Abstract
Cord blood stem cells are an attractive starting source for the production of red blood cells in vitro for therapy because of additional expansion potential compared with adult peripheral blood progenitors and cord blood banks usually being more representative of national populations than blood donors. Consequently, it is important to establish how similar cord RBCs are to adult cells. In this study, we used multiplex tandem mass tag labeling combined with nano-LC-MS/MS to compare the proteome of adult and cord RBCs and reticulocytes. 2838 unique proteins were identified, providing the most comprehensive compendium of RBC proteins to date. Using stringent criteria, 1674 proteins were quantified, and only a small number differed in amount between adult and cord RBC. We focused on proteins critical for RBC function. Of these, only the expected differences in globin subunits, along with higher levels of carbonic anhydrase 1 and 2 and aquaporin-1 in adult RBCs would be expected to have a phenotypic effect since they are associated with the differences in gaseous exchange between adults and neonates. Since the RBC and reticulocyte samples used were autologous, we catalogue the change in proteome following reticulocyte maturation. The majority of proteins (>60% of the 1671 quantified) reduced in abundance between 2- and 100-fold following maturation. However, ∼5% were at a higher level in RBCs, localized almost exclusively to cell membranes, in keeping with the known clearance of intracellular recycling pools during reticulocyte maturation. Overall, these data suggest that, with respect to the proteome, there is no barrier to the use of cord progenitors for the in vitro generation of RBCs for transfusion to adults other than the expression of fetal, not adult, hemoglobin.
The generation of human red blood cells (RBCs)1 in vitro for transfusion purposes is a major goal of health services globally. In recent years, advances in the development of systems for the generation of erythrocytes in vitro have progressed rapidly using progenitor cells isolated from adult peripheral blood (PB) (1) umbilical cord blood (2, 3) and human pluripotent stem cells (4–6). Cells from adult PB and cord can be expanded and induced to differentiate efficiently down the erythroid pathway to generate significant numbers of enucleated reticulocytes as an end point (1). However, adult PB progenitors have a more limited proliferative capacity than cord, which restricts the number of red cells that can be obtained by in vitro culture methods and greatly impacts the economic viability of producing therapeutic quantities of red cells from this source. Therefore, progenitors isolated from cord are attractive as a starting material for in vitro blood production because of their potential for greater expansion capacity (3, 7). In addition cord stem cell banks are generally more representative of blood group diversity in the population compared with adult donor blood banks.
Although cord progenitors offer a realistic potential for generating therapeutic quantities of erythroid cells, these cells appear to maintain a fetal, rather than adult, phenotype. The most obvious difference between erythroblasts generated from cord compared with adult PB is their expression of predominately γ- (fetal) rather than β- (adult) globin. Other differences have been reported (8, 9), including expression of i rather than I antigen and weak expression of ABH antigens on cord cells. However, to date, a comprehensive, comparative analysis of the proteome of cord cells compared with adult cells has not been undertaken, although such information is essential before such cells can be considered for therapeutic use.
Another poorly defined feature of erythropoiesis is the change in proteome as reticulocytes undergo extensive transformation to create mature erythrocytes. Alterations in membrane proteins have been observed to occur during enucleation (10), and the role of autophagic vesicles in reticulocyte maturation has recently been described (1, 11). However, a more comprehensive understanding of the final stages of reticulocyte maturation is now required in order to facilitate the study of inherited and acquired anemias exhibiting reticulocytosis (12) and aid in extending in vitro culture systems to create mature erythrocytes.
We have undertaken multiplex tandem mass tag (TMT) labeling and nanoLC-MS/MS to compare the proteome of adult and cord endogenous RBCs and also of reticulocytes generated in vitro from adult PB and cord progenitors. In addition, as our culture system generates functional, mature reticulocytes (1), we were able to compare the proteome of these cells with that of the original donors own mature RBCs, mimicking as close as possible the in vivo maturation process.
MATERIALS AND METHODS
Isolation of Adult and Cord Blood RBCs and CD34+ cells
Leukocyte reduction system (LRS) cones and cord blood (CB) units were obtained from healthy donors with written informed consent for research use in accordance with the Declaration of Helsinki and approved by the local Research Ethics Committees (Southmead Research Ethics Committee reference 08/H0102/26 and Bristol Research Ethics Committee reference 12/SW/0199). Adult RBCs and CD34+ cells, and CB RBCs and CD34+ cells were isolated from the same leukocyte reduction system cones and cord blood units, respectively. CD34+ cells were separated as described by Griffiths et al. (1).
Erythroid Differentiation of CD34+ Cells
CD34+ cells were differentiated in our three-stage culture system (1), harvested on day 19, and passed through a WBF leukocyte filter (Pall life sciences, Portsmouth, UK) (1) to isolate mature reticulocytes.
Preparation of Membrane and Cytosol Fractions
The RBCs and reticulocytes were separated into membrane and cytoplasmic fractions to reduce protein complexity for mass spectrometry (MS). Cells were washed twice in cold PBS, followed by two washes with iso-osmotic buffer (Na2HPO4 103 mm, NaH2PO4 155 mm, pH 7.4). The cells were then lysed in lysis buffer (1 in 20 dilution of iso-osmotic buffer with water, 1x complete protease inhibitor (Roche, Basel, Switzerland) and 0.5 mm PMSF (Sigma-Aldrich, Poole, UK)) at 50x packed cell volume, followed by centrifugation at 15,000 rpm for 10 min at 4 °C. The first supernatant was collected and kept as a cytoplasmic fraction. The lysis step was repeated an identical number of times for the adult and cord cells until the membrane pellet of both become white. The pellets were solubilized in sodium phosphate buffer with 1x complete protease inhibitor and 0.5 mm PMSF.
SDS-PAGE and Western Blotting
Washed cell pellets were resuspended in solubilization buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 10% glycerol, 1% Triton, 0.1% SDS) containing 1x complete protease inhibitor and 2 mm PMSF at 200 μl of buffer to 1 × 107 cells. After 1 h incubation on ice, the protein samples were treated with 25 Uml−1 Bensonase (Sigma-Aldrich) for 1 h on ice and then centrifuged at 17,000 g for 5 min, at 4 °C. Cytoplasmic and nuclear fractions were prepared in the same way as samples for mass spectrometry (above). Proteins were resolved by SDS-PAGE and transferred to PVDF by Western blotting. Membranes were blocked with 10% milk powder for 1 h, followed by incubation with primary antibodies overnight at 4 °C. Primary antibodies used were aquaporin I (CHIP28) and glycophorin A (CVDP) 1:1000 dilution, ankyrin 1 (BRIC274) 1:100 dilution, and band 3 (BRIC170) 1:2000 dilution, all validated and supplied by IBGRL (http://ibgrl.blood.co.uk/ResearchProducts/ResProdHome.htm). BCL11A[14B5] (ab19487) and CAII (ab6621) 1:1000 dilution, CAI (ab34978) and myosin IIB (ab684) 1:2000 dilution, and tropomodulin 4 (ab67776) 1:500 dilution, all from Abcam (Cambridge, UK); β–globin (37–8; sc21757) 1:5000 dilution and γ-globin (51–7; sc21756) and α-globin (d-16; sc31110) 1:1000 dilution all from Santa Cruz Biotechnology (Dallas, Texas, USA); β–actin (A1978; Sigma-Aldrich) 1:2000 dilution. Specificity of antibodies has been previously demonstrated (13–15).
Lentiviral Constructs and Transduction of Erythroblasts
pLKO.1-TRC BCL11A short hairpin (sh) RNA plasmids (B1 and B5) were designed by the Broad Institute (Cambridge, MA, USA) and purchased from Open Biosystems (GE Healthcare Life Sciences, Little Chalfont, UK). The sequence of hairpin B1 is 5′-CCGG-CGCACAGAACACTCATGGATT-CTCGAG-AATCCATGAGTGTTCTGTGCG-TTTTTG-3′, targeting nucleotides 792–812, and the sequence of B5 is 5′-CCGG-GCATAGACGATGGCACTGTTA-CTCGAG-TAACAGTGCCATCGTCTATGC-TTTTTG-3′, targeting nucleotides 2015–35 of BCL11A-XL (accession number NM_022893.3). Transduction of erythroblasts was performed as described by Satchwell et al. and Trakarnsanga et al. (15,16).
Polymerase Chain Reaction (PCR)
RNA (400 ng) was reverse transcribed into cDNA using SuperScript II reverse transcriptase (Invitrogen, ThermoFisher Scientific, Waltham, MA, USA). Globin expression was analyzed using primers to β-globin 5′ CTTTAGTGATGGCCTGGCTC and 5′ GGCAGAATCCAGATGCTCAA, and γ-globin 5′ GGGCAAGGTGAATGTGGAAGAT and 5′ GGGTCCATGGGTAGACAACCA as described previously (15).
Tandem Mass Tag Labeling, Preparation of Samples for Mass Spectrometry, Database Search Parameters and Acceptance Criteria for Identifications
For multiplexed comparative proteomics 100 μg of each cell lysate was digested with trypsin and labeled with tandem mass tag (TMT) reagents according to the manufacturer's protocol (Thermo Fisher Scientific, Waltham, Massachusetts, USA). After labeling, samples were combined in equal amounts and 50 μg of pooled sample fractionated by strong cation exchange using an Ettan LC system (GE Healthcare, Little Chalfont. UK) prior to analysis by nanoLC-MS/MS. The raw data files were processed and quantified using Proteome Discoverer software v1.2 and v1.4 (Thermo Scientific) and searched against the UniProt/SwissProt Human database release version 57.3 (20,326 entries) using the SEQUEST (Ver. 28 Rev. 13) algorithm. Peptide precursor mass tolerance was set at 10 ppm, and MS/MS tolerance was set at 0.8 Da. Search criteria included oxidation of methionine (+15.9949) as a variable modification and carbamidomethylation of cysteine (+57.0214) and the addition of isobaric mass tags (+229.163) to peptide N termini and lysine as fixed modifications. Searches were performed with full tryptic digestion and a maximum of one missed cleavage was allowed. The reverse database search option was enabled and all peptide data was filtered to satisfy false discovery rate of 5%. The Proteome Discoverer software generates a reverse “decoy” database from the same protein database, and any peptides passing the initial filtering parameters that were derived from this decoy database are defined as false positive identifications. The minimum cross-correlation factor (Xcorr) filter was readjusted for each individual charge state separately to optimally meet the predetermined target false discovery rate of 5% based on the number of random false positive matches from the reverse decoy database. Thus, each data set has its own passing parameters. Quantitation was performed using a peak integration window tolerance of 0.0075 Da with the integration method set as the most confident centroid. Protein ratios represent the median of the raw measured peptide ratios for each protein. These methods are described in Trakarnsanga et al. (5).The mass spectrometry (MS) proteomics data have been deposited to the ProteomeXchange Consortium (17) via the PRIDE partner repository with the dataset identifier PXD003276.
For analysis, only rank 1 peptides were used, and only quantifications obtained using two or more unique peptides with high/medium confidence per protein were considered (although for additional information proteins quantified from one unique peptide are sometimes included). Proteins recorded as uncharacterized by the software were returned with a gene identifier. We selected a comparative protein threshold of 2, such that proteins that differed in level by 2.00-fold or more between adult and cord samples were considered differentially expressed. For classification by cellular component and molecular function, proteins were analyzed using WebGestalt GSAT V2 (35).
Experimental Design and Rationale
The human samples for this study were provided as a pool of autologous CD34+ cells and RBCs from 16 adults and a pool of autologous CD34+ cells and RBCs from four cord samples, minimizing intrinsic variability between individuals. To ensure robustness of our comparative data, the level of proteins in the pooled adult sample were further compared with an additional cord RBC and reticulocyte sample, and only proteins that also differed between the adult and these second cord samples are reported as differentially expressed. For comparative analysis between adult and cord erythroid cells, internal controls included proteins known to be consistent in level, e.g. α-globin, and to differ in level, e.g. β- and γ-globin, between these cells, as described. For comparative analysis between reticulocytes and mature RBCs, internal controls included proteins known to change in level following reticulocyte maturation, as described.
RESULTS AND DISCUSSION
Membrane and cytosol fractions of the adult and cord RBC and reticulocytes were each labeled with a different TMT, pooled, and analyzed by MS. Using this methodology, 1880 proteins were identified in the membrane fractions, and 1602 in the cytosolic fractions (Supplemental Tables 1A and 1B and 2A and 2B). Certain proteins were detected in both fraction, hence overall we identified 2838 unique proteins, which represents the most comprehensive identification of RBC proteins to date. No membrane or cytosol protein detected was unique to just adult or cord or just RBCs or reticulocytes.
We detected 26 RBC blood group protein antigens in these data (Table I). As stated previously (5), Duffy is a large glycosylated protein with few trypsin sites, resulting in a large peptide that is not compatible with MS identification. We have previously shown that our culture system does not alter the blood group protein expression profile of cultured adult erythroid cells (1). In the present study, we further compared the abundance of the RBC blood group proteins between the endogenous RBCs and autologous cultured cells. The levels were either consistent or lower in the cultured cells (Table I), reassuring that the cultured cells are not more immunogenic than endogenous cells. We performed the same analysis for RBCs and autologous cultured cells from a single cord blood donor, confirming abundance of the RBC blood group proteins was either consistent or lower in the cultured cells (Supplemental Table 3). We also performed serological analysis of cord blood cells, again comparing the expression of blood group antigens between endogenous and erythroid cells differentiated from CD34+ cells from the same donor (Supplemental Fig. 1). As expected, Ch and Rg were not detected on the cultured cells as these antigens are expressed on complement component C4, absorbed onto red cells from plasma. When blood group antigens defined by carbohydrate structures were examined (ABH, Ii, P1, P, Pk, LKE), cultured cord reticulocytes showed stronger expression of P1 and I antigens and weaker expression of H and LKE antigens. Weaker expression of ABH antigens and stronger expression of i antigen on cord RBCs when compared with adult is well known (8, 18, 19). Such variations in the degree of N-glycosylation are unlikely to impact the use of cultured cord reticulocytes for therapy, although the possibility of exposure of novel cryptantigens cannot be entirely ruled out.
Table I. RBC blood group proteins in pooled erythroid cell samples.
Endogenous adult and cord RBCs and reticulocytes (Retic) differentiated in vitro from adult peripheral blood and cord CD34+ cells (RBCs and CD34+ cells pooled from 16 and 4 individuals, respectively) were fractionated and proteins subjected to trypsin digest, with resultant peptides labelled with TMTs for nanoLC-MS/MS based quantitation. Values show the ratio of protein levels between adult RBCs and reticulocytes (Retic) and cord RBCs and reticulocytes. Proteins were quantified from at least two unique peptides. Peptides and unique peptides; the total number of peptide sequences and number of unique peptides identified for that protein. Proteome Discoverer software v1.4 was used for analysis.
| Accession | Description | System | Unique peptides | Peptides | Adult RBC/retic | Cord RBC/Retic |
|---|---|---|---|---|---|---|
| C9JGQ9 | ACHE | Yt | 5 | 5 | 2.742 | 1.406 |
| E9PC21 | AQP1 | Colton | 2 | 2 | 5.165 | 3.412 |
| Q9NP58 | ATP-binding cassette subfamily B member 6 | LAN | 14 | 15 | 0.816 | 1.166 |
| Q9UNQ0 | ATP-binding cassette subfamily G member 2 | JR | 3 | 3 | 2.204 | 2.023 |
| P02730 | Band 3 anion transport protein | Dieago | 27 | 27 | 3.826 | 3.604 |
| P50895 | Basal cell adhesion molecule | Lutheran | 10 | 10 | 2.373 | 1.155 |
| Q54A51 | Basigin | Ok | 11 | 11 | 0.954 | 1.260 |
| Q02161 | Blood group Rh(D) polypeptide | Rh | 1 | 2 | 2.872 | 8.032 |
| A6H8M8 | C4A protein | Chido/Rodgers | 1 | 1 | 6.252 | 1.575 |
| B6EAT9 | CD44 | Indian | 6 | 6 | 1.946 | 1.188 |
| E9PNW4 | CD59 | CD59 | 3 | 3 | 1.259 | 2.748 |
| A6NIW1 | CD99 antigen | Xg | 1 | 1 | 2.014 | 0.741 |
| E9PDY4 | CR1 | knops | 2 | 2 | 1.885 | 1.083 |
| Q14UF5 | Decay-accelerating factor | Cromer | 11 | 11 | 2.113 | 2.026 |
| Q93070 | Ecto-ADP-ribosyltransferase 4 | Dombrock | 3 | 3 | 3.086 | 3.262 |
| Q96PL5 | Erythroid membrane-associated protein | Scianna | 10 | 10 | 3.127 | 2.423 |
| B8Q185 | Glycophorin A MNS blood group | MNS | 3 | 3 | 12.251 | 11.878 |
| P04921 | Glycophorin-C | Gerbich | 4 | 4 | 2.685 | 2.892 |
| Q14773 | Intercellular adhesion molecule 4 | Landsteiner-Wiener | 5 | 5 | 2.414 | 4.418 |
| P23276 | Kell blood group glycoprotein | Kell | 8 | 8 | 3.245 | 2.189 |
| P51811 | Membrane transport protein XK | Kx | 6 | 6 | 2.411 | 2.520 |
| F5H250 | RHAG | Rh associated glycoprotein | 1 | 1 | 1.431 | 5.235 |
| E7EWZ5 | RHCE | Rh | 1 | 2 | 1.431 | 5.235 |
| O75326 | Semaphorin-7A | John Milton Hagen | 8 | 8 | 5.814 | 3.986 |
| E9PR61 | SLC14A1 | Kidd | 1 | 5 | 2.675 | 1.397 |
| Q13336 | Urea transporter 1 SLC14A1 | Kidd | 3 | 7 | 2.844 | 2.518 |
Previous reports of the RBC proteome include studies by Kakhniashvili et al., who identified 181 proteins (20); Pasini et al. (21), who reported 534 proteins with unique IDs; and Roux-Dalvai et al. (22), who used hexapeptide libraries to reduce the signal from hemoglobin during MS and thus maximize identification of RBC cytosolic proteins, detecting 1578 proteins. Recently, Heqedus et al. (23) performed MS for RBC membranes, identifying 419 proteins, pooling these data with that available in the literature to generate a database for the RBC membrane containing 846 proteins. D'Alessandro et al. (24) also interrogated the literature, compiling a list of 1989 RBC proteins, pathway and network analysis of which supported the concept that RBCs suffer exacerbated oxidative stress. Basu et al. (25) recently catalogued proteins of the RBC membrane skeleton identifying unexpected components such as myosin-9, lipid raft proteins, and multiple chaperone proteins. Such studies clearly reveal the power of proteomic technologies. Our data represent a significant advance in the number of erythroid proteins identified to date and was achieved for both cytosolic and membrane proteins simultaneously from the same cells. The depth of our coverage is confirmed by identification of the nine hemoglobin chains, including those low-expression chains highlighted by Roux-Dalvai et al. (22) as normally hidden by the α- and β-globin chains.
Proteomic Comparison of Protein Levels between Adult and Cord Erythroid Cells
We compared the level of proteins between the adult and cord RBCs and adult and cord reticulocytes.
Of the identified proteins, 943 membrane proteins and 731 cytosolic proteins were quantified (Supplemental Tables 4A and 4B). Of these, only 2.6% varied in level between adult and cord reticulocytes and 6% between adult and cord RBCs.
As expected, the level of β- and δ-globin was higher in adult than cord RBCs (5- and 12-fold, respectively) and reticulocytes (2.2- and 3.4-fold), whereas the level of γ-globin 1 and 2 was higher in cord than adult RBCs (12- and 66-fold, respectively) and reticulocytes (three- and ninefold). The level of ε- and ξ-globin was also higher in cord than adult RBCs (23- and 56-fold, respectively) and reticulocytes (both fourfold). The level of α-globin was consistent between adult and cord RBCs and between adult and cord reticulocytes.
We interrogated the data to ensure inclusion of proteins critical for RBC function. All proteins of the band 3-ankyrin macro-complex and 4.1R junctional complex (26–28) were quantified. Rh antigens and RhAG were identified, but as they were from only one unique peptide, their quantification was not considered. Protein levels were consistent between the membrane fraction of adult and cord RBCs and adult and cord reticulocytes (Supplemental Table 5).
We next searched for proteins with known adhesion or transport function, metabolic enzymes, and cytoskeleton proteins essential for RBC structure, identifying 137 and quantifying 92 (Supplemental Table 5).
The overall abundance of all was equivalent between adult and cord reticulocytes.
In RBCs, five proteins (carbonic anhydrase 1 and 2, aquaporin 1, BCAM [Lutheran], and semaphorin-7A) were at a higher level in the adult cells (Table II). The higher level of carbonic anhydrase 1 and 2 and aquaporin-1, along with the expression of β-globin in adult RBCs, can be attributed to known differences in gaseous exchange requirement between adults and neonates. We previously showed that transduction of cord-derived erythroid cells with transcription factors KLF1 and BCL11A-XL induces the switch from γ- to β-globin (15). Conversely, knockdown of BCL11A in adult erythroblasts reverses the globin switch, increasing the expression of γ-globin (29). We knocked down the level of BCL11A in adult erythroid cells using two different shRNA, B1 and B5 (Supplemental Fig. 2). There was a greater increase in γ-globin expression following transduction with shRNA B5, we therefore compared the protein expression profile of these cells with that of control cells using TMT labeling and MS. The level of β-globin decreased as expected, but interestingly, the level of carbonic anhydrase 1 and 2 and aquaporin-1 (Table III) was also reduced, suggesting coregulatory mechanisms for these proteins. Variation in abundance of semaphorin 7A, which carries the JMH blood group antigens, and BCAM, which carries the Lutheran blood group antigens, would not be expected to have any clinical sequalae.
Table II. Proteins that differ in amount between adult and cord RBCs.
See legend for Table I for experimental details. Values show the ratio of protein levels between adult and cord RBCs and adult and cord reticulocytes.
| Accession | Description | Unique Peptides | Peptides | RBC adult/cord | Retic adult/cord |
|---|---|---|---|---|---|
| E9PC21 | Aquaporin 1 | 2 | 2 | 2.104 | 1.565 |
| P50895 | Basal cell adhesion molecule | 10 | 10 | 3.236 | 1.560 |
| P00915 | Carbonic anhydrase 1 | 11 | 11 | 8.706 | 1.075 |
| P00918 | Carbonic anhydrase 2 | 11 | 11 | 4.976 | 1.470 |
| O75326 | Semaphorin-7A | 8 | 8 | 2.152 | 1.399 |
| P19105 | Myosin regulatory light chain 12A | 4 | 4 | 0.339 | 0.918 |
| P35579 | Myosin-9 | 83 | 96 | 0.375 | 0.796 |
| P35580 | Myosin-10 | 74 | 88 | 0.175 | 0.737 |
Table III. Alteration in protein levels following knockdown of BCL11A in adult erythroid cells.
Adult erythroid cells differentiated from peripheral blood CD34+ cells were transduced with a BCL11A shRNA at day 3 in culture, with cells collected on day 8. Cells were lysed and protein levels analysed as described in legend for Table I. Values show the ratio of protein levels between control erythroid cells and those transduced with BCL11A shRNA (BCL11A KD). Glycophorin A and band 3 were included to show that changes in protein expression following BCL11A knockdown were not generic or due to delayed differentiation. Globin subunits were quantified using high stringency, all other proteins were quantified using medium stringency peptide ID.
| Description | Unique peptides | Peptides | Control/BCL11A KD |
|---|---|---|---|
| β-globin | 2 | 13 | 7 |
| γ-globin | 7 | 8 | 0.3 |
| ϵ-globin | 2 | 3 | 0.09 |
| Aquaporin 1 | 3 | 3 | 3 |
| Carbonic anhydrase 1 | 10 | 10 | 11 |
| Carbonic anhydrase 2 | 14 | 14 | 3 |
| Band 3 | 28 | 30 | 1 |
| Glycophorin A | 2 | 3 | 1 |
Three proteins, myosin heavy chain 9 and 10 and myosin regulatory light chain 12A, all subunits of the myosin cellular motor proteins that interact with actin, were at a higher level in cord than adult RBCs (Table II). A higher level of myosin 9 has previously been reported in neonatal RBCs (30). Differences in the level of these proteins could contribute to differences in membrane deformability between cord and adult RBCs, although data pertaining to such are conflicting (31, 32). However, 40 other cytoskeleton proteins, including those critical for RBC stability, were at equivalent abundance, suggesting an overall similarity between the cell's resistance to mechanical stress.
Quantification of Western blots of adult and cord RBC lysates probed with antibodies to selected proteins corroborated the MS data (Supplemental Fig. 3). Glycophorin A and α-globin, which were consistent in level between adult and cord RBCs in the MS data, served as controls.
The similarity between the proteome of adult and cord erythroid cells makes their distinct globin expression profiles even more striking, as clearly their differences in oxygen-binding capacity are not associated with significant differences in the expression of other proteins.
Analysis of the Change in Proteome Following Reticulocyte Maturation to RBC
Detailed analysis of the change in proteome during maturation of human reticulocytes to mature RBCs has been hampered by the difficulty isolating a suitable population and amount of reticulocytes. As we were able to generate a large number of mature functional reticulocytes by in vitro culture, we were able to compare the proteome of these cells with that of the autologous endogenous RBCs using our MS data.
We first confirmed that specific proteins known to decrease in level during maturation of reticulocytes to RBCs (1, 33) did so in our study. Transferrin receptor 1, integrin β1, CD98, 65 ribosome subunits, all subunits of the sodium potassium ATPase detected, and all isoforms of tubulin detected were at a lower level in both adult and cord RBCs compared with reticulocytes.
We next looked at the change in proteome between the adult autologous reticulocytes and RBCs. A total of 943 proteins were quantified in the membrane and 728 in the cytosol fraction (Supplemental Tables 6A and 6B).
In the membrane fraction, 500 proteins were at a lower level in RBCs compared with reticulocytes; of these, 370 were reduced by 2–10-fold and 130 by >10-fold. There were just 86 proteins at a higher level in RBCs, and the number of proteins with a large magnitude of change was far less with only three proteins (glycophorin A, sorbitol dehydrogenase, and cathepsin E) >10-fold higher. In the cytosol fraction, 588 proteins were at a lower level in RBCs, comprising 464 proteins reduced by 2–10-fold and 124 by >10-fold. Only four proteins were at a higher level in RBCs compared with reticulocytes, with just carbonic anhydrase 1 > 10-fold higher. All proteins that differed in level by 10-fold or more in the membrane and cytosol fraction are shown in Supplemental Tables 7A and 7B, respectively.
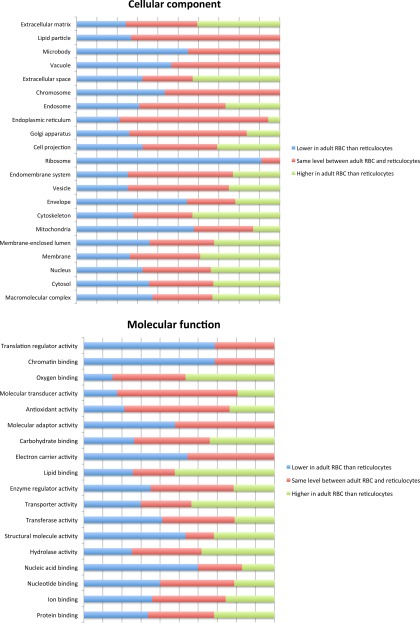
We classified the proteins by cellular component and molecular function (Fig. 1). Examples from these analyses show the majority of ribosomal and mitochondrial proteins are lost from reticulocytes as expected. The majority of proteins involved in translation regulation and chromatin binding were also lost from reticulocytes, whereas proteins involved in lipid and oxygen binding were at a higher level in RBCs than reticulocytes.
Fig. 1.
Cellular component and molecular function of proteins that decrease, increase and remain at the same level during maturation of adult reticulocytes to RBCs. Proteins from endogenous adult RBCs and reticulocytes differentiated in vitro from adult peripheral blood CD34+ cells were subjected to trypsin digest and resultant peptides labeled with TMTs for nano-LC-MS/MS based quantitation. Proteins were quantified using at least two unique peptides and analyzed using WebGestalt GSAT V2. The proportion of proteins as a percentage of the total in each of the groups, cellular component, and molecular function, was calculated.
As there was a distinct difference in the number of proteins that increased in level in the membrane compared with cytosol fraction of RBCs, we questioned whether some proteins underwent differential partitioning between the cytosol to the membrane during maturation. To address this, we interrogated our MS data for proteins at a higher level in the RBC than reticulocyte membrane fraction, with a corresponding decrease in level in the RBC cytosol (Supplemental Table 8). Notably, this included a disproportionate number of proteins belonging to the band 3-ankyrin and 4.1R junctional membrane complexes. To determine whether other proteins in these complexes displayed the same trend, we employed reduced stringency of one unique peptide, which, although below our normal robust stringency of two unique peptides per protein for comparative analysis, still ensures that the peptide is specific to that protein and gives an indication of abundance. Using two or more unique peptides reassures consistency between comparisons. Using this approach, we found that all proteins detected displayed such partitioning (Supplemental Table 9) in both adult and cord cells. This may be due to proportional loss of other membrane proteins, but more likely, re-localization of proteins from the cytosol to the membrane occurs during reticulocyte maturation reflecting the known loss of intracellular endocytic pools of proteins during maturation (1, 34) Data for selected proteins were confirmed by quantification of Western blots (Supplemental Fig. 4).
Using the reduced stringency, we also found all 14 α and β proteasome subunits at a higher level in RBCs than reticulocytes, which, along with the identification of other proteasome subunits, support a function for proteasome in RBCs.
Finally, we examined the profile of all globin subunits in the cytosol fraction (Table IV). In adult cells, the level of β- and δ-globin was consistent between reticulocytes and RBCs; however, the level of gamma 1 and 2, and ε- and ζ-globin was lower in RBCs than reticulocytes. In cord cells, the level of both γ subunits and ε- and ζ-globin was consistent between reticulocytes and RBCs, but the level of β- and δ-globin was lower in RBCs than reticulocytes. The level of α-globin was consistent. These data suggest selective loss of globin subunits during reticulocyte maturation or differences in the stability of different mRNAs resulting in the continued translation of some but not other transcripts. Similar mechanisms may function for other proteins, as overall levels were more consistent between adult and cord reticulocytes than RBCs.
Table IV. Comparing the level of globin subunits between adult and cord erythroid cells, and between RBCs and reticulocytes.
See legend for Table I for experimental details. Proteins were analysed in the cytosol fraction of cells. Values show the ratio of protein levels between RBCs and reticulocytes (Retic), and between adult and cord cells.
| Accession | Globin subunit | Unique peptides | Peptides | RBC adult/cord | Retic adult/cord | Adult RBC/retic | Cord RBC/retic |
|---|---|---|---|---|---|---|---|
| P69905 | Alpha | 13 | 14 | 1.115 | 0.951 | 0.894 | 0.725 |
| P68871 | Beta | 8 | 16 | 4.842 | 2.203 | 0.970 | 0.450 |
| P02042 | Delta | 6 | 14 | 11.660 | 3.431 | 0.901 | 0.268 |
| D9YZU8 | Gamma-1 | 4 | 15 | 0.085 | 0.328 | 0.253 | 0.933 |
| P69892 | Gamma-2 | 3 | 14 | 0.015 | 0.106 | 0.174 | 1.171 |
| P02100 | Epsilon | 3 | 6 | 0.044 | 0.281 | 0.123 | 0.968 |
| P02008 | Zeta | 8 | 9 | 0.018 | 0.231 | 0.119 | 1.291 |
| Q6B0K9 | Mu | 5 | 5 | 0.206 | 0.362 | 0.161 | 0.496 |
| P09105 | Theta-1 | 3 | 3 | 0.627 | 0.690 | 0.720 | 0.652 |
CONCLUSION
Our data provide the most comprehensive identification to date of the RBC proteome, and insight into the change in proteome during maturation, which will serve as a useful resource. Apart from the known differences in globin expression and key proteins known to be involved in gaseous exchange (aquaporin 1 and carbonic anhydrase 1 and 2), these data indicate the proteome of adult and cord erythroid cells are reassuringly similar. Thus, with respect to the proteome, there is no detectable barrier to using cord progenitors for the in vitro generation of RBCs for adult therapeutics other than the known differences in oxygen uptake and release of HbF and HbA.
Supplementary Material
Footnotes
Author contributions: D.J.A. and J.F. designed the research; M.C.W., K.T., K.J.H., N.C., and C.G. performed the research; M.C.W., K.T., A.M.T., D.J.A., and J.F. analyzed data; J.F. and A.M.T. wrote the paper; and S.F.P. advised research.
* This research was funded by the National Institute for Health Research Blood and Transplant Unit (NIHR BTRU) in Red Blood Cell Products at the University of Bristol in Partnership with NHS Blood and Transplant (NHSBT). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
 This article contains supplemental material.
This article contains supplemental material.
The accession number F5H250 for RhAG has been updated to Q02094.
The authors declare no competing financial interests.
1 The abbreviations used are:
- RBC
- red blood cell
- PB
- peripheral blood
- TMT
- tandem mass tag
- PMSF
- phenylmethylsulfonyl fluoride
- PBS
- phosphate buffered saline
- MS/MS
- tandem mass spectrometry
- nanoLC
- nano liquid chromatography
- MS
- mass spectrometry
- ABH
- blood group antigens
- KLF
- Kruppel-like factor
- BCL
- B-cell lymphoma
- BCAM
- basal cell adhesion molecule
- JMH
- John Milton Hagen
- HbF
- fetal hemoglobin
- HbA
- adult hemoglobin.
REFERENCES
- 1. Griffiths R. E., Kupzig S., Cogan N., Mankelow T. J., Betin V. M., Trakarnsanga K., Massey E. J., Lane J. D., Parsons S. F., and Anstee D. J. (2012) Maturing reticulocytes internalize plasma membrane in glycophorin A-containing vesicles that fuse with autophagosomes before exocytosis. Blood 119, 6296–6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fujimi A., Matsunaga T., Kobune M., Kawano Y., Nagaya T., Tanaka I., Iyama S., Hayashi T., Sato T., Miyanishi K., Sagawa T., Sato Y., Takimoto R., Takayama T., Kato J., Gasa S., Sakai H., Tsuchida E., Ikebuchi K., Hamada H., and Niitsu Y. (2008) Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int. J. Hematol. 87, 339–350 [DOI] [PubMed] [Google Scholar]
- 3. Giarratana M. C., Kobari L., Lapillonne H., Chalmers D., Kiger L., Cynober T., Marden M. C., Wajcman H., and Douay L. (2005) Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat. Biotechnol. 23, 69–74 [DOI] [PubMed] [Google Scholar]
- 4. Dias J., Gumenyuk M., Kang H., Vodyanik M., Yu J., Thomson J. A., and Slukvin II. (2011) Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Develop. 20, 1639–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trakarnsanga K., Wilson M. C., Griffiths R. E., Toye A. M., Carpenter L., Heesom K. J., Parsons S. F., Anstee D. J., and Frayne J. (2014) Qualitative and quantitative comparison of the proteome of erythroid cells differentiated from human iPSCs and adult erythroid cells by multiplex TMT labelling and nanoLC-MS/MS. PloS One 9, e100874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu S. J., Feng Q., Park J. S., Vida L., Lee B. S., Strausbauch M., Wettstein P. J., Honig G. R., and Lanza R. (2008) Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood 112, 4475–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanavde V. M., Malehorn M. T., Lumkul R., Gao Z., Wingard J., Garrett E. S., and Civin C. I. (2002) Human stem-progenitor cells from neonatal cord blood have greater hematopoietic expansion capacity than those from mobilized adult blood. Exp. Hematol. 30, 816–823 [DOI] [PubMed] [Google Scholar]
- 8. Bhattacharya N., and Stubble P. (2009) Frontiers of Cord Blood Science. London: Springer-Verlag [Google Scholar]
- 9. Greer J. P., Arber D. A., Glader B., List A. F., Means R. T. J., Paraskevas F., Rodgers G. M., and Foerster J. (2014) Fetal and neonatal erythrocyte membrane and metabolism. In Wintrobes Clinical Hematology, 13th ed Walters Kluwer, Lippincott Williams & Wilkins: Philadelphia [Google Scholar]
- 10. Bell A. J., Satchwell T. J., Heesom K. J., Hawley B. R., Kupzig S., Hazell M., Mushens R., Herman A., and Toye A. M. (2013) Protein distribution during human erythroblast enucleation in vitro. PloS One 8, e60300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mankelow T. J., Griffiths R. E., Trompeter S., Flatt J. F., Cogan N. M., Massey E. J., and Anstee D. J. (2015) Autophagic vesicles on mature human reticulocytes explain phosphatidylserine-positive red cells in sickle cell disease. Blood 126, 1831–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu J., Guo X., Mohandas N., Chasis J. A., and An X. (2010) Membrane remodeling during reticulocyte maturation. Blood 115, 2021–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richardson B. M., Heesom K. J., Parsons S. F., Anstee D. J., and Frayne J. (2009) Analysis of the differential proteome of human erythroblasts during in vitro erythropoiesis by 2-D DIGE. Proteomics Clin. Appl. 3, 1123–1134 [DOI] [PubMed] [Google Scholar]
- 14. Senol O., Schaaij-Visser T. B., Erkan E. P., Dorfer C., Lewandrowski G., Pham T. V., Piersma S. R., Peerdeman S. M., Ströbel T., Tannous B., Saydam N., Slavc I., Knosp E., Jimenez C. R., and Saydam O. (2015) miR-200a-mediated suppression of non-muscle heavy chain IIb inhibits meningioma cell migration and tumor growth in vivo. Oncogene 34, 1790–1798 [DOI] [PubMed] [Google Scholar]
- 15. Trakarnsanga K., Wilson M. C., Lau W., Singleton B. K., Parsons S. F., Sakuntanaga P., Kurita R., Nakamura Y., Anstee D. J., and Frayne J. (2014) Induction of adult levels of beta-globin in human erythroid cells that intrinsically express embryonic or fetal globin by transduction with KLF1 and BCL11A-XL. Haematologica 99, 1677–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Satchwell T. J., Hawley B. R., Bell A. J., Ribeiro M. L., and Toye A. M. (2015) The cytoskeletal binding domain of band 3 is required for multiprotein complex formation and retention during erythropoiesis. Haematologica 100, 133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vizcaíno J. A., Deutsch E. W., Wang R., Csordas A., Reisinger F., Rios D., Dianes J. A., Sun Z., Farrah T., Bandeira N., Binz P. A., Xenarios I., Eisenacher M., Mayer G., Gatto L., Campos A., Chalkley R. J., Kraus H. J., Albar J. P., Martinez-Bartolomé S., Apweiler R., Omenn G. S., Martens L., Jones A. R., and Hermjakob H. (2014) ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 32, 223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marsh W. L. (1961) Anti-i: A cold antibody defining the Ii relationship in human red cells. Br. J. Haematol 7, 200–209 [DOI] [PubMed] [Google Scholar]
- 19. Marsh W. L., Nichols M. E., and Reid M. E. (1971) The definition of two I antigen components. Vox Sanguinis 20, 209–217 [DOI] [PubMed] [Google Scholar]
- 20. Kakhniashvili D. G., Bulla L. A. Jr., and Goodman S. R. (2004) The human erythrocyte proteome: Analysis by ion trap mass spectrometry. Mol. Cell. Proteomics 3, 501–509 [DOI] [PubMed] [Google Scholar]
- 21. Pasini E. M., Kirkegaard M., Mortensen P., Lutz H. U., Thomas A. W., and Mann M. (2006) In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood 108, 791–801 [DOI] [PubMed] [Google Scholar]
- 22. Roux-Dalvai F., Gonzalez de Peredo A., Simó C., Guerrier L., Bouyssié D., Zanella A., Citterio A., Burlet-Schiltz O., Boschetti E., Righetti P. G., and Monsarrat B. (2008) Extensive analysis of the cytoplasmic proteome of human erythrocytes using the peptide ligand library technology and advanced mass spectrometry. Mol. Cell. Proteomics 7, 2254–2269 [DOI] [PubMed] [Google Scholar]
- 23. Hegedus T., Chaubey P. M., Varady G., Szabo E., Saranko H., Hofstetter L., Roschitzki B., Stieger B., and Sarkadi B. (2015) Inconsistencies in the red blood cell membrane proteome analysis: Generation of a database for research and diagnostic applications. Database (Oxford), bav056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'Alessandro A., Righetti P. G., and Zolla L. (2010) The red blood cell proteome and interactome: An update. J. Proteome Res. 9, 144–163 [DOI] [PubMed] [Google Scholar]
- 25. Basu A., Harper S., Pesciotta E. N., Speicher K. D., Chakrabarti A., and Speicher D. W. (2015) Proteome analysis of the triton-insoluble erythrocyte membrane skeleton. J. Proteomics 128, 298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bruce L. J., Beckmann R., Ribeiro M. L., Peters L. L., Chasis J. A., Delaunay J., Mohandas N., Anstee D. J., and Tanner M. J. (2003) A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 101, 4180–4188 [DOI] [PubMed] [Google Scholar]
- 27. Mohandas N., and Gallagher P. G. (2008) Red cell membrane: Past, present, and future. Blood 112, 3939–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van den Akker E., Satchwell T. J., Williamson R. C., and Toye A. M. (2010) Band 3 multiprotein complexes in the red cell membrane; of mice and men. Blood Cells Mols Dis 45, 1–8 [DOI] [PubMed] [Google Scholar]
- 29. Sankaran V. G., Menne T. F., Xu J., Akie T. E., Lettre G., Van Handel B., Mikkola H. K., Hirschhorn J. N., Cantor A. B., and Orkin S. H. (2008) Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322, 1839–1842 [DOI] [PubMed] [Google Scholar]
- 30. Matovcik L. M., Gröschel-Stewart U., and Schrier S. L. (1986) Myosin in adult and neonatal human erythrocyte membranes. Blood 67, 1668–1674 [PubMed] [Google Scholar]
- 31. Gross G. P., and Hathaway W. E. (1972) Fetal erythrocyte deformability. Pediatric Res. 6, 593–599 [PubMed] [Google Scholar]
- 32. Johnson R. M., Panchoosingh H., Goyette G. Jr., and Ravindranath Y. (1999) Increased erythrocyte deformability in fetal erythropoiesis and in erythrocytes deficient in glucose-6-phosphate dehydrogenase and other glycolytic enzymes. Pediatric Res. 45, 106–113 [DOI] [PubMed] [Google Scholar]
- 33. Ney P. A. (2011) Normal and disordered reticulocyte maturation. Curr. Opin. Hematol. 18, 152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Satchwell T. J., Bell A. J., Pellegrin S., Kupzig S., Ridgwell K., Daniels G., Anstee D. J., van den Akker E., and Toye A. M. (2011) Critical band 3 multiprotein complex interactions establish early during human erythropoiesis. Blood 118, 182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang B., Kirov S. A., Snoddy J. R. (2005). WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res, 33 (Web server issue), W741–W748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.