Abstract
Mutations of the truncated cytoplasmic domain of human erythropoietin receptor (EPOR) result in gain-of-function of erythropoietin (EPO) signaling and a dominantly inherited polycythemia, primary familial and congenital polycythemia (PFCP). We interrogated the unexplained transient absence of perinatal polycythemia observed in PFCP patients using an animal model of PFCP to examine its erythropoiesis during embryonic, perinatal, and early postnatal periods. In this model, we replaced the murine EpoR gene (mEpoR) with the wild-type human EPOR (wtHEPOR) or mutant human EPOR gene (mtHEPOR) and previously reported that the gain-of-function mtHEPOR mice become polycythemic at 3~6 weeks of age, but not at birth, similar to the phenotype of PFCP patients. In contrast wtHEPOR mice had sustained anemia. We report that the mtHEPOR fetuses are polycythemic, but their polycythemia is abrogated in the perinatal period and reappears again at 3 weeks after birth. mtHEPOR fetuses have a delayed switch from primitive to definitive erythropoiesis, augmented erythropoietin signaling, and prolonged Stat5 phosphorylation while the wtHEPOR fetuses are anemic. Our study demonstrates the in vivo effect of excessive EPO/EPOR signaling on developmental erythropoiesis switch and describes that fetal polycythemia in this PFCP model is followed by transient correction of polycythemia in perinatal life associated with low Epo levels and increased expression of erythrocytes’ phosphatidylserine. We suggest that neocytolysis contributes to the observed perinatal correction of polycythemia in mtHEPOR newborns as embryos leaving the hypoxic uterus are exposed to normoxia at birth.
Keywords: Human EPOR mutation, fetal polycythemia, prolonged primitive erythropoiesis, augmented Stat5 signaling, neocytolysis
Introduction
Erythropoietin (EPO)/EPO receptor (EPOR) signaling plays a central role in survival, proliferation, and differentiation of committed erythroid progenitors in definitive erythropoiesis. Epo- and EpoR-null mutant mice die at ~E13.5 due to severe anemia [1]. These loss-of-function mutations of Epo and EpoR genes permit production of primitive erythroblasts during E10~11, albeit at low levels, but definitive fetal liver (FL) erythropoiesis is blocked at the more differentiated erythropoiesis stage (CFU-E) [1]. The gain-of-function of EPOR mutations resulting from truncation mutations of the cytoplasmic domain of EPOR, are associated with primary familial and congenital polycythemia (PFCP) characterized by augmented EPO/EPOR signaling and hypersensitivity of erythroid progenitors to EPO [2].
The yolk sac is the first site of erythropoiesis during mouse and human ontogeny, followed by FL erythropoiesis in developing fetuses. In the mouse, the first blood cells arise in the yolk sac around embryonic days (E)7~8. These are unipotential progenitors giving rise to nucleated primitive erythroblasts, which synthesize embryonic hemoglobins. After E8 these primitive erythroblasts enter the circulation [3]. The definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are also the products of the yolk sac [3,4]. Definitive hematopoiesis is then established in the FL and produces enucleated erythrocytes from E11.5, when these cells first enter the bloodstream. Definitive erythroid cells predominate in the circulation from E14 [4]. There is a temporal overlap of the appearance of primitive and definitive erythrocytes in the circulation, as primitive erythroid cells undergo progressive enucleation between E12.5–16.5 and form mature primitive erythrocytes in the circulation [5]. Around birth, the mouse spleen and bone marrow become the principal sites of adult erythropoiesis, producing definitive erythroid cells; however, primitive erythrocytes circulate as late as 5 days after birth [5].
The switch from embryonic to adult erythropoiesis coincides with the differential use of globin genes; in the mouse, the β globin cluster is composed of 4 functional β-globin genes (εγ-, βH1-, β1-, and β2-globins). The εγ- and βH1-globins are expressed in the primitive erythroid lineage and the β1- and β2-globins are expressed in definitive erythroid cells. A transient wave of early definitive erythroid lineage in murine FL that originates from yolk sac-derived erythromyeloid progenitors expresses adult β-globins along with embryonic βH1-globin [4]. In contrast, in the human β globin cluster, ε gene expression is followed by expression of γ globin genes in fetal life (Aγ, and Gγ), while adult δ- and β- globins are expressed after birth. The α globin cluster is characterized by the expression of ζ-globin gene in primitive erythropoiesis in both humans and mice, while fetal and adult erythropoiesis is characterized by the expression of α1- and α2- globin genes [5].
We have previously shown that animal model of PFCP - the mice having gain-of-function EPOR mutation (mtHEPOR) - become polycythemic at 3~6 weeks of age, but not at birth similar to the polycythemic phenotype of affected humans and that the mice with wild type human EPOR (wtHEPOR) mice are anemic [6]. Here, we report that PFCP mouse embryos have fetal polycythemia associated with a delayed switch from embryonic to adult erythropoiesis. This fetal polycythemia is transiently overcorrected in the perinatal period and reappears again at 3 weeks of postnatal life. The observed perinatal fall of hematocrit in the mtHEPOR mice could be a parallel to transient anemia in normal human newborns associated with neocytolysis; i.e. a selective destruction (hemolysis) of young (hypoxia-made) erythrocytes [7].
Material and Methods
Mice
The mice (wtHEPOR - homozygous wild-type human EPOR knock-in, mtHEPOR - homozygous gain-of-function human EPOR knock-in (substitution c.1278C>G; designation of the mutation according to LOVD database, www.lovd.nl), and mEpoR - wild-type mouse control) were bred and maintained in an IACUC-approved CCM facility at University of Utah and in the Center for Laboratory Animals, Palacky University (PU) in accordance with the regulations of PU Institutional Animal Care and Use Committee, Olomouc, Czech Republic.
The wtHEPOR or mtHEPOR knock-in mice were generated by gene targeting [6], and then backcrossed onto C57BL/6 strain at least 6 times. Thereafter, the heterozygous knock-in offspring were intercrossed to produce all experimental mice, i.e. the model homozygous wtHEPOR and homozygous mtHEPOR as well as the control mEpoR mice.
Hematocrit assessment and isolation of fetal liver cells (FLCs) from fetuses
Mice aged 10 weeks or older were used for timed mating. When the vaginal plug was detected, noon of that day was considered E0.5. Fetuses were removed from the uterus at the indicated time-points. Fetal blood was immediately drawn from carotid arteries by a Drummond 2 lambda microcap (Fisher Scientific; Pittsburgh, PA) for hematocrit measurement and by hand-drawn pipette for other analyses. Hematocrits were recorded with a hematocrit reader (International Equipment Company; Needham Heights, MA). FLs were dissected and dispersed by repeated pipetting in cold PBS. Red blood cells (RBCs) were lysed by RBC lysis buffer (0.15M NH4Cl, 1mM KHCO3, 0.1mM EDTA).
Immunostaining and flow cytometry analysis of erythroid progenitors and erythroblasts
Fetal liver cells (FLCs) or peripheral blood (PB) were stained with phycoerythrin (PE)-conjugated anti-CD71 and fluorescein isothiocyanate (FITC)-conjugated anti-TER119 (BD Biosciences; San Diego, CA) antibodies as described [8], followed by 1% formaldehyde fixation. The cells were analyzed by flow cytometry on a Coulter Epics Profile instrument (Beckman/Coulter; Miami, FL); 5 different regions were analyzed based on the expression levels of CD71/TER119 [8]. Each region represented differentiation cell stage as previously described [8].
Real-time PCR analysis of globin genes
For details of quantifications including specific primer sequences see Supplementary Material.
Assessment of enucleation
PB cells were washed twice with cold PBS, then stained with 10μM DRAQ5 (Biostatus, Leicestershire, UK) for 15 minutes at ambient temperature, followed by FACS analysis.
Stat5 phosphorylation analysis
Antibodies specific to phospho-Stat5 and total Stat5 were purchased from Cell Signaling Technology (Beverly, MA) and Santa Cruz Biotechnology, respectively. After 2 hours of starvation in RPMI with 10% fetal calf serum (FCS), FLCs were treated with recombinant human (rh) EPO (Amgen Co., Thousand Oaks, CA), and cell lysates were prepared as described [9]. Proteins were separated on 7.5% SDS-PAGE and transferred to PVDF membranes (Bio-Rad; Hercules, CA).
Hypoxia treatment
Mice of all genotypes (8 to 12 weeks old) were placed in a hypobaric chamber (BioSpherix, Lacona, NY) for 10 days at 12 % O2. Mice were then returned to ambient condition for 29 days, and hematocrits (using 10 μL of blood) were measured at selected time-points. The consecutive blood samplings did not materially influence hematocrits (data not shown).
Quantification of Epo
The serum levels of Epo in neonatal (7 days old) and adult (8 to 12 weeks old) mice were quantified according to the manufacturer’s instructions for the Mouse Erythropoietin Quantikine ELISA Kit (R&D Systems). The expression of Epo mRNA was determined in the liver and kidney by qRT-PCR (see Supplementary Material for details). The statistical significance of relative expression changes in target mRNA levels was analyzed using the REST© 2009 software [10].
Erythrocyte Annexin V binding
PB erythrocytes were labeled using Annexin V/FITC kit (BD Biosciences) as described [11]. Fluorescence intensity was measured by FACS Calibur (BD Biosciences).
Statistical analysis
Students’ test and ANOVA test were used for statistical analyses. Significant differences were assumed when p<0.05. The softwares GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA) or Origin 6.1 (OriginLab Corporation, Northampton, MA) were used for the statistical calculations.
Results
Truncated human EPOR causes polycythemia, while wild-type human EPOR causes anemia at mid- to late-gestation mouse embryonic stages
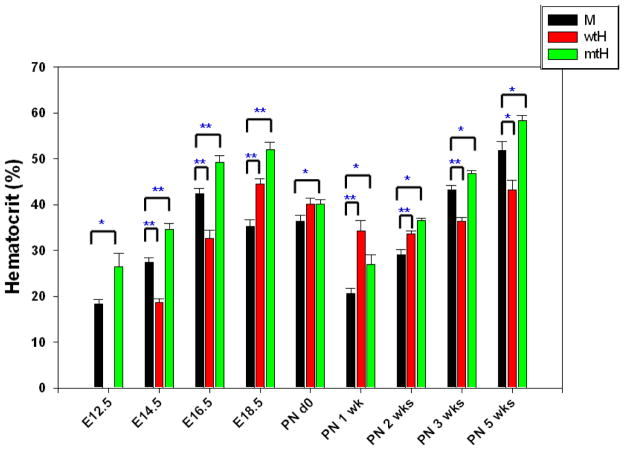
Our previous studies demonstrated that polycythemia in the mtHEPOR mice was not apparent until 3 to 6 weeks after birth [6] thus the effect of the gain-of-function of EPOR mutation in mtHEPOR fetuses was not clear. We examined wtHEPOR, mtHEPOR and mEpoR fetuses at various time-points of mouse development: E12.5, E14.5, E16.5, E18.5; and mice at following postnatal (PN) time-points: 0 day, 1 week, 2 weeks, 3 weeks, and 5 weeks. Unexpectedly, as shown in Fig. 1 and similarly to what we reported in adult mice [6, 12], mtHEPOR fetuses were polycythemic and wtHEPOR were anemic from E12.5 to E16.5 as compared to mEpoR fetuses. However, all genotypes had significantly reduced hematocrits at perinatal time; mEpoR from E18.5 to PN 2 weeks, mtHEPOR and wtHEPOR from PN 0 day to PN 2 weeks. In wtHEPOR and mtHEPOR, the peaks of hematocrit levels were reached at E18.5 in contrast to E16.5 of mEpoR. From 3 weeks of PN, hematocrits from mtHEPOR (polycythemic) and wtHEPOR (anemic) were comparable to those at the mid-gestation. At these examined developmental stages, there were no apparent size differences of the fetuses and volumes of FLs among the studied genotypes (not shown).
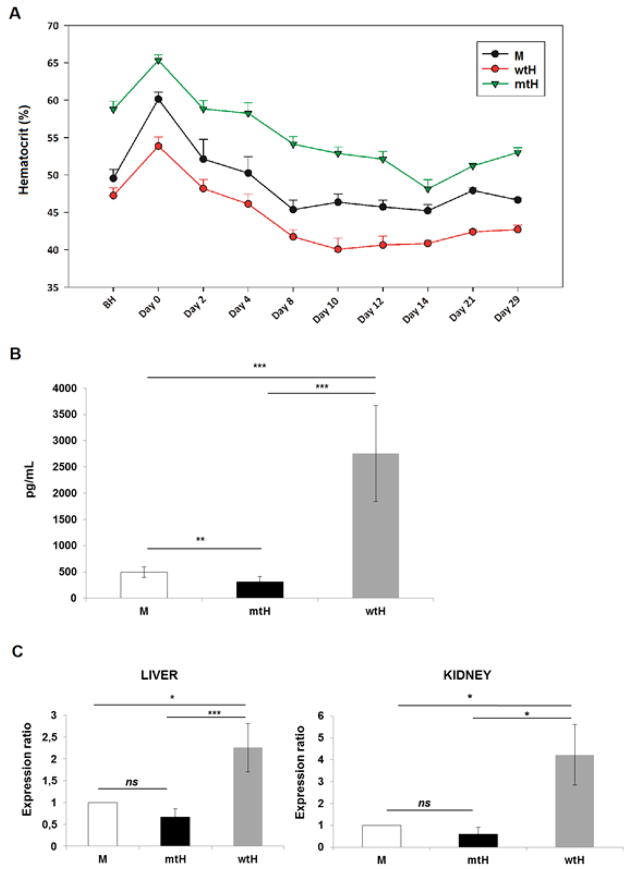
Figure 1. The mtHEPOR fetuses are polycythemic and wtHEPOR fetuses are anemic compared to mEpoR fetuses at mid- to late-gestation (E12.5~E16.5). These phenotypes transiently disappear around perinatal period, and then reappear at 3 weeks after birth.
M, wtH, mtH denote mEpoR, wtHEPOR, and mtHEPOR genotypes, respectively. * and ** denote P<0.05 and P<0.001 for mEpoR versus wtHEPOR, and mtHEPOR, respectively. The mean and standard error of hematocrit levels from at least 10 embryos/neonatal pups for each time-point are shown.
wtHEPOR and mtHEPOR have a greater proportion of immature circulating erythroid progenitors than mEpoR
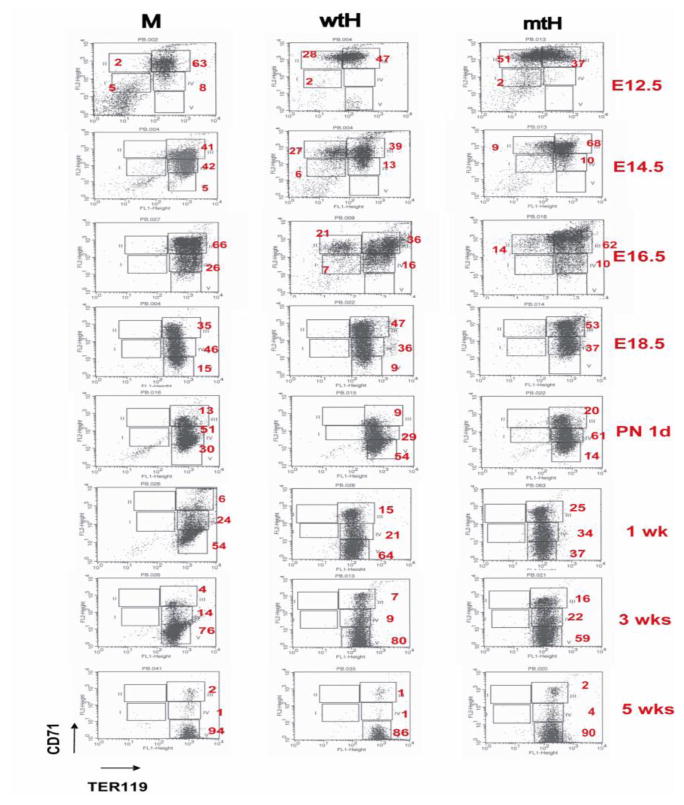
We hypothesized that polycythemia of mtHEPOR and anemia of wtHEPOR in embryos could be influenced by an aberrant erythroid differentiation. Therefore, PB erythroid progenitors were analyzed at various time-points of mouse development using two differentially regulated erythroid specific surface markers, CD71 and TER119 by FACS [8]. The relative proportion of the erythroid R2 region (proerythroblasts and early basophilic erythroblasts) in mtHEPOR rapidly decreased (51% at E12.5 versus 14% at E16.5), while wtHEPOR had only a small reduction of this population until E16.5 (28% at E12.5 versus 21% at E16.5) (Fig. 2). At E18.5, immature erythroid cells (R2 region) from PB of all genotypes were no longer detectable. At birth, all genotypes had circulated erythroid cells of a comparable differentiation stage.
Figure 2. Peripheral blood (PB) from all genotypes contains early erythroid cells while wtHEPOR and mtHEPOR mice have delayed maturation.
PB was obtained from all homozygous fetuses/neonatal pups at the indicated time-points, stained with CD71/TER119 antibodies and analyzed by FACS. Each differentiation stage (R1~R5) was gated as previously described [8]; R1 (CD71mid/TER119negative)-primitive progenitor cells and proerythroblasts, R2 (CD71high/TER119negative)-proerythroblasts and early basophilic erythroblasts, R3 (CD71high/TER119positive)-early and late basophilic erythroblasts, R4 (CD71mid/TER119positive)-chromatophilic and orthochromatophilic erythroblasts, and R5 (CD71low/TER119positive)-late orthochromatophilic erythroblasts and reticulocytes. Numbers are the averages of each differentiation stage in mice derived from multiple litters. M, wtH, mtH denote mEpoR, wtHEPOR, and mtHEPOR genotype, respectively. * indicates p<0.05.
All genotypes had immature circulating erythroid progenitors and underwent their ongoing differentiation from E12.5 to PN 3 weeks (Fig. 2). However, PB from wtHEPOR and mtHEPOR at late-gestation and the perinatal period contained significantly higher proportions of immature erythroid progenitors compared to mEpoR until PN 3 weeks, suggesting delayed ongoing differentiation. Erythroid FLCs had no significant differences of their maturation pattern at any time-point (not shown).
PB of mid- to late-gestation mtHEPOR embryos contain more primitive erythroid cells in circulation
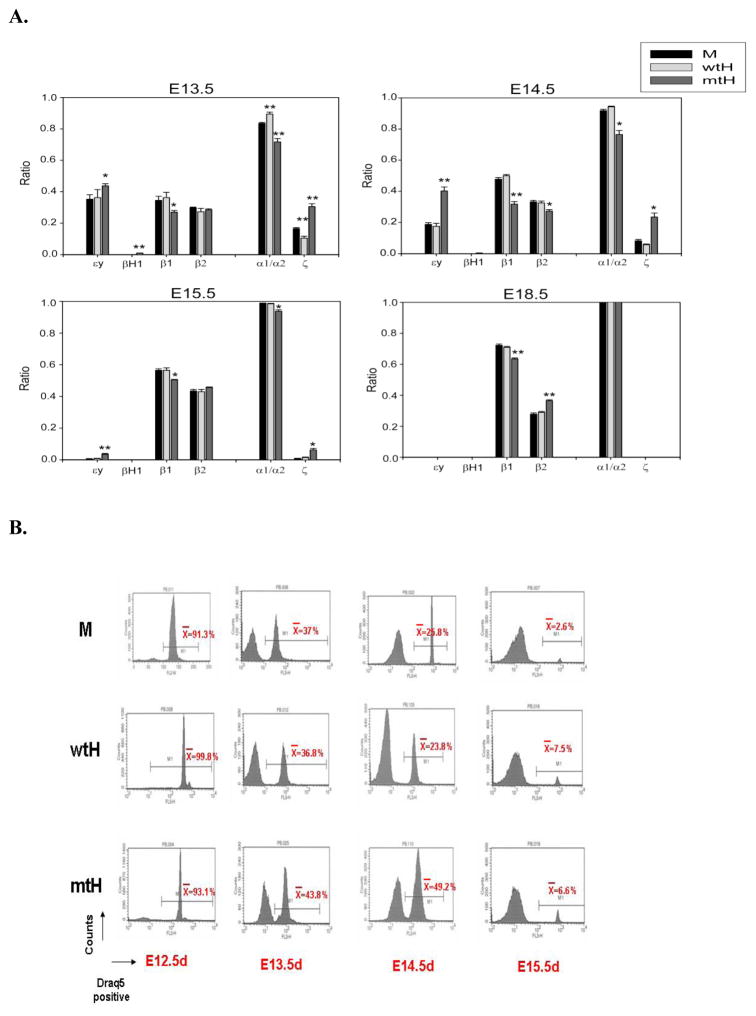
Lux et al. showed that primitive and definitive hematopoietic progenitor cells coexist and emerge in the mouse embryo before E10 [3]. Progressive maturation of immature primitive erythroid cells occurs in the circulation, as evaluated by changes of globin isotypes [5]. We postulated that the higher proportion of circulating immature erythroid cells from mtHEPOR embryos during mid- to late-gestation embryogenesis may be due to the presence of a greater proportion of primitive erythroid cells. As shown in Fig. 3A, fetal PB from mtHEPOR embryos had higher levels of embryonic globin transcripts (εy/βH1 and ζ) than mEpoR, while fetal PB from wtHEPOR embryos had comparable levels of embryonic globin transcripts to mEpoR controls. We also evaluated the enucleation status of fetal PB using a DNA dye, DRAQ5. In agreement with globin analysis, mtHEPOR fetuses from E13.5 and 14.5 had higher proportion of nucleated erythroblasts than other genotypes (Fig. 3B). These data indicate that the mtHEPOR mice have prolonged primitive erythropoiesis, i.e. delayed switch from primitive to definitive erythropoiesis.
Figure 3. mtHEPOR fetuses have more embryonic globin transcripts than other two genotypes.
A. Peripheral blood (PB) was obtained from carotid arteries of fetuses at indicated time-points and total RNA was extracted using manufacture’s protocol. Embryonic and adult globin transcripts were analyzed by realtime PCR using SYBR green dye with primer sets as described in the Methods section. M, wtH, mtH denote mEpoR, wtHEPOR, and mtHEPOR genotype, respectively. B. PB was obtained and stained by DRAQ5 dye. Nucleated and enucleated cells in PB were analyzed by FACS. Average numbers were calculated from multiple embryos (at least 10 for each genotype). M, wtH, mtH denote mEpoR, wtHEPOR, and mtHEPOR genotype, respectively.
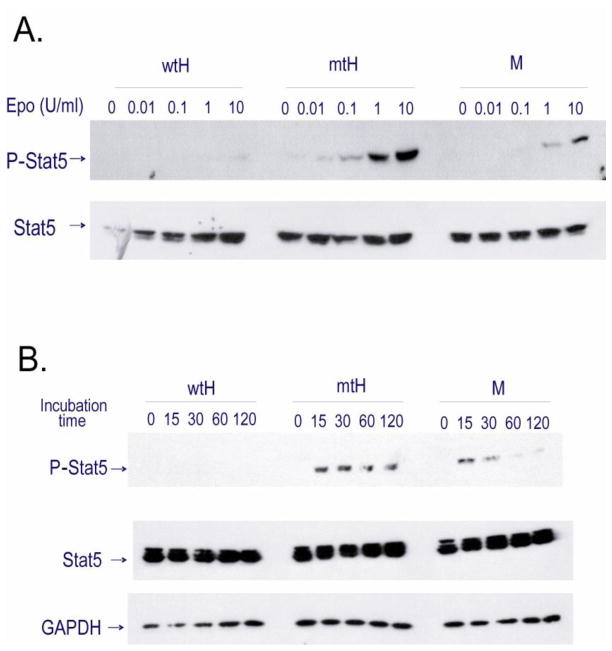
Stat5 phosphorylation is increased and sustained upon EPO stimulation in mtHEPOR FLCs
To evaluate the EPO signal transduction, we tested the EPO dose response and kinetics of Stat5 phosphorylation in FLCs. FLCs were obtained from all genotypes at E14.5 and E18.5, starved for 2 hours in RMPI + 10% FCS, and stimulated with recombinant human EPO (rhEPO) at ambient temperature. The results were identical for E14.5 and E18.5. As shown in Fig. 4A, mtHEPOR FLCs at 0.01U/ml of EPO had detectable phosphorylation of Stat5, whereas mEpoR FLCs had detectable phosphorylation of Stat5 only at 1 and 10 U/ml of EPO. The wtHEPOR FLCs had only a barely detectable level of Stat5 phosphorylation at 10 U/ml of rhEPO (Fig. 4A). As shown in Fig. 4B and Supplementary Fig. 1, phosphorylation of Stat5 in mEpoR was seen after 15 minutes of stimulation and almost disappeared after 60 minutes, whereas in mtHEPOR FLCs Stat5 was comparably phosphorylated after 15 minutes of stimulation but only minor decline in Stat5 phosphorylation was detected during the 120-minute observation time period.
Figure 4. EPO-induced Stat5 phosphorylation in mtHEPOR fetal liver erythroid progenitors is augmented and sustained.
FLCs were obtained from mEpoR (M), wtHEPOR (wtH), and mtHEPOR (mtH) E14.5 fetuses and starved for 2 hours in RPMI + 10% FCS prior to stimulation with recombinant human (rh) EPO. The cell lysates (equivalent to 106 cells) were immunoblotted with a phopho-Stat5 (p-Stat5) antibody, followed by re-probing with antibodies specific for total Stat5 and GAPDH. A. EPO dose-response test of Stat5 activation. FLCs were stimulated with indicated concentrations of EPO for 15 minutes. B. Stat5 phosphorylation kinetic study. Analysis of p-Stat5 in FLCs stimulated with 1 U/ml of EPO for indicated periods of time. The relative quantification of p-Stat5 for mtHEPOR and mEpoR FLCs is presented graphically in Supplementary Fig. 1.
mtHEPOR mice have greater decrease of hematocrit upon normoxic return from hypoxia
We interrogated the observed fall of hematocrit in the mtHEPOR neonates. Since mouse pups underwent significant change of oxygen tension at delivery (from the uterus to ambient atmosphere) we tested whether it may account for the decrease in perinatal hematocrit levels (Fig. 1). Mice exposed to hypoxia at hypobaric chamber for 10 days had their hematocrit level followed for 29 days after returning to normoxia. As shown in Fig 5A, hypoxia increased the hematocrit levels by a similar degree in individual genotypes; i.e. mEpoR=60.0 (1.2-times); wtHEPOR=53.9 (1.13-times); mtHEPOR=65.0 (1.12-times). However, upon normoxic return the reduction of hematocrits differ among individual genotypes. The hematocrits reached the lowest levels between days 10 to 14 and decreased to mEpoR=45.2 (drop of 14.8%), wtHEPOR=40.1 (drop of 13.8%), and mtHEPOR=48.1(drop of 16.9%). The most pronounced relative hematocrit drop was observed for mtHEPOR mice and showed statistical significance compared to wtHEPOR (P=0.0071) and mEpoR (P=0.0291) mice, respectively. Thus, the mtHEPOR mice undergo greater changes of hematocrit than other genotypes in response to changes in oxygen tension.
Figure 5. Upon return from hypoxia to normoxia, mtHEPOR mice undergo greater changes of hematocrit than other genotypes. Neonatal mice of individual genotypes have different Epo levels.
A. Mice mEpoR (M), wtHEPOR (wtH), and mtHEPOR (mtH)) were treated in a hypobaric chamber for 10 days at 12% O2, and their hematocrit measured at indicated timepoints for 29 days after returning to normoxia. The mean and standard error of hematocrit levels (n≥4) are shown. BH denotes before hypoxia. B. ELISA measurements of Epo levels in neonatal mice (7 days old) of the indicated genotypes. Epo levels [pg/ml] in individual mouse genotypes are expressed as the mean value ± SD of measurements for 7 mEpoR mice (M), 6 mtHEPOR (mtH), and 4 wtHEPOR (wtH) mice. C. qRT-PCR analysis of Epo expression in the liver and kidney of day 7 neonates using Epo Taqman probe. The results were normalized to the expression of beta-actin and to mRNA levels of wild-type mouse control (M) using REST© 2009 software. n = 6 for mEpoR mice (M) and n = 4 for mtHEPOR (mtH), and wtHEPOR (wtH) mice. ***P<0.001, **P<0.01, *P<0.05, ns - not significant.
Neonatal and adult mice of individual mouse genotypes differ in Epo production
We reasoned that possible differences in Epo concentrations might reflect mtHEPOR and wtHEPOR perinatal phenotypes. As Epo levels of mtHEPOR and wtHEPOR mice have not been previously reported, we measured their Epo concentrations. wtHEPOR newborns had higher Epo levels in relative to those of mtHEPOR and their wild type mEpoR counterparts (Fig. 5B). Serum Epo levels correlated with changes of Epo mRNA expression in liver and kidney as determined by qRT-PCR (Fig. 5C). We also assessed the serum Epo levels and Epo mRNA expression in adult animals in normoxia and during exposure to hypoxia. We observed that serum Epo levels are highest in wtHEPOR mice and lowest in mtHEPOR mice in both normoxic and hypoxic conditions (Supplementary Fig. 2A). The differences in serum Epo levels between the individual genotypes were paralleled by differences in Epo mRNA expression in the kidney; the highest was detected for wtHEPOR mice and the lowest for mtHEPOR mice, again in both normoxic and hypoxic conditions (Supplementary Fig. 2B), suggesting that the regulation of Epo levels is appropriate and commensurate to differences to hematocrit.
mtHEPOR erythrocytes have increased surface exposure of phosphatidylserine in perinatal period
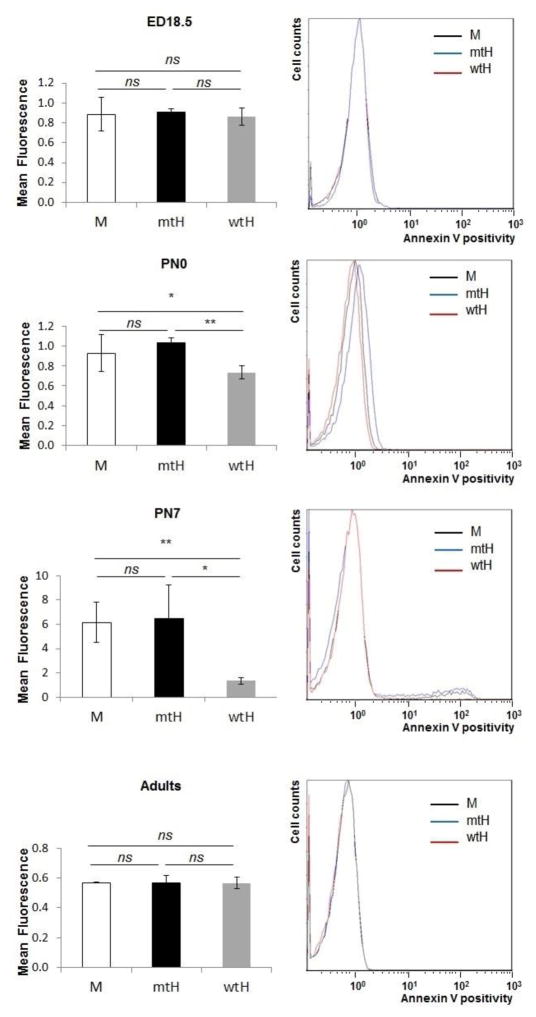
We next measured the expression of phosphatidylserine on the erythrocytes’ membranes at E18.5, PN 0, PN 7 days, and in adult mice using Annexin V staining and flow cytometry. The expression of phosphatidylserines (determined by mean fluorescence values) at E18.5 was comparable among the different genotypes (mean fluorescence around 0.8) (Figure 6) during their adult life. In contrast there was a gradual increase in phosphatidylserine expression on the mtHEPOR erythrocytes after birth and, to a lower extent, also on mEpoR erythrocytes between PN 0 and PN 7. The maximum increase of surface expression of phosphatidylserines on erythrocytes was PN 7 (mean fluorescence of 6.5±2.8 and 6.2±1.7, respectively). This corresponded to a maximal drop of hematocrit and lowest Epo levels at PN 7 in polycythemic mtHEPOR, in contrast to a milder reduction of hematocrit and slightly higher Epo levels in non-polycythemic mEpoR mice. While there was a trend to higher lever of surface expression of phosphatidylserines on the mtHEPOR erythrocytes compared to mEpoR erythrocytes it did not achieve statistical significance. The anemic wtHEPOR’s erythrocytes had the lowest expression of phosphatidylserines at all analyzed time points and the increase between PN 0 and PN 7 was only subtle (from 0.7±0.1 to 1.3±0.3) consistent with the lowest reduction in perinatal hematocrit and highest Epo levels (Fig. 6). The expression of phosphatidylserines on erythrocytes’ membranes then declined and comparable levels were detected in adult mice of all three genotypes (mean fluorescence around 0.6). Thus the increased expression of phosphatidylserines in the perinatal period likely contributes to enhanced recognition and destruction of mtHEPOR erythrocytes by reticuloendothelial macrophages paralleling the dramatic drop of hematocrit in the neonatal period [13].
Figure 6. mtHEPOR erythrocytes show increased phosphatidylserine exposure on erythrocytes membrane.
Annexin V binding to exposed phosphatidylserines on the membrane of erythrocytes isolated from embryos at day 18.5 (ED18.5), neonatal mice at postnatal day 0 (PN0) and 7 (PN7), and adult mice (8–12 weeks old) was determined by flow cytometry. The mean intensity of fluorescence gradually increased for mtHEPOR mice (mtH, n = 9 for PN7, n = 3 for E18.5, PN0, and adults) and mEpoR mice (M, n = 5 for PN7, n = 3 for E18.5, PN0, and adults). The lowest phosphatidylserine expression was detected for wtHEPOR mice; with subtle changes in perinatal period (wtH, n = 4 for PN7, n = 3 for E18.5, PN0, and adults). The overlays of original histograms of representative measurements are also shown (right). The results are expressed as the mean value ± SD. ***P<0.001, **P<0.01, ns - not significant.
Discussion
We previously reported the PFCP phenotype of gain-of-function EPOR gene (mtHEPOR) resulting from the EPOR truncation and the loss of negative regulatory domain [2] and shown that mtHEPOR mouse mimics human disease [6]. At 3–6 weeks after birth both heterozygous wtHEPOR/mtHEPOR and homozygous gain-of-function mtHEPOR animals develop polycythemia while homozygous wtHEPOR become anemic [6, 12]. The lack of perinatal polycythemic phenotype in this mouse model eluded an obvious explanation and suggested that polycythemia may be only confined to adult hematopoiesis. However, we now show that at the mid- and late gestation stages of erythropoiesis (E12.4~E16.5), mtHEPOR fetuses are polycythemic while the wtHEPOR fetuses are anemic, similar to what we observed in adult mice (Fig. 1). These phenotypes temporarily disappear during the perinatal period (E16.5~PN 2 weeks), then reappear at the adult stage (>PN 3 weeks).
To better understand how these phenotypes occur during mid- to late-gestation (E12.5~16.5) and in the perinatal and neonatal periods, we analyzed distribution of maturation stages of erythroid precursors, proportion of nucleated/enucleated erythroid cells, and expression of globin genes in fetuses and neonates. It has been previously demonstrated that immature primitive erythroid cells enter the embryonic circulation and progressively mature in the circulation during embryogenesis [14]. It was also shown that EPO/EPOR signaling promotes survival and delays maturation of primitive erythroblasts [15]. In agreement, our data show the presence of immature erythroid cells in the circulation of mouse fetuses as well as newborns until 3 weeks after birth; the mtHEPOR mouse with augmented EPOR signaling has a larger proportion of immature erythroblasts than wtHEPOR and mEpoR mice; predominantly in the mid- and late-gestation period (Fig. 2). Since most of the progeny of primitive erythropoiesis in wild-type mouse disappear around E15.5 [14, 16], the circulating immature erythroid cells from E15.5 and thereafter, were expected to be the progeny of definitive erythropoiesis. The globin gene expression analysis suggested that this applies only to the mEpoR and wtHEPOR genotypes. Interestingly, in the mtHEPOR mice, the observed delayed maturation paralleled with delayed hemoglobin switching (Fig. 3). This delayed switch from embryonic to fetal/adult globins could be attributed to a slower clearance of primitive erythroid cells from the circulation in mtHEPOR fetuses and/or to a delayed “maturational” switch in the mtHEPOR primitive erythroblasts, as supported by data in Fig. 2. This conclusion is also supported by assessment of the enucleation status of erythroid cells in PB using DRAQ5. In aggregate our results suggest that at mid- to late-gestation, a significantly higher proportion of primitive erythroid cells was present in mtHEPOR mouse PB compared to wtHEPOR and mEpoR embryos.
EPO signaling has a unique role in primitive erythropoiesis. Primitive erythropoiesis in EpoR-deficient mouse and zebrafish models is impaired, but not completely abolished [1, 17, 18]. The proliferative maturation of primitive erythroblasts is highly dependent on EPO/EPOR mediated signaling [15]. These combined results demonstrate that EPO signaling does not fully affect primitive erythroid cell differeniationand formation, but plays a role in the proliferation of primitive erythroid progenitors and modulates primitive erythroblasts maturation which, in turn, influences the abundance of primitive erythroid cells in embryonic circulation. Our data support following concept – gain-of-function EPOR mutation causes enhanced EPO/EPOR signaling and results in delayed terminal maturation of primitive erythroid cells.
We have previously investigated the effects of recombinant mouse (rm) Epo on adult bone marrow-derived mtHEPOR and wtHEPOR erythroid progenitors in vitro and showed that while mtHEPOR BFU-Es were hypersensitive to rmEpo, the wtHEPOR were hyposensitive to rmEpo in these culture conditions [6]. Because these findings could be influenced by interaction differences between mouse Epo with human EPOR, we examined in this study of mtHEPOR and wtHEPOR FLCs the effects of rhEPO on EPOR signal transduction pathway. The data shown here are consistent with our previous studies. Here we demonstrate augmented and sustained activation of Stat5 in mtHEPOR FLCs and significantly reduced Stat5 activation in wtHEPOR FLCs (Fig. 4A and B). Collectively, these data are in agreement with the observed polycythemia of mtHEPOR and yet not fully explained anemia of wtHEPOR adult mice [6, 12] and fetuses (this study). We show here that the Epo production in wtHEPOR neonatal mice (day 7) was significantly elevated (Fig. 5B and 5C). These data likely reflect weaker signaling downstream human EPOR when compared to murine EpoR [19] leading to anemia and chronic hypoxia resulting in increased Epo production in wtHEPOR mice.
The causes of observed perinatal fall of hematocrit in the mtHEPOR neonates could be associated transient anemia in normal human newborns [20]. We reported that neocytolysis, a selective destruction (hemolysis) of young (hypoxia-made) erythrocytes, first described in astronauts [13] but also observed in people acclimatized to hypoxic high-altitude upon their return to normoxic sea levels [7, 21], can account for the perinatal fall of hematocrit in mice [7]. We propose that the same phenomenon contributes to the observed perinatal correction of polycythemia in mtHEPOR newborns as embryos leaving the hypoxic uterus are exposed to normoxia at birth. We report here differential decrease of hematocrits in perinatal period in studied genotypes but to a greater degree in mtHEPOR mice (Fig. 1). We could then demonstrate that hypoxic exposure of adult mice bearing these three different genotypes when followed by rapid normoxic change was also accompanied by similar changes of hematocrits mimicking those we observed in the perinatal life of these mice (Fig. 5A). We can only speculate that the relatively moderate hematocrit decrease in the wtHEPOR neonates could be a result of a protective effect of Epo on neocytolytic process of young erythrocytes [21] – and we show here anemic wtHEPOR newborns have greatly increased Epo levels (Fig. 5B). In contrast, mtHEPOR mice have low Epo levels and exhibit greater decrease of hematocrit following their birth. Increased phosphatidylserine exposure on the membrane of mtHEPOR erythrocytes (Fig. 6) in the perinatal period is congruent with accelerated destruction of these cells by macrophages [22, 23], thus contributing to the fall of hematocrit in these neonatal mice. However, because the hematocrit in perinatal period is significantly lower in mEpoR mice compared to mtHEPOR mice notwithstanding comparable degree of phosphatidylserine exposure on their RBCs, it is likely that other biochemical features besides phosphatidylserine exposure are involved in the clearance of hypoxia-born RBCs [24].
In conclusion, we demonstrate that the human gain-of-function EPOR causes fetal polycythemia associated with prolonged primitive erythropoiesis and delayed switch from primitive to definitive erythropoiesis and transient lack of polycythemia in perinatal life. In addition, we demonstrate that wtHEPOR embryos are anemic, and that this anemia is a consequence of decreased STAT5 activation in wtHEPOR FLCs. A transient correction of polycythemia in mtHEPOR newborns may result from neocytolysis.
Supplementary Material
Key Messages.
Human gain-of-function EPOR (mtHEPOR) causes fetal polycythemia in knock-in mice.
Wild-type human EPOR causes fetal anemia in knock-in mouse model.
mtHEPOR mice have delayed switch from primitive to definitive erythropoiesis.
Polycythemia of mtHEPOR mice is transiently corrected in perinatal life.
mtHEPOR newborns have low Epo and increased erythrocytes’ phosphatidylserine.
Acknowledgments
The study was supported by VAH Merit Review Award (DY and JTP). VD and MH were supported by the Czech Science Foundation (project P301-12-1503) and by the European Commission (project CZ.1.07/2.3.00/20.0164). We thank Merav Socolovsky (University of Massachusetts), Paul Kingsley, and James Palis (Rochester University) for helpful advice and Zuzana Korbasova (Palacky University) for mouse genotyping.
Footnotes
Disclosure Statement:
The authors declare no conflict of interest.
References
- 1.Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 2.Kralovics R, Prchal JT. Congenital and inherited polycythemia. Curr Opin Pediatr. 2000;12:29–34. doi: 10.1097/00008480-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrath KE, Frame JM, Fromm GJ, Koniski AD, Kingsley PD, Little J, Bulger M, Palis J. A transient definitive erythroid lineage with unique regulation of the β-globin locus in the mammalian embryo. Blood. 2011;117:4600–4608. doi: 10.1182/blood-2010-12-325357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingsley PD, Malik J, Emerson RL, Bushnell TP, McGrath KE, Bloedorn LA, Bulger M, Palis J. “Maturational” globin switching in primary primitive erythroid cells. Blood. 2006;107:1665–1672. doi: 10.1182/blood-2005-08-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Divoky V, Liu Z, Ryan TM, Prchal JF, Townes TM, Prchal JT. Mouse model of congenital polycythemia: Homologous replacement of murine gene by mutant human erythropoietin receptor gene. Proc Natl Acad Sci U S A. 2001;98:986–991. doi: 10.1073/pnas.98.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song J, Yoon D, Christensen RD, Horvathova M, Thiagarajan P, Prchal JT. HIF-mediated increased ROS from reduced mitophagy and decreased catalase causes neocytolysis. J Mol Med (Berl) 2015;93:857–66. doi: 10.1007/s00109-015-1294-y. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 9.Yoon D, Watowich SS. Hematopoietic cell survival signals are elicited through non-tyrosine-containing sequences in the membrane-proximal region of the erythropoietin receptor (EPOR) by a Stat5-dependent pathway. Exp Hematol. 2003;31:1310–1316. doi: 10.1016/j.exphem.2003.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang KS, Roll B, Myssina S, Schittenhelm M, Scheel-Walter HG, Kanz L, Fritz J, Lang F, Huber SM, Wieder T. Enhanced erythrocyte apoptosis in sickle cell anemia, thalassemia and glucose-6-phosphate dehydrogenase deficiency. Cell Physiol Biochem. 2002;12:365–372. doi: 10.1159/000067907. [DOI] [PubMed] [Google Scholar]
- 12.Divoky V, Prchal JT. Mouse surviving solely on human erythropoietin receptor (EpoR): model of human EpoR-linked disease. Blood. 2002;99:3873–3874. doi: 10.1182/blood-2002-01-0301. author reply 3874–3875. [DOI] [PubMed] [Google Scholar]
- 13.Rice L, Alfrey CP. The negative regulation of red cell mass by neocytolysis: physiologic and pathophysiologic manifestations. Cell Physiol Biochem. 2005;15:245–250. doi: 10.1159/000087234. [DOI] [PubMed] [Google Scholar]
- 14.Kingsley PD, Malik J, Fantauzzo KA, Palis J. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004;104:19–25. doi: 10.1182/blood-2003-12-4162. [DOI] [PubMed] [Google Scholar]
- 15.Malik J, Kim AR, Tyre KA, Cherukuri AR, Palis J. Erythropoietin critically regulates murine and human primitive erythroblast survival and terminal maturation. Haematologica. 2013;98:1778–1787. doi: 10.3324/haematol.2013.087361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser ST, Isern J, Baron MH. Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression. Blood. 2007;109:343–352. doi: 10.1182/blood-2006-03-006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin CS, Lim SK, D’Agati V, Costantini F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 18.Paffett-Lugassy N, Hsia N, Fraenkel PG, Paw B, Leshinsky I, Barut B, Bahary N, Caro J, Handin R, Zon LI. Functional conservation of erythropoietin signaling in zebrafish. Blood. 2007;110:2718–2726. doi: 10.1182/blood-2006-04-016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebie AZ, Fleming KG. Dimerization of the erythropoietin receptor transmembrane domain in micelles. J Mol Biol. 2007;366:517–524. doi: 10.1016/j.jmb.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Halvorsen S, Bechensteen AG. Physiology of erythropoietin during mammalian development. Acta Paediatr Suppl. 2002;91:17–26. doi: 10.1111/j.1651-2227.2002.tb02901.x. [DOI] [PubMed] [Google Scholar]
- 21.Risso A, Ciana A, Achilli C, Minetti G. Survival and senescence of human young red cells in vitro. Cell Physiol Biochem. 2014;34:1038–1049. doi: 10.1159/000366319. [DOI] [PubMed] [Google Scholar]
- 22.Kuypers FA, de Jong K. The role of phosphatidylserine in recognition and removal of erythrocytes. Cell Mol Biol (Noisy-le-grand) 2004;50:147–58. [PubMed] [Google Scholar]
- 23.Risso A, Turello M, Biffoni F, Antonutto G. Red blood cell senescence and neocytolysis in humans after high altitude acclimatization. Blood Cells Mol Dis. 2007;38:83–92. doi: 10.1016/j.bcmd.2006.10.161. [DOI] [PubMed] [Google Scholar]
- 24.Risso A, Ciana A, Achilli C, Antonutto G, Minetti G. Neocytolysis: none, one or many? A reappraisal and future perspectives. Front Physiol. 2014;14:54. doi: 10.3389/fphys.2014.00054. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.