Abstract
IMPORTANCE
Depression has been identified as a risk factor for dementia. However, most studies have measured depressive symptoms at only one time point, and older adults may show different patterns of depressive symptoms over time.
OBJECTIVE
To investigate the association between trajectories of depressive symptoms and risk of dementia in older adults.
DESIGN, SETTING, AND PARTICIPANTS
This was a prospective cohort investigation of black and white community-dwelling older adults in the Health, Aging, and Body Composition study. Participants were enrolled between May 1997 and June 1998 and followed up through 2001–2002. The dates of this analysis were September 2014 to December 2015. The setting was community research centers in Memphis, Tennessee, and Pittsburgh, Pennsylvania. Trajectories of depressive symptoms were assessed from baseline to year 5. Symptoms were measured with the Center for Epidemiologic Studies Depression Scale Short Form, and trajectories were calculated using latent class growth curve analysis.
MAIN OUTCOMES AND MEASURES
Incident dementia through year 11, determined by dementia medication use, hospital records, or significant cognitive decline (≥1.5 SD race-specific decline on the Modified Mini-Mental State Examination). We examined the association between depressive symptom trajectories and dementia incidence using Cox proportional hazards regression models adjusted for demographics, health factors that differed between groups, and cognition during the depressive symptom assessment period (baseline to year 5).
RESULTS
The analytic cohort included 2488 black and white older adults with repeated depressive symptom assessments from baseline to year 5 who were free of dementia throughout that period. Their mean (SD) age at baseline was 74.0 (2.8) years, and 53.1% (n = 1322) were female. The following 3 depressive symptom trajectories were identified: consistently minimal symptoms (62.0% [n = 1542] of participants), moderate and increasing symptoms (32.2%[n = 801] of participants), and high and increasing symptoms (5.8% [n = 145] of participants). Compared with the consistently minimal trajectory, having a high and increasing depressive symptom trajectory was associated with significantly increased risk of dementia (fully adjusted hazard ratio, 1.94; 95% CI, 1.30–2.90), while the moderate and increasing trajectory was not associated with risk of dementia after full adjustment. Sensitivity analyses indicated that the high and increasing trajectory was associated with dementia incidence, while depressive symptoms at individual time points were not.
CONCLUSIONS AND RELEVANCE
Older adults with a longitudinal pattern of high and increasing depressive symptoms are at high risk for dementia. Individuals’ trajectory of depressive symptoms may inform dementia risk more accurately than one-time assessment of depressive symptoms.
The relationship between depression and cognition in aging is complex, with evidence supporting different temporal associations between depressive symptoms and the onset of cognitive decline and dementia.1 Some studies2,3 suggest older adults may develop depressive symptoms in reaction to experiencing cognitive decline, other researchers identify depression as a risk factor for dementia,4 and others theorize that depressive symptoms and cognitive decline may both be symptoms of an underlying neurodegenerative process.5
Most studies investigating the association between depressive symptoms and development of dementia have been limited by measuring depressive symptoms at only one time point. This approach does not capture intraindividual variability in symptoms or the longitudinal course of depressive symptoms, which may be particularly important because older individuals appear to experience different patterns of depressive symptoms over time.6–8 For example, a study7 of older women followed up for approximately 20 years showed that individuals tended to exhibit the following 4 longitudinal patterns of depressive symptoms: trajectories of “minimal,” “persistently low,” “increasing,” and “persistently high” symptoms. It remains unclear whether different trajectories of depressive symptoms confer differential risk for dementia.
We investigated the association between trajectories of depressive symptoms and risk of dementia among black and white older adults followed up prospectively in the Health, Aging, and Body Composition (Health ABC) study. We hypothesized that individuals with particularly deleterious depressive symptom trajectories (eg, increasing or persistently high symptoms)would be most likely to develop dementia. We also investigated whether the effect of depressive symptom trajectories on dementia risk differed by sex, race, or APOE (OMIM 107741) ε4 carrier status because some evidence suggests that depression or its association with dementia may differ by these factors.9–11 In addition, we investigated whether depressive symptom trajectories provide information regarding risk of dementia beyond that captured by one-time assessment of depressive symptoms.
Methods
Population
Participants were from the Health ABC prospective cohort study of community-dwelling older adults conducted in Memphis, Tennessee, and Pittsburgh, Pennsylvania. Potential participants were identified and contacted based on a random sample of white and all black Medicare-eligible older adults within designated zip codes. Eligibility criteria included self-report of no difficulties performing activities of daily living, walking a quarter of a mile, or climbing 10 steps without resting and no plans to leave the are a within 3 years. Of 22 999 individuals identified, 8695 could not be contacted, 7250 declined, 3082 were ineligible, and 897 were deceased, institutionalized, or had moved away. A total of 3075 adults (age range, 70–79 years)were enrolled between May 1997 and June 1998 and followed up through 2001–2002 for the present study. The dates of the analysis were September 2014 to December 2015. Institutional review boards at the University of Pittsburgh, University of Tennessee(Memphis), and University of California (San Francisco) approved the study. All participants provided written informed consent. Prior publications give additional details about the Health ABC study.12–15
Key Points.
Question
Are different trajectories of depressive symptoms in older adults associated with risk of dementia?
Findings
In this prospective cohort study of 2488 older adults, a trajectory of high and increasing depressive symptoms was associated with significantly increased risk of dementia even when accounting for other factors and severity of depressive symptoms measured at single time points.
Meaning
Older adults who exhibit a pattern of chronically high and increasing depressive symptoms over time may be at higher risk of subsequently developing dementia.
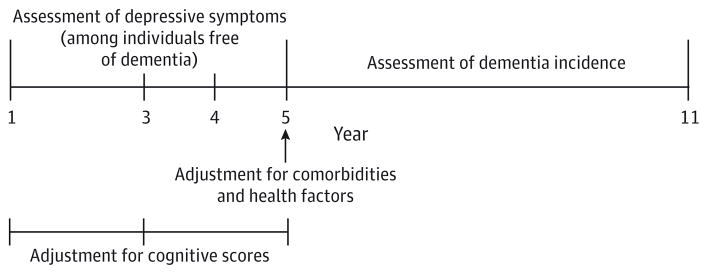
For the present study, we investigated whether depressive symptom trajectories from baseline (year 1) to year 5 (1997–1998 through 2001–2002)were associated with subsequent development of dementia through year 11 (2007–2008). This analysis time line (Figure 1) was specified to assess depressive symptom trajectories over approximately the first half of the study and investigate whether these trajectories were associated with development of dementia during later years. Because we aimed to characterize longitudinal patterns of depressive symptoms from baseline to year 5, we excluded participants with fewer than 2 depression assessments during that interval (193 individuals met this criteria). To investigate whether depressive symptom trajectories were associated with later development of dementia, we excluded participants whom et criteria for dementia during the depressive symptom assessment period (142 individuals met this criteria), and we also excluded participants who did not complete the year 5 visit or have follow-up for dementia after year 5 (432 individuals met these criteria). After these exclusions, the analytic cohort consisted of 2488 participants (mean [SD] age at baseline, 74.0 [2.8] years; 38.4% [n = 956] black; and 53.1%[n = 1322] female), who had a mean (SD) of 3.9 (0.3) depression assessments between baseline and year 5 and a mean (SD) of 4.9 (1.9) years of follow-up for dementia after year 5.
Figure 1. Study Design Showing the Time Line of Analysis.
The time line was used to investigate the association between depressive symptom trajectories over years 1 through 5 and subsequent risk of dementia after year 5. Comorbidities and other health factor variables were assessed at year 5 to adjust models for health factors that had developed by the end of the depressive symptom assessment period. To adjust for differences in overall level of cognitive functioning and cognitive change during the depressive symptom assessment period, models were adjusted for the mean Modified Mini-Mental State Examination score over years 1 through 5 and the Modified Mini-Mental State Examination difference score (year 5 minus year 1).
Measures
Depressive Symptoms
Depressive symptoms were measured at years 1, 3, 4, and 5 with the Center for Epidemiologic Studies Depression Scale Short Form(CES-D-10),16 a 10-item self-report scale of symptoms over the past week. The maximal total score is 30, with higher scores reflecting greater symptoms and a score of 10 or higher indicating clinically significant depression.16
Incident Dementia
Incident dementia was determined based on hospital records, medication use, and decline in global cognitive functioning, as in previous Health ABC studies.17–19 Participants were queried every 6 months about the occurrence of hospitalizations, and Health ABC staff requested records associated with the hospitalizations. To obtain medication information, participants were asked to bring their medications to each clinic visit for study staff to record. To assess global cognitive functioning, participants were repeatedly administered the Modified Mini-Mental State Examination (3MS),20 a cognitive screening measure that assesses orientation, concentration, language, praxis, and immediate and delayed memory. The 3MS, with a score range of 0 to 100 points, has been shown to be more sensitive in detecting dementia than other cognitive screening instruments.21
Dementia incidence was determined based on a combination of criteria, with the date of dementia onset defined as the date a participant first met any of the following criteria: (1) record of hospitalization indicating dementia as a primary or secondary diagnosis, (2) record of a prescription for dementia medication (eg, galantamine hydrobromide, rivastigmine, memantine hydrochloride, donepezil hydrochloride, or tacrine hydrochlroide), or (3) evidence of clinically significant decline in global cognitive functioning based on the 3MS scores (≥1.5S Drace-specific decline from an individual’s baseline 3MS score to the 3MS score at his/her last available visit). The date of dementia diagnosis by the latter criterion was considered to be when an individual’s 3MS score first fell below the cutoff for a 1.5 SD race-specific decline in comparison with his or her baseline 3MS score. Time to event was defined as the time between a participant’s year 5 visit and when the participant was either classified as having incident dementia or censored from observation at the last available contact.
Other Variables
Age, sex, race, and education were self-reported at baseline. Literacy, in terms of reading grade level, was assessed shortly after baseline using the Rapid Estimate of Adult Literacy in Medicine (REALM).22 Cardiovascular comorbidities, including diabetes mellitus, hypertension, history of stroke or transient ischemic attack, and history of myocardial infarction, were determined at baseline and follow-up visits based on a combination of self-report data, physician diagnosis, medications, and laboratory values. Body mass index (calculated as weight in kilograms divided by height in meters squared) was recorded at baseline and follow-up visits from direct height and weight measurements. Participants self-reported cigarette smoking. APOE genotype testing was conducted via standard single-nucleotide polymorphism analyses, and participants were coded as ε4 carriers vs noncarriers. Treatment for depression with antidepressant medication was determined based on medication information collected at clinic visits, taking into account that antidepressant use was related to depression rather than other indications.
Statistical Analysis
We evaluated trajectories of depressive symptoms over the first 5 years using latent class growth curve analysis, a semipara-metric analysis that differentiates groups of individuals based on their probability of following a similar trajectory on an outcome overtime.23 We conducted latent class growth curve analysis using a statistical procedure (SAS Proc Traj; SAS Institute Inc) to estimate the mean trajectories of CES-D-10 scores (measured at years 1, 3, 4, and 5) across visits. The repeated CES-D-10 scores were modeled as censored normal.24 We evaluated the appropriate number of trajectories and trajectory shape following recommended procedures.24 We required that each trajectory group had to include at least 5% of participants.24 Model selection proceeded in the following manner. For each model specifying a given number of trajectories, we tested linear, quadratic, and cubic terms to determine what shapes best fit the data (terms were considered significant if P < .05). Using the resulting models, we evaluated the appropriate number of trajectories by calculating the estimated log Bayes factor, which compares Bayesian information criterion values between models.23 Log Bayes factor values exceeding 10 are considered a “very strong” indicator that the more complex model better fits the data.23 These procedures indicated that a model with 3 trajectories (2 cubic and 1 quadratic) was superior to models with fewer trajectories (log Bayes factor for 2-trajectory model vs 1-trajectory model was 2368.06, and log Bayes factor for 3-trajectory model vs 2-trajectory model was 607.34). Although a model with 4 trajectories was associated with further improvement in model fit (log Bayes factor, 228.52), this model yielded a trajectory with less than 5% of participants. Therefore, we selected the 3-trajectory model for use in subsequent analyses.
We used χ2 tests and analyses of variance to explore whether participant characteristics differed between depressive trajectory groups. To examine the association between depressive symptom trajectories and risk of dementia, we plotted Kaplan-Meier survival curves and conducted Cox proportional hazards regression models that specifically investigated the association between depressive symptom trajectories (from baseline to year 5) and dementia incidence after year 5. Model 1 was unadjusted. Model 2 adjusted for demographics (age, sex, and race), education, and literacy. Model 3 additionally adjusted for comorbidities and other health factors that differed between trajectory groups (P < .10). These variables were assessed at year 5 to enable investigation of the effect of depressive symptom trajectories on dementia risk independent of other health factors that had developed by the end of the depressive symptom assessment period. Model 4 additionally adjusted for individuals’ mean 3MS score over year 1 to year 5 and the 3MS difference score (year 5 minus year 1) to account for differences in overall level of cognitive functioning and cognitive change during the depressive symptom assessment period. Using model 4, we tested for interactions between depressive symptom trajectory with sex, race, and APOE ε4 carrier status in predicting risk of dementia.
We conducted sensitivity analyses to investigate whether depressive symptom trajectories were associated with dementia risk above and beyond the effect of depressive symptoms captured by one-time assessment. To investigate whether trajectories were more informative than individuals’ baseline depressive symptoms, we additionally adjusted model 4 for baseline CES-D-10 score. To investigate whether depressive trajectories were more informative than individuals’ final depressive symptom score, we additionally adjusted model 4 for year 5 CES-D-10 score.
Statistical analyses were conducted using software programs( SAS, version 9.4; SAS Institute Inc and Stata, version 13.1; Stata Corp LP). Statistical significance was set at P < .05(2 tailed).
Results
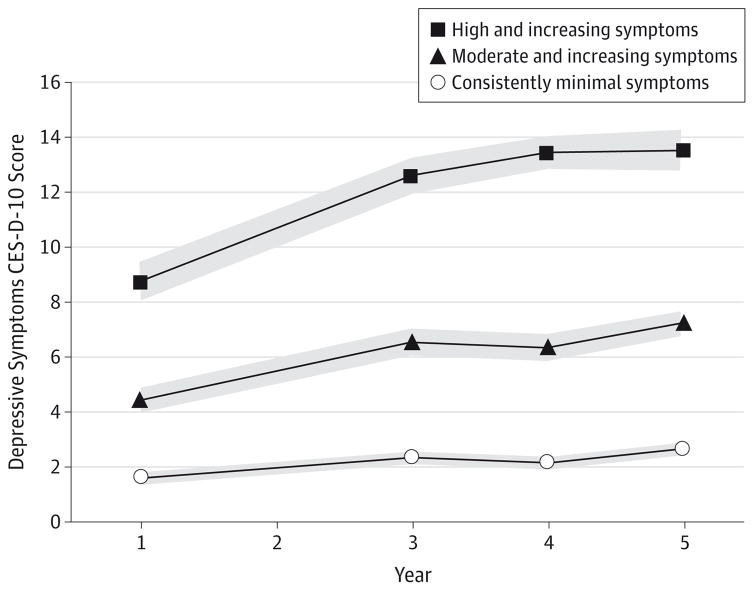
As shown in Figure 2, the 3 depressive symptom trajectories reflected patterns of minimal, moderate, or high symptoms regarding overall symptom level. Symptoms also tended to increase over time in each trajectory but particularly so in the latter 2; therefore, we labeled the 3 trajectories as consistently minimal, moderate and increasing, and high and increasing symptoms. Among 2488 older adults, predicted group membership in the 3 trajectories was as follows: 62.0%(n = 1542)with consistently minimal symptoms, 32.2% (n = 801)with moderate and increasing symptoms, and 5.8%(n = 145)with high and increasing symptoms. The mean posterior probabilities of group membership for each trajectory (0.92 for consistently minimal, 0.87 for moderate and increasing, and 0.91 for high and increasing) suggested strong reliability. As summarized in Table 1, sex, race, education, and literacy differed across trajectory groups. The presence of the following health factors, as assessed at year 5 (by the end of the depressive symptom assessment period), also differed between trajectory groups: history of stroke or transient ischemic attack, history of myocardial infarction, hypertension, and cigarette smoking. In addition, trajectory groups differed in the mean 3MS score and the 3MS score change from year 1 to year 5.
Figure 2. Depressive Symptom Trajectories From Baseline to Year 5 Among 2488 Older Adults.
Shown are results of the latent class growth curve analysis used to identify groups of individuals following a similar trajectory of depressive symptoms over time, as assessed with the Center for Epidemiologic Studies Depression Scale Short Form (CES-D-10) score at years 1, 3, 4, and 5. The 3 depressive symptom trajectories that were identified are shown with 95% CIs (gray shaded area).
Table 1.
Participant Characteristics by Depressive Symptom Trajectory Group Among 2488 Older Adults
| Characteristic | Consistently Minimal Symptoms (n = 1542) | Moderate and Increasing Symptoms (n = 801) | High and Increasing Symptoms (n = 145) | P Value |
|---|---|---|---|---|
| Age at year 1, mean (SD), y | 74.0 (2.8) | 74.0 (2.8) | 74.3 (2.9) | .52 |
| Female sex, No. (%) | 736 (47.7) | 487 (60.8) | 99 (68.3) | <.001 |
| Black race, No. (%) | 554 (35.9) | 348 (43.5) | 54 (37.2) | .002 |
| Education less than high school, No./total No. (%) | 299/1540 (19.4) | 232/799 (29.0) | 43/145 (29.7) | <.001 |
| Ninth grade literacy or higher, No. (%) | 1120 (72.6) | 534 (66.7) | 94 (64.8) | .004 |
| APOE ε4 carrier status, No./total No. (%) | 377/1463 (25.8) | 219/757 (28.9) | 39/136 (28.7) | .25 |
| History of stroke or TIA at year 5, No. (%) | 367 (23.8) | 227 (28.3) | 47 (32.4) | .01 |
| History of myocardial infarction at year 5, No. (%) | 299 (19.4) | 176 (22.0) | 41 (28.3) | .02 |
| Hypertension at year 5, No./total No. (%) | 1254/1540 (81.4) | 686/801 (85.6) | 122/145 (84.1) | .03 |
| Diabetes mellitus at year 5, No. (%) | 325 (21.1) | 190 (23.7) | 34 (23.5) | .31 |
| Body mass index at year 5, mean (SD)a | 27.2 (4.7) | 27.4 (4.9) | 27.7 (5.5) | .39 |
| Current cigarette smoking at year 5, No./total No. (%) | 69/1332 (5.2) | 50/678 (7.4) | 15/130 (11.5) | .006 |
| 3MS score over year 1 to year 5, mean (SD) | 91.5 (6.8) | 89.1 (8.4) | 89.2 (8.0) | <.001 |
| 3MS difference score, year 5 minus year 1, mean (SD) | 0.2 (4.9) | −0.2 (5.4) | −0.8 (5.2) | .05 |
Abbreviations: 3MS, Modified Mini-Mental State Examination; TIA, transient ischemic attack.
Calculated as weight in kilograms divided by height in meters squared.
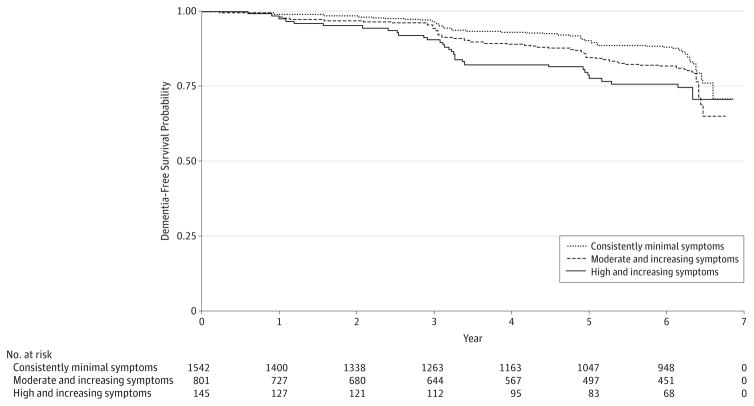
Overall, 353 participants (14.2%) developed dementia and did so a mean (SD) of 3.9 (1.7) years after the depressive symptom assessment period (28.7 cases per 1000 person-years). Kaplan-Meier survival curves by depressive symptom trajectory group (Figure 3) indicated that the groups significantly differed in dementia incidence (P < .001 by log-rank test). Results of unadjusted and adjusted Cox proportional hazards regression models are summarized in Table 2. Compared with individuals with consistently minimal depressive symptoms, those with high and increasing symptoms were significantly more likely to develop dementia even in the fully adjusted model 4 (adjusted hazard ratio [HR], 1.94; 95% CI, 1.30–2.90). While the moderate and increasing symptom trajectory was associated with increased risk of dementia in models 1 through 3, this association was reduced and became nonsignificant in model 4 after adjusting for mean cognitive functioning and cognitive change during the depressive symptom assessment period (adjusted HR, 1.16; 95% CI, 0.91–1.49). There were no interactions between depressive symptom trajectory and sex, race, or APOE ε4 carrier status (P > .05 for all).
Figure 3. Association Between Depressive Symptom Trajectory Group and Dementia-Free Survival Among 2488 Older Adults.
Kaplan-Meier survival curves show dementia incidence after year 5 by depressive symptom trajectory group (P < .001 by log-rank test).
Table 2.
Association Between Depressive Symptom Trajectory Group and Risk of Dementia Among 2488 Older Adultsa
| Depressive Symptom Trajectory Group | No./Total No. (%) With Incident Dementia | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Consistently minimal symptoms | 190/1542 (12.3) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Moderate and increasing symptoms | 132/801 (16.5) | 1.41 (1.13–1.76) | 1.41 (1.13–1.77) | 1.29 (1.01–1.64) | 1.16 (0.91–1.49) |
| High and increasing symptoms | 31/145 (21.4) | 1.93 (1.32–2.82) | 1.94 (1.32–2.85) | 2.09 (1.41–3.10) | 1.94 (1.30–2.90) |
Model 1 is unadjusted. Model 2 is adjusted for demographics, education, and literacy. Model 3 is model 2 plus adjusted for history of stroke or transient ischemic attack and myocardial infarction, hypertension, and cigarette smoking status. Model 4 is model 3 plus adjusted for the mean Modified Mini-Mental State Examination score over year 1 to year 5 and the Modified Mini-Mental State Examination difference score (year 5 minus year 1).
Sensitivity analyses examining whether depressive symptom trajectories were associated with risk of dementia above and beyond depressive symptoms at one time point yielded results similar to our primary findings. After adding adjustment for year 1 CES-D-10 score to model 4, the high and increasing depressive symptom trajectory was significantly associated with dementia incidence (adjusted HR, 1.75; 95% CI, 1.04–2.93), while the moderate and increasing symptoms trajectory was not (adjusted HR, 1.13; 95% CI, 0.85–1.48). There was no association between year 1 CES-D-10 score and dementia incidence in this model (adjusted HR, 1.01; 95% CI, 0.97–1.06). This same pattern was apparent when adding adjustment for year 5 CES-D-10 score to model 4, with adjusted HRs of 2.08 (95% CI, 1.17–3.70) for high and increasing symptoms, 1.20(95% CI, 0.88–1.63) for moderate and increasing symptoms, and 0.99 (95% CI, 0.96–1.03) for year 5 CES-D-10 score.
To explore whether treatment with antidepressant medication influenced findings, we added adjustment for antidepressant medication use during the depressive symptom assessment period to model 4, and results remained similar. However, only 6.2% (n = 154) of the cohort took antidepressant medication, limiting our ability to further explore medication effects.
Discussion
We found that older adults with patterns of moderate and increasing and high and increasing depressive symptoms were more likely to develop dementia than those with consistently minimal symptoms. After adjusting for individuals’ cognitive functioning at the time of their depressive symptom trajectories, only the high and increasing trajectory remained associated with increased risk of dementia. Whether depression is a risk factor for dementia vs a symptom of an underlying neurodegenerative process is a complex question. Although our study cannot fully disentangle these possibilities, our results suggest the nature of the depression-dementia relationship may differ depending on the pattern of depressive symptoms. Given that predementia cognitive functioning at least partially explained the association we observed between the moderate and increasing depressive symptom trajectory and dementia risk, it is possible that older adults with that trajectory were experiencing depressive symptoms as an emotional reaction to already being in the early stages of cognitive decline. Alternatively, the same underlying disease process may give rise to both cognitive decline and moderate and increasing depressive symptoms. In contrast, our results suggest that a trajectory of high and increasing depressive symptoms is an independent risk factor for dementia. Older adults following that trajectory were almost twice as likely to develop dementia even after adjusting for cognitive functioning at the time of their depressive trajectories. Moreover, dementia risk associated with that trajectory was not simply due to high baseline depressive symptoms or high symptoms at the most recent time point. Instead, a pattern of depressive symptoms that are both chronically high and increasing over time appears particularly impactful in increasing dementia risk.
There are many potential mechanisms by which depressive symptoms may either lead to dementia or be a prodromal phase of dementia. Both major depression and depressive symptoms in older adults are associated with reductions in hippocampal volume,25,26 and the neurotoxicity hypothesis posits that glucocorticoids released in response to stress may be responsible for this atrophy.27 Associations between depression and β-amyloid levels28 raise the possibility that depressive symptoms may be an early indication of preclinical Alzheimer disease or that depression may more directly lead to development of Alzheimer neuropathology.29 Cerebrovascular disease30,31 and inflammation32 may also underlie the depression-dementia relationship because these processes are associated with both depressive symptoms and cognitive decline. Future research is needed to clarify whether mechanisms driving associations between depressive symptoms and dementia may differ depending on the pattern of depressive symptoms that individuals experience over time.
Our results suggest that tracking older persons’ depressive symptoms over time in clinical settings may help identify individuals at greatest risk for dementia. This recommendation is in line with previous findings of related cohort studies of older women (primarily individuals of white race), which found that women with high depressive symptoms at 2 time points33 or greater cumulative burden of depressive symptoms over time34 were most likely to develop cognitive impairment. Building on this work, our study suggests that the clinical importance of tracking older adults’ depressive symptoms over time extends to more diverse older adult populations because associations we observed were similar across men and women, black and white individuals, and regardless of APOE ε4 status.
Strengths of our study include a novel analysis to identify different depressive symptom trajectories and explore associations with dementia risk. Our study benefits from the ability to divide follow-up time into 2 segments, which helps establish temporal precedence of the observed relationships. Limitations include that our analysis time line was somewhat arbitrary, and we cannot confirm causality, nor can we be certain of when the earliest stages of neurodegeneration first began among individuals who developed dementia, particularly because dementia can have a long prodromal period.35 Relatedly, because our dementia out come was based on an algorithm rather than a clinical evaluation, misclassification is possible, and we cannot know if or how this design influenced our results. Our study also does not investigate risk of cognitive decline that does not reach the threshold of dementia. In addition, we studied a cohort of relatively healthy, mostly non depressed older adults rather than a clinical sample; therefore, results may differ for older adults who are seen in clinical settings for depression care.
Conclusions
In summary, we identified a pattern of high and increasing depressive symptoms as an independent risk factor for dementia, while moderate and increasing depressive symptoms may be associated with incident dementia in relation to an underlying neurodegenerative disease process already in progress. Our results suggest that individuals’ trajectory of depressive symptoms may inform dementia risk above and beyond assessment of depressive symptoms at one time point alone. Because few participants in our study were taking antidepressant medication and no information about nonpharmacologic interventions was known, we were unable to thoroughly investigate the potential effect of treatment for depression on our findings. Future studies are needed to determine whether depression interventions, particularly for individuals with high and increasing symptoms, may help improve older adults’ depressive symptom trajectories and in turn reduce dementia risk.
Acknowledgments
Funding/Support: This research was supported by contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106 from the National Institute on Aging; by grant R01-AG028050 from the National Institute on Aging; by grant R01-NR012459 from the National Institute for Nursing Research; and in part by the Intramural Research Program of the National Institute on Aging. The research described herein was also supported in part by Career Development Award 1IK2RX001629 from the US Department of Veterans Affairs, Rehabilitation Research and Development Service (Dr Kaup); by research training grant T32 MH019986 from the National Institute of Mental Health (Dr Smagula); by grant K24AG031155 from the National Institute on Aging (Dr Yaffe); and by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the San Francisco Veterans Affairs Medical Center, and the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center.
Role of the Funder/Sponsor: The funding sources supported the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation and review of the manuscript; approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Kaup reported being given access to software programs and tablet devices by Akili Interactive Labs for use in research separate from and unrelated to the present study. Dr Yaffe reported being a consultant for Novartis and Pfizer, reported serving on data and safety monitoring boards for Takeda Inc and a National Institute on Aging–sponsored study, and reported serving on the Beeson Scientific Advisory Board. No other disclosures were reported.
Disclaimer: The contents of the article do not represent the views of the US Department of Veterans Affairs or the US government.
Author Contributions: Dr Kaup had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kaup, Yaffe.
Acquisition, analysis, or interpretation of data: Kaup, Byers, Falvey, Yaffe.
Drafting of the manuscript: Kaup, Falvey.
Critical revision of the manuscript for important intellectual content: Kaup, Byers, Simonsick, Satterfield, Ayonayon, Smagula, Rubin, Yaffe.
Study supervision: Yaffe.
Administrative, technical, or material support: All authors.
References
- 1.Bennett S, Thomas AJ. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79(2):184–190. doi: 10.1016/j.maturitas.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Jajodia A, Borders A. Memory predicts changes in depressive symptoms in older adults: a bidirectional longitudinal analysis. J Gerontol B Psychol Sci Soc Sci. 2011;66(5):571–581. doi: 10.1093/geronb/gbr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinkers DJ, Gussekloo J, Stek ML, Westendorp RG, van der Mast RC. Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ. 2004;329(7471):881. doi: 10.1136/bmj.38216.604664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panza F, Frisardi V, Capurso C, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18(2):98–116. doi: 10.1097/JGP.0b013e3181b0fa13. [DOI] [PubMed] [Google Scholar]
- 6.Cui X, Lyness JM, Tang W, Tu X, Conwell Y. Outcomes and predictors of late-life depression trajectories in older primary care patients. Am J Geriatr Psychiatry. 2008;16(5):406–415. doi: 10.1097/JGP.0b013e3181693264. [DOI] [PubMed] [Google Scholar]
- 7.Byers AL, Vittinghoff E, Lui LY, et al. Twenty-year depressive trajectories among older women. Arch Gen Psychiatry. 2012;69(10):1073–1079. doi: 10.1001/archgenpsychiatry.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuchibhatla MN, Fillenbaum GG, Hybels CF, Blazer DG. Trajectory classes of depressive symptoms in a community sample of older adults. Acta Psychiatr Scand. 2012;125(6):492–501. doi: 10.1111/j.1600-0447.2011.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsson IK, Bennet AM, Ploner A, et al. Apolipoprotein E ε4 genotype and the temporal relationship between depression and dementia. Neurobiol Aging. 2015;36(4):1751–1756. doi: 10.1016/j.neurobiolaging.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol. 2005;57(3):381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 11.Skarupski KA, Mendes de Leon CF, Bienias JL, et al. Black-white differences in depressive symptoms among older adults over time. J Gerontol B Psychol Sci Soc Sci. 2005;60(3):136–142. doi: 10.1093/geronb/60.3.p136. [DOI] [PubMed] [Google Scholar]
- 12.Harris TB, Visser M, Everhart J, et al. Waist circumference and sagittal diameter reflect total body fat better than visceral fat in older men and women: the Health, Aging, and Body Composition study. Ann N Y Acad Sci. 2000;904(1):462–473. doi: 10.1111/j.1749-6632.2000.tb06501.x. [DOI] [PubMed] [Google Scholar]
- 13.Rooks RN, Simonsick EM, Miles T, et al. The association of race and socioeconomic status with cardiovascular disease indicators among older adults in the Health, Aging, and Body Composition study. J Gerontol B Psychol Sci Soc Sci. 2002;57(4):S247–S256. doi: 10.1093/geronb/57.4.s247. [DOI] [PubMed] [Google Scholar]
- 14.Yaffe K, Barnes D, Lindquist K, et al. Health ABC Investigators. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28(2):171–178. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Yaffe K, Fiocco AJ, Lindquist K, et al. Health ABC Study. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72(23):2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 17.Hong CH, Falvey C, Harris TB, et al. Anemia and risk of dementia in older adults: findings from the Health ABC study. Neurology. 2013;81(6):528–533. doi: 10.1212/WNL.0b013e31829e701d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaup AR, Simonsick EM, Harris TB, et al. Older adults with limited literacy are at increased risk for likely dementia. J Gerontol A Biol Sci Med Sci. 2014;69(7):900–906. doi: 10.1093/gerona/glt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaffe K, Falvey C, Harris TB, et al. Health ABC Study. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. doi: 10.1136/bmj.f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 21.Holsinger T, Plassman BL, Stechuchak KM, Burke JR, Coffman CJ, Williams JW., Jr Screening for cognitive impairment: comparing the performance of four instruments in primary care. J Am Geriatr Soc. 2012;60(6):1027–1036. doi: 10.1111/j.1532-5415.2012.03967.x. [DOI] [PubMed] [Google Scholar]
- 22.Davis TC, Long SW, Jackson RH, et al. Rapid Estimate of Adult Literacy in Medicine: a shortened screening instrument. Fam Med. 1993;25(6):391–395. [PubMed] [Google Scholar]
- 23.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–393. [Google Scholar]
- 24.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent class growth modelling: a tutorial. Tutor Quant Methods Psychol. 2009;5(1):11–24. [Google Scholar]
- 25.Sawyer K, Corsentino E, Sachs-Ericsson N, Steffens DC. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging Ment Health. 2012;16(6):753–762. doi: 10.1080/13607863.2012.678478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donovan NJ, Hsu DC, Dagley AS, et al. Depressive symptoms and biomarkers of Alzheimer’s disease in cognitively normal older adults. J Alzheimers Dis. 2015;46(1):63–73. doi: 10.3233/JAD-142940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 28.Harrington KD, Lim YY, Gould E, Maruff P. Amyloid-β and depression in healthy older adults: a systematic review. Aust N Z J Psychiatry. 2015;49(1):36–46. doi: 10.1177/0004867414557161. [DOI] [PubMed] [Google Scholar]
- 29.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18(9):963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorelick PB, Scuteri A, Black SE, et al. American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32(10):1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 33.Goveas JS, Espeland MA, Hogan PE, et al. Depressive symptoms and longitudinal changes in cognition: Women’s Health Initiative Study of Cognitive Aging. J Geriatr Psychiatry Neurol. 2014;27(2):94–102. doi: 10.1177/0891988714522697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeki Al Hazzouri A, Vittinghoff E, Byers A, et al. Long-term cumulative depressive symptom burden and risk of cognitive decline and dementia among very old women. J Gerontol A Biol Sci Med Sci. 2014;69(5):595–601. doi: 10.1093/gerona/glt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68(3):351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]