Abstract
We assessed regional differences in potentially discretionary [<3 units of red blood cell (RBC)] transfusions across 56 medical centers and 11,200 patients undergoing isolated non-emergent coronary artery bypass (CABG) surgery. Regional variation in overall RBC rates remained after risk adjustment, perhaps due to differences in regional practice environments.
Objective
A number of established regional quality improvement collaboratives have partnered to assess and improve care across their regions under the umbrella of the “Cardiac Surgery Quality IMPROVEment (IMPROVE) Network”. The first effort of the IMPROVE Network has been to assess regional differences in potentially discretionary [<3 units of red blood cell (RBC)] transfusions.
Methods
We examined 11,200 patients undergoing isolated non-emergent coronary artery bypass (CABG) surgery across 56 medical centers in four IMPROVE Network regions between January 2008 and June 2012. Each center submitted the most recent 200 patients who received 0, 1, or 2 units of RBC transfusion during the index admission. Patient and disease characteristics, intra-operative practices, and percentage of cases receiving RBC transfusions were collected. Region-specific transfusion rates were calculated, after adjusting for pre- and intra-operative factors among region-specific centers.
Results
There were small, but significant, differences in patient case mix across regions. RBC transfusions of 1 or 2 units occurred among 25.2% (2,826/11,200) of CABG procedures. Significant variation in use and number of RBCs existed across regions [None: 74.8% (min:max 70.0%, 84.1%), 1 unit: 9.7% (5.1%, 11.8%), 2 units: 15.5% (9.1%, 18.2%)], p<0.001. Variation in overall transfusion rates remained after adjustment (9.1% – 31.7%, p<0.001).
Conclusions
Delivery of small volumes of RBC transfusions was common, yet varied across geographic regions. These data suggest that differences in regional practice environments, including transfusion triggers and anemia management, may contribute to variability in RBC transfusion rates.
Keywords: Transfusion, Coronary artery bypass grafts, CABG
For more than two decades the Society of Thoracic Surgeons (and more recently, public and private payers) has encouraged cardiothoracic surgeons to participate in a national cardiac surgical registry designed to facilitate the assessment of clinical practice and outcomes from these surgical procedures. Data from this registry have been used for research, as well as to support quality assurance and improvement. While contributing surgeons receive detailed reports describing the process and outcomes of their care, comparative outcomes reporting alone often fails to positively transform care.
Regional voluntary collaboratives for open-heart surgery have also emerged over the last several decades. Unlike a registry, a surgical collaborative is a consortium of institutions that discusses opportunities for working together to improve the quality and safety of care. Within cardiac surgery, such collaboratives are typically state-based with the majority using Society of Thoracic Surgeons (STS) data elements. Uniquely, many of the regional collaboratives have leveraged physician champions to convene surgeons and allied health professionals on a regular basis to focus their energy and intellect on a broad variety of quality improvement initiatives. In this fashion, these groups have turned registry data into information useful for targeted action, thus demonstrating improvement in the process and outcomes of cardiovascular surgical care in each of their regions.
Representatives from several of these regional collaboratives recently convened to discuss opportunities to drive quality assurance and improvement on a much larger scale. While each collaborative has a similar mission, namely the improvement of cardiothoracic surgical care and outcomes, no formal process has existed to benchmark performance across these groups. The groups have agreed to share aggregated data and expertise to further evaluate and improve the quality, safety, and cost of cardiac surgical care both within and across regions under the umbrella of the Cardiac Surgery Quality IMPROVEment (IMPROVE) Network.
Given prior work in and outside of collaboratives, coupled with growing interest on the topic within the surgical community, members of the IMPROVE Network have agreed to use clinical registry data to benchmark red blood cell (RBC) transfusion practices. In particular, the IMPROVE Network seeks to use these data to assess variability in rates of potentially discretionary RBC transfusions. We report our findings from a prospective, observational study of patients undergoing isolated CABG surgery across 56 medical centers in four IMPROVE Network regions.
METHODS
Study Population
The Cardiac Surgery Quality IMPROVEment (IMPROVE) Network [http://www.improvenetwork.org] is composed of five regional quality improvement collaboratives: The Clinical Outcomes Assessment Program, Maritime Cardiovascular Quality Initiative, Michigan Society of Thoracic and Cardiovascular Surgeons, Northern New England Cardiovascular Disease Study Group, and Providence Health & Services Cardiovascular Disease Study Group. The IMPROVE Network has developed a set of bylaws to govern its work, along with the following mission: to improve the value of cardiovascular surgical care by developing and sharing best practice knowledge, coordinating, undertaking, evaluating and disseminating quality improvement projects across our member organizations.
We examined patients undergoing isolated, non-emergent CABG surgery at any of 56 medical centers across four of the IMPROVE Network regions between January 1, 2008 and June 30, 2012.
We excluded any case in which a patient was transfused ≥3 RBC units during the intra- or postoperative period. After excluding patients with emergent status and those missing data on gender, the last 200 isolated CABG cases in each center were retained for analysis, leaving a final sample of 11,200 patients from 56 centers and four regions. Centers with fewer than 200 cases meeting the criteria were excluded from the analysis. Due to privacy concerns, only aggregate, de-identified data from each center were used.
The University of Michigan's institutional review board deemed that the use of such data was not regulated (HUM00071282).
Data Collection
All data were collected locally at each medical center and aggregated to means or percentages at the region level by each collaborative using standardized definitions.1 Information specific to pre-operative care included: patient demographics (age, sex, weight), comorbid disease (diabetes, peripheral vascular disease, hypertension, dialysis, last pre-operative serum creatinine, chronic lung disease, last pre-operative hematocrit), medications (aspirin within 5 days of surgery, and other antiplatelet agents), cardiac history (cardiovascular disease, heart failure within 2 weeks of surgery, myocardial infarction), days from cardiac catheterization to CABG surgery, cardiac anatomy and function (left main disease, ejection fraction, number of diseased vessels), prior CABG surgery, urgent status. We additionally collected intra-operative factors, including: use of off-pump procedure, cross-clamp duration, and cardiopulmonary bypass duration.
Statistical Analysis
Given that four medical centers participated simultaneously in two regional collaboratives, each center was randomly allocated to contribute data to only one collaborative for analysis.
Demographics are presented as the means of all center means within each region. We similarly present the means within each category for categorical variables. Comparisons between regions were made using analysis of variance.
A logistic regression model using aggregated data from each center was used to compute risk-adjusted rates of transfusion for each region. We explored the effect of pre- and intra-operative factors in our modeling. We include in the logistic regression model a class variable corresponding to the region and pre- and intra-operative center characteristics. Pre- and intra-operative center characteristics were chosen via backward selection with the goal of minimizing Akaike information criterion.2 The risk-adjusted rate for each region is calculated by fixing each pre- and intra-operative center characteristic at the same level (i.e., the overall mean across all regions) and therefore the risk-adjusted rates are adjusted for differences in pre- and intra-operative factors. Observed rates and asymptotic standard errors were computed from the raw data.
For each region, these rates and their 95% confidence intervals were plotted. Differences between risk-adjusted regional rates were further assessed by testing for the significance of region as a fixed effect in the adjusted model.
We conducted a sensitivity analysis specifically focused on the contribution of the highest transfusion rate region (i.e., Region 4) to our overall findings. Specifically, we compared the adjusted transfusion rates with and without Region 4.
Analyses were performed using R 3.0.1 and SAS 9.3.
RESULTS
Comparisons of pre- and intra-operative characteristics across the four regional collaboratives are displayed in Table 1. Clinically relevant differences did not exist exclusive of some noteworthy exceptions. Region 4 had the highest proportion of patients with severe chronic lung disease (4.9%). Region 2 had the highest proportion of myocardial infarctions within 7 days (39.4%), urgent status (73.5%), aspirin use (97.8%), and off-pump procedures (28.1%). The use of off-pump procedures in Region 2 was predominantly driven by one center.
Table 1.
Characteristics associated with intra- or post-operative transfusions across the four regional collaboratives (56 centers).
| Region | ||||||
|---|---|---|---|---|---|---|
| Variables | Overall | 1 | 2 | 3 | 4 | p |
| Pre-operative Factors | ||||||
| Age (years) | 64.5 (1.3) | 64.2 (2.0) | 65.8 (1.0) | 65 (1.3) | 64.1 (1.1) | 0.024 |
| Male (Mean %, SD) | 77.9 (4.5) | 80.4 (4.5) | 78.4 (1.5) | 78.2 (5.1) | 77.3 (4.4) | 0.454 |
| BSA (m2) | 2.1 (0.03) | 2 (0.03) | 2.1 (0.02) | 2.1 (0.01) | 2 (0.03) | < 0.001 |
| CVD (%) | 13.5 (3.2) | 11.8 (2.4) | 12.5 (4.8) | 13.1 (3.6) | 14.1 (2.9) | 0.365 |
| PVD (%) | 13.5 (4.3) | 13.3 (5.9) | 16.6 (3.8) | 11.6 (3.1) | 14 (4.3) | 0.142 |
| Chronic lung disease (%) | ||||||
| Mild | 14.8 (6.3) | 11.3 (5.0) | 12.7 (2.6) | 13.7 (4.8) | 16.2 (7.0) | 0.220 |
| Moderate | 5 (3.6) | 1.7 (1.3) | 4.5 (5.8) | 5.4 (5.4) | 5.5 (2.6) | 0.099 |
| Severe | 3.8 (3.2) | 2.2 (1.8) | 3.6 (1.6) | 2.2 (2.2) | 4.9 (3.6) | 0.024 |
| Diabetes (%) | 40.6 (5.0) | 40 (5.3) | 38.6 (5.7) | 38.6 (5.4) | 41.9 (4.6) | 0.180 |
| Dialysis (%) | 1.5 (1.4) | 1.9 (0.80) | 2.8 (3.8) | 1.4 (0.9) | 1.3 (1.1) | 0.169 |
| Number of diseased vessels (%) | ||||||
| None or One | 4.8 (2.7) | 4.8 (2.09) | 4 (3.1) | 5 (3.0) | 4.7 (2.7) | 0.931 |
| Two | 20.9 (5.8) | 21.2 (6.8) | 20.6 (5.9) | 21.6 (7.4) | 20.6 (5.0) | 0.961 |
| Three | 74.4 (7.3) | 74 (7.8) | 75.4 (4.3) | 73.4 (9.6) | 74.7 (6.6) | 0.941 |
| Ejection fraction | ||||||
| < 40 | 12 (3.8) | 13.2 (4.3) | 14 (1.9) | 10.5 (3.3) | 12.3 (4.0) | 0.261 |
| 40 - 49 | 15.2 (3.9) | 13.5 (3.0) | 13.9 (1.9) | 15.3 (3.3) | 15.7 (4.4) | 0.556 |
| 50 - 59 | 31.7 (7.8) | 25.7 (5.8) | 26.7 (6.6) | 29.7 (5.6) | 34.3 (8.1) | 0.016 |
| ≥ 60 | 41 (9.7) | 47.7 (8.1) | 45.4 (9.6) | 44.5 (8.3) | 37.7 (9.5) | 0.021 |
| Heart failure within 2 weeks (%) | 11.1 (7.6) | 11.8 (3.7) | 10.7 (4.3) | 14.9 (11.3) | 9.3 (5.8) | 0.155 |
| Hematocrit (%) | ||||||
| < 36 | 20.4 (5.7) | 18.8 (4.8) | 23.8 (4.3) | 20.3 (5.0) | 20.4 (6.4) | 0.597 |
| 36 - 39 | 29.5 (3.7) | 31.2 (3.5) | 31.4 (3.8) | 28.4 (3.7) | 29.5 (3.8) | 0.321 |
| 40 - 42 | 26 (3.8) | 26.5 (4.6) | 25.4 (2.7) | 26 (3.9) | 26.1 (3.9) | 0.978 |
| ≥ 43 | 24 (5.6) | 23.6 (4.2) | 19.4 (4.4) | 25.3 (5.2) | 24 (6.0) | 0.320 |
| Hypertension (%) | 86.4 (6.2) | 84.8 (5.7) | 88.6 (3.6) | 84 (7.5) | 87.5 (6.5) | 0.252 |
| Left main disease ≥ 50% | 33.5 (5.9) | 36.1 (6.4) | 33.9 (5.4) | 34.3 (4.7) | 32.7 (6.3) | 0.585 |
| MI (%) | 50.9 (7.7) | 51.1 (11.3) | 61.8 (8.1) | 53.6 (5.5) | 48.3 (13.5) | 0.002 |
| Recent MI (≤ 7 days) | 25.3 (7.7) | 24.6 (6.4) | 39.4 (7.7) | 25 (8.3) | 23.9 (6.1) | 0.001 |
| Urgent status (%) | 59.6 (14.6) | 47.1 (13.3) | 73.5 (5.9) | 54.4 (14.3) | 62.5 (13.5) | 0.008 |
| Redo CABG (%) | 2.2 (1.4) | 3.1 (1.9) | 2 (1.4) | 1.9 (1.2) | 2.3 (1.4) | 0.389 |
| Days from Cardiac | 12.3 (6.1) | 10.9 (5.0) | 15.9 (10.8) | 12.9 (5.0) | 11.9 (6.2) | 0.59 |
| Catheterization to Surgery (Mean %, SD) | ||||||
| Medication Use within 5 days | ||||||
| Anti-platelet Agents (%) | 2.9 (7.8%) | 9.5 (22.0) | 9.3 (6.0) | 1.1 (1.0) | 1.6 (3.1) | 0.03 |
| Aspirin (%) | 90.9 (6.8) | 83.0 (9.0) | 97.8 (1.0) | 86.7 (6.3) | 93.5 (4.4) | < 0.001 |
| Last serum creatinine (mg/dl) | 1.1 (0.1) | 1.4 (0.08) | 1.1 (0.5) | 1.1 (0.1) | 1.1 (0.1) | < 0.001 |
| Intra-operative Factors | ||||||
| Off-pump procedure (%) | 11.5 (19.0) | 5.1 (8.2) | 28.1 (46.3) | 12.2 (21.8) | 10.2 (15.5) | 0.32 |
| Cross-clamp duration (Mean % minutes, SD) | 74.3 (17.2) | 68.7 (18.2) | 67.3 (8.3) | 76.8 (18.4) | 75.2 (17.5) | 0.65 |
| Bypass duration (Mean % minutes, SD) | 100.2 (17.0) | 93.7 (17.1) | 101.7 (6.4) | 101.3 (19.6) | 99.9 (13.0) | 0.80 |
BSA: body surface area; CVD: cardiovascular disease; PVD: peripheral vascular disease; MI: myocardial infarction; CABG: coronary artery bypass grafting
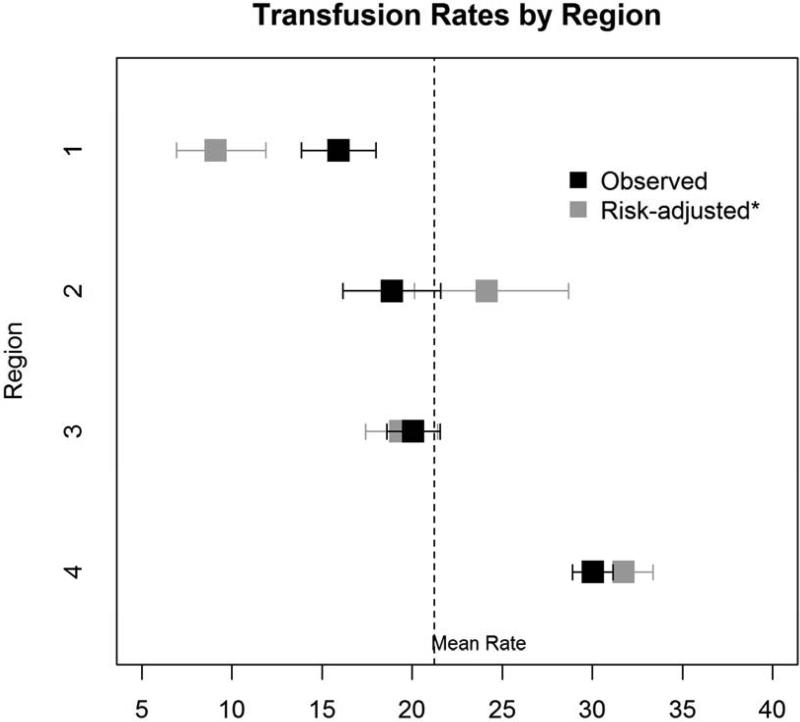
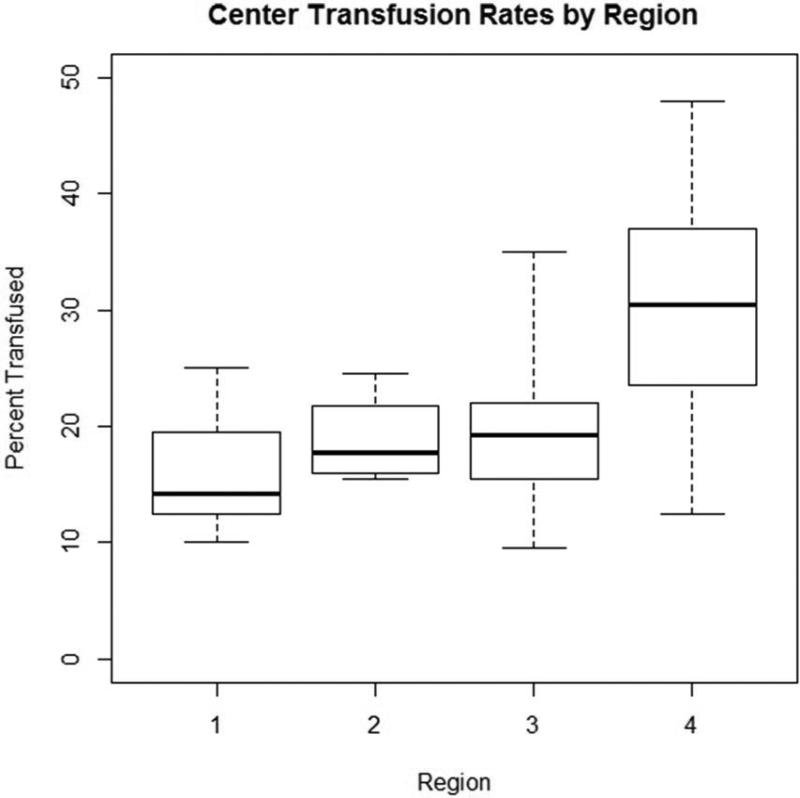
Within our cohort, 25.2% (2,826/11,200) of patients received one or two units of RBC transfusions. Of these, 9.7% received only one unit, whereas 15.5% received two units (Figure 1). Significant variation in use and number of RBCs existed across regions [None: 74.8% (min:max 70.0%, 84.1%), 1 unit: 9.7% (5.1%, 11.8%), 2 units: 15.5% (9.1%, 18.2%)], p<0.001 (Table 2). Variation in overall transfusion rates remained after adjusting for pre- and intra-operative center characteristics (9.1% – 31.7%, p<0.001), as depicted in Figure 2. These findings were qualitatively unchanged when excluding Region 4 (p=0.004).
Figure 1. Observed delivery of transfusion of 1 or 2 units of RBCs across regions.
Boxes represent the interquartile range within each center, whereas the “whiskers” represent the minimum and maximum center-level transfusion rate within each region.
Table 2.
Observed transfusion of 1 or 2 units by region.
| Region | |||||
|---|---|---|---|---|---|
| Number of Transfusions | Overall | 1 | 2 | 3 | 4 |
| 0 Units | 74.8 | 84.1 (75.0, 90.0) | 81.1 (75.5, 84.5) | 80.0 (65.0, 90.5) | 70.0 (52.0, 87.5) |
| 1 Unit | 9.7 | 5.1 (2.5, 11.0) | 9.8 (5.0, 15.0) | 6.8 (1.0, 22.5) | 11.8 (2.5, 24.0) |
| 2 Units | 15.5 | 10.8 (7.5, 19.0) | 9.1 (2.5, 11.0) | 13.3 (6.5, 19.5) | 18.2 (4.0, 29.5) |
Values represent the mean across centers, along with the minimum and maximum center values (in parentheses) within each region.
Figure 2. Observed and risk-adjusted delivery of transfusion of 1-2 units of RBCs across regions.
Black boxes represent observed rates of 1-2 units of RBC transfusions, while gray boxes represent risk-adjusted rates. Error bars represent 95% confidence intervals for observed and risk-adjusted rates.
*Adjusted for age, sex, body surface area, hematocrit, dialysis, creatinine, three-vessel disease,cross-clamp duration, bypass duration, anti-platelet agents, and aspirin use.
DISCUSSION
Within our cohort, 25.2% (2,826/11,200) patients received one or two units of RBC transfusions. Rates of one-unit transfusions as well as two-unit transfusions varied more than twofold across regions. There was a 22.6% absolute difference (9.1% – 31.7%) in rates across regions after adjustment. These findings suggest that there is a lack of consensus across geographical regions in use of small volumes of RBCs.
While in some circumstances blood transfusions are life-preserving (replacement of volume in the setting of acute blood loss), evidence suggests that some transfusions may be associated with harm.3 Major bleeding is a rare phenomenon, although one that is associated with increased risk of mortality.4 In order to address confounding by indication, some investigators have studied the short and long-term impact of small units (i.e., 1–2 units) of RBCs, as these are likely not given to address active bleeding. Parallel work from several investigative teams have found that transfusions of 1–2 units of RBCs are associated with a 90% increased odds of operative mortality5, and a 16% increased hazard of long-term3 mortality after cardiac surgery. These compelling data suggest that small units of RBCs may have an important impact on patient outcomes, and identifying determinants of their use is worthy of further investigation. While not discounting the potential impact of larger volumes of RBCs, we have deliberately excluded patients who have received units of RBCs (>3 units), which may have been given to address active bleeding.
Beyond acute blood loss, small units of RBC transfusions may be given in cardiac surgery for a number of reasons, including decreased hematocrit levels and poor oxygen carrying capacity. Falling hematocrit levels may occur in patients experiencing hemodilutional anemia during cardiopulmonary bypass (CPB).6 Clinical practice guidelines may help guide transfusion decisions.7 In its most recent guidelines, the STS, in conjunction with the Society of Cardiovascular Anesthesiologists and The International Consortium for Evidence-Based Perfusion, states that transfusions are reasonable when Hcts are ≤18%, Class IIa/Level C recommendation. The clinical team must balance the benefits of treating anemia with RBCs against the potential harm associated with anemia and the RBC units themselves.4 Unfortunately, for this analysis we did not have data from each collaborative concerning the Hct prior to the transfusion decisions, nor the type of prophylactic strategies that may have been undertaken to prevent anemia, including reduction of circuit prime volume, ultrafiltration, or the use of retrograde autologous priming.7, 8 As such, our current study is unable to determine the extent to which these and other strategies are differentially adopted across regions, or how their use may help explain the apparent variation in transfusion rates.
For some time now, researchers at the Dartmouth Atlas of Health Care have used Medicare claims data to reveal that the choice of treatment is invariably driven by geographically-distinct styles of practice, rather than differences in patient presentation.9 Additionally, others have found that practice patterns may also be driven by factors at the provider level. For instance, Salem-Schatz and colleagues found that a physician's knowledge concerning clinical indications for transfusions as well as his/her receptivity to input from colleagues may influence the appropriateness of transfusions.10 In fact, a recent survey of cardiac team members suggests that only 20% had institutional discussions concerning the STS blood management guidelines, while only 14% had formed institutionally-based working groups.11 Jin et al. reported a prospective study across 12 hospitals participating in the Providence Health & Services Cardiovascular Disease Study Group,12 in which they sought to disentangle physician and institutional contributors to transfusion decisions. They found that the variance of transfusions across institutions was more than twice that of surgeons practicing within a facility; surgeons contributed to 30% of the variation in transfusion practice, while the institution contributed the remaining 70%. From this the authors concluded that the hospital, rather than the individual surgeon, predominantly drives transfusion practice. While not restricted to small volumes of RBCs, Jin's work suggests that hospitals have their own transfusion “signature.” Our present finding complements this growing body of literature by suggesting that geographic regions themselves may have their own transfusion signature. Within cardiac surgery, these regions are represented by quality improvement collaboratives whose members meet internally to discuss their own quality and outcomes, and define practices to drive quality improvement. Until recently these regional collaboratives have not had a forum to share benchmarking data with each other. Ideally, the partnering of the collaboratives through the IMPROVE Network will be a means for sharing benchmarking data to drive quality improvement on a larger geographic scale.
Our finding of geographic variability in RBC transfusion rates is supported by previous studies. Using national STS data, Bennett-Guerrero and colleagues observed geographic differences in RBC (as well as other blood products) utilization at 798 institutions performing 102,470 primary isolated CABG procedures.13 Utilization was highest (61%) in the West South Central region (Oklahoma, Louisiana, Arkansas, and Texas) of the United States, while lowest (50%) in the Mountain region (Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, and Wyoming). Similarly, wide variability occurred in rates of transfusion of RBCs and other blood products among patients undergoing CABG surgery with cardiopulmonary bypass in U.S. hospitals. Maddux and colleagues used a dataset from a perfusion services provider to document rates of RBC transfusions across 17,252 isolated CABG procedures conducted at one of any 144 institutions located in 32 states and Puerto Rico.14 The authors reported transfusion rates of 40.8% across institutions, although varied (Midwest: 33.7%, South: 36.1%, West: 40.5%, Northeast: 43.1%). Together, these reports suggest that a patient's risk of transfusion may in part be driven by factors other than patient presentation, including the practice patterns of the region in which they seek care.
As is the case with any observational study, our findings are subject to confounding at the level of the patient, provider, medical center, and region. While we adjusted for many common risk factors for blood transfusions including pre-operative hematocrit, lingering confounding may exist, including the reasons underlying the transfusion decision. While we are not able to fully rule out the influence of unmeasured confounding, we have made efforts to address these confounding factors both in our study design and analysis, including limiting our cohort to patients receiving only small increments of blood transfusions and undergoing non-emergent operations, and performing multivariable logistic regression analyses. We used center-level aggregate data to describe and adjust for differences in pre- or intra-operative practices. These data may in some circumstances not appropriately reflect variation at the patient or center level. Last, we hypothesize that apparent regional differences in transfusion practices across the IMPROVE Network may in part be explained by regional supply and demand of blood products, or more (or less) intensive marketing efforts by agencies (e.g. American Red Cross) to limit potentially unnecessary transfusion practices. While we were unable to account for these regional effects, we anticipate that they would have a marginal effect, although would drive our findings toward the null.
Our findings suggest that there is wide geographic variability in the use of potentially discretionary RBC transfusions. Our findings persisted even after adjusting for known factors that may impact transfusion rates. Further investigation is warranted to improve our understanding of why such variability in transfusion rates persists. Partnerships across geographic regions, including through the IMPROVE Network, may serve as a vehicle for undertaking and evaluating the effectiveness of such investigations, including those seeking to reduce unwarranted variability in practice.
Acknowledgments
Funding Sources
Dr. Likosky received funds from the Agency for Healthcare Research and Quality under a 1R13HS020562-01 grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Society of Thoracic Surgeons . STS Adult Cardiac Database Data Specifications, 2.73. [Google Scholar]
- 2.Agresti A. Categorical data analysis. xv. Wiley-Interscience; New York: 2002. p. 710. [Google Scholar]
- 3.Surgenor SD, Kramer RS, Olmstead EM, Ross CS, Sellke FW, Likosky DS, et al. The association of perioperative red blood cell transfusions and decreased long-term survival after cardiac surgery. Anesthesia and analgesia. 2009;108:1741–6. doi: 10.1213/ane.0b013e3181a2a696. [DOI] [PubMed] [Google Scholar]
- 4.Ranucci M, Baryshnikova E, Castelvecchio S, Pelissero G. Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann Thorac Surg. 2013;96:478–85. doi: 10.1016/j.athoracsur.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Paone G, Likosky DS, Brewer R, Theurer PF, Bell GF, Cogan CM, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg. 2014;97:87–93. doi: 10.1016/j.athoracsur.2013.07.020. discussion 94. [DOI] [PubMed] [Google Scholar]
- 6.Surgenor SD, DeFoe GR, Fillinger MP, Likosky DS, Groom RC, Clark C, et al. Intraoperative red blood cell transfusion during coronary artery bypass graft surgery increases the risk of postoperative low-output heart failure. Circulation. 2006;114:I43–8. doi: 10.1161/CIRCULATIONAHA.105.001271. [DOI] [PubMed] [Google Scholar]
- 7.Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 8.Shann KG, Likosky DS, Murkin JM, Baker RA, Baribeau YR, DeFoe GR, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: a focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132:283–90. doi: 10.1016/j.jtcvs.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Dartmouth Medical School. Center for the Evaluative Clinical Sciences . The Dartmouth atlas of health care, 1998 [1 atlas (305 p )] American Hospital Publishing; Chicago, Ill.: 1998. [Google Scholar]
- 10.Salem-Schatz SR, Avorn J, Soumerai SB. Influence of knowledge and attitudes on the quality of physicians' transfusion practice. Med Care. 1993;31:868–78. doi: 10.1097/00005650-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Likosky DS, FitzGerald DC, Groom RC, Jones DK, Baker RA, Shann KG, et al. Effect of the perioperative blood transfusion and blood conservation in cardiac surgery clinical practice guidelines of the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists upon clinical practices. Anesthesia and analgesia. 2010;111:316–23. doi: 10.1213/ANE.0b013e3181e329f1. [DOI] [PubMed] [Google Scholar]
- 12.Jin R, Zelinka ES, McDonald J, Byrnes T, Grunkemeier GL, Brevig J. Effect of hospital culture on blood transfusion in cardiac procedures. Ann Thorac Surg. 2013;95:1269–74. doi: 10.1016/j.athoracsur.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Bennett-Guerrero E, Zhao Y, O'Brien SM, Ferguson TB, Jr., Peterson ED, Gammie JS, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA: the journal of the American Medical Association. 2010;304:1568–75. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- 14.Maddux FW, Dickinson TA, Rilla D, Kamienski RW, Saha SP, Eales F, et al. Institutional variability of intraoperative red blood cell utilization in coronary artery bypass graft surgery. Am J Med Qual. 2009;24:403–11. doi: 10.1177/1062860609339384. [DOI] [PubMed] [Google Scholar]