Abstract
Background
Current treatment strategies for antibody-mediated renal allograft rejection (AMR) are not sufficiently effective. In most centers, “standard of care” treatment includes plasmapheresis (PPH) and IVIG preparations. Since several years, modern therapeutics targeting B cells and plasma cells have become available. We investigated, whether combined administration of rituximab and bortezomib in addition to PPH and high-dose IVIG is useful.
Methods
Between November 2011 and January 2013, we treated 10 consecutive patients with biopsy-proven AMR with rituximab (500 mg), bortezomib (4× 1.3 mg/m2), PPH (6×), and high-dose IVIG (1.5 g/kg) (group A). This group was compared with a group of 11 consecutive patients treated with an identical regimen without rituximab between July 2010 and November 2011 (group B).
Results
Median follow-up was 41(33-46) months in group A and 55(47-63) months in group B. At 40 months after treatment, graft survival was 60% in group A and 64% in group B, respectively (P = 0.87). Before and after treatment, serum creatinine, estimated glomerular filtration rate, and proteinuria were not different between groups. A significant reduction in donor-specific HLA antibody mean fluorescence intensity was observed in group A (25.2%, P = 0.046) and B (38.3%, P = 0.01) at 3 months posttreatment. In group A, more patients suffered from side effects compared with group B (infections: 70% vs 18%, P = 0.02).
Conclusions
The addition of rituximab to bortezomib, PPH, and high-dose IVIG did not further improve graft survival. Instead, we observed an increase of side effects. Therefore, combined administration of bortezomib and rituximab in addition to PPH and IVIG should be regarded with caution.
Since the implementation of a pathology-based definition for antibody-mediated rejection (AMR)1 and the introduction of solid-phase immunoassays to detect circulating donor-specific HLA antibodies (DSA), AMR has turned out to be one of the major causes of renal allograft failure.2-4 The fact that these profound changes concerning allograft pathology occurred “only” about 1 decade before, may explain—at least in part—why the existing evidence on the treatment of AMR is not satisfactory.5 To date, treatment is largely based on 2 old, but obviously not sufficiently effective, principles, namely, the removal of antibodies via plasmapheresis (PPH) and the administration of IVIG preparations.6 For a long period, the effects of IVIG preparations, containing the pooled serum IgG fractions from thousands of donors, have not been completely understood. Meanwhile, it has become clear that much of the immunosuppressive effect is mediated via the Fc fragment.7 Interestingly, Fc fragment glycosylation including terminal sialic acid residues seems to be crucial for the effectiveness of IVIG.8 The fact that this important structure is present only in a minority of the total serum IgG pool8 explains why high doses of IVIG are necessary to achieve therapeutic efficacy.
During the past years, the available therapeutic repertoire against humoral immune reactions has been expanded by the introduction of agents directly targeting B cells and plasma cells. In one of the first clinical studies in this field, we analyzed 19 patients with AMR retrospectively. We observed a trend toward an improved graft survival in patients, who received bortezomib-based treatment in combination with PPH and IVIG as compared with patients, who received fixed dose (500 mg) rituximab-based treatment in combination with PPH and IVIG.9 However, even bortezomib-based treatment had only limited efficacy. Because rituximab and bortezomib target different stages of B-cell development, we here hypothesize that it might be reasonable to combine both agents to take advantage of a synergistic antihumoral effect of both substances without increasing substance-specific side effects.
MATERIALS AND METHODS
Between November 2011 and January 2013, we treated 10 consecutive patients with biopsy-proven AMR with rituximab (500 mg intravenous [IV], fixed dose), bortezomib (1.3 mg/m2 IV, days 1, 4, 8, 11), 6 sessions of PPH (2.5 L/session, 4% albumin), and high-dose (1.5 g/kg) polyvalent human IVIG (KIOVIG®) (group A) (Figure 1). This group was compared with a group of 11 consecutive patients treated with the identical regimen without rituximab between July 2010 and November 2011 (group B). Patients of both groups additionally received 3 methylprednisolone pulses (500 mg/d IV). All patients received prophylaxis with trimethoprim-sulfamethoxazole (80/400 mg daily) and valganciclovir (adapted to estimated glomerular filtration rate [eGFR]) for 3 months. In both groups, all patients received the same treatment protocol irrespective of the individual antibody level and pathology scoring. After discharge, all patients were regularly monitored in our outpatient clinic.
FIGURE 1.
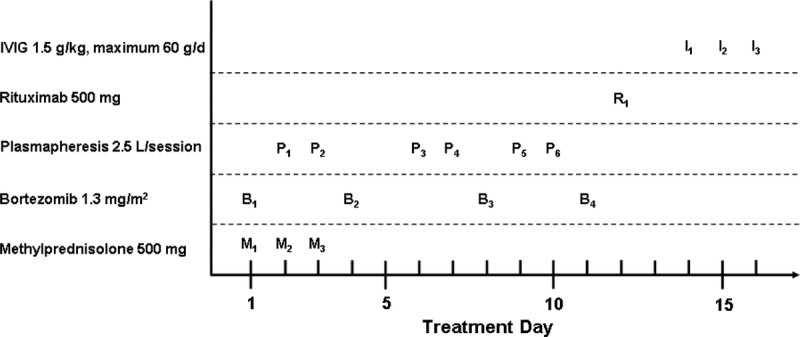
Treatment protocol of group A. Bortezomib was applied on days 1, 4, 8, and 11. In group B, patients received no rituximab, and IVIG treatment was started on day 12.
Renal transplantation was performed at the Charité Hospital based on a negative complement-dependent cytotoxicity crossmatch with and without dithiothreitol using T and B lymphocytes with current and historical serum. In addition, graft allocation was based on a negative virtual crossmatch by considering current and historical unacceptable antigens as defined by solid phase assays (ELISA and Luminex). Consequently, only patients with de novo DSA were included.
Renal biopsies were taken on indication only. All patients presented with clinically relevant allograft dysfunction posttransplant manifesting as an otherwise unexplained increase of serum creatinine (≥0.3 mg/dL), proteinuria (≥1 g/d), or primary nonfunction in the early phase after transplantation. Renal allograft pathology was carried out by 2 experienced nephropathologists (B.R., K.W.). The diagnosis of AMR was based on the presence of circulating DSA and significant allograft pathology according to the definitions of the current Banff classification.10 C4d staining was done by indirect immunofluorescence on paraffin sections using a polyclonal rabbit antihuman C4d IgG antibody (Biomedica, Vienna, Austria). Only patients who gave their written informed consent were considered eligible for enrollment.
Serum samples before and after treatment were screened for HLA antibodies (HLAab) by the Luminex bead-based assay LABScreen Mixed (One Lambda, Canoga Park, CA). In addition, HLAab specificities were determined by LABScreen Single Antigen beads assay (One Lambda). As an indicator for the antibody level, the normalized mean fluorescence intensity (MFI) was used. HLAab were considered positive when exceeding an MFI value of 500. The DSA showing the highest MFI at the time of AMR diagnosis (DSAmax) was tracked to indicate the effectiveness of treatment.
End of follow-up was October 31, 2015. Renal allograft survival was defined as the interval between treatment of AMR and return to chronic dialysis treatment or end of follow-up. The eGFR was calculated according to the chronic kidney disease epidemiology collaboration formula.11 Adherence to immunosuppressive medication was evaluated by self-assessment of all 19 patients alive. In addition, all 21 patients were independently assessed by 4 experienced nephrologists continuously involved in posttransplant care. Based on both assessments, patients were grouped into high, intermediate, and low adherence. Adverse events occurring during the first year after treatment were documented and graded according to the Common Terminology Criteria for Adverse Events version 3.0.12 Comparison between groups was carried out using χ2 test for categorical variables and Mann-Whitney U test for continuous variables. Correlation between ordinal variables was assessed using Spearman rank correlation. Pretreatment and posttreatment comparisons were performed by Wilcoxon signed-rank test. Graft survival was analysed according to Kaplan-Meier with a log-rank test. A probability of less than 0.05 was considered as statistically significant. Statistical analysis was carried out using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY) and STATA 11 IC software (StataCorp., College Station, TX).
RESULTS
Relevant patient characteristics are shown in Table 1. Median observation time after treatment was significantly longer in group B as compared with group A (55 [47-63] vs 41 [33-46] months, P < 0.01). This difference was caused by the sequential nature of our treatment protocol modifications. All other parameters including the scoring of allograft pathology at diagnosis were not different between groups. No statistically significant difference between both groups was found with respect to the interval between transplantation and diagnosis. In both groups, there were each 2 patients with early AMR occurring during the first year after transplantation. All patients had received induction therapy with basiliximab. Before diagnosis, 6 patients in each group were on triple maintenance immunosuppression, all other patients were on double immunosuppression. After diagnosis, all but 2 patients received triple drug maintenance immunosuppression including tacrolimus. One patient in group A was converted from tacrolimus to cyclosporine A at 1 month after treatment because of a diagnosis of posterior reversible encephalopathy syndrome, which was attributed to tacrolimus treatment. One patient in group B refused conversion from cyclosporine A to tacrolimus. In both groups, 3 patients with clinically ongoing biopsy-proven acute AMR received a second methylprednisolone pulse (3×, 500 mg IV) together with PPH (6×). Of these, 1 patient in each group with ongoing severe acute AMR additionally received a second course of IVIG (1.5 g/kg) together with bortezomib (4×, 1.3 mg/m2). At the end of follow-up, 2 of these 6 kidneys (1 in each group) were functioning, whereas 4 patients (2 in each group) resumed maintenance dialysis at 19 (11-27) months after the second treatment course. Graft survival in these 6 patients was not different between both groups (P = 0.59). Graft survival of patients with no evidence for ongoing acute AMR (n = 15) was also similar in both groups (P = 0.98). Taken together, the percentage of patients with ongoing acute AMR as well as their response to treatment was similar in both groups and did not influence the results of our study.
TABLE 1.
Patient characteristics

Adherence to immunosuppression as judged by patients and physicians was similar in both groups (Table 1). Also, the percentage of tacrolimus/cyclosporine A/everolimus trough levels below the lower limit of target range (tacrolimus <4 ng/mL, cyclosporine A <80 ng/mL, everolimus <3 ng/mL) during a period of 3 months before diagnosis was similar in both groups (Table 1). No correlation was found between adherence scoring and the percentage of trough levels below the lower limit of target range (r = 0.15, P = 0.53). After diagnosis, all patients received repeated and intense education on the importance of regular medication intake accompanied by close-meshed appointments in our outpatient clinic. We defined a target range of 5 to 8 ng/mL for tacrolimus and 100 to 150 ng/mL for cyclosporine A after diagnosis. The mean trough levels during the first 3 months after diagnosis are shown in Table 1.
Compared with 6 months before diagnosis, serum creatinine had increased by 0.7 ± 0.4 mg/dL (P = 0.01) in group A and 0.6 ± 0.5 mg/dL (P = 0.03) in group B, respectively. The increase was not different between both groups (P = 0.80). At baseline, serum creatinine (2.4 ± 0.6 vs 2.4 ± 0.7 mg/dL, P = 0.86), eGFR (32.1 ± 10.5 vs 31.4 ± 12.4 mL/min, P = 0.92), and proteinuria (1.4 ± 1.3 vs 1.4 ± 1.0 g/day, P = 0.94) were not different between groups A and B. After treatment, serum creatinine and eGFR among patients with a functioning graft remained comparable in both groups (Figure 2). Also, proteinuria was not different between groups A and B during follow-up (12 months: 0.9 ± 0.9 vs 0.9 ± 1.2 g/d, P = 0.68).
FIGURE 2.
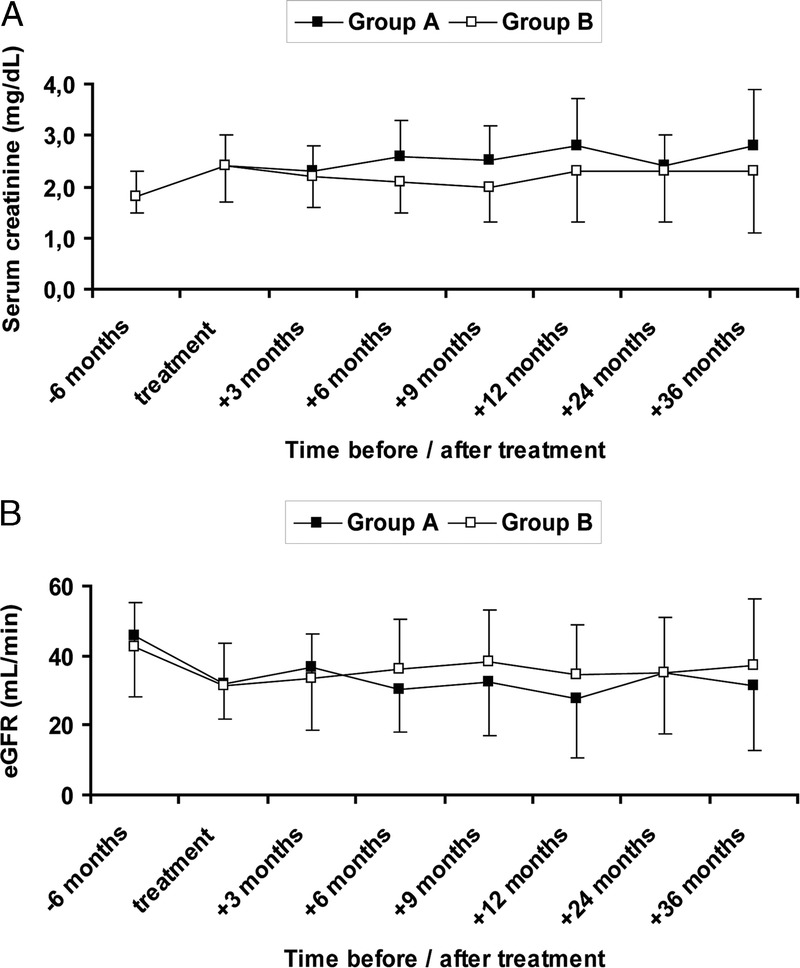
Renal function of patients with a functioning graft before, during and after treatment. Differences between groups were calculated by Mann-Whitney U test. Group A: rituximab + bortezomib + plasmapheresis + high-dose IVIG; group B: bortezomib + plasmapheresis + high-dose IVIG. Statistically significant differences between both groups were not observed. A, Serum creatinine. B, eGFR calculated according to the CKD-EPI formula.11 CKD-EPI, chronic kidney disease epidemiology.
Graft survival (including death) at 12 and 40 months after treatment was 90% and 60% in group A as compared with 91% and 64% in group B, respectively (P = 0.87) (Figure 3). None of the patients died with functioning graft. Two patients died because of cardiac events after graft loss. In group A, a 70-year-old man died because of an acute cardiac event a few hours after left heart catheterization at 37 months after treatment and 24 months after graft failure. In group B, a 67-year-old man died because of an acute cardiovascular event between 2 ambulatory haemodialysis sessions at 37 months after treatment and 10 months after graft failure.
FIGURE 3.
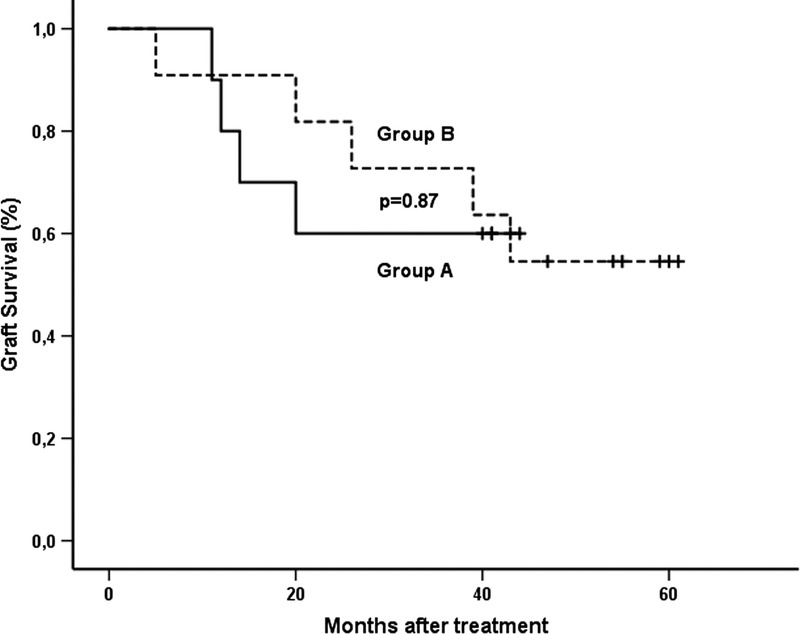
Graft survival after treatment was analyzed according to Kaplan-Meier. The difference between both groups was calculated by log-rank test. Group A: rituximab + bortezomib + plasmapheresis + high-dose IVIG; group B: bortezomib + plasmapheresis + high-dose IVIG. +, end of follow-up.
HLA mismatches and HLAab are summarized in Table 2. The number of HLA mismatches was equally distributed between groups. Notably, all DSA were de novo DSA. Concerning the level of all DSA and DSAmax according to MFI pretreatment, there was no statistically significant difference between both groups. In group A, there were 2 patients with DSA values below 500 MFI. At 3 months after treatment, DSAmax could be decreased significantly as compared with the pretreatment status in both groups. Before treatment, there was no significant difference between class I and II DSA (group A: P = 0.06; group B: P = 0.21). After treatment, no significant difference in the reduction of DSA was found between class I and class II (70.3% [49.4-91.1] vs 60.9% [43.9-77.8], P = 0.23). In both groups, there were patients with a sustained DSA level, that is, with no decrease of DSAmax greater than 10% MFI at 1 year after treatment as compared with baseline (group A: n = 3, group B: n = 2). Graft survival in these 5 patients was not different as compared to patients (n = 16) with a decreased DSA level (P = 0.75). In 1 patient of group A and in 2 patients of group B DSA levels dropped to undetectable levels (MFI = 0) after treatment. In these 3 patients, graft failure was not observed.
TABLE 2.
HLA mismatches and HLA antibodies

The observed adverse events are shown in Table 3. During the first year after treatment, the most frequent adverse event was hematologic toxicity resulting in a decrease of haemoglobin (100% of patients), thrombocytopenia (81%), and leukopenia (57%). Anemia was successfully treated by increasing erythropoietin doses. The incidence of thrombocytopenia (100% vs 64%, P = 0.03) was significantly higher in group A as compared with group B. Both thrombocytopenia and leukopenia were spontaneously reversible in all patients. A transient increase of serum transaminase levels was observed in 6 of 10 patients of group A and in 3 of 11 patients in group B (P = 0.13).
TABLE 3.
Adverse events
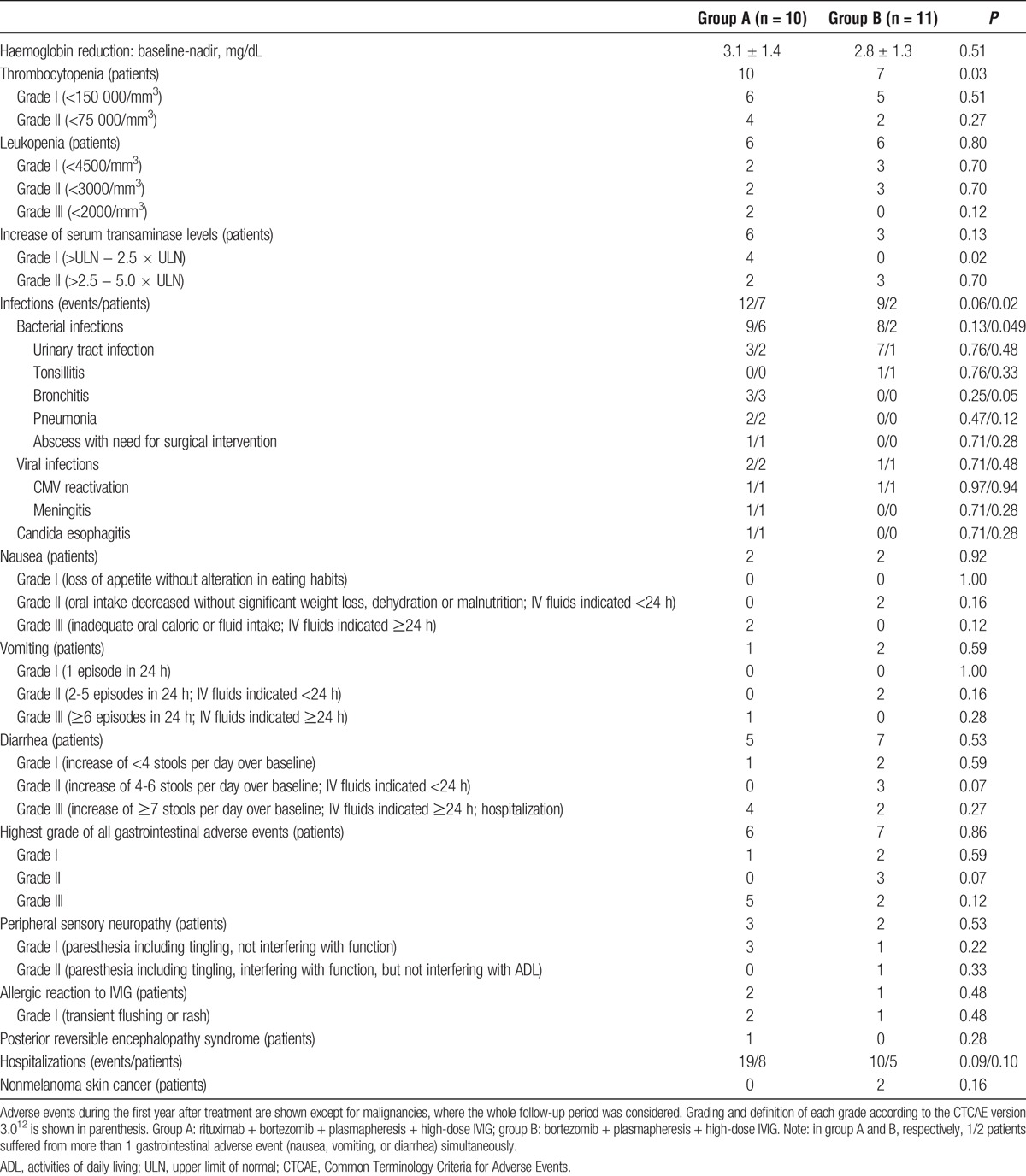
In group A, more patients suffered from infections (70% vs 18%, P = 0.02), especially bacterial infections (60% vs 18%, P = 0.049) after treatment as compared with group B. A summary of all episodes of infection is shown in Table 3. Notably, all 7 episodes of urinary tract infection (UTI) in group B occurred in a single patient. His underlying renal disease was diabetic nephropathy. Despite extensive diagnostics, a urologic cause was not detected. Finally, both native kidneys were nephrectomized at 14 months after treatment of AMR. Subsequently, the frequency of infections decreased. The pathogen was identified in 4 episodes of group A (UTI: 3× Escherichia coli, pneumonia: 1× Pneumocystis jirovecii) and in all 7 episodes of UTI in group B (4× Escherichia coli, 3× Escherichia coli together with Enterococcus faecalis). All patients without an identified pathogen presented with typical signs and symptoms of the respective bacterial infection. All of these patients received antibiotic treatment and responded to treatment. Bacterial infections occurred before completion of cotrimoxazole prophylaxis, that is, within 3 months after AMR therapy, in 1 case of group A (UTI), and in 3 cases of group B (all UTI). Cytomegalovirus (CMV) reactivation occurred in 1 patient of each group (both D+/R+) during the first 3 months after treatment, that is, during valganciclovir prophylaxis and was treated with oral valganciclovir. In both patients, the applied valganciclovir prophylaxis was not adequately adapted to increasing GFR, and therefore underdosed. Altogether, 62% of all patients experienced gastrointestinal side effects including nausea, vomiting, and diarrhea. Of these, 7 patients had to be hospitalized to restore fluid losses. The need for hospitalization and prolonged IV fluid administration was responsible for classification as grade III adverse event. Mild-to-moderate sensory peripheral neuropathy occurred in 24% of all patients with no differences between groups. Symptoms were reversible in all cases during the first year after treatment. A mild allergic reaction during IVIG administration was observed in 3 patients and successfully treated with antihistamines and prednisolone. The number of hospitalizations (19 vs 10, P = 0.09) as well as the number of hospitalized patients (8 vs 5, P = 0.10) was higher in group A as compared with group B, although these differences were not statistically significant. Notably, 1 patient in group A was hospitalized at 1 week after treatment because of headache, malaise, and fever. Analysis of the cerebrospinal fluid after lumbar puncture was interpreted as viral meningitis. Despite thorough diagnostics, the infectious cause could not be identified. The patient was empirically treated with a combination of acyclovir, ceftriaxone, and sultamicillin. Under this regimen, all symptoms disappeared, and he was discharged after 10 days. Another patient of group A was admitted to our stroke unit with signs of a cerebral stroke at 1 month after treatment. After exclusion of a cerebral stroke, a diagnosis of posterior reversible encephalopathy syndrome was established, and the patient was converted from tacrolimus to cyclosporine A. Subsequently, he recovered completely within 10 days. Importantly, none of the patients developed malignancy during follow-up except for 2 cases of nonmelanoma skin cancer in group B. Both patients were successfully treated by local excision.
DISCUSSION
A diagnosis of AMR is associated with an unfavorable prognosis for graft survival in most cases. Both the existing evidence and the efficacy of treatment are not satisfactory to date.5 Since 2005, all patients of our center with a diagnosis of AMR have been treated according to standardized protocols. These protocols have been stepwise adapted and modified after treatment and outcome analysis of groups of 10 to 12 patients. In our previously published retrospective analysis of patients treated with 1 cycle of bortezomib in combination with PPH and IVIG versus a fixed dose (500 mg) of rituximab in combination with PPH and IVIG, we observed a trend toward an improved graft survival in the bortezomib group, which led to the implementation of a bortezomib-based treatment regimen for AMR in our center since February 2009.9
Bortezomib and rituximab target the humoral immune response at different stages of B-cell development. Thus, it was tempting to combine both substances in an attempt to increase therapeutic efficacy without increasing substance-specific side effects. Therefore, we compared 2 groups of patients with biopsy-proven AMR: group A was treated with rituximab in addition to bortezomib, PPH, and high-dose IVIG, group B was treated with the identical regimen without rituximab. Both groups were well comparable concerning the underlying patient characteristics including allograft pathology and renal function at diagnosis. In both groups, treatment resulted in a decrease in the level of DSAmax (MFI) at 3 months after treatment. At 40 months after treatment, graft survival and graft function were similar in both groups. The frequency of ongoing acute AMR as well as the response to treatment was also similar in both groups and did not influence results. Thus, our results do not support the hypothesis that the addition of rituximab to treatment with bortezomib, PPH, and high-dose IVIG does increase therapeutic efficacy in patients with AMR.
The fixed single dose of 500 mg rituximab in our protocol could be criticized as too low. However, Vieira et al13 showed that peripheral B cell depletion can be achieved even with low doses of 50 or 150 mg/m2 rituximab. In addition, Mulley et al14 described successful salvage treatment of patients with refractory AMR with a single fixed dose of 500 mg rituximab. Although there is considerable evidence indicating that treatment of AMR with rituximab is effective,14-16 the efficacy of rituximab is not undoubted. First, rituximab treatment was mostly accompanied by significant antihumoral comedication in multimodal regimens so that its additional efficacy cannot be safely discriminated. Second, the RITUX ERAH study, a multicenter randomized controlled trial showed no additional effect of rituximab (375 mg/m2) compared with placebo in patients with acute AMR during the first year after transplantation treated with PPH, IVIG, and corticosteroids.17 However, as the authors themselves state, the results are limited by the fact that (i) the study was underpowered (only 38 instead of 64 patients were included), (ii) the number of patients, who received only placebo, was decreased by rituximab rescue treatment (8/19 patients), (iii) the primary endpoint (day 12) captured only short-term effects, and (iv) the follow-up period (1 year) was too short. Recently, Immenschuh et al18 reported in a retrospective analysis of 20 patients that increased proteinuria and decreased graft function are negative predictors for the responsiveness to rituximab therapy. According to our results, (i) fixed dose rituximab-based treatment seems to be not as effective as bortezomib-based treatment,9 and (ii) the addition of a fixed dose of rituximab to bortezomib, high-dose IVIG, and PPH seems to have no additional effect. Taken together, the efficacy of rituximab, its potential dose dependency, and the pretreatment identification of potential responders need to be further elucidated.
Similarly, the existing evidence on the efficacy of bortezomib is still not satisfactory. Our own results indicate that bortezomib might be more effective compared with rituximab.9 The fact that graft survival after bortezomib-based treatment ranged between 60% and 65% at 40 months after treatment here sheds some light to the limited prognosis of AMR under current treatment options. At least, DSA intensity could be significantly reduced under both protocols, although DSA were still present despite intense therapy. Meanwhile, it became evident that bortezomib-based treatment is not a cure for all patients with AMR.19 To discern potential responders from nonresponders before treatment would be helpful to avoid treatment-associated adverse events in patients without a reasonable chance to respond. The BORTEJECT study,20,21 an ongoing randomized controlled trial investigating the effect of 2 cycles of bortezomib on late AMR (≥180 days after transplantation) will hopefully further expand our knowledge on the efficacy of bortezomib treatment.
The toxicity profile of bortezomib in patients treated for AMR or desensitization was recently summarized by Schmidt et al.22 The most important adverse events are anemia, leukopenia, thrombocytopenia, peripheral neuropathy, and gastrointestinal side effects including nausea, vomiting, and diarrhea. In our present study, hemoglobin reduction occurred in all patients of both groups and was treated by close blood count monitoring in addition to erythropoietin substitution. Thrombocytopenia and leukopenia were transient and did not cause clinically relevant adverse events. Notably, the addition of rituximab increased the incidence of thrombocytopenia in bortezomib-treated patients. Transient elevation of serum transaminase levels was monitored but did not require therapeutic intervention.
Importantly, more patients in group A experienced infection, especially bacterial infections, as compared with group B, indicating that the addition of rituximab increased overall immunosuppression. One patient in group A experienced infectious meningitis. Whether, this serious adverse event was caused by overimmunosuppression should be discussed with caution in consideration of the relatively small number of patients in both groups. In each group, we observed 1 episode of CMV reactivation obviously caused by underdosing of valganciclovir prophylaxis. Therefore, extensive CMV prophylaxis adapted to GFR seems to be recommendable. The frequency of gastrointestinal adverse events was similar in both groups, although there was a trend toward more severe episodes necessitating hospitalization in group A. Notably, gastrointestinal adverse events, especially diarrhea, caused 7 hospitalizations in 21 patients. Generally, there was a trend toward an increased number of hospitalizations in group A as compared with group B. Peripheral sensory neuropathy was reversible in all cases. However, some patients mentioned numbness or tingling in the upper or lower extremities for several months after treatment. Polyneuropathy after bortezomib treatment is known to be dose-dependent,23 limiting its use in patients with AMR. In our view, preexisting polyneuropathy must be considered and should be regarded as a potential contraindication for bortezomib treatment. Finally, it is important to mention that none of our patients died because of treatment-associated adverse events, and none of our patients developed malignancy except for nonmelanoma skin cancer.
Our study has some limitations. First of all, it is a nonrandomized retrospective study with a limited number of patients. In addition, both groups comprise patients with “early” (<12 months after transplantation) and “late” AMR. “Early” and “late” AMR were described to exhibit distinct immunologic characteristics and to respond differentially to bortezomib treatment.24 Notably, graft survival between patients (n = 4) with “early” AMR was not different as compared with patients (n = 17) with “late” AMR (P = 0.46). Furthermore, we found histological evidence for both acute and chronic AMR in 14 (66.6%) of 21 patients (group A: n = 8, group B: n = 6). This may be important because acute lesions seem to respond better to treatment than chronic lesions.25 However, in the present study, graft survival was not different in patients with acute and chronic lesions as compared with patients (7/21) with exclusively acute lesions (P = 0.35).
To our knowledge, this is the first study investigating the efficacy and the side effect profile of treatment with a combination of rituximab and bortezomib in renal allograft recipients with AMR. Our results indicate that the addition of rituximab to a treatment regimen consisting of bortezomib, PPH, and IVIG may not further improve graft function or graft survival in renal allograft recipients with AMR. The increased incidence of adverse events indicates that combined treatment with bortezomib and rituximab should be regarded with caution. Because of the small sample size and the retrospective nature of our study, further investigations are necessary to confirm our findings.
Footnotes
Published online 1 July 2016.
The authors declare no funding or conflicts of interest.
J.W. and M.D. contributed equally to the study.
J.W. participated in the conception, data acquisition and analysis, preparation, and writing of the article. M.D. participated in conception, data acquisition and analysis, preparation and writing of the article. C.S. participated in conception, review, and proofreading of the article. B.R. participated in data acquisition and analysis, review, and proofreading of the article. K.W. participated in data acquisition and analysis, review, and proofreading of the article. F.H. participated in data acquisition and analysis, review, and proofreading of the article. K.B. participated in conception, review and proofreading of the article. N.L. participated in conception, data acquisition and analysis, preparation, and writing of the article.
REFERENCES
- 1.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. [DOI] [PubMed] [Google Scholar]
- 2.Lachmann N, Terasaki PI, Budde K, et al. Anti-human leukocyte antigen and donor-specific antibodies detected by Luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation. 2009;87:1505–1513. [DOI] [PubMed] [Google Scholar]
- 3.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. [DOI] [PubMed] [Google Scholar]
- 4.Lefaucheur C, Suberbielle-Boissel C, Hill GS, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8:324–331. [DOI] [PubMed] [Google Scholar]
- 5.Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients—a systematic review. Transplantation. 2012;94:775–783. [DOI] [PubMed] [Google Scholar]
- 6.Burton SA, Amir N, Asbury A, et al. Treatment of antibody-mediated rejection in renal transplant patients: a clinical practice survey. Clin Transplant. 2015;29:118–123. [DOI] [PubMed] [Google Scholar]
- 7.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176–189. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. [DOI] [PubMed] [Google Scholar]
- 9.Waiser J, Budde K, Schütz M, et al. Comparison between bortezomib and rituximab in the treatment of antibody-mediated renal allograft rejection. Nephrol Dial Transplant. 2012;27:1246–1251. [DOI] [PubMed] [Google Scholar]
- 10.Sis B, Mengel M, Haas M, et al. Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–471. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed October, 15, 2015.
- 13.Vieira CA, Agarwal A, Book BK, et al. Rituximab for reduction of anti-HLA antibodies in patients awaiting renal transplantation: 1. Safety, pharmacodynamics, and pharmacokinetics. Transplantation. 2004;77:542–548. [DOI] [PubMed] [Google Scholar]
- 14.Mulley WR, Hudson FJ, Tait BD, et al. A single low-fixed dose of rituximab to salvage renal transplants from refractory antibody-mediated rejection. Transplantation. 2009;87:286–289. [DOI] [PubMed] [Google Scholar]
- 15.Kaposztas Z, Podder H, Mauiyyedi S, et al. Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant. 2009;23:63–73. [DOI] [PubMed] [Google Scholar]
- 16.Lefaucheur C, Nochy D, Andrade J, et al. Comparison of combination plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9:1099–1107. [DOI] [PubMed] [Google Scholar]
- 17.Sautenet B, Blancho G, Büchler M, et al. One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation. 2016;100:391–399. [DOI] [PubMed] [Google Scholar]
- 18.Immenschuh S, Zilian E, Dämmrich ME, et al. Indicators of treatment responsiveness to rituximab and plasmapheresis in antibody-mediated rejection after kidney transplantation. Transplantation. 2015;99:56–62. [DOI] [PubMed] [Google Scholar]
- 19.Ejaz NS, Alloway RR, Halleck F, et al. Review of bortezomib treatment of antibody-mediated rejection in renal transplantation. Antioxid Redox Signal. 2014;21:2401–2418. [DOI] [PubMed] [Google Scholar]
- 20.Eskandary F, Bond G, Schwaiger E, et al. Bortezomib in late antibody-mediated kidney transplant rejection (BORTEJECT Study): study protocol for a randomized controlled trial. Trials. 2014;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eskandary F, Bond G, Regele H, et al. Late antibody-mediated rejection in a large prospective cross-sectional study of kidney allograft recipients—preliminary results of the screening phase of the BORTEJECT Trial. Clin Transpl. 2014:189–195. [PubMed] [Google Scholar]
- 22.Schmidt N, Alloway RR, Walsh RC, et al. Prospective evaluation of the toxicity profile of proteasome inhibitor-based therapy in renal transplant candidates and recipients. Transplantation. 2012;94:352–361. [DOI] [PubMed] [Google Scholar]
- 23.Argyriou AA, Cavaletti G, Bruna J, et al. Bortezomib-induced peripheral neurotoxicity: an update. Arch Toxicol. 2014;88:1669–1679. [DOI] [PubMed] [Google Scholar]
- 24.Walsh RC, Brailey P, Girnita A, et al. Early and late acute antibody-mediated rejection differ immunologically and in response to proteasome inhibition. Transplantation. 2011;91:1218–1226. [DOI] [PubMed] [Google Scholar]
- 25.Sadaka B, Ejaz NS, Shields AR, et al. A Banff component scoring-based histologic assessment of bortezomib-based antibody-mediated rejection therapy. Transplantation. 2015;99:1691–1699. [DOI] [PubMed] [Google Scholar]


