Abstract
Background
Hyperpolarized 129Xe is a promising contrast agent for MRI of pediatric lung function but its safety and tolerability in children have not been rigorously assessed.
Objective
To assess the feasibility, safety and tolerability of hyperpolarized 129Xe gas as an inhaled contrast agent for pediatric pulmonary MRI in healthy control subjects and in children with cystic fibrosis.
Materials and methods
Seventeen healthy control subjects (ages 6–15 years, 11 boys) and 11 children with cystic fibrosis (ages 8–16 years, 4 boys) underwent 129Xe MRI, receiving up to three doses of 129Xe gas prepared by either a commercially available or a homebuilt 129Xe polarizer. Subject heart rate and SpO2 were monitored for 2 minutes post inhalation and compared to resting baseline values. Adverse events were reported via follow-up phone call at days 1 and 30 (range ±7 days) post-MRI.
Results
All children tolerated multiple doses of 129Xe, and no children withdrew from the study. Relative to baseline, most children who received a full dose of gas for imaging (10 of 12 controls and 8 of 11 children with cystic fibrosis) experienced a nadir in SpO2 (mean −6.0 ± standard deviation 7.2%, P≤0.001); however within 2 minutes post inhalation SpO2 values showed no significant difference to baseline (P=0.11). There was a slight elevation in heart rate (mean +6.6 ± 13.9 beats per minute [bpm], P=0.021), which returned to baseline within 2 minutes post inhalation (P=0.35). Brief side effects related to the anesthetic properties of xenon were mild and quickly resolved without intervention. No serious or severe adverse events were observed; in total, four minor adverse events (14.3%) were reported following 129Xe MRI, but all were deemed unrelated to the study.
Conclusion
The feasibility, safety and tolerability of 129Xe MRI has been assessed in a small group of children as young as 6 years. SpO2 changes were consistent with the expected physiological effects of a short anoxic breath-hold, and other mild side effects were consistent with the known anesthetic properties of xenon and with previous safety assessments of 129Xe MRI in adults. Hyperpolarized 129Xe is a safe and well-tolerated inhaled contrast agent for pulmonary MR imaging in healthy children and in children with cystic fibrosis who have mild to moderate lung disease.
Keywords: Children, Cystic fibrosis, Hyperpolarized xenon, Lungs, Magnetic resonance imaging
Introduction
Driven by improved solid-state laser technology in the mid-1990s, it became possible to generate highly non-equilibrium total nuclear magnetic moment in the noble gas isotopes 3He and 129Xe. These hyperpolarized gases displayed approximately 10,000-fold enhanced MRI signal intensities, making it possible to generate MR images in seconds. As a result, hyperpolarized noble gases quickly emerged as promising inhaled contrast agents for pulmonary MRI [1–3], and since then the utility of hyperpolarized-gas MRI to visualize and quantify regional ventilation [4, 5] and assess alveolar airspace microstructural dimensions [6–9] has been demonstrated both in healthy volunteers and in subjects with a range of pulmonary diseases, including chronic obstructive pulmonary disease (COPD) [10, 11], cystic fibrosis (CF) [12, 13] and asthma [14–16]. It is important to note that unlike chest radiographs, scintigraphic ventilation/perfusion scanning and CT, hyperpolarized-gas MRI involves no ionizing radiation, and in stark contrast to lung biopsy (currently the gold standard for assessing alveolar structure), hyperpolarized-gas MRI is noninvasive, making the modality attractive for longitudinally assessing lung pathology. Reviews of hyperpolarized gas preparation [17, 18] and the translational applications in MRI are available elsewhere [19–22].
Hyperpolarized 3He has historically been the preferred hyperpolarized-gas MRI contrast agent for pediatric pulmonary imaging [23–26], and indeed 3He MRI has an established safety record for assessing pulmonary function in pediatric lung diseases, including CF [27–31] and asthma [32, 33], and quantifying normal alveolar growth during childhood and adolescence [34, 35]. Driven by the increasing scarcity and cost of 3He [36] and substantial improvements in 129Xe polarization technology, there has been a renewed interest in 129Xe MRI [37–41]. A growing number of studies have demonstrated that hyperpolarized 129Xe can provide regional measures of pulmonary ventilation (an assessment of lung function) and alveolar airspace microstructure (via 129Xe diffusion imaging) comparable to those obtained from 3He, providing a sustainable path for clinical translation of 129Xe MRI [10, 42, 43]. Furthermore, xenon is moderately soluble in pulmonary tissues (Ostwald solubility ~10% [44]) and exhibits a large (>200 parts per million [ppm]) chemical shift range in the lungs, thus allowing hyperpolarized 129Xe to serve as a regional probe of gas-exchange dynamics [45–49]. However, its tissue solubility also makes the safety and tolerability of 129Xe a greater concern than 3He. Specifically, dissolved xenon can give rise to neurological effects that include anesthesia at sustained, elevated alveolar concentrations (alveolar concentration >63% [50]) and milder effects including euphoria and dizziness at lower alveolar concentrations [51].
Although reports demonstrate that hyperpolarized 129Xe is a safe contrast agent for pulmonary imaging in adults [52, 53], the safety and feasibility of 129Xe MRI in children has not been assessed. However there are reports of sub-anesthetic doses of xenon gas being safely administered to children undergoing xenon-enhanced CT [54, 55]. The most relevant work documenting the safety of xenon gas in children is that of Goo et al. [56], who performed Xe-enhanced CT in 17 pediatric patients (age range 7–18 years) with bronchiolitis obliterans. Subjects inhaled multiple breaths of 30% xenon to establish a steady-state exhaled Xe concentration of 25–30% (wash-in times ranged 36–90 s) before a breath-hold and CT acquisition (approximately 10 s), and the authors observed no significant changes in subject heart rate, SpO2 or blood pressure. Transient, minor side effects related to the anesthetic properties of xenon, including peripheral numbness and dizziness, were reported in most subjects (70.6%); however these effects were deemed mild and resolved within a few minutes and without intervention [56].
This study assesses the safety of hyperpolarized 129Xe MRI in a small group of children. For this initial assessment, we performed 129Xe MRI in a cohort children with cystic fibrosis (n=11) and in healthy age-matched children (n=17) ranging 6–16 years old. In doing so, we investigated the safety and tolerability of xenon gas in children and also demonstrated the feasibility of 129Xe MRI techniques in relatively young children with mild to moderate lung disease.
Materials and methods
Research subjects
Following U.S. Food and Drug Administration investigational new drug (IND 123,577) and institutional review board approvals, we enrolled 28 children (age range 6–16 years) in the study. Inclusion criteria included the ability to perform a 16-s breath-hold and, for the children with cystic fibrosis, a clinical diagnosis of CF based on standard guidelines [57]. Exclusion criteria included a history of poorly controlled asthma, history of heart defect, use of an asthma rescue inhaler ≥2 times in the last month, symptoms of respiratory or sinus infection 1 week prior to imaging, baseline pulse oximetry <95% at the time of MRI, pregnancy or positive pregnancy test, and standard MRI exclusions. We obtained written parental consent and age-appropriate subject assent for all subjects. Table 1 details demographics and clinical information for our subjects. We enrolled 17 healthy control subjects (11 boys, 6 girls, age range 6–16 years) and 11 children with diagnosed CF [58] (4 boys, 7 girls, age range 8–16 years). CF patient genotypes and CF-related pathogens are also provided in Table 1. In children for whom recent (within 6 months) clinical pulmonary function tests were not available, spirometry was performed immediately prior to MR imaging using a Koko portable handheld spirometer (nSpire Health, Longmont, CO) according to American Thoracic Society (ATS) guidelines [57].
Table 1.
Subject demographics and clinical information
| Subject ID | Age (years) | Gender | Weight (kg) | Height (cm) | FEV1% predicted | Predicted TLC (L) | Number of 129Xe doses | CF genotype | CF pathogensb |
|---|---|---|---|---|---|---|---|---|---|
| Healthy volunteers (n=17) | |||||||||
| HV-1a | 11 | F | 35.1 | 148.5 | 100% | 3.59 | 2 | - | - |
| HV-2a | 14 | M | 49.1 | 164.6 | NA | 4.94 | 2 | - | - |
| HV-3a | 10 | M | 100.7 | 148.7 | 127% | 3.81 | 2 | - | - |
| HV-4a | 10 | M | 39.6 | 138.8 | 91% | 3.19 | 2 | - | - |
| HV-5a | 7 | F | 27.4 | 122.5 | 106% | 2.19 | 2 | - | - |
| HV-6 | 7 | F | 33 | 128.4 | 115% | 2.47 | 2 | - | - |
| HV-7 | 7 | M | 24.9 | 123.2 | 99% | 2.35 | 2 | - | - |
| HV-8 | 14 | M | 40.6 | 150 | 108% | 3.89 | 2 | - | - |
| HV-9 | 14 | M | 64.8 | 176.8 | 106% | 5.94 | 2 | - | - |
| HV-10 | 15 | F | 51.5 | 158.5 | 110% | 4.25 | 2 | - | - |
| HV-11 | 12 | M | 34.8 | 140.9 | 103% | 3.32 | 2 | - | - |
| HV-12 | 13 | M | 57.2 | 171.2 | 109% | 5.47 | 2 | - | - |
| HV-13 | 13 | M | 45.8 | 160 | 89% | 4.60 | 2 | - | - |
| HV-14 | 12 | M | 56.6 | 159.9 | 92% | 4.59 | 2 | - | - |
| HV-15 | 8 | M | 35.2 | 136 | 91% | 3.03 | 2 | - | - |
| HV-16 | 6 | F | 20.8 | 115.5 | 95% | 1.88 | 3 | - | - |
| HV-17 | 16 | F | 49.6 | 169.2 | 110% | 5.03 | 3 | - | - |
| Children with cystic fibrosis (n=11) | |||||||||
| CF-1 | 14 | M | 54.4 | 167 | 97% | 5.13 | 2 | F508Δ / F508Δ | Sa, A, Ca |
| CF-2 | 12 | F | 43.6 | 162 | 77% | 4.50 | 2 | F508Δ / F508Δ | A, Sp |
| CF-3 | 13 | F | 48.9 | 161 | 96% | 4.43 | 2 | G551D / F508Δ | - |
| CF-4 | 14 | F | 51.2 | 156 | 106% | 4.08 | 2 | L206W / F508Δ | Sa, Hi |
| CF-5 | 16 | M | 65.5 | 179 | 120% | 6.13 | 2 | F508Δ / F508Δ | Sm |
| CF-6 | 8 | M | 27.8 | 126 | 118% | 2.49 | 3 | F508Δ / F508Δ | Hi |
| CF-7 | 11 | M | 34.5 | 141.8 | 102% | 3.37 | 3 | R1066H / F508Δ | - |
| CF-8 | 13 | F | 46.5 | 157 | 114% | 4.15 | 2 | G178R / F508Δ | Hi |
| CF-9 | 15 | F | 43.7 | 159 | 72% | 4.29 | 2 | F508Δ / F508Δ | Sa, Pa |
| CF-10 | 11 | F | 40.1 | 152 | 89% | 3.82 | 2 | F508Δ / F508Δ | Sa |
| CF-11 | 11 | F | 34.3 | 147 | 86% | 3.50 | 2 | F508Δ / F508Δ | Pa |
Subject was imaged with half dose of xenon gas (1/12th predicted TLC)
Pathogens grown in respiratory cultures within 1 year prior to study
A Achromobacter, Ca Candida albicans, CF cystic fibrosis, F female, FEV1 forced expiratory volume in 1 second, Hi Haemophilus influenzae, HV healthy volunteers, ID identification, M male, NA not obtained for this child, Pa Pseudomonas aeruginosa, Sa Staphylococcus aureus, Sm Stenotrophomonas maltophilia, Sp Scedosporium prolificans, TLC total lung capacity
Hyperpolarized 129Xe gas preparation
Hyperpolarized 129Xe gas was prepared using either a commercially available polarizer (Model 9800; Polarean, Durham, NC) or our center’s homebuilt polarizer [59, 60]. 129Xe was dispensed into a Tedlar delivery bag (600 ml or 1,000 ml, the latter if the dose was >600 ml; Jensen Inert Products, Coral Springs, FL) through 3/8″ tubing (Tygon; Saint-Gobain, Akron, OH). A mouthpiece (part number DK100191; Epsilon Medical Devices, Penang, Malaysia) or a custom mouthpiece designed by Teleflex (Morrisville, NC) was fitted to the tubing before delivery to the child. All experiments used isotopically enriched xenon gas (86% 129Xe), and 129Xe polarizations ranged 10–30% at the time of imaging. Xenon polarization was measured using a polarization measurement station (Model 2881; Polarean, Durham, NC) before gas administration.
MR imaging protocol
Children were imaged with a Philips 3-T Achieva MRI scanner using one of two homebuilt 129Xe dual-looped saddle coils designed to fit either small or large subjects [61]. At the time of imaging, a visual assessment was made to determine which coil best matched a child’s thoracic volume. After 3-plane 1H localization scans, children received a bag of air with a volume approximately equal to one-sixth total lung capacity (TLC) to practice the inhalation and breath-hold maneuver (~16 s) during a conventional 1H MRI gradient-echo scan. For each 129Xe dose (calibration and imaging), the child was coached by a team member to exhale to functional residual capacity (FRC) before inhaling the 129Xe gas mixture.
To set the scanner flip angle, α, a 129Xe calibration dose consisting of ~250 ml of xenon and ~250 ml of N2 (99.9999%, Praxair) was administered using the same breath-hold maneuver as described to determine the in vivo flip angle. Calibration was performed for each child individually by fitting the signal decay from 64 constant flip-angle whole-lung excitations (total duration ~2 s) to Sn=S0cosn−1(α), where Sn is the signal magnitude of the nth excitation.
Hyperpolarized 129Xe ventilation images were acquired with linear phase encoding and a flip angle optimized for maximum signal at k-zero [62]. Additional imaging parameters included flip angle of 9–12°, repetition time/echo time [TR/TE] 8/4 ms, matrix size 52–96 × 96–144, voxel size 3 × 3 × 15 mm3, 9–14 slices, with a total scan time of less than 16 s. For eight children, 129Xe diffusion images were acquired using multiple b values (b = 6.25, 12.5, 18.75, 25 s/cm2) bi-polar diffusion-sensitizing gradient-echo sequence [63] (flip angle 5–10°, diffusion time [Δ] 3.5 ms, lobe duration [δ] 3.1 ms, TR/TE 15/10 ms, matrix size 25–96 × 48–96, voxel size 3–7 × 3–7 × 15–30 mm3, 4–10 slices), using a maximum scan duration of 16 s. 129Xe apparent diffusion coefficient (ADC) maps were generated from the diffusion images using code written in MATLAB (MathWorks, Natick, MA) and the R language. Hyperpolarized 129Xe chemical shift saturation recovery (CSSR) MR spectroscopy [48, 49] was performed using a variable delay time ranging from 3.5 ms to 900 ms and a 2-ms Gaussian radiofrequency excitation pulse to generate a free induction decay, which was repeated 32 times during a single breath-hold. The resulting 129Xe spectra were processed using code written in MATLAB.
Safety monitoring and xenon dosing
Hyperpolarized 129Xe gas was administered in the presence of a registered nurse or physician. The subject heart rate (beats per minute, or bpm) and blood oxygenation (SpO2) were monitored via an MR-compatible pulse oximeter (model 865353 MRI Patient Monitor; InVivo, Orlando, FL). Subject resting baseline heart rate and SpO2 were recorded prior to inhaling 129Xe, and heart rate and SpO2 were monitored for 2 min post-inhalation for every dose of 129Xe gas. We recorded nadir SpO2 (i.e. the lowest blood oxygenation post inhalation), subject heart rate at the time of the SpO2 nadir, and the duration of the SpO2 nadir. Immediately following each dose, the gas administrator performed a brief assessment to evaluate the central nervous system effects of hyperpolarized 129Xe. In this assessment, the child was asked to describe any sensations such as dizziness, light-headedness, numbness or euphoria. Prior to additional doses of 129Xe gas, the child breathed room air for at least 2 min. We administered a maximum of three 129Xe doses per child, including the calibration dose. Adverse events were assessed at the time of imaging and during two follow-up phone calls at day 1 and day 30 (range ±7 days) post imaging.
As an initial test of tolerability and feasibility, the first five healthy volunteers (subjects HV-1 through HV-5) were imaged with a half-dose of gas (approximately equal to one-twelfth of their predicted TLC volume as calculated from the ATS plethysmography-based guidelines for pediatrics [64, 65]). Briefly, TLC is given by the equations TLC (L) = 9.96 × h2.5698 × 10−6 for boys and TLC (L) = 9.17 × h2.5755 × 10−6 for girls, where h is the subject’s height in centimeters. Predicted TLC values were used (as opposed to performing plethysmography in each individual subject), because plethysmography overestimates functional volumes in cases of significant airway obstruction and air trapping [66] — a specific concern for children with cystic fibrosis. The remaining healthy volunteers (HV-6 through HV-17) and all children with CF received a full imaging dose of up to one-sixth of the predicted TLC, with a maximum dose of 1 liter. In subsequent text, these larger-volume doses are referred to as full doses to distinguish them from the initial one-twelfth TLC doses (half-doses) and the calibration doses.
Statistical analysis
Nadir SpO2 and heart rate at the time of SpO2 nadir were compared to resting baseline values to determine changes post inhalation of xenon gas. Baseline SpO2 and heart rate were also compared to SpO2 and heart rate at 2-min post inhalation. For these comparisons, two-sided paired t-tests with unequal variance were used to determine statistically significant (P-value≤0.05) changes in heart rate and SpO2. For the purposes of comparing children with CF and healthy controls, we performed Welch two-sample t-tests with 95% confidence interval. We used Bland–Altman analysis to evaluate the difference between the SpO2 nadirs for the calibration and imaging doses.
Results
Tolerability and physiological monitoring
The first five healthy children received and tolerated a half-dose of gas, or 1/12 of the predicted TLC (HV-1 through HV-5), and no child withdrew from the study. Table 2 summarizes the changes in SpO2 and heart rate for these children during the 129Xe gas administration (subject HV-1 was excluded from the analysis because nadir SpO2 and heart rate at the SpO2 nadir were not captured). The SpO2 nadir post inhalation was −2.5 ± 2.4% (mean ± standard deviation) for the calibration dose and −4.3 ± 4.6% for the imaging dose; however this drop was not significantly different from the resting baseline value (P=0.13 and P=0.16 for the calibration and imaging doses, respectively). For the calibration dose, there was no significant difference in SpO2 at 2 minutes post inhalation relative to baseline (P=0.22). For the imaging dose (a half-dose), subject SpO2 at 2 minutes post inhalation was lower compared to baseline (97.4±1.3%, P=0.034); however the change (a reduction of 1% below baseline) was not physiologically significant. Table 2 also summarizes changes in heart rate for these five children throughout the 129Xe imaging; there were no significant changes in heart rate for any time-point comparison for either the calibration or imaging doses, with P-values ≥ 0.22.
Table 2.
Summary of SpO2 and heart rate changes (mean ± standard deviation) during 129Xe MRI breath-hold for children receiving the initial one-twelfth of a TLC dose of hyperpolarized gas (n=5; HV-1 through HV-5)
| Baseline | Nadir | Mean difference from baseline [95% CI]a | P-valueb (baseline vs. nadir) | 2-minute recovery | Mean difference from baseline [95% CI]a | P-valueb (baseline vs. 2-minute recovery) | |
|---|---|---|---|---|---|---|---|
| Calibration dose | |||||||
| SpO2 (%) | 98.4 ± 1.5 | 96.0 ± 1.6 | −2.5 [−6.3, 1.3] | 0.13 | 98.0 ± 1.6 | −0.4 [−1.8, 1.0] | 0.48 |
| HR (bpm) | 75.8 ± 20.5 | 74.3 ± 19.0 | −4.5 [−13.7, 4.7] | 0.22 | 78.3 ± 13.8 | −2.4 [−7.6, 2.8] | 0.27 |
| Imaging dose | |||||||
| SpO2 (%) | 98.4 ± 1.5 | 94.0 ± 5.6 | −4.3 [−11.6, 3.1] | 0.16 | 97.4 ± 1.3 | −1.0 [−1.9, −0.1] | 0.034 |
| HR (bpm) | 72.2 ± 14.2 | 75.5 ± 11.0 | 2.0 [−16.4, 20.5] | 0.75 | 74.2 ± 16.8 | 2.0 [−5.2, 9.2] | 0.48 |
Mean differences from baseline and 95% confidence intervals ([lower limit, upper limit])
P-value ≤ 0.05 is significant
bpm beats per minute, CI confidence interval, HR heart rate, HV healthy volunteer, SpO2 blood oxygenation, TLC total lung capacity
After reviewing the safety data and adverse events for the initial five healthy volunteers, full imaging doses of gas were administered to the remaining controls (HV-6 through HV-17) and all children with CF. Again, no children withdrew from this stage of the study and no medical intervention was required at any time for any child. Table 3 summarizes the changes in SpO2 during the gas administration. Relative to baseline, the average change in SpO2 was −2.9 ± 2.9% (P≤0.001) for the calibration doses and −6.0 ± 7.2% (P≤0.001) for the imaging doses. Although there were no significant differences in SpO2 at 2 minutes post inhalation relative to baseline, a slight trend toward increased values was observed (calibration dose at P=0.069 and imaging dose at P=0.11). Additionally, of the 50 total doses administered to these 23 children, either no change or an increase in SpO2 was reported for six doses, likely representing normal physiological variability in SpO2.
Table 3.
Summary of mean ± standard deviation SpO2 changes during HP 129Xe imaging for children receiving full doses of xenon gas
| All children (n=23) | Healthy controls (n=12; HV-6 through HV-17) | Children with CF (n=11) | |
|---|---|---|---|
| Calibration dose | |||
| Baseline SpO2 (%) | 98.0 ± 1.1 | 98.4 ± 1.0 | 97.6 ± 1.2 |
| Nadir SpO2 (%) | 95.1 ± 2.8 | 95.4 ± 2.4 | 94.8 ± 3.2 |
| Mean difference from baseline SpO2 (%) [95% CI] | −2.9 [−4.2, −1.7] | −3.0 −4.6, −1.4] | −2.8 [−5.0, −0.6] |
| P-value (baseline vs. nadir)a | ≤0.001 | ≤0.01 | 0.017 |
| 2-minute SpO2 (%) | 97.5 ± 1.5 | 97.8 ± 1.8 | 97.3 ± 1.1 |
| Mean difference from baseline SpO2 (%) [95% CI] | −0.5 [−1.1, 0.0] | −0.7 [−1.8, 0.4] | −0.4 [−0.82, 0.1] |
| P-value (baseline vs. 2-minute)a | 0.069 | 0.21 | 0.10 |
| Imaging dose | |||
| Baseline SpO2 (%) | 97.9 ± 1.4 | 98.5 ± 1.2 | 97.3 ± 1.6 |
| Nadir SpO2 (%) | 92.0 ± 7.5 | 93.9 ± 4.0 | 89.9 ± 9.8 |
| Mean difference from baseline SpO2 (%) [95% CI] | −6.0 [−8.8, −3.1] | −4.6 [−2.3, −7.0] | −7.4 [−13.1, −1.7] |
| P-value (baseline vs. nadir)a | ≤0.001 | ≤0.001 | 0.015 |
| 2-minute SpO2 (%) | 97.4 ± 1.7 | 97.5 ± 1.7 | 97.3 ± 1.8 |
| Mean difference from baseline SpO2 (%) [95% CI] | −0.5 [−1.2, 0.1] | −1.0 [−0.2, −1.8] | 0.0 [−1.0, 1.0] |
| P-value (baseline vs. 2-minute)a | 0.11 | 0.024 | 1.0 |
P-value ≤ 0.05 is significant
CF cystic fibrosis, CI confidence interval, HV healthy volunteer, SpO2 blood oxygenation
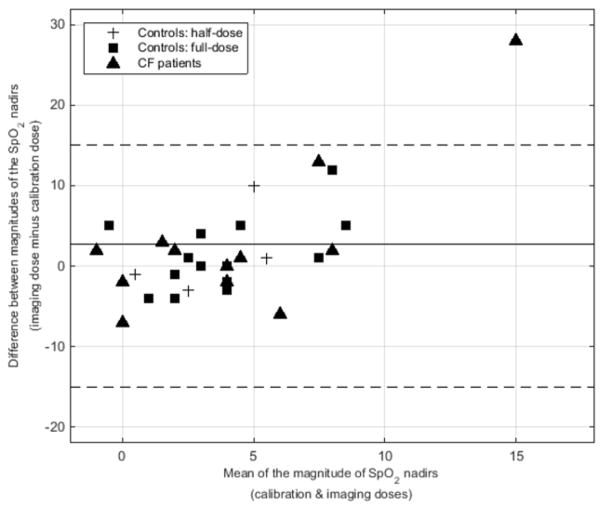
Figure 1 is a Bland–Altman plot comparing the magnitudes of the SpO2 nadirs for the calibration and imaging doses for all three groups of subjects (i.e. controls with half-dosing, controls with full-dosing, and children with CF). For all doses of gas for all children, the mean difference in the SpO2 nadirs between the imaging and calibration doses was −2.7 ± 7.7%, meaning that the SpO2 nadir for the imaging dose was slightly larger. For subject CF-7, the SpO2 nadir for the calibration dose was only −1%; however the nadir SpO2 for the imaging dose was −29% (nadir of 67%, with a baseline of 96%). This nadir spontaneously resolved, and the SpO2 quickly recovered without intervention (97% at 2 minutes post inhalation).
Fig. 1.
Bland–Altman plot shows a comparison of the magnitude of the SpO2 nadirs for the calibration and imaging doses for all three subject groups (controls with half-doses of hyperpolarized gas, controls with full doses, and children with cystic fibrosis, who all received full doses. Across all subjects and all doses, the mean difference in SpO2 nadir for the imaging and calibration doses was −2.7±7.7% (solid line; magnitude is plotted). Note the 95% confidence intervals (dashed lines). CF cystic fibrosis
Table 4 shows the heart rate changes at the same four time-points for these children. There were no significant changes in subject heart rate throughout the calibration dose (P-values ≥ 0.46). For the imaging dose, there was a significant elevation in heart rate (captured at the time of the SpO2 nadir) to 83.5±16.6 bpm (P=0.021 relative to a baseline value of 76.9±13.1 bpm), which returned to resting baseline by 2 minutes post inhalation (P=0.35).
Table 4.
Summary of mean ± standard deviation heart rate changes during HP 129Xe imaging for children receiving full dosing of xenon gas
| All children (n=23) | Controls, up to 1/6 TLC dose (n=12) | CF subjects, up to 1/6 TLC dose (n=11) | |
|---|---|---|---|
| Calibration dose | |||
| Baseline HR (bpm) | 78.7 ± 14.3 | 79.2 ± 15.1 | 78.3 ± 14.0 |
| Nadir HRa (bpm) | 80.3 ± 13.1 | 79.9 ± 16.3 | 80.6 ± 9.3 |
| Mean difference from baseline (bpm) [95% CI] | 1.5 [−2.7, 5.7] | 0.8 [−5.2, 6.7] | 2.4 [−4.6, 9.4] |
| P-valueb (baseline vs. nadir) | 0.46 | 0.79 | 0.47 |
| 2-minute HR (bpm) | 78.4 ± 13.6 | 78.3 ± 13.8 | 78.6 ± 14.1 |
| Mean difference from baseline (bpm) [95% CI] | −0.3 [−3.3, 2.7] | −1.0 [−3.8, 1.9] | 0.4 [−5.8, 6.6] |
| P-valueb (baseline vs. 2-minute) | 0.84 | 0.50 | 0.90 |
| Imaging dose | |||
| Baseline HR (bpm) | 76.9 ± 13.1 | 77.8 ± 15.0 | 76.0 ± 11.3 |
| Nadir HRa (bpm) | 83.5 ± 16.6 | 85.3 ± 17.3 | 80.4 ± 16.3 |
| Mean difference from baseline (bpm) [95% CI] | 6.6 [1.1, 12.1] | 7.5 [−0.7, 15.7] | 5.5 [−3.0, 14.1] |
| P-valueb (baseline vs. nadir) | 0.021 | 0.070 | 0.18 |
| 2-minute HR (bpm) | 78.0 ± 12.8 | 79.3 ± 14.4 | 76.7 ± 11.3 |
| Mean difference from baseline (bpm) [95% CI] | 1.1 [−1.3, 3.5] | 1.5 [−2.0, 5.0] | 0.7 [−3.1, 4.4] |
| P-valueb (baseline vs. 2-minute) | 0.35 | 0.37 | 0.69 |
bpm beats per minute, CF cystic fibrosis, CI confidence interval, HR heart rate, TLC total lung capacity
Nadir HR is the heart rate recorded at the time of the SpO2 nadir
P-value ≤ 0.05 is significant
Control- versus CF-group comparisons
For the purposes of comparing changes in vital signs between children with CF and healthy controls, only the children who received full doses of gas were considered (n=23, subjects HV-6 through HV-17 and all children with CF). There were no significant differences in age (P=0.36), weight (P=0.74), height (P=0.40) or forced expiratory volume in 1 second (FEV1) predicted (P=0.44) between these controls and the children with CF. The SpO2 nadir was not significantly different between controls and children with CF for the calibration dose (−3.0 ± 2.6%, and −2.8 ± 3.3% for the controls and children with CF, respectively; P=0.89). Likewise, there was no significant difference in the SpO2 nadir for the imaging doses between the control and CF groups (−4.6 ± 4.1% and −7.4 ± 9.4%, respectively; P=0.35).
Following inhalation of the calibration dose, all control subjects except HV-9 experienced a nadir in SpO2 (P≤0.01 versus baseline), but SpO2 returned to baseline within 2 minutes post inhalation (P=0.21 versus baseline). Similar SpO2 changes were observed in the children with CF (P=0.017 for the SpO2 nadir; P=0.10 at 2 minutes post inhalation). Prior to inhaling a full imaging dose of gas, control subjects had a baseline SpO2 of 98.5±1.2%, which was significantly reduced following inhalation (nadir of 93.9±4.0%, P=≤0.001). At 2 minutes post inhalation, SpO2 remained slightly decreased in these children (97.5±1.7%, P=0.024 relative to baseline), but this change was not physiologically relevant. Children with CF experienced a similarly significant SpO2 nadir relative to baseline (97.3±1.6% at baseline, 89.9±9.8% at the nadir, P=0.015). However, SpO2 returned to baseline values by 2 minutes post inhalation (97.3±1.8%, P=1.0 compared to baseline).
For the calibration doses, there was no significant change in heart rate (i.e. the difference between resting baseline and the heart rate recorded at the SpO2 nadir post inhalation), with the mean for control subjects being +0.8 ± 9.4 bpm compared to +2.4 ± 10.4 bpm for the children with CF (P=0.70). Likewise, for the imaging doses of gas, there was no significant change in mean heart rate for the children with CF (+5.5 ± 14 bpm) or the controls (+7.5 ± 14.2 bpm; P=0.72). There were also no statistically significant changes in heart rate for any time-point comparison for the control group (P≥0.070). However there was a trend toward significance for an elevation in heart rate at the time of the SpO2 nadir in children with CF (P≥0.18) in both imaging and calibration doses of gas.
Neurological symptoms and adverse events
None of the half-dose cohort (one-twelfth TLC) reported central nervous system effects, while in general all children receiving the full dose of gas (one-sixth TLC) experienced central nervous system effects to some degree. However all side effects were mild and consistent with the sub-anesthetic properties of xenon (e.g., tingling, dizziness, euphoria) at these relatively low alveolar xenon concentrations. Moreover, all side effects resolved quickly (<30 s) and spontaneously after breathing room air for 2 minutes. In total, four adverse events were reported, but none was serious, and all were determined to be unrelated to the study. At the day 1 follow-up phone call, the parent of subject HV-5 reported that the child ate food too quickly and vomited later in the day of the imaging procedure. However because of the child’s reported history of this behavior, this event was deemed unrelated to the study. Three of the four adverse events were reported at the day 30 follow-up phone call: cough (subject CF-2), influenza B (CF-1), and upper respiratory tract infection (CF-3), and each of the three cases reported a decrease in pulmonary function tests. Given the common susceptibility of children with CF to decreases in pulmonary function, these adverse events were also considered to be unrelated to the study.
Pediatric 129Xe MRI feasibility
All children were able to perform the up to 16 s breath-hold maneuver required for MR studies. Moreover, diagnostically meaningful 129Xe MR images were successfully obtained from all children. Illustrative examples of the most promising of these potential 129Xe MR applications (i.e. spectroscopy, diffusion-weighted MRI, and ventilation imaging) are demonstrated in Figs. 2, 3 and 4.
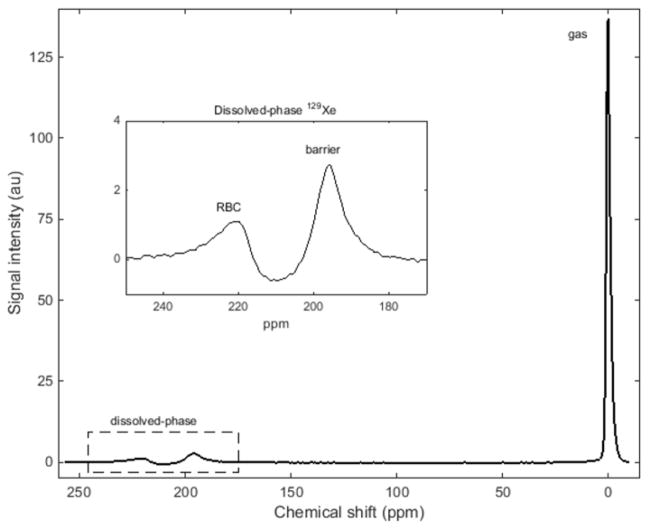
Fig. 2.
129Xe MR spectrum from a healthy 16-year-old girl, subject HV-17. Three spectral peaks are observed: the bulk gas-phase peak (0 ppm) and two dissolved-phase peaks (enlarged in the inset). The other two peaks originate from hyperpolarized 129Xe dissolved in the red blood cells and the barrier tissues (blood plasma and lung parenchymal tissue) peaks at ~212 ppm and ~197 ppm, respectively. ppm parts per minute
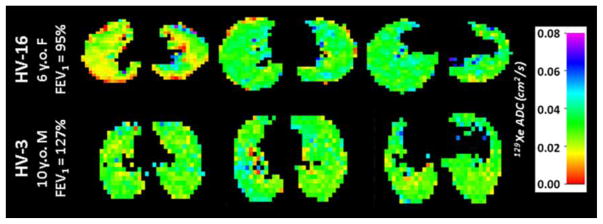
Fig. 3.
129Xe apparent diffusion coefficient (ADC) maps generated via diffusion imaging in two healthy control subjects, a 6-year-old girl (HV-16, top row) and a 10-year-old boy (HV-3, bottom row) for three axial slices through the lungs. The mean ± standard deviation 129Xe ADC was 0.029±0.01 cm2/s and 0.031±0.02 cm2/s for HV-16 and HV-3, respectively
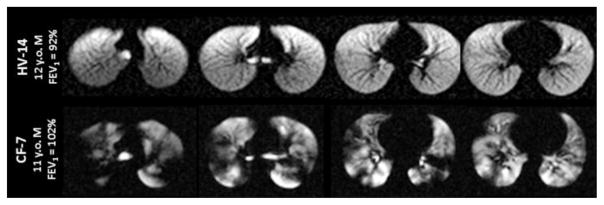
Fig. 4.
129Xe ventilation images for a healthy 12-year-old boy (HV-14, top row) and an 11-year-old boy with cystic fibrosis (CF-7, bottom row). The homogeneous signal seen throughout the lungs of the healthy control indicates that all regions of the lung are well ventilated. In contrast, the hyperpolarized 129Xe images of the child with cystic fibrosis display numerous regions of low signal intensity, indicating that ventilation is substantially impaired in multiple regions of the lungs
Discussion
Safety and tolerability
In this study we examined the safety, tolerability and feasibility of hyperpolarized 129Xe gas as an inhaled contrast agent for pulmonary MR of children with and without lung disease (cystic fibrosis). Although most children did experience a statistically significant nadir in SpO2 after inhaling the 129Xe gas, it is important to note that these changes were clinically insignificant and fully consistent with expectations from previous hyperpolarized-gas MRI studies [52, 53, 67]. In general an increase in heart rate was noted at the time of the SpO2 nadir; however this increase was only statistically significant for one comparison (i.e. the aggregate analysis for children receiving the full-volume imaging dose of gas, P=0.021). Moreover these changes in SpO2 and heart rate were small and transient and resolved quickly without medical intervention, within 2 minutes of normal breathing. At the 2-minute recovery time-point, subject SpO2 remained decreased relative to baseline for one comparison (controls with full imaging doses of gas, P=0.024), but this decrease was not clinically significant (approximately a 1% change from 98.5% at baseline to 97.5% at 2 minutes post inhalation). For the aggregate analysis of children receiving the full imaging dose of gas, there was a mean increase in heart rate of 6.6 bpm after gas inhalation; while this increase was statistically significant, it was clinically insignificant and subject heart rate returned to resting baseline values within 2 minutes.
Our results were in good agreement with previous assessments of 129Xe MRI safety in adults [52, 53]. In a phase 1 clinical trial of 129Xe MRI conducted at Duke University Medical Center [52] involving 44 adults who inhaled up to five 1-liter doses of xenon gas, no significant changes in vital signs were observed within 10 minutes post inhalation (note, the presence of a nadir SpO2 was not explicitly examined in this study). No subjects withdrew from that study or experienced any serious or severe adverse events. Although most subjects experienced transient symptoms related to the known anesthetic properties of xenon, all symptoms resolved without clinical intervention in 1.6±0.9 min [52]. In a second study of 129Xe tolerability in adults, conducted at Robarts Research Institute [53], 33 adults were imaged with 0.5 l 129Xe gas mixed with 0.5 l 4He. All subjects tolerated these doses well, no subjects withdrew from the study, and no severe adverse events were observed. Two subjects reported mild adverse events (light-headedness), likely related to xenon administration, but these events resolved within 2 minutes and without intervention. Similar to our results, they reported statistically significant changes in SpO2 and heart rate immediately post inhalation. However these changes were also clinically insignificant and returned to resting baseline within 1 minute and without intervention [53].
Because 129Xe MRI was performed during a short breath-hold using sub-anesthetic doses of Xe gas, all central nervous system effects experienced by the children in this study were mild and resolved spontaneously with normal breathing of room air. Note, in earlier assessments of hyperpolarized 129Xe safety in adults [52, 53], central nervous system effects were described in great detail, with subjects differentiating between nuanced sensations such as euphoria and light-headedness. However, we anticipated that making similar distinctions would be challenging for some younger pediatric subjects, thus we combined central nervous system effects into a single category.
The Xe dosing strategy of one-sixth of the predicted TLC dose resulted in an alveolar concentration ~17% for a single breath as compared to ~30% concentration sustained over minutes for Xe-enhanced CT [56] and ~63% to initiate anesthesia [50]. Even if the predicted TLC was overestimated by 30%, the alveolar concentration would only have reached 24% for a single breath. The SpO2 nadir was larger for the full imaging doses of 129Xe gas relative to the calibration doses for most children, which is likely related to the longer anoxic breath-hold duration (i.e. ~2 s and up to 16 s for the calibration and imaging doses, respectively). For all children except subject CF-7, the magnitude of the SpO2 nadirs fell within the 95% confidence interval, suggesting the change in SpO2 was not related to the volume of xenon gas inhaled but rather to the anoxic breath-hold itself (i.e. the effects of the Xe gas are not likely to manifest during a single 16-s breath-hold, as opposed to minutes-long exposure at high concentrations for anesthesia). While 129Xe MRI generally was well tolerated by all children, one child with CF (CF-7) had an SpO2 drop from 96% to 67% during the imaging dose breath-hold, which quickly recovered to baseline without intervention (97% at 2 min post inhalation). This child did not clearly differ from other children in terms of age or lung function (FEV1=102%), highlighting the value of SpO2 motoring during the anoxic xenon breath-holds.
For this initial assessment of 129Xe MRI, our cohort comprised both healthy children and children with CF who had mild to moderate lung disease. CF was chosen for this initial study (as opposed to asthma, for example) because of its known genetic etiology. While no significant differences in vital sign changes were observed between these groups, it is possible that children with other pulmonary diseases or with more severe disease may respond differently to the anoxic 129Xe gas inhalation. Children with more severe disease might also have difficulty performing the 16-s breath-hold employed for our 129Xe imaging studies. However the use of more efficient imaging sequences (e.g., acquisitions with spiral k-space trajectories [68, 69]) that enable faster imaging and reduced breath-hold durations and the addition of O2 to the inhaled gas mixture (via a second bag to mitigate T1 relaxation [45]) would mitigate the majority of these obstacles.
Compliance with the inhalation maneuvers
Compliance with breathing maneuvers can be challenging for children compared to adults. To address this issue, the team member responsible for 129Xe gas administration worked to build a good rapport with the children, which helped to avoid complications and increased compliance with imaging. Children performed a practice breath-hold with a bag of air during a conventional 1H gradient-echo sequence (with the same duration as the 129Xe scan) to acclimate to the scanner environment. Practice breath-holds were repeated until a child could comfortably perform the maneuver before Xe was administered. Typically only one to two practice attempts were required to achieve full compliance during the 129Xe scans.
Feasibility of pediatric studies with hyperpolarized 129Xe
Hyperpolarized 129Xe MRI has the potential to provide unique regional insights into gas exchange, lung microstructure and ventilation at little or no risk to the child, even in younger children with significant underlying lung disease. A 129Xe magnetic resonance spectrum for subject HV-17 is presented in Fig. 2. Although the majority of the 129Xe MR signal originates from gaseous 129Xe in the alveolar spaces (by convention, reference to 0 ppm), two small peaks are observed near ~200 ppm, demonstrating the high sensitivity of the 129Xe resonance frequency to differences in chemical environment. Previously, these unique physical and MR properties of 129Xe (i.e. the ability to dissolve in lung tissues and produce resonances specific to red blood cells and barrier tissues) have been exploited to visualized dynamic gas uptake and regionally impaired exchange in adults [11, 45, 46, 48, 49].
In apparent diffusion coefficient (ADC) maps obtained from diffusion-weighted 129Xe images (Fig. 3), the mean 129Xe ADC was 0.029±0.01 cm2/s for HV-15 and 0.031±0.02 cm2/s for HV-3. Notably, these mean ADC values are smaller than those previously reported for healthy adults (~0.04 cm2/s [70]). Consistent with previous observation using hyperpolarized 3He, these differences likely arise from the smaller alveoli present in these young subjects and demonstrates the high sensitivity of 129Xe to even minute differences in alveolar microstructure [24].
As expected from 129Xe ventilation imaging of healthy adults [15, 71, 72], the control subject in Fig. 4 displayed qualitatively uniform signal intensity throughout the lungs. The variability that is observed, aside from gravitationally dependent gradients in ventilation, results from shading caused by imperfect coil uniformity. In contrast, the ventilation pattern of the child with CF is highly heterogeneous and contains regions of little to no 129Xe signal despite this child having a normal FEV1, which was higher than that of the control. These so-called ventilation defects are well-known features of hyperpolarized-gas lung images from individuals with various lung diseases and are indicative of regionally impaired ventilation [10, 71].
Conclusion
We report the assessment of the safety, tolerability and feasibility of 129Xe MRI in a small cohort of children with and without lung disease. Our data indicate that 129Xe is a safe and easily tolerated inhaled contrast agent for pulmonary imaging, paving the way for studies of other pulmonary diseases as well as larger-scale clinical trials with pediatric subjects. 129Xe MRI has a well-established track record in imaging adult lung disease and has repeatedly demonstrated its utility in quantifying regional ventilation and acinar microstructural parameters noninvasively and without the use of ionizing radiation. In particular, applying 129Xe MRI techniques to pediatric lung disease might provide a means to quantify subtle longitudinal changes in gas exchange, ventilation and alveolar microstructure associated with pulmonary disease progression and to assess response to treatment.
Acknowledgments
The authors would like to acknowledge Bastiaan Driehuys, Leslie Korbee, Jenny Jeffries, Lisa McCord, Colleen Murphy and Jeanne Dahlquist for regulatory assistance; Laurie Vanderah, Beth Decker and Emily Bell for subject monitoring; Matthew Lanier, Brynne Williams and Lacey Haas for subject scanning; Dan Dwyer of Teleflex Inc. for the gift of the custom gas-delivery mouthpieces; and Matthew Freeman, Nara Higano, Jim Wild, Charles Dumoulin and the CCHMC IRC Coil Engineering Lab for technical assistance.
This work was supported by the National Institutes of Health (T32HL007752, R01HL116226), Cystic Fibrosis Foundation (CLANCY 15R0), and University of Cincinnati, Center for Clinical and Translational Science and Training (T1 Core).
Footnotes
Compliance with ethical standards
Conflicts of interest None
References
- 1.Mugler JP, 3rd, Driehuys B, Brookeman JR, et al. MR imaging and spectroscopy using hyperpolarized 129Xe gas: preliminary human results. Magn Reson Med. 1997;37:809–815. doi: 10.1002/mrm.1910370602. [DOI] [PubMed] [Google Scholar]
- 2.Black RD, Middleton HL, Cates GD, et al. In vivo He-3 MR images of guinea pig lungs. Radiology. 1996;199:867–870. doi: 10.1148/radiology.199.3.8638019. [DOI] [PubMed] [Google Scholar]
- 3.MacFall JR, Charles HC, Black RD, et al. Human lung air spaces: potential for MR imaging with hyperpolarized He-3. Radiology. 1996;200:553–558. doi: 10.1148/radiology.200.2.8685356. [DOI] [PubMed] [Google Scholar]
- 4.Spector ZZ, Emami K, Fischer MC, et al. Quantitative assessment of emphysema using hyperpolarized 3He magnetic resonance imaging. Magn Reson Med. 2005;53:1341–1346. doi: 10.1002/mrm.20514. [DOI] [PubMed] [Google Scholar]
- 5.Emami K, Kadlecek SJ, Woodburn JM, et al. Improved technique for measurement of regional fractional ventilation by hyperpolarized 3He MRI. Magn Reson Med. 2010;63:137–150. doi: 10.1002/mrm.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yablonskiy DA, Sukstanskii AL, Leawoods JC, et al. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized He-3 diffusion MRI. Proc Natl Acad Sci USA. 2002;99:3111–3116. doi: 10.1073/pnas.052594699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saam BT, Yablonskiy DA, Kodibagkar VD, et al. MR imaging of diffusion of He-3 gas in healthy and diseased lungs. Magn Reson Med. 2000;44:174–179. doi: 10.1002/1522-2594(200008)44:2<174::aid-mrm2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Nguyen NM, Yablonskiy DA, et al. Imaging lung microstructure in mice with hyperpolarized He-3 diffusion MRI. Magn Reson Med. 2011;65:620–626. doi: 10.1002/mrm.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yablonskiy DA, Sukstanskii AL, Quirk JD, et al. Probing lung microstructure with hyperpolarized noble gas diffusion MRI: theoretical models and experimental results. Magn Reson Med. 2014;71:486. doi: 10.1002/mrm.24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirby M, Svenningsen S, Kanhere N, et al. Pulmonary ventilation visualized using hyperpolarized helium-3 and xenon-129 magnetic resonance imaging: differences in COPD and relationship to emphysema. J Appl Physiol. 1985;114:707–715. doi: 10.1152/japplphysiol.01206.2012. [DOI] [PubMed] [Google Scholar]
- 11.Dregely I, Mugler JP, III, Ruset IC, et al. Hyperpolarized xenon-129 gas-exchange imaging of lung microstructure: first case studies in subjects with obstructive lung disease. J Magn Reson Imaging. 2011;33:1052–1062. doi: 10.1002/jmri.22533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahon CJ, Dodd JD, Hill C, et al. Hyperpolarized 3helium magnetic resonance ventilation imaging of the lung in cystic fibrosis: comparison with high resolution CT and spirometry. Eur Radiol. 2006;16:2483–2490. doi: 10.1007/s00330-006-0311-5. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly LF, MacFall JR, McAdams HP, et al. Cystic fibrosis: combined hyperpolarized 3He-enhanced and conventional proton MR imaging in the lung — preliminary observations. Radiology. 1999;212:885–889. doi: 10.1148/radiology.212.3.r99se20885. [DOI] [PubMed] [Google Scholar]
- 14.de Lange EE, Altes TA, Patrie JT, et al. Evaluation of asthma with hyperpolarized helium-3 MRI: correlation with clinical severity and spirometry. Chest. 2006;130:1055–1062. doi: 10.1378/chest.130.4.1055. [DOI] [PubMed] [Google Scholar]
- 15.Altes TA, Powers PL, Knight-Scott J, et al. Hyperpolarized He-3 MR lung ventilation imaging in asthmatics: preliminary findings. J Magn Reson Imaging. 2001;13:378–384. doi: 10.1002/jmri.1054. [DOI] [PubMed] [Google Scholar]
- 16.Thomen RP, Sheshadri A, Quirk JD, et al. Regional ventilation changes in severe asthma after bronchial thermoplasty with (3)He MR imaging and CT. Radiology. 2015;274:250–259. doi: 10.1148/radiol.14140080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appelt S, Baranga AB, Erickson CJ, et al. Theory of spin-exchange optical pumping of He-3 and Xe-129. Phys Rev A. 1998;58:1412–1439. [Google Scholar]
- 18.Walker TG, Happer W. Spin-exchange optical pumping of noble-gas nuclei. Rev Mod Phys. 1997;69:629–642. [Google Scholar]
- 19.Fain S, Schiebler ML, McCormack DG, Parraga G. Imaging of lung function using hyperpolarized helium-3 magnetic resonance imaging: review of current and emerging translational methods and applications. J Magn Reson Imaging. 2010;32:1398–1408. doi: 10.1002/jmri.22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mugler JP, 3rd, Altes TA. Hyperpolarized 129Xe MRI of the human lung. J Magn Reson Imaging. 2013;37:313–331. doi: 10.1002/jmri.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lilburn DM, Pavlovskaya GE, Meersmann T. Perspectives of hyperpolarized noble gas MRI beyond 3He. J Magn Reson. 2013;229:173–186. doi: 10.1016/j.jmr.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walkup LL, Woods JC. Translational applications of hyperpolarized 3He and 129Xe. NMR Biomed. 2014;27:1429–1438. doi: 10.1002/nbm.3151. [DOI] [PubMed] [Google Scholar]
- 23.Altes TA, de Lange EE. Applications of hyperpolarized helium-3 gas magnetic resonance imaging in pediatric lung disease. TMRI. 2003;14:231–236. doi: 10.1097/00002142-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Altes TA, Mata J, de Lange EE, et al. Assessment of lung development using hyperpolarized helium-3 diffusion MR imaging. JMRI. 2006;24:1277–1283. doi: 10.1002/jmri.20723. [DOI] [PubMed] [Google Scholar]
- 25.Altes TA, Mata J, Froh DK, et al. Abnormalities of lung structure in children with brochopulmonary dysplasia as assessed by diffusion hyperpolarized helium-3 MRI. Proc Intl Soc Magn Reson Med. 2006;14:86. [Google Scholar]
- 26.Kirby M, Coxson HO, Parraga G. Pulmonary functional magnetic resonance imaging for paediatric lung disease. Paediatr Respir Rev. 2013;14:180–189. doi: 10.1016/j.prrv.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 27.van Beek EJ, Hill C, Woodhouse N, et al. Assessment of lung disease in children with cystic fibrosis using hyperpolarized 3-Helium MRI: comparison with Shwachman score, Chrispin-Norman score and spirometry. Eur Radiol. 2007;17:1018–1024. doi: 10.1007/s00330-006-0392-1. [DOI] [PubMed] [Google Scholar]
- 28.Woodhouse N, Wild JM, van Beek EJ, et al. Assessment of hyperpolarized 3He lung MRI for regional evaluation of interventional therapy: a pilot study in pediatric cystic fibrosis. JMRI. 2009;30:981–988. doi: 10.1002/jmri.21949. [DOI] [PubMed] [Google Scholar]
- 29.Koumellis P, van Beek EJ, Woodhouse N, et al. Quantitative analysis of regional airways obstruction using dynamic hyperpolarized 3He MRI-preliminary results in children with cystic fibrosis. J Magn Reson Imaging. 2005;22:420–426. doi: 10.1002/jmri.20402. [DOI] [PubMed] [Google Scholar]
- 30.McMahon CJ, Dodd JD, Hill C, et al. Hyperpolarized (3)helium magnetic resonance ventilation imaging of the lung in cystic fibrosis: comparison with high resolution CT and spirometry. Eur Radiol. 2006;16:2483–2490. doi: 10.1007/s00330-006-0311-5. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, O’Sullivan BP, Roche JP, et al. Using hyperpolarized 3He MRI to evaluate treatment efficacy in cystic fibrosis patients. J Magn Reson Imaging. 2011;34:1206–1211. doi: 10.1002/jmri.22724. [DOI] [PubMed] [Google Scholar]
- 32.Cadman RV, Lemanske RF, Evans MD, et al. Pulmonary He-3 magnetic resonance imaging of childhood asthma. J Allergy Clin Immunol. 2013;131:369–376. doi: 10.1016/j.jaci.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lange EE, Altes TA, Patrie JT, et al. The variability of regional airflow obstruction within the lungs of patients with asthma: assessment with hyperpolarized helium-3 magnetic resonance imaging. J Allergy Clin Immunol. 2007;119:1072–1078. doi: 10.1016/j.jaci.2006.12.659. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan M, Owers-Bradley J, Beardsmore CS, et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med. 2012;185:186–191. doi: 10.1164/rccm.201107-1348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayanan M, Beardsmore CS, Owers-Bradley J, et al. Catch-up alveolarization in ex-preterm children: evidence from (3)He magnetic resonance. Am J Respir Crit Care Med. 2013;187:1104–1109. doi: 10.1164/rccm.201210-1850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anglister J, Grzesiek S, Ren H, et al. Isotope-edited multidimensional NMR of calcineurin B in the presence of the non-deuterated detergent CHAPS. J Biomol NMR. 1993;3:121–126. doi: 10.1007/BF00242480. [DOI] [PubMed] [Google Scholar]
- 37.Nikolaou P, Coffey AM, Walkup LL, et al. XeNA: An automated ‘open-source’ (129)Xe hyperpolarizer for clinical use. Magn Reson Imaging. 2014;32:541–550. doi: 10.1016/j.mri.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikolaou P, Coffey AM, Walkup LL, et al. A 3D-printed high power nuclear spin polarizer. J Am Chem Soc. 2014;136:1636–1642. doi: 10.1021/ja412093d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korchak SE, Kilian W, Mitschang L. Configuration and performance of a mobile (129)Xe polarizer. Appl Magn Reson. 2013;44:65–80. doi: 10.1007/s00723-012-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart NJ, Norquay G, Griffiths PD, Wild JM. Feasibility of human lung ventilation imaging using highly polarized naturally abundant xenon and optimized three-dimensional steady-state free precession. Magn Reson Med. 2015;74:346–352. doi: 10.1002/mrm.25732. [DOI] [PubMed] [Google Scholar]
- 41.Hersman FW, Ruset IC, Ketel S, et al. Large production system for hyperpolarized 129Xe for human lung imaging studies. Acad Radiol. 2008;15:683–692. doi: 10.1016/j.acra.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirby M, Svenningsen S, Owrangi A, et al. Hyperpolarized 3He and 129Xe MR imaging in healthy volunteers and patients with chronic obstructive pulmonary disease. Radiology. 2012;265:600–610. doi: 10.1148/radiol.12120485. [DOI] [PubMed] [Google Scholar]
- 43.Patz S, Hersman FW, Muradian I, et al. Hyperpolarized (129)Xe MRI: a viable functional lung imaging modality? Eur J Radiol. 2007;64:335–344. doi: 10.1016/j.ejrad.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen RY, Fan FC, Kim S, et al. Tissue-blood partition coefficient for xenon: temperature and hematocrit dependence. J Appl Physiol Respir Environ Exerc Physiol. 1980;49:178–183. doi: 10.1152/jappl.1980.49.2.178. [DOI] [PubMed] [Google Scholar]
- 45.Mugler JP, 3rd, Altes TA, Ruset IC, et al. Simultaneous magnetic resonance imaging of ventilation distribution and gas uptake in the human lung using hyperpolarized xenon-129. Proc Natl Acad Sci USA. 2010;107:21707–21712. doi: 10.1073/pnas.1011912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cleveland ZI, Cofer GP, Metz G, et al. Hyperpolarized Xe MR imaging of alveolar gas uptake in humans. PLoS One. 2010;5:e12192. doi: 10.1371/journal.pone.0012192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaushik SS, Freeman MS, Cleveland ZI, et al. Probing the regional distribution of pulmonary gas exchange through single-breath gas- and dissolved-phase 129Xe MR imaging. J Appl Physiol (1985) 2013;115:850–860. doi: 10.1152/japplphysiol.00092.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qing K, Mugler JP, 3rd, Altes TA, et al. Assessment of lung function in asthma and COPD using hyperpolarized 129Xe chemical shift saturation recovery spectroscopy and dissolved-phase MRI. NMR Biomed. 2014;27:1490–1501. doi: 10.1002/nbm.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruppert K, Altes TA, Mata JF, et al. Detecting pulmonary capillary blood pulsations using hyperpolarized xenon-129 chemical shift saturation recovery (CSSR) MR spectroscopy. Magn Reson Med. 2016;75:1771–1780. doi: 10.1002/mrm.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakata Y, Goto T, Ishiguro Y, et al. Minimum alveolar concentration (MAC) of xenon with sevoflurane in humans. Anesthesiology. 2001;94:611–614. doi: 10.1097/00000542-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Sanders RD, Franks NP, Maze M. Xenon: no stranger to anaesthesia. Br J Anaesth. 2003;91:709–717. doi: 10.1093/bja/aeg232. [DOI] [PubMed] [Google Scholar]
- 52.Driehuys B, Martinez-Jimenez S, Cleveland ZI, et al. Chronic obstructive pulmonary disease: safety and tolerability of hyperpolarized Xe-129 MR imaging in healthy volunteers and patients. Radiology. 2012;262:279–289. doi: 10.1148/radiol.11102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shukla Y, Wheatley A, Kirby M, et al. Hyperpolarized 129Xe magnetic resonance imaging: tolerability in healthy volunteers and subjects with pulmonary disease. Acad Radiol. 2012;19:941–951. doi: 10.1016/j.acra.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Chae EJ, Seo JB, Goo HW, et al. Xenon ventilation CT with a dual-energy technique of dual-source CT: initial experience. Radiology. 2008;248:615–624. doi: 10.1148/radiol.2482071482. [DOI] [PubMed] [Google Scholar]
- 55.Goo HW, Chae EJ, Seo JB, Hong SJ. Xenon ventilation CT using a dual-source dual-energy technique: dynamic ventilation abnormality in a child with bronchial atresia. Pediatr Radiol. 2008;38:1113–1116. doi: 10.1007/s00247-008-0914-x. [DOI] [PubMed] [Google Scholar]
- 56.Goo HW, Yang DH, Hong SJ, et al. Xenon ventilation CT using dual-source and dual-energy technique in children with bronchiolitis obliterans: correlation of xenon and CT density values with pulmonary function test results. Pediatr Radiol. 2010;40:1490–1497. doi: 10.1007/s00247-010-1645-3. [DOI] [PubMed] [Google Scholar]
- 57.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 58.Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walkup L, Higano N, Ellis-Caleo T, et al. Scaling-up an ‘open-source’ 129Xe hyperpolarizer for human pulmonary imaging applications. Presented at the Experimental Nuclear Magnetic Resonance Conference; Boston. 2014. [Google Scholar]
- 60.Walkup LL, Thomen RP, Higano NS, et al. Initial experience performing hyperpolarized 129Xe MRI in young, pediatric subjects using a homebuilt Xe polarizer. Presented at the Experimental Nuclear Magnetic Resonance Conference; Pittsburgh. 2016. [Google Scholar]
- 61.Loew W, Thomen R, Pratt R, et al. A volume saddle coil for hyperpolarized 129Xe lung imaging. Proc Intl Soc Magn Reson Med. 2015;23:1507. [Google Scholar]
- 62.Miller GW, Altes TA, Brookeman JR, et al. Hyperpolarized 3He lung ventilation imaging with B1-inhomogeneity correction in a single breath-hold scan. Magma. 2004;16:218–226. doi: 10.1007/s10334-003-0028-2. [DOI] [PubMed] [Google Scholar]
- 63.Yablonskiy DA, Sukstanskii AL, Quirk JD. Diffusion lung imaging with hyperpolarized gas MRI. NMR in Biomed. 2015 doi: 10.1002/nbm.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS workshop on lung volume measurements. Official statement of the European Respiratory Society. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 65.Zapletal A, Paul T, Samanek M. Significance of contemporary methods of lung function testing for the detection of airway obstruction in children and adolescents (author’s transl) Z Erkr Atmungsorgane. 1977;149:343–371. [PubMed] [Google Scholar]
- 66.West JB. Respiratory physiology: the essentials. 9. Lippincott Williams & Wilkins; Baltimore: 2012. [Google Scholar]
- 67.Lutey BA, Lefrak SS, Woods JC, et al. Hyperpolarized 3He MR imaging: physiologic monitoring observations and safety considerations in 100 consecutive subjects. Radiology. 2008;248:655–661. doi: 10.1148/radiol.2482071838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salerno M, Altes TA, Brookeman JR, et al. Rapid hyperpolarized 3He diffusion MRI of healthy and emphysematous human lungs using an optimized interleaved-spiral pulse sequence. J Magn Reson Imaging. 2003;17:581–588. doi: 10.1002/jmri.10303. [DOI] [PubMed] [Google Scholar]
- 69.Salerno M, Altes TA, Brookeman JR, et al. Dynamic spiral MRI of pulmonary gas flow using hyperpolarized (3)He: preliminary studies in healthy and diseased lungs. Magn Reson Med. 2001;46:667–677. doi: 10.1002/mrm.1244. [DOI] [PubMed] [Google Scholar]
- 70.Kaushik SS, Cleveland ZI, Cofer GP, et al. Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med. 2011;65:1154–1165. doi: 10.1002/mrm.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Virgincar RS, Cleveland ZI, Kaushik SS, et al. Quantitative analysis of hyperpolarized 129Xe ventilation imaging in healthy volunteers and subjects with chronic obstructive pulmonary disease. NMR Biomed. 2013;26:424–435. doi: 10.1002/nbm.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kauczor HU, Hofmann D, Kreitner KF, et al. Normal and abnormal pulmonary ventilation: visualization at hyperpolarized He-3 MR imaging. Radiology. 1996;201:564–568. doi: 10.1148/radiology.201.2.8888259. [DOI] [PubMed] [Google Scholar]