Abstract
Background
The 2012 Kidney Dialysis Initiative Global Outcomes chronic kidney disease (CKD) classification scheme subdivides stage 3 CKD and incorporates the urinary albumin-to-creatinine ratio (ACR). The aim of this study was to evaluate whether the novel scheme provides graded risk in kidney transplant recipients (KTRs).
Methods
Prevalent KTRs with available laboratory data were included. The primary outcome was a composite of doubling of serum creatinine, graft failure, or death. Patients were stratified using the CKD-Epidemiolgic Collaboration equation, and ACR and the event rate per 1000 patient-years in each CKD category were calculated.
Results
There were 269 KTRs with a mean follow-up of 4.5 ± 2.0 years. There was a graded increase in outcomes with increasing ACR and decreasing estimated glomerular filtration rate (eGFR). For the primary outcome, the event rate was 15.3 (95% confidence interval, 4.2-39.2) per 1000 patient-years for those with an eGFR greater than 60 mL/min per 1.73 m2 and an ACR less than 30 mg/g, whereas it was 375 (95% confidence interval, 193.8-655.1) for those with an eGFR less than 30 mL/min per 1.73 m2 and an ACR greater than 300 mg/g.
Conclusions
The novel Kidney Dialysis Initiative Global Outcomes classification scheme provides graded risk for important clinical events in KTRs. This information can be used to identify high-risk patients and to tailor follow-up and management strategies aimed at improving outcomes.
In 2002, the National Kidney Foundation Kidney Dialysis Outcomes Quality Initiative published guidelines on the classification of chronic kidney disease (CKD).1 The proposed scheme classified patients into CKD stages 1 to 5 based on the glomerular filtration rate (GFR). The guidelines were widely adopted internationally, and their merits and pitfalls have been widely discussed.2-7 In 2004, the Kidney Dialysis Initiative Global Outcomes (KDIGO) work group endorsed the guidelines and recommended that transplant patients be further identified by including a “T”.8
One of the major criticisms of the guideline classification scheme was its failure to adequately reflect prognosis.2,9 Evidence had accumulated revealing proteinuria as an important predictor of outcome and that the risk of adverse outcomes varied greatly within the same CKD stage depending on degree of proteinuria.4,5,10-13 In response to ongoing debate and with new evidence, KDIGO has refined the original scheme with subdivision of stage 3 CKD into 2 substages and incorporation of a measure of proteinuria (urinary albumin-to-creatinine ratio [ACR]).14
The new guidelines also recommend that patients be classified by diagnosis including transplantation.14 Transplant recipients, however, were not included in the study populations analyzed by KDIGO,15 and it is not known whether the new classification system reflects risk in this unique population as it does in other populations. The purpose of this study was to evaluate whether the novel KDIGO CKD classification scheme provides graded risk for the important clinical outcomes of mortality, graft function decline, and graft loss in kidney transplant recipients.
MATERIALS AND METHODS
Study Population
The study received approval from the Ottawa Hospital Research Ethics Board. The study population included adult kidney transplant recipients followed up at the Ottawa Hospital who were at least 6 months posttransplantation and who had participated in either a GFR measurement study16 or in a randomized controlled study examining the use of angiotensin-converting enzyme (ACE) inhibitors.17 For the GFR measurement study, patients provided a serum sample for creatinine (Cr) and a urine sample for ACR at the first study visit. For patients in the ACE inhibitor study, Cr was obtained on the day of randomization and the ACR was abstracted from the medical records (all within 1 month of randomization).
Laboratory Assessment
Serum and urine Cr was measured using the modified Jaffe reaction on a Beckman Coulter LX20 Pro Clinical System using manufacturer's reagents (Beckman Coulter Inc. Brea CA). The coefficient of variation for serum Cr was 4.9% at 0.6 mg/dL (55 μmol/L), 1.7% at 1.7 mg/dL (150 μmol/L), and 1.3% at 6.8 mg/dL (600 μmol/L). Creatinine values were adjusted to the isotope dilution mass spectrometry standard. Urine albumin was measured by an immunoturbidimetric method. Coefficient of variation for urine albumin was 4.1% at 2.7 mg/dL (27 mg/L) and 3.1% at 12 mg/dL (120 mg/L). The coefficient of variation for urine Cr was 1.7% at 69 mg/dL (6.1 mmol/L) and 1.5% at 167 mg/dL (14.8 mmol/L).
Outcomes
Graft failure (need for dialysis or repeat transplantation), patient death, and doubling of serum Cr were extracted retrospectively from the medical records of patients in the GFR measurement study and from data collected prospectively by the Ottawa Hospital kidney program of all transplant recipient deaths and new dialysis starts. The same data were collected prospectively for the ACE inhibitor study participants by the research study coordinator. Data were collected until the first occurrence of doubling of serum Cr, graft failure, or death. The primary outcome was a composite measure consisting of the first occurrence of doubling Cr, graft failure, or death. The secondary outcome was an allograft composite consisting of doubling of serum Cr or graft loss. Tertiary outcome was patient death. These outcomes were selected as they are standard and meaningful outcomes in clinical trials in kidney transplantation.17-19
Analysis
Patients were stratified according to the new classification scheme using the CKD-Epidemiolgic Collaboration (CKD-EPI) estimated GFR (eGFR) equation20 and the ACR. For this analysis, GFR categories G1 and G2 were combined as were G4 and G5 because numbers were small, and these share the same KDIGO risk categories at all levels of albuminuria (ie, G1 and G2 both are low risk for A1, intermediate for A2 and high risk for A3). Unadjusted Poisson regression was used to determine event rates (with 95% confidence intervals) expressed per 1000 patient-years for each of the outcome measures.
RESULTS
Patient Characteristics
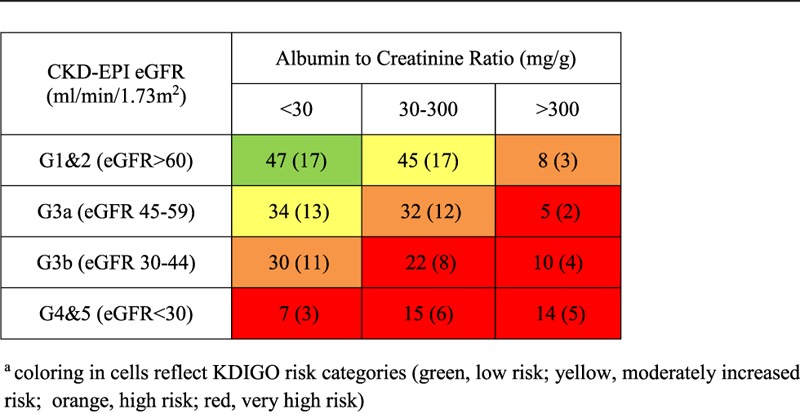
Two hundred sixty-nine patients were included: 172 from the GFR measurement study and 97 from the ACE inhibitor study. The baseline characteristics of the cohort are presented in Table 1. Mean (SD) follow-up time was 4.5 (2.0) years. The majority (91%) of patients were white. The mean calculated CKD-EPI GFR was 53.8 (21.5) mL/min per 1.73 m2. There were subjects in all 5 GFR categories. Mean ACR was 199.8 (462) mg/g with 44% of the cohort having an ACR less than 30 mg/g. Table 2 shows the distribution of the cohort within the risk groups. There were fewer patients with either the highest degree of proteinuria (14%, ACR > 300 mg/g) or the lowest GFR category (13%, eGFR < 30 mL/min per 1.73 m2).
TABLE 1.
Patient characteristics
TABLE 2.
Distribution of cohort within CKD groups, n (%of cohort)
Outcome Occurrence
The primary outcome occurred in 70 (26%) patients. The secondary outcome occurred in 41 (15%) patients; 24 (9%) patients who experienced doubling of Cr and 17 (6%) who experienced graft loss. Twenty-nine (11%) patients died.
Event Rates by Risk Category
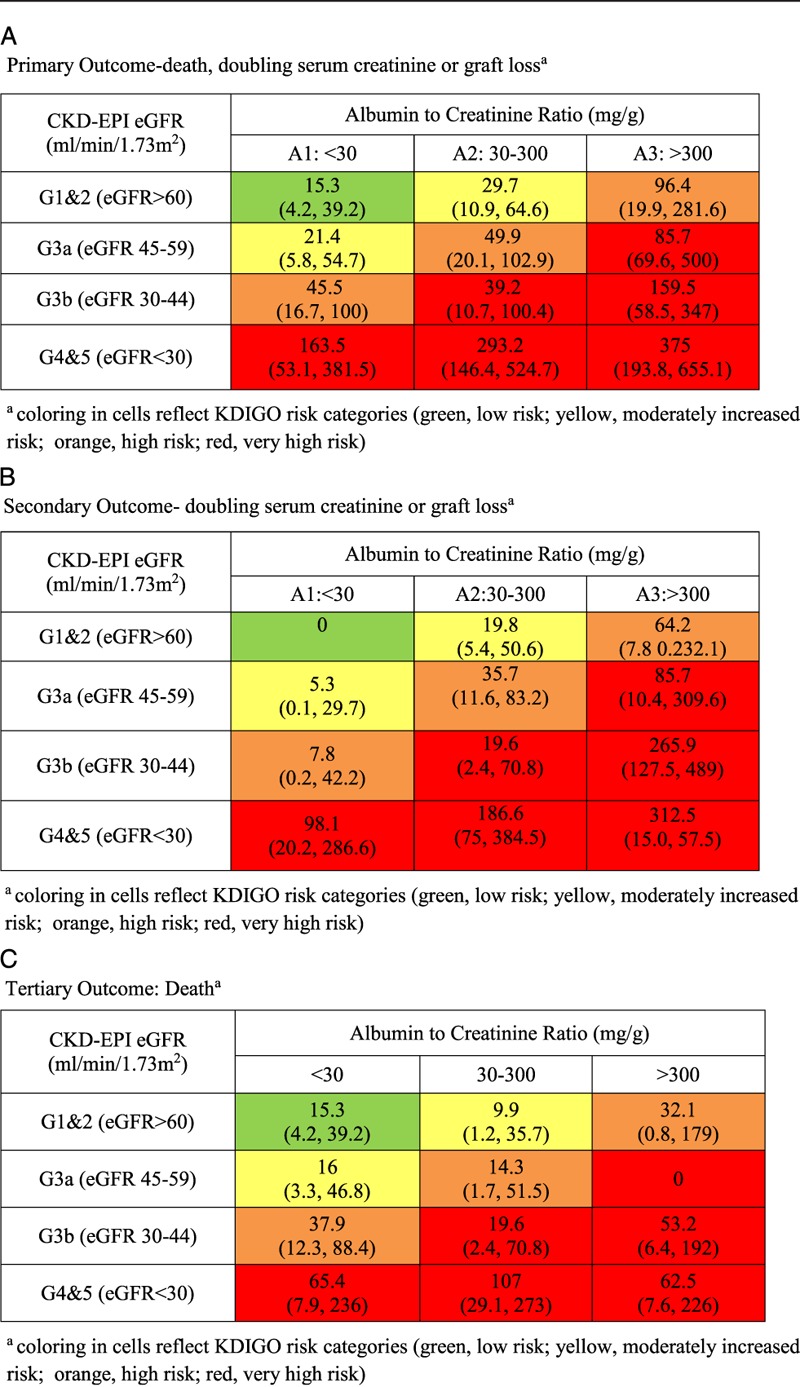
The event rates per 1000 patient-years are shown in Table 3. The number of events and person years followed are shown in supplementary Table S1 (SDC, http://links.lww.com/TXD/A28). For the primary and secondary outcomes (Table 3A and B), there was an increased and graded risk within eGFR categories as ACR increased. Within each ACR category, there was increased and graded risk of events as eGFR declined. The primary outcome rate for those with eGFR greater than 60 mL/min per 1.73 m2 was 15.3 (95% confidence interval [95% CI], 4.2-39.2) for those in the lowest ACR category and 96.4 (95% CI, 19.9-281.6) for those in the highest ACR category (Table 3A). Similarly, the primary outcome rate for those in the lowest ACR category was 15.3 (95% CI, 4.2-39.2) for those with eGFR greater than 60 mL/min per 1.73 m2 and 163.5 (95% CI, 53.1 to 381.5) for those with eGFR less than 30 mL/min per 1.73 m2. The highest primary outcome rate was 375 (95% CI, 193.8-655.1), seen in those in the highest ACR and lowest eGFR categories combined. This is 25-fold higher than the rate seen in the lowest risk group (highest eGFR and lowest ACR categories combined). Similar patterns are seen for the secondary and tertiary outcomes (Table 3B and C). Death has a less consistent graded risk relationship than the other outcomes and seems more closely related to eGFR than it does to ACR.
TABLE 3.
Event rate per risk group (expressed as event per 1000 patient-years)
DISCUSSION
In this study, we found that the new KDIGO classification scheme provides a graded risk assessment for kidney transplant recipients similar to what is seen in the general population. Patients with heavy proteinuria and significantly reduced eGFR had the highest event rates. For the highest eGFR category, there was a 4-fold increase in the occurrence of the primary outcome between the lowest and highest ACR categories. In the lowest eGFR category, there was an 11-fold increase. The classification scheme is simple to administer and does not require multiple additional variables included in more complicated risk prediction scores available.21,22
Our findings in kidney transplant recipients are consistent with previous analyses in the nontransplant population.5,23,24 There are, however, a few notable differences. First, the event rate was higher in our lowest risk group (eGFR > 60 mL/min per 1.73 m2, ACR < 30 mg/g) compared with similarly categorized nontransplant patients. Tonelli et al5 report a rate of renal outcomes (end-stage renal disease [ESRD] or registration for transplant) and of death of 0.7 events and 8.9 per 1000 patient-years in patients with combined eGFR greater than 60 mL/min per 1.73 m2 and ACR less than 30 mg/g in a Canadian provincial registry. In pooled analyses of populations considered at high risk for CKD, rates of ESRD and all-cause mortality were 0.22 and approximately 10 per 1000 patient-years, respectively, in the combined low ACR and high eGFR category.23,24 These event rates are much lower than the rates in our study. The finding of higher event rates in transplant recipients is not surprising given that most of these patients have already survived a phase of CKD and dialysis before undergoing transplantation and have additional risks, such as immunosuppressant exposure and toxicity.
The second major difference in our study is that the event rates in the combined moderately increased ACR and moderately to severely decreased eGFR (G3bA2) group, designated as a “very high risk” group by KDIGO are more similar to those of the KDIGO “high risk” groups (ie, G3a, A2) than to the rates observed in the other “very high risk” groups (Table 3). In contrast, in the cohorts studied by the KDIGO consortium, the event rates in G3bA2 are significantly higher than that of G3aA2.4,23,24 This suggests that the relationship between GFR and outcomes may be different in transplantation as compared with native kidney disease at this moderate reduction in kidney function frequently seen in kidney transplantation.
This concept is supported by the findings that eGFR alone is not robustly predictive of graft survival.21,25,26 In 1 study using a 6-month posttransplant eGFR, He et al25 found an area under the curve by receiver operating curve analysis of only 0.6 for 5-year graft failure. In other words, by randomly selecting 2 individuals, 1 from each of the 2 groups (those who developed graft failure and those who did not), the individual who developed graft failure will have had the lower eGFR only 60% of the time. This is only slightly improved over the results you would obtain by random chance. Other groups have also reported similar findings, suggesting that there are other important determinants of graft failure/death.21,26 The results of our study suggest that incorporating proteinuria may improve predictive ability of the eGFR.
Two scoring systems have been proposed incorporating eGFR and proteinuria among multiple other variable to predict graft failure.21,27 These however are very cumbersome and were developed using data from one year post transplant which limit their clinical utility.21,27 Bucsa et al28 examined the association between GFR categories, proteinuria, and outcomes in 231 renal transplant recipients but used 24-hour urine total protein collections which were then converted to estimated albumin excretion and the 4 variable MDRD equation. The composite outcome studied was death, ESRD, or a greater than 30% decline in eGFR from 6 months posttransplant. Patients were observed from study enrollment for a total of 30 months. There were no deaths, 13 patients developed ESRD, and 51 reached the composite endpoint. They do not however present event rates by eGFR/ACR group and those that did not. This study is also hampered by the short follow-up, lack of ACR, and low number of outcomes during the prospective study time. As a result, it is very difficult to draw any real conclusions about the utility of the KDIGO CKD risk stratification in transplant recipients from this study.
Strengths of our study include the comprehensive assessment of important outcomes, the measurement of ACR, and eGFR on the same day in the majority of study subjects and within 1 month in the remainder, and the presence of subjects with all 3 albuminuria categories. Study weaknesses include a relatively small number of events contributing to wide confidence intervals for some categories. Although the precision would have improved with more events, we still found a graded relationship between worsening eGFR/ACR and outcomes. Other comorbid conditions of the GFR measurement study patients were not known, so we could not provide an adjusted analysis. It is possible that confounding may have influenced the study results as patients in the highest risk categories may have had other characteristics (ie, heart disease) that put them at risk of adverse outcomes. Information about the use of medications that inhibit the renin-angiotensin system are not known for the GFR measurement study cohort. The results from the ACE inhibitor randomized controlled trial revealed that Ace inhibition had no impact on graft failure, patient death, or doubling of serum Cr.17 There should therefore be no impact of Ace inhibitor use on our current findings. Finally, the mean time posttransplant was 7.0 ± 6.6 years, so results may be affected by survival bias. However, prevalent populations (prone to survivor bias) were also used in the validation of the new KDIGO classification system in nontransplant patients.
The results of this study suggests that the 2012 KDIGO CKD classification scheme provides graded risk in kidney transplant recipients with worse outcomes observed in patients with increasing degrees of proteinuria within each GFR category. The classification scheme also supports the KDIGO Guidelines for the Care of Kidney Transplant Recipients' recommendation of yearly urinary protein excretion assessment.29 Confirmation of study findings in a larger cohort is required.
ACKNOWLEDGMENT
The authors would like to acknowledge the Ottawa Hospital Kidney Transplant Program staff for providing some of the outcome data.
Footnotes
Published online 25 July 2016.
The Angiotensin Converting Enzyme (ACE) inhibition for the preservation of renal function and patient survival in kidney transplantation was funded by the Canadian Institute of Health Research. The accurate determination of GFR in renal transplantation GFR measurement study was funded by the Physician Services' Incorporated Foundation and Astellas Pharma Canada.
The authors declare no conflicts of interest.
Canadian Institute of Health Research. ISRCTN, number 78129473.
Drs. White and Akbari contributed equally to this work and both should be regarded as co-first authors.
C.W., A.A., and G.A. contributed to study idea, design, data analysis, and article preparation. H.T. and N.L. contributed to data acquisition and article preparation.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 2.Eckardt KU, Berns JS, Rocco MV, et al. Definition and classification of CKD: the debate should be about patient prognosis—a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53:915–920. [DOI] [PubMed] [Google Scholar]
- 3.Botev R, Mallié JP, Wetzels JF, et al. The clinician and estimation of glomerular filtration rate by creatinine-based formulas: current limitations and quo vadis. Clin J Am Soc Nephrol. 2011;6:937–950. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. [DOI] [PubMed] [Google Scholar]
- 5.Tonelli M, Muntner P, Lloyd A, et al. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154:12–21. [DOI] [PubMed] [Google Scholar]
- 6.Poggio ED, Rule AD. Can we do better than a single estimated GFR threshold when screening for chronic kidney disease? Kidney Int. 2007;72:534–536. [DOI] [PubMed] [Google Scholar]
- 7.Glassock RJ, Winearls C. Screening for CKD with eGFR: doubts and dangers. Clin J Am Soc Nephrol. 2008;3:1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2005;67:2089–2100. [DOI] [PubMed] [Google Scholar]
- 9.de Jong PE, Gansevoort RT. Fact or fiction of the epidemic of chronic kidney disease—let us not squabble about estimated GFR only, but also focus on albuminuria. Nephrol Dial Transplant. 2008;23:1092–1095. [DOI] [PubMed] [Google Scholar]
- 10.Brantsma AH, Bakker SJ, Hillege HL, et al. Cardiovascular and renal outcome in subjects with K/DOQI stage 1-3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant. 2008;23:3851–3858. [DOI] [PubMed] [Google Scholar]
- 11.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. [DOI] [PubMed] [Google Scholar]
- 12.Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. [DOI] [PubMed] [Google Scholar]
- 13.Hallan SI, Ritz E, Lydersen S, et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) Work Group. KDIGO 2012 Clinical practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2012;3:1–150. [DOI] [PubMed] [Google Scholar]
- 15.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White CA, Akbari A, Doucette S, et al. Estimating glomerular filtration rate in kidney transplantation: is the new chronic kidney disease epidemiology collaboration equation any better? 2010. Clin Chem. 2010;56:474–477. [DOI] [PubMed] [Google Scholar]
- 17.Knoll GA, Fergusson D, Chassé M, et al. Ramipril versus placebo in kidney transplant patients with proteinuria: a multicentre, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:318–326. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Archdeacon P, Dixon C, Belen O, et al. Summary of the US FDA approval of belatacept. Am J Transplant. 2012;12:554–562. [DOI] [PubMed] [Google Scholar]
- 19.Schold JD, Kaplan B. The elephant in the room: failings of current clinical endpoints in kidney transplantation. Am J Transplant. 2010;10(5):1163–1166. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foucher Y, Daguin P, Akl A, et al. A clinical scoring system highly predictive of long-term kidney graft survival. Kidney Int. 2010;78:1288–1294. [DOI] [PubMed] [Google Scholar]
- 22.Shabir S, Halimi JM, Cherukuri A, et al. Predicting 5-year risk of kidney transplant failure: a prediction instrument using data available at 1 year posttransplantation. Am J Kidney Dis. 2014;63:643–651. [DOI] [PubMed] [Google Scholar]
- 23.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van d V, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. [DOI] [PubMed] [Google Scholar]
- 25.He X, Moore J, Shabir S, et al. Comparison of the predictive performance of eGFR formulae for mortality and graft failure in renal transplant recipients. Transplantation. 2009;87:384–392. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan B, Schold J, Meier-Kriesche HU. Poor predictive value of serum creatinine for renal allograft loss. Am J Transplant. 2003;3:1560–1565. [DOI] [PubMed] [Google Scholar]
- 27.Chand S, Shabir S, Chan W, et al. β cell glucotoxic-associated single nucleotide polymorphisms in impaired glucose tolerance and new-onset diabetes after transplantation. Transplantation. 2014;98:e19–e20. [DOI] [PubMed] [Google Scholar]
- 28.Bucşa C, Stefan G, Tacu D, et al. Does the KDIGO CKD risk stratification based on GFR and proteinuria predict kidney graft failure? Int Urol Nephrol. 2014;46:1857–1865. [DOI] [PubMed] [Google Scholar]
- 29.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1–S157. [DOI] [PubMed] [Google Scholar]


