Abstract
Background
Invasive aspergillosis (IA) is a major cause of invasive fungal infection in kidney transplant recipients (KTR), and it has a high mortality rate. However, its impact on patients and graft survival has not been well defined in the current era of voriconazole first-line therapy.
Methods
We retrospectively collected all cases of KTR-associated IA occurring at Necker Enfants Malades Hospital, Paris, from 2003 to 2013. These cases were compared with a group of controls (1:3) who were matched by age, year of kidney transplantation, and sex. The characteristics of IA were also studied.
Results
Sixteen patients developed IA after KTR. Most IA cases were limited to the lungs (81.3%), with mild respiratory symptoms in only 53% of the patients. The patients were administered voriconazole (n = 15, 94%) and/or posaconazole (n = 2, 13%). The 12-week and 1-year postinfection survival rates were 94% and 81%, respectively. Compared with the controls (n = 46), patients and death-censored graft survivals rates were significantly lower after IA (P = 0.017 and 0.001, respectively). In the patients with IA, the occurrences of cardiovascular diseases before transplantation (P < 0.0001), delayed graft function (P < 0.0001), and infectious complications (0.0018) were significantly more frequent.
Conclusions
Even with voriconazole therapy, the prognosis of patients with IA after kidney transplantation is still poor. When the patients survive to IA, they have a high risk of graft loss.
Invasive aspergillosis (IA) is a leading cause of opportunistic infections in immunocompromised patients. In kidney transplant recipients (KTR), it is the third leading cause of fungal infection after Candida infections and cryptococcosis,1 with an estimated prevalence of 0.5% to 4%.2 It has been associated with a high mortality rate, ranging from 40% to 70%.3,4 After 2002, voriconazole, a broad-spectrum triazole that is active against Aspergillus species, and improved diagnostic tools (eg, galactomannan antigen and Aspergillus PCR) have dramatically improved the prognosis for the patients with IA,5 particularly for hematological neutropenic patients. Two recently published studies provided discordant information regarding IA prognosis in KTR.4,6 Heylen et al6 reported a decrease in the 12 weeks mortality after IA diagnosis, from 73% before 2003 to 19% after 2003. However, in the study of Hoyo et al,4 still 70% (7/10) of KTR with IA occurring after 2003 died. No study has specifically studied both short- and long-term survivals after IA, nor has compared it with the survival of patients without IA. Furthermore, the reduction of the immunosuppressive regimen required to control the infectious process may lead to graft rejection and can impact graft survival. Recent studies have focused on IA risk factors in KTR,6,7 but there are no data concerning kidney allograft outcomes after IA.
Thus, we performed a retrospective case control study of all IA cases occurring at our center from 2003 to 2013. We aimed to determine the impact of IA on patients and graft survival. We also described the clinical and radiological presentations, diagnostic methods, and factors associated with IA.
MATERIALS AND METHODS
Patients
We retrospectively analyzed all patients who developed proven or probable IA in our kidney transplant unit at Necker-Enfants Malades University Hospital, Paris, between January 2003 and December 2013. Invasive aspergillosis was defined according to the 2008 European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group criteria.8 We excluded patients with possible IA.
According to EORTC criteria, aspergillosis diagnosis was done as follows:
– Proven IA was based on the presence of Aspergillosis on microscopic analysis of a sterile material or positive cultures of a sterile material.
– Probable IA was defined by the presence of a host factor (recent history of neutropenia, receipt of an allogeneic stem cell transplant, prolonged use of corticosteroids, immunosuppressants, or inherited severe immunodeficiency), a clinical criterion, and a mycological criterion (cytology, direct microscopy, culture or indirect tests, that is, detection of Galactomannan antigen in plasma, serum, bronchoalveolar lavage fluid, or CSF or β-d-glucan detected in serum).
– Cases that met the criteria for a host factor and a clinical criterion but for which mycological criteria were absent were considered possible IA and were therefore excluded of our study.
For each patient with IA (case), 3 control patients were chosen in our cohort. Controls and cases were matched by the year of transplantation, age (±3 years), and sex. The controls were alive, with a functional graft, when their matched cases developed IA.
For each patient, we collected demographic and therapeutic data and outcomes.
For the patients with IA, we collected the clinical and radiological characteristics, microbiological data (including the results of tissue and fluid sample cultures, serum Platelia Aspergillus Galactomannan EIA (a galactomannan index of ≥0.5 was used to define positivity) and Aspergillus fumigatus quantitative PCR (already evaluated and published9), IA treatment and outcomes and graft and patients outcomes. All radiological examinations (including tomodensitometry and magnetic resonance imagery) were reviewed by the same radiologist who described the initial elementary lesions and their evolutions.
Exhaustive microbiological investigations were performed for each patient with bronchoalveolar fluid or bronchial aspiration analysis (direct examination and bacterial cultures [including nocardia and actinomyces], direct examination and cultures of mycobacteria, search for pneumocystis and fungal analysis, as well as viral PCR including influenza, parainfluenza, RSV, metapneumovirus, adenovirus, and CMV), as well as CMV viremia.
The complete clinical remission of IA was defined by the resolution of symptoms and weight loss. Radiological complete remission was defined by the complete resolution of radiological lesions or the persistence of sequelae and unmeasurable lesions. Radiological partial remission was defined by the persistence of lesions that measured less than 70% of the initial lesions.
Delayed graft function was defined by the requirement of dialysis within the first 7 days after kidney transplantation.
Immunosuppressive Regimen
The induction immunosuppressive regimen consisted of a biologic induction agent (ie, basiliximab 20 mg on days 0 and 4 [Simulect; Novartis Pharma, Basel, Switzerland]) or a 5- to 8-day course of rabbit thymoglobulin, 75 mg/d (thymoglobulin; Genzyme, Saint-Germain-en-Laye, France) and intravenous corticosteroids (methylprednisolone 500 mg on day 0 and 125 mg on day 1) relayed by oral tapered corticosteroids. The maintenance immunosuppression included a calcineurin inhibitor (tacrolimus or cyclosporine), azathioprine (2 mg/kg daily), or mycophenolate mofetil (MMF) (1 g twice per day) or mycophenolate sodium (720 mg twice per day) and prednisone. The doses were adjusted to obtain blood concentrations for the appropriate objectives, according to the delay from the graft. After 2006, patients with pretransplant donor-specific antibodies also received intravenous immunoglobulins (2 g/kg) on posttransplantation days 1, 21, 42, and 63, and some patients also underwent plasmapheresis.
All patients were given Pneumocystis jirovecii prophylaxis (trimethoprim-sulfamethoxazole) for 3 to 12 months after transplantation, CMV prophylaxis (valacyclovir 1.5 g 4 times per day until 2006, and then valganciclovir 450 mg daily for 3 to 6 months).
Statistical Methods
Quantitative variables were expressed as the mean (standard deviation) or the median (interquartile range, 25-75). Categorical variables were expressed as numbers and percentages. Quantitative parameters were compared using the nonparametric Mann-Whitney U test. Qualitative parameters were compared using χ2 tests or the Fisher exact test. Survival curves for the patients and grafts were determined using the Kaplan-Meier method. Patient survival among the 2 groups was compared using the log-rank test, and a P value of less than 0.05 was considered to be statistically significant. JMP9.0 software (SAS Institute, Inc., Cary, NC) was used for the statistical analysis.
RESULTS
Patients and Transplantation-Related Data
Sixteen patients developed proven or probable IA between 2003 and 2013, and they were compared with 46 control patients. The participants' demographic data and pretransplantation and posttransplantation characteristics are detailed in Table 1.
TABLE 1.
Characteristics of patients with and without IA
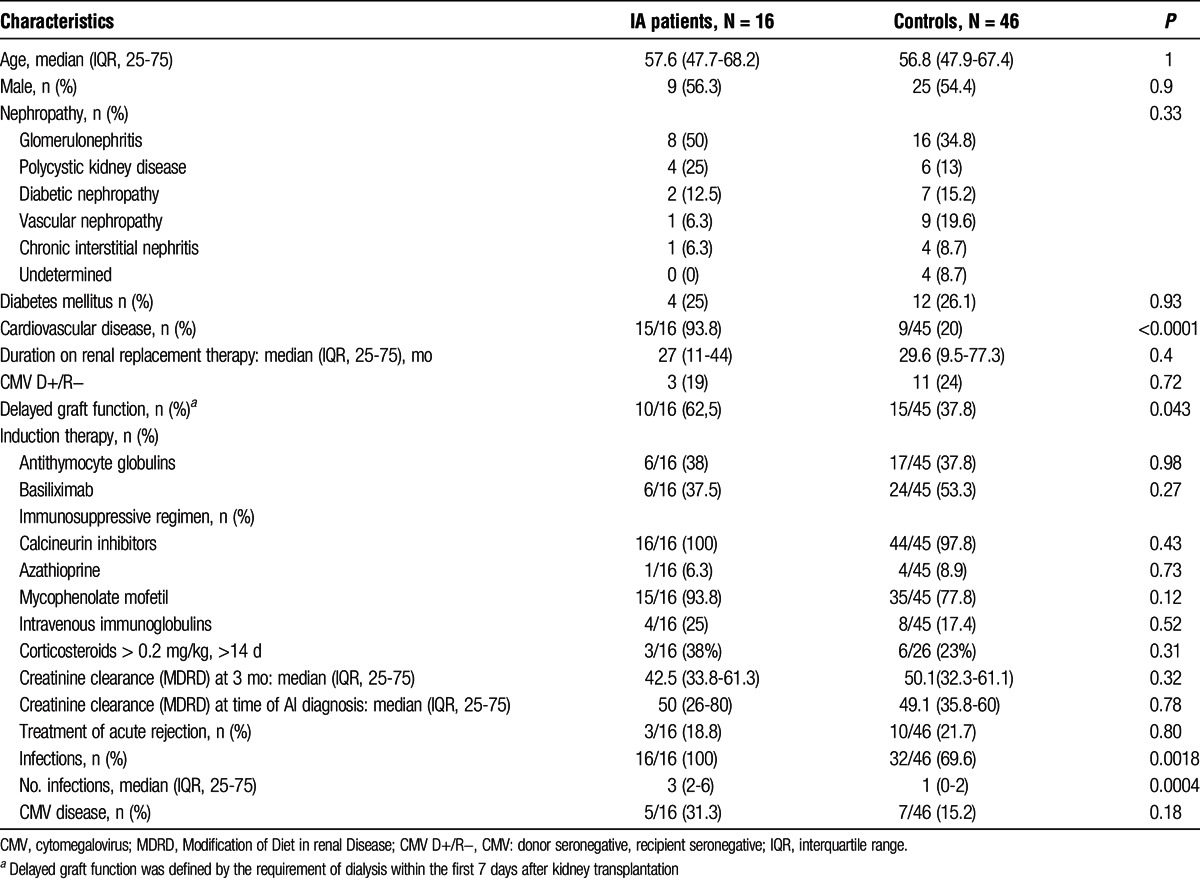
Compared with the controls, the patients with IA had a significantly higher rate of cardiovascular diseases (93.8% vs 20%, P < 0.0001) and more delayed graft function (ie, the need for renal replacement therapy after kidney transplantation, 62.5 vs 37.8%, P = 0.043). All patients with IA developed other infections, compared with 32 of the 46 controls (69.6%, P = 0.0018), and the median number of infections was also significantly higher in the patients with IA compared with the controls (3 vs 1, P = 0.0004).
IA Clinical Presentation
Invasive aspergillosis was proven in 2 patients, and it was probable in 14 patients. After kidney transplantation, IA occurred a median time of 34 months (range, 1-181 months). Only 1 patient developed early IA (within the first 3 months after transplantation), and 5 (31%) of the 16 patients developed IA within the first 6 months after transplantation. At the time of diagnosis, 15 patients (94%) received prednisone (median daily dosage, 10 mg; range, 0-70 mg), 12 (75%) calcineurin inhibitors, 12 (75%) MMF, and 2 (12.5%) mTOR inhibitors. Invasive aspergillosis was unifocal in 15 (94%) patients (lung, n = 13; sinus, n = 1), sinus and central nervous system (CNS) (n = 1), and it was disseminated (ie, ≥ 2 noncontiguous sites) in 1 (6.3%) patient (lung, CNS, and skin).
All patients, except for one, were symptomatic. The symptoms included fever (n = 11, 69%), altered clinical status (n = 9, 56%), weight loss greater than 5% of total body weight (n = 11, 69%), respiratory symptoms (n = 7, ie, 44% of patients with pulmonary lesions), and hemoptysis (n = 2,12.5%). The 2 patients with CNS involvement presented with headache, impaired consciousness, and neurological deficiency. Five (31%) patients were admitted to the intensive care unit for IA management related to respiratory failure (n = 4) or neurological failure (n = 1).
Microbiological and Radiological Data
The results of IA diagnostic procedures are detailed in Table 2. Aspergillus fumigatus was identified in all patients with available species identification (n = 13). It was recovered from 10 of 12 (83%) bronchoalveolar fluid or bronchial aspiration cultures and from 6 (86%) of 7 sputum cultures. Serum galactomannan (GM) was detected in 6 (43%) of 14 cultures within 19 ± 8 days after the first symptoms appeared, and Aspergillus fumigatus quantitative polymerase chain reaction (aPCR) was used for 5 (62.5%) of 8 patients. When both GM and aPCR were performed (8 patients), at least 1 of the 2 tests was positive in 7 (87.5%) of the 8 patients. In 2 cases, standard histopathologic analysis of tissue biopsies revealed features that were compatible with IA (skin biopsy and lung mass), but A. fumigatus was recovered only from the skin biopsy culture.
TABLE 2.
Microbiological diagnostic tools and their diagnostic value
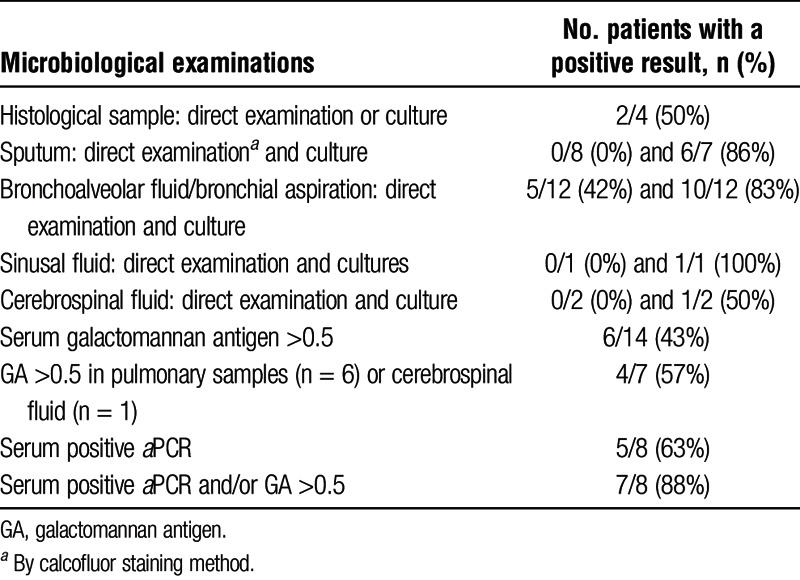
Only 1 patient had another infection at the time of IA diagnosis: he had an asymptomatic CMV without symptoms that resolved without relapse after 21 days of valgancyclovir therapy.
Chest CT scans were available for 14 patients. Sinus and brain MRIs were available for 1 patient, and a brain MRI was available for 1 patient. The pulmonary radiological lesions included alveolar consolidations (n = 8, 57%), nodular opacities (n = 7, 50%), ground glass opacities (n = 7, 50%), micronodules (n = 6, 43%), cavities (n = 2, 14%), and halo signs (n = 2, 14%). The lung lesions were multifocal in all patients and bilateral in 10 (67%) patients. The sinus and cerebral lesions consisted of a sinus mass and mucosal thickening with osteolysis and vascular sheathing for 1 patient and anterior communicating artery aneurysm with meningeal hemorrhage for another patient.
Treatment Regimens
All patients received voriconazole (n = 15, 94%) and/or posaconazole (n = 2, 13%) for a median duration of 6.3 months (range, 0.2-23 months). Seven (44%) patients also received caspofungin for 1 month (range, 0.5-11 months), and 1 patient received liposomal amphotericin B during the first 4 weeks of IA treatment. Antifungal combination therapy was indicated by IA severity (n = 3), slow recovery (n = 2), and unknown (n = 3). The immunosuppressive regimen was reduced in 15 (94%) patients. Surgery was required and successfully performed for 1 patient with IA sinusitis because he demonstrated an unfavorable outcome under medical treatment.
Outcome
The median follow-up period after kidney transplantation was 69 months (range, 5-219 months) for the patients with IA and 97 months (range, 1-216 months) for the controls. The median follow-up time after IA was 41 months (range, 1-104 months).
In the patients with IA, the clinical outcomes were favorable in 14 of the 15 patients (1 patient was asymptomatic but had radiologic lesions). The median times to apyrexia and weight recovery were 12 days (range, 1-37 days) and 39 days (range, 2-274), respectively. GM and aPCR were negative within 18 days (range, 1-10 days) and 4 days (range, 1-179 days), respectively. Radiological partial remission was observed in 4 of the 15 (27%) patients after 61.5 days (range, 35-178 days), and complete remission was observed in 10 (67%) patients after 195 days (range, 44-1089 days). The radiological lesions did not improve in 1 patient. One patient with CNS aspergillosis could not be analyzed because he died within 15 days of the IA diagnosis. Three of 4 patients with partial remission were classified as such because nodular sequelae persisted on their last imaging. These patients had favorable clinical outcomes.
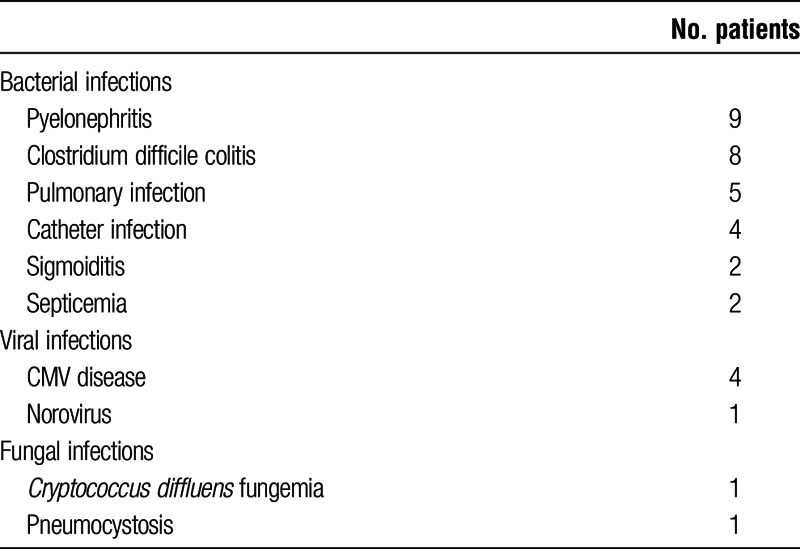
After IA diagnosis, and despite immunosuppression reduction, severe infectious complications still affected 11 (69%) of the 16 patients after IA. These infections are listed Table 3.
TABLE 3.
Infections occurring after IA diagnosis
After IA diagnosis, the patient survival rate was 94%, 81%, and 67% at 12 weeks, 1 year, and 3 years, respectively. No patient was lost of follow-up. Only 1 death was directly attributed to IA. The causes of death were other infectious complications (n = 2), cardiovascular disease (n = 2), acute leukemia after retransplantation (n = 1), and unknown (n = 3). Compared with the controls, the overall mortality rate was significantly higher in the patients with IA (P = 0.017, Figure 1A).
FIGURE 1.
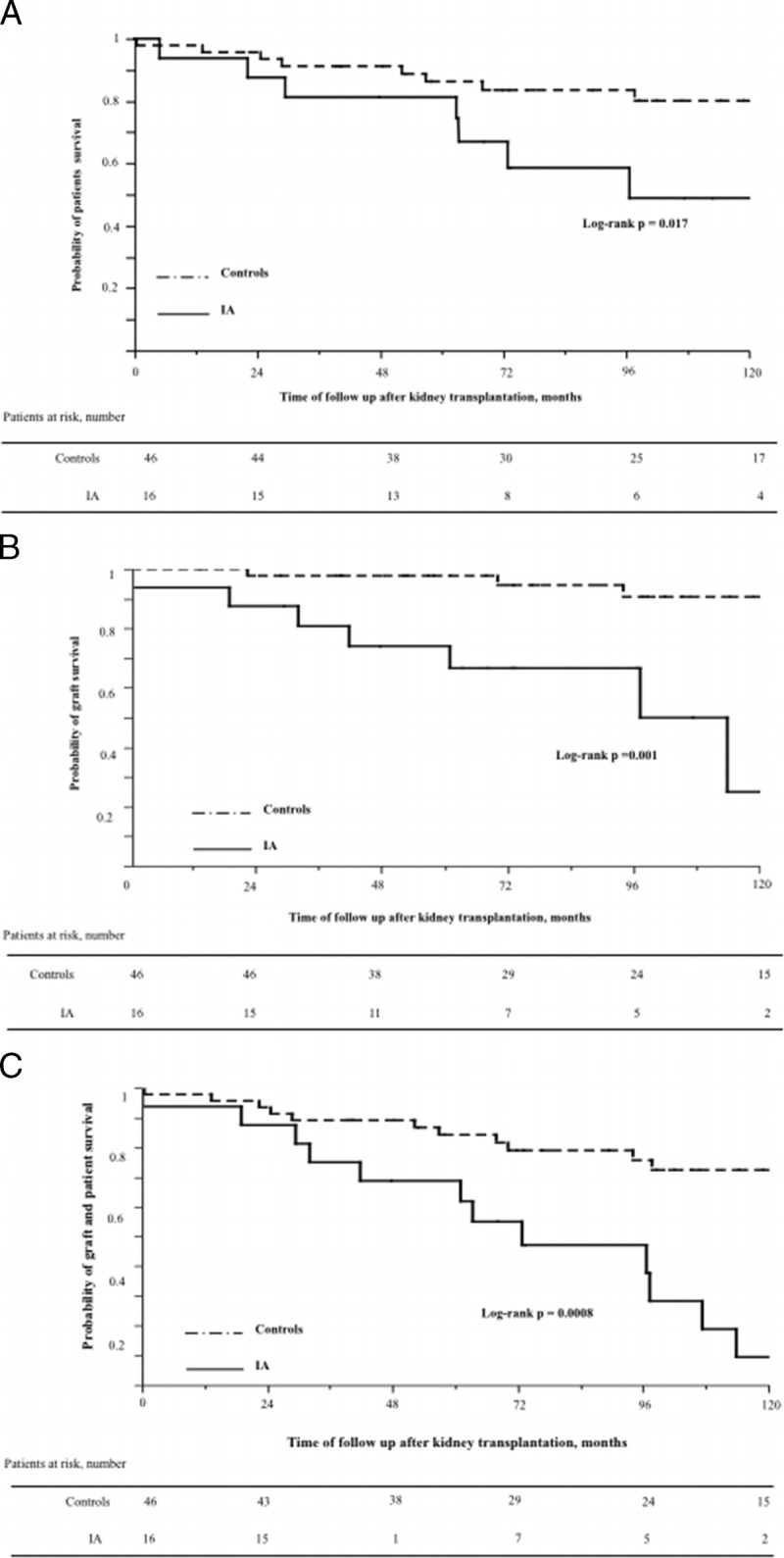
Kaplan-Meier analysis based on time to kidney transplantation. A, Patient survival in IA patients compared to controls. B, Death censored graft survival in IA patients compared with controls. C, Non–death-censored graft survival in IA patients compared to controls.
Two patients developed biopsy proven antibody-mediated rejection at 202 and 279 days after IA (in both cases after discontinuing antifungal therapy). The therapy consisted of 6 courses of IVIg and increased tacrolimus targeted doses for one patient and 3 methylprednisolone pulses with reintroduction of MMF for the other patient. No graft loss was observed in these patients.
Fourteen months (range, 1-54 months) after IA, 6 (37.5%) of the 16 patients lost their graft because of transplant glomerulopathy, including 2 patients in the month after IA. Compared with the controls, death censored graft survival was significantly reduced in the patients with IA (P = 0.001), as shown in Figure 1B.
Graft failure, defined as graft loss, or death, was significantly higher in the patients with IA (P = 0.0008, Figure 1C).
Finally, 3 patients with IA were eligible for retransplantation. Only one of these patients was given aspergillosis secondary prophylaxis during the 12 months after retransplantation. No IA relapse was observed in these patients.
DISCUSSION
Invasive aspergillosis remains rare in KTR, which explains the paucity of data in this population. From 2003 to 2013, 16 patients developed IA in our cohort of 2000 KTR, which is consistent with previous results.3,10 Thus, despite an increase in transplants among elderly and highly immunized patients, no IA breakthrough was observed. Although early IA was described in half of the IA cases in solid organ transplantation (SOT) 10 years ago,3,11 our study, in accordance with other recent studies,4,6 demonstrated that late IA (>6 months after transplantation) is more frequent in KTR, occurring in 69% of patients.
Our study is the first to precisely describe the outcomes of the patients with IA, who still have poor prognoses. Indeed, previous studies did not evaluate long-term survival and did not compare KTR with IA to KTR without IA. Our study shows for the first time that, despite voriconazole and improved 12-weeks survival, long-term survival remains significantly decreased in KTR with IA. Voriconazole has been associated with significantly improved survival rates5,12-14 among immunocompromised patients in various settings; however, no study has focused on KTR. We found that the global mortality rate was still high: the overall mortality rate was 56.3% (9 of 16 patients) compared with 70% in 2 retrospective studies without voriconazole.3,4 However, the 12-week mortality rate was lower than that reported in other studies of patients receiving voriconazole (ie, 6% in our study compared to 19% in the recent study of Heylen et al6 and 34.4% among SOT recipients.7 Similarly, in the Veroux et al10 study, none of 4 patients receiving voriconazole died within 13 months after IA. Improved diagnostic methods, including a galactomannan assay,15 the use of quantitative Aspergillus PCR and the isolation of Aspergillus species, may also shorten the time to diagnosis and treatment initiation, thereby improving survival rates.
We demonstrated that patient survival rates are lower after IA compared with controls, which reflects disease severity, patient comorbidities, and intense immunodeficiency. Despite immunosuppression reductions, severe infectious complications remained frequent after IA, accounting for at least 2 deaths. Compared with the controls, the infections were also significantly more frequent in the patients with IA. Screening for infectious risk factors, including hypogammaglobulinemia and lymphopenia, and the use of anti-infectious prophylaxis and vaccines should, therefore, be encouraged when KTR develop IA.
Few studies have specifically evaluated allograft outcomes after IA. In 2 small retrospective studies of 4 and 8 patients with fungal infections, graft loss was observed in 25% to 30% of the patients.10,16 We found that the graft prognosis was significantly poorer in the patients with IA compared to the controls. Graft loss was attributed to transplant glomerulopathy in all cases of IA, but no biopsies were performed in these patients. The other primary causes of graft dysfunction were drug nephrotoxicity and acute renal failure following sepsis. Immunosuppression reduction may partly explain the graft losses. Thus, the net immunosuppressive state of the patients with IA is complex, and is of particular interest: despite immunosuppression reduction and probable chronic rejection, patients still develop severe infections. Previous studies have suggested that infections might trigger rejection17 despite profound immunosuppression, which could explain our observations.
In our study, the clinical presentation of IA was consistent with that in previous studies,3,6 primarily pulmonary symptoms. We found that KTR might have mild pulmonary symptoms, as respiratory signs were found in only 53% of the patients compared with 86% in a study of non-neutropenic patients, with only 2 SOT recipients.18 The radiological presentation was as expected.3,19 Interestingly, there were multiple radiological lesions in all patients and bilateral lesions in two thirds of the patients; these observations should prompt clinicians to consider an IA diagnosis.
In a recent case control study of 41 KTR with IA, leukopenia and duration of renal replacement therapy were risk factors for early IA, and donor CMV seropositivity increased the risk of late onset IA.6 In our study, the patients with IA underwent renal replacement therapy more often after IA. We also demonstrated that cardiovascular disease occurred more frequently in the patients with IA. This association has previously been described: in the study of Hoyo et al4 that studied 27 solid organ transplant recipients, including 10 KTRs, KTRs with IA more frequently had chronic heart failure (33% vs 6%). This surprising finding may reflect the altered conditions (including malnutrition and prolonged hospitalization) and inflammatory process. This association needs to be confirmed in further studies.
Our study has several limitations. It is a single-center retrospective study, with a low number of patients. However, we provided new data concerning modern mycological diagnostic tools and important results related to IA prognosis.
In conclusion, IA occurs after kidney transplantation in heavily immunocompromised patients. It remains a serious infection despite voriconazole use, and it has a poor prognosis, in terms of patient and graft survival compared to control patients.
Footnotes
Published online 1 July 2016.
The authors declare no funding or conflicts of interest.
A.C.D. helped perform the research, write and edit the article. S.P. helped perform the research and edit the article. R.S. helped perform the research and edit the article. M.-E.B. helped perform the research and edit the article. R.S.-S. helped perform the research and edit the article. F.L. helped perform the research and edit the article. C.L. helped perform the research and edit the article. O.L. helped perform the research and edit the article. A.S. helped perform the research, write and edit the article.
REFERENCES
- 1.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101–1111. [DOI] [PubMed] [Google Scholar]
- 2.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18:44–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavalda J, Len O, San Juan R, et al. Risk factors for invasive aspergillosis in solid-organ transplant recipients: a case-control study. Clin Infect Dis. 2005;41:52–59. [DOI] [PubMed] [Google Scholar]
- 4.Hoyo I, Sanclemente G, de la Bellacasa JP, et al. Epidemiology, clinical characteristics, and outcome of invasive aspergillosis in renal transplant patients. Transpl Infect Dis. 2014;16:951–957. [DOI] [PubMed] [Google Scholar]
- 5.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. [DOI] [PubMed] [Google Scholar]
- 6.Heylen L, Maertens J, Naesens M, et al. Invasive aspergillosis after kidney transplantation: case-control study. Clin Infect Dis. 2015;60:1505–1511. [DOI] [PubMed] [Google Scholar]
- 7.Baddley JW, Andes DR, Marr KA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis. 2010;50:1559–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suarez F, Lortholary O, Buland S, et al. Detection of circulating Aspergillus fumigatus DNA by real-time PCR assay of large serum improves early diagnosis of invasive aspergillosis in high-risk adult patients under hematologic surveillance. J Clin Microbiol. 2008;46:3772–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veroux M, Corona D, Gagliano M, et al. Voriconazole in the treatment of invasive aspergillosis in kidney transplant recipients. Transplant Proc. 2007;39:1838–1840. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Avery RK, Munoz P, et al. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin Infect Dis. 2003;36:46–52. [DOI] [PubMed] [Google Scholar]
- 12.Lortholary O, Gangneux JP, Sitbon K, et al. French Mycosis Study Group. Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005-2007). Clin Microbiol Infect. 2011;17:1882–1889. [DOI] [PubMed] [Google Scholar]
- 13.Nivoix Y, Velten M, Letscher-Bru V, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47:1176–1184. [DOI] [PubMed] [Google Scholar]
- 14.Denis B, Guiguet M, de Castro N, et al. French Hospital Database on HIV Agence Nationale de Recherche sur le SIDA et les hépatites virales, France CO4. Relevance of EORTC criteria for the diagnosis of invasive aspergillosis in HIV-infected patients, and survival trends over a 20-year period in France. Clin Infect Dis 2015 Jun 29. [Epub ahead of print] [DOI] [PubMed]
- 15.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42:1417–1427. [DOI] [PubMed] [Google Scholar]
- 16.Sharifipour F, Rezaeetalab F, Naghibi M. Pulmonary fungal infections in kidney transplant recipients: an 8-year study. Transplant Proc. 2009;41:1654–1656. [DOI] [PubMed] [Google Scholar]
- 17.Cainelli F, Vento S. Infections and solid organ transplant rejection: a cause-and-effect relationship? Lancet Infect Dis. 2002;2:539–549. [DOI] [PubMed] [Google Scholar]
- 18.Dai Z, Zhao H, Cai S, Lv Y, et al. Invasive pulmonary aspergillosis in non-neutropenic patients with and without underlying disease : a single-centre retrospective analysis of 52 subjects. Respirology. 2013;18:323–331. [DOI] [PubMed] [Google Scholar]
- 19.Lim C, Seo JB, Park SY, et al. Analysis of initial and follow up CT findings in patients with invasive pulmonary aspergillosis after solid organ transplantation. Clin Radiol. 2012;67:1179–1186. [DOI] [PubMed] [Google Scholar]


