Abstract
Objective
Grip strength predicts functional decline and death, and is regarded as a biomarker of biological aging. The primary objective of this manuscript was to assess differences in the rate of decline in grip strength in persons aging with and without HIV.
Design
Grip strength was assessed in 1,552 (716 HIV+ and 836 HIV−) men ≥ 50 years participating in the Multicenter AIDS Cohort Study between 2007 and 2014.
Methods
Grip strength decline was modeled longitudinally, adjusting for serostatus, demographics, comorbidities, and conditions. In HIV-specific models, coefficients were included for cumulative viral load and history of AIDS.
Results
Grip strength at age 50 averaged 37.9 kg and 38.2 kg for HIV+ and HIV− men, respectively (p = 0.70). In fully adjusted models, grip strength declined 0.33 kg/yr in HIV− men (p <0.001) and 0.42 kg/yr in HIV+ men (p = 0.01). In HIV-stratified models, higher cumulative viral load indicated greater strength decline (−0.884 kg for 3.1 – 4.0 log10 copies-years/mL and −1.077 kg for ≥ 4.1 log10 copies-years/mL) relative to men with consistently low viral load (≤ 3.0 log10 copies-years/mL). Adjusted Cox proportional hazard models revealed a 70% greater risk of clinically weak grip strength in HIV+ men (aHR 1.70; 95% CI, 1.22 – 2.40).
Conclusions
Grip strength decline is accelerated in HIV-infected men, which may contribute to decreased life expectancy and lower quality of life with aging. Greater cumulative viral load exposure appears to be an important driver of this decline and underscores the importance of early initiation of therapy.
Keywords: Cumulative viral load, grip strength, clinical weakness, functional decline, aging
Introduction
In 2014, approximately 48% of the 1.2 million people living in the United States with HIV were aged 50 or older[1]. Recent evidence indicates that those aging with HIV may be at an increased risk of poor functional outcomes compared to the general aging population, regardless of virologic suppression[2–4], due to a higher pro-inflammatory state[5] and greater comorbidity burden[6]. As life expectancy of those living with HIV continues to increase[7], these factors may contribute to increased morbidity and an accelerated rate of disability in this population.
Muscle strength is an essential component of physical fitness and a predictor of functional decline, frailty, and sarcopenia in older adults[8]. Grip strength assessment is a simple and valid test that correlates with whole body strength, and has been proposed as a biomarker of aging across the life course[9]. It has been shown to predict disability, morbidity, and mortality in both middle-and-older aged populations, and has gained clinical recognition in recent years, with newly defined thresholds from the Foundation for the National Institutes of Health Sarcopenia Project[8]. These thresholds were defined using pooled analyses from 11 cross-sectional studies, and stratified by sex to identify normal grip strength, intermediate grip strength, and clinically weak grip strength, further establishing the utility of grip strength as a clinically relevant biomarker[8].
Among HIV-infected (HIV+) persons, substantial research has demonstrated the development of lipodystrophy and lipoatrophy in mid-to-late life, particularly with older HIV treatment regimens, which may hasten the development of sarcopenia and frailty through poor muscle strength and quality[10, 11]. However, data on the utility of grip strength as a marker of functional decline in those aging with HIV have been conflicting [12–14], which may be at least partially attributable to the relatively young ages of many HIV-infected study populations. Accordingly, a large sample of longitudinal data that includes middle- and older-aged HIV+ and comparable HIV− people is needed to determine the effect of HIV status on the trajectory of decline in grip strength, and the risk of clinical weakness with aging.
The purpose of this study was to examine the onset and rate of grip strength decline in a large population of HIV+ middle- and older-aged adults, relative to HIV− adults of similar demographics and lifestyle behaviors. To this end, we analyzed grip strength measurements collected over a seven-year period in the Multicenter AIDS Cohort Study (MACS), an ongoing study of the natural and treated history of HIV infection that includes HIV+ and HIV− men who have sex with men (MSM).
Methods
Study Population
The MACS includes over 7,000 HIV+ and demographically similar HIV− MSM enrolled in Baltimore/Washington DC, Chicago, Los Angeles, and Pittsburgh/Columbus in 1984–1985 (n=4,954), 1987–1991 (n=668), 2001–2003 (n=1,350), and 2010–2015 (n= 371). Specific details of the study have been published[15]. Briefly, participants complete semiannual study visits consisting of standardized interviews, physical examination, lifestyle questionnaires, and collection of blood for laboratory testing and storage. Informed consent is obtained from all study participants, and the institutional review boards at each study site approved the study protocol.
Grip strength
Grip strength has been measured at each MACS study visit as part of a frailty assessment since October 1, 2007. Using a Jamar® hydraulic hand held dynamometer, participants are asked to squeeze the dynamometer “as hard as you can” three separate times using their dominant hand, with a brief recovery between trials. The average of the three measures was used for the present analysis. Participants with acute arthritis, tendonitis, carpal tunnel syndrome, or recent hand or wrist surgery in their dominant hand were not tested.
HIV status
All men were assessed for HIV antibodies by ELISA and confirmed by Western blot.
Covariates
Date of birth, race, and education were self-reported at enrollment. Cigarette smoking, drug and alcohol use, and comorbidities were self-reported at each study visit during the analysis period. For analyses, smoking and drug use were dichotomized as “ever” or “never.” Alcohol use was assessed continuously as number of drinks per week. Race was dichotomized into white (non-Hispanic) or non-white. Education was dichotomized into college degree or no college degree. Hepatitis C infection was defined by detectable hepatitis C RNA in serum; and hepatitis B infection was defined by positive hepatitis B surface antigen in serum. Mental health was assessed using the mental component summary (MCS) score of the SF-36, and was dichotomized as a score of < or ≥ 42 for the analysis[16]. Height and weight were measured using standard procedures, and body mass index (BMI) was calculated as [mass (kg)]/[height (m)]2. Hypertension was defined as systolic pressure ≥ 140 mm Hg, diastolic pressure ≥ 90 mm Hg, or self-reported diagnosis of hypertension with use of antihypertensive medications. Diabetes mellitus was defined as fasting glucose ≥ 126 mg/dL or self-reported previous diagnosis with use of diabetes medication. Kidney disease was defined as estimated glomerular filtration rate < 60 ml/min/1.73 m2 BSA or urine protein-to-creatinine ratio ≥ 200 g/gCr. Arthritis was defined as prior or current self-reported arthritis pain. Peripheral neuropathy was defined as current or past report of pain, burning, numbness, or pins and needles sensation in the feet or legs, or measured inability to detect a vibratory sensation in either foot with a 128-Hz tuning fork.
T-lymphocyte subsets were measured at each MACS visit using standardized 3-color flow cytometry[17]. Plasma HIV RNA concentrations (viral load) were measured using the Roche ultrasensitive assay (limit of detection = 50 copies/mL; Roche Diagnostics, Nutley, NJ). HAART was defined according to the U.S. Department of Health and Human Services Kaiser Panel guidelines[18] as three or more antiretroviral drugs including either: (i) a protease inhibitor; (ii) a nonnucleoside reverse transcriptase inhibitor; (iii) an entry or integrase inhibitor; or (iv) three nucleoside reverse transcriptase inhibitors, including abacavir or tenofovir. AIDS was defined using the Centers for Disease Control and Prevention’s 1993 definition, excluding cases defined only by a CD4 T-cell count <200 cells/mm[19], and confirmed by review of medical records.
Statistical Analysis
Two-sample t-test and chi-square test statistics were used to evaluate differences in continuous and categorical variables by HIV status, respectively. Exploratory data analyses included locally weighted regression smoothers, quadratic fit plots, and histograms to assess the normality of the grip strength distribution.
Based on the appearance of these plots (Figure 1), the longitudinal association between grip strength and age was modeled using generalized linear models with generalized estimating equations (GEE) and an exchangeable working correlation matrix to account for correlation of repeated measures of grip strength. Combined (Table 2a) and HIV-stratified models (Table 2b) were created to assess differences in the rate of change in grip strength by HIV status. Covariates in these models included age, BMI, race, education, smoking status, MCS score, history of drug and alcohol use, diabetes, kidney disease, hypertension, arthritis, peripheral neuropathy, hepatitis B, and hepatitis C. The model restricted to HIV+ men (Table 2b) also included nadir CD4 T-cell count, history of AIDS, and cumulative viral load. Cumulative viral load is a time-varying measure of cumulative plasma HIV burden which uses area under the curve analyses calculated using the trapezoidal rule to generate a single measure of viral burden that captures the effect of chronic HIV-infection better than concurrent visit measures[20]. Cumulative viral load was calculated for the same period during which grip strength was measured (2007 – 2014). If a viral load measure was missing (n= 20), the last available viral load observation was carried forward. Differences in the rate of grip strength decline between those with and without exposure to mono- or combination therapy were tested using stratified linear models. Variables included in the final models were restricted to those with statistical significance in the multivariate model (p < 0.05) and/or face validity.
Figure 1.
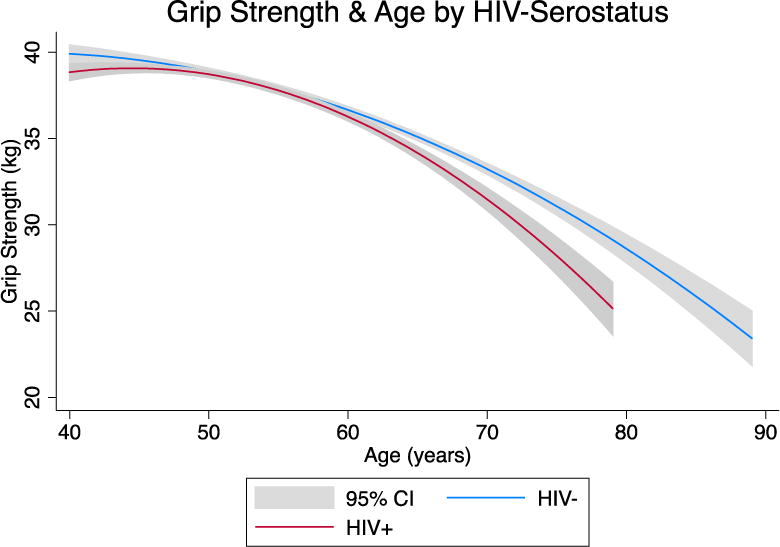
Quadratic fit plot of the unadjusted association (mean and 95% C.I.) between grip strength (kg) and age (years) by HIV status.
Table 2a.
Continuous, longitudinal association between age and grip strength, adjusted for HIV serostatus and other confounding variables, from October 1, 2007 – September 30, 2014 (N = 1,552)
| Dependent Variable: Grip strength (kg) Independent variables: |
Coefficient | S.E. | p-value |
|---|---|---|---|
| Age (per year centered at 50 years) | −0.330 | .065 | < 0.001 |
| Age squared | −0.005 | .002 | < 0.01 |
| HIV infection | 0.293 | .467 | 0.53 |
| HIV * Age | − 0.093 | .034 | 0.01 |
| Body Mass Index (kg/m2) | 0.121 | .028 | < 0.001 |
| Non-White Racea | −1.440 | .458 | < 0.01 |
| College educationb | 1.818 | .412 | < 0.001 |
| Kidney diseasec | −0.351 | .121 | < 0.01 |
| Peripheral Neuropathyd | −0.230 | .126 | 0.07 |
Table 2 shows the final longitudinal model assessing the associations among age, HIV, and grip strength decline. Smoking, history of drug and alcohol use, liver disease, hypertension, arthritis, mental quality of life, hepatitis B, and hepatitis C were not significant and were not included in the final model.
Black (non-Hispanic), Black Hispanic, American Indian or Alaskan Native, Asian or Pacific Islander, other Hispanic, or other
Completed college degree or more
Estimated Glomerular Filtration Rate < 60 or Urine Protein to Creatinine Ratio ≥ 200
Current or past report of pain, burning, numbness, or pins and needles sensation in the feet or legs, or measured inability to detect vibratory sensation in either foot
Table 2b.
Continuous, longitudinal association between age and grip strength stratified by HIV status. October 1, 2007 – September 30, 2014 (N=1,552)
| Dependent variable: Grip strength (kg) |
HIV+ | HIV− | ||||
|---|---|---|---|---|---|---|
| N = 716 | N = 836 | |||||
|
| ||||||
| Independent variables: | Coefficient | SE | p-value | Coefficient | SE | p-value |
| Age (centered at 50 years) | −0.525 | .060 | < 0.001 | −0.394 | .044 | < 0.001 |
|
| ||||||
| Age squared | −0.002 | .003 | 0.49 | −0.006 | .002 | < 0.001 |
|
| ||||||
| Body mass index (kg/m2) | 0.233 | .046 | < 0.001 | 0.047 | .035 | 0.178 |
|
| ||||||
| Non-White Racea | −1.233 | .639 | 0.06 | −1.466 | .689 | 0.03 |
|
| ||||||
| College educationb | 2.293 | .600 | < 0.001 | 1.243 | .569 | 0.03 |
|
| ||||||
| Kidney diseasec | −0.189 | .183 | 0.30 | −0.502 | .164 | < 0.01 |
|
| ||||||
| Peripheral Neuropathyd | −0.316 | .194 | 0.10 | −0.132 | .167 | 0.43 |
|
| ||||||
| Hepatitis Ce | −1.597 | .681 | 0.02 | 1.338 | .852 | 0.12 |
|
| ||||||
| History of AIDSf | −1.081 | .657 | 0.10 | |||
|
| ||||||
| Cumulative Viral Load ≤ 3.0g (log10 copy-years/mL) | Ref. | |||||
|
| ||||||
| Cumulative Viral Load 3.1 – 4.0 (log10 copy-years/mL) | − 0.884 | .427 | 0.04 | |||
|
| ||||||
| Cumulative Viral Load ≥ 4.1(log10 copy-years/mL) | − 1.077 | .399 | 0.01 | |||
Table 3 shows the final longitudinal model assessing the associations among age and grip strength decline, stratified by HIV serostatus. Smoking, history of drug and alcohol use, hypertension, arthritis, mental quality of life, and hepatitis B were not significant and were not included in the final model.
Black (non-Hispanic), Black Hispanic, American Indian or Alaskan Native, Asian or Pacific Islander, other Hispanic, or other
Completed college degree or more
Estimated Glomerular Filtration Rate < 60 or Urine Protein to Creatinine Ratio ≥ 200
Current or past report of pain, burning, numbness, or pins and needles sensation in the feet or legs, or measured inability to detect vibratory sensation in either foot
Detectable hepatitis C RNA in serum
First AIDS diagnosis at or before current visit
A measure of cumulative HIV burden that estimates the area under each participant’s longitudinal viral load curve
Kaplan-Meier survival estimates and adjusted Cox proportional hazard models were used to estimate differences in time from age 50 to clinical weakness by HIV status. Grip strength of < 26 kg was defined as “weak,” in accordance with the Foundation for the National Institutes of Health Sarcopenia Project[8]. Separate analyses of HIV+ men were performed to assess differences between those with and without clinical weakness by exposure to “d-drugs” (didanosine, stavudine), AZT (zidovudine), and efavirenz, and by time since seroconversion and time on HAART, using two-sample t-test and chi-square test statistics. All analyses were conducted using Stata MP version 14 (Statacorp, College Station, TX).
Results
The study population consisted of the 1,552 men (716 HIV+ and 836 HIV−) aged 50 and older who had two or more study visits between October 1, 2007 and September 30, 2014. These men contributed 15,590 person-visits (6,654 HIV+ and 8,936 HIV−) to the analysis. The mean number of visits per participant was 9.3 (range: 2–18 visits) for HIV+ men and 10.7 (range 2–18 visits) for HIV− men (p < 0.05). Baseline characteristics of these men are shown in Table 1. HIV+ participants were on average 2.6 years younger than HIV− participants and had lower BMIs (p-values <0.001). HIV+ men were more likely to have kidney disease, peripheral neuropathy, hepatitis B and hepatitis C infection, be non-white, report a history of drug use and have fewer years of education, and were less likely to consume alcohol than HIV− men (p-values <0.001). There was a wide range of grip strength values, from less than 10 kg to more than 60 kg, which is consistent with previous studies[8].
Table 1.
Baseline characteristics of study participants October 1, 2007 – September 30, 2014 (N = 1,552), shown as mean and standard deviation (continuous variables) or number and percent (categorical variables).
| HIV+ | n = 716 | HIV− | n = 836 | ||
|---|---|---|---|---|---|
| Mean, n | (SD)/% | Mean, n | (SD)/% | p-value | |
| Age (years) | 53.4 | (4.6) | 56.0 | (6.3) | < 0.001 |
| Body Mass Index (kg/m2) a | 25.6 | (4.2) | 27.2 | (5.0) | < 0.001 |
| Non-whiteb | 247 | 34.5% | 173 | 20.7% | < 0.001 |
| College Educationc | 375 | 52.4% | 553 | 66.1% | < 0.001 |
| Smokingd | 181 | 25.3% | 237 | 28.3% | 0.17 |
| History of drug usee | 362 | 50.6% | 333 | 39.8% | < 0.001 |
| Alcohol usef | 3.9 | (8.6) | 4.9 | (8.3) | 0.02 |
| Diabetesg | 142 | 19.7% | 158 | 18.9% | 0.12 |
| Kidney diseaseh | 210 | 29.3% | 98 | 11.7% | < 0.001 |
| Hypertensioni | 341 | 47.6% | 368 | 44.0% | 0.19 |
| Arthritisj | 35 | 4.9% | 37 | 4.4% | 0.53 |
| Peripheral neuropathyk | 259 | 36.2% | 215 | 25.7% | < 0.001 |
| SF36 MCSl | 47.9 | (12.4) | 49.8 | (12.4) | 0.05 |
| Hepatitis B infectionm | 30 | 3.6% | 7 | <1.0% | <.001 |
| Hepatitis C infectionn | 126 | 15.4% | 86 | 8.8% | < 0.001 |
| Grip strength at age 50 (kg) | 37.9 | (9.3) | 38.2 | (8.8) | 0.70 |
| Years since seroconversiono | 14.9 | (8.3) | |||
| Years of HAARTp | 9.1 | (3.8) | |||
| CD4 nadir (cells/ul)q | 269.1 | (179.1) | |||
| Suppressed viral loadr | 562 | 78.5% |
BMI, body mass index, calculated as weight in kilograms divided by height in meters squared
Black (non-Hispanic), Black Hispanic, American Indian or Alaskan Native, Asian or Pacific Islander, other Hispanic, or other
Completed college degree or more
Current or former smoker
History of any drug use
Number of drinks per week
Fasting glucose ≥ 126 mg/dL or diagnosed with diabetes and use of medications
Estimated Glomerular Filtration Rate < 60 or Urine Protein to Creatinine Ratio ≥ 200
Systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or diagnosed with hypertension and use of medications
Current or past report of arthritis pain
Current or past report of pain, burning, numbness, or pins and needles sensation in the feet or legs, or measured inability to detect vibratory sensation in either foot
Short form 36 mental component summary score
Positive hepatitis B surface antigen
Detectable hepatitis C RNA in serum
Reported years from HIV diagnosis
Reported years from HAART initiation
Lowest CD4 T-cell count, as measured or reported and confirmed with medical records
< 200 copies HIV RNA/ml
Figure 1 displays the unadjusted mean grip strength by age according to HIV status using a quadratic fit plot. Grip strength was similar between the two groups at age 50, averaging 37.9 kg in HIV+ participants and 38.2 kg in HIV− participants (p = 0.70), but after age 50, the curves for the HIV− and HIV+ men diverged significantly. Among the HIV− men, grip strength declined 0.33 kg for each one-year increase in age (p < 0.001), whereas in the HIV+ men, this decline was significantly faster at 0.42 kg per year (p = 0.01 for interaction between age and HIV status (Table 2a)). Other significant predictors of the rate of grip strength decline included: lower BMI, non-white race, fewer years of education, history of kidney disease, and peripheral neuropathy. Smoking, history of drug and alcohol use, diabetes, hypertension, arthritis, MCS score, and hepatitis B and hepatitis C infections were not statistically significant in the multivariate models and therefore were not included in the final model.
In analyses stratified by HIV status (Table 2b), education contributed significantly to both the HIV+ and HIV− models. In the HIV− model, those with a history of kidney disease and those of non-white race had a faster rate of decline in grip strength (p < 0.05 for both). In the HIV+ model, there were significant associations between the rate of grip strength decline and BMI, hepatitis C infection, and cumulative viral load (p < 0.05 for all). Stratified sensitivity analyses revealed an increased rate of grip strength decline according to number of unsuppressed visits and mean viral load during the analysis period (Table 3). Specifically, grip strength decline was progressively worse among those with a higher mean viral load, as shown by declines of 0.31 kg and 1.91 kg more per year in those with mean viral loads of 3.83 log10 copies/mL (n = 60) and ≥ 4.01 log10 copies/mL (n = 27), respectively, compared to those with a mean viral load of ≤ 1.3 log10 copies/ml (n = 629).
Table 3.
Viral load (Log10 copies/mL) and rate of decline in grip strength (kg/year) stratified by HIV status and number of non-suppressed visits
| Strata | N | Mean Viral Load (Log10 copies/mL) | S.D. | Mean Cumulative Viral Load (Log10 copy-years/mL) | S.D. | Rate of decline kg/year |
|---|---|---|---|---|---|---|
| HIV− | 836 | N/A | N/A | N/A | N/A | − 0.39 |
| HIV+ suppressed | 629 | 1.33 | 0.33 | 2.14 | 1.21 | − 0.48 |
| HIV+ 1 – 6 non-suppressed visits | 60 | 3.83 | 0.99 | 2.92 | 1.90 | − 0.79 |
| HIV+ 7 or more non-suppressed visits | 27 | 4.01 | 0.82 | 4.07 | 1.44 | − 2.39 |
To provide clinical perspective, we examined the effect of HIV status on the time to clinical weakness (< 26 kg) [8]. As shown in Figure 2, the trajectories of time to clinical weakness were significantly different between HIV+ and HIV− men (p < 0.001), with 25% of the HIV+ men exhibiting clinical weakness by age 60 compared to 25% of HIV− men by age 66. In Cox proportional hazard models using age as the time metric and adjusting for the variables that were significant in the combined continuous analysis (BMI, race, education, peripheral neuropathy, and hepatitis C), the hazard of developing clinical weakness was 70% greater for HIV+ compared with HIV− men (aHR 1.70, p = 0.002; 95% CI, 1.22 – 2.40).
Figure 2.
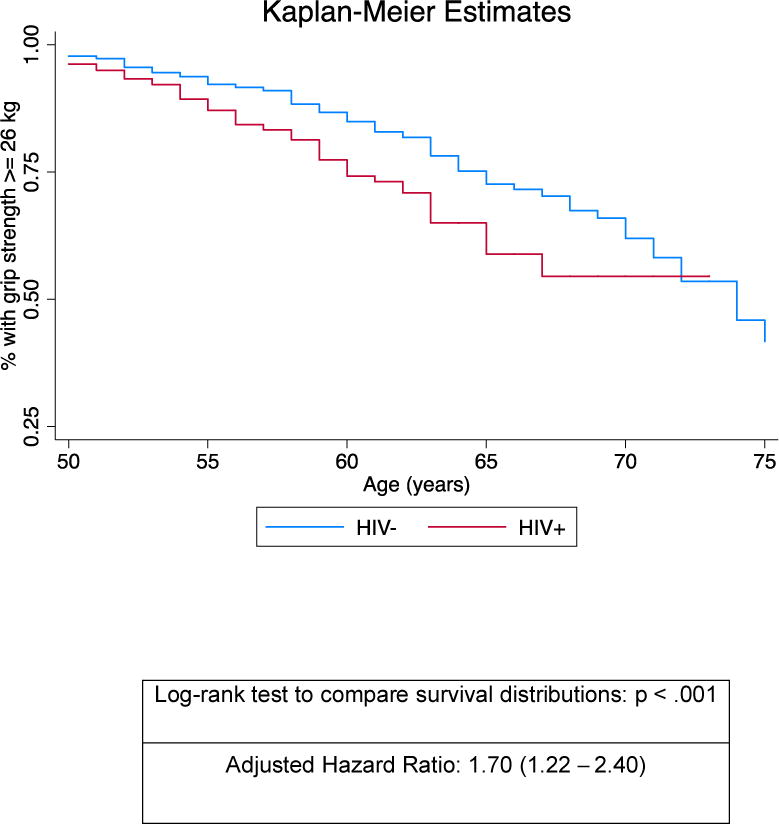
Kaplan-Meier estimates of the proportion of participants with grip strength <26 kg of force (“clinically weak”) by years of age (x-axis), stratified by HIV status (log rank to compare survival distributions p <.001). The adjusted hazard ratio was derived from Cox proportional hazards regression models detailed in the Methods and Results sections.
Finally, to examine potential contributors to the heightened risk of clinical weakness, HIV+ men were stratified into: (1) those who reached clinical weakness (< 26 kg, n = 106) and (2) those who maintained normal to intermediate grip strength ≥ 26 kg (n = 610). Those in the weak group were on average 2.2 years older, had a lower BMI, and were more likely to report a history of diabetes, kidney disease, or peripheral neuropathy, and had a higher cumulative viral load (p < 0.05 for all). Although there were no biologically meaningful or statistically significant differences between weak and not-weak men by concurrently measured viral load, years since seroconversion, or by cumulative years on HAART, ddI, d4T, AZT, or efavirenz, when stratified by history of therapy, men who reported ever taking mono- or combination therapy showed a faster rate of grip strength decline than men who only reported taking ART (0.67 kg/yr vs 0.43 kg/yr).
Discussion
Muscle strength is a central component of functional independence that is essential to maintaining quality of life with aging. To our knowledge, this study is the first to: a) evaluate grip strength decline prospectively in a large HIV+ population and b) compare these observations to a demographically and behaviorally similar HIV− population. We observed that HIV+ men experienced a more rapid decline in grip strength after age 50 and an earlier occurrence of clinical weakness, despite having similar strength levels at age 50. Thus, the findings from the present study strongly support the hypothesis that HIV+ men are at higher risk for poor health outcomes with aging.
Previous research has shown that grip strength tends to peak in early adult life, and decline more rapidly after age 60[21]. Multiple factors have been associated with this decline including changes in body composition (e.g., sarcopenia) and inflammation, signifying that reduced grip strength may reflect underlying biological and physiological challenges that develop with aging[9, 22]. A typical 65-year-old lives with two or more chronic conditions[23]. The addition of chronic HIV infection to this disease burden adds another layer of complexity to an aging system, which may hasten the onset of functional decline, aggravating the risk of future disability and death[2, 3]. Further, our results suggest a strong psychosocial component to rate of decline in grip strength, as demonstrated by the significant contributions of race and education. Further research is needed to provide a better understanding of the underlying factors contributing to these differences, and to develop future interventions and clinical recommendations for preserving strength in persons aging with HIV.
In the current study, mean grip strength at age 50 of 37.9 kg and 38.2 kg in HIV+ and HIV− men, respectively, indicates normal strength at the study population level[8]. However, the prevalence of those with intermediate grip strength (26 – 32 kg) was greater in those with HIV, as was the risk of clinical weakness over time[8]. Although normative data for longitudinal changes in grip strength are not well established, mean annual loss in grip strength among healthy people aged 30 to 70 is estimated to be between 0.5 – 1.0%[24]. The stratified results of 0.394 kg/yr decline for HIV− men are comparable to these estimates (1.0%), but the 0.525 kg/yr loss for HIV+ men exceeds these estimates (1.5%). Combined with the significant negative effect of a greater cumulative viral load and the borderline significant effect of a history of AIDS on grip strength, these analyses are consistent with previous research linking a greater degree of immunosuppression with an increased propensity for frailty[25] and underscore the importance of early initiation of antiretroviral therapy.
Although a link between reduced functional performance and HIV infection has been hypothesized, the majority of previous research has focused on the syndrome of frailty[25, 26], aerobic capacity[27], or composite measures of performance[3]. Moreover, previous research limited to differences in grip strength by HIV serostatus has been cross sectional, focused on participants with lipodystrophy, or largely inconclusive[12, 13, 28], leaving the possibility of differences in the rate of strength decline undefined. The current study demonstrates a statistically significant difference in the trajectory of grip strength decline between men aging with HIV and demographically similar HIV− men. Combined with our previous work defining differences in the rate of gait speed decline by HIV status[2], the current analysis helps quantify the underlying mechanisms contributing to the greater risk of frailty in the HIV+ population. Further, among the HIV+ men, grip strength was significantly correlated with gait speed (r = 22.9%), the VACS index (17.8%), unintentional weight loss (4.9%), and reported energy and fatigue levels (8.6%, p< 0.05 for all).
The heightened risk of age-related diseases, HCV co-infection, and other conditions in people with chronic HIV infection may also affect grip strength[6]. The presence of clinical weakness was associated with HCV, diabetes, history of kidney disease, and peripheral neuropathy, many of which aggravate inflammatory burden. The association between kidney disease and lower grip strength among the HIV− participants may in part be explained by increased inflammatory burden, and also warrants future investigation[29]. Although the current analysis did not include longitudinal measures of inflammation, stored samples provide opportunities for future research.
The HIV+ men in the MACS may not be generalizable to other aging HIV+ populations, as longstanding participants of HIV cohort studies are different from the general HIV+ population. Moreover, MACS participants differ from persons living with HIV in the United States in age (17% are age 65 or older compared with 5%[30], respectively), and in having experienced more time without effective ART (i.e., prior to 1996) and/or exposure to less effective and more toxic ART medications. Our results do not show a difference in clinically weak grip strength by experiences prior to effective treatment (specific d-drug usage), but do show an overall difference in the rate of grip strength decline by history of exposure to mono- or combination therapy. This indicates that the difference in grip strength decline by HIV status may be smaller with newer, less toxic ART regimens that are initiated at higher CD4 cell counts. Further, the current study did not include women, limiting its generalizability to women aging with HIV.
As the treatment and management of HIV as a chronic condition continues to expand, the need to manage and treat age-related conditions in persons living with HIV will grow exponentially. The 70% increased hazard of developing clinical weakness holds significant implications for the care of those aging with HIV who may be at increased risk of sarcopenia, mobility limitations, hospitalization, and death. Grip strength is an important biomarker of aging that is clinically relevant and easy to measure and interpret. Accordingly, efforts to prevent and treat strength loss in those aging with HIV should be a clinical and public health focus.
Acknowledgments
Dr. Schrack had full access to all of the data in the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Sources:
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay H. Bream, Todd Brown, Barbara Crain, Adrian Dobs, Lisette Johnson-Hill, Sean Leng, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Maurice O’Gorman, David Ostrow, Frank Palella, Ann Ragin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-P I), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Ross D. Cranston, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; and the Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (Co-PI), Alison Abraham, Keri Althoff, Christopher Cox, Jennifer Deal, Priya Duggal, Sabina Haberlen, Heather McKay, Alvaro Munoz, Eric C. Seaberg, Sol Su, Pamela Surkan, Nikolas Wada. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), National Institute for Drug Abuse (NIDA), and the National Institute for Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR000424 (JHU CTSA). Website located at http://www.statepi.jhsph.edu/macs/macs.html. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). JA Schrack was supported by K01AG048765 from NIA. KN Althoff was supported by K01AI093197 from NIAID. KM Erlandson was support by K23AG050260 from NIA. TT Brown was supported in part by R01AI093520 and K24AI120834 from the NIAID. This work was also supported by the JHU CFAR (1P30AI094189).
Footnotes
Author contributions:
Study design: JBM, LPJ, JP, SK, BDJ, KME, TTB, JAS. Participant recruitment: JBM, LPJ, JP, SK, BDJ. Data analysis and results interpretation: JAS, KNA, LPJ, JBM. Drafted manuscript: JAS. Critical Review: JBM, LPJ, JP, SK, BDJ, KME, TTB. All authors reviewed and approved the final version of the manuscript.
Conflicts of interest:
None of the authors has an association that may pose a conflict of interest for the present work.
References
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2014. 2015:26. [Google Scholar]
- 2.Schrack JA, Althoff KN, Jacobson LP, Erlandson KM, Jamieson BD, Koletar SL, et al. Accelerated Longitudinal Gait Speed Decline in HIV-Infected Older Men. J Acquir Immune Defic Syndr. 2015;70:370–376. doi: 10.1097/QAI.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene M, Covinsky K, Astemborski J, Piggott DA, Brown TT, Leng SX, et al. The relationship of physical performance with HIV disease and mortality. AIDS. 2014;28:2711–2719. doi: 10.1097/QAD.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS Rep. 2014;11:279–290. doi: 10.1007/s11904-014-0215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunology and allergy clinics of North America. 2003;23(1):15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 6.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis. 2015;60:627–638. doi: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alley DE, Shardell MD, Peters KW, McLean RR, Dam TT, Kenny AM, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayer AA, Kirkwood TB. Grip strength and mortality: a biomarker of ageing? Lancet. 2015 doi: 10.1016/S0140-6736(14)62349-7. [DOI] [PubMed] [Google Scholar]
- 10.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A New Frailty Syndrome: Central Obesity and Frailty in Older Adults with the Human Immunodeficiency Virus. J Am Geriatr Soc. 2012;60:545–549. doi: 10.1111/j.1532-5415.2011.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richert L, Brault M, Mercie P, Dauchy FA, Bruyand M, Greib C, et al. Handgrip strength is only weakly correlated with physical function in well-controlled HIV infection: ANRS CO3 Aquitaine Cohort. J Acquir Immune Defic Syndr. 2014;65:e25–7. doi: 10.1097/QAI.0b013e3182a03db8. [DOI] [PubMed] [Google Scholar]
- 13.Raso V, Shephard RJ, do Rosario Casseb JS, da Silva Duarte AJ, D’Andrea Greve JM. Handgrip force offers a measure of physical function in individuals living with HIV/AIDS. J Acquir Immune Defic Syndr. 2013;63:e30–2. doi: 10.1097/QAI.0b013e31828c42bb. [DOI] [PubMed] [Google Scholar]
- 14.Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE, et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis. 2013;208:249–259. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995;142:323–330. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE., Jr SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Hultin LE, Menendez FA, Hultin PM, Jamieson BD, O’Gorman MR, Borowski L, et al. Assessing immunophenotyping performance: proficiency-validation for adopting improved flow cytometry methods. Cytometry B Clin Cytom. 2007;72:249–255. doi: 10.1002/cyto.b.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed on June 27, 2014.
- 19.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 20.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ, Jr, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. 2010;171:198–205. doi: 10.1093/aje/kwp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9:e113637. doi: 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesari M, Penninx BWJH, Pahor M, Lauretani F, Corsi AM, Williams GR, et al. Inflammatory Markers and Physical Performance in Older Persons: The InCHIANTI Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2004;59:M242–M248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 23.Federal Interagency Forum on Aging-Related Statistics. Older Americans 2012: Key Indicators of Well-Being. 2012 Jun; [Google Scholar]
- 24.Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol. 2006;16:554–562. doi: 10.1016/j.annepidem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Althoff KN, Jacobson LP, Cranson R, Detels R, Phair J, Xiuhong L, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. Journal of Gerontology: Medical Sciences. 2013 Oct; doi: 10.1093/gerona/glt148. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One. 2013;8:e54910. doi: 10.1371/journal.pone.0054910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Research and Human Retroviruses. 2006;22(11):1113–21. doi: 10.1089/aid.2006.22.1113. [DOI] [PubMed] [Google Scholar]
- 28.Crawford KW, Li X, Xu X, Abraham AG, Dobs AS, Margolick JB, et al. Lipodystrophy and inflammation predict later grip strength in HIV-infected men: the MACS Body Composition substudy. AIDS Res Hum Retroviruses. 2013;29:1138–1145. doi: 10.1089/aid.2013.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartmann EL, Kitzman D, Rocco M, Leng X, Klepin H, Gordon M, et al. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. 2009;4:588–594. doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Center for Disease Control and Prevention. HIV Among Older Americans. CDC Reports. 2013 Nov [Google Scholar]


