Abstract
Rapid-acting bronchodilators, systemic corticosteroids, and antibiotics are among the keys to managing exacerbations of chronic obstructive pulmonary disease. Preventing exacerbations should also be a component of therapy for the disease.
Keywords: chronic obstructive pulmonary disease (COPD), acute exacerbations, bronchodilators, systemic steroids
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by chronic airflow limitation and airway inflammation that is not fully reversible and is progressive in nature.1 COPD is the third-leading cause of death and a considerable cause of disability in the United States.2 In 2011, an estimated 13.7 million Americans had been diagnosed with COPD.2 The disease led to 10.3 million office visits, 1.5 million emergency department (ED) visits, and 699,000 hospitalizations in 2010.2 The economic burden continues to rise; approximately $50 billion was spent in 2010, including $20 billion in indirect costs and $30 billion in direct health care costs.3 A significant portion (50% to 70%) of the direct health care costs related to COPD are attributed to exacerbations.1,3 A recent report indicated an increase in cost with each COPD readmission, ranging from $8,400 to $11,100 based on principal diagnosis and all-cause COPD readmissions, respectively.3
The overall goals of COPD management are to optimize pulmonary function, to prevent progression, to improve quality of life, and to prevent and reduce the frequency and severity of exacerbations.1
An exacerbation of COPD is defined as an event in the natural course of the disease characterized by a change in the patient’s baseline dyspnea, cough, and/or sputum that is beyond normal day-to-day variations; is acute in onset; and may require a change in medication regimen.1 The most common etiologies of COPD exacerbations are bacterial and viral infections. Air pollutants, cigarette smoke, and noncompliance with medication can also result in exacerbations, although the cause is never identified in about one-third of exacerbations. At present, exacerbations are diagnosed based upon the patient’s clinical presentation.
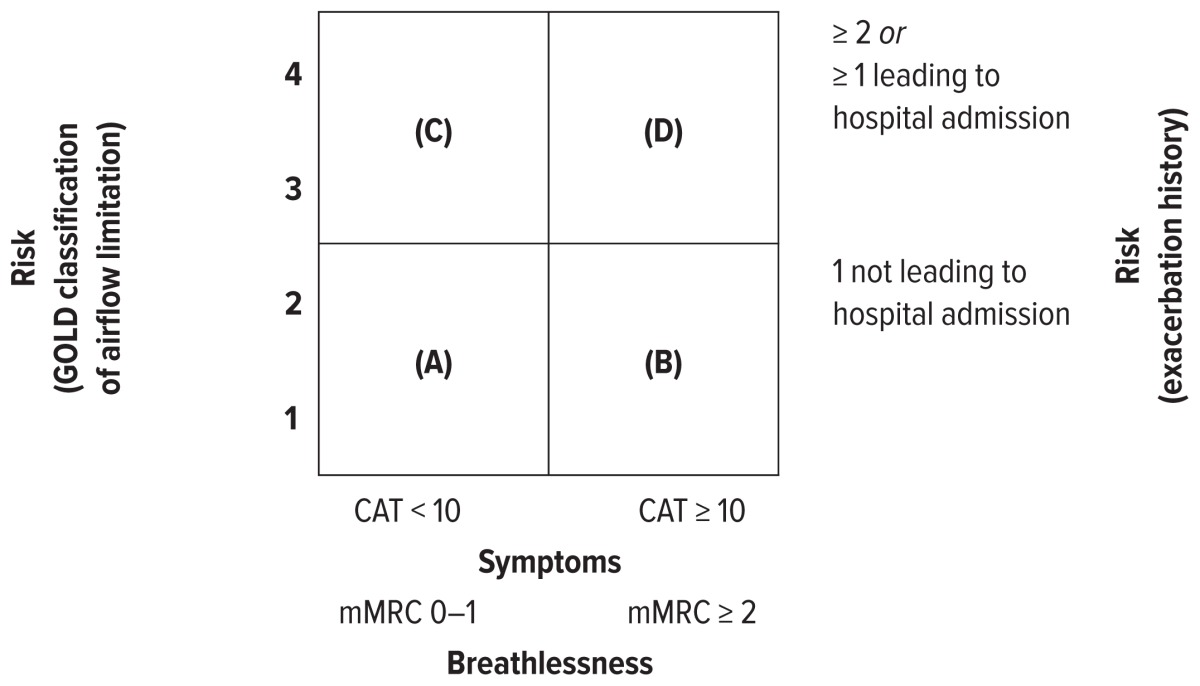
The three cardinal symptoms of COPD exacerbation are increased dyspnea, cough, and purulent sputum production.1 The frequency and severity of exacerbations are among the factors that determine the prognosis of COPD. Exacerbations become more frequent and severe as COPD severity increases (Table 1).1 It is estimated that patients with moderate COPD experience an average of 1.3 exacerbations per year; those with severe COPD experience an average of two exacerbations per year.4 The risk of exacerbations varies greatly among patients. Recent trials have identified several risk factors for exacerbations (Table 2).5,6 It is critical to identify patients at risk for frequent exacerbations and to prevent associated hospital readmissions; this has implications for targeting of exacerbation-prevention strategies across the spectrum of disease severity.5–7
Table 1.
Model of Symptom/Risk Evaluation of COPD1
| When assessing risk, choose the highest risk according to GOLD grade or exacerbation history. One or more hospitalizations for COPD exacerbations should be considered high risk. | |||||
|---|---|---|---|---|---|
| |||||
| Patient Category | Characteristic | Spirometric Classification | Exacerbations Per year | CAT Score | mMRC Score |
| A | Low risk, less symptoms | GOLD 1–2 | ≤ 1 | < 10 | 0–1 |
| B | Low risk, more symptoms | GOLD 1–2 | ≤ 1 | ≥ 10 | ≥ 2 |
| C | High risk, less symptoms | GOLD 3–4 | ≥ 2 | < 10 | 0–1 |
| D | High risk, more symptoms | GOLD 3–4 | ≥ 2 | ≥ 10 | ≥ 2 |
CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; mMRC = Modified Medical Research Council Dyspnea Scale
Table 2.
|
Pharmacological management of an acute exacerbation of COPD (AECOPD) includes rapid-acting bronchodilators; systemic corticosteroids; and, in select patients, antimicrobials. The goals of therapy are prevention of hospitalization or reduction in length of hospital stay, prevention of acute respiratory failure and mortality, resolution of exacerbation symptoms, and resumption of baseline clinical status and quality of life. Recommended strategies for preventing AECOPD include selection of and adherence to optimal pharmacological therapy, smoking cessation, pulmonary rehabilitation, and influenza and pneumococcal vaccination.1
As part of its Hospital Readmissions Reduction Program, the Centers for Medicare and Medicaid Services in 2015 began measuring all-cause readmissions for patients hospitalized for AECOPD, in addition to heart failure, acute myocardial infarction, and pneumonia; hospitals are penalized for excess unplanned 30-day readmissions for exacerbations of COPD.8 However, according to the 2015 American College of Chest Physicians (ACCP) and Canadian Thoracic Society (CTS) guidelines, hospitals currently have little guidance on how to reduce readmissions related to COPD. The 30-day readmission rate may be reduced by pharmacist involvement in transitions of care, medication reconciliation, patient counseling, tracking outpatient adherence, and postdischarge outpatient follow-up.7
This review highlights findings from the recent body of evidence on pharmacological interventions, including bronchodilators, corticosteroids, antibiotics, and preventive strategies for AECOPD.
PHARMACOLOGICAL TREATMENT
Bronchodilators
Bronchodilator therapy is considered to be one of the cornerstones of treating COPD exacerbation. In stable COPD, inhaled antimuscarinic agents and beta2 agonists are often used in combination on either a scheduled and/or as-needed basis for symptom management. Long-acting inhaled bronchodilators, such as tiotropium and salmeterol, offer minimal advantages in AECOPD, with limited published data to support their use; frequently dosed short-acting inhaled bronchodilators are generally used instead. Beta agonists’ onset of action is faster than antimuscarinics with a marginally shorter duration of action. To date, studies have not demonstrated a difference in efficacy between these two classes of drugs; treatment is usually initiated with beta agonists because of their faster onset, with antimuscarinics added if beta agonist monotherapy results in an inadequate response.1,9 Furthermore, trials have shown that beta2 agonists provide benefits similar to antimuscarinic agents with regard to change in forced expiratory volume of air in one second (FEV1); however, optimal doses for AECOPD have yet to be established.9 Bronchodilator dosing during AECOPD includes increasing the dose and/or frequency (every two to four hours) and utilizing combination therapy to optimize symptomatic relief (Table 3).10,11
Table 3.
| Agent | Metered-Dose Inhaler | Nebulizer |
|---|---|---|
| Albuterol | 4–8 puffs every 30 minutes up to 4 hours, then every 1–4 hours as needed | 2.5–5 mg every 20 minutes for 3 doses, then 2.5–10 mg every 1–4 hours as needed, or 10–15 mg per hour continuously |
| Terbutaline | Same as albuterol | N/A |
| Levalbuterol | 4–8 puffs every 20 minutes up to 4 hours, then every 1–4 hours as needed | 0.63–1.25 mg at the same dosing frequency as albuterol |
| Ipratropium | 4–8 puffs as needed | 0.5 mg every 30 minutes for 3 doses, then every 2–4 hours as needed |
Delivery of bronchodilators during AECOPD via metered-dose inhalers with a spacer/holding chamber is considered equivalent to nebulization. Several factors, such as patient preference, disease severity, and ability and willingness to comply with instructions and technique, usually guide the choice of delivery method. According to the recent guidelines, methylxanthines are considered second-line therapy for AECOPD management due to significant side effects and a narrow therapeutic window. Methylxanthines may be considered in patients with insufficient response to inhaled short-acting bronchodilators.1
Corticosteroids
Systemic corticosteroids (sCS) have been shown to improve symptoms and lung function (FEV1), reduce risk of treatment failure and relapse, and reduce the length of hospitalization,1,12,13 although there is a lack of benefit in reduction of mortality rate related to COPD exacerbation.1,12,13 To date, studies suggest that lower-dose corticosteroids and shorter treatment duration are associated with a lower incidence of treatment failures and a lower rate of side effects.12–14 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend oral prednisone 40 mg daily for five days, based on the findings from an observational analysis and the Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial.1,12,13
The REDUCE trial was a prospective, randomized, non-inferiority, multicenter study that included 314 patients with a mean age of 69 years; more than 50% of the participants in both groups (prednisone 40 mg daily for five days or for 14 days) had moderate-severity disease (GOLD stage IV), and many of the patients had experienced prior exacerbations.14 The primary endpoint was time to next COPD exacerbation within six months. The investigators found that a five-day course of prednisone 40 mg daily was noninferior to a 14-day course with respect to re-exacerbation within six months. In light of the recent robust body of evidence, it appears that the optimal corticosteroid regimen in hospitalized patients may be a low-dose, short course of oral prednisone 40 mg per day for five days, concurrently reducing the risks associated with steroid exposure.
In contrast, the body of evidence supporting the efficacy and safety of sCS among critically ill patients with AECOPD on mechanical ventilation is scant. Nearly all studies have excluded patients requiring mechanical ventilation or intensive care unit (ICU) admission for AECOPD, leading to uncertainty as to whether the results from the aforementioned studies are applicable to patients on mechanical ventilators. However, extrapolation of data from non-ICU AECOPD has led to sCS use in critically ill patients with AECOPD. Despite evidence demonstrating that higher doses are not superior to lower doses in hospitalized patients for AECOPD,14,15 a recent retrospective cohort study suggests that patients admitted to the ICU for AECOPD are managed with higher doses of sCS than inpatient non-ICU patients, predisposing them to potential side effects without a clear benefit. Side effects associated with cumulative high-dose sCS include muscle weakness, myopathy, sleep disturbances, immune suppression, tremors, susceptibility to infections, hyperglycemia, and other metabolic disorders.16 Clinicians should consider the risks and benefits of prescribing high doses of sCS in ICU patients with AECOPD.
Only a few randomized clinical trials have evaluated the efficacy of sCS in patients with AECOPD requiring ventilator support.17,18 A prospective, randomized controlled trial was conducted that included 217 patients with severe COPD exacerbations requiring ventilator support.18 Study investigators compared open-label use of prednisone 1 mg/kg per day (oral or via enteral tube) for a maximum of 10 days or usual care. The primary endpoint was ICU mortality, and secondary endpoints were days on ventilator support and ICU length of stay (LOS). The results showed no significant differences between the prednisone versus the usual care groups in ICU mortality (15% versus 14%; P = 0.81), median duration of ventilation (six days versus six days; P = 0.87), noninvasive ventilation failure (16% versus 13%; P = 0.59), or ICU LOS (nine days versus eight days; P = 0.88). Insulin treatment for managing hyperglycemia was significantly higher in patients receiving prednisone (50% versus 33%; P = 0.01). The results suggest that prednisone did not exhibit a reduction in ICU mortality rate or patient-centered outcomes.
Although the studies above offer insight into efficacy and treatment regimens for critically ill patients with AECOPD, immediate change in clinical practice seems unlikely based on these findings due to the small, retrospective, observational nature of the studies.
Recently a multicenter, retrospective, observational cohort study evaluated the efficacy and safety of lower versus higher doses of corticosteroids in critically ill patients with AECOPD.15 The trial included 17,239 patients (77% of them more than 60 years of age) using a quality and health care utilization database from 400 U.S. hospitals between 2003 and 2008. Patients were included if they were admitted with a diagnosis of AECOPD and received oral or intravenous (IV) sCS within the first two days of admission. The study subjects were split into two groups according to the dosing regimen: high dose (more than 240 mg per day methylprednisolone equivalent) and low dose (240 mg per day or less methylprednisolone equivalent). The primary endpoint was hospital mortality; secondary endpoints were ICU LOS, hospital LOS, and total hospital costs. Patients were matched by propensity scoring. The analysis included 11,083 (64%) in the high-dose group and 6,156 (36%) in the low-dose group. Though there was only a trend toward reduction in hospital mortality in the low-dose group (P = 0.06), the lower dose was associated with a decrease in hospital LOS (P < 0.01), ICU LOS (P < 0.01), hospital costs (P = 0.01), length of invasive ventilation (P = 0.01), insulin therapy (22.7% in the low-dose group versus 25.1% in the high-dose group; P < 0.01), and fungal infections (3.3% in the low-dose group versus 4.4% in the high-dose group; P < 0.01). Even though this study did not ascertain the optimal dose, route of administration, or duration of sCS therapy in critically ill patients, study findings suggest that lower doses of steroids (no more than 240 mg per day methylprednisolone equivalent) should be promoted for patients in the ICU for AECOPD until future clinical trials delineate the optimal dosing regimen.
Despite study limitations, the results from these trials are noteworthy and offer some insight into steroid treatment for critically ill patients, especially because none of the guidelines or systematic reviews provide guidance on dosing and duration of sCS therapy in patients with exacerbations requiring ventilator support. In light of recent publications, it appears that the evidence weighs against the practice of using high doses of sCS in patients with AECOPD requiring mechanical ventilation. Large randomized clinical trials comparing the safety and efficacy of high-dose versus low-dose steroids are required to determine the most effective dosing regimen to manage patients with severe AECOPD. Owing to sCS’s excellent bioavailability, ease of administration, lower costs, and similar efficacy in head-to-head equivalence trials of oral versus IV sCS, oral sCS should be used in the inpatient management of AECOPD when oral or enteral feeding tube administration is feasible.19,20
As an alternative to sCS, nebulized corticosteroids have minimal bioavailability, absorption, and systemic adverse effects. The GOLD guidelines recommend nebulized budesonide as an alternative to oral corticosteroids for the treatment of AECOPD.1 Only a few nonblinded studies have examined nebulized corticosteroids in AECOPD. Investigators compared 4 mg to 8 mg of nebulized budesonide with various doses of oral or IV prednisolone.21,22 The results showed no difference between the groups, and interpretation was hindered by small sample sizes, heterogeneous populations, and exclusion of severe exacerbation. Larger studies are needed to confirm the long-term impact of clinical outcomes, optimal agent, and dosage of nebulized corticosteroids for AECOPD.
Antibiotics
Utilization of antibiotics in COPD exacerbations remains a part of management, despite overestimation of bacteria in older studies and newer evidence of viruses as causative pathogens. More recent studies with improved study design and methodology estimate that only 50% of exacerbations have a bacterial source.23 At least a third of cases are caused by viruses, including influenza, parainfluenza, respiratory syncytial virus, human metapneumonia virus, picomaviruses, and coronavirus.23–25
Nevertheless, COPD guidelines recommend empiric antibiotic treatment in certain patient populations, particularly in patients with moderate-to-severe exacerbations.1 This determination is characterized by presence of the Anthonisen criteria of increased sputum purulence and at least one of the following: increased dyspnea and increased sputum volume.1,26 Employment of this classification to initiate therapy in patients with moderate-to-severe exacerbations is largely based on a systematic review by Ram et al.27 In this evaluation, antibiotics showed a decrease in short-term mortality by 77%, treatment failure by 53%, and sputum purulence by 44%.
Production of purulent versus mucoid sputum is indicative of patients who will benefit from antibiotics as determined by Stockley et al. In their study, purulent sputum resulted in positive bacterial cultures in 84% of patients, compared with 38% of those producing mucoid sputum (P < 0.0001). The former was found to be 94.4% sensitive and 77% specific for the yield of high bacterial load. When evaluating resolution, all patients with mucoid sputum improved without antibiotic treatment.28
Utilization of sputum purulence as a cornerstone in the decision to initiate antibiotics is not entirely reliable. Patients may not routinely assess expectorated sputum, and the appearance may change quickly. In a study by Daniels et al., sputum colors reported by patients were compared to those assessed using a validated color chart as markers for bacterial load and systemic inflammation. The sensitivity and specificity for reported sputum color were 73% and 39% respectively, compared to 90% and 52% in assessed sputum, indicating that patient-reported color was unreliable.29
Biomarkers have been identified as a means of determining etiology of COPD exacerbations in addition to playing a role in diagnosing and assessing severity.30 Most recently, the COPD Biomarker Qualification Consortium received Food and Drug Administration approval for plasma fibrinogen as a COPD biomarker.31 Pertaining to infection-related biomarkers, procalcitonin is utilized as a marker of sepsis due to bacteria. As it is an indicator of systemic bacterial infection, it can therefore be employed to identify exacerbations caused by such pathogens to determine if antibiotics should be administered. This was evaluated by Stolz et al., who randomized patients into standard-care or procalcitonin-guided treatment groups.32 Antibiotic use in patients with procalcitonin levels up to 0.1 mcg/L was discouraged. Levels between 0.1 and 0.25 mcg/L indicated a possible bacterial infection, and antibiotic treatment was to be based upon clinical condition. In patients with procalcitonin levels greater than 0.25 mcg/L, antibiotic use was strongly supported. The study indicated no difference in clinical success, hospital LOS, and ICU LOS despite decreased use of antibiotics in patients with low procalcitonin levels. Furthermore, when patients were evaluated at six months, there were no statistically significant differences in exacerbation rates, rehospitalization rates, and time to next exacerbation.
Procalcitonin has also been studied in assessing treatment response. In a systematic review of 14 trials, Schuetz et al. evaluated the effect of using a procalcitonin algorithm to guide initiation and discontinuation of antibiotics in acute respiratory tract infections.33 In this analysis, there was no statistically significant difference in treatment failure or mortality at 30 days when procalcitonin was used to guide initiation and duration of antibiotic treatment. Furthermore, mean duration of antibiotic exposure was 34.8% lower in the procalcitonin group compared with the standard-care group (95% confidence interval [CI], −40.3 to −28.7).
If antibiotics are initiated in patients with COPD exacerbations, the regimen should be based upon severity, likely pathogens, and local susceptibility patterns.1 While cultures may be drawn, therapy is generally empiric. Common bacterial pathogens include Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, and Staphylococcus aureus.24 Although uncommon, atypical pathogens, including Chlamydophila pneumoniae and Mycoplasma pneumoniae, can also be infectious causes of exacerbations.24 Moderately to severely ill patients may be divided into uncomplicated and complicated exacerbations based on certain parameters to help guide therapy. Generally, patients with uncomplicated exacerbations include those younger than 65 years of age with FEV1 more than 50% of predicted who experience fewer than three exacerbations per year.23,24 These patients may be treated with an advanced macrolide or a second- or third-generation cephalosporin. While amoxicillin was once considered an option for first-line therapy, it should no longer be used due to the incidence of beta-lactamase production among H. influenzae and M. catarrhalis. Similarly, resistance to doxycycline and trimethoprim/sulfamethoxazole has emerged, and therefore, these medications may not be considered first line.34 Complicated exacerbations should be treated with a respiratory fluoroquinolone or amoxicillin/clavulanate (Table 4).23,24
Table 4.
| Moderate-to-Severe Exacerbations | Severe Exacerbations | |
|---|---|---|
| Uncomplicated | Complicated | |
|
|
|
This list is not all-inclusive.
Covers Pseudomonas depending on local suceptiblities
Among various agents, the literature does not strongly support the utilization of one antibiotic over another. In the MAESTRAL study, moxifloxacin administered for five days was compared with amoxicillin/clavulanate given over seven days. Clinical failure at eight weeks post-therapy was found to be 20.6% and 22% (95% CI, −5.89 to 3.83) for moxifloxacin and amoxicillin/clavulanate, respectively, indicating noninferiority.35 Similarly, in a study conducted by Yoon et al., levofloxacin was compared with cefuroxime in AECOPD, stratified by severity. Clinical efficacy was found to be comparable between the two agents (90.4% in the levofloxacin group compared to 90.6% in the cefuroxime group; 95% CI, −9.40 to 10.91).36
In patients with severe exacerbations or in those with frequent antibiotic usage or recent hospitalization, Pseudomonas aeruginosa and other gram-negative bacilli must also be considered, particularly if such pathogens were previously isolated.34 In this subset of patients, depending on local susceptibilities, ciprofloxacin, levofloxacin, pipercillin/tazobactam, ceftazidime, or cefepime should be initiated empirically and sputum cultures should be obtained.24,34 Patients with recent exposure to antibiotics should be initiated on an agent of a different class for the treatment of exacerbation (Table 4).34
Once patients are deemed eligible, treatment should be initiated in a timely manner, because antibiotics have been associated with a longer median time to the next exacerbation. In a study conducted in 49,599 Dutch patients, those who received antibiotics for the first or second exacerbation had a longer median time to subsequent exacerbations by 97 and 113 days, respectively (P < 0.001).37 In addition, early antibiotic administration has been associated with improved outcomes in hospitalized patients. An observational study of 84,621 patients by Rothberg et al. identified a decrease in treatment failure, inpatient mortality, and late mechanical ventilation in patients started on antibiotics by day 2 of hospitalization.38 Regardless of the empiric regimen and severity of exacerbation, patients should be reassessed in 48 to 72 hours. If the patient exhibits signs of clinical improvement, conversion to oral therapy may occur or the regimen may be de-escalated based on sputum culture and susceptibility results.39 Duration of treatment can range from five to 10 days.1
STRATEGIES FOR PREVENTING AECOPD
Medications available for the treatment of COPD have not been shown consistently to modify the underlying pulmonary architecture or reverse airway destruction; thus, the goals of comprehensive disease management include alleviating acute symptoms and reducing the severity and frequency of exacerbations, as well as improving quality of life and exercise tolerance.1,40,41 Nonpharmacological interventions, such as prevention of influenza or pneumococcal disease via immunization, smoking cessation, pulmonary rehabilitation, and education, along with specific pharmacological approaches, including antibiotics, bronchodilators, and corticosteroids, may reduce or prevent exacerbations of disease.1,7
IMMUNIZATION RECOMMENDATIONS AND NONPHARMACOLOGICAL STRATEGIES
Vaccination
According to current recommendations from the Centers for Disease Control and Prevention, all individuals 6 months of age or older should receive the influenza vaccine annually. Pooled results of randomized clinical trials suggest immunization against influenza may be associated with a reduction in exacerbations of COPD.42 Adults 19 to 64 years of age who have chronic lung diseases, including COPD, should also receive a one-time dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23), followed by a single dose of the 13-valent pneumococcal conjugate vaccine (PCV13) at age 65 years or older, with a single subsequent dose of PPSV23 administered at least one year after PCV13 and at least five years after the last dose of PPSV23.43 The most recent ACCP/CTS guidelines suggest that PPSV23 be administered to all COPD patients as part of disease management, although evidence from clinical trials does not suggest vaccination to reduce exacerbations of disease.44
Smoking Cessation
Smoking cessation is the single most effective intervention for altering the progressive decline in lung function that is characteristic of this disease.1 Every encounter with a clinician, regardless of care setting, represents an opportunity to evaluate the patient’s readiness to quit and provide education on strategies to reduce nicotine cravings. Various pharmacological and behavioral strategies exist to support quitting attempts, including a variety of nicotine replacement therapies (gum, lozenge, patch, inhaler, or nasal spray), varenicline or sustained-release bupropion, and behavioral approaches, including support through counseling and motivational interviewing, print-based self-help materials, or computer-based interventions.1,45,46 Specifically regarding efforts to reduce COPD exacerbations, there is low-level evidence to suggest that smoking cessation has a direct effect on reducing exacerbations of pulmonary disease; regardless, most recent clinical guidelines emphasize that multimodal smoking cessation should be offered as part of a comprehensive strategy to reduce exacerbations of disease.7
Pulmonary Rehabilitation
The effects of pulmonary rehabilitation—a comprehensive and multidisciplinary intervention that includes provision of exercise, education, and behavioral approaches to improve physical and psychological function of patients living with COPD—have been evaluated in clinical trials and are well established.1,7,47 Although not completely elucidated, the effects of pulmonary rehabilitation include improvement in exercise tolerance, improved health-related quality of life, and reduced number and duration of hospitalizations for exacerbations, as well as improved recovery after hospitalization for COPD exacerbation.1 Current guidelines suggest that pulmonary rehabilitation may have particular benefit for individuals with moderate, severe, or very severe COPD who have had an exacerbation of disease requiring hospitalization within the previous four weeks to prevent subsequent exacerbations and hospitalizations.7 The frequency with which this therapy should be provided is not specified in current clinical guidelines, and there may be heterogeneity among rehabilitation programs and centers. Most programs meet three times a week for at least six weeks after the acute-care hospitalization and typically include two to three hours of both education and exercise at each session.48
Education, Case Management, and Telemonitoring
Recent clinical guidelines underscore the importance of the general principles of chronic disease management, including patient empowerment and education regarding the chronic disease as well as a coordinated team-based effort to meet the patient’s comprehensive health care needs.7 Evidence exists to support the position that a comprehensive disease self-management strategy, including monthly access to a health care specialist to prevent disease exacerbations, may influence and reduce exacerbations of COPD.7 In addition, the need to reduce readmissions after COPD hospitalizations under the Centers for Medicare and Medicaid Services’ Hospital Readmission Reduction Program has mandated that hospitals increasingly focus on ensuring that patients receive optimal medical treatment and therapy based on evidence-based guidelines and on medication reconciliation at the time of hospital admission and discharge.49 Although telemedicine and telemonitoring of disease are a rapidly developing area of clinical medicine services, evidence at this time does not suggest telemonitoring to be associated with a reduced incidence of exacerbations of disease compared with usual care.7
PHARMACOLOGICAL STRATEGIES
Bronchodilators
As previously described, long-acting bronchodilators are generally preferred for management of chronic broncho-constriction in COPD; both long-acting beta agonists (LABAs) and long-acting muscarinic antagonists (LAMAs), when compared with placebo, are likely to reduce moderate-to-severe acute exacerbations of disease.7 A systematic review encompassing seven clinical trials and 12,223 participants with COPD designed to compare the effects of tiotropium to LABAs (salmeterol, formoterol, and indacaterol) concluded there is equivocal evidence regarding the effects of LABAs and tiotropium on patients’ quality of life. Tiotropium use was associated with a reduction in the number of participants experiencing one or more exacerbations compared with use of a LABA (odds ratio [OR], 0.86; 95% CI, 0.79–0.93), with no differences observed among the different types of LABAs. No statistical difference in mortality was observed between the treatment groups. As a secondary outcome, tiotropium was associated with a reduction in the number of COPD exacerbations leading to hospitalization compared with LABA treatment (OR, 0.87; 95% CI, 0.77–0.99), but not in the overall rate of all-cause hospitalizations; thus, in moderate-to-severe COPD, use of tiotropium may be associated with a lower rate of exacerbation and hospitalization compared with LABAs.50 More recent trials suggest that combination LABA/LAMA therapy (indacaterol/glycopyrronium) is more effective than a LABA with an inhaled corticosteroid (ICS) (salmeterol/fluticasone) at preventing COPD exacerbations in patients with a history of exacerbation in the previous year and that withdrawal of use of an ICS in patients receiving LABA/LAMA combination therapy (tiotropium plus salmeterol) is not associated with exacerbation of disease.51,52
Corticosteroids
Although COPD is characterized by chronic broncho-constriction, the anti-inflammatory effects of systemically administered corticosteroids may be beneficial not only in alleviating acute shortness of breath and reversing broncho-constriction when an asthmatic component exists, but may also reduce exacerbations of disease in a 30-day window following an initial exacerbation, as well as having a preventive effect on hospitalization.7 There are numerous well-established risks associated with chronic systemic corticosteroid therapy, including hyperglycemia, hypertension, weight gain, mood disturbances, skeletal adverse effects, and insomnia, and long-term use (beyond 30 days) has not been demonstrated to have a greater effect on exacerbation reduction.7
While associated with fewer systemic adverse effects, ICS should only be used in conjunction with long-acting bronchodilator therapy to reduce exacerbations of disease and may be utilized in individuals with moderate to very severe disease.7 The availability of newer classes and types of inhaled bronchodilators, including LABAs and LAMAs; emerging data on comparative efficacy of various bronchodilator regimens versus ICS combinations; and data suggesting that stepwise and gradual withdrawal of ICS therapy will not worsen exacerbations of disease, have prompted reconsideration of the role of ICS therapy to prevent exacerbations of disease.52
Antibiotics
There has been much interest regarding long-term antimicrobial use to reduce exacerbations of pulmonary disease. Specifically, the macrolide antibiotic azithromycin is not only thought to have antimicrobial effects but may also reduce inflammation and modulate the immune response in the upper airways. Several clinical trials have evaluated different dosing and administration strategies for azithromycin in reducing exacerbations of disease, including azithromycin 250 mg administered orally once daily for one year versus placebo for patients with moderate-to-severe COPD who either experienced an exacerbation within the year prior to study enrollment or required long-term oxygen therapy.53 Investigators found azithromycin use to be associated with a significant reduction in exacerbations of COPD, from 1.83 to 1.48 acute exacerbations per patient-year (relative risk, 0.83; 95% CI, 0.72–0.95; P = 0.01).53 Based on this data, current guidelines suggest use of long-term macrolide therapy for patients with one or more moderate-to-severe exacerbations of disease in the previous year despite optimization of inhaler use, although clinicians must weigh the risks of QTc prolongation (including history of cardiovascular disease or dysrhythmia, concomitant medication use, and presence of electrolyte disturbances), emergence of bacterial resistance, and patient cost when considering this type of treatment.7
Other Pharmacological Treatment Options
Other therapies, including theophylline, the phospho-diesterase-4 inhibitor roflumilast, oral N-acetylcysteine, and mucolytics—as well as emerging treatment options for COPD—may have effects on reducing exacerbations of COPD, but they are beyond the scope of this article. The reader is encouraged to evaluate clinical guideline evidence to gain additional insight into the role of these complementary therapies in modulating the course of disease.7
CONCLUSION
Acute exacerbations of COPD are associated with substantial negative impact on pulmonary function, morbidity, mortality, and increased health care costs. Rapid-acting bronchodilators, systemic corticosteroids, and antibiotics are considered cornerstones of therapy for managing COPD exacerbations. Prevention of COPD exacerbations should be a key component of management. Recent guidelines emphasize several preventive pharmacological and nonpharmacological strategies to reduce or prevent exacerbations of COPD.
Footnotes
Disclosures: The authors report no commercial or financial interests in regard to this article.
REFERENCES
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2016. [Accessed March 27, 2016]. Available at: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016.
- 2.Ford ES, Croft JB, Mannino DM, et al. COPD surveillance–United States, 1999–2011. Chest. 2013;144(1):284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston AK, Mannino DM. Epidemiology of COPD exacerbations. In: Wedzicha JA, Martinez FJ, editors. Exacerbations of Chronic Obstructive Pulmonary Disease. London, England: Informa Healthcare; 2008. pp. 15–26. [Google Scholar]
- 5.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 6.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894–942. doi: 10.1378/chest.14-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services. Readmissions reduction program. Apr 20, 2013. [Accessed July 20, 2016]. Available at: www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html.
- 9.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 10.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(5 Pt 2):S77–S121. [PubMed] [Google Scholar]
- 11.Schatz M, Kazzi A, Brenner B, et al. Introduction. Proc Am Thorac Soc. 2009;6:353–356. doi: 10.1513/pats.P09ST1. [DOI] [PubMed] [Google Scholar]
- 12.Walters JA, Tan DJ, White CJ, et al. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;9:CD001288. doi: 10.1002/14651858.CD001288.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindenauer PK, Pekow PS, Lahti MC, et al. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303:2359–2367. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 14.Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs. conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223–2231. doi: 10.1001/jama.2013.5023. [DOI] [PubMed] [Google Scholar]
- 15.Kiser TH, Allen RR, Valuck RJ, et al. Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:1052–1064. doi: 10.1164/rccm.201401-0058OC. [DOI] [PubMed] [Google Scholar]
- 16.Amaya-Villar R, Garnacho-Montero J, Garcia-Garmendıa JL, et al. Steroid-induced myopathy in patients intubated due to exacerbation of chronic obstructive pulmonary disease. Intensive Care Med. 2005;31:157–161. doi: 10.1007/s00134-004-2509-9. [DOI] [PubMed] [Google Scholar]
- 17.Alía I, de la Cal MA, Esteban A, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Arch Intern Med. 2011;171:1939–1946. doi: 10.1001/archinternmed.2011.530. [DOI] [PubMed] [Google Scholar]
- 18.Abroug F, Ouanes-Besbes L, Fkih-Hassen M, et al. Prednisone in COPD exacerbation requiring ventilatory support: an open label randomized evaluation. Eur Respir J. 2014;43:717–724. doi: 10.1183/09031936.00002913. [DOI] [PubMed] [Google Scholar]
- 19.Al-Habet S, Rogers HJ. Pharmacokinetics of intravenous and oral prednisolone. Br J Clin Pharmacol. 1980;10(5):503–508. doi: 10.1111/j.1365-2125.1980.tb01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Jong YP, Uil SM, Grotjohan HP, et al. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double-blind study. Chest. 2007;132(6):1741–1747. doi: 10.1378/chest.07-0208. [DOI] [PubMed] [Google Scholar]
- 21.Yilmazel Ucar E, Araz O, Meral M, et al. Two different dosages of nebulized steroid versus parenteral steroid in the management of COPD exacerbations: A randomized control trial. Med Sci Monit. 2014;20:513–520. doi: 10.12659/MSM.890210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaude GS, Nadagouda S. Nebulized corticosteroids in the management of acute exacerbation of COPD. Lung India. 2009;26:S11–S12. doi: 10.4103/0970-2113.71957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sethi S, Murphy TM. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 24.Sykes A, Mallia P, Johnston SL. Diagnosis of pathogens of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(8):642–646. doi: 10.1513/pats.200707-101TH. [DOI] [PubMed] [Google Scholar]
- 25.Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalization: a case-control study. Thorax. 2003;58:37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 27.Ram FS, Rodriguez-Roisin R, Granados-Navarrete A, et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(2):CD004403. doi: 10.1002/14651858.CD004403.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Stockley RA, O’Brien C, Pye A, et al. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117(6):1638–1645. doi: 10.1378/chest.117.6.1638. [DOI] [PubMed] [Google Scholar]
- 29.Daniels JM, de Graaf CS, Vlaspolder F, et al. Sputum colour reported by patients is not a reliable marker of the presence of bacteria in acute exacerbations of chronic obstructive pulmonary disease. Clin Microbiol Infect. 2010;16:583–588. doi: 10.1111/j.1469-0691.2009.02892.x. [DOI] [PubMed] [Google Scholar]
- 30.Lacoma A, Prat C, Andreo F, et al. Biomarkers in the management of COPD. Eur Respir Rev. 2009;18(112):96–104. doi: 10.1183/09059180.00000609. [DOI] [PubMed] [Google Scholar]
- 31.Miller BE, Tal-Singer R, Rennard SI, et al. Plasma fibrinogen qualification as a drug development tool in chronic obstructive pulmonary disease. Perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Am J Respir Crit Care Med. 2016;193(6):607–613. doi: 10.1164/rccm.201509-1722PP. [DOI] [PubMed] [Google Scholar]
- 32.Stoltz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD. Chest. 2007;131(1):9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]
- 33.Schuetz P, Müller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;12(9):CD007498. doi: 10.1002/14651858.CD007498.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sponsler KC, Markley JD, LaBrin J. What is the appropriate use of antibiotics in acute exacerbations of COPD? The Hospitalist. Jan 26, 2012. [Accessed July 20, 2016]. Available at: www.the-hospitalist.org/article/what-is-the-appropriate-use-of-antibiotics-in-acute-exacerbations-of-copd.
- 35.Wilson R, Anzuelo A, Miravitlles M, et al. Moxifloxacin versus amoxicillin/clavulanate in outpatient acute exacerbations of COPD: MAESTRAL results. Eur Respir J. 2012;40(1):17–27. doi: 10.1183/09031936.00090311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon HI, Lee CH, Kim DK, et al. Efficacy of levofloxacin versus cefuroxime in treating exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:329–334. doi: 10.2147/COPD.S41749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roede BM, Bresser P, Bindels PJ, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population-based cohort study. Thorax. 2008;63(11):968–973. doi: 10.1136/thx.2008.095349. [DOI] [PubMed] [Google Scholar]
- 38.Rothberg MB, Pekow PS, Lahti M, et al. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303(20):2035–2042. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqi A, Sethi S. Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulm Dis. 2008;3(1):31–44. doi: 10.2147/copd.s1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmaco-therapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 41.Decramer M, Celli B, Kesten S, et al. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374:1171–1178. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23) MMWR Morb Mortal Wkly Rep. 2010;59(34):1102–1106. [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. Adult immunization schedule—United States—2016. [Accessed July 19, 2016]. Available at: www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf.
- 44.Walters JS, Smith S, Poole P, et al. Injectable vaccine for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010;(11):CD001390. doi: 10.1002/14651858.CD001390.pub3. [DOI] [PubMed] [Google Scholar]
- 45.Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;163:608–621. doi: 10.7326/M15-0171. [DOI] [PubMed] [Google Scholar]
- 46.Siu AL. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:622–634. doi: 10.7326/M15-2023. [DOI] [PubMed] [Google Scholar]
- 47.Spruit MA, Singh SJ, Garvey C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 48.Corhay J-L, Dang DN, Van Cauwenberge H, et al. Pulmonary rehabilitation and COPD: providing patients a good environment for optimizing therapy. Int J Chron Obstruct Pulmon Dis. 2014;9:27–39. doi: 10.2147/COPD.S52012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnan JA, Gussin HA, Prieto-Centurion V, et al. Integrating COPD into patient-centered hospital readmissions reduction programs. Chronic Obstr Pulm Dis. 2015;2:70–80. doi: 10.15326/jcopdf.2.1.2014.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(9):CD009157. doi: 10.1002/14651858.CD009157.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222–2234. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 52.Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371:1285–1294. doi: 10.1056/NEJMoa1407154. [DOI] [PubMed] [Google Scholar]
- 53.Albert RK, Connett J, Bailey WC, et al. COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]


