Abstract
Humans and other species explore a visual scene by rapidly shifting their gaze 2-3 times every second. Although the eyes may appear immobile in the brief intervals in between saccades, microscopic (fixational) eye movements are always present, even when attending to a single point. These movements occur during the very periods in which visual information is acquired and processed and their functions have long been debated. Recent technical advances in controlling retinal stimulation during normal oculomotor activity have shed new light on the visual contributions of fixational eye movements and their degree of control. The emerging body of evidence, reviewed in this article, indicates that fixational eye movements are important components of the strategy by which the visual system processes fine spatial details, enabling both precise positioning of the stimulus on the retina and encoding of spatial information into the joint space-time domain.
Keywords: Ocular drift, microsaccade, saccade, retina, ganglion cell, neural encoding, visual acuity
1. Vision and eye movements
Vision is an active process. Humans collect visual information from a remarkably broad field of view, covering an angle larger than 180° . Monitoring such a wide area, however, comes at a cost: a variety of visual functions including acuity are not uniform throughout the visual field, but progressively deteriorate with increasing distance from a small region, approximately the size of the full moon in the sky (Weymouth et al. 1928; Jacobs 1979; Legge & Kersten 1987; Hansen, Pracejus & Gegenfurtner 2009; Nandy & Tjan 2012). This is the portion of the visual field that projects onto the foveola, the tiny region of the retina where rods receptors are absent and cones most densely packed. Thus, to efficiently examine a visual scene, humans must move their eyes.
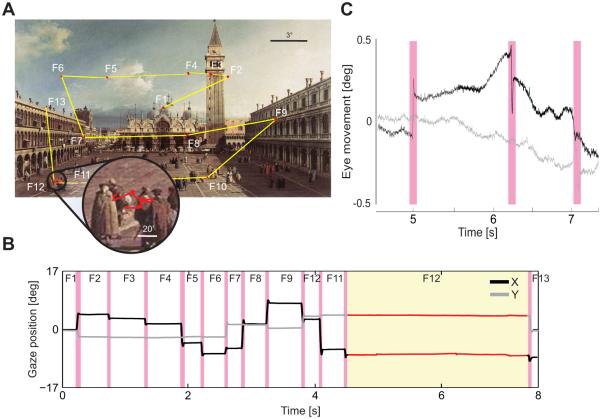
Rapid gaze shifts (saccades) that enable inspection of the objects of interest with the high-acuity foveola normally occur 2-3 times every second (Figure 1A). It is in the brief “fixation” intervals in between these movements that visual information is acquired and the targets for successive saccades selected, effectively establishing a tight loop between perception and action. The fixational sequence enabled by saccades is only the most evident aspect of a deep sensory-motor coupling (Kowler 2011). Close examination of gaze position reveals the presence of incessant microscopic eye movements during fixation (Figure 1B). We are normally not aware of these movements, but they yield speeds of retinal motion which would be immediately visible had they originated from objects in the scene rather than our own eyes.
Figure 1.
Normal eye movements. (A) As an observer explores a scene, saccades (yellow segments) separate brief periods of “fixation” in which visual information is acquired (red). As shown in the enlargement, small eye movements continually occur during fixation. (B) Horizontal and vertical traces of the oculomotor sequence in A. Magenta bars indicate saccades. (C) Traces of the fixational eye movements present during one fixation (circle in A and yellow region in B). The eye wanders with a seemingly random trajectory (ocular drift) occasionally interrupted by microscopic saccades (microsaccades; magenta).
The study of fixational eye movements is often considered an area of specialty for oculomotor researchers. These movements are commonly regarded as a nuisance by experimentalists, rarely taken into account in the explanation of experimental results, and generally ignored by theories of visual functions. Yet they have been observed in a wide variety of species including the owl, a predator which was believed not to move its eyes (Pritchard & Heron 1960; Skavenski et al. 1975; Collewijn & Van Der Mark 1972; Greschner et al. 2002; Steinbach 2004). Furthermore, fixational eye movements can be regarded as the ultimate token of behavior necessary to enable normal vision: percepts tend to fade away when retinal image motion is eliminated, whereas visual functions appear normal when fixational instability is the only source of input modulations (Ditchburn & Ginsborg 1952; Riggs & Ratliff 1952; Tulunay-Keesey 1982). Thus, fixational eye movements are sufficient for enabling vision of stationary scenes, but how they do so remains unknown. Given the temporal integration windows of neurons, it also remains unclear how the visual system establishes fine spatial representations (Burak et al. 2010) and avoids perceptual blurring of the image (Packer & Williams 1992) despite the incessant presence of these movements.
Although traditionally regarded as a simple means to refresh neural activity and prevent perceptual fading, a plethora of mounting evidence indicates that fixational eye movements play a more central role in vision. It is now known that these movements modulate neural responses in various cortical areas (Martinez-Conde, Macknik & Hubel 2000; Snodderly, Kagan & Gur 2001; Kagan, Gur & Snodderly 2008; Herrington et al. 2009; Hafed, Goffart & Krauzlis 2009; Hohl & Lisberger 2011), and multiple findings support the idea that they contribute not just to the acquisition but also the processing of visual information (Ahissar & Arieli 2001; Rucci 2008; Rolfs 2009). In this article, we summarize the main characteristics of fixational eye movements (Sec. 2) and review some recent findings within this emerging body of evidence. We focus first on the smooth inter-saccadic movements (Sec. 3) and then on microscopic saccades (Sec. 4).
2. Types of fixational eye movements
Although multiple indirect observations have long provided evidence that the eyes are always in motion, the first direct measurements of microscopic eye movements only became possible in the middle of last century, when eye-tracking methods with sufficient resolution were first developed (Ratliff & Riggs 1950; Barlow 1952; Ditchburn & Ginsborg 1953). These recordings have revealed the existence of a rich and diverse oculomotor activity (Figure 1B).
Fixational eye movements are traditionally subdivided into three categories: microsaccades, ocular drift, and tremor (Kowler 2011). But classification becomes challenging for the movements with the smallest amplitudes, and the boundaries between categories are not as marked as one may assume, particularly between drift and tremor. For this reason, in later chapters in this article we will often use the term ocular drift (or, for brevity, just drift) to refer to the inter-saccadic motion of the eye in general, without attempting to subdivide this motion into separate categories. The following two sections summarize the general characteristics of fixational eye movements.
2.1. Inter-saccadic movements
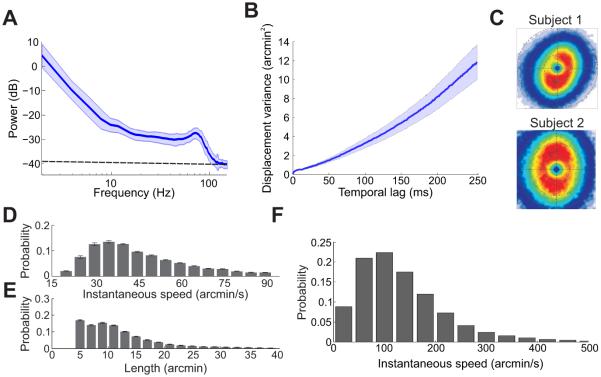
In the periods in between the abrupt gaze shifts caused by saccades, the eyes move in a smooth but jittery way. As shown in Figure 1C, a slow meandering motion and a smaller superimposed jiggle incessantly shift the projection of the stimulus over many retinal receptors. The slow motion occupies the frequency range from 0 to approximately 40 Hz, and is most prominent at low temporal frequencies. The faster component possesses smaller amplitude and a spectral peak in the range 40-100 Hz. These two components are often indicated by the term ocular drift (Cornsweet 1956; Fiorentini & Ercoles 1966) and tremor (Adler & Fliegelman 1934; Eizenman, Hallett & Frecker 1985), but no clear-cut separation exists in terms of their frequency bandwidths (Figure 2A). Also note that the amplitude of tremor (~1′; the symbol ′ represents minutes of arc) is at the very limit of the resolution of the most sophisticated eyetrackers (dashed line in Figure 2C), and its frequency bandwidth includes the AC power line frequency. Both factors contribute to the difficulty of distinguishing tremor from recording noise, so that little is known about the characteristics and possible functions of this oculomotor behavior.
Figure 2.
General characteristics of ocular drifts and tremor. (A) Power spectrum of the oculomotor activity recorded in the periods between saccades/microsaccades. The dashed line represents the level of the eyetracker noise. (B) Variance of the displacement in the line of sight as a function of time. The increment is approximately linear, as distinctive of Brownian motion. (C) Probability distributions of instantaneous drift velocity for two observers during sustained fixation on a marker (Cherici et al. 2012). (D-E) Average distributions of ocular drift speed and path length during free viewing of natural scenes (Kuang et al. 2012). (F) Drift speed distribution for one observer during normal head-free viewing (Aytekin, Victor & Rucci 2014).
Most of what we know about fixational eye movements comes from experiments in which the subject’s head is immobilized, a standard practice for resolving very small eye movements. Under these conditions, the eyes appear to move in an erratic fashion during fixation, yielding trajectories that resemble the random motion of a particle in a fluid. The variance of gaze displacement increases approximately linearly with time, a behavior characteristic of Brownian motion (Engbert et al. 2011) (Figure 2B). This behavior carries the beneficial consequence that the standard deviation of the eye position increases at speed slower than linear, so that the target’s projection remains within a relatively narrow retinal region during the naturally brief periods of inter-saccadic fixation. A resemblance with Brownian motion should not be taken to imply lack of oculomotor control. Control could be exerted in several ways; for example, by changing the diffusion coefficient, the parameter that regulates the speed of the Brownian process. Considerable evidence indicates that drift is indeed controlled (Nachmias 1961; Steinman et al. 1973; Kowler 2011) and the term slow-control is sometimes used in the literature to refer to this motion.
Ocular drift is commonly believed to move the eyes very little and very slowly. Classical studies typically reported amplitude values ranging from about 1.5′ to 4′, with median velocities around 4′ /s (Ditchburn 1973). However, these numbers severely underestimate the real drift displacement and speed for multiple reasons. They represent measurements from highly experienced observers attempting to maintain strict fixation on a single point, which are not representative of the faster motion occurring during normal inter-saccadic fixation. Furthermore, they are average estimates obtained over long intervals (and commonly on a single axis), a procedure which implicitly selects only low temporal frequencies. But ocular drift has a broad sprectrum (Figure 2A) and changes direction very frequently (Figure 2C). Figure 2D-E show the mean 2D characteristics of ocular drift low-pass filtered at ~30 Hz, as subjects freely examined natural scenes. The resulting speed (mean ~50′/s) is more than one order of magnitude larger than classical estimates, covering a considerable length in visual space.
Special equipment is necessary to resolve ocular drift during normal head-free viewing. One device with demonstrated sufficient resolution is the coil-based Revolving Field Monitor developed by Steinman and collaborators (Steinman, Kowler & Collewijn 1990). Studies with this system have shown that ocular drift becomes much faster when the head is not immobilized. An example is shown in Figure 2F for one observer. Note that the median drift speed is approximately three times higher than that measured under head immobilization. Under these conditions, drift also shows clear sign of control, partially compensating for the instability of the head, so to preserve the spatio-temporal characteristics of retinal stimulation (Skavenski et al. 1979; Aytekin, Victor & Rucci 2014).
2.2. Microsaccades
Microsaccades are miniature replicas of the rapid gaze shifts (saccades) that humans normally use to explore a visual scene. These are the fixational eye movements that can be most easily detected and have thus been the focus of extensive research. For these reasons, microsaccades commonly come first to mind in discussions of fixational instability. But, unlike the smooth inter-saccadic motion described in Section 2.1, they are episodic events, with rates generally lower than 1-2 events per second.
Much of the literature on microsaccades has focused on experiments of sustained fixation, a a frequent condition in vision research experiments in which observers maintain steady gaze on a cue for a prolonged period of time. Under these conditions, microsaccades appear as involuntary—subjects are usually not aware of their occurrence—and unnecessary movements, and their function has remained unclear. However, it is important to keep in mind that sustained fixation rarely, if ever, happens in natural tasks, when fixations only last for a fraction of a second. Thus, it is not surprising if saccades cannot be suppressed for too long (Kowler & Steinman 1980). It has also long been known that sustained fixation affects the pattern of fixational eye movements (Steinman et al. 1973) including microsaccades (Cornsweet 1956).
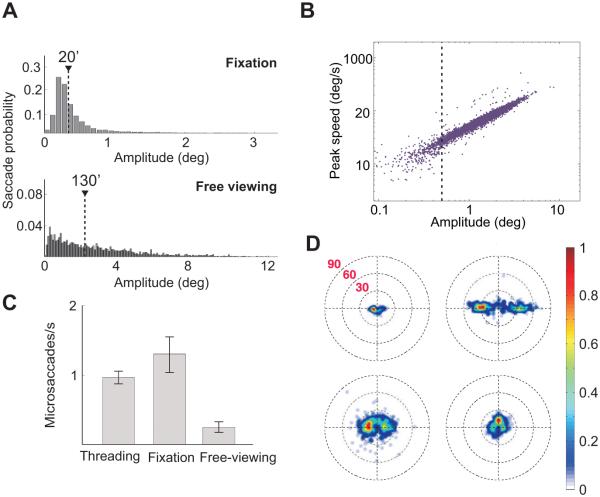
Under more natural conditions in which observers are not requested to maintain fixation, the term microsaccade has been traditionally used to refer to the very small saccades that minimally change the stimulus within the foveola (Collewijn & Kowler 2008). These are the saccades with amplitudes smaller than a predefined threshold, but the actual threshold value has varied across studies. Since saccade amplitude distributions are normally unimodal with no separation between small and large movements (Figure 3A), a common practical approach has been to set the threshold to encompass the range of saccade amplitudes measured under sustained fixation. Following this or similar criteria, classical studies usually restricted the use of the term to tiny saccades, smaller than 30′ (e.g., Zuber, Stark & Cook 1965; Fiorentini & Ercoles 1966) or even smaller than 12′ (e.g., Ditchburn & Ginsborg 1953; Boyce 1967; St.Cyr & Fender 1969).
Figure 3.
General characteristics of microsaccades. (A) Saccade amplitude distribution during sustained fixation and free viewing. Data represent average distributions across six observers. Triangles mark the medians of the distributions. (B) Saccade peak speed of as a function of saccade amplitude during free viewing. The dashed line marks saccades smaller than half a degree. (C) Microsaccade rates in three different tasks: needle threading, sustained fixation on a single dot, and free viewing of a natural scene (Ko, Poletti & Rucci 2010). (D) Individual variability in microsaccades during sustained fixation. Each panel shows the two-dimensional probability distribution of microsaccade displacements for an individual observer (Cherici et al. 2012).
It should be noted that several recent studies have reported broader amplitude distributions during sustained fixation and consequently raised the microsaccade threshold to include much larger movements, up to 1.5° or even 2° (Rolfs 2009). Various factors may have contributed to this discrepancy, and the use of different recording methods is likely a key element. However, such a large increment in threshold alters the nature of traditional debates on microsaccades (Collewijn & Kowler 2008): since the average radius of the foveola is approximately 30′ (Curcio et al. 1990), saccades with amplitudes above 1° completely change the portion of the visual field within this region.
In this article, we will focus on conditions in which fixation is not explicitly enforced and continue the classical tradition of using the term microsaccade to indicate the very small saccades that maintain the stimulus within the foveola. All data reported here refer to saccades with amplitudes smaller than 30′ (in some cases 20′), which give an overlap between pre- and post-saccadic images larger than 50%. This threshold also conveniently coincides with the 90th percentile of the average saccade distribution measured during sustained fixation by means of an eyetracker with demonstrated high resolution (Cherici et al. 2012).
Since microsaccades are saccades, they share many characteristics with their larger counterparts. For example, it has long been known that the main sequence, the linear relationship between amplitude and velocity observed for saccades also extends to the microsaccade range (Bahill, Clark & Stark 1975) (see Figure 3B). Like for larger saccades, microsaccade amplitudes, directions, and frequencies vary significantly according to the experimental conditions (e.g., Malinov et al. 2000), the task (e.g., Ko, Poletti & Rucci 2010), the stimulus (e.g., Thaler et al. 2013), and attention (e.g., Hafed & Clark 2002). As shown in Figure 3C, microsaccades tend to be frequent during sustained fixation as well as in tasks that require high visual acuity and are usually rare in tasks that favor larger saccades, like free viewing of a scene. However, rates vary both with the specific conditions (e.g., the type of fixation marker and the characteristics of the scene) and the instructions given to the subject. Interestingly, microsaccade characteristics also vary considerably across observers (Figure 3D), but the reason for such large individual variability remains unclear.
When fixation is not enforced, microsaccades also show clear signs of voluntary control. For example, they can be made in response to small displacements of a target (Timberlake et al. 1972; Wyman & Steinman 1973; Havermann et al. 2014) and to look in specified directions (Haddad & Steinman 1973; Ko, Poletti & Rucci 2010). Even under sustained fixation, microsaccades become less frequent by simply changing the instruction from “fixate” to “hold the eyes still” (Steinman et al. 1967), and it has long been suggested that they may correct for fixational displacements (Cornsweet 1956). In Section 4, we will argue that even microsaccades smaller than 20′, which yield a 66% overlap in the pre- and post-saccadic images, serve the same exploratory function as larger saccades.
3. Visual functions of inter-saccadic fixational movements
Considerable evidence indicates that the smooth motion of inter-saccadic fixation helps stabilizing the image on the retina (Skavenski et al. 1979; Epelboim & Kowler 1993). This stabilization is far from perfect, leaving a residual motion with approximately Brownian characteristics (Aytekin, Victor & Rucci 2014), which appears to be generated on purpose (Collewijn, Martins & Steinman 1981). The resulting retinal image motion is commonly thought to “refresh” neural responses in order to prevent the image fading experienced under retinal stabilization. But examination of the luminance signals impinging onto retinal receptors reveals that this description is misleading: fixational instability implements a much more interesting reformatting of the visual input.
3.1. A space-time conversion
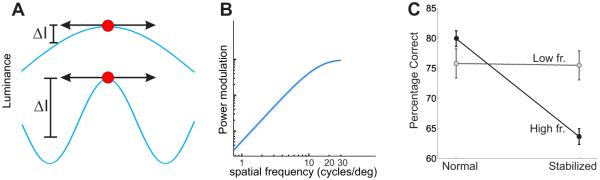
All eye movements convert a spatial scene into a spatiotemporal input signal to the retina, but the amplitude of the resulting luminance modulations depends on both the characteristics of the scene and how the eye moves. Rather than just refreshing the retinal image, inter-saccadic fixational eye movements restructure spatial information into a spatiotemporal format which emphasizes high spatial frequencies. As shown in Figure 4, this amplification occurs up to a cut-off frequency close to the spatial resolution of the photoreceptor array.
Figure 4.
Enhancement of high spatial frequencies resulting from fixational drift. (A) Exposure of retinal receptors (circles) to low (top) and high (bottom) spatial frequency gratings during fixational instability (arrows). The grating at higher frequency gives a larger change in luminance (ΔI). (B) Mean amplification resulting from ocular drift as a function of spatial frequency. Data represent averages across N =5 observers (Mostofi, Boi & Rucci 2014). (C) Results of experiments in which subjects judged the orientation of a noisy grating (±45° either at low or high spatial frequency) in the presence (normal) and absence (stabilized) of normal fixational eye movements. In the stabilized condition, the position of the stimulus was continually updated according to the subject’s eye movements so to eliminate retinal image motion. Removal of fixational modulations via retinal stabilization selectively impaired high spatial frequency vision (Rucci et al. 2007).
An intuitive understanding of this phenomenon can be gained by considering the luminance change experienced by a retinal receptor during an infinitesimally brief interval: in this period, ocular drift can be regarded as uniform motion (a constant-speed translation), and the amplitude of the modulation is determined by the spatial gradient of image. For a sinusoidal pattern of luminance, like the ones shown in Figure 4A, the gradient is proportional to the spatial frequency of the stimulus. Consequently, gratings at higher spatial frequencies yield larger fixational modulations. For longer periods of time, ocular drift can no longer be approximated by uniform motion, and its Brownian character needs to be taken into account. The main implication is that amplification will only occur at spatial frequencies for which drift covers a small fraction of the period. For sufficiently high spatial frequencies for which the eyes move over a larger fraction of the period, there will instead be attenuation.
Figure 4B shows the actual spatial frequency amplification resulting from ocular drift averaged over several observers. Up to approximately 15 cycles/deg, the amplitude of the modulations (or, equivalently, the amount of spatial power that spreads into the temporal domain) tends to increase proportionally with the square of the spatial frequency. Above this range the effect starts to be attenuated, but an enhancement can be observed up to 30 cycles/deg.
3.2. Perceptual consequences
The spatiotemporal conversion shown in Figure 4B makes a counter-intuitive prediction. It is usually assumed that fixational instability is primarily helpful at low spatial frequencies, the range in which fading is most pronounced when stimuli are stabilized on the retina for long periods of time (Kelly 1979; Koenderink 1972; Tulunay-Keesey 1982). In contrast, the amplification in Figure 4B suggests that inter-saccadic fixational eye movements enhance high spatial frequency vision, a proposal with a very long history (Averill & Weymouth 1925; Marshall & Talbot 1942; Arend 1973; Ahissar & Arieli 2012).
To test this prediction, we developed a new method of retinal stabilization, which enabled selective elimination of retinal image motion during natural post-saccadic fixation (Santini et al. 2007). Figure 4C shows the results of an experiment in which subjects reported whether a noisy grating was tilted by 45° clockwise or counter-clockwise. Following the predictions of Figure 4B, we presented two stimuli: one in which the target (a grating) was at a higher spatial frequency than the noise and one in which it was at a lower spatial frequency. These two stimuli yield different predictions: fixational modulations are expected to enhance visibility of the high-spatial-frequency target, but not the low one.
Figure 4C compares the average percentages of correct discrimination measured in the presence of the normal fixational modulations to those reported when they were stabilized on the retina by counteracting the effects of eye movements. Results confirm the predictions of Figure 4B: eliminating retinal image motion drastically impaired discrimination of the high-frequency gratings, but had little effect on the low spatial frequency gratings.
3.3. Temporal reformatting of natural images
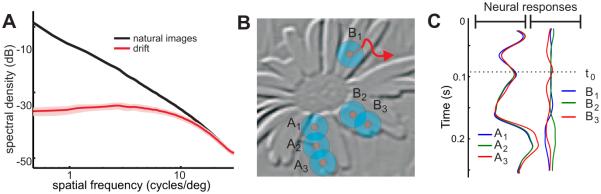
Natural scenes provide highly structured visual input signals. Statistical regularities are present at multiple scales, and it has long been argued that sensory systems exploit them in establishing neural representations (Barlow 1961). A well known feature of natural images is the very specific spectral density, which declines approximately as the square of the spatial frequency (Field 1987, see Figure 5A), and a considerable amount of work has focused on the consequences of this spectral distribution on early visual representations (Simoncelli & Olshausen 2001; Hyvärinen, Hurri & Hoyer 2009). How does the space-time conversion shown in Figure 4B interacts with the characteristics of natural images?
Figure 5.
Interaction with natural images and possible neural consequences of ocular drift. (A) Comparison between the power of a set of natural images and the temporal power (the sum over all nonzero temporal frequencies) in the modulations caused by ocular drift. Normal drift equalizes power over a broad spatial frequency range (Kuang et al. 2012). (B) Activity in a simulated array of retinal ganglion cells. Each pixel represents the mean instantaneous firing rate at time to of a simulated neuron with receptive field (circles) centered on the pixel. (C) Time-course of the responses of the six neurons shown in B. Note the enhancement of edges in the synchronous modulations.
To address this question, Figure 5A analyzes the consequences of eye movements during fixation on a natural image. The specific power spectrum of natural images implies that the contrast of each frequency component decreases as its spatial frequency increases, i.e., gratings at high spatial frequencies are fainter than those at low spatial frequencies. Note that this contrast attenuation goes in the opposite direction of the oculomotor amplification in Figure 4: whereas the motion of the eye enhances high spatial frequencies, natural images possess most power at low spatial frequencies.
Remarkably the two effects counterbalance each other. Figure 5A compares the power spectrum of a set of images to the average temporal power made available in the form of modulations by normal inter-saccadic fixational instability. The net effect of the interaction between ocular drift and the spectral distribution of natural images is to yield modulations with approximately equal amplitude over a wide spatial frequency range (Kuang et al. 2012). Very similar results were also obtained during head-free viewing, a condition in which eye and head movements combine to form a retinal stimulus with almost identical characteristics (Aytekin, Victor & Rucci 2014).
These data show that ocular drift causes a very specific input reformatting during natural fixation. Fixational fluctuations of luminance at different spatial frequencies possess similar power. This equalization—a transformation known as “spectral whitening”—, requires both images with the spectral density of natural scenes and normal oculomotor activity. It reveals a form of matching between the characteristics of the natural world and those of normal eye movements.
3.4. Neural consequences
The spatial and temporal reformatting the viual input described in the previous sections has deep implications for the mechanisms of neural encoding. In the temporal domain, this transformation creates power at temporal frequencies within the range of peak sensitivity of retinal neurons (Kaplan & Benardete 2001). Thus, fixational luminance fluctuations are likely to strongly influence neural responses (Snodderly, Kagan & Gur 2001). In the spatial domain, the frequency enhancement shown in Figure 4B implies that neurons will respond less to low spatial frequencies and more to high spatial frequencies than their contrast sensitivity functions measured with immobile retinas would suggest.
Further important consequences follow from the interaction between eye movements and natural images shown in Figure 5A. Since an equalization of power in frequency is equivalent to the removal of correlations in space, the spectral distribution in Figure 5A implies that pairs of retinal receptors will experience uncorrelated luminance fluctuations during fixation on a natural image (Rucci & Casile 2004; Desbordes & Rucci 2007). A similar decorrelation has long been argued to be an important function of retinal processing, as it removes predictable regularities from the input signals and enables the visual system to focus resources on more informative input components (Attneave 1954; Barlow 1961). The center-surround organization of the receptive fields of ganglion cell has been implicated in this process (Srinivasan, Laughlin & Dubs 1982; van Hateren 1992; Atick & Redlich 1992), but these proposals did not consider the impact of eye movements. Thus, the incessant presence of ocular drift implies a reexamination of well-known theories of retinal encoding.
Since decorrelation is already accomplished by eye movements, the amplification of high spatial frequencies operated by the contrast sensitivity of ganglion cells must serve a different function than counterbalancing the spectral distribution of natural scenes. An interesting possibility is that this neural filtering combines with fixational instability to start the process of feature extraction commonly believed to take place at higher stages in the visual system. Figure 5B-C shows an example of activity in an array of modeled retinal ganglion cells. The interaction among eye movements, natural images, and the spatiotemporal sensitivity of ganglion cells yields synchronous responses, which emphasize luminance discontinuities. This edge enhancement occurs even if modeled neurons in Figure 5C are circularly symmetric and do not possess a preference for oriented stimuli. Thus, ocular drift may allow for a neural code which uses synchrony for encoding edges (Greschner et al. 2002; Poletti & Rucci 2008) and which can take advantage of the robustness of temporally synchronous responses in propagating through neural networks (Dan et al. 1998; Bruno & Sakmann 2006).
4. Visual functions of microsaccades
The possible visual functions of microsaccades have been intensely debated. Much confusion seems to have originated from the use of unnatural viewing conditions (Steinman 2003) as well as the chronic difficulty in spatially localizing the line of sight: whereas eyetrackers can resolve very small changes in eye position, absolute determination of the line of sight in the scene is usually only approximate (Holmqvist et al. 2011).
A widespread proposal— originally derived from false assumptions on the characteristics of ocular drift (Ditchburn & Ginsborg 1953)—is that microsaccade may serve a special function in preventing perceptual fading (Ditchburn, Fender & Mayne 1959; Martinez-Conde et al. 2006). However, multiple observations argue against this idea (Collewijn & Kowler 2008; Poletti & Rucci 2010; Kagan 2012), including the recent report that no fading occurs under total paralysis (Whitham et al. 2011). Under sustained fixation, another prominent idea has been that microsaccades help centering gaze, a notion supported by a considerable body of evidence (Cornsweet 1956; Engbert & Kliegl 2004; Cherici et al. 2012). Here we focus on the function of microsaccades under more natural conditions, when fixation is not explicitly enforced and observers are free to normally move their eyes.
4.1. Exploration of fine spatial details
It was first proposed by Cunitz & Steinman (1969) that, by precisely redirecting a tiny preferred locus of fixation, microsaccades could be helpful in the examination of fine spatial detail. This idea was later abandoned following the observation that microsaccades appear to be suppressed in high-acuity tasks (Winterson & Collewijn 1976; Bridgeman & Palca 1980). Specifically, it was noted that microsaccade rates tend to progressively decrease during the execution of finely guided visuomotor tasks, such as threading a sewing needle or aiming a rifle, even when the task is successfully accomplished. Microsaccades also tend to be less frequent in these tasks than during sustained fixation on a small cue.
These findings were taken to imply that microsaccades are detrimental for high visual acuity. However, several considerations caution that this conclusion might have been premature. First, a reduction in the rate of microsaccades toward the end of an experimental trial does not imply that microsaccades did not provide helpful information at earlier times. Second, as pointed out before, sustained fixation is an unnatural condition which elicits a particularly high number of microsaccades and may therefore not constitute an optimal reference baseline. Moreover, analysis of the rate of microsaccades alone might not be the best way to determine whether microsaccades serve a useful function. The specific patterns of gaze shifts resulting from microsaccades are more informative, but examination of how exactly microsaccades position the stimulus on the retina requires absolute localization of the line of sight to a level beyond the accuracy of most eyetrackers.
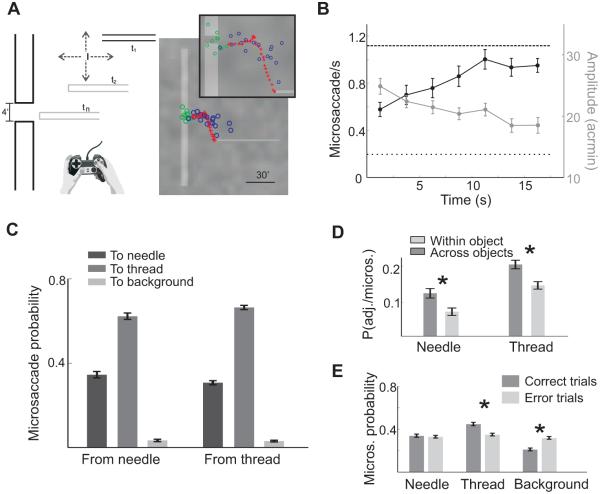
To circumvent this problem, we have recently developed a gaze-contingent calibration procedure coupled with a high resolution DPI eyetracker, which effectively improves gaze localization by almost one order of magnitude compared to standard methods (Poletti, Listorti & Rucci 2013, see their supplementary information). This approach has enabled us to reexamine how microsaccades move the center of gaze during execution of a high-acuity visuomotor task similar to that of the previous studies: the threading of a needle. To better control experimental variables, we created a virtual environment (Figure 6A). Rather than threading a real needle, subjects controlled the motion of a thin horizontal bar (the thread) by means of a joystick, and were asked to insert the thread into a small aperture at the center of a stationary vertical bar (the needle). Like the real threading of a needle, the stimulus covered a very small visual angle, smaller than 1 deg2, so that it could entirely fit within the foveola.
Figure 6.
Microsaccades in a high-acuity task. (A) Threading a needle in a virtual environment. Subjects moved a horizontal bar (the thread) toward the small gap in a vertical bar (the needle). The panel on the right and its enlargement show the spatial distribution of fixations in a trial. Fixations were primarily allocated to the thread (blue circles) and the eye of the needle (green circles). The red crosses mark the thread trajectory. (B) Mean instantaneous frequency and amplitude of microsaccades. The two horizontal lines represent mean rates during sustained fixation (dashed line) and free viewing (dotted line). (C) Probabilities of various types of microsaccades, classified according to their starting and landing points. Microsaccades almost always brought the line of sight on the thread and needle, rarely on the background. (D) Conditional probabilities of realigning the thread following different types of microsaccades. Adjustments were more likely to occur immediately after microsaccades across different objects. (E) Microsaccade landing probability in successful and unsuccessful trials. Microsaccades were more precise in the trials in which the thread was correctly aligned with the needle. All data refer to saccades smaller than 20′. Modified from Ko, Poletti & Rucci (2010).
Our experiments confirmed previous findings (Winterson & Collewijn 1976; Bridgeman & Palca 1980). The microsaccade rate was on average lower than that measured during sustained fixation (Figure 3C) and decreased toward the end of the trial when the specific conditions of previous experiments were also replicated. However, microsaccades were frequent at earlier times in a trial, when subjects adjusted the alignment between the thread and the needle, yielding a mean rate five times higher than that measured during free viewing of natural images (Figure 3C).
To examine the functions of these microsaccades, we (a) terminated each trial when the thread was close to, but still had not reached, the needle; and (b) restricted control of the thread to the vertical axis, moving it at constant speed toward the needle on the horizontal axis. This approach ensured that adjustments in the thread’s position reflected a perceived misalignment and excluded possible changes in attention toward the end of the trial.
The main results of our experiment are summarized in Figure 6. Microsaccades were clearly influenced by the characteristics of the stimulus and the ongoing demands of the task: they became progressively smaller and more frequent as the thread approached the needle (Figure 6B). Furthermore, careful localization of the line of sight revealed that microsaccades shifted gaze in a very precise manner. Fixations were clustered at salient locations of the scene. Most fixations were either on the thread or around the eye of the needle, and very few fixations fell far from these two regions. That is, microsaccades precisely shifted the gaze between the thread and eye of the needle and very rarely landed on other task-irrelevant regions of the scene (Figure 6C).
These results suggest that observers used microsaccades to acquire useful information from the scene. To confirm this hypothesis, we examined whether a link existed between microsaccades and adjustments in the thread’s position. We estimated the probabilities of correcting the thread/needle alignment immediately after a microsaccade and the probabilities of performing microsaccades immediately after changing the thread’s position. We found that subjects were more likely to realign the thread after executing a microsaccade that shifted the center of gaze from one object (the thread or the needle) to the other (see Figure 6D). In contrast, microsaccades were likely to move the line of sight toward the thread after adjusting its position. Furthermore, microsaccades were less precise in the trials in which subjects failed to thread the needle (Figure 6E). These data corroborate the idea that subjects performed microsaccades to judge the alignment between the thread and the needle.
4.2. Implications for foveal vision
The oculomotor strategy followed by our subjects in Figure 6 may appear paradoxical. In principle, given the small size of the stimulus, a fixation at the center of the display would have enabled the entire stimulus to fall within the high-acuity foveola without any need for eye movements. But this was not the approach taken by the observers, who preferred instead to execute precisely-directed microsaccades. What was the benefit of this strategy?
Anatomical examinations of the retina have revealed the presence of considerable nonhomogeneity within the foveola. Although substantial individual variability exists, it has been observed that cone density falls with increasing eccentricity not just outside the central fovea, but also within the foveola itself, with the region of maximum cone concentration restricted on average to an area smaller than 0.032 deg2 (Curcio et al. 1990). Thus, mi-crosaccades may contribute to fine spatial vision by properly centering this region or a similar preferred fixation locus—not necessarily coincident with the region of highest cone density (Putnam et al. 2005)— which facilitates fine spatial judgments.
Previous studies that attempted to map systematically visual acuity within the central fovea have given conflicting results. Some studies have reported a decline in performance with eccentricity, whereas others have found minimal changes (Weymouth et al. 1928; Adler & Meyer 1935; Millodot 1966). However, it is critical to realize that testing visual acuity at very small eccentricities is extremely challenging. This operation requires both: (a) very high accuracy in localizing the line of sight, which is necessary to reliably determine the eccentricity of retinal stimulation; and (b) during stimulus exposure, real-time compensation for fixational eye movements, which would otherwise move the stimulus on the retina. Both the uncertainty in gaze localization and the fixational motion of the retinal image effectively prevent isolation of closely spaced regions on the retina and are likely to homogenize measurements at adjacent locations. These factors may have contributed to previous reports of approximately uniform acuity across the foveola.
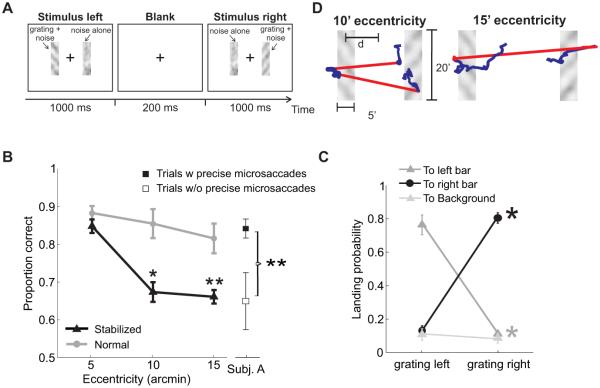
The gray line in Figure 7B shows the consequence of not properly controlling for the presence of fixational eye movements. In this experiment, subjects were confronted with a forced-choice paradigm designed to confine stimulation at fixed eccentricities. They reported whether two gratings displayed within narrow rectangular noise fields were parallel or orthogonal (Figure 7A). The two noise bars were located symmetrically around the point of fixation at three possible—very small—distances (5′, 10′, and 15′), and gratings were presented sequentially, first in the left bar, then in the right one. The data summarized by the gray line were collected following the same procedure of most previous experiments. Subjects were simply asked to maintain strict fixation at the center of the display, while performance was measured with stimuli at different eccentricities. Under these conditions, performance varied little as the distance of the two bars increased from 5′ to 15′, a result which could be mistakenly interpreted as supporting the notion of uniform vision within the foveola.
Figure 7.
Consequences of microsaccades for foveal vision. (A) Subjects reported whether two sequentially-presented noisy gratings were parallel or orthogonal. Gratings (11 cycles/deg) were tilted by ±45° and appeared within two rectangular noise bars centered at the desired eccentricity d, first in the left and then in the right bar. (B) Stimuli were either displayed at fixed positions on the screen (Normal) and normally moved on the retina because of fixational eye movements, or at fixed locations on the retina (Stabilized) and moved on the display under computer control to compensate for the subject’s eye movements. Performance decreased sharply with eccentricity under retinal stabilization. Discrimination was also impaired in the trials in which microsaccades did occur but were less precise ((★) p <0.05; (★★) p <0.005; two-tailed paired t-test). (C) Proportions of microsaccades landing on different regions of the display for stimuli at 15′ eccentricity. Most microsaccades moved the line of sight on the bar containing the stimulus. (D) Eye movements in two example trials. Red and blue segments represent microsaccades and drifts, respectively. Subjects were asked to maintain fixation at the center of the display (cross in A). Modified from Poletti, Listorti & Rucci (2013).
In reality, eye movements continually occurred during the course of the trial, as observers attempted to maintain fixation. Like in the study of Figure 6, careful examination of oculomotor activity reveals that microsaccades were frequent and not random. As shown in Figure 7C microsaccades closely followed the sequence of experimental events: they first shifted the center of gaze to the left of the fixation point when the grating appeared in the left bar, and then relocated the line of sight to the right when the grating was displayed in the right bar. Therefore, because of microsaccades, stimuli did not remain at a fixed eccentricity on the retina, but systematically moved toward the preferred fixation locus (see examples in Figure 7D).
The black line in Figure 7B shows results obtained in the same experiment when proper precautions were taken to control for the effects of eye movements. In this case, rather than the distance of the bar from the fixation cue on the display, eccentricity represents the distance of the stimulus from the center of the preferred locus on the retina. The position of this locus (or equivalently, the line of sight) was measured during a preliminary gaze-contingent calibration procedure similar to that of the experiments in Figure 6. Furthermore, stimuli were maintained at fixed retinal eccentricities throughout the course of the trial by continually updating their position on the display according to the subjects eye movements. Perceptual fading was not an issue in this experiment, as gratings were flashed for brief periods of time.
Note the difference in results between the two conditions. Performance decreased sharply with eccentricity after eliminating the consequences of eye movements, an effect which was visible even at just 10′ eccentricity. This impairment was not the consequence of retinal stabilization: a similar drop in performance was also observed during normal viewing (no retinal stabilization) in the rare trials in which microsaccades did not occur or were not precise, thus failing to bring the projection of the stimulus onto the preferred retinal locus (Figure 7B). These results support the existence of a narrow retinal locus, smaller than the foveola, which observers use in tasks involving high visual acuity.
4.3. A finely controlled oculomotor strategy
In sum, accurate gaze localization during high-acuity visual and visuomotor tasks has revealed a surprisingly fine level of control in microsaccades and a clear dependence on the ongoing task. Microsaccades precisely move the eye to center the retinal projection of the stimulus onto a preferred fixation locus, a behavioral strategy which would give the false impression of a broader high-acuity region, if oculomotor activity is not monitored at high resolution.
Together with recent findings highlighting the similarity between saccades and microsaccades in terms of production mechanisms and extraretinal influences (Hafed, Goffart & Krauzlis 2009; Hafed 2013; Havermann et al. 2014; Snodderly 2015), the results of Figures 6 and 7 provide strong support to Cunitz and Steinman’s (1969) proposal that microsaccades are exploratory movements like larger saccades. They indicate that outside of the laboratory, when fixation is not enforced, a subdivision between saccades and microsaccades is not warranted. All saccades, independent of their amplitudes, enable inspection of regions of interest by properly positioning the stimulus on the retina.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant EY18363 and National Science Foundation grants 1127216 and 1420212
Footnotes
DISCLOSURE STATEMENT
The authors have nothing to disclose.
LITERATURE CITED
- Adler FH, Fliegelman M. Influence of fixation on the visual acuity. Arch. Ophthalmol. 1934;12:475483. [Google Scholar]
- Adler FH, Meyer GP. The mechanism of the fovea. Trans. Am. Ophthalmol. Soc. 1935;33:266–280. [PMC free article] [PubMed] [Google Scholar]
- Ahissar E, Arieli A. Figuring space by time. Neuron. 2001;32:185–201. doi: 10.1016/s0896-6273(01)00466-4. [DOI] [PubMed] [Google Scholar]
- Ahissar E, Arieli A. Seeing via miniature eye movements: A dynamic hypothesis for vision. Front. Comp. Neurosci. 2012;6:1–27. doi: 10.3389/fncom.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend LE. Spatial differential and integral operations in human vision: Implications of stabilized retinal image fading. Psychol. Rev. 1973;80:374–395. doi: 10.1037/h0020072. [DOI] [PubMed] [Google Scholar]
- Atick J, Redlich A. What does the retina know about natural scenes? Neural Comput. 1992;4:196–210. [Google Scholar]
- Attneave F. Some informational aspects of visual perception. Psychol. Rev. 1954;61:183–193. doi: 10.1037/h0054663. [DOI] [PubMed] [Google Scholar]
- Averill HI, Weymouth FW. Visual perception and the retinal mosaic. II. The influence of eye movements on the displacement threshold. J. Comp. Psychol. 1925;5:147–176. [Google Scholar]
- Aytekin M, Victor JD, Rucci M. The visual input to the retina during natural head-free fixation. J. Neurosci. 2014;34:12701–12715. doi: 10.1523/JNEUROSCI.0229-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math. Biosci. 1975;24:191–204. [Google Scholar]
- Barlow HB. Eye movements during fixation. J. Physiol. 1952;116:290–306. doi: 10.1113/jphysiol.1952.sp004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB. In: Sensory Communication. Rosenblith WA, editor. MIT Press; Cambridge, MA: 1961. pp. 217–234. [Google Scholar]
- Boyce PR. Monocular fixation in human eye movement. P. R. Soc. Lond. B. Bio. 1967;167:293–315. doi: 10.1098/rspb.1967.0028. [DOI] [PubMed] [Google Scholar]
- Bridgeman B, Palca J. The role of microsaccades in high acuity observational tasks. Vision Res. 1980;20:813–817. doi: 10.1016/0042-6989(80)90013-9. [DOI] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- Burak Y, Rokni U, Meister M, Sompolinsky H. Bayesian model of dynamic image stabilization in the visual system. Proc. Natl. Acad. Sci. USA. 2010;107:19525–19530. doi: 10.1073/pnas.1006076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherici C, Kuang X, Poletti M, Rucci M. Precision of sustained fixation in trained and untrained observers. J. Vis. 2012;12:1–16. doi: 10.1167/12.6.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H, Kowler E. The significance of microsaccades for vision and oculomotor control. J. Vis. 2008;8:1–21. doi: 10.1167/8.14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H, Martins AJ, Steinman RM. Natural retinal image motion: Origin and change. Ann. NY. Acad. Sci. 1981;374:312–329. doi: 10.1111/j.1749-6632.1981.tb30879.x. [DOI] [PubMed] [Google Scholar]
- Collewijn H, Van Der Mark F. Ocular stability in variable feedback conditions in the rabbit. Vision Res. 1972;36:47–57. doi: 10.1016/0006-8993(72)90765-2. [DOI] [PubMed] [Google Scholar]
- Cornsweet TN. Determination of the stimuli for involuntary drifts and saccadic eye movements. J. Opt. Soc. Am. 1956;46:987–988. doi: 10.1364/josa.46.000987. [DOI] [PubMed] [Google Scholar]
- Cunitz RJ, Steinman RM. Comparison of saccadic eye movements during fixation and reading. Vision Res. 1969;9:683–693. doi: 10.1016/0042-6989(69)90125-4. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J. Comp. Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Dan Y, Alonso JM, Usrey WM, Reid RC. Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nat. Neurosci. 1998;6:501–507. doi: 10.1038/2217. [DOI] [PubMed] [Google Scholar]
- Desbordes G, Rucci M. A model of the dynamics of retinal activity during natural visual fixation. Visual Neurosci. 2007;24:217–230. doi: 10.1017/S0952523807070460. [DOI] [PubMed] [Google Scholar]
- Ditchburn RW. Eye movements and visual perception. Clarendon Press; Oxford, UK: 1973. [Google Scholar]
- Ditchburn RW, Fender DH, Mayne S. Vision with controlled movements of the retinal image. J. Physiol. 1959;145:98–107. doi: 10.1113/jphysiol.1959.sp006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchburn RW, Ginsborg BL. Vision with a stabilized retinal image. Nature. 1952;170:36–37. doi: 10.1038/170036a0. [DOI] [PubMed] [Google Scholar]
- Ditchburn RW, Ginsborg BL. Involuntary eye movements during fixation. J. Physiol. 1953;119:1–17. doi: 10.1113/jphysiol.1953.sp004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizenman M, Hallett PE, Frecker RC. Power spectra for ocular drift and tremor. Vision Res. 1985;24:1635–1640. doi: 10.1016/0042-6989(85)90134-8. [DOI] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades keep the eyes’ balance during fixation. Psychol. Sci. 2004;15:431–436. doi: 10.1111/j.0956-7976.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- Engbert R, Mergenthaler K, Sinn P, Pikovsky A. An integrated model of fixational eye movements and microsaccades. Proc. Natl. Acad. Sci. USA. 2011;108:765–770. doi: 10.1073/pnas.1102730108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelboim J, Kowler E. Slow control with eccentric targets: Evidence against a position-corrective model. Vision Res. 1993;33:361–380. doi: 10.1016/0042-6989(93)90092-b. [DOI] [PubMed] [Google Scholar]
- Field DJ. Relations between the statistics of natural images and the response properties of cortical cells. J. Opt. Soc. Am. A. 1987;4:2379–2394. doi: 10.1364/josaa.4.002379. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Ercoles AM. Involuntary eye movements during attempted monocular fixation. Atti Fondazione Giorgio Ronchi. 1966:199–217. [Google Scholar]
- Greschner M, Bongard M, Rujan P, Ammermuller J. Retinal ganglion cell synchronization by fixational eye movements improves feature estimation. Nat. Neurosci. 2002;5:341–347. doi: 10.1038/nn821. [DOI] [PubMed] [Google Scholar]
- Haddad GM, Steinman RM. The smallest voluntary saccade: Implications for fixation. Vision Res. 1973;13:1075–1086. doi: 10.1016/0042-6989(73)90145-4. [DOI] [PubMed] [Google Scholar]
- Hafed ZM. Alteration of visual perception prior to microsaccades. Neuron. 2013;77:775–786. doi: 10.1016/j.neuron.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Clark JJ. Microsaccades as an overt measure of covert attention shifts. Vision Res. 2002;42:2533–2545. doi: 10.1016/s0042-6989(02)00263-8. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. A neural mechanism for microsaccade generation in the primate superior colliculus. Science. 2009;323:940–943. doi: 10.1126/science.1166112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T, Pracejus L, Gegenfurtner KR. Color perception in the intermediate periphery of the visual field. J. Vis. 2009;9:1–12. doi: 10.1167/9.4.26. [DOI] [PubMed] [Google Scholar]
- Havermann K, Cherici C, Rucci M, Lappe M. Fine-scale plasticity of microscopic saccades. J. Neurosci. 2014;34:11665–11672. doi: 10.1523/JNEUROSCI.5277-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington TM, Masse NY, Hachmeh KJ, Smith JE, Assad JA, Cook EP. The effect of microsaccades on the correlation between neural activity and behavior in middle temporal, ventral intraparietal, and lateral intraparietal areas. J. Neurosci. 2009;29:5793–57805. doi: 10.1523/JNEUROSCI.4412-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl SS, Lisberger SG. Representation of perceptually invisible image motion in extrastriate visual area MT of macaque monkeys. J. Neurosci. 2011;31:16561–16569. doi: 10.1523/JNEUROSCI.3166-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist K, Nystrm M, Andersson R, Dewhurst R, Jarodzka H, de Weijer JV. Eye tracking: A comprehensive guide to methods and measures. Oxford University Press; 2011. [Google Scholar]
- Hyväarinen A, Hurri J, Hoyer PO. A probabilistic approach to early computational vision. Springer-Verlag; New York: 2009. Natural image statistics. [Google Scholar]
- Jacobs RJ. Visual resolution and contour interaction in the fovea and periphery. Vision Res. 1979;19:1187–1195. doi: 10.1016/0042-6989(79)90183-4. [DOI] [PubMed] [Google Scholar]
- Kagan I. Microsaccades and image fading during natural vision. Response to McCamy et al. Microsaccadic efficacy and contribution to foveal and peripheral vision. J. Neurosci. 2012;32:9194–9204. doi: 10.1523/JNEUROSCI.0515-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan I, Gur M, Snodderly DM. Saccades and drifts differentially modulate neuronal activity in V1: Effects of retinal image motion, position, and extraretinal influences. J. Vis. 2008;8:1–25. doi: 10.1167/8.14.19. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Benardete E. The dynamics of primate retinal ganglion cells. Prog. Brain. Res. 2001;134:17–34. doi: 10.1016/s0079-6123(01)34003-7. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Motion and vision. I. Stabilized images of stationary gratings. J. Opt. Soc. Am. 1979;69:1266–1274. doi: 10.1364/josa.69.001266. [DOI] [PubMed] [Google Scholar]
- Ko HK, Poletti M, Rucci M. Microsaccades precisely relocate gaze in a high visual acuity task. Nat. Neurosci. 2010;13:1549–1553. doi: 10.1038/nn.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenderink JJ. Contrast enhancement and the negative afterimage. J. Opt. Soc. Am. 1972;62:685–689. doi: 10.1364/josa.62.000685. [DOI] [PubMed] [Google Scholar]
- Kowler E. Eye movements: The past 25 years. Vision Res. 2011;51:1457–1483. doi: 10.1016/j.visres.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowler E, Steinman RM. Small saccades serve no useful purpose: Reply to a letter by R. W. Ditchburn. Vision Res. 1980;20:273–276. doi: 10.1016/0042-6989(80)90113-3. [DOI] [PubMed] [Google Scholar]
- Kuang X, Poletti M, Victor JD, Rucci M. Temporal encoding of spatial information during active visual fixation. Curr. Biol. 2012;20:510–514. doi: 10.1016/j.cub.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge GE, Kersten D. Contrast discrimination in peripheral vision. J. Opt. Soc. Am. A. 1987;4:1594–1598. doi: 10.1364/josaa.4.001594. [DOI] [PubMed] [Google Scholar]
- Malinov IV, Epelboim J, Herst AN, Steinman RM. Characteristics of saccades and vergence in two kinds of sequential looking tasks. Vision Res. 2000;40:2083–2090. doi: 10.1016/s0042-6989(00)00063-8. [DOI] [PubMed] [Google Scholar]
- Marshall WH, Talbot SA. In: Biological Symposia—Visual Mechanisms. Kluver H, editor. Vol. 7. Cattel; Lancaster, PA: 1942. pp. 117–164. [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nat. Neurosci. 2000;3:251–258. doi: 10.1038/72961. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Troncoso XG, Dyar TA. Microsaccades counteract fading during fixation. Neuron. 2006;49:297–305. doi: 10.1016/j.neuron.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Millodot M. Foveal and extra-foveal acuity with and without stabilized retinal images. Br. J. Physiol. Optic. 1966;23:75–106. [PubMed] [Google Scholar]
- Mostofi N, Boi M, Rucci M. Influence of microsaccades on contrast sensitivity: Theoretical analysis and experimental results. J. Vis. 2014;14:109. [Google Scholar]
- Nachmias J. Determiners of the drift of the eye during monocular fixation. J. Opt. Soc. Am. 1961;51:761–766. doi: 10.1364/josa.51.000761. [DOI] [PubMed] [Google Scholar]
- Nandy AS, Tjan BS. Saccade-confounded image statistics explain visual crowding. Nat. Neurosci. 2012;15:463–469. doi: 10.1038/nn.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer O, Williams DR. Blurring by fixational eye movements. Vision Res. 1992;32:1931–1939. doi: 10.1016/0042-6989(92)90052-k. [DOI] [PubMed] [Google Scholar]
- Poletti M, Listorti C, Rucci M. Microscopic eye movements compensate for nonhomogeneous vision within the fovea. Curr. Biol. 2013;23:1691–1695. doi: 10.1016/j.cub.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti M, Rucci M. Oculomotor synchronization of visual responses in modeled populations of retinal ganglion cells. J. Vis. 2008;8:1–15. doi: 10.1167/8.14.4. [DOI] [PubMed] [Google Scholar]
- Poletti M, Rucci M. Fixational eye movements under various conditions of image fading. J. Vis. 2010;10(3):1–18. doi: 10.1167/10.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard RM, Heron W. Small eye movements of the cat. Canad. J. Psychol. 1960;40:131–137. doi: 10.1037/h0083176. [DOI] [PubMed] [Google Scholar]
- Putnam NM, Hofer HJ, Doble N, Chen L, Carroll J, Williams DR. The locus of fixation and the foveal cone mosaic. J. Vis. 2005;7:632–639. doi: 10.1167/5.7.3. [DOI] [PubMed] [Google Scholar]
- Ratliff F, Riggs LA. Involuntary motions of the eye during monocular fixation. J. Exp. Psychol. 1950;40:687–701. doi: 10.1037/h0057754. [DOI] [PubMed] [Google Scholar]
- Riggs LA, Ratliff F. The effects of counteracting the normal movements of the eye. J. Opt. Soc. Am. 1952;42:872–873. [Google Scholar]
- Rolfs M. Microsaccades: Small steps on a long way. Vision Res. 2009;49:2415–2441. doi: 10.1016/j.visres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Rucci M. Fixational eye movements, natural image statistics, and fine spatial vision. Network: Comp. Neural. 2008;19:253–285. doi: 10.1080/09548980802520992. [DOI] [PubMed] [Google Scholar]
- Rucci M, Casile A. Decorrelation of neural activity during fixational instability: Possible implications for the refinement of V1 receptive fields. Visual Neurosci. 2004;21:725–738. doi: 10.1017/S0952523804215073. [DOI] [PubMed] [Google Scholar]
- Rucci M, Iovin R, Poletti M, Santini F. Miniature eye movements enhance fine spatial detail. Nature. 2007;447:852–855. doi: 10.1038/nature05866. [DOI] [PubMed] [Google Scholar]
- Santini F, Redner G, Iovin R, Rucci M. EyeRIS: A general-purpose system for eye movement contingent display control. Behav. Res. Methods. 2007;39:350–364. doi: 10.3758/bf03193003. [DOI] [PubMed] [Google Scholar]
- Simoncelli E, Olshausen B. Natural image statistics and neural representation. Annu. Rev. Neurosci. 2001;24:1193–1216. doi: 10.1146/annurev.neuro.24.1.1193. [DOI] [PubMed] [Google Scholar]
- Skavenski AA, Hansen RM, Steinman RM, Winterson BJ. Quality of retinal image stabilization during small natural and artificial body rotations in man. Vision Res. 1979;19:675–683. doi: 10.1016/0042-6989(79)90243-8. [DOI] [PubMed] [Google Scholar]
- Skavenski AA, Robinson DA, Steinman RM, Timberlake GT. Miniature eye movements of fixation in rhesus monkey. Vision Res. 1975;15:1269–1273. doi: 10.1016/0042-6989(75)90173-x. [DOI] [PubMed] [Google Scholar]
- Snodderly DM. A physiological perspective on fixational eye movements. Vision Res. 2015 doi: 10.1016/j.visres.2014.12.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodderly DM, Kagan I, Gur M. Selective activation of visual cortex neurons by fixational eye movements: Implications for neural coding. Visual Neurosci. 2001;18:259–277. doi: 10.1017/s0952523801182118. [DOI] [PubMed] [Google Scholar]
- Srinivasan MV, Laughlin SB, Dubs A. Predictive coding: A fresh view of inhibition in the retina. P. R. Soc. Lond. B. Bio. 1982;216:427–459. doi: 10.1098/rspb.1982.0085. [DOI] [PubMed] [Google Scholar]
- St.Cyr GJ, Fender DH. The interplay of drifts and flicks in binocular fixation. Vision Res. 1969;9:245–265. doi: 10.1016/0042-6989(69)90004-2. [DOI] [PubMed] [Google Scholar]
- Steinbach MJ. Owls’ eyes move. Br. J. Ophthal. 2004;88:1103. doi: 10.1136/bjo.2004.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R, Cunitz R, Timberlake G, Herman M. Voluntary control of microsaccades during maintained monocular fixation. Science. 1967;155:1577–1579. doi: 10.1126/science.155.3769.1577. [DOI] [PubMed] [Google Scholar]
- Steinman RM. In: The Visual Neurosciences. Chalupa LM, Werner JS, editors. MIT Press; Cambridge: 2003. pp. 1339–1356. [Google Scholar]
- Steinman RM, Haddad GM, Skavenski AA, Wyman D. Miniature eye movement. Science. 1973;181:810–819. doi: 10.1126/science.181.4102.810. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Kowler E, Collewijn H. New directions for oculomotor research. Vision Res. 1990;30:1845–1864. doi: 10.1016/0042-6989(90)90163-f. [DOI] [PubMed] [Google Scholar]
- Thaler L, Schtz AC, Goodale MA, Gegenfurtner KR. What is the best fixation target? The effect of target shape on stability of fixational eye movements. Vision Res. 2013;76:31–42. doi: 10.1016/j.visres.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Timberlake GT, Wyman D, Skavenski AA, Steinman RM. The oculomotor error signal in the fovea. Vision Res. 1972;12:1059–1064. doi: 10.1016/0042-6989(72)90027-2. [DOI] [PubMed] [Google Scholar]
- Tulunay-Keesey U. Fading of stabilized retinal images. J. Opt. Soc. Am. 1982;72:440–447. doi: 10.1364/josa.72.000440. [DOI] [PubMed] [Google Scholar]
- van Hateren JH. A theory of maximizing sensory information. Biol. Cybern. 1992;68:23–29. doi: 10.1007/BF00203134. [DOI] [PubMed] [Google Scholar]
- Weymouth FW, Hines DC, Acres LH, Raaf JE, Wheeler MC. Visual acuity within the area centralis and its relation to eye movements and fixation. Am. J. Ophthalmol. 1928;11:947–960. [Google Scholar]
- Whitham EM, Fitzgibbon SP, Lewis TW, Pope KJ, Delosangeles D, et al. Visual experiences during paralysis. Front. Hum. Neurosci. 2011:160. doi: 10.3389/fnhum.2011.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterson B, Collewijn H. Microsaccades during finely guided visuomotor tasks. Vision Res. 1976;16:1387–1390. doi: 10.1016/0042-6989(76)90156-5. [DOI] [PubMed] [Google Scholar]
- Wyman D, Steinman RM. Latency characteristics of small saccades. Vision Res. 1973;13:2173–2175. doi: 10.1016/0042-6989(73)90195-8. [DOI] [PubMed] [Google Scholar]
- Zuber BL, Stark L, Cook G. Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science. 1965;150:1459–1460. doi: 10.1126/science.150.3702.1459. [DOI] [PubMed] [Google Scholar]