Abstract
Introduction
Bevacizumab plus carboplatin-paclitaxel (BCP) chemotherapy has FDA approval for advanced non-squamous, non-small cell lung cancer (NS-NSCLC) based upon improved survival in a clinical trial. However, sub-group analyses of this and other studies have suggested variable results by age and gender.
Methods
1,605 HMO NS-NSCLC patients aged ≥ 21 years, diagnosed 2002–2010, who received carboplatin-paclitaxel (CP), with and without bevacizumab for first-line treatment of stage IIIB/IV disease were identified. Patients were categorized into three groups based on year of diagnosis and regimen during 120 days post-diagnosis: 1) diagnosed 2005–2010 and received BCP; 2) 2005–2010, CP (CP2005), and 3) 2002–2004, CP (CP2002). Survival differences between groups were estimated using Cox proportional hazard models with several propensity score adjustments for demographic, comorbidity, and tumor characteristics. Multi-variable sub-analyses were also estimated.
Results
Median survival was 12.3 months (inter quartile range [IQR] 6.0–29.1) for BCP patients versus 8.8 months (IQR 3.7–21.3) for CP2005 patients, and 7.5 months (IQR 3.8–15.6) for CP2002 patients. In the propensity score adjusted models, BCP demonstrated a significant survival benefit with a hazard ratio of BCP relative to CP2005 and CP2002 patients of 0.79 (95% CI 0.66–0.94) and 0.63 (95% CI 0.52–0.75) respectively. In the multivariable-adjusted sub-analyses, relative to the CP2005 cohort, the BCP hazard ratios for patients age <65 years, age ≥65 years, and females were 0.78 (95% CI 0.62–1.00), 0.74 (95% CI 0.54–1.00) and 0.77 (95% CI 0.58–1.00).
Conclusions
In this community-based, comparative effectiveness analysis, we found an overall survival benefit for adults receiving BCP compared to CP.
Keywords: non-squamous, non-small cell, chemotherapy, comparative effectiveness, overall survival
INTRODUCTION
Non-squamous, non-small cell lung cancer (NS-NSCLC) accounts for more than half of lung cancers in the United States, and the majority of these cases are diagnosed in advanced stages after a surgical cure is no longer feasible.1;2 Chemotherapy incrementally has improved both response and survival rates in patients with advanced, incurable lung cancer.3 Various chemotherapy regimens, mostly based on platinum-based doublets with and without third generation agents, have been shown to increase survival by upwards of two months in patients with advanced (stage IIIB–IV) NSCLC.4 In this context, where there are no realistic goals of cure, the gains in survival and potential for palliation must be carefully balanced against the significant toxicities and costs of chemotherapy.
The anti-angiogenic monoclonal antibody bevacizumab (Avastin®, Genentech/Roche, San Francisco, California) was initially approved by the FDA in 2004 for metastatic colorectal cancer. In October 2006, bevacizumab received a label extension for administration in combination with carboplatin-paclitaxel (CP) for first-line treatment of advanced lung cancer.5 Approval was granted based on a randomized trial, conducted by the Eastern Cooperative Oncology Group (ECOG 4599) of 878 patients with advanced NS- NSCLC, that demonstrated a significant survival benefit with hazard ratio of 0.79 (95% CI, 0.67–0.92) and a progression-free-survival benefit.5–7 In unplanned sub-group analyses, the original trial did not find a significant survival benefit for patients age ≥65 years or for female patients6;8;9 However, a European Union randomized study showed no improvement in overall survival (OS) by adding bevacizumab to other platinum based therapy,10 and a recent study phase II study (JO19907) found that the addition of bevacizumab to first-line CP significantly improved progression free survival (PFS) in Japanese patients with advanced non-squamous NSCLC, but the addition of bevacizumab did not translate into an OS benefit.11 Moreover, a recent retrospective cohort comparative effectiveness study using SEER-Medicare linked data did not find a survival benefit when bevacizumab was added to combination CP for elderly patients with NS-NSCLC.12
Studies using the SEER-Medicare data link suggest that only 25% to 38% of older patients diagnosed with advanced NSCLC receive chemotherapy.12–14 Given the modest improvement in survival probabilities associated with most chemotherapy agents coupled with the associated treatment toxicity, treatment for advanced NSCLC may be considered a “preference sensitive” decision. However, a study examining the use of chemotherapy for patients with advanced NSCLC who receive their care in four HMOs that participate in the Cancer Research Network (CRN) found that for the years 2000–2007, 64% of patients age <65 years, and 46% of patients age ≥65 years received chemotherapy.15 This study also found that doublet regimens containing cisplatin or carboplatin plus a taxane (docetaxel/paclitaxel) were the most common first-line chemotherapy regimens across all study years, but significant increases were found in triplet regimen use after 2005. By 2007, bevacizumab-carboplatin-paclitaxel (BCP) was the most common triplet, and comprised 11% of all first-line treatment.
U.S studies that describe treatment patterns for patients younger than those captured in SEER-Medicare, or for those that receive their care outside of a fee-for-service setting are lacking. While clinical trial data provide information on treatment efficacy and toxicity of these agents, variation in uptake, use, and outcomes for patients with NSCLC has primarily been studied in the SEER-Medicare patient population, using data prior to 2007. HMO-based community practice comparative effectiveness research (CER) studies, juxtaposed relative to SEER-Medicare studies, can provide needed data that may be more generalizable than clinical trial data where strict inclusion/exclusion criteria and standardized treatment protocols limit the populations under study. In addition, because of the very different financial incentives experienced by providers in the HMO and the fee-for-service settings, parallel analyses conducted in both settings may be of significant clinical and policy relevance.
By employing methods and models consistent with those used by Zhu and colleagues,12 this study complements their SEER-Medicare based study and bridges the gap in the literature associated with patients across all adult ages with advanced NS-NSCLC who receive care in an HMO setting. Using data derived from the Virtual Data Warehouse (VDW) at four HMOs participating in the National Cancer Institute (NCI)-funded Cancer Research Network (CRN) (http://crn.cancer.gov/), and employing methods to address selection bias, we examined whether the addition of bevacizumab to first-line carboplatin-paclitaxel therapy was associated with a significant survival benefit for adult patients with stage IIIB–IV NS-NSCLC.
METHODS
Research Setting
Patients included in this study received their care at four non-profit HMOs (the Colorado, Northern California, and Northwest regions of Kaiser Permanente, and Group Health Cooperative). In each of these health plans, the majority of cancer care was delivered by salaried physicians in plan-owned facilities. This study was approved by the Institutional Review Boards of the four participating HMOs.
Data Sources
As described in detail elsewhere, the VDW is a standardized data model that was developed for research use across the CRN.15;16 Within the VDW, the Virtual Tumor Registry (VTR) contains data consistent with the North American Association of Central Cancer Registries standards.17 VTR data are derived from manual reviews of cancer patients’ medical charts by trained abstractors and include coded clinical data associated with inpatient and outpatient events, date of diagnosis, first-course definitive treatment (surgery, radiotherapy, chemotherapy, etc.), tumor characteristics, and patient demographic characteristics—including race and ethnicity. VDW diagnosis and procedure files include coded diagnoses and procedures associated with inpatient and outpatient encounters or events, including cancer treatment-related surgery, radiotherapy, and chemotherapy that were extracted from EMRs and other claims databases. Codes were based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), Healthcare Common Procedure Coding System (HCPCS), and the Fourth Edition of the Common Procedure Terminology codes (CPT-4). The VDW pharmacy and infusion files capture National Drug Code (NDC) based prescription drugs dispensed from both outpatient pharmacies and infusion centers. The VDW Census files include ecological surrogates for median family income and education derived from mapping patients’ residential addresses to census data. VDW Death data are derived from the tumor registries, membership data, state level (CA, CO, OR, WA) death datasets, and data from the Social Security Administration.
Study Sample
The study sample included patients identified in the VTR as aged 21 years and older, diagnosed with stage IIIB/IV NSCLC between 01/01/2002 and 12/31/2010, and followed through December 31, 2011. Consistent with the criteria described in Zhu and colleagues,12 eligibility for these analyses was limited to patients with health plan enrollment at the time of cancer diagnosis, first cancer diagnosis, survival of at least 1 month after cancer diagnosis, and a pathologically confirmed diagnosis. Patients were excluded if they were diagnosed with squamous cell types or if they were receiving concurrent radiation and chemotherapy. Patients were followed from cancer diagnosis until death, health plan termination, or the end of the study; whichever came first.
Our previous research15 showed use of bevacizumab began in 2005; after FDA approval for use metastatic colorectal cancer, but prior to the 2006 label extension for administration in combination with carboplatin-paclitaxel (CP) for first-line treatment of advanced lung cancer. Therefore, our primary comparison groups were patients diagnosed in 2005–2010 who received first-line CP (CP2005), and patients diagnosed in 2005–2010 who received first-line BCP. Using methods consistent with Zhu and colleagues,12 we defined a second control group composed of patients diagnosed in 2002–2004 (CP2002) who received CP.12 This second control group was created to minimize selection bias caused by the exposure of patients to either BCP or CP in 2005–2010 that may have been based on unobserved patient characteristics associated with survival outcomes.
Identification of First-line Carboplatin, Paclitaxel, and Bevacizumab
Eligible patients receiving first-line CP with or without bevacizumab were identified using VDW Pharmacy, Procedure, and Infusion files using methods described previously.15;16;18 First-line therapy was defined as the first chemotherapy regimen identified within 120 days of cancer diagnosis. All chemotherapy agents administered within eight days of the chemotherapy start date were considered to be part of the same regimen. Patients with concurrent administration of chemotherapy and radiotherapy, defined as start dates within 14 days of each other, were excluded. Patients receiving CP or BCP as a second line regimen were not included in this analysis.
Survival Outcomes
The primary outcome was all-cause mortality, defined as the number of months from the start of first-line chemotherapy until death. The VDW Death files for the HMOs included in this study captured death through December 31, 2011. Patients who dis-enrolled or were alive at the end of the study period were censored.
Baseline Characteristics
The VTR was used to identify the age of the patient at diagnosis, sex, race/ethnicity, tumor grade, and stage. The presence of specific comorbidities was determined using the Quan adaptation of the Charlson comorbidity index, modified to exclude cancer diagnoses.19 The algorithm was applied to diagnoses associated with inpatient and outpatient events that occurred in the 13 months prior to cancer diagnosis. Surrogate patient-level measures of socioeconomic status were obtained from VDW Census files. For patients diagnosed after 2005, we also captured other known pre-treatment negative predictor factors or possible exclusion criteria for BCP treatment6 including bleeding, thrombosis, use of anti-coagulants, brain metastasis, active cardio-vascular disease, etc.
Statistical Analysis
Analyses were performed comparing the BCP group to both the CP2005 group and the CP2002 group. Differences in baseline characteristics between the groups were evaluated using the χ2 test. Medians and interquartile ranges of time to death were estimated using the Kaplan-Meier method and compared between groups with log rank tests.20 Given that one goal of this study was to juxtapose CRN VDW findings relative to those derived using SEER-Medicare data, we used models consistent with those described in Zhu et al,12 we initially estimated unadjusted and multivariate-adjusted Cox proportional hazard models, adjusting for similar baseline characteristics, to evaluate the effect of the addition of bevacizumab on survival. We also performed several propensity score analyses to balance the observed characteristics of patients non-randomly assigned to different treatments.21–23 Logistic regression was used to obtain a propensity score adjusted for age, sex, gender, health plan, education, AJCC stage, tumor grade, and comorbidity score. To adjust for the differences between groups, we estimated Cox proportional hazard models that included the propensity score.24 BCP and CP patients were also matched 1:1 on the logit of the propensity score using greedy nearest-neighbor matching with a 0.02 caliper.25;26 Lastly, a stabilized inverse probability weighting (IPW) analysis by creating stabilized inverse probability weights from the preliminary inverse probability weights to reduce the possibility of large changes to estimates being caused by a few, unusual observations.21;27 These weights were then incorporated into the Cox models.
Several different multivariable sub-group analyses were performed by limiting the cohort to a) patients less than 65 years; b) patients 65 years and older; c) female patients only, consistent with Brahmer and colleagues;9 d) stage IV patients in order to investigate the imbalance in stage, which was more prevalent in the BCP group; e) patients with no comorbidities to indirectly address issues related selection bias; and f) patients diagnosed prior to 2010 to address any potential issues related to immortal time bias given the secular increase in the use bevazicumab.28 A second set of propensity score models were also re-estimated comparing the BCP group to the CP2005 group to to include additional negative predictor factors for BCP treatment. In addition, models and subgroup analyses were also re-estimated separately for patients less than 65 years and patients 65 years and older. All analyses were performed using SAS 9.2 (SAS Software Inc, Cary, NC).
RESULTS
Cohort Description and Baseline Characteristics
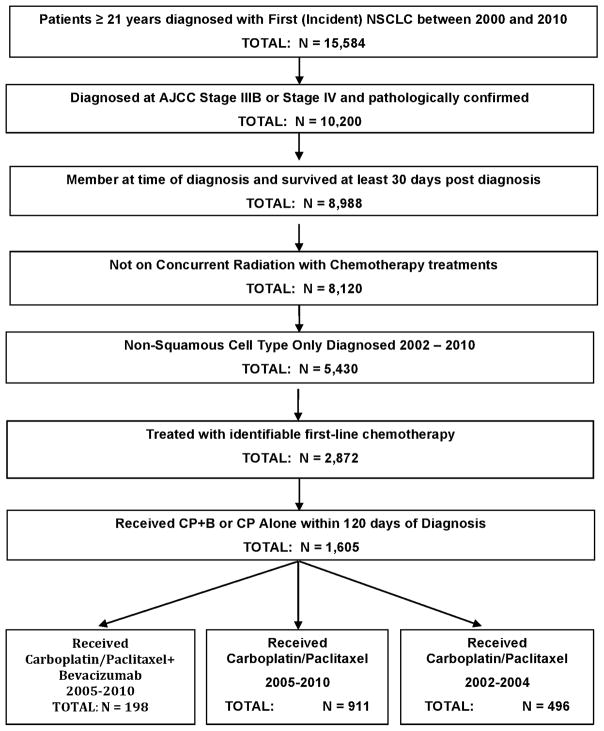
Of the 5,430 patients diagnosed with stage IIIB or IV NS-NSCLC between 2002 and 2010 who met the inclusion/exclusion criteria (see Figure 1), 2,872 (52.9%) received identifiable chemotherapy, and 1,605 patients (55.9% of those treated) received first-line treatment with CP or BCP. Within this cohort, 496 patients (31%) were diagnosed between 2002 and 2004 and received CP (CP2002 group); 911 patients (57%) were diagnosed between 2005 and 2010 and received CP (CP2005 group); and 198 patients (12%) were diagnosed between 2005 and 2010 and received bevacizumab plus CP (BCP group). As noted in Table 1, relative to either CP-only group, patients receiving BCP were significantly younger, more likely to have stage IV disease, and more likely to have a well—or moderately—differentiated tumors. There were no significant differences in the distribution of race/ethnicity, gender, comorbidities, income, and education, between the BCP and CP groups. For the negative predictor factors that were noted as exclusion criteria in the original ECOG4599 trial,6 statistically significant differences in three criteria or diagnoses were noted between the BCP and CP2005 groups. Specifically, the BCP group had a higher proportion of patients diagnosed with ischemic heart disease (3.0% vs 1.1%, p = 0.05), atrial fibrillation (3.0 vs 7.0, p = 0.04), and hypertension (42.4 vs 52.0 p = 0.01). No differences were found for pre-treatment diagnosis of hemoptysis, CNS metastases, history of hemorrhagic diathesis, coagulopathy or therapeutic anticoagulation.
FIGURE 1.
Selection of Patients for Lung Cancer Treatment Cohort
TABLE 1.
Characteristics of IIIB/IV Non-Squamous NSCLC patients in the three treatment cohorts
| Bevacizumab Carboplatin-Paclitaxel 2005–2010 (n = 198) | Carboplatin-Paclitaxel 2005–2010 (n = 911) | Carboplatin-Paclitaxel 2002–2004 (n = 496) | |||
|---|---|---|---|---|---|
|
| |||||
| Characteristics | No. (%) | No. (%) | P Value* | No. (%) | P Value* |
| Age at diagnosis, years | |||||
| < 60 | 89 (44.9) | 281 (30.8) | < 0.001 | 164 (33.1) | 0.02 |
| 60 – 64 | 37 (18.7) | 163 (17.9) | 90 (18.1) | ||
| 65 – 69 | 37 (18.7) | 170 (18.7) | 111 (22.4) | ||
| 70 – 74 | 20 (10.1) | 143 (15.7) | 63 (12.7) | ||
| 75 + | 15 (7.6) | 154 (16.9) | 68 (13.7) | ||
| Age at diagnosis, years | |||||
| < 65 | 126 (63.6) | 444 (48.7) | < 0.0001 | 254 (51.2) | 0.003 |
| 65 + | 72 (36.4) | 467 (51.3) | 242 (48.8) | ||
| Gender | |||||
| Female | 96 (48.5) | 448 (49.2) | 0.85 | 253 (51.0) | 0.54 |
| Male | 102 (51.5) | 463 (50.8) | 243 (49.0) | ||
| Race Ethnicity | |||||
| White | 155 (78.3) | 691 (75.9) | 0.43 | 352 (71.0) | 0.06 |
| Hispanic | < 6 | 38 (4.2) | 36 (7.3) | ||
| Black | 11 (5.6) | 69 (7.6) | 39 (7.9) | ||
| Asian/Pacific Islander | 22 (11.1) | 94 (10.3) | 53 (10.7) | ||
| Other race | < 6 | 19 (2.1) | 16 (3.2) | ||
| % college educated (census tract quintile) | |||||
| 1 (lowest) | 30 (15.2) | 194 (21.3) | 0.17 | 96 (19.4) | 0.41 |
| 2 | 50 (25.3) | 176 (19.3) | 96 (19.4) | ||
| 3 | 38 (19.2) | 186 (20.4) | 97 (19.6) | ||
| 4 | 42 (21.2) | 175 (19.2) | 104 (21.0) | ||
| 5 (highest) | 38 (19.2) | 180 (19.8) | 103 (20.8) | ||
| Median income (census tract quintile) | |||||
| 1 (lowest) | 36 (18.2) | 183 (20.1) | 0.47 | 102 (20.6) | 0.46 |
| 2 | 44 (22.2) | 184 (20.2) | 93 (18.8) | ||
| 3 | 47 (23.7) | 174 (19.1) | 99 (20.0) | ||
| 4 | 38 (19.2) | 186 (20.4) | 98 (19.8) | ||
| 5 (highest) | 33 (16.7) | 184 (20.2) | 104 (21.0) | ||
| Modified Charlson comorbidity score | |||||
| 0 | 121 (61.1) | 505 (55.4) | 0.16 | 329 (66.3) | 0.37 |
| 1 | 49 (24.7) | 227 (24.9) | 112 (22.6) | ||
| 2+ | 28 (14.1) | 179 (19.6) | 55 (11.1) | ||
| AJCC Stage at Diagnosis | |||||
| IIIB | 30 (15.2) | 189 (20.7) | 0.07 | 135 (27.2) | < 0.01 |
| IV | 168 (84.8) | 722 (79.3) | 361 (72.8) | ||
| Level of differentiation (tumor grade) | |||||
| Well-moderately | 44 (22.2) | 110 (12.1) | < 0.001 | 67 (13.5) | < 0.01 |
| Poorly/undifferentiated | 32 (16.2) | 201 (22.1) | 118 (23.8) | ||
| Unknown | 122 (61.6) | 600 (65.9) | 311 (62.7) | ||
|
| |||||
| Exclusion criteria noted in the ECOG4599 Trial6 that were captured prior to chemotherapy initiation** | |||||
|
| |||||
| Hemoptysis | 7 (3.5) | 53 (5.8) | 0.20 | N/A | N/A |
| CNS Metastases | < 6 | < 6 | 0.83 | N/A | N/A |
| History of Hemorrhagic Diathesis, Coagulopathy or Therapeutic Anticoagulation | <6 | 9 (1.0) | 0.61 | N/A | N/A |
| Use of Aspirin | 6 (3.0) | 43 (4.7) | 0.29 | N/A | N/A |
| Stroke | <6 | 41 (4.5) | 0.21 | N/A | N/A |
| Ischemic Heart Disease | 6 (3.0) | 10 (1.1) | 0.05 | N/A | N/A |
| Atrial Fibrillation | 6 (3.0) | 64 (7.0) | 0.04 | N/A | N/A |
| Angina | 6 (3.0) | 37 (4.1) | 0.50 | N/A | N/A |
| Hypertension | 84 (42.4) | 474 (52.0) | 0.01 | N/A | N/A |
p-values for comparisons with bevacizumab-carboplatin-paclitaxel cohort
Specific ICD9 diagnosis codes used are available from the author upon request.
Survival Outcomes
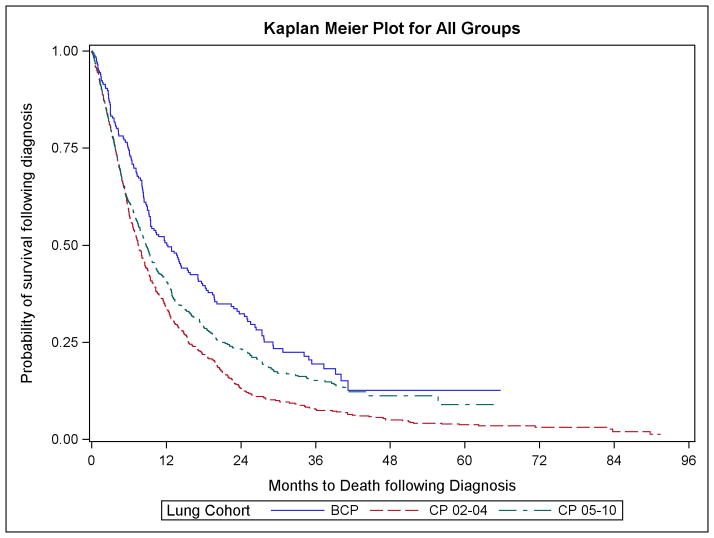
Kaplan-Meier survival curves are shown in Figure 2. Cude median survival estimates by patient characteristics are noted in Table 2. Overall median survival was 12.3 months (inter quartile range [IQR] 6.0 – 29.1) for the BCP patients as compared to 8.8 months (IQR 3.7–21.3) for the CP2005 group and 7.5 months (IQR 3.8–15.6) for the CP2002 group.
FIGURE 2.
Kaplan Meier Curves for All Groups
TABLE 2.
Crude Median Survival Among Patients in the 3 Treatment Cohorts
| Characteristic | Crude Median Survival (IQR), Months | ||
|---|---|---|---|
| Bevacizumab Carboplatin-Paclitaxel 2005–2010 (n = 198) | Carboplatin-Paclitaxel 2005–2010 (n =911) | Carboplatin-Paclitaxel 2002–2004 (n = 496) | |
| Chemotherapy regimen | |||
| Carboplatin-paclitaxel | 8.8 (3.7–21.3) | 7.5 (3.8–15.6) | |
| Beva-carboplatin-paclitaxel | 12.3 (6.0–29.1) | ||
| Age at Diagnosis | |||
| < 60 | 11.8 (5.8–25.5) | 9.2 (4.1–25.1) | 7.4 (3.7–15.4) |
| 60–64 | 13.8 (4.3–30.7) | 6.7 (2.7–20.0) | 6.3 (3.1–15.7) |
| 65–69 | 19.7 (8.9–41.2) | 8.4 (3.7–19.9) | 7.6 (4.3–13.3) |
| 70–74 | 9.5 (6.1–18.3) | 8.1 (4.3–18.6) | 8.5 (4.6–19.8) |
| >= 75 | 7.2 (1.8–18.7) | 10.7 (4.4–23.2) | 8.4 (2.7–20.0) |
| Sex | |||
| Female | 14.4 (7.3–34.1) | 10.1 (4.1–26.5) | 9.2 (4.5–19.8) |
| Male | 9.5 (3.4–25.1) | 7.6 (3.6–17.5) | 6.5 (3.5–12.7) |
| Race/Ethnicity | |||
| Non-Hispanic White | 12.9 (8.9–17.2) | 8.1 (7.3–8.9) | 7.4 (6.2–8.5) |
| Other | 12.1 (8.3–19.7) | 10.8 (9.3–13.4) | 8.0 (6.5–10.6) |
| Modified Charlson comorbidity Score | |||
| 0 | 12.9 (6.0–27.7) | 8.8 (3.8–20.7) | 7.6 (3.7–16.1) |
| 1 | 12.1 (5.7–27.6) | 10.1 (4.3–24.4) | 6.7 (4.0–15.4) |
| >= 2 | 13.2 (5.2–40.1) | 7.6 (3.1–18.2) | 9.1 (3.8–15.4) |
| Level of Differentiation (tumor grading) | |||
| Well/moderately | 20.2 (8.1–29.2) | 13.8 (5.1–38.6) | 7.4 (3.8–22.1) |
| Poor/Undifferentiated | 8.4 (3.0–34.9) | 7.8 (3.7–17.5) | 6.8 (3.7–16.1) |
| Unknown | 10.8 (5.2–25.5) | 8.7 (3.6–19.6) | 8.0 (3.8–15.2) |
| AJCC Stage | |||
| IIIB | 10.8 (7.1–22.4) | 12.3 (5.7–27.5) | 11.5 (4.8–21.0) |
| IV | 12.9 (5.4–29.1) | 8.0 (3.5–19.4) | 6.7 (3.6–13.3) |
Consistent with the Kaplan Meier curves in Figure 2, and as described in Table 3, for all adult patients, the adjusted Cox proportional hazards models consistent with those used by Zhu et al, showed a significant protective survival effect for patients receiving the bevacizumab combination relative to both the CP2005 group (HR 0.79, CI 0.66–0.94) and the CP2002 group (HR 0.63, CI 0.52–0.75). Similar estimates were found for the propensity score adjusted models. The protective and statistically significant survival effect of BCP was robust across all model specifications as compared to the CP2002 group. However, as compared to the CP2005 group the protective effect of adding bevacizumab was no longer statically significant for the propensity score stabilized IPTW models. These findings were robust to the model specification of using only patients diagnosed prior to 2010 (i.e., all patients had the opportunity for at least 2 years of follow-up).
TABLE 3.
Effect of Bevacizumab Added to Carboplatin and Paclitaxel Chemotherapy on Hazard Ratios for Overall Survival for Adults with Stage IIIB/IV, Non-squamous NSCLC
| Models | Hazard Ratio (95% CI)
|
|
|---|---|---|
| Bevacizumab Carboplatin-Paclitaxel- vs Carboplatin-Paclitaxel 2005–2010 |
Bevacizumab Carboplatin- Paclitaxel- vs Carboplatin- Paclitaxel 2002–2004 |
|
| Unadjusted modela | 0.79 (0.66–0.94) | 0.63 (0.52–0.75) |
| Multivariable-adjusted modela,b | 0.76 (0.63–0.92) | 0.60 (0.50–0.74) |
| Propensity score-adjusted model a,c | 0.79 (0.66–0.95) | 0.64 (0.52–0.77) |
| Weighting (stabilized IPW) a, d | 0.78 (0.56–1.08) | 0.62 (0.48–0.78) |
| Matching 1:1e | 0.70 (0.49–0.98) | 0.61 (0.47–0.78) |
| Multivariable-adjusted subgroup analyses | ||
| Age < 65 years f, b | 0.78 (0.62–1.00) | 0.62 (0.48–0.80) |
| Age ≥ 65 years g, b | 0.74 (0.54–1.00) | 0.64 (0.46–0.88) |
| Females Only h, b | 0.77 (0.58–1.00) | 0.61 (0.46–0.82) |
| Stage IV i,b | 0.75 (0.61–0.91) | 0.59 (0.48–0.74) |
| Estimated comorbidity score of 0 j,b | 0.76 (0.60–0.97) | 0.67 (0.52–0.85) |
Sample sizes: BCP vs CP 2005–2010 = 198 and 911, BCP vs CP 2002–2004 =198 and 496
The model was adjusted for age at diagnosis, sex, race/ethnicity, health plan, tumor grading, census tract education, modified Charlson comorbidities, and American Joint Commission on Cancer (AJCC) stage.
The propensity of receiving BCP was estimated using a multivariable logistic regression model that included age at diagnosis, sex, race/ethnicity, healthplan, tumor grading, census tract education, modified Charlson comorbidities, and AJCC stage. The propensity score was then included as a predictor in the survival model.
The propensity score was used to create stabilized weights
Sample sizes: BCP vs CP 2005–2010 = 192 and 192, BCP vs CP 2002–2004 =192 and 192, BCP and CP patients were matched based on their propensity score.
Sample sizes: BCP vs CP 2005–2010 = 126 and 444, BCP vs CP 2002–2004 = 126 and 254
Sample sizes: BCP vs CP 2005–2010 = 72 and 467, BCP vs CP 2002–2004= 72 and 242
Sample sizes: BCP vs CP 2005–2010 = 102 and 463, BCP vs CP 2002–2004 =102 and 243 and the model was
Sample sizes: BCP vs CP 2005–2010 = 168 and 722, BCP vs CP 2002–2004 =168 and 361
Sample sizes: BCP vs CP 2005–2010 = 121 and 505, BCP vs CP 2002–2004 =121 and 329
Controlling for demographic and clinical characteristics in the multivariable adjusted Cox proportional hazards models, we also found that relative to CP2005 or CP2002, that BCP was associated with a consistent protective effect, with hazard ratios of less than 1.0, in all sub-analyses including those limited to age less than 65, age 65 years and older, females only, stage IV, and to patients with no comorbidities. Specifically, for patients age less than 65 years, relative to CP2005 and CP2002, the estimated hazard ratios associated with BCP were 0.78 (95% CI 0.62–1.00), and 0.62 (95% CI 0.48–0.80). Similarly, for patients age 65 years and older, relative to CP2005 and CP2002, the estimated hazard ratios associated with BCP were 0.74 (95% CI 0.54–1.00), and 0.64 (95% CI 0.46–0.88).
As noted in Table 4, when the propensity score models comparing BCP to CP2005 were re-estimated to include the negative predictor factors for BCP that were noted as exclusion criteria in the ECOG4599 trial,6 the results noted above held. With exception of the propensity score stabilized IPTW models, the addition of bevacizumab again, showed a statistically significant protective effect with the estimated hazard ratios associated with BCP for the multivariable-adjusted model, the propensity score adjusted model, and the propensity score matching model of 0.80 (95% CI 0.66–0.98), 0.82 (95% CI 0.66–0.999), and 0.63 (95% CI 0.45–0.89), respectively.
Table 4.
Effect of Bevacizumab Added to Carboplatin and Paclitaxel Chemotherapy on Hazard Ratios for Overall Survival for all Adults with Stage IIIB/IV, Non-squamous NSCLC for Patients Diagnosed from 2005–2009.
| Re-estimated Propensity Score Using Exclusion Criteria Noted in Sander et al.6
| |
|---|---|
| Models | Hazard Ratio (95% CI)
|
| Bevacizumab Carboplatin-Paclitaxel- vs Carboplatin-Paclitaxel 2005–2010 | |
| Multivariable-adjusted modela,b | 0.80 (0.66–0.98) |
| Propensity score-adjusted modela,c | 0.82 (0.68–0.99) |
| Weighting (stabilized IPW) a, d | 0.76 (0.55–1.05) |
| Matching 1:1e | 0.63 (0.45–0.89) |
Sample sizes: BCP vs CP 2005–2010 = 198 and 911.
The model was adjusted for age at diagnosis, sex, race/ethnicity, health plan, tumor grading, census tract education, modified Charlson comorbidities, American Joint Commission on Cancer (AJCC) stage, and diagnoses noted in the Sandler exclusion criteria that are noted in Table 2.
The propensity of receiving BCP was estimated using a multivariable logistic regression model that included age at diagnosis, sex, race/ethnicity, health plan, tumor grading, census tract education, modified Charlson comorbidities, and AJCC stage and diagnoses noted in the Sandler exclusion criteria noted in Table 2. The propensity score was then added as a predictor in the survival model.
The propensity score was used to create stabilized weights
Sample sizes: BCP vs CP 2005–2010 = 193 and 193, BCP and CP patients were matched based on their propensity score.
When all multivariate and propensity score adjusted models and subgroup analyses were re-estimated separately for patients less than 65 years and patients 65 years and older, we found similar results (See Supplementary Tables 3 and 4, available on-line). All hazard ratios across all models estimated were less than 1.0. However, we did not find a statistically significant overall survival effect for either age stratification relative to the CP2005 in any of the multivariate or propensity score adjusted models. Relative to the earlier CP2002 group, the protective survival effect was statistically significant for both age stratifications in all adjusted models except for the propensity score matched sample.
DISCUSSION
Using clinical data from four CRN sites, we compared survival outcomes for patients with advanced NS-NSCLC who were treated with carboplatin and paclitaxel with and without the addition of bevacizumab. Our findings echo the results of the ECOG 4599 trial, where bevacizumab was found to improve overall survival in the studied population. This study, while not without limitations, is an important confirmation of the original trial findings in all adult NSCLC patients.
We believe that this study provides important evidence for the selected use of bevacizumab in the community setting. Since the benefit of adding bevacizumab in the ECOG 4599 study was relatively modest, one could hypothesize that outcomes in community practices, where patient selection may be less stringent and monitoring less frequent, that the benefit of bevacizumab might disappear or be diluted. To the contrary, our data shows fairly robust benefit that closely approximates the survival curves seen in ECOG 4599.6
Further, this study represents another important proof of concept for our ability to merge chemotherapy, staging, and survival data from the electronic health records of a large HMO-based population. Inasmuch as these results approximate results seen from a large randomized controlled trial, we believe this validates the use of these tools for future health outcomes and comparative effectiveness research.
Our findings also complement the sub-group analysis from ECOG 4599 and the work of Zhu and colleagues who found that BCP was not associated with better survival among Medicare-aged patients with advanced NS-NSCLC.12 While our estimated hazard ratios for patients age 65 years and older were consistently less than 1.0, our estimates did not reach statistical significance in all models. However, our sample only included 72 patients age 65 years and older who received bevacizumab. Consistent with the SEER-Medicare study, elderly patients who received BCP (versus CP) in the HMO/CRN setting were more likely to be diagnosed with stage IV disease. Conversely, between group differences in the comorbidity burden were not consistent between the two studies. While Zhu and colleagues found that bevacizumab treated patients were less likely to have two or more comorbidities (6.3% vs 16.3%; P< .001), no significant differences were found for elderly HMO/CRN patients receiving bevacizumab versus CP alone (18.1% vs 16.5%; P=0.23). While our findings are somewhat inconclusive regarding bevacizumab in older patients, probably due to the small sample size, they do suggest that in conjunction with the results from the original ECOG 4599 trial, the Yang et al. meta-analysis,29 and the Zhu et al.12 comparative effectiveness study, that consideration may be needed when the question of whether to prescribe bevacizumab in an elderly patient presents itself.
We also found a protective, and in most cases, a significant effect in our sub-group analyses that were limited to females only. There are a number of possible explanations for the lack of efficacy for bevacizumab for females in the ECOG 4599 study, including known and unknown baseline prognostic or clinical factors (e.g., differences in hormone levels, smoking status, or creatinine clearance) or second line therapy.9;30;31 In addition, a recent re-analyses of the ECPG 4599 data found that while women ≥60 years old, treated with chemotherapy live longer than men and younger women, the bevacizumab survival benefit is more pronounced in men of any age and in women age 60 years or younger.32 However, our findings support the current community standard practice of administering adjunctive bevacizumab regardless of gender.
Our findings from sensitivity analyses were robust to the propensity score model inclusion of known negative predictive factors for BCP treatment. However, given that the overall outcome in CP-treated patients is our study was much worse than in the ECOG 4599 trial (8.8 vs 10.3 months), coupled with the fact that eligibility for bevacizumab itself represents a powerful prognostic factor for patients with NS-NSCLC,33 limitations of this study include not being able to determine from the available data whether some of the improvements in overall survival seen in the BCP group should be attributed instead to differences in known (or unknown) but unmeasured treatment and prognostic factors. Specifically, limitations of this study include the absence of measures including weight loss, ECOG performance status, biomarkers, baseline pulmonary function, or the location or number of sites of disease, dose of each chemotherapeutic regimen, median number of cycles of each regiment, use of bevacizumab as maintenance therapy, second line or salvage therapy after CP or BCP, achievement of parity in toxicity-related deaths between the regimens, or disproportionate use of other services, such as additional lines of therapy (i.e., pemetrexed, EGFR-receptor inhibitors, etc.) and/or earlier adoption of palliative care. In addition, variation in OS may be related to stage migration that occurred during the study period. For example, because a PET scan are more able to find micro-metastasis, and PET scans have been more frequently used in clinical practice in recent years,34 stage-shifts between CP2002 and CP2005 might have possibly occurred. Consequently, more minimal stage IV diseases may have been diagnosed in CP2005 than CP2002, resulting in greater improvement in the OS in BCP, when compared to CP2002.
As with the prior comparative effectiveness study employing SEER-Medicare data that was conducted by Zhu and colleagues, this study has other limitations that are consistent with most retrospective, observational studies.35 The study was limited adult insured patients who receive their care in four HMOs located in the western United States. While this cohort may not be representative of all adult NS-NSCLC patients in the United States, relative to the sample of clinical trial participants, it is likely to better reflect the characteristics of patients receiving care in community practices. In addition, with the exception of tumor registry data, all diagnostic data used in this study were derived from coded medical recorded and claims data; not manual chart abstraction.
In conclusion, our study supports the existence of a survival benefit associated with use of bevacizumab among NS-NSCLC in adult patients, regardless of gender. However, this was an observational study, not a replication of the original clinical trial. Bevacizumab is a very expensive drug, with known toxicities and variable response rates. Additional analyses need to be conducted to examine the impact of specific, treatment-related toxicities resulting hospital events and deaths. In addition, future research should include the linkage of patient reported preference and outcome data to detailed treatment and survival data.
Supplementary Material
Acknowledgments
Sources of Funding: Funding for this research was provided by NCI Grant No. RC2 CA148185, Building CER Capacity: Aligning CRN, CMS, and State Resources to Map Cancer Care, Co-PIs: Jane C. Weeks, MD and Debra P. Ritzwoller, PhD; NCI Grant No. R01 CA114204, Medical Care Burden of Cancer: System and Data Issues, PI: Mark C. Hornbrook, PhD; and NCI Cooperative Agreement No. U19 CA79689, Increasing Effectiveness of Cancer Control Interventions (Cancer Research Network), PI: Edward H. Wagner, MD; and from internal operational funds provided by the Kaiser Permanente Center for Effectiveness & Safety Research (CESR).
Funding for this research was provided by NCI Grant No. RC2 CA148185, Building CER Capacity: Aligning CRN, CMS, and State Resources to Map Cancer Care, Co-PIs: Jane C. Weeks, MD and Debra P. Ritzwoller, PhD; NCI Grant No. R01 CA114204, Medical Care Burden of Cancer: System and Data Issues, PI: Mark C. Hornbrook, PhD; and NCI Cooperative Agreement No. U19 CA79689, Increasing Effectiveness of Cancer Control Interventions (Cancer Research Network), PI: Edward H. Wagner, MD, and from internal operational funds provided by the Kaiser Permanente Center for Effectiveness & Safety Research (CESR).
The following staff members provided data processing support for this study: KP Northwest: Jenny Stabb and Michael Zimmerman, KP Northern California: Karl Huang and Valarie Lee; Group Health: Arvind Ramaprasan. Barbara McCray provided editorial assistance. We would like to acknowledge the contributions of Drs. Jane Weeks and Deborah Schrag for their comments and suggestions associated with earlier drafts of this manuscript.
Footnotes
The funders did not have any involvements in the design of the study; the collection, analysis, and interpretation of the data; the writing of the article; or the decision to submit the article for publication.
CONFLICT OF INTEREST
There is no conflict of interest by any of the authors of this paper.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. [Accessed August 6, 2013];Lung Cancer (Non-Small Cell) 2010 [Google Scholar]
- 3.NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bareschino MA, Schettino C, Rossi A, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3:122–133. doi: 10.3978/j.issn.2072-1439.2010.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute. [Accessed August 6, 2013];Cancer Drug Information: FDA approval for Bevacizumab. 2012 [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 8.Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26:60–65. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- 9.Brahmer JR, Dahlberg SE, Gray RJ, et al. Sex differences in outcome with bevacizumab therapy: analysis of patients with advanced-stage non-small cell lung cancer treated with or without bevacizumab in combination with paclitaxel and carboplatin in the Eastern Cooperative Oncology Group Trial 4599. J Thorac Oncol. 2011;6:103–108. doi: 10.1097/JTO.0b013e3181fa8efd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reck M, von PJ, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 11.Niho S, Kunitoh H, Nokihara H, et al. Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer. 2012;76:362–367. doi: 10.1016/j.lungcan.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Sharma DB, Gray SW, Chen AB, Weeks JC, Schrag D. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA. 2012;307:1593–1601. doi: 10.1001/jama.2012.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsey SD, Howlader N, Etzioni RD, Donato B. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from surveillance, epidemiology and end results-Medicare. J Clin Oncol. 2004;22:4971–4978. doi: 10.1200/JCO.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Lang K, Marciniak MD, Faries D, et al. Trends and predictors of first-line chemotherapy use among elderly patients with advanced non-small cell lung cancer in the United States. Lung Cancer. 2009;63:264–270. doi: 10.1016/j.lungcan.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Ritzwoller DP, Carroll NM, Delate T, et al. Patterns and predictors of first-line chemotherapy use among adults with advanced non-small cell lung cancer in the cancer research network. Lung Cancer. 2012 Dec;78:245–252. doi: 10.1016/j.lungcan.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritzwoller DP, Carroll N, Delate T, et al. Validation of Electronic Data on Chemotherapy and Hormone Therapy Use in HMOs. Med Care. 2012 Apr 23; doi: 10.1097/MLR.0b013e31824def85. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North American Association of Central Cancer Registries. [Accessed 8-26-11];NAACCR Strategic Management Plan. 2011 [Google Scholar]
- 18.Delate T, Bowles EJ, Pardee R, et al. Validity of Eight Integrated Healthcare Delivery Organizations’ Administrative Clinical Data to Capture Breast Cancer Chemotherapy Exposure. Cancer Epidemiol Biomarkers Prev. 2012 Apr 21;:637–680. doi: 10.1158/1055-9965.EPI-11-1075. Epub 2012 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 20.Cox DR. Partial likelihood. Biometrika. 1975;62:269–276. [Google Scholar]
- 21.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Imai K, Van Dyk AD. Causal inference with general treatment regimes: Generalizing the propensity score. J Am Stat Assoc. 2004;99:854–866. [Google Scholar]
- 23.Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol. 2012;30:4215–4222. doi: 10.1200/JCO.2012.41.6701. [DOI] [PubMed] [Google Scholar]
- 24.Hosmer DW, Jr, Lemeshow S. Applied survival analysis: Regression modeling of time to event data. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- 25.Austin PC, Mamdani MM. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006;25:2084–2106. doi: 10.1002/sim.2328. [DOI] [PubMed] [Google Scholar]
- 26.Gou S, Barth RP, Gibbons C. Propensity score matching strategies for evaluating substance abuse services for child welfare clients. Child Youth Serv Rev. 2006;18:357–383. [Google Scholar]
- 27.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 28.Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 29.Yang K, Wang YJ, Chen XR, Chen HN. Effectiveness and safety of bevacizumab for unresectable non-small-cell lung cancer: a meta-analysis. Clin Drug Investig. 2010;30:229–241. doi: 10.2165/11532260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Wakelee HA, Dahlberg SE, Brahmer JR, et al. Increased benefit from bevaczumab (BEV) in younger women with advanced NSCLC on Eastern Cooperative Oncology Group (ECOG), Abstract #E4599. J Thor Onc Proc. 2008;3:S282. [Google Scholar]
- 31.Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–786. doi: 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 32.Wakelee HA, Dahlberg SE, Brahmer JR, et al. Differential effect of age on survival in advanced NSCLC in women versus men: analysis of recent Eastern Cooperative Oncology Group (ECOG) studies, with and without bevacizumab. Lung Cancer. 2012;76:410–415. doi: 10.1016/j.lungcan.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takagi Y, Toriihara A, Nakahara Y, et al. Eligibility for bevacizumab as an independent prognostic factor for patients with advanced non-squamous non-small cell lung cancer: a retrospective cohort study. PLoS One. 2013;8:e59700. doi: 10.1371/journal.pone.0059700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loggers ET, Wagner EH, Weeks JC, Ritzwoller DP. Advanced imaging among health maintenance organization enrollees with cancer. J Onc Pract. doi: 10.1200/JOP.2013.001258. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsey SD, Sullivan SD, Reed SD, et al. Oncology comparative effectiveness research: a multistakeholder perspective on principles for conduct and reporting. Oncologist. 2013;18:760–767. doi: 10.1634/theoncologist.2012-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.