Abstract
Gastroesophageal reflux disease (GERD) has become the most commonly seen gastrointestinal disorder in outpatient clinics. In the United States, around 20% of the general population experience heartburn on a weekly basis. Although clinical complaints can be mild or moderate, patients with GERD may develop further complications, such as peptic strictures, Barrett's esophagus (BE), and even esophageal adenocarcinoma (EAC). Pathologically, GERD is developed as a result of chronic and enhanced exposure of the esophageal epithelium to noxious gastric refluxate. In this review article, we provide an overview of GERD, and then focus on the roles of stromal cells, interleukin 4 (IL-4), and adiponectin in GERD and BE. The importance of inflammation and immunomodulators in GERD pathogenesis is highlighted. Targeting the immunomodulators or inflammation in general may improve the therapeutic outcome of GERD, in particular, in those refractory to proton pump inhibitors.
Keywords: gastroesophageal reflux disease, Barrett's esophagus, interleukin 4, adiponectin, stromal cells
Gastroesophageal reflux disease (GERD) is a common disorder, affecting 18.1–27.8% of the population in the United States.1,a Epidemiologic studies strongly suggest that the prevalence of GERD is increasing, especially compared with the prevalence found in studies published in 1992 (approximately 13.2%).2 GERD is also increasingly prevalent in Asia, with a prevalence of approximately 8%.1 GERD is mainly treated with lifestyle modifications, pharmacotherapy, and occasionally antireflux surgery. A recent meta-analysis of the role of lifestyle intervention has shown that weight loss and tobacco smoking cessation significantly reduced esophageal acid exposure. Avoiding late evening meals and head-of-the-bed elevation also modified supine acid exposure.3
Pharmacotherapy, especially antacid therapy with proton pump inhibitors (PPIs), remains the mainstay in the management of GERD4,5 (Fig. 1). Ever since their introduction in the late 1980s, PPIs have revolutionized the management of GERD and peptic ulcer disease, with substantial reductions in morbidity and mortality. Their efficacy and safety have been proved in long-term clinical follow-up studies.6,7 In the past three decades, PPIs have been among the most commonly prescribed medications worldwide. In fact, PPIs tend to be overused and overdosed due to the over-the-counter availability. However, as many as 10–40% of patients find their reflux symptoms inadequately controlled, especially at night.8,9 Poor patient compliance, reduced PPI bioavailability, weakly acidic or non-acidic reflux, nocturnal breakthrough, esophageal hypersensitivity, functional gastrointestinal disorders, and even mental disorders may all contribute to PPI failures.8,10,11 For those who do respond to PPI therapy, long-term treatment with PPIs not only generates a financial burden, but may also produce side effects. For example, PPIs have been showed to promote cancer promotion in animal models owing to hypergastrinemia12,13 or CYP1A1 induction.14 Hypochlorhydria is likely to cause nutritional malabsorption and, subsequently, increased risks of bone fracture, bacterial infections, and interstitial nephritis. Drug–drug interactions, for example, interference with the antiplatelet effect of clopidogrel, may also be a concern of clinical significance.15–17 Therefore, it is still quite important to study the mechanisms of GERD and its complications, Barrett's esophagus (BE) and esophageal adenocarcinoma (EAC), and to develop additional treatment modalities. It should be noted that putative risks associated with PPIs are often based on observational epidemiological studies with low odds ratios, and should not be exaggerated.
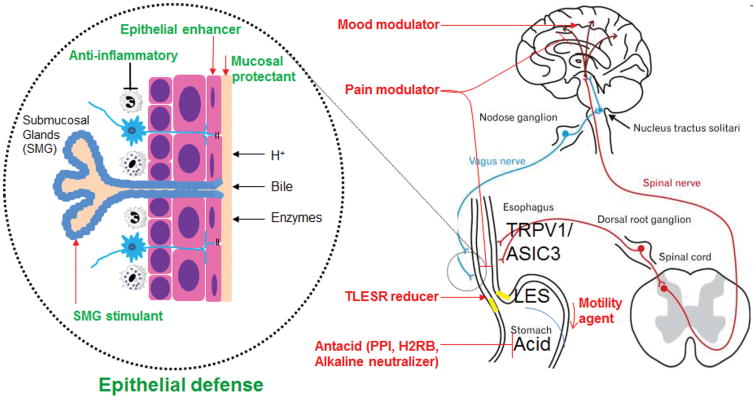
Figure 1.
Current and potential pharmacotherapy of GERD. TRPV1 and ASIC3 are two major receptors for chemical and mechanical stimuli leading to heartburn. Transient lower esophageal sphincter relaxation reducers failed in clinical trials. Mucosal protectants (e.g., Esoxx) are of limited efficacy.117 Epithelial enhancers, anti-inflammatory agents or SMG stimulants have not yet been developed to strengthen esophageal defense against GERD. Repamipide, an antiulcer agent acting through temporary stimulation of endogenous prostaglandins, has not been well studied for its potentially protective effect on esophageal epithelium
The esophageal defense mechanism is made up of pre-epithelial defense, epithelial defense, and postepithelial defense. Pre-epithelial defense relies on esophageal clearance by peristalsis and gravity and the buffer supplied by the saliva and esophageal submucosal glands. The combination of apical junction complexes and apical cell membranes of cells within the stratum corneum largely accounts for the defensive ability of esophageal epithelium.18 The apical junction complexes are highly complex organelles, including tight junctions, adherens junctions, and desmosomes.19,20 For tight junctions in esophageal epithelium, occludin and claudins (CLDNs) are the major proteins bridging the intercellular space, with CLDN1 and CLDN4 being the most highly expressed.21,22 In adherens junctions, E-cadherin is the major bridging protein,23 and, in desmosomes, desmogleins and desmocollin are the major ones.24
In the presence of gastroesophageal reflux, the esophageal epithelium responds to the acidic injury with altered apical junction complexes, increased paracellular permeability, and dilated intercellular space.25 These morphological changes are often due to changes in the expression and localization of tight junction proteins (occludin, CLDN1, CLDN4), the number of desmosomes, and cleavage of the adherens junction protein E-cadherin.26–30 CLDN4 is believed to be a major tight junction protein contributing to barrier function in esophageal squamous epithelium, because CLDN4 overexpression in MDCK II cells increases epithelial resistance and lowers paracellular permeability for cations.21,31 CLDN4 deficiency causes urothelial hyperplasia and progressive hydronephrosis.32 CLDN1 deficiency causes postnatal death due to skin barrier defect to the water loss.33 However, esophageal phenotypes in CLDN1- or CLDN4-deficient mice have not been reported.
Following the initial change in the esophageal epithelium, inflammation takes place in the esophageal epithelium.34,35 Using surgical models of gastroesophageal reflux in rats, we have clearly shown that anti-inflammatory agents have chemopreventive effects on adenocarcinoma.36–38 In humans, the use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with lower risk of BE and EAC, although long-term double-blind clinical trials are still needed to validate this conclusion.39 Mechanistically, inflammation is triggered by the release of various immunomodulators when cells are stimulated by reflux. Inflammation promotes and perpetuates injury of the esophageal epithelium directly by attenuating epithelial barrier function and indirectly by altering neuromuscular transmission of esophageal smooth muscle. In addition to inflammation, oxidative stress subsequent to reflux also induces dilated intercellular space and impairs the barrier function.40–42 Once inflammation becomes chronic, it reduces lower esophageal sphincter pressure, impairs peristaltic contractility, contracts the longitudinal muscle, and promotes the formation of hiatal hernia. It is very likely that a vicious cycle is formed between inflammation and epithelial injury to facilitate GERD pathogenesis (Fig. 2).
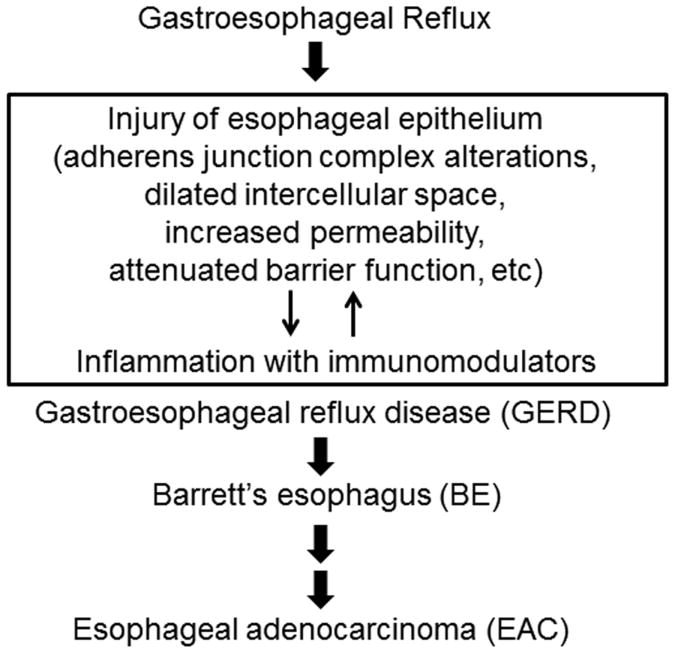
Figure 2.
Critical role of inflammation in the development of GERD and subsequent complications. This mechanistic model suggests that agents targeting immunomodulators or inflammation in general can be potentially used for GERD therapy.
In this review, we focus on three major players in the pathogenesis of GERD and its complications. Stromal cells are major producers of immunomodulators apart from epithelial cells. Interleukin 4 (IL-4) is one of the proinflammatory cytokines that participates in intestinal metaplasia. As an anti-inflammatory adipokine secreted by adipose tissues, low adiponectin partially explains how obesity contributes to GERD and its complications.
Stromal cells
The importance of the stroma in maintaining epithelial integrity, contributing to wound healing, and promoting the tumor microenvironment has been established in a number of tissues. The complex stromal microenvironment contains several cell types, including fibroblasts, smooth muscle cells, immune and inflammatory cells, and endothelial cells.43 Under normal conditions, fibroblasts have a low proliferative index and primarily secrete factors necessary to maintain tissue homeostasis. In the wound-repair response, activated fibroblasts are characterized by high proliferative index, synthesis of extracellular matrix, and secretion of growth factors and remodeling enzymes necessary for tissue repair. This activation is self-limited, however, and once the wound is healed, activated fibroblasts revert to their quiescent state. In cancer, the stroma remains reactive and promotes tumor growth and invasion.43–45 The cellular and molecular mechanisms mediating wound healing, repair, and malignancy in the esophagus are topics of ongoing investigation.
Several lines of evidence support a broad range of activity for esophageal stromal cells, including modulation of mucosal damage and effects on esophageal motility. Evidence from work in other organ systems suggests that the main effector cells in the esophagus are likely mesenchymal cell derivatives, such as fibroblasts, myofibroblasts, and smooth muscle cells.46 In response to an inflammatory environment, stromal cells, such as fibroblasts, expand in number (proliferate) and migrate toward a chemotactic gradient.46 Molecules inducing migration include fibronectin, platelet-derived grown factor (PDGF)-A and PDGF-B, insulin-like growth factor 1 (IGF-1), and epidermal growth factor (EGF).46 In eosinophilic esophagitis, subepithelial fibrosis is thought to be a major cause of dysphagia. Elevated T helper 2 cytokines, the profibrotic mediator TGF-β1, and eosinophil products induce fibrogenesis via induction of esophageal fibroblast production and secretion of fibronectin and collagen I.47 In addition, incubation of fibroblasts with eosinophil products promotes eosinophil binding via increased expression of vascular cell adhesion molecule 1 (VCAM-1).48 An increase in a large number of cytokines and chemokines has been detected in mucosal biopsy specimens of GERD patients, including IL-1β, IL-6, IL-8, IL-10, interferon γ (IFNγ), monocyte chemoattractant protein 1 (MCP-1), and RANTES.46 Cellular sources of these cytokines include epithelial cells, immune cells, and a heterogeneous population of stromal cells.
Myofibroblasts are a subpopulation of stromal cells best characterized in the lower gastrointestinal tract located at the interface between the epithelium and the lamina propria. Distinguishing features of these cells are a spindle-shaped morphology and expression of α-SMA and vimentin and weak or inconsistent desmin expression.49 They participate in injury and repair, inflammation, and carcinogenesis, at least in part via paracrine mechanisms.50 The importance of this population of stromal cells and the critical role of epithelial–stromal interactions is further demonstrated by their role in regulating epithelial cell proliferation and in contributing to the stem cell niche.50,51 Spindle-shaped α-SMA– and vimentin-expressing, non-immune, non-endothelial cells have been observed near the stratified squamous epithelium of neonatal and adult murine esophagus52 and normal adult human esophagectomy specimens.53 On the basis of morphology and protein expression, these cells were termed myofibroblasts. Vimentin-expressing fibroblasts and endothelial cells populated the remainder of the subepithelial stroma of the normal human esophagus. In GERD esophageal biopsies with sufficient lamina propria, an increase in α-SMA+ non-endothelial cells, along with an increase in extracellular stromal IL-6 and nuclear factor κB (NF-κB) activation, was observed.53 These findings further implicated these cells in disease pathogenesis.
The molecular mechanisms regulating the role of esophageal stromal cells have been investigated in primary cultures of fibroblasts and myofibroblasts. Primary fibroblast cultures derived from freshly resected human histologically normal esophagectomy specimens48 and subcultured in medium used for skin fibroblasts produce proinflammatory cytokines. Interestingly, these cells spontaneously produced low IL-6 levels, which markedly increased after stimulation with IFN-ɣ+ PMA and particularly with IL-1β stimulation. IL-1β and tumor necrosis factor α (TNF-α) were not detected in unstimulated or stimulated cell supernatants.48
In a separate study, esophageal stromal cells were derived from the mucosa of histologically normal human esophagectomy specimens. These cells were spindle shaped, expressed α-SMA and vimentin, and lacked expression of endothelial and hematopoietic markers53 in primary culture and were therefore termed myofibroblasts. Immunocytochemistry and immunoblotting showed that human esophageal myofibroblasts expressed the putative acid receptor transient receptor potential channel vanilloid subfamily 1 (TRPV1).53 Increased TRPV1 gene and protein expression has been reported in nonerosive reflux disease and erosive esophagitis biopsies.54 Primary cultures of human esophageal myofibroblasts responded to non-cytotoxic pH-4 acidified media with IL-6 and IL-8 secretion.53 Treatment of esophageal myofibroblasts with the TRPV1 competitive antagonist AMG9810 inhibited IL-6 secretion in response to acidified media.53
Human esophageal myofibroblasts in primary culture also express the innate immune system activator toll-like receptor 4 (TLR4) mRNA and protein and proinflammatory cytokines in response to TLR4 ligands LPS and HMGB1.53 These cells secrete IL-6 in response to acid and TLR4 ligand injurious agents encountered in GERD via NF-κB activation.53 In this study, human esophageal myofibroblast secretion of IL-1β was not detected in response to acidified media or to TLR4 ligands.53
Expression of TLR4 has been evaluated in the human esophagus. TLR4 expression progressively increased from reflux esophagitis patients, to patients with BE, to those with EAC.44 Examination of TLR4 expression in normal and reflux esophagitis biopsies with quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and in situ hybridization showed a 1.9-fold increase in TLR4 expression in reflux esophagitis, largely confined to the basal layer of the epithelium.55 Immunostaining of normal squamous epithelium showed cytoplasmic expression of TLR4 in keratinocytes located at the basal layer of the epithelium.55 In reflux esophagitis biopsies, expression was also observed in more superficial epithelial layers.55 Protein expression was shown in images of lamina propria papillae, although this has not been explored further. The presence of lamina propria was not required in these biopsies, however, and therefore not specifically evaluated. In biopsies from BE and EAC patients, where lamina propria was readily available, however, nuclear and cytoplasmic TLR4 expression was present in a variety of cells, including inflammatory cells (plasma cells, lymphocytes), fibroblasts, muscle cells, and endothelial cells.55
The potential physiologic consequences of esophageal stromal cell proinflammatory mediator production have also been investigated. For example, the proinflammatory cytokines IL-1β and IL-6 reduce esophageal circular muscle contraction in an experimental cat esophagitis model.56 Endoscopic mucosal biopsies from the distal human esophagus with esophagitis cultured on Transwell inserts demonstrate enhanced secretion of IL-1β and IL-6 compared with non-esophagitis mucosal biopsies.48 Furthermore, soluble products of mucosal organ cultures from the lower third of the esophagus from esophagitis patients reduced esophageal circular muscle contraction, an effect not observed using mucosa of patients with GERD symptoms only.48 To investigate the cellular source of these mediators, the effect of supernatants from untreated primary esophageal cells, including epithelial cells, muscle cells, and fibroblasts, on the inhibition of contraction of esophageal circular muscle strips was evaluated.48 Supernatants from epithelial cells were most effective at reducing muscle cell contractility, followed by muscle cells. Interestingly, supernatants from unstimulated fibroblasts had no effect on muscle cell contractility in this study. The effects of supernatants isolated from stimulated fibroblasts on muscle contractility were not examined. Furthermore, sample limitations precluded definitive identification of the secreted factor responsible for the effects on muscle contractility.
The concept that the esophageal stroma is more than a bystander has also been investigated in esophageal cancer, with evidence that the stroma is an active participant in the regulation of tumor invasion and progression. Fibroblasts in the tumor microenvironment are termed cancer-associated fibroblasts (CAFs). While the cellular origins of esophageal CAFs remain a topic of ongoing inquiry, potential sources are thought to include circulating mesenchymal stem cells, pericytes, resident tissue fibroblasts, and transdifferentiation of epithelial and endothelial cells.57 Studies from a number of malignancies have demonstrated that CAFs affect tumor growth, survival, progression, and dissemination through a variety of processes, including supporting tumor cell proliferation and survival through secretion of growth factors, tumor cell dissemination through formation of a metastatic niche, angiogenesis, extracellular matrix remodeling, and regulation of the innate and adaptive immune system. Although de novo expression of α-SMA is frequently used as a marker of CAFs, α-SMA does not exclusively label CAFs, and definitive markers for CAFs are still lacking.57 In the esophagus, the source of stromal fibroblasts (adult versus fetal) and the state of activation of AKT signaling are determinants of whether esophageal squamous cell tumor cells are invasive.58 Gene expression analysis has demonstrated that 43% of the deregulated genes from CAFs isolated from esophageal squamous cell carcinoma compared to adjacent non-tumorous esophageal tissue stromal genes were associated with cell proliferation, extracellular matrix remodeling, and immune response, including several members of the Wnt family, such as WNT2.59 Wnt2 secreted from CAFs has subsequently been shown to promote the growth and invasion of esophageal cancer cells through activation of the canonical Wnt/β-catenin signaling pathway and by inducing the epithelial–mesenchymal transition.60 Additional studies have demonstrated that esophageal stromal fibroblasts mediate proliferation, angiogenesis, and invasion via autocrine and paracrine mechanisms involving secretion of a number of other mediators, including fibroblast grown factor (FGF), hepatocyte grown factor (HGF), vascular endothelial growth factors (VEGFs), TGF-β1, periostin, and podoplanin.44
In conclusion, several lines of evidence suggest that esophageal stromal cells have a broader range of activity than a simple structural role. Secreted factors from esophageal stromal cells have been implicated in the pathogenesis of inflammatory esophageal disorders, such as GERD, eosinophilic esophagitis, and esophageal malignancy. The role of esophageal stromal cells as potential immunomodulators of the inflammatory response in these disorders therefore remains an area of intense investigation.
Interleukin 4
The prevalence of GERD is around 10%, and 2–4% of GERD patients develop BE every 10 years.61 BE is characterized by the replacement of normal esophageal squamous epithelium with intestinalized columnar epithelium. BE is a premalignant condition, and BE progresses into EAC at a rate of 0.12–0.14% per year. There has been substantial controversy over the years regarding the origin of the cells of BE. The metaplastic conversion of squamous to columnar epithelium may develop through transdifferentiation of native squamous cells or through transcommitment of resident squamous stem cells. According to the stem cell hypothesis, stem cells may come from at least three sources: resident squamous stem cells, bone marrow–derived stem cells, and submucosal glandular stem cells.62 The dominant concept is that squamous stem cells may differentiate into columnar lineages with intestinal characteristics.63 The multilayered epithelium that is observed in BE has histological features of both squamous and columnar epithelia, and represents an early or intermediate stage in the development of BE, suggesting that the stratified squamous epithelium can give rise to BE.
Triggers of metaplastic induction of BE
Gastroesophageal reflux of acid and bile acids induces esophageal mucosal damage and an increase in permeability due to tight junction damage.27,64 Exposure of the stem cells to acid and bile acids may induce the activation of the columnar differentiation pathway. However, in animal models, acid reflux by itself did not induce BE.65 As reflux of acid with bile acids increases in BE rather than in GERD without BE, chronic reflux appears to play a crucial role in the development of BE.66 Previous reports indicate that acid and/or bile acids induce CDX2 in esophageal epithelial cells.63 The regurgitation of acid with bile acids can induce BE-like lesions in animal models.65
TH1 and TH2 cytokines in GERD and BE
In addition to direct stimulation by intraluminal reflux contents, the esophageal epithelial layer is also exposed to factors produced by chronic inflammatory cells from the basal side. Mucosal inflammation is a common mechanism of epithelial metaplasia in many tissues, and induces phenotypical changes in the cells.67 Studies have shown an increase in proinflammatory TH1 cytokines, including IL-1β, IL-8, IFN-γ, and TNF-α, in GERD compared to BE,68 whereas TH2 cytokines are predominant in BE.68,69 There is a fourfold increase in IL-4 in BE only. If the IL-4 level is normalized for T cell receptor expression, IL-4 expression increases by 100-fold in BE.68 In addition, the proportion of TH2 effector cells, including plasma cells and mast cells, is higher in BE than in GERD patients.69 However, these data are observational and cannot explain the causality of IL-4 in the development of BE.
A recent study using an animal model of BE showed that upregulation of IL-4 mRNA preceded the increase in CDX2 mRNA.70 These data indicate that IL-4 plays an important role in the early phase of the development of BE. It has also been reported that IL-4–producing CD4+ T cells cause goblet cell transformation in the small intestine,71 and that T-cells within BE tissue are mainly IL-4+CD8− cells.72 As IL-4 inhibits TH1 responses by inhibiting IFN-γ production and TH1 differentiation, an increase in IL-4–producing T cells may play a role in switching the balance from a TH1 response in GERD to a TH2 response in BE. However, the detailed role of these cytokines, including IL-4, in the development of BE remains unknown.
The roles of TH1 and TH2 cytokines in the differentiation of esophageal epithelial cells had not been assessed because of the lack of a research model for assessment of esophageal epithelial differentiation. Previous in vitro studies of esophageal epithelial differentiation were performed using monolayers of immortalized or cancer cell lines73 that could not assess metaplastic change. To address this problem, we recently developed a primary cell culture model of esophageal squamous epithelial cells that represents human esophageal epithelial layers in vivo with respect to morphology, molecular marker expression, and barrier function.64,74 As the origin of the cells is normal esophageal epithelium, squamous stem cells in the basal layers may potentially be transcommitted to columnar epithelial cells under certain conditions. In addition, this model makes cellular changes, such as an increase in cell layers, a change in basal cell morphology, and localization of specific markers, readily visible. We evaluated the effects of TH1 and TH2 cytokines on esophageal epithelial differentiation using this model. IL-4 induced columnar differentiation of the esophageal stratified squamous layers and induced the expression of the columnar cell markers KRT7 and KRT8 through the JAK-PI3K pathway.75 In contrast, IL-4 decreased the expression of the squamous cell markers involucrin and KRT13. The columnar cell markers KRT7 and KRT8 are known to be expressed in BE.76 KRT7 is an early marker of GERD-related columnar mucosa without intestinal metaplasia.76 These data are reminiscent of the multilayered epithelium observed in BE patients that has both squamous and columnar histological features.77
Based on our in vitro study, IL-4 downregulates squamous cell differentiation and induces columnar-like differentiation. However, IL-4 does not induce the markers of BE, including CDX-2, MUC5AC, and MUC2, or the complete transdifferentiation of BE in the stratified squamous epithelial layers. This finding is similar to findings obtained with other factors that may affect the development of BE, for instance bone morphogenetic protein 4,78 which induces the expression of columnar types of genes in esophageal cells but does not lead to intestinal metaplasia.79 These data indicate that, in addition to a chronic inflammatory environment, other triggers, including acid and bile acids, might cooperatively induce metaplastic transformation.63
It is still not clear what modifies the cytokine profiles from TH1-predominant GERD to TH2-predominant BE. An alteration of the microbiome at the distal esophagus may be one of the mechanisms. IL-4 induces columnar-like differentiation of esophageal squamous epithelial cells, irrespective of whether TH2 cytokines are the initial factors or the maintaining factors in BE. Therefore, clarifying the mechanisms involved in the transition of TH1 to TH2 cytokines in the development of BE may give us new insights for preventing BE.
In conclusion, IL-4 induces columnar-like differentiation of esophageal squamous epithelial cells, and IL-4 is a causal factor for the development of BE at an early phase. Therapeutic strategies that modulate the production of IL-4 may provide a good solution to prevent the development of BE and EAC.
Adiponectin
Obesity is one of the most serious public health problems worldwide, especially in developed countries. Obesity is defined as a body mass index equal to or higher than 30 kg/m2.80 Previous studies have found a link between obesity and GERD, and the increasing incidence of GERD may be subsequent to the rising prevalence of obesity.81 More specifically, abdominal obesity (central obesity) has been shown to be a risk factor for GERD.82–87 It is believed that abdominal obesity is likely to increase intra-abdominal pressure owing to transmission of gravitational force of the adipose tissue to the abdominal cavity.88 The high abdominal pressure may lead to prolonged esophageal acid exposure compared with normal-weight individuals.89,90 In addition to these physical factors, obesity may also contribute to GERD through secretion of adipokines. Adipose tissue can secret a number of biologically active adipokines, such as leptin, resistin, and adiponectin.91,92
Adiponectin is an adipokine that has recently attracted much attention. There are two types of adiponectin: full-length adiponectin and globular adiponectin.93 Full-length adiponectin consists of an N-terminal collagen-like sequence and a C-terminal globular region, whereas globular adiponectin consists of a globular domain that is cleaved from the full-length protein by proteolysis.94 Almost all adiponectin appears to exist as full-length adiponectin in plasma. The physiological level of adiponectin in human serum has been reported to range from 5 to 30 μg/mL and is typically found at levels 35% lower in men than in women.95 There is a tendency for reduced adiponectin levels in obese subjects.96–98 It is believed that a plasma level of adiponectin less than 6 μg/mL is related to obesity.97,99,100
Epidemiology
Almers et al.101 conducted a case-control study evaluating the association between adiponectin (total, high molecular weight, and low/medium molecular weight) and BE in the Kaiser Permanente Northern California population. Patients with a new diagnosis of BE were matched to patients with GERD (without BE) and to population controls. Serum adiponectin levels were found to be positively related to the risk of BE among patients with GERD, but not among population controls without GERD. Higher adiponectin levels were independently associated with aberrant healing of esophageal injury in GERD.
On the contrary, another recent study summarized data from nine observational studies (10 independent cohorts; 1432 patients with BE and 3550 controls).102 When patients with BE were matched to population controls in five studies, subjects within the highest tertile of serum adiponectin level showed decreased odds of BE (OR 0.65; 95% CI 0.31–1.36). This association was even more pronounced in males. After adjustment for potential confounders, such as age, sex, and race, the observed associations of adiponectin with BE remained significant.
In a third study from Taiwan, patients that had experienced typical GERD symptoms (heartburn and/or acid regurgitation) for at least three episodes per week in the past 3 months received a baseline assessment of symptom severity and frequency with the Reflux Disease Questionnaire and an upper endoscopy.103 GERD was classified into erosive esophagitis (n = 67), nonerosive esophagitis (n = 37), and BE (n = 8). These patients were measured for the serum levels of adipokines (adiponectin and leptin). No statistical difference was found in circulating adiponectin levels between the GERD and control groups, whereas BE patients had significantly lower adiponectin levels than those without BE.
Conflicting results derived from these studies may be due to the use of different controls and populations. In addition, the first study included only newly diagnosed BE, whereas the second study included both prevalent and newly diagnosed BE. Overall, a negative association between adiponectin and BE was found in the most epidemiological studies,103–105 suggesting that low adiponectin is associated with BE and may play a biological role in the pathogenesis of metaplasia.
Potential mechanisms
Numerous studies demonstrated that adiponectin might be a protective factor against mucosal inflammation and symptoms of GERD.106,107 Two types of adiponectin play different roles in inflammatory responses: full-length adiponectin has an anti-inflammatory effect, whereas globular adiponectin seems to have a proinflammatory effect in a reactive oxygen species (ROS)/NF-κB/p65–dependent manner.93 Globular adiponectin regulates proinflammatory cytokines, such as IL-6 and TNF-α.108 Full-length adiponectin reduces the activity of cytokine signaling and NF-κB.98,109,110 Previous studies found that adiponectin can inhibit leptin-induced signaling and inflammatory factors.111
Adiponectin receptors (AdipoR1 and AdipoR2) are expressed in normal squamous epithelium, BE, and EAC biopsies and cells.112–114 Globular adiponectin seems to mainly activate AdipoR1, whereas AdipoR2 engages mainly with full-length adiponectin.11. Konturek et al. examined expression of AdipoR1 and AdipoR2 in BE and matched normal esophageal biopsies from 15 patients. They found reduced expression of receptors in Barrett's mucosa relative to normal esophageal tissue. This observation suggests that the biological effect of adiponectin on the Barrett's mucosa of obese individuals may be attenuated.116
It is likely that, in obese patients with GERD, low adiponectin levels contribute to increased expression of proinflammatory cytokines, resulting in chronic inflammation.108 This persistent state of chronic inflammation gives rise to the release of proinflammatory mediators, which promote cell growth and invasion, thus supporting metaplasia and transformation. To summarize, the association between adiponectin and the metaplastic healing response (i.e., BE) suggests a potential mechanism linking obesity and esophageal injury.
Future perspectives.
We reviewed the roles of immunomodulators in GERD and BE by focusing on stromal cells, IL-4, and adiponectin. It has long been believed that targeting the immunomodulators or inflammation in general may improve therapeutic outcomes of GERD, particularly in patients refractory to PPIs. However the mechanisms are still not well understood. This is partially due to the complexity of molecular mechanisms of GERD. Isolating one factor out of many in mechanistic studies often endangers the physiological relevance of the discovery. The real challenge is how to translate laboratory research into the clinical setting, in particular, for diagnosis and treatment of GERD and its complications.
Acknowledgments
The section on stromal cells was written by Anisa Shaker; the interleukin 4 section was written by Tadayuki Oshima, Jing Shan, and Hiroto Miwa; and the section on adiponectin was written by Cheng Feng and Jun Zhang.
Footnotes
The first five paragraphs of this manuscript share the research idea in our previous review article published as Ref. 118.
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talley NJ, Zinsmeister AR, Schleck CD, et al. Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–1268. [PubMed] [Google Scholar]
- 3.Ness-Jensen E, Hveem K, El-Serag H, et al. Lifestyle Intervention in Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2016;14:175–182 e173. doi: 10.1016/j.cgh.2015.04.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong D, Sifrim D. New pharmacologic approaches in gastroesophageal reflux disease. Gastroenterol Clin North Am. 2010;39:393–418. doi: 10.1016/j.gtc.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Vela MF. Medical treatments of GERD: the old and new. Gastroenterol Clin North Am. 2014;43:121–133. doi: 10.1016/j.gtc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Bateman DN, Colin-Jones D, Hartz S, et al. Mortality study of 18 000 patients treated with omeprazole. Gut. 2003;52:942–946. doi: 10.1136/gut.52.7.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia Rodriguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut. 2006;55:1538–1544. doi: 10.1136/gut.2005.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain ZH, Henderson EE, Maradey-Romerao C, et al. The Proton Pump Inhibitor Non-Responder: A Clinical Conundrum. Clin Transl Gastroenterol. 2015;6:e115. doi: 10.1038/ctg.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershcovici T, Fass R. Management of gastroesophageal reflux disease that does not respond well to proton pump inhibitors. Curr Opin Gastroenterol. 2010;26:367–378. doi: 10.1097/MOG.0b013e32833ae2be. [DOI] [PubMed] [Google Scholar]
- 10.Ang D, Sifrim D, Tack J. Mechanisms of heartburn. Nat Clin Pract Gastroenterol Hepatol. 2008;5:383–392. doi: 10.1038/ncpgasthep1160. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Y, Hunt RH. Evolving issues in the management of reflux disease? Curr Opin Gastroenterol. 2009;25:342–351. doi: 10.1097/MOG.0b013e32832c1504. [DOI] [PubMed] [Google Scholar]
- 12.Haigh CR, Attwood SE, Thompson DG, et al. Gastrin induces proliferation in Barrett's metaplasia through activation of the CCK2 receptor. Gastroenterology. 2003;124:615–625. doi: 10.1053/gast.2003.50091. [DOI] [PubMed] [Google Scholar]
- 13.Ireland AP, Peters JH, Smyrk TC, et al. Gastric juice protects against the development of esophageal adenocarcinoma in the rat. Ann Surg. 1996;224:358–370. doi: 10.1097/00000658-199609000-00012. discussion 370-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi H, Shimamoto K, Taniai E, et al. Liver tumor promoting effect of omeprazole in rats and its possible mechanism of action. J Toxicol Sci. 2012;37:491–501. doi: 10.2131/jts.37.491. [DOI] [PubMed] [Google Scholar]
- 15.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139:1115–1127. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Yuan YC, Leontiadis GI, et al. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol. 2012;46:93–114. doi: 10.1097/MCG.0b013e3182333820. [DOI] [PubMed] [Google Scholar]
- 17.Schinke T, Schilling AF, Baranowsky A, et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat Med. 2009;15:674–681. doi: 10.1038/nm.1963. [DOI] [PubMed] [Google Scholar]
- 18.Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol. 2010;24:873–882. doi: 10.1016/j.bpg.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodgers LS, Fanning AS. Regulation of epithelial permeability by the actin cytoskeleton. Cytoskeleton (Hoboken) 2011;68:653–660. doi: 10.1002/cm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jovov B, Van Itallie CM, Shaheen NJ, et al. Claudin-18: a dominant tight junction protein in Barrett's esophagus and likely contributor to its acid resistance. American journal of physiology Gastrointestinal and liver physiology. 2007;293:G1106–1113. doi: 10.1152/ajpgi.00158.2007. [DOI] [PubMed] [Google Scholar]
- 22.Oshima T, Gedda K, Koseki J, et al. Establishment of esophageal-like non-keratinized stratified epithelium using normal human bronchial epithelial cells. American journal of physiology Cell physiology. 2011;300:C1422–1429. doi: 10.1152/ajpcell.00376.2010. [DOI] [PubMed] [Google Scholar]
- 23.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomason HA, Scothern A, McHarg S, et al. Desmosomes: adhesive strength and signalling in health and disease. Biochem J. 2010;429:419–433. doi: 10.1042/BJ20100567. [DOI] [PubMed] [Google Scholar]
- 25.Tobey NA, Carson JL, Alkiek RA, et al. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111:1200–1205. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 26.Jovov B, Que J, Tobey NA, et al. Role of E-cadherin in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1039–1047. doi: 10.1038/ajg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshima T, Koseki J, Chen X, et al. Acid modulates the squamous epithelial barrier function by modulating the localization of claudins in the superficial layers. Lab Invest. 2012;92:22–31. doi: 10.1038/labinvest.2011.139. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Oshima T, Shan J, et al. Bile salts disrupt human esophageal squamous epithelial barrier function by modulating tight junction proteins. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G199–208. doi: 10.1152/ajpgi.00454.2011. [DOI] [PubMed] [Google Scholar]
- 29.Asaoka D, Miwa H, Hirai S, et al. Altered localization and expression of tight-junction proteins in a rat model with chronic acid reflux esophagitis. Journal of gastroenterology. 2005;40:781–790. doi: 10.1007/s00535-005-1628-6. [DOI] [PubMed] [Google Scholar]
- 30.Oguro M, Koike M, Ueno T, et al. Dissociation and dispersion of claudin-3 from the tight junction could be one of the most sensitive indicators of reflux esophagitis in a rat model of the disease. Journal of gastroenterology. 2011;46:629–638. doi: 10.1007/s00535-011-0390-1. [DOI] [PubMed] [Google Scholar]
- 31.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 32.Fujita H, Hamazaki Y, Noda Y, et al. Claudin-4 deficiency results in urothelial hyperplasia and lethal hydronephrosis. PLoS One. 2012;7:e52272. doi: 10.1371/journal.pone.0052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida N. Inflammation and oxidative stress in gastroesophageal reflux disease. J Clin Biochem Nutr. 2007;40:13–23. doi: 10.3164/jcbn.40.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Yang CS. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis. 2001;22:1119–1129. doi: 10.1093/carcin/22.8.1119. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Li N, Wang S, et al. Aberrant arachidonic acid metabolism in esophageal adenocarcinogenesis, and the effects of sulindac, nordihydroguaiaretic acid, and alpha-difluoromethylornithine on tumorigenesis in a rat surgical model. Carcinogenesis. 2002;23:2095–2102. doi: 10.1093/carcin/23.12.2095. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Li N, Wang S, et al. Leukotriene A4 hydrolase in rat and human esophageal adenocarcinomas and inhibitory effects of bestatin. Journal of the National Cancer Institute. 2003;95:1053–1061. doi: 10.1093/jnci/95.14.1053. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Wang S, Wu N, et al. Overexpression of 5-lipoxygenase in rat and human esophageal adenocarcinoma and inhibitory effects of zileuton and celecoxib on carcinogenesis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:6703–6709. doi: 10.1158/1078-0432.CCR-04-0838. [DOI] [PubMed] [Google Scholar]
- 39.Zeb MH, Baruah A, Kossak SK, et al. Chemoprevention in Barrett's Esophagus: Current Status. Gastroenterol Clin North Am. 2015;44:391–413. doi: 10.1016/j.gtc.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Ito H, Iijima K, Ara N, et al. Reactive nitrogen oxide species induce dilatation of the intercellular space of rat esophagus. Scand J Gastroenterol. 2010;45:282–291. doi: 10.3109/00365520903469956. [DOI] [PubMed] [Google Scholar]
- 41.Lin BR, Hsieh HT, Lee JM, et al. Luminal hydrochloric acid stimulates rapid transepithelial ion fluxes in rodent esophageal stratified squamous epithelium. J Physiol Pharmacol. 2008;59:525–542. [PubMed] [Google Scholar]
- 42.Farre R, Fornari F, Blondeau K, et al. Acid and weakly acidic solutions impair mucosal integrity of distal exposed and proximal non-exposed human oesophagus. Gut. 2010;59:164–169. doi: 10.1136/gut.2009.194191. [DOI] [PubMed] [Google Scholar]
- 43.Beacham DA, Cukierman E. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Seminars in cancer biology. 2005;15:329–341. doi: 10.1016/j.semcancer.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Zhang G, Wang J, et al. The role of cancer-associated fibroblasts in esophageal cancer. Journal of translational medicine. 2016;14:30. doi: 10.1186/s12967-016-0788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. The Journal of urology. 2001;166:2472–2483. [PubMed] [Google Scholar]
- 46.Rieder F, Biancani P, Harnett K, et al. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. American journal of physiology Gastrointestinal and liver physiology. 2010;298:G571–581. doi: 10.1152/ajpgi.00454.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rieder F, Nonevski I, Ma J, et al. T-Helper 2 Cytokines, Transforming Growth Factor beta1, and Eosinophil Products Induce Fibrogenesis and Alter Muscle Motility in Patients With Eosinophilic Esophagitis. Gastroenterology. 2014;146:1266–1277. doi: 10.1053/j.gastro.2014.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rieder F, Cheng L, Harnett KM, et al. Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology. 2007;132:154–165. doi: 10.1053/j.gastro.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Powell DW, Mifflin RC, Valentich JD, et al. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 50.Shaker A, Rubin DC. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl Res. 2010;156:180–187. doi: 10.1016/j.trsl.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vermeulen L, De Sousa EMF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 52.Shaker A, Binkley J, Darwech I, et al. Stromal cells participate in the murine esophageal mucosal injury response. American journal of physiology Gastrointestinal and liver physiology. 2013;304:G662–672. doi: 10.1152/ajpgi.00225.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gargus M, Niu C, Vallone JG, et al. Human esophageal myofibroblasts secrete proinflammatory cytokines in response to acid and Toll-like receptor 4 ligands. American journal of physiology Gastrointestinal and liver physiology. 2015;308:G904–923. doi: 10.1152/ajpgi.00333.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guarino MP, Cheng L, Ma J, et al. Increased TRPV1 gene expression in esophageal mucosa of patients with non-erosive and erosive reflux disease. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22:746–751, e219. doi: 10.1111/j.1365-2982.2010.01514.x. [DOI] [PubMed] [Google Scholar]
- 55.Verbeek RE, Siersema PD, Ten Kate FJ, et al. Toll-like receptor 4 activation in Barrett's esophagus results in a strong increase in COX-2 expression. Journal of gastroenterology. 2014;49:1121–1134. doi: 10.1007/s00535-013-0862-6. [DOI] [PubMed] [Google Scholar]
- 56.Cao W, Cheng L, Behar J, et al. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. American journal of physiology Gastrointestinal and liver physiology. 2004;287:G1131–1139. doi: 10.1152/ajpgi.00216.2004. [DOI] [PubMed] [Google Scholar]
- 57.Harper J, Sainson RC. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Seminars in cancer biology. 2014;25:69–77. doi: 10.1016/j.semcancer.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Okawa T, Michaylira CZ, Kalabis J, et al. The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes & development. 2007;21:2788–2803. doi: 10.1101/gad.1544507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang C, Fu L, Fu J, et al. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:4017–4027. doi: 10.1158/1078-0432.CCR-08-2824. [DOI] [PubMed] [Google Scholar]
- 60.Fu L, Zhang C, Zhang LY, et al. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/beta-catenin signalling pathway. Gut. 2011;60:1635–1643. doi: 10.1136/gut.2011.241638. [DOI] [PubMed] [Google Scholar]
- 61.de Jonge PJ, van Blankenstein M, Grady WM, et al. Barrett's oesophagus: epidemiology, cancer risk and implications for management. Gut. 2014;63:191–202. doi: 10.1136/gutjnl-2013-305490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kapoor H, Agrawal DK, Mittal SK. Barrett's esophagus: recent insights into pathogenesis and cellular ontogeny. Transl Res. 2015;166:28–40. doi: 10.1016/j.trsl.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett's metaplasia. American journal of physiology Gastrointestinal and liver physiology. 2008;295:G211–218. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 64.Chen X, Oshima T, Tomita T, et al. Acidic bile salts modulate the squamous epithelial barrier function by modulating tight junction proteins. American journal of physiology Gastrointestinal and liver physiology. 2011;301:G203–209. doi: 10.1152/ajpgi.00096.2011. [DOI] [PubMed] [Google Scholar]
- 65.Kapoor H, Lohani KR, Lee TH, et al. Animal Models of Barrett's Esophagus and Esophageal Adenocarcinoma-Past, Present, and Future. Clin Transl Sci. 2015;8:841–847. doi: 10.1111/cts.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nehra D, Howell P, Williams CP, et al. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;44:598–602. doi: 10.1136/gut.44.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slack JM. Stem cells in epithelial tissues. Science. 2000;287:1431–1433. doi: 10.1126/science.287.5457.1431. [DOI] [PubMed] [Google Scholar]
- 68.Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, et al. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002;50:451–459. doi: 10.1136/gut.50.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moons LM, Kusters JG, Bultman E, et al. Barrett's oesophagus is characterized by a predominantly humoral inflammatory response. J Pathol. 2005;207:269–276. doi: 10.1002/path.1847. [DOI] [PubMed] [Google Scholar]
- 70.Kohata Y, Fujiwara Y, Machida H, et al. Role of Th-2 cytokines in the development of Barrett's esophagus in rats. Journal of gastroenterology. 2011;46:883–893. doi: 10.1007/s00535-011-0405-y. [DOI] [PubMed] [Google Scholar]
- 71.Dohi T, Fujihashi K, Koga T, et al. T helper type-2 cells induce ileal villus atrophy, goblet cell metaplasia, and wasting disease in T cell-deficient mice. Gastroenterology. 2003;124:672–682. doi: 10.1053/gast.2003.50092. [DOI] [PubMed] [Google Scholar]
- 72.Kavanagh ME, Conroy MJ, Clarke NE, et al. Impact of the inflammatory microenvironment on T-cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinoma. Cancer Lett. 2016;370:117–124. doi: 10.1016/j.canlet.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 73.Huo X, Zhang HY, Zhang XI, et al. Acid and bile salt-induced CDX2 expression differs in esophageal squamous cells from patients with and without Barrett's esophagus. Gastroenterology. 2010;139:194–203 e191. doi: 10.1053/j.gastro.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shan J, Oshima T, Muto T, et al. Epithelial-derived nuclear IL-33 aggravates inflammation in the pathogenesis of reflux esophagitis. Journal of gastroenterology. 2015;50:414–423. doi: 10.1007/s00535-014-0988-1. [DOI] [PubMed] [Google Scholar]
- 75.Shan J, Oshima T, Farre R, et al. IL-4 induces columnar-like differentiation of esophageal squamous epithelium through JAK/PI3K pathway: possible role in pathogenesis of Barrett's esophagus. American journal of physiology Gastrointestinal and liver physiology. 2014;306:G641–649. doi: 10.1152/ajpgi.00386.2013. [DOI] [PubMed] [Google Scholar]
- 76.Cabibi D, Fiorentino E, Pantuso G, et al. Keratin 7 expression as an early marker of reflux-related columnar mucosa without intestinal metaplasia in the esophagus. Med Sci Monit. 2009;15:CR203–210. [PubMed] [Google Scholar]
- 77.Glickman JN, Chen YY, Wang HH, et al. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett's esophagus. Am J Surg Pathol. 2001;25:569–578. doi: 10.1097/00000478-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Mari L, Milano F, Parikh K, et al. A pSMAD/CDX2 complex is essential for the intestinalization of epithelial metaplasia. Cell Rep. 2014;7:1197–1210. doi: 10.1016/j.celrep.2014.03.074. [DOI] [PubMed] [Google Scholar]
- 79.Milano F, van Baal JW, Buttar NS, et al. Bone morphogenetic protein 4 expressed in esophagitis induces a columnar phenotype in esophageal squamous cells. Gastroenterology. 2007;132:2412–2421. doi: 10.1053/j.gastro.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 80.Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang P, Friedenberg F. Obesity and GERD. Gastroenterol Clin North Am. 2014;43:161–173. doi: 10.1016/j.gtc.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111:30–50. doi: 10.1038/ajg.2015.322. quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chandar AK, Iyer PG. Role of Obesity in the Pathogenesis and Progression of Barrett's Esophagus. Gastroenterol Clin North Am. 2015;44:249–264. doi: 10.1016/j.gtc.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 84.El-Serag HB, Hashmi A, Garcia J, et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett's oesophagus: a case-control study. Gut. 2014;63:220–229. doi: 10.1136/gutjnl-2012-304189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett's oesophagus: a pooled analysis from the international BEACON consortium. Gut. 2013;62:1684–1691. doi: 10.1136/gutjnl-2012-303753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kramer JR, Fischbach LA, Richardson P, et al. Waist-to-hip ratio, but not body mass index, is associated with an increased risk of Barrett's esophagus in white men. Clin Gastroenterol Hepatol. 2013;11:373–381 e371. doi: 10.1016/j.cgh.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 88.Lambert DM, Marceau S, Forse RA. Intra-abdominal pressure in the morbidly obese. Obes Surg. 2005;15:1225–1232. doi: 10.1381/096089205774512546. [DOI] [PubMed] [Google Scholar]
- 89.Varela JE, Hinojosa M, Nguyen N. Correlations between intra-abdominal pressure and obesity-related co-morbidities. Surg Obes Relat Dis. 2009;5:524–528. doi: 10.1016/j.soard.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 90.El-Serag HB, Tran T, Richardson P, et al. Anthropometric correlates of intragastric pressure. Scand J Gastroenterol. 2006;41:887–891. doi: 10.1080/00365520500535402. [DOI] [PubMed] [Google Scholar]
- 91.Gibson MK, Dhaliwal AS, Clemons NJ, et al. Barrett's esophagus: cancer and molecular biology. Ann N Y Acad Sci. 2013;1300:296–314. doi: 10.1111/nyas.12252. [DOI] [PubMed] [Google Scholar]
- 92.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang R, Yin X, Shi H, et al. Adiponectin modulates DCA-induced inflammation via the ROS/NF-kappa B signaling pathway in esophageal adenocarcinoma cells. Dig Dis Sci. 2014;59:89–97. doi: 10.1007/s10620-013-2877-5. [DOI] [PubMed] [Google Scholar]
- 94.Whitehead JP, Richards AA, Hickman IJ, et al. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 95.Nagaraju GP, Rajitha B, Aliya S, et al. The role of adiponectin in obesity-associated female-specific carcinogenesis. Cytokine Growth Factor Rev. 2016 doi: 10.1016/j.cytogfr.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 96.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999. Biochem Biophys Res Commun. 2012;425:560–564. doi: 10.1016/j.bbrc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 97.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 98.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 99.Fasshauer M, Klein J, Neumann S, et al. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 100.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 101.Almers LM, Graham JE, Havel PJ, et al. Adiponectin May Modify the Risk of Barrett's Esophagus in Patients With Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2015;13:2256–2264 e2251. 2253. doi: 10.1016/j.cgh.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chandar AK, Devanna S, Lu C, et al. Association of Serum Levels of Adipokines and Insulin With Risk of Barrett's Esophagus: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2015;13:2241–2255 e2241. 2244. doi: 10.1016/j.cgh.2015.06.041. quiz e2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tseng PH, Yang WS, Liou JM, et al. Associations of Circulating Gut Hormone and Adipocytokine Levels with the Spectrum of Gastroesophageal Reflux Disease. PLoS One. 2015;10:e0141410. doi: 10.1371/journal.pone.0141410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 105.Greer KB, Falk GW, Bednarchik B, et al. Associations of Serum Adiponectin and Leptin With Barrett's Esophagus. Clin Gastroenterol Hepatol. 2015;13:2265–2272. doi: 10.1016/j.cgh.2015.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karmiris K, Koutroubakis IE, Xidakis C, et al. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 107.Schaffler A, Scholmerich J. The role of adiponectin in inflammatory gastrointestinal diseases. Gut. 2009;58:317–322. doi: 10.1136/gut.2008.159210. [DOI] [PubMed] [Google Scholar]
- 108.Garcia JM, Splenser AE, Kramer J, et al. Circulating inflammatory cytokines and adipokines are associated with increased risk of Barrett's esophagus: a case-control study. Clin Gastroenterol Hepatol. 2014;12:229–238 e223. doi: 10.1016/j.cgh.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 110.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beales IL, Garcia-Morales C, Ogunwobi OO, et al. Adiponectin inhibits leptin-induced oncogenic signalling in oesophageal cancer cells by activation of PTP1B. Mol Cell Endocrinol. 2014;382:150–158. doi: 10.1016/j.mce.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 112.Mokrowiecka A, Sokolowska M, Luczak E, et al. Adiponectin and leptin receptors expression in Barrett's esophagus and normal squamous epithelium in relation to central obesity status. J Physiol Pharmacol. 2013;64:193–199. [PubMed] [Google Scholar]
- 113.Howard JM, Cathcart MC, Healy L, et al. Leptin and adiponectin receptor expression in oesophageal cancer. Br J Surg. 2014;101:643–652. doi: 10.1002/bjs.9469. [DOI] [PubMed] [Google Scholar]
- 114.Howard JM, Beddy P, Ennis D, et al. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg. 2010;97:1020–1027. doi: 10.1002/bjs.7072. [DOI] [PubMed] [Google Scholar]
- 115.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 116.Konturek PC, Burnat G, Rau T, et al. Effect of adiponectin and ghrelin on apoptosis of Barrett adenocarcinoma cell line. Dig Dis Sci. 2008;53:597–605. doi: 10.1007/s10620-007-9922-1. [DOI] [PubMed] [Google Scholar]
- 117.Di Simone MP, Baldi F, Vasina V, et al. Barrier effect of Esoxx((R)) on esophageal mucosal damage: experimental study on ex-vivo swine model. Clin Exp Gastroenterol. 2012;5:103–107. doi: 10.2147/CEG.S31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen C, Fang Y, Li W, et al. NFkB and Nrf2 in esophageal epithelial barrier function. Tissue Barriers. 2013;1:e27463. doi: 10.4161/tisb.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]