repolarisation, defined by the interval for mMS and the interval vgate ≤ vm ≤ 1/2 for MS, and the recovery, when h returns to its initial value, defined by vm < vgate.
For both models, during the repolarisation vm approaches vgate at a rate proportional to 1/τout. Since vm ≥ vgate, h decreases at a rate proportional to 1/τclose and the phase portrait moves away from the nullcline branch lying in the region of the phase plane where no depolarisation can occur.
When vm < vgate, h recovers at a rate proportional to 1/τopen, while the time derivative of vm is still negative, as depicted in Fig. 2B. The system moves towards Point 0. Since in mMS, the phase portrait will never cross the nullcline branch independently of the value of τopen. Unless an external stimulus is applied, the system evolves towards Point 0, and no pacemaker behaviour will occur. In contrast, the MS model can cross the nullcline branch if τopen is small enough, since there is a non-empty region where .
We tested the robustness of the MS and the mMS models to pacemaker behaviour on a regular grid set of 57,800 parameters chosen with the range and the spacing reported in Table 1. The set of parameters characterised by were excluded since this choice does not produce an action potential. The statistics of the pacemaker behaviour was thus evaluated on a total of 52598 samples. For both models, an external stimulus was applied at ms and the numerical solution was evaluated over 1200 ms; parameter sets presenting more than one depolarisation were considered pacemaker. For 3042 combinations (≃6% of the cases) MS displayed pacemaker behaviour, while the mMS was robust in all of the 52,598 tests.
Table 1.
Parameter set ranges and step adopted for testing the robustness to pacemaker behaviour of the mMS model. For the same set of parameters the behaviour of MS was also evaluated.
| τin [ms] | τout [ms] | τopen [ms] | τclose [ms] | |
|---|---|---|---|---|
| min | 0.05 | 0.5 | 60 | 60 |
| max | 0.5 | 10 | 220 | 220 |
| step | 0.05 | 0.5 | 10 | 10 |
4. Asymptotic derivation of the restitution curve
Following the same procedure adopted in [16], it is possible to derive an explicit leading order asymptotic approximation for the APD restitution curve of mMS, based on the assumption
| (9) |
From assumption (9) it follows that the time constants of Eq. (3) are infinitesimal if compared to the time constants of Eq. (4) and thus the duration of the depolarisation and repolarisation phases can be neglected when compared to the APD and recovery durations.
4.1. Single stimulus: maximum APD (APDmax)
In this paper we adopt the same definition introduced in [16] for APD as the elapsed time during which vm ≥ vgate.
Due to the separation of the time scale introduced by assumption (9) and considering a cell membrane initially in the rest state (), then the action potential generated by an externally applied stimulus is decomposed into the following 4 phases:
-
1.
Depolarisation: when the external stimulus is applied with sufficient duration Tstim and intensity such that vm(Tstim) > vgate, the fast inward current dominates and the potential rises quickly to () on the right nullcline with a characteristic time proportional to τin.
-
2.
APD: when the cell membrane is fully depolarised and the gate progressively closes, the inward and the outward ionic currents balance each other. The system evolves following the right nullcline on a time scale proportional to τclose.
-
3.
Repolarisation: when the solution falls off the nullcline, the outward current dominates, and the potential drops toward on a time scale proportional to τout.
-
4.
Recovery: when the gate variable slowly re-opens and recovers with a characteristic time scale of τopen. Since the fast outward current, with characteristic time scale of τin, dominates, the potential drops to rapidly, due to assumption (9).
The durations of the depolarisation and repolarisation are negligible if compared to the APD and recovery duration: thus, at leading order, APD can be approximated as the time elapsed to evolve from to obtained from (4) as follows:
| (10) |
4.2. Multiple stimuli: restitution curve
In this section we derive a leading order approximation of the APD when two (or more) stimuli are applied. The first stimulus is applied at to a fully recovered tissue; the second stimulus is applied at time to a system not fully recovered.
Denoting by APD1 the APD following the first stimulus and by DI = S-APD1 the diastolic interval (DI), that is the time elapsed between the end of the APD and the second pacing stimulus, from Eq. (4) there follows
| (11) |
Due to assumption (9), the depolarisation duration is negligible if compared to APD and recovery duration; thus, from (4) and the initial condition (11), the following relation between APD and DI, called restitution, holds:
| (12) |
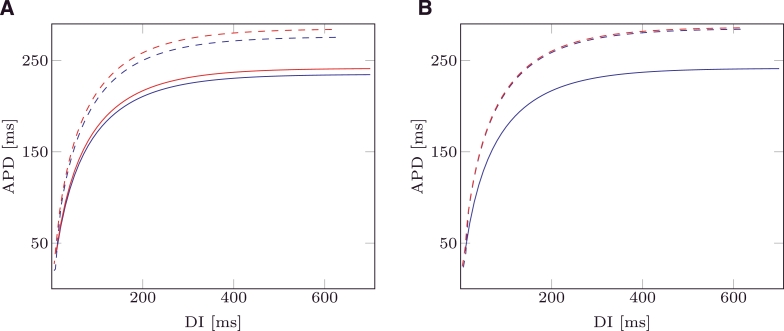
In Fig. 3A the analytical APD restitutions are depicted for mMS ( ) and MS (
) and MS ( ) and . In the same figure dashed lines depict the APD restitution computed by numerically solving MS and mMS. In Fig. 3B, the same restitutions are plotted for : this value is evaluated by imposing and produces identical analytical restitution curves for both models. This choice of the activation threshold also produced similar computed restitution curves for the two models (dashed lines, Fig. 3B).
) and . In the same figure dashed lines depict the APD restitution computed by numerically solving MS and mMS. In Fig. 3B, the same restitutions are plotted for : this value is evaluated by imposing and produces identical analytical restitution curves for both models. This choice of the activation threshold also produced similar computed restitution curves for the two models (dashed lines, Fig. 3B).
Fig. 3.
Analytic APD restitution curves for mMS ( ) and MS (
) and MS ( ) models. Evaluated APD restitution curves for mMS (
) models. Evaluated APD restitution curves for mMS ( ) and MS (
) and MS ( ) models. The values of the ionic parameters were taken from [16], and are . (A) ; (B) .
) models. The values of the ionic parameters were taken from [16], and are . (A) ; (B) .
The restitution curves were computed by applying an protocol [17]. Briefly, the model is repetitively stimulated with an inter-pacing interval s1 until a steady state is achieved, (in the present paper, applied 100 times to achieve a limit cycle), and then a single stimulus is applied at an interval s2 < s1 and the system is left to evolve. This procedure is applied for different values of s2 and the APD is measured after the premature stimulus is applied. In the present paper, the values of the premature stimulus s2 < s1 were chosen with the following sequence:
Remark
Expressions (10) and (12) are formally equivalent to those obtained in [16] for MS and differ only in the expression of Point 2 defined in Section 3.1. While in the MS model this point is defined by (5) and does not depend on vgate, in the mMS model this point is defined by expression (7) which introduces a dependence on vgate in expressions (10) and (12).
Since the APD is defined as the elapsed time during which vm ≥ vgate, expressions (10) and (12) are able to account for this dependency. As a demonstration, if is chosen, one expects no APD will be generated, independently of the choice of the other 4 ionic parameters. The analytic restitution expression of the MS model still will furnish a non-zero APD in this case. In contrast, from expressions (10) and (11) the restitution equation for the mMS model predicts an APD of 0 ms.
5. Numerical examples
To compare the functional characteristics of the MS and mMS models we compare model simulations for a stable action potential, an action potential affected by pacemaker behaviour, a dynamic restitution protocol and pacemaker behaviour in a tissue simulation.
Time discretisation was performed by a backward Euler method and non-linearities were treated by Newton iterations.
A time step was chosen; to test the accuracy of the chosen time step we evaluated the numerical solution for the set of parameters reported in [16] with a time step and a time step . The maximum and the L2 differences between the solutions were equal to 0.004 and 0.005 respectively, (i.e., ≃ 0.5% of the maximum value of vm). The externally applied stimulus is characterised by an intensity of and a duration of 0.4 ms.
In Section 5.1 we deal with the ionic parameters taken from [16] that do not provide any pacemaker activity for MS.
In Section 5.2 we adopt the parameters reported in Table 2 that yield pacemaker behaviour for the MS model. We will show that while the MS model will furnish a pacemaker solution, the mMS model will be robust to this behaviour.
Table 2.
Parameter values used for testing pacemaker stability.
| vgate | τin [ms] | τout [ms] | τopen [ms] | τclose [ms] |
|---|---|---|---|---|
| 0.13 | 0.1 | 9.0 | 100 | 120 |
In Section 5.3 we evaluate the dynamic restitution curves for both models with the parameter set defined in [16] and we show that the differences between the two models in the dynamic APD restitutions are small.
In Section 5.4 we consider a homogeneous tissue slab characterised by the parameters reported in Table 3, stimulated by a cross-field protocol. This set of parameters did not produce any pacemaker behaviour for the MS model in a single cell, nor in a 1D tissue string for the same parameter set and model conditions, but did show pacemaker behaviour in two dimensions. We show that the mMS model is robust to pacemaker behaviour under these conditions.
Table 3.
Parameter values used for testing pacemaker stability.
| vgate | τin [ms] | τout [ms] | τopen [ms] | τclose [ms] | conductivity [cm2/s] |
|---|---|---|---|---|---|
| 0.13 | 0.15 | 6.5 | 90 | 85 | 1.75 |
5.1. Example 1: pacemaker free MS
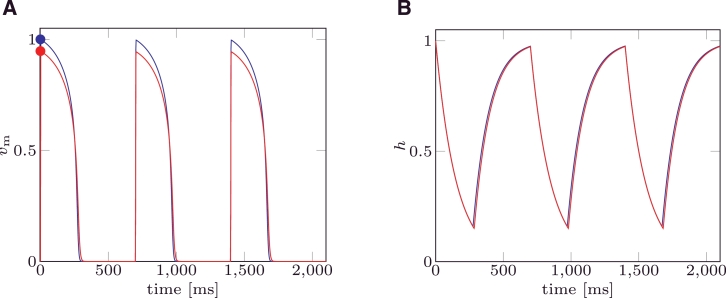
In the first example the MS and the mMS models were solved with the parameter set taken from [16]. This choice yields a pacemaker free MS model. Three stimuli were applied at an inter-pacing interval . For both models the trans-membrane potential (Fig. 4A) and the gate variable (Fig. 4B) functions of time are depicted.
Fig. 4.
(A) Trans-membrane potential (vm) for mMS ( ) and MS (
) and MS ( ) models. Circles (
) models. Circles ( ) represent the values () characterising Point 1 for mMS and MS, respectively. (B) Gate variable for mMS (
) represent the values () characterising Point 1 for mMS and MS, respectively. (B) Gate variable for mMS ( ) and MS (
) and MS ( ) models. Parameter values were taken from [16], and are .
) models. Parameter values were taken from [16], and are .
The dynamics of the gate variable h do not significantly differ between the two models, while the trans-membrane potential vm obtained by solving the mMS model only differs in the maximum value reached at the end of the depolarisation.
In Fig. 5 the trans-membrane potential vm and the gate variable h are plotted when a value of is chosen. This choice of vgate yields the same analytical restitution curves for the mMS and the MS models.
Fig. 5.
(A) Trans-membrane potential (vm) for mMS ( ) and MS (
) and MS ( ) models. Circles (
) models. Circles ( ) represent values () characterising Point 1 for mMS and MS, respectively. (B) Gate variable for mMS (
) represent values () characterising Point 1 for mMS and MS, respectively. (B) Gate variable for mMS ( ) and MS (
) and MS ( ) models. Values for the ionic parameters were taken from [16], and are while .
) models. Values for the ionic parameters were taken from [16], and are while .
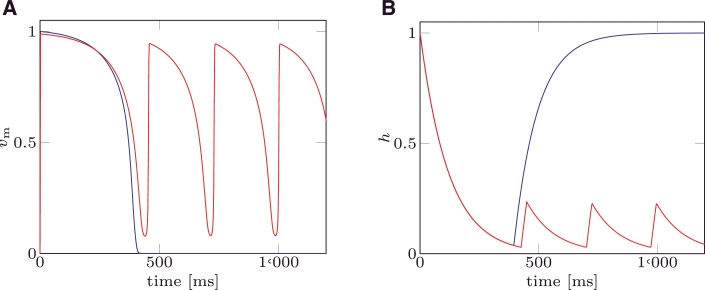
5.2. Example 2: MS with pacemaker activity
In the second example we characterise both models with the parameter set summarised in Table 2. This choice leads to a pacemaker solution in the MS model. The system is paced once at and the solution is evaluated until . The results are plotted in Fig. 6; while the mMS model presents only one action potential, the MS cyclically depolarises (with 4 activations during the simulation period).
Fig. 6.
(A) Trans-membrane potential (vm) for mMS ( ) and MS (
) and MS ( ) models. (B) Gate variable (h) for mMS (
) models. (B) Gate variable (h) for mMS ( ) and MS (
) and MS ( ) models. Parameter values are reported in Table 2.
) models. Parameter values are reported in Table 2.
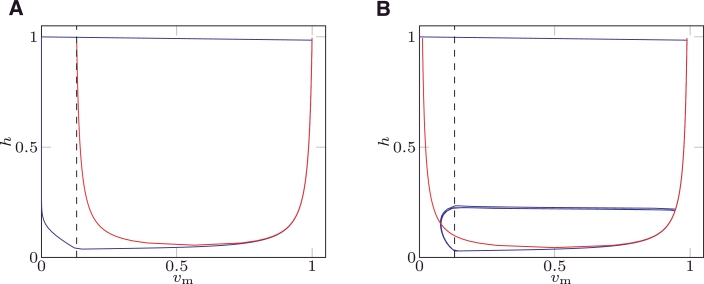
In Fig. 7 the nullclines and the phase portrait are depicted for both models. The mMS model (Fig. 7A) recovers without crossing the left nullcline, since the recovery of h is far from the nullcline. The MS model (Fig. 7B) crosses the left nullcline during recovery and thus re-depolarises entering into a periodic limit cycle.
Fig. 7.
Phase portrait ( ) and nullclines (
) and nullclines ( ) for the modified MS model (A) and the standard MS model (B). Black dashed line () represents the threshold where h switches to recovering. Parameter values reported in Table 2 were taken.
) for the modified MS model (A) and the standard MS model (B). Black dashed line () represents the threshold where h switches to recovering. Parameter values reported in Table 2 were taken.
5.3. Example 3: dynamic restitution curves
Dynamic restitution curves are used to characterise the value of the APD when the tissue is periodically paced with a pacing period S. As discussed in [16], at larger values of S, each stimulus produces an action potential, yielding a 1:1 correspondence between S and APD, called 1:1 behaviour. As S decreases, this 1:1 behaviour eventually becomes unstable; as a result, two possible behaviours can be presented:
-
•
A 2:1 behaviour: only every other stimulus produces an action potential;
-
•
A 2:2 behaviour (alternans): each stimulus will produce an action potential, but the APD periodically alternates between short and long.
For both models, dynamic restitution curves were numerically evaluated. For each pacing period S, the system was periodically paced by applying 104 external stimuli and the APD was computed for each of the last 4 applied stimuli. When the APD values of the last 4 stimuli all coincide, the behaviour was considered of type 1:1. If the APD presented two different alternating values and all of the 4 stimuli produced an action potential the behaviour was considered of type 2:2 (alternans). Lastly, if the APD presented only one value and the 4 stimuli produced only two activations, the behaviour was considered to be of type 2:1. The examples tested did not present any type 2:1 behaviour.
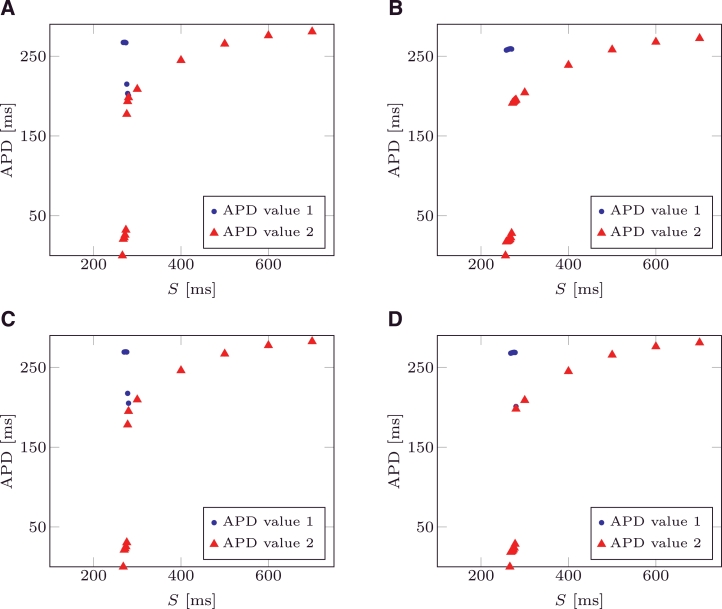
Dynamic restitutions were evaluated for the parameters of Table 2 and for as defined in Section 4.2. The pacing interval S was decremented from 700 ms to 300 ms with a step of 100 ms and then from 280 ms with a step of 2 ms to the first value that did not produce APD. For no APD were produced for S = 256 ms for the mMS model and S = 266 ms for the MS model; the dynamic restitution of the MS model bifurcates at S = 278 ms with two APD values differing by 10 ms, while the mMS model bifurcates at 270 ms with two APD values differing by 230 ms. For no APD was produced for S = 266 ms for the mMS model and S = 268 ms for the MS model, while bifurcations occurred at S = 280 ms with two APD values differing by 10 ms in the MS model, and at S = 278 ms with two APD values differing by 240 ms in the MS model. The pacing periods where either APD bifurcates or no APD was produced differ by 2 ms between both models, a difference comparable with the step used to decrement S.
In Fig. 8A the dynamic restitution of the MS model is depicted, while in Fig. 8B the same curve is depicted for the mMS model. In Fig. 8C and D the dynamic restitutions are depicted for the mMS and the MS models respectively. The two different APD values are depicted with a blue circle and a red triangle (second stimulus). When the circles and triangles are no longer superimposed, bifurcation occurs.
Fig. 8.
(A) Dynamic APD restitution for MS model, . (B) Dynamic APD restitution for the mMS model, . For two subsequent pacings, APD value are depicted as ( ) and (
) and ( ). When circles and triangles are no longer superimposed, bifurcation occurs. (C) Dynamic APD restitution for MS model, . (D) Dynamic APD restitution for the mMS model, . (For interpretation of the references to colour in the text, the reader is referred to the web version of this article.)
). When circles and triangles are no longer superimposed, bifurcation occurs. (C) Dynamic APD restitution for MS model, . (D) Dynamic APD restitution for the mMS model, . (For interpretation of the references to colour in the text, the reader is referred to the web version of this article.)
5.4. Example 4: 2D model with a cross-field stimulus
In this example we consider an homogeneous isotropic tissue slab measuring 5 cm × 5 cm; the tissue electrophysiology is described by the mono-domain model [13] and solved with the CARP [18], [26] finite element code. The equations were discretised in space with a triangular mesh with a characteristic size and in time with a time step of 0.1 ms. The model was simulated for .
The tissue electrophysiology was characterised by the parameters reported in Table 3. These parameters did not generate any pacemaker activity in simulations of an isolated single cell or in a 1D domain.
The tissue was stimulated by the cross field stimulation protocol, [19]. Briefly, an external stimulus was applied to two orthogonal regions at two different stimulation times to trigger a spiral activation pattern. In this work the first stimulus s1 was applied at on the left edge of the slab between and 0.2 cm, and s2 was applied at on the bottom edge of the slab between and 0.25 cm. For both stimuli, a current intensity of with a duration of 0.6 ms were employed.
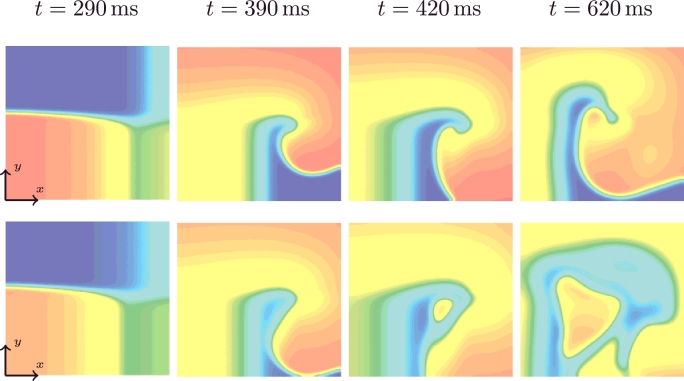
The transmembrane potentials of the mMS (top row) and the MS (bottom row) models are depicted in Fig. 9 for (s2 is applied), (a spiral wave is generated, no pacemaker activity is present), (pacemaker behaviour appears in the MS model) and (presence of pacemaker behaviour in the MS model). A video of the whole simulation can be found in the online supplement.
Fig. 9.
Trans-membrane potential followed by a cross-field stimulus for the mMS (top) and the MS (bottom) models at times t = 290, 390, 420 and 620 ms.
The mMS model generated a spiral re-entry wave that broke up at and terminated at whereas the MS model showed a pacemaker behaviour, initiated by a pacemaker beat at (Fig. 9, third column) and which persisted over the entire simulation period.
6. Discussion and conclusions
In this paper we introduced a novel two state-variable ionic model, obtained by modifying the standard Mitchell–Schaeffer (MS) ionic model. The new model has the same benefits as the standard MS model and is proven to be robust to pacemaker behaviour. Previously the MS model had been adapted to introduce pacemaker behaviour [6]. The nonlinear term characterising the inward ionic current was modified by introducing two new parameters. In this paper, we presented a model that is robust to pacemaker behaviour by modifying the inward current in a similar manner. Unlike [6], in this paper we also gated the outward current. This yields three advantages: first, the threshold value of the transmembrane voltage above which an action potential is triggered corresponds to vgate; second, at the end of the depolarisation, ; third, the analytical solution of the mMS model coincides with the analytical solution of the MS model for the same set of ionic parameters and .
We then introduced an asymptotic derivation of the restitution curves, obtaining a relation formally equivalent to that obtained in [16]. We also showed, through numerical examples, that there are only small differences in the action potential introduced by the modified MS (mMS) equations compared to the original MS model when the same set of parameters are adopted. We also compared the APD dynamic restitution curves obtained by numerically solving both models; we showed that the mMS is able to reproduce the APD alternans and that these occur under a similar pacing regime in both models. Last, we demonstrated the robustness of the new model even when incorporated into a mono-domain tissue simulation, confirming its applicability in tissue scale patient-specific modelling.
Acknowledgements
This work was supported by the British Heart Foundation (PG\13\37\30280) and the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Footnotes
Supplementary material associated with this article can be found, in the online version, at 10.1016/j.mbs.2016.08.010
Contributor Information
Cesare Corrado, Email: cesare.corrado@kcl.ac.uk.
Steven A. Niederer, Email: steven.niederer@kcl.ac.uk.
Appendix A. Supplementary materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
References
- 1.Aliev R.R., Panfilov A.V. A simple two-variable model of cardiac excitation. Chaos, Solitons Fractals. 1996;7(3):293–301. [Google Scholar]
- 2.Beeler G.W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J. Physiol. 1977;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z. Bayesian filtering: from Kalman filters to particle filters, and beyond. Statistics. 2003;182(1):1–69. [Google Scholar]
- 4.Corrado C., Gerbeau J.-F., Moireau P. Identification of weakly coupled multiphysics problems. Application to the inverse problem of electrocardiography. J. Comput. Phys. 2015;283(0):271–298. http://dx.doi.org/10.1016/j.jcp.2014.11.041 [Google Scholar]
- 5.Corrado C., Whitaker J., Chubb H., William S., Wright M., Gill J., O’Neill M., Niederer S. Personalized models of human atrial electrophysiology derived from endocardial electrograms. IEEE Trans. Biomed. Eng. 2016;PP(99) doi: 10.1109/TBME.2016.2574619. [DOI] [PubMed] [Google Scholar]
- 6.Djabella K., Landau M., Sorine M. 2007 46th IEEE Conference on Decision and Control. 2007. A two-variable model of cardiac action potential with controlled pacemaker activity and ionic current interpretation; pp. 5186–5191. [Google Scholar]
- 7.Fenton F., Karma A. Vortex dynamics in three-dimensional continuous myocardium with fiber rotation: filament instability and fibrillation. Chaos: An Interdiscip. J. Nonlinear Sci. 1998;8(1):20–47. doi: 10.1063/1.166311. [DOI] [PubMed] [Google Scholar]
- 8.FitzHugh R. Impulses and physiological states in theoretical models of nerve membrane. Biophys. J. 1961;1(6):445. doi: 10.1016/s0006-3495(61)86902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houtekamer P.L., Mitchell H.L. Data assimilation using an ensemble Kalman filter technique. Mon. Weather Rev. 1998;126(3):796–811. [Google Scholar]
- 10.Jensen A.C., Ditlevsen S., Kessler M., Papaspiliopoulos O. Markov chain Monte Carlo approach to parameter estimation in the Fitzhugh-Nagumo model. Phys. Rev. E. 2012;86:041114. doi: 10.1103/PhysRevE.86.041114. [DOI] [PubMed] [Google Scholar]
- 11.Julier S.J., Uhlmann J.K. Unscented filtering and nonlinear estimation. Proc. IEEE. 2004;92(3):401–422. [Google Scholar]
- 12.Karma A. Spiral breakup in model equations of action potential propagation in cardiac tissue. Phys. Rev. Lett. 1993;71(7):1103. doi: 10.1103/PhysRevLett.71.1103. [DOI] [PubMed] [Google Scholar]
- 13.Keener J.P., Sneyd J. Mathematical Physiology. vol. 1. Springer; 1998. [Google Scholar]
- 14.Koller M.L., Riccio M.L., Jr. R.F.G. Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am. J. Physiol. - Heart Circ. Physiol. 1998;275(5):H1635–H1642. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 15.Luo C.-h., Rudy Y. A model of the ventricular cardiac action potential. depolarization, repolarization, and their interaction. Circ. Res. 1991;68(6):1501–1526. doi: 10.1161/01.res.68.6.1501. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell C., Schaeffer D. A two-current model for the dynamics of cardiac membrane. Bull. Math. Bio. 2003;65:767–793. doi: 10.1016/S0092-8240(03)00041-7. [DOI] [PubMed] [Google Scholar]
- 17.Murgatroyd F.D. Handbook of Cardiac Electrophysiology: A Practical Guide to Invasive EP Studies and Catheter Ablation. Remedica; 2002. [Google Scholar]
- 18.Niederer S., Mitchell L., Smith N., Plank G. Simulating human cardiac electrophysiology on clinical time-scales. Front Physiol. 2011;2:14. doi: 10.3389/fphys.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pertsov A.M., Davidenko J.M., Salomonsz R., Baxter W.T., Jalife J. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ. Res. 1993;72(3):631–650. doi: 10.1161/01.res.72.3.631. [DOI] [PubMed] [Google Scholar]
- 20.Relan J., Sermesant M., Delingette H., Pop M., Wright G.A., Ayache N. IEEE International Symposium on Biomedical Imaging: From Nano to Macro, 2009. ISBI’09. IEEE; 2009. Quantitative comparison of two cardiac electrophysiology models using personalisation to optical and MRI data; pp. 1027–1030. [Google Scholar]
- 21.Relan J., Sermesant M., Pop M., Delingette H., Sorine M., Wright G.A., Ayache N. World Congress on Medical Physics and Biomedical Engineering, September 7–12, 2009, Munich, Germany. Springer; 2010. Parameter estimation of a 3D cardiac electrophysiology model including the restitution curve using optical and MRI data; pp. 1716–1719. [Google Scholar]
- 22.Rioux M., Bourgault Y. A predictive method allowing the use of a single ionic model in numerical cardiac electrophysiology. ESAIM: Math. Modell. Numer. Anal. 2013;47:987–1016. [Google Scholar]
- 23.Talbot H., Cotin S., Razavi R., Rinaldi C., Delingette H. CARS 2015. 2015. Personalization of cardiac electrophysiology model using the unscented Kalman filtering. [Google Scholar]
- 24.Ten Tusscher K., Noble D., Noble P., Panfilov A. A model for human ventricular tissue. Am. J. Physiol. - Heart Circ. Physiol. 2004;286(4):H1573–H1589. doi: 10.1152/ajpheart.00794.2003. [DOI] [PubMed] [Google Scholar]
- 25.Van Capelle F., Durrer D. Computer simulation of arrhythmias in a network of coupled excitable elements. Circ. Res. 1980;47(3):454–466. doi: 10.1161/01.res.47.3.454. [DOI] [PubMed] [Google Scholar]
- 26.Vigmond E., Dos Santos R.W., Prassl A., Deo M., Plank G. Solvers for the cardiac bidomain equations. Prog. Biophys. Mol. Biol. 2008;96(1):3–18. doi: 10.1016/j.pbiomolbio.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L., Wong K., Zhang H., Liu H., Shi P. A statistical physiological-model-constrained framework for computational imaging of subject-specific volumetric cardiac electrophysiology using optical imaging and MRI data. In: Camara O., Pop M., Rhode K., Sermesant M., Smith N., Young A., editors. Statistical Atlases and Computational Models of the Heart. vol. 6364. Springer; Berlin, Heidelberg: 2010. pp. 261–269. (Lecture Notes in Computer Science). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/