Abstract
Purpose
The purpose of this study was to define the hematologic response to total splenectomy (TS) or partial splenectomy (PS) in children with hereditary spherocytosis (HS) or sickle cell disease (SCD).
Methods
The Splenectomy in Congenital Hemolytic Anemia (SICHA) consortium registry collected hematologic outcomes of children with CHA undergoing TS or PS to 1 year after surgery. Using random effects mixed modeling, we evaluated the association of operative type with change in hemoglobin, reticulocyte counts, and bilirubin. We also compared laparoscopic to open splenectomy.
Results
The analysis included 130 children, with 62.3% (n = 81) undergoing TS. For children with HS, all hematologic measures improved after TS, including a 4.1 g/dl increase in hemoglobin. Hematologic parameters also improved after PS, although the response was less robust (hemoglobin increase 2.4 g/dl, p < 0.001). For children with SCD, there was no change in hemoglobin. Laparoscopy was not associated with differences in hematologic outcomes compared to open. TS and laparoscopy were associated with shorter length of stay.
Conclusion
Children with HS have an excellent hematologic response after TS or PS, although the hematologic response is more robust following TS. Children with SCD have smaller changes in their hematologic parameters. These data offer guidance to families and clinicians considering TS or PS.
Keywords: Congenital hemolytic anemia, Splenectomy, Hematologic outcomes, Surgical technique
Splenectomy can control select clinical symptoms in severely affected children with congenital hemolytic anemias (CHA) such as hereditary spherocytosis (HS) or sickle cell disease (SCD) [1–5]. However, the risks associated with total splenectomy (TS) such as postsplenectomy sepsis and venous thromboembolism remain major concerns for patients and clinicians [6]. Advances with splenectomy for CHA in the last 2 decades have included laparoscopy [4,7–9] and partial splenectomy (PS) [2,3,5,10]. Although the advantages of laparoscopy are becoming more clear, studying the impact of partial splenectomy is limited by small sample size, rare clinical events, lack of comparison groups, narrowly focused patient populations, and use of nonstandardized data [5,11]. To address this gap in understanding of splenectomy, we formed a clinical research consortium entitled Splenectomy in Congenital Hemolytic Anemia (SICHA), composed of pediatric surgeons and hematologists at 16 sites in North America [12].
With no reports focused on a direct comparison of TS vs. PS, the goal of the current study was to examine the differences between these procedures in a head-to-head evaluation. Our consortium has operated an observational, multicenter registry for children with CHA undergoing different types of splenectomy, and has recently demonstrated excellent clinical outcomes and low risk of adverse events in children undergoing TS or PS [3]. However, the low rate of clinical events limits the ability to identify subtle but important differences between procedures given multiple clinical confounders; therefore, the current report uses random effects mixed modeling to account for confounding variables in this heterogeneous population. We hypothesized that TS and PS would not result in significantly different hematologic outcomes in children with HS or SCD, and used a random effects model to compare outcomes of TS and PS in children with HS and SCD. We also examined hematologic responses over time, and compared outcomes between laparoscopic and open procedures.
1. Methods
1.1. SICHA
The SICHA clinical research consortium was established to provide a research infrastructure for high-quality, standardized data collection to support the study of children with CHA. After input from multiple stakeholders, a data dictionary, data collection system, and prospective study protocol were implemented to develop a Web-linked, observational, prospective patient registry collecting high-fidelity outcomes of children with CHA undergoing different types of splenectomy. The details of registry operations have been previously published [3,12].
1.2. Study population
Children aged 2–17 years with HS or SCD undergoing PS or TS from 2005 to 2014 at one of the 16 SICHA sites were reviewed for the study (n = 130). Patients with CHA classified as “other thalassemia or “other congenital hemolytic anemia” were excluded. As an observational registry, no care was dictated by this study, and the decision for TS or PS was left to the discretion of the family and primary clinicians. Patients with splenectomy for trauma or idiopathic thrombocytopenia purpura were not enrolled. Institutional Review Board approval was obtained from each site, and informed consent was required.
1.3. Study design
We analyzed demographic and disease characteristics, operative techniques, and hematologic outcomes, with hematologic variables collected at baseline and 4, 24, and 52 weeks after surgery. Our main comparison groups were TS vs. PS. Patients converted from PS to TS as well as from laparoscopic to open approach were analyzed in an intention to treat manner. A sensitivity analysis examining PS converted to TS as TS was also performed. Other variables of interest included gender, race/ethnicity, laparoscopic vs. open technique and diagnosis [2,18,19].
1.4. Outcomes
The primary hematologic outcomes were hemoglobin, reticulocyte count, and bilirubin. Secondary outcomes included remnant splenic volume (estimated by surgeon intraoperatively and by follow-up ultrasound), estimated blood loss, length of stay (LOS), postoperative and long-term blood transfusion requirements, postsplenectomy sepsis, and death. All variables have been previously defined [3].
1.5. Statistical analysis
We expressed outcomes using count and percentages for categorical variables and median and interquartile range for continuous variables. To determine significant differences between cohorts, Pearson’s chi-squared or Fisher’s exact test were used as appropriate for categorical data and the Mann–Whitney U test was used for continuous data.
To account for confounding factors and better understand expected hematologic outcomes following different procedures, a random effects mixed model was applied to hematologic outcomes. This allowed us to use multiple time points for each patient, increasing the power to identify associations between case characteristics and hematologic changes [13]. The random effects mixed model included the following variables: gender, race/ethnicity, laparoscopic vs. open technique, PS vs TS, diagnosis, baseline laboratory measures, and weeks from surgery. In addition to the factors above, the association of the hematologic outcomes with weeks after surgery was examined to evaluate for temporal trends in the hematologic response. Results of the mixed model are described as the point estimate in the difference between 2 groups and the 95% confidence interval (CI) around that estimate. It was determined a priori to include an interaction term between diagnosis and response to surgery. If a significant interaction existed, these populations would be analyzed separately. p-Values <0.05 were considered statistically significant, with type I error controlled at the level of comparison. All statistical analyses were performed using R (v. 3.02; R Foundation for Statistical Computing, Vienna, Austria).
2. Results
2.1. Patient characteristics
Of 130 eligible cases in the SICHA registry 120 children (92%) had 4-week follow-up and 81 children (62%) had 1 year follow-up. Most patients in all groups had follow-up through 1 year, with mean follow-up (standard deviation) for each group of interest as follows: total splenectomy, 34 (21) weeks; partial splenectomy, 42 (18) weeks; open splenectomy, 47 (13 weeks); and laparoscopic splenectomy, 33 (21) weeks. The majority of children underwent TS (62%), with the remaining children undergoing PS. SCD made up 53% of children, while 47% had HS (Table 1). The use of PS did not differ by diagnosis; however, patients with splenic sequestration as an indication for surgery were more likely to undergo TS.
Table 1.
Demographic, disease, and operative characteristics.
| Variable | Overall | Total splenectomy | Partial splenectomy | p-Value |
|---|---|---|---|---|
| N | 130 | 81 (62.3%) | 49 (37.7%) | |
| Demographics | ||||
| Gender | 0.93 | |||
| Male | 67 (51.5%) | 42 (51.9%) | 25 (51%) | |
| Female | 63 (48.5%) | 39 (48.1%) | 24 (49%) | |
| Race/Ethnicity | 0.05 | |||
| White | 52 (40%) | 26 (32.1%) | 26 (53.1%) | |
| Black | 69 (53.1%) | 50 (61.7%) | 19 (38.8%) | |
| Hispanic | 7 (5.4%) | 4 (4.9%) | 3 (6.1%) | |
| Other | 2 (1.5%) | 1 (1.2%) | 1 (2%) | |
| Diagnosis | 0.07 | |||
| Hereditary spherocytosis | 61 (46.9%) | 33 (40.7%) | 28 (57.1%) | |
| Sickle cell disease | 69 (53.1%) | 48 (59.3%) | 21 (42.9%) | |
| Indication for surgery | ||||
| Splenic sequestration | 74 (56.9%) | 54 (66.7%) | 20 (40.8%) | <0.01 |
| Transfusion dependence | 28 (21.5%) | 19 (23.5%) | 9 (18.4%) | 0.64 |
| Splenomegaly | 22 (16.9%) | 9 (11.1%) | 13 (26.5%) | 0.04 |
| Hypersplenism | 4 (3.1%) | 2 (2.5%) | 2 (4.1%) | 0.63 |
| Aplastic crisis or anemia | 7 (5.4%) | 4 (4.9%) | 3 (6.1%) | 0.99 |
| Poor growth | 6 (4.6%) | 6 (7.4%) | 0 (0%) | 0.08 |
| Operative characteristics | ||||
| Partial converted to total | 2 (4.2%) | 0 (0%) | 2 (4.2%) | |
| Initial approach | <0.001 | |||
| Open | 37 (28.5%) | 9 (11.1%) | 28 (57.1%) | |
| Laparoscopic | 93 (71.5%) | 72 (88.9%) | 21 (42.9%) | |
| Laparoscopic converted to open | 9 (9.9%) | 3 (4.2%) | 6 (30.0%) | <0.01 |
2.2. Hematologic outcomes
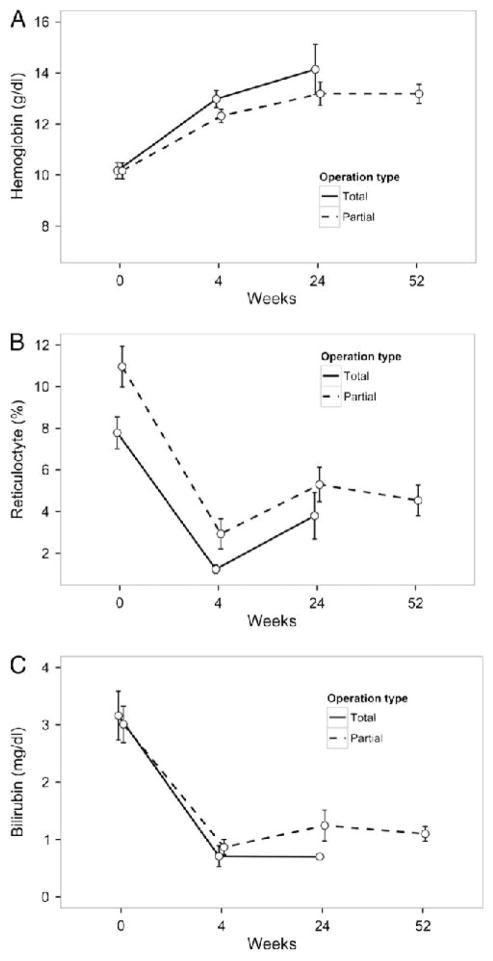
Unadjusted hematologic outcomes are described in Figs. 1 and 2. Using adjusted mixed modelling analysis, we found differences in the hematologic response to surgery between SCD and HS (interaction p < 0.001 for all parameters) and analyzed these groups separately. Children with HS experienced increased hemoglobin (4.1 g/dl; 95% CI: 3.1–5.1 g/dl; p < 0.001), decreased reticulocytes (8.3%; 5.2%–11.4%; p < 0.001), and decreased bilirubin (1.9 mg/dl; 0.5–3.4 mg/dl; p = 0.01) after splenectomy (Fig. 3). Children with HS undergoing laparoscopic surgery (TS or PS) trended toward a smaller increase in hemoglobin compared to open surgery (p = 0.08), increasing their hemoglobin by only 3.3 g/dl. Children with HS who underwent PS had a significantly smaller hemoglobin rise compared to TS (p < 0.001), increasing by only 2.4 g/dl postoperatively. When examining the hemoglobin response over time, we found no changes through 1 year of follow-up, indicating that after the initial increase associated with surgery values remained steady (p = 0.10).
Fig. 1.
Unadjusted hematologic outcomes after partial or total splenectomy in children with hereditary spherocytosis. Data represent hemoglobin (A), reticulocyte count (B), and serum bilirubin (C), at baseline, 4 weeks, 24 weeks, and 52 weeks. Circles represent mean and error bars represent standard error. Because of limited data points available at 52 weeks in children with HS undergoing total splenectomy, data are not displayed.
Fig. 2.
Unadjusted hematologic outcomes after partial or total splenectomy in children with sickle cell disease. Data represent hemoglobin (A), reticulocyte count (B), and serum bilirubin (C), at baseline, 4 weeks, 24 weeks, and 52 weeks. Circles represent mean and error bars represent standard error.
Fig. 3.
Postsplenectomy changes in hematologic parameters for children with congenital hemolytic anemias. Results represent differences in postoperative hematologic values at 4, 24, and 52 weeks (versus baseline), laparoscopic (versus open) splenectomy, and partial (versus total) splenectomy as indicated. Results produced from random-effects mixed modeling including the following covariates: gender, race/ethnicity, laparoscopic vs. open technique, partial vs. total splenectomy. Hereditary spherocytosis and sickle cell disease were analyzed separately. Blue squares represent point estimates and black lines represent 95% confidence intervals. Results are additive such that estimating the change in postoperative hemoglobin after laparoscopic partial splenectomy, the point estimates for postoperative, laparoscopy, and partial splenectomy would need to be added. For example, the postoperative hemoglobin increase in HS is estimated at 4.1 g/dl in open, total splenectomy. This estimate is decreased by 1.7 g/dl in partial splenectomy compared to total splenectomy (estimate displayed) indicating that the expected postoperative hemoglobin in partial splenectomy would only be 2.4 g/dl (equal to 4.1–1.7; not displayed).
In HS, the change in reticulocyte count after surgery was not associated with either PS vs. TS or laparoscopic vs. open technique, meaning that the >8% decrease in reticulocytes was not significantly different among any subgroup. After the initial 8.3% drop in reticulocytes, there was evidence of a rebound of 2.4% (0.7%–4.3%; p = 0.01) over the first 6 months; however, reticulocytes stabilized from 6 months to 1 year (p = 0.87). Similar to the decrease in reticulocytes, the change in bilirubin was not associated with splenectomy type or surgical technique in HS. These changes showed no trends over time (p = 0.34) through 1 year. No HS patients converted from partial to total splenectomy, so no sensitivity analysis was required.
In children with SCD, there was no significant increase in hemoglobin (0.1 g/dl; −0.8 to 1.9 g/dl; p = 0.77) after surgery and no differences when comparing PS to TS or laparoscopy to open splenectomy. Reticulocytes did decrease by 4.2% (0.8%–7.6%; p = 0.02). Use of laparoscopy was not associated with a difference in reticulocytes compared to open surgery; however, PS was associated with a smaller reticulocyte improvement by 3.4% (0.3%–6.5%; p = 0.03) compared to TS. In addition, there was a small decrease in bilirubin by 1.0 mg/dl (0.3–1.7 mg/dl; p = 0.01) after surgery. Laparoscopy showed no difference, although PS resulted in a smaller bilirubin decrease by 0.8 mg/dl (0.1–1.4 mg/dl; p = 0.03) compared to TS. No changes were found over time. In a sensitivity analysis grouping children by procedure performed rather than intention to treat, the same trends were seen; however, the difference in reticulocytes was not statistically significant (p = 0.07), indicating that these results may be sensitive to the rate of conversion from PS to TS.
2.3. Secondary outcomes
PS appeared to have greater estimated blood loss than TS (28% with EBL ≥100 ml vs. 14%), but perioperative transfusions were similar (6.2% vs. 3.8%; p = 0.67) (Table 2). LOS was 1.5 days longer in the PS group (3.5 vs. 2 days, p < 0.001). Long-term transfusion requirements through 1 year were similar between PS and TS (2.1% vs. 5.6%; p = 0.65). By surgeon estimate, patients undergoing PS were left with a median splenic volume of 15% of original (IQR: 10%–15%), with two children (4.2%) converted from PS to TS. PS was also less likely to be done laparoscopic (43% vs. 89%, p < 0.001) and more likely to be converted to open if started laparoscopic compared to TS (30% vs. 4%, p = 0.003). Although there were no deaths in this cohort through 1 year of follow-up, 3 children (4.2%) undergoing total splenectomy experienced postsplenectomy sepsis compared to 0 children undergoing partial splenectomy (p = 0.28).
Table 2.
Secondary outcomes — total vs. partial splenectomy.
| Variable | Overall | Total splenectomy | Partial splenectomy | p-Value |
|---|---|---|---|---|
| N | 130 | 81 (62.3%) | 49 (37.7%) | |
| Estimated blood loss (ml) | 15 (10, 50) | 15 (10, 30) | 25 (10, 100) | 0.03 |
| Estimated blood loss | 0.05 | |||
| <30 ml | 81 (65.3%) | 57 (74%) | 24 (51.1%) | |
| 30–99 ml | 19 (15.3%) | 9 (11.7%) | 10 (21.3%) | |
| 100–199 ml | 11 (8.9%) | 6 (7.8%) | 5 (10.6%) | |
| ≥200 ml | 13 (10.5%) | 5 (6.5%) | 8 (17%) | |
| Perioperative blood transfusion | 6 (4.8%) | 3 (3.8%) | 3 (6.2%) | 0.67 |
| Splenic volume retained, surgeon estimate (%) | 0 (0, 10) | 0 (0,0) | 15 (10,15) | <0.001 |
| Length of stay (days) | 3 (2, 4) | 2 (1,3) | 3.5 (3,4) | <0.001 |
| Long-term transfusion (through 1 year) | 5 (4.2%) | 4 (5.6%) | 1 (2.1%) | 0.65 |
| Sepsis (through 1 year) | 3 (2.5%) | 3 (4.2%) | 0 (0%) | 0.27 |
Continuous data represented by median and interquartile range (IQR).
Laparoscopic splenectomy had higher blood loss than open splenectomy (24% vs. 5.8% of children losing ≥100 ml of blood), but perioperative transfusions showed no difference with 5.6% vs. 2.8% requiring transfusion in laparoscopic and open approaches, respectively (p = 0.67; Table 3). The LOS was longer with use of open technique (3 days) compared to laparoscopy (2 days; p = 0.002). With a median of 15% of retained spleen, there were no differences between remnant volume in laparoscopy vs open technique (p = 0.38). These results were confirmed in 4, 24, and 52 week assessments of splenic remnant by ultrasound.
Table 3.
Secondary outcomes — laparoscopic vs. open splenectomy.
| Variable | Overall | Open splenectomy | Laparoscopic splenectomy | p-Value |
|---|---|---|---|---|
| N | 130 | 37 (28.5%) | 93 (71.5%) | |
| Estimated blood loss (ml) | 15 (10, 50) | 10 (10, 12.5) | 20 (10, 75) | <0.01 |
| Estimated blood loss | 0.03 | |||
| <30 ml | 81 (65.3%) | 30 (85.7%) | 51 (57.3%) | |
| 30–99 ml | 19 (15.3%) | 3 (8.6%) | 16 (18%) | |
| 100–199 ml | 11 (8.9%) | 1 (2.9%) | 10 (11.2%) | |
| ≥200 ml | 13 (10.5%) | 1 (2.9%) | 12 (13.5%) | |
| Perioperative blood transfusion | 6 (4.8%) | 1 (2.8%) | 5 (5.6%) | 0.67 |
| Splenic volume retained, surgeon estimate (%) | 15 (10, 15) | 15 (10, 20) | 15 (10, 15) | 0.38 |
| Length of stay (days) | 3 (2, 4) | 3 (3, 4) | 2 (1, 4) | <0.01 |
| Splenic volume retained, 4 weeks (%) | 16 (10, 22) | 16 (10, 25) | 15 (11, 19) | 0.78 |
| Splenic volume retained, 24 weeks (%) | 15 (11, 27) | 15 (8, 26) | 15 (13, 24) | 0.78 |
| Splenic volume retained, 52 weeks (%) | 17 (10, 29) | 22 (9, 31) | 12 (12, 12) | 0.77 |
| Long-term transfusion (through 1 year) | 5 (4.2%) | 1 (2.7%) | 4 (4.8%) | 0.99 |
Continuous data represented by median and interquartile range (IQR). Patients with total splenectomies were excluded from analysis on retained splenic volume.
3. Discussion
The outcomes of both total and partial splenectomy remain incompletely defined, with most prior studies limited by small samples or lack of comparison groups [12]. Our consortium has developed a multicenter patient registry to better understand the outcomes of these procedures, and we have previously demonstrated excellent clinical outcomes after all types of splenectomy in these children [3]. However, the low rates of clinical events allow limited sensitivity to identify subtle differences between procedures; therefore, we used random effects mixed models to examine these differences and better understand expected hematologic outcomes from various procedures and diagnoses.
All types of splenectomy improve hemoglobin, reticulocyte, and bilirubin measures in children with HS. The hematologic outcomes from the mixed modeling analysis (Fig. 3) correlate well with those displayed in the unadjusted analysis (Figs. 1 and 2). Our current findings confirm most prior studies examining PS for children with HS, which have shown good clinical and hematologic responses to surgery, although the hematologic response to TS is more robust than PS [2,5,10,14–16]. This difference between TS and PS is not unexpected given the remaining splenic remnant effect on hemolysis following PS. In addition, both adjusted and unadjusted analyses demonstrated initial postoperative improvements that remained stable over time. The exception was reticulocyte count which demonstrated a rebound after initial improvement in HS; however, the 6-month and 1-year values continued to be lower than baseline.
Although no significant difference was found in postsplenectomy sepsis, the higher rate of this complication following TS underlines the complexity of the decision to undergo PS or TS. The most appropriate procedure for a given child depends on many variables, such as age, disease severity, genotype, risk of adverse events, and long-term hematologic control, and not all were addressed in this study. The decision between TS and PS is dependent on clinician and family preferences, taking into account all expected risks and benefits. Our current report provides estimates of hematologic response following TS or PS, which should help all stakeholders through this decision-making process.
For children with SCD, our findings confirm most studies which suggest that hemoglobin levels do not change after TS or PS [12]. However these findings are likely confounded by artificially elevated hemoglobin preoperatively because of the common practice of chronic transfusions following sequestration events in very young children with SCD. While reticulocyte count and bilirubin may be more sensitive measures for hematologic improvement in SCD, the small differences that we found in these measures support previous studies in suggesting that splenectomy in SCD should focus on outcomes such as quality of life or adverse events rather than hematologic parameters. We and others have previously confirmed control of important select clinical symptoms in these children with regard to transfusion dependence and sequestration following either TS or PS, which is a major goal for both families and clinicians caring for these children [3,12,17].
Numerous previous studies have compared laparoscopic to open splenectomy in children, finding advantages in postoperative pain control and decreased length of stay (LOS) for children undergoing laparoscopy [4,7–9]. The single study comparing hematologic outcomes for laparoscopic vs. open PS showed similar postoperative hemoglobin but less favorable results in reticulocytes [11]. This prior study also demonstrated a larger splenic remnant in laparoscopy vs open splenectomy, raising concerns that hematologic benefits may depend on the amount of spleen resected, and that extensive resection may not be as easily achieved in laparoscopic technique. Our current study offers reassurance that the hematologic outcomes following laparoscopy for TS or PS are similar to an open approach, and that the retained spleen size is not different between approaches for children undergoing PS. We did confirm the longer LOS for open splenectomy compared to laparoscopy as previously reported [4]. PS has a low but real risk of conversion to total splenectomy estimated at 4%. In addition, this study demonstrates a significantly higher conversion from laparoscopic to open surgery in PS compared to TS (30% vs. 4%), which surgeons and families need to understand when considering these procedures.
This study has the advantage of generalizable results due its prospective and multi-institutional design. Because random effects models allow the use of multiple data points per patient [13], this analytic approach can identify differences among subgroups, as we used an average of 250 data points per hematologic outcome and accounted for important confounding factors. In a therapeutic area that is limited by sample size and rare clinical events, this tool provides sensitive assessment of differences that may exist between patient groups and procedure types.
Despite the use of a prospective, multi-institutional database and multivariable analysis to better define expected hematologic outcomes of TS or PS, our report remains limited by the inherent flaws of observational research and small sample size. The confirmatory nature of our findings to previous studies adds to their validity, but additional unmeasured confounders that impact the decision to perform TS or PS may bias our results. Although mixed modeling allows robust treatment of missing or incomplete data among other advantages previously mentioned, this analytic technique increases the uncertainty around estimates making real differences more difficult to detect. Mixed modeling results can also be challenging to interpret as they rely on comparisons rather than the more intuitive absolute values. Finally, results were limited by patients missing or not yet achieving 1-year follow-up and results being more heavily weighted by earlier time points. The consortium has added follow-up through 5 years to the protocol and hopes that continued contact with these patient and the longer follow-up period will improve future studies.
4. Conclusions
Children with HS undergoing either TS or PS can expect improvements in all hematologic outcomes that are durable through one year, with TS providing a more robust response compared to PS. For children with SCD, hematologic parameters showed lesser changes, although previous reports demonstrate acceptable control of select clinical symptoms after TS or PS. We did not find any difference in splenic remnant volumes or hematologic outcomes after laparoscopic vs. open surgery. These results suggest that total splenectomy may need stronger consideration in children with the most severe hemolytic disorders. Future research will need to examine these outcomes in the context of clinical benefits for children with different diseases.
Acknowledgments
In addition to the authors, the SICHA consortium collaborators include: Brittany Herzberg, Jeffrey M. Ferranti, Sofia Mouttalib, Meredith Nahm, Rachel Richesson, Denise C. Snyder. We thank Terri Ainsworth, Mark Bettger, Ceci Chamorro, Phyllis Kennel, Justin Levens, Joan Wilson and the Duke Office of Clinical Research (DOCR) their assistance. The DOCR is supported by the Duke School of Medicine, made possible through CTSA Grant Number UL1TR001117 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not represent the official view of NCATS or NIH. This work was supported in part by the Duke University Office of Clinical Research and the Duke School of Medicine, made possible through Clinical and Translational Science Awards Grant Number UL1TR001117 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
Footnotes
Level of Evidence: II.
IRB approval: Duke Medicine IRB for Clinical Investigations; IRB ID: Pro00020000; Approval Date: 10/22/2009.
References
- 1.Gallagher PG. Red cell membrane disorders. Hematology Am Soc Hematol Educ Program. 2005:13–8. doi: 10.1182/asheducation-2005.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Bader-Meunier B, Gauthier F, Archambaud F, et al. Long-term evaluation of the beneficial effect of subtotal splenectomy for management of hereditary spherocytosis. Blood. 2001;97:399–403. doi: 10.1182/blood.v97.2.399. [DOI] [PubMed] [Google Scholar]
- 3.Rice HE, Englum BR, Rothman J, et al. Clinical outcomes of splenectomy in children: report of the splenectomy in congenital hemolytic anemia registry. Am J Hematol. 2015;90:187–92. doi: 10.1002/ajh.23888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goers T, Panepinto J, Debaun M, et al. Laparoscopic versus open abdominal surgery in children with sickle cell disease is associated with a shorter hospital stay. Pediatr Blood Cancer. 2008;50:603–6. doi: 10.1002/pbc.21245. [DOI] [PubMed] [Google Scholar]
- 5.Seims AD, Breckler FD, Hardacker KD, et al. Partial versus total splenectomy in children with hereditary spherocytosis. Surgery. 2013;154:849–53. doi: 10.1016/j.surg.2013.07.019. discussion 53–5. [DOI] [PubMed] [Google Scholar]
- 6.Bisharat N, Omari H, Lavi I, et al. Risk of infection and death among postsplenectomy patients. J Infect. 2001;43:182–6. doi: 10.1053/jinf.2001.0904. [DOI] [PubMed] [Google Scholar]
- 7.Reddy VS, Phan HH, O’Neill JA, et al. Laparoscopic versus open splenectomy in the pediatric population: a contemporary single-center experience. Am Surg. 2001;67:859–63. discussion 63–4. [PubMed] [Google Scholar]
- 8.Rescorla FJ, Engum SA, West KW, et al. Laparoscopic splenectomy has become the gold standard in children. Am Surg. 2002;68:297–301. discussion -2. [PubMed] [Google Scholar]
- 9.Farah RA, Rogers ZR, Thompson WR, et al. Comparison of laparoscopic and open splenectomy in children with hematologic disorders. J Pediatr. 1997;131:41–6. doi: 10.1016/s0022-3476(97)70122-7. [DOI] [PubMed] [Google Scholar]
- 10.Rice HE, Oldham KT, Hillery CA, et al. Clinical and hematologic benefits of partial splenectomy for congenital hemolytic anemias in children. Ann Surg. 2003;237:281–8. doi: 10.1097/01.SLA.0000048453.61168.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buesing KL, Tracy ET, Kiernan C, et al. Partial splenectomy for hereditary spherocytosis: a multi-institutional review. J Pediatr Surg. 2011;46:178–83. doi: 10.1016/j.jpedsurg.2010.09.090. [DOI] [PubMed] [Google Scholar]
- 12.Rice HE, Crary SE, Langer JC, et al. Comparative effectiveness of different types of splenectomy for children with congenital hemolytic anemias. J Pediatr. 2012;160:684–9. e13. doi: 10.1016/j.jpeds.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. John Wiley & Sons; 2012. [Google Scholar]
- 14.Idowu O, Hayes-Jordan A. Partial splenectomy in children under 4 years of age with hemoglobinopathy. J Pediatr Surg. 1998;33:1251–3. doi: 10.1016/s0022-3468(98)90161-0. [DOI] [PubMed] [Google Scholar]
- 15.Mouttalib S, Rice HE, Snyder D, et al. Evaluation of partial and total splenectomy in children with sickle cell disease using an Internet-based registry. Pediatr Blood Cancer. 2012;59:100–4. doi: 10.1002/pbc.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morinis J, Dutta S, Blanchette V, et al. Laparoscopic partial vs total splenectomy in children with hereditary spherocytosis. J Pediatr Surg. 2008;43:1649–52. doi: 10.1016/j.jpedsurg.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 17.al-Salem AH, Qaisaruddin S, Nasserallah Z, et al. Splenectomy in patients with sickle-cell disease. Am J Surg. 1996;172:254–8. doi: 10.1016/S0002-9610(96)00158-4. [DOI] [PubMed] [Google Scholar]