Abstract
Background
There have been no previous reports how Kupffer cells affect the outcome of living donor liver transplantation (LDLT) with an elderly donor. The aim of this study was to elucidate the influence of Kupffer cells on LDLT.
Methods
A total of 161 adult recipients underwent LDLT. The graft survival, prognostic factors for survival, and graft failure after LDLT were examined between cases with a young donor (<50, n = 112) and an elderly donor (≥50, N = 49). The Kupffer cells, represented by CD68-positive cell in the graft, were examined in the young and elderly donors.
Results
In a multivariable analysis, a donor older than 50 years, sepsis, and diabetes mellitus were significant predictors of graft failure after LDLT. The CD68 in younger donors was significantly more expressed than that in elderly donors. The group with a less number of CD68-positive cells in the graft had a significantly poor survival in the elderly donor group and prognostic factor for graft failure.
Conclusions
The worse outcome of LDLT with elderly donors might be related to the lower number of Kupffer cells in the graft, which can lead to impaired recovery of the liver function and may predispose patients to infectious diseases after LDLT.
Liver transplantation (LT) has been performed for end-stage liver disease.1 To overcome the scarcity of deceased donors, living donor LT (LDLT) has been accepted as an alternative option, especially in Asian countries including Japan.2-6 Moreover, to expand the donor pool for LT, the use of marginal donors, such as those with a steatotic liver, grafts with a prolonged ischemic time, small-for-size grafts, and elderly donors, has been considered.7
With regard to the donor age at LT, some centers have reported that the outcome of diseased donor LT (DDLT) using an elderly donor was worse than that of procedures using a younger donor. On the other hand, other centers have reported that elderly donor grafts were acceptable in terms of the outcomes after DDLT under some conditions.8-16 The registry data from United States and Europe showed that the graft survival of DDLT from elderly donor was worse than that from younger donors.8,9 With regard to LDLT, the Japanese Liver Transplantation Registry in 2011 reported that 18.1% and 4% of the donors were older than 50 and 60 years. The survival of recipients with an elderly donor was significantly worse than that of recipients with a younger donor.10
The reasons for the worse outcome were estimated to include that the quality of the graft liver in elderly donors might be associated with problems even if the liver function tests were normal. The ischemic-reperfusion–induced liver injury might cause the impaired liver function after LT.7
Kupffer cells are located in the sinusoidal region in the liver and are responsible for the phagocytosis of bacteria-like macrophages in the blood. Kupffer cells were activated in the process of hepatitis B virus and hepatitis C virus (HCV) infection of the liver and also in a mouse model of acute liver failure.17,18 However, it has been unclear how many Kupffer cells are located in the liver in young and elderly human liver and whether Kupffer cells affect the graft survival after LDLT.
The aim of this study was to investigate the outcomes of elderly donor graft survival and to determine the influence of Kupffer cell activity on the graft survival.
MATERIALS AND METHODS
A total of 208 patients underwent LDLT from August 1997 to October 2014 at Nagasaki University Hospital. Among these recipients, 161 adult recipients were enrolled in this study and had a follow-up period over 1 year. These patients all had indications for LDLT, as defined by the ethics committee of our hospital. The management of LDLT recipients and the donor selection was described previously.6 The donor selection criteria were defined as follows, and the estimated liver volume had been calculated by multidetector computed tomography: our policy for graft selection is the use of an extended left lobe graft if the estimated graft volume (GV)/standard liver volume (SLV) of the recipient was over 30%.6 If the estimated GV/SLV was under 30%, the right lobe graft was chosen in cases that the remnant lobe of the donor was over 35% as estimated by multidetector computed tomography. After LDLT, recipients were managed in the intensive care unit until the general status had recovered. The immunosuppression after LDLT was managed by steroid and tacrolimus where trough level was adjusted to be between 10 and 15 ng/mL within 1 month and between 5 and 10 ng/mL after 1 month. In cases with acute kidney dysfunction after LDLT, the mycofenolate mofetil was administered to the recipient to avoid severe kidney dysfunction.19 The steroid consumption was tapered within the first 3 months. We have performed ABO-incompatible LT since 2004; and rituximab was used for the treatment before LDLT in these cases, with local treatment performed via the portal vein or hepatic artery until 2009. The early enteral feeding nutrition has been administrated to all recipients though an enteral feeding tube by tube jejunostomy from 2004 in an early period from the day after LDLT to the day when the recipient can orally eat so much.
The treatment for the recipients with HCV recurrence was decided based on the status of fibrosis on liver biopsy, which was pathologically diagnosed based on an Ishak fibrosis stage over 3. The treatment administered for HCV before 2013 was PEGylated interferon (PEG-IFN) plus ribavirin, whereas the PEGylated interferon α 2a monotherapy was administered for the recipients who had relapsed HCV.20 Since 2013, we have administered triple therapy which contains PEG-INF with ribavirin, a protease inhibitor (telaprevir), and simeprevir.
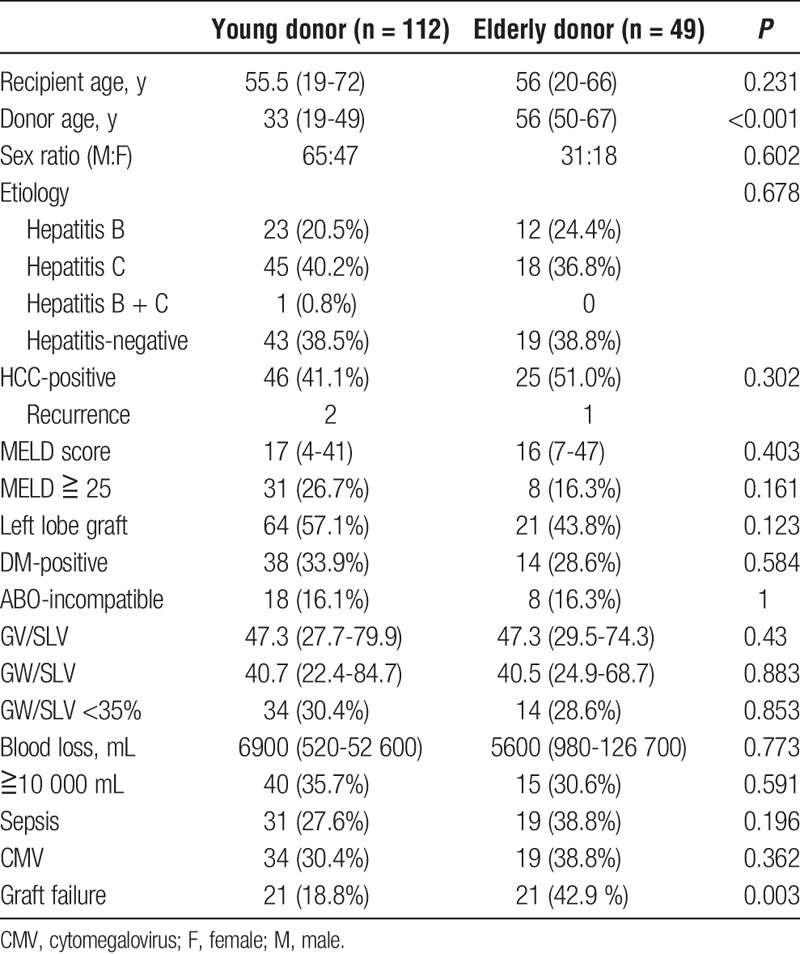
The graft survival, causes of death by donor age (young donor [n = 112]: younger than 50 years, elderly donor [n = 49]: ≥50 years) after LDLT, and the sinusoidal function represented by Kupffer cells in the graft by donor age were examined. The clinical background data of the recipients between the young and elderly donors are described in Table 1. There were no significant differences in the clinical data in the recipients between the young and elderly donor groups. Graft failure was defined as follows: graft loss was caused by liver dysfunction without any causes, liver dysfunction related to or after any infection, liver dysfunction by biliary complications, and HCV-related liver dysfunction. The other causes of death (other malignancy and recurrence of hepatocellular carcinoma [HCC]) were excluded from the graft failure.
TABLE 1.
A comparison of the background data in recipients between the elderly and young donor groups
The Expression of CD68 in the Graft by Immunohistochemical Examination
The biopsy of the graft liver (time zero biopsy) was performed on the back table after reperfusion and before the implantation of the graft. These biopsy samples were examined for the degree of steatosis by a pathologist. We also examined the time zero biopsies for CD68-positive cells in the graft as the number of Kupffer cells. Immunohistochemical examinations were performed as follows: sections were deparaffinized using graded ethanol concentrations and were washed in tris-phosphate–buffered saline. Then, the sections were place in Pepsin solution for 10 minutes at 37°C for antigen retrieval.
After being washed, their sections were treated with 0.1% H2O2 at room temperature for 10 minutes. The sections were reacted at room temperature for 30 minutes with primary monoclonal antibodies against CD68 (1:200) (cat. no. M0814, Dako, Japan), after incubation with the secondary antibody (Envision; DAKO, Chicago, Ill) for 30 minutes at room temperature. The sections were stained with 3,3-diaminobenzidine tetrahydrochloride for visualization. The sections were counterstained with Mayer hematoxylin.
The expression and number of the CD68-positive cells were examined by pathologist T.K. and H.M., coauthor, who did not have any information about the outcome in the recipients and the donors. They counted these cells independently with a 6 high-power field which they randomly selected in the specimen. The average of 6 high-power fields was calculated, and the number of CD68-positive cells was compared between younger donor (in their 20s) and elderly donors (older than 50 years).
Analyses and Statistics
The survival was analyzed from the day of LT to the most recent follow-up. The overall survival and graft survival rates were assessed with the Kaplan-Meier method using the log-rank test. To clarify the prognostic factors for survival and graft failure, 10 clinical variables were determined. The preoperative and postoperative parameters examined included the donor age, recipient age, model for end-stage liver disease (MELD) score, diabetes mellitus (DM), etiology (hepatitis B, hepatitis C, non-BnonC), HCC status, the rate of ABO-incompatible recipients, the GW/SLV, blood loss, sepsis, and cytomegalovirus infection after LDLT. The univariable and multivariable analyses of the prognostic factors for graft failure were performed using a logistic regression analysis. Differences were considered to be statistically significant for a P value less than 0.05. Statistical analyses were performed using the SPSS Version 18.0 software package (Tokyo, Japan).
RESULTS
Patient Characteristics and the Differences Between Elderly and Young Donor
Table 1 shows that there were no significant differences in the patient characteristics and perioperative parameters between the young and elderly donor groups except for graft failure after LDLT. Twenty-one (42.9%) patients with elderly donors developed graft failure after LDLT compared with 21 (18.8%) patients with young donors despite no differences in the occurrence in sepsis after LDLT between the 2 groups. The causes of graft failure in the younger donor group were as follows: graft failure without any particular cause in 14 patients, and graft failure related to infection in 7 patients. The cause of graft failure in the elderly donor group was graft failure without any particular cause in 17 patients, in addition to graft failure related to infection, biliary complication, HCV, and chronic rejection in each patient
The Differences in Patient Survival Between Young and Elderly Donor
The median follow-up of all patients was 45.8 months (range, 0.3-166.2). The causes of death in the recipients with donors older 50 years after LDLT were graft failure in 14 patients, infection in 5 patients, and HCC recurrence, other carcinoma, and chronic rejection in each patient.
The 1-, 3- and 5-year overall survival rates of recipients in the young donor group were 83.1%, 79.2%, and 73.8%, respectively. This was significantly better than the corresponding rats of 73.1%, 59.3%, and 50.5% in the elderly donor group (P = 0.01) (Figure 1).
FIGURE 1.
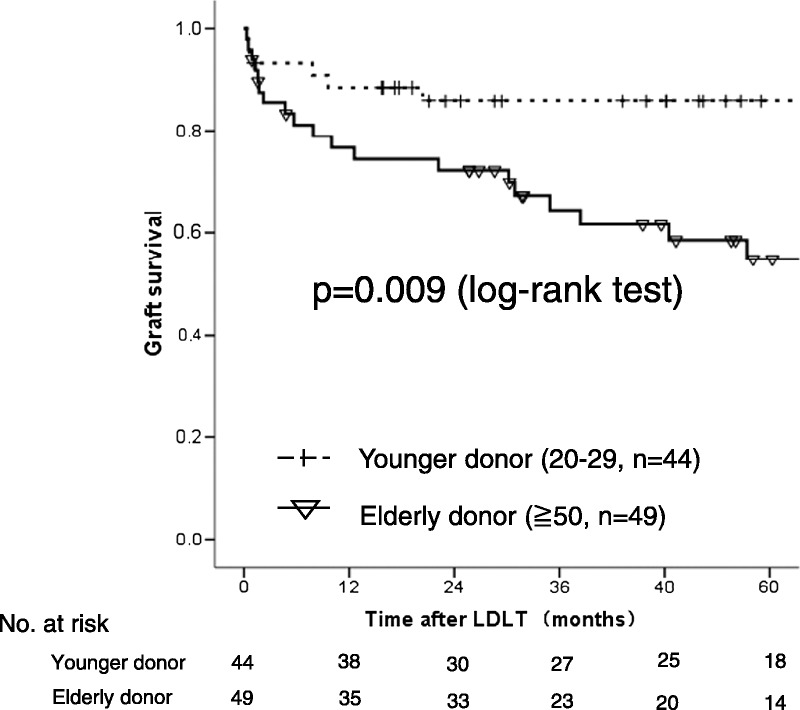
A comparison of the overall survival in recipients with elderly and young donor after LDLT. P = 0.01 (log-rank test).
The Results of the Univariable and Multivariable Analyses for Survival and Graft Failure
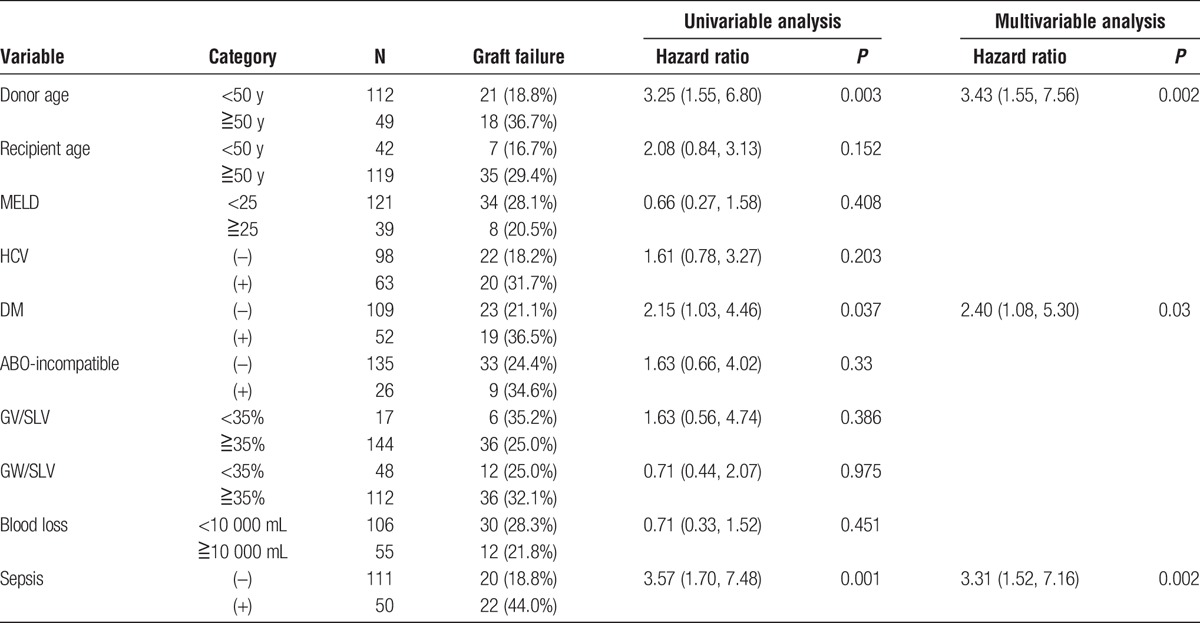
The univariable analysis identified 3 significant prognostic factors for survival: donor older than 50 years, recipient older than 50 years, and complication with sepsis after LDLT. A multivariable analysis based on the significant variables in the univariable analysis revealed that sepsis after LDLT (hazard ratio [HR], 2.59) and a donor older than 50 years (HR, 1.973) were the independent prognostic factor for survival.
Table 2 shows the prognostic factors for graft failure in the univariable analysis. The significant poor prognostic factors for graft failure were sepsis after LDLT, donor older than 50 years, and DM before LDLT. A donor older than 50 years (HR, 3.43), sepsis after LDLT (HR, 3.31), and DM before LDLT (HR, 2.40) were identified as independent prognostic indicators for graft failure in the multivariable analysis.
TABLE 2.
The results of the univariable and multivariable logistic regression analyses of the prognostic factors for graft failure after LDLT
The Expression of CD68-Positive Cells (Kupffer Cells) in the Grafts From Elderly and Younger Donors
Figure 2A1 shows the typical expression of CD68-positive cells in an elderly donor biopsy. The expression was weak and had less expression in the sinusoidal region. In the young donors (in their 20s), numerous CD68-positive cells were present in the sinusoidal region (Figure 2A2). There were significantly fewer Kupffer cells in the donor liver from the elderly donor (19.0/high power field [HPF], n = 49) than in the young donor livers (46.5/HPF, n = 44) (Figure 2B) (P = 0.001).
FIGURE 2.
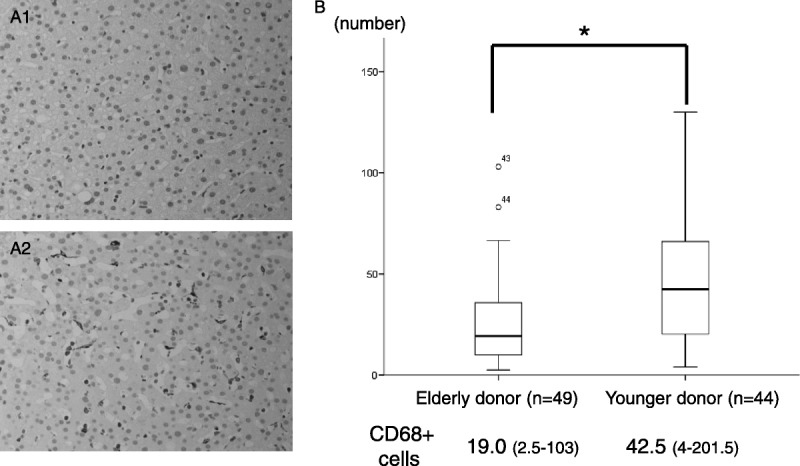
The expression of CD68-positive cells in elderly donors (older than 50 years, A1) and younger donors (twenties, A2) in the time zero biopsy. B, A comparison of the average number of CD68-positive cells between elderly and young donors. (*P = 0.01).
The Influence of Kupffer Cells on the Graft Survival
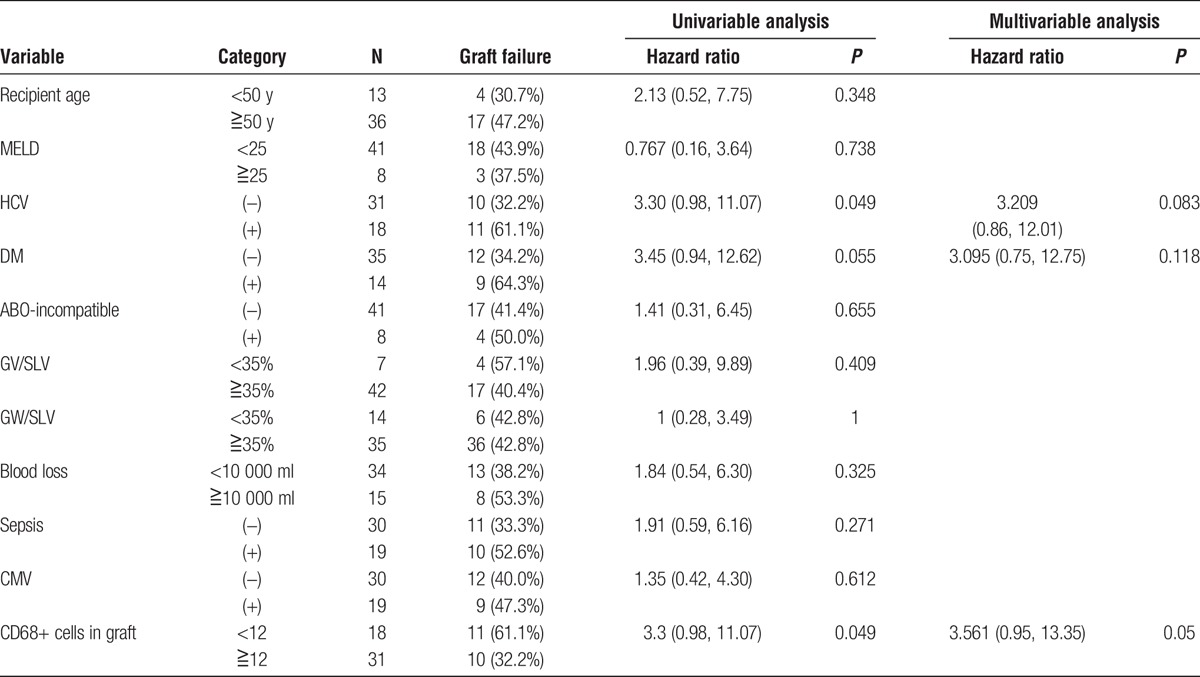
The median number of CD68-positive cells in the samples from younger and elderly donors (n = 93) was 28.5/HPF (range, 2.5-201.5). The multivariable analysis showed that sepsis after LDLT was an independent prognostic marker for the patient survival and graft failure. The receiver operating characteristic curve shows how the number of CD68-positive cells was associated with the development of sepsis. The results showed that the presence of fewer than 12 CD68-positive cells associated with the development of sepsis. The sensitivity, specificity, and area under curve were 0.44, 0.76 and 0.618, respectively. The graft survival of the recipients with a donor older than 50 years with CD68-positive cells less than 12 had significantly worse than those over 12 in recipient with a donor older than 50 years (P = 0.015) (Figure 3A). There were no significant differences of graft survival between CD68-positive cells less than 12 and over 12 in the recipient with younger donor (N.S.) (Figure 3B). The graft survival of the recipients with a donor older than 50 years was significantly worse than those with younger donors among the group with CD68-positive cells over 12 (P = 0.001) (Figure 4A). There were no significant differences of the graft survival between younger donor and elderly donor in the group with CD68-positive cells less than 12 (P = 0.37) (Figure 4B). The significant poor prognostic factors in recipient with elderly donor for graft failure after LDLT were HCV-positive status and fewer than 12 CD68-positive cells in the univariable analysis (Table 3). The multivariable analysis showed fewer CD68-positive cells was the only prognostic factor in the recipient with elderly donors.
FIGURE 3.
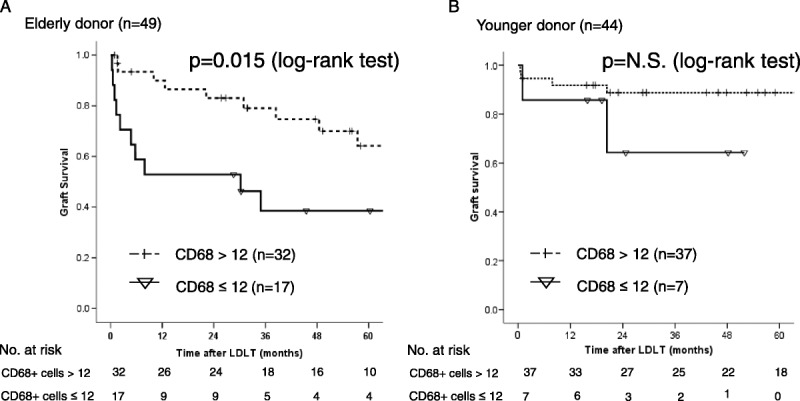
A, A comparison of the graft survival in recipients with donors older than 50 years (n = 49) between those with more than and fewer than 12 CD68-positive cells. P = 0.015 (log-rank test), B, A comparison of the graft survival in recipients with younger donors (n = 44) between those with more than and fewer than 12 CD68-positive cells. N.S. (log-rank test).
FIGURE 4.
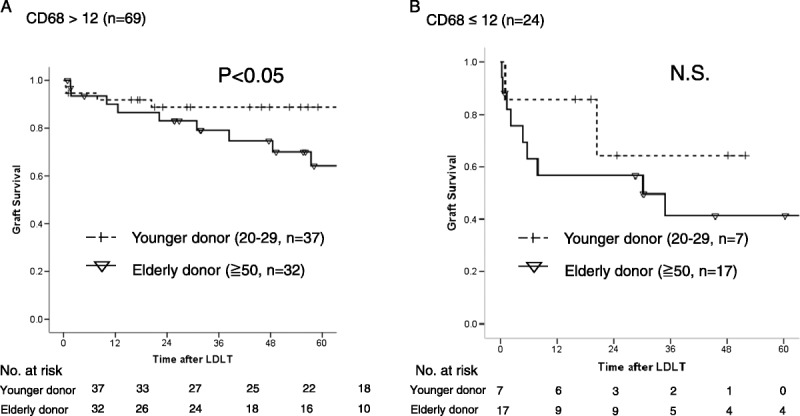
A, A comparison of the graft survival in recipients using donor graft with CD68 more than 12 between younger and elderly donor, P = 0.01. B, A comparison of the graft survival in recipients using donor graft with CD68 less than 12 between younger and elderly donor, N.S.
TABLE 3.
The results of the univariable and multivariable logistic regression analyses of the prognostic factors for graft failure after LDLT in recipients with elderly donors
The Patient Survival in the HCV-Positive Recipients by Donor Age
There were no significant differences in the survival between the HCV-positive and HCV-negative group with young donors (HCV(+) 80.0%/77.7%/74.7%, HCV(−) 83.6%/80.1%/73.9%). On the other hand, 1-, 3- and 5-year survival rate of the recipients with HCV in the elderly donor were 59.0%, 33.2%, and 24.9%, respectively. This was significantly worse than the corresponding rates of 77.4%, 73.5%, and 65.3%, respectively, in recipients without HCV in the elderly donor (P = 0.021).
The cause of death after LDLT in the recipient with elderly donor was graft failure in 5 patients during the early period after LDLT with 3 patients with bacteremia, delayed graft failure in 5 cases, HCC recurrence, and chronic rejection in each patient. Only 2 recipients with HCV of the 18 recipients with elderly donors achieved clearance of HCV after LDLT, and 9 recipients experienced HCV recurrence after LDLT. The median number of CD68-positive cells in HCV recipients who were nonresponders and had elderly donors was 14.5 (range, 5-36.5), which was less than that in the 2 cases with successful HCV eradication (CD68 cell number: 103 and 47.5).
DISCUSSION
The outcome of LT has greatly improved during the past few decades.1,8 However, there are still some problems that affect the survival after LT. Especially in the case of DDLT, several reports have indicated that the outcome after DDLT was worse in the cases with elderly donors than with young donors.11-13 However, other groups have reported that the donor age did not affect the survival after DDLT.14-16 The use of elderly donor grafts has been increasing due to the scarcity of donor organs worldwide despite the increase in the candidates for LT; however, this remains controversial.
Regarding the morphological change due to aging, older livers are smaller, darker-colored, and suffer atrophy, which has been attributed to the increased accumulation of lipofuscin and fibrous thickening of Glisson capsule.21,22 Mclean et al23 reported that the number of endothelial cell fenestrations according to increasing age was decreased to 80%, and the endothelial lining decreased to 60% thickness in human livers. Liver biopsy samples obtained from healthy subjects and those with chronic liver disease showed decreases in telomere length with age.24 Recently, changes in the hepatic sinusoid with age have been identified which probably contribute to the substantial age-related changes in liver function. These changes included pseudocapillarization, thickening, and defenestration of the liver sinusoidal endothelial cells and sporadic deposition of basal lamina in the extracellular space of Disse. These age-related changes in the hepatic sinusoid may have important systemic implications for age-related diseases.25
There were no significant differences in the patient survival in patients with young donors based on their HCV status. However, the patient survival in those with elderly donor was significantly worse in HCV-positive cases than that in HCV-negative cases. A decrease in survival was observed within 36 months after LDLT in the cases with elderly donors and HCV-positive status. This result indicated that the initial graft function in HCV-positive cases was worse than that in HCV-negative. The combination of an old donor liver and HCV-positive recipient might be avoided in these cases, especially those with low MELD score.26
Our present results showed that the prognostic factors for survival and graft failure were the donor age and sepsis in the multivariable analysis. With regard to the outcomes of LDLT with an elderly donor, the registry report of the Japanese Liver Transplantation Society showed that the patient survival after LDLT gradually worsened as the donor age increased.10 Ikegami and colleagues27 reported that the donor age and MELD score affected the patient survival, as did the D-MELD score in a prognostic estimation of the LDLT focused on the left lobe liver grafts. Our results also showed a poorer outcome in recipients with elderly donors older than 50 years) compared to those with young donors.
The causes of the poorer outcome using elderly donors were uncertain in the previous reports. Tanemura and coworkers28 reported that a donor older than 50 years was independently correlated with impaired liver regeneration at 6 months in the right lobe in the donor. In addition, a donor older than 50 years independently correlated with the graft liver regeneration at 1 week in the right and left lobes in LDLT. Iwamoto et al29 compared the signaling molecules in young and elderly donors for LDLT, and their results suggested that lower expression of signal transducer and activator of transcription 3 after reperfusion in the older donor grafts was associated with cell apoptosis and oxidative injury.
Regarding the relationship between gene senescence and liver regeneration, our investigation showed that the elderly donor grafts could not undergo rejuvenation after LT. SMP-30, a marker of rejuvenation, was not expressed in the young recipients with elderly donor at 6 to 8 years after LDLT.30 Zhu et al31 reported that the liver regeneration of elderly patient was worse than that of a young patient because the senescence-related genes were expressed at lower levels than in young patients.
The key to the outcome after LDLT was the liver regeneration and recovery of liver function during the early period after LDLT. Small-for-size syndrome is related to a worse outcome, and portal hypertension might also be related to impaired liver regeneration. The modulation of the portal venous pressure was associated with a good prognosis of small grafts after LDLT.32 However, our policy is that portal venous modulation, such as port-systemic shunt and splenectomy, was not mandatory for a small graft. We decided to perform splenectomy in the recipients with a platelet count less than 50 000/mL before LDLT and HCV recipients who would receive antiviral treatment after LDLT to avoid pancytopenia. Ligation of shunt vessel to obtain portal flow to the graft was also not mandatory.33
The multivariable analysis revealed that the donor age and sepsis after LDLT were prognostic factors for survival and graft failure after LDLT (Table 2). A lower number of Kupffer cells, indicated as CD68-positive cells, around the sinusoidal region in liver biopsy samples was found in the elderly donors. Previous studies showed that Kupffer cells have been observed in increased numbers in older subjects,34,35 whereas another study reported a reduction in the volume density with age.36 The function of Kupffer cells in normal aging is not well understood. In this study, the number of CD68-positive cells in the elderly donors was lower than that in younger donors. We hypothesized that the reasons why the number of CD68-positive cells tended to be small in the liver with elderly donor might be due to the aging process. However, Findor and Schaffner mentioned that Kupffer cells have also been observed in increased numbers in older subjects in their study; however, their survey was carried out in the 1950s to 1970s.34,35 These findings were based on pathological evaluations without any immunohistochemical examination.
Area under curve of 0.5 to 0.7 is weak; however, we decided the cutoff level of the number of CD68-positive cells in this investigation was a significant factor for the graft failure in patients receiving grafts from elderly donors after LDLT. The median number of CD68-positive cells in the group with sepsis was 14 (3-53). This definition of the cutoff number of CD68-positive cells was thought to be appropriate. This study showed that the prognosis of recipients with elderly donors was worse than that in recipients with younger donors, with an especially poorer outcome in those with elderly donors with a lower Kupffer cell number. In the total number of recipients, a multivariate analysis for prognostic factor showed the donor age and sepsis after LDLT. When focusing on the group of elderly donors in Table 3, the results showed that graft failure without sepsis was significantly found in the elderly donor group. This result was related to the findings of a multivariable analysis which indicated less than 12 CD68-positive cells to be a prognostic factor for graft failure in the elderly group. Figure 4 indicated that CD68-positive cells of the graft affect the outcome in the graft survival because there were no significant differences between younger and elderly donors in this group. This phenomenon might indicate that less CD68-positive cells in the graft related to the impaired liver regeneration and graft function after; LDLT. Figures 3 and 4 indicated that the survival curve declined during the perioperative period. After 1 year, the survival curve was the same between the groups with less than 12 CD68-positive cells and the group with more than 12 CD68-positive cells. This indicated that the number of CD68-positive cells might affect the liver function and liver regeneration immediately after LDLT. The number CD68-positive cells might change over time; however, the effect of the number of CD68-positive cells might be associated with an early recovery of the liver graft which might be related to ischemic-reperfusion injury.
Moreover, the multivariable analysis indicated that a lower number of Kupffer cell was significantly associated with the graft survival in recipients with elderly donor after LDLT. Kupffer cells are tissue macrophages localized within the liver sinusoid and serve as mediators that promote homeostatic liver regeneration, as well as gatekeepers of this regeneration.37,38 Kupffer cells produce major growth mediators and influence hepatocyte proliferation. The depletion of Kupffer cells led to a failure of liver regeneration after partial hepatectomy due to the decreases in TNF-α and IL-6.37,39
In this study, the result has some limitation, thus further investigation will be needed. We hypothesized that the impaired graft function and liver regeneration in elderly donors might be sustained, which would lead the elderly liver graft to be fragile under stress, such as that due to infection or HCV infection after LDLT.
In conclusion, the outcome of LDLT with elderly donors was significantly worse than that with younger donors. These results might be related to the lower number of Kupffer cells in the graft, which might lead to delayed liver regeneration and an increased risk of infectious disease after LDLT.
ACKNOWLEDGMENT
The authors wish to thank our colleagues in the Department of Surgery, Graduate School of Biomedical Sciences, Nagasaki University, for their kind cooperation and support.
Footnotes
Published online 22 July 2016.
This study was supported by the JSPS KAKENHI grant 26861987.
The authors declare no conflicts of interest.
REFERENCES
- 1.Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic hemotransplantation of the human liver. Ann Surg. 1968;168:392–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morioka D, Egawa H, Kasahara M, et al. Outcomes of adult-to-adult living donor liver transplantation. A single institusion's experience with 335 consecutive cases. Ann Surg. 2007;245:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaido T, Egawa H, Tsuji H, et al. In-hospital mortality in adult recipients of living donor liver transplantation: experience of 576 consecutive cases at a single center. Liver Transpl. 2009;15:1420–1425. [DOI] [PubMed] [Google Scholar]
- 4.Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2014;20:425–436. [DOI] [PubMed] [Google Scholar]
- 5.Reichman TW, Katchman H, Tanaka T, et al. Living donor versus deceased donor liver transplantation: a surgeon-matched comparison of recipient morbidity and outcomes. Transpl Int. 2013;26:780–787. [DOI] [PubMed] [Google Scholar]
- 6.Eguchi S, Takatsuki M, Hidaka M, et al. Evolution of living donor liver transplantation over 10 years: experience of a single center. Surg Today. 2008;38:795–800. [DOI] [PubMed] [Google Scholar]
- 7.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. [DOI] [PubMed] [Google Scholar]
- 8.Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675–688. [DOI] [PubMed] [Google Scholar]
- 9.Organ Procurement & Transplantation Network. http://optn.transplant.hrsa.gov/.
- 10.Japanese Liver Transplantation Society. Liver Transplantation in Japan—Registry by the Japanese Liver Transplantation Society. 2011;47:416–428. [Google Scholar]
- 11.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. [DOI] [PubMed] [Google Scholar]
- 12.Selzner M, Kashfi A, Selzner N, et al. Recipient age affects long-term outcome and hepatitis C recurrence in old donor livers following transplantation. Liver Transpl. 2009;15:1288–1295. [DOI] [PubMed] [Google Scholar]
- 13.Cuende N, Miranda B, Cañón JF, et al. Donor characteristics associated with liver graft survival. Transplantation. 2005;79:1445–1452. [DOI] [PubMed] [Google Scholar]
- 14.Ravaioli M, Grazi GL, Cescon M, et al. Liver transplantations with donors aged 60 years and above: the low liver damage strategy. Transpl Int. 2009;22:423–433. [DOI] [PubMed] [Google Scholar]
- 15.Cescon M, Grazi GL, Cucchetti A, et al. Improving the outcome of liver transplantation with very old donors with updated selection and management criteria. Liver Transpl. 2008;14:672–679. [DOI] [PubMed] [Google Scholar]
- 16.Jiménez-Romero C, Clemares-Lama M, Manrique-Municio A, et al. Long-term results using old liver grafts for transplantation: sexagenerian versus liver donors older than 70 years. World J Surg. 2013;37:2211–2221. [DOI] [PubMed] [Google Scholar]
- 17.Boltjes A, Movita D, Boonstra A, et al. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J Hepatol. 2014;61:660–671. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsui H, Nishiguhi S. Importance of Kupffer cells in the development of acute liver injuries in mice. Int J Mol Sci. 2014;15:7711–7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue Y, Soyama A, Takatsuki M, et al. Acute kidney injury following living donor liver transplantation. Clin Transplant. 2012;26:E530–E535. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa T, Taura N, Miyaaki H, et al. Successful pegylated interferon alpha2a monotherapy for hepatitis C virus infection in a transplanted patient who relapsed after the preceding course. Transpl Infect Dis. 2011;13:438–440. [DOI] [PubMed] [Google Scholar]
- 21.Wynne HA, Cope LH, Mutch E, et al. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology. 1989;9:297–301. [DOI] [PubMed] [Google Scholar]
- 22.Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci. 2007;1119:97–111. [DOI] [PubMed] [Google Scholar]
- 23.McLean AJ, Cogger VC, Chong GC, et al. Age-related pseudocapillarization of the human liver. J Pathol. 2003;200:112–117. [DOI] [PubMed] [Google Scholar]
- 24.Aikata H, Takaishi H, Kawakami Y, et al. Telomere reduction in human liver tissues with age and chronic inflammation. Exp Cell Res. 2000;256:578–582. [DOI] [PubMed] [Google Scholar]
- 25.Le Couteur DG, Warren A, Cogger VC, et al. Old age and the hepatic sinusoid. Anat Rec (Hoboken). 2008;291:672–683. [DOI] [PubMed] [Google Scholar]
- 26.Uemura T, Nikkel LE, Hollenbeak CS, et al. How can we utilize livers from advanced aged donors for liver transplantation for hepatitis C? Transpl Int. 2012;25:671–679. [DOI] [PubMed] [Google Scholar]
- 27.Ikegami T, Imai D, Wang H, et al. D-MELD as a predictor of early graft mortality in adult-to-adult living-donor liver transplantation. Transplantation. 2014;97:457–462. [DOI] [PubMed] [Google Scholar]
- 28.Tanemura A, Mizuno S, Wada H, et al. Donor age affects liver regeneration during early period in the graft liver and late period in the remnant liver after living donor liver transplantation. World J Surg. 2012;36:1102–1111. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto T, Yagi T, Umeda Y, et al. The impact of donor age on the outcome of adult living donor liver transplantation. Transplantation. 2008;85:1240–1245. [DOI] [PubMed] [Google Scholar]
- 30.Eguchi S, Takatsuki M, Hidaka M, et al. Lack of grafted liver rejuvenation in adult-to-pediatric liver transplantation. Dig Dis Sci. 2011;56:1542–1547. [DOI] [PubMed] [Google Scholar]
- 31.Zhu C, Ikemoto T, Utsunomiya T, et al. Senescence-related genes possibly responsible for poor liver regeneration after hepatectomy in elderly patients. J Gastroenterol Hepatol. 2014;29:1102–1108. [DOI] [PubMed] [Google Scholar]
- 32.Ogura Y, Hori T, El Moghazy WM, et al. Portal pressure <15 mm Hg is a key for successful adult living donor liver transplantation utilizing smaller grafts than before. Liver Transpl. 2010;16:718–728. [DOI] [PubMed] [Google Scholar]
- 33.Takatsuki M, Baimakhanov Z, Soyama A, et al. Obstructing spontaneous major shunt vessels might not be mandatory to maintain adequate portal inflow in living donor liver transplantation. Transplantation. 2014;97:e52–e53. [DOI] [PubMed] [Google Scholar]
- 34.Findor J, Perez V, Igartua EB, et al. Structure and ultrastructure of the liver in aged persons. Acta Hepatogastroenterol (Stuttg). 1973;20:200–204. [PubMed] [Google Scholar]
- 35.Schaffner F, Popper H. Nonspecific reactive hepatitis in aged and infirm people. Am J Dig Dis. 1959;4:389–399. [DOI] [PubMed] [Google Scholar]
- 36.Martin G, Sewell RB, Yeomans ND, et al. Ageing has no effect on the volume density of hepatocytes, reticulo-endothelial cells or the extracellular space in livers of female Sprague-Dawley rats. Clin Exp Pharmacol Physiol. 1992;19:537–539. [DOI] [PubMed] [Google Scholar]
- 37.Meijer C, Wiezer MJ, Diehl AM, et al. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77. [DOI] [PubMed] [Google Scholar]
- 38.Takeishi T, Hirano K, Kobayashi T, et al. The role of Kupffer cells in liver regeneration. Arch Histol Cytol. 1999;62:413–422. [DOI] [PubMed] [Google Scholar]
- 39.Seki E, Tsutsui H, Iimuro Y, et al. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology. 2005;41:443–450. [DOI] [PubMed] [Google Scholar]


