Abstract
Objective
Chronic hypertension induces detrimental changes in the structure and function of surface cerebral arteries. Very little is known about parenchymal arterioles (PAs), which perfuse distinct neuronal populations in the cortex and may play a role in cerebrovascular disorders. We investigated the effect of deoxycorticosterone acetate (DOCA)-salt induced hypertension on endothelial function and artery structure in PAs and middle cerebral arteries (MCAs).
Methods
Uninephrectomized male Sprague-Dawley rats were implanted with a subcutaneous pellet containing DOCA (150 mg/kg b.w.) and drank salt-water (1% NaCl and 0.2% KCl) for 4 weeks. Sham rats were uninephrectomized and drank tap water. Vasoreactivity and passive structure in the MCAs and the PAs were assessed by pressure myography.
Results
Both MCAs and PAs from DOCA-salt rats exhibited impaired endothelium dependent dilation (p<0.05). In the PAs, addition of NO and COX inhibitors enhanced dilation in DOCA-salt rats (p<0.05) suggesting that dysfunctional NO and COX-dependent signaling could contribute to impaired endothelium-mediated dilation. MCAs from DOCA-salt rats exhibited inward remodeling (p<0.05).
Conclusions
Hypertension-induced MCA remodeling coupled with impaired endothelium dependent dilation in both the MCAs and PAs may exacerbate the risk of cerebrovascular accidents and the associated morbidity and mortality.
Keywords: Cerebral microcirculation, endothelium-dependent dilation, hypertension, parenchymal arterioles, deoxycorticosterone acetate
INTRODUCTION
Hypertension-induced pathologies in the cerebral vasculature have been linked to cerebrovascular disorders such as stroke [33], vascular dementia [15,18] and Alzheimer’s disease [23]. Cerebral vascular changes associated with hypertension are well-documented [16,47]. Chronic administration of deoxycorticosterone acetate (DOCA)-salt leads to hypertension, analogous to the salt-sensitive hypertension seen in some patients [32]. Despite their common use for cardiovascular studies, little is known about the cerebral vasculature in DOCA-salt hypertensive rats. A previous study from our laboratory reported detrimental remodeling in the middle cerebral artery (MCA) with DOCA administration without salt [11], however the dilatory capacity of the MCA was not assessed. Remodeling or alterations in the structure of the blood vessels can be either inward (reduction in lumen diameter) or outward (increase in lumen diameter). Remodeling along with impaired dilation [25,57,60], and enhanced constriction [35], are observed in peripheral and large cerebral arteries, in various forms of hypertension [47].
Significant differences between the peripheral and cerebral circulation exist [4] thus it is important to study the cerebral arteries directly and not just rely on extrapolations from the peripheral circulation. Studies of pial arteries cannot be extended to the parenchymal arterioles (PAs) due to differences in function, size, innervation and microenvironment [47]. PAs are a bottleneck between the pial arteries and the cerebral microcirculation, and recent studies have linked PA dysfunction to the pathogenesis of cerebrovascular disorders [40,41]. PAs perfuse discrete neuronal populations, and proper functioning of the PAs is essential to the neurovascular unit [46]. As hypertension progresses into the established phase, the percent of the cerebrovascular resistance carried by the microcirculation increases [6]. We previously demonstrated that PAs from stroke prone spontaneously hypertensive rats (SHRSP) have increased myogenic tone and inward remodeling [49]. Changes associated with chronic hypertension could lead to alterations in the structure and vasoreactivity of PAs and cause significant alterations in regional cerebral blood flow. We hypothesized that DOCA-salt hypertension would impair endothelium-dependent dilation in the MCAs and PAs. We further hypothesized that these changes in vascular function would be accompanied by a reduced lumen diameter and an increased wall-to-lumen ratio in both vessel types.
METHODS
Animals and DOCA-pellet implantation
Male Sprague-Dawley rats weighing 250–275g were purchased from Charles River Inc. (Portage, MI). Under isoflurane anesthesia the rats were uninephrectomized, and implanted with a subcutaneous pellet containing DOCA (150mg/ kg of body weight). DOCA treated rats had free access to regular rat chow and water containing 1% NaCl and 0.2% KCl [56]. Sham rats were uninephrectomized and drank tap water. Blood pressure was measured during the last week of treatment by tail-cuff plethysmography using a RTBP1001 tail-cuff blood pressure system (Kent Scientific, Torrington CT) as described previously by our laboratory[48]. The experimental protocol was approved by the Michigan State University Institutional Animal Care & Use Committee and was in accordance with the National Research Council’s Guiding Principles in the Care and Use of Animals (2011).
MCA isolation and cannulation
After 4 weeks of DOCA-salt treatment, rats were euthanized by an overdose of sodium pentobarbital. Rats were decapitated and the brain was removed and placed in ice-cold Ca2+-free physiological saline solution (PSS, in mM: NaCl 140, KCl 5, MgCl2•7H2O 1, HEPES 10, Dextrose 10) for isolation of the MCA and PAs. The MCA was carefully dissected from the brain and transferred to the pressure myograph chamber. A branchless segment of the MCA was cannulated between two glass micropipettes. The outer diameter of the MCA was constantly tracked and recorded using MyoView 2.0 software (Danish Myo Technology, Aarhus, Denmark). The MCA was allowed to equilibrate in PSS, at an intraluminal pressure of 80mmHg and 37°C until the development of spontaneous myogenic tone. Myogenic tone was calculated using the following formula: % tone = [1−(stable diameter with Ca2+ PSS/ diameter in Ca2+ free PSS with EGTA and SNP) ×100]. Arteries that generated less than 20% tone were discarded [44]. Once stable tone was generated, endothelial function was assessed by a cumulative addition of adenosine diphosphate (ADP: 10−10 to 10−5 M) applied intraluminally at physiological flow rates (20 dynes/cm3); the outer diameter of the MCA was continuously tracked and recorded. The MCA was then placed in Ca-free PSS containing 2mM ethylene glycol tetra acetic acid (EGTA) + 100μM sodium nitroprusside (SNP) to assess the passive structure of the artery as described previously [52]. Intraluminal pressure was increased from 3 to 180 mmHg in 20 mmHg increments. MCA outer and lumen diameters were continuously tracked and recorded after 5 minutes at each pressure step. Wall thickness was calculated as (outer diameter − lumen diameter)/2. The wall-to-lumen ratio {[(outer diameter− lumen diameter)/2]/lumen diameter}, passive distensibility {[ (lumen diameter at 3mmHg/lumen diameter at intraluminal pressure)−1] × 100} were calculated as previously described [9]. Circumferential wall stress were calculated as [(arteriolar pressure x arteriolar diameter)/(2x wall thickness)] [1]. While elastic modulus (β-coefficient) was calculated from the stress/strain curves using an exponential model (y=aeβx) where β is the slope of the curve and is directly correlated to vascular stiffness.
PA isolation and cannulation
A 5 x 3 x 3 mm (length x width x depth) segment of brain tissue containing the MCA was removed and placed in Ca2+-free PSS at 4°C, with 1% bovine serum albumin (BSA) + 10μM diltiazem + 10μM SNP. The pia with the MCA was gently separated from the tissue, and PAs branching from the MCA were transferred to a custom-made cannulation chamber using a glass micropipette (Wiretrol, Drummond Scientific Company, Broomall, PA). PAs were cannulated between two glass pipettes, bathed in warm (37°C) PSS containing 1.8mM Ca2+, and pressurized to 60mmHg until spontaneous myogenic tone developed [34]. PA outer and lumen diameters were constantly tracked and recorded using MyoView 2.0 software (Danish Myo Technology, Aarhus, Denmark).
Assessment of myogenic tone and reactivity in the PAs
Myogenic reactivity in the PAs was assessed by changing intraluminal pressure from 25 to 50, 75 and 100mmHg. PAs were equilibrated at each pressure for 15 minutes. At the end of the pressure response curve the PAs were bathed in Ca-free PSS containing 2mM EGTA + 100μM SNP, the pressure curve was repeated to obtain the passive structure of the PAs to calculate % myogenic tone.
Assessment of endothelium-dependent dilation in PAs
To assess endothelium dependent dilation, PAs were incubated with increasing concentrations of the muscarinic receptor agonist acetylcholine (ACh, 1nM to 100μM) in the bath. Due to the small size of the lumen of the cannulas (<40 μm) used for PA studies, ACh was added abluminally. For the PAs, ACh was used instead of ADP since abluminal application of ADP activates P2X purinoceptors in the vascular smooth muscle cells (VSMC) and results in constriction or only slight dilation [10,63]. ACh causes the generation of nitric oxide (NO), cyclooxygenase (COX) metabolites and endothelium-derived hyperpolarizing factor (EDHF) [16]. To assess the role played by each dilator in the ACh-mediated response, PAs were incubated with the NO synthase (NOS) inhibitor N(G)-nitro-L-arginine methyl ester (L-NAME,100μM) for 30 minutes, with the COX inhibitor indomethacin (Indo, 10μM) for 30 minutes, or with L-NAME and Indo together, prior to performing an ACh concentration-response experiment. Endothelium-independent dilation was studied by incubating PAs with increasing concentrations of SNP (1nM to 100μM). Only one concentration-response experiment was performed in each cannulated PA. Dilation data are shown as change in diameter from baseline (ΔDiameter) and as % vasodilation, calculated as follows: ((lumen diameter after dilation − lumen diameter at baseline) / (passive lumen diameter−lumen diameter at baseline)) * 100 [13].
Assessment of the structural and mechanical properties in PAs
The structural and mechanical properties of PAs were assessed after incubation with 0Ca2+-PSS supplemented with 2mM EGTA + 100μM SNP. Intraluminal pressure was increased from 3 to 180mmHg in 20mmHg increments, and arterioles were allowed to equilibrate for 5 minutes before intraluminal pressure was increased. The passive structure and mechanical properties of the PAs were calculated as described for the MCA.
Statistical Analyses
Body weight, blood pressure, myogenic tone and resting lumen diameter data were analyzed by Student’s t-test or a non-parametric alternative if the data was not normally distributed. Endothelium-dependent dilation, passive and mechanical properties were analyzed by two-way ANOVA followed by Sidak correction for multiple comparisons, or a non-parametric alternative. In cases of unequal variance data was transformed to homogenize variance using the following formula: y= √y. A two-way ANOVA was then performed on the transformed data. Analyses were performed using the software GraphPad Prism 6.0 (La Jolla, CA, USA).
Chemicals and Reagents
Unless otherwise stated, all chemical and reagents were purchased from Sigma-Aldrich (Saint Louis, MO, USA).
RESULTS
Physiological parameters and blood pressure
There was no significant difference in body weight (Sham vs DOCA-salt: 407±16.8 vs 375.5±12.3 g), measured before euthanasia. Four weeks of DOCA-salt treatment caused a significant increase in blood pressure (systolic pressure, Sham vs DOCA-salt: 130.2±1.2 vs 193.4±2.5 mmHg, p<0.05) as shown previously [59].
Myogenic tone and impaired dilation in MCAs
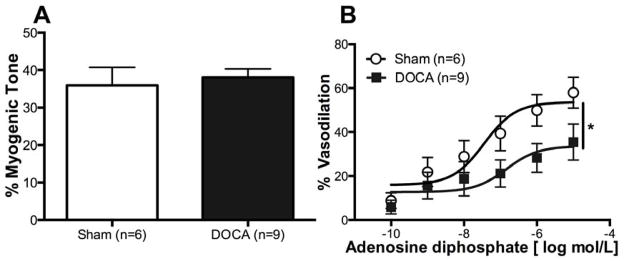
There was no difference in myogenic tone generation between MCAs from Sham and DOCA-salt rats (Fig 1A). MCA’s from DOCA-salt rats exhibited a smaller percent dilation with no change in EC50 (logEC50, Sham vs DOCA-salt: −7.4±0.4 vs −6.8±0.7) in response to intraluminal ADP (Fig 1B), suggesting endothelial dysfunction.
Figure 1. DOCA-salt treatment impairs endothelium dependent dilation in the MCA.
Endothelium functions in the MCAs were measured using ADP. There was no difference in percent myogenic tone between the two groups (A). Percent change in dilation was reduced in DOCA-salt MCAs (B). *p <0.05, Sham vs DOCA, two-way ANOVA. Values are mean± SEM. MCAs were mounted in a pressure myograph and kept in warm calcium PSS.
Structural and mechanical properties of the MCAs
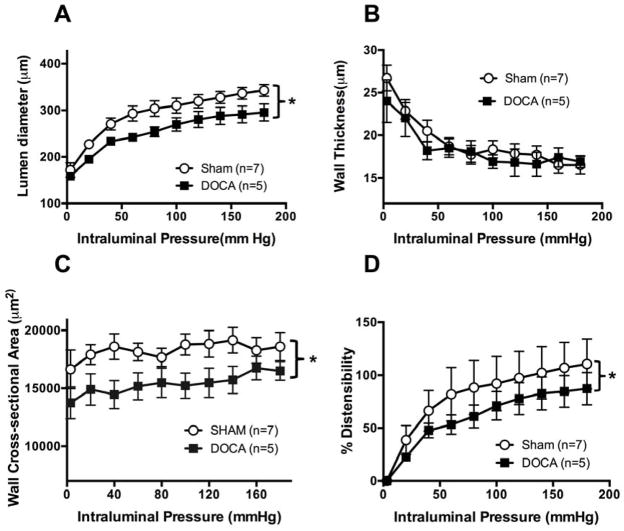
MCA structure was assessed under zero flow and calcium free conditions. MCA’s from DOCA-salt rats had smaller lumen diameters compared to Sham rats (Fig 2A). There were no differences in wall thickness (Fig 2B) between the MCAs from the two groups. However, wall cross-sectional area was reduced in the MCAs from DOCA-salt hypertensive rats (Fig 2C). Distensibility was reduced in the MCAs from DOCA-salt rats compared to Sham rats (Fig 2D). There was no difference in β coefficient between the two groups (SHAM vs DOCA-salt: 4.9±0.7 vs 7.0±1.2, p=0.15).
Figure 2. DOCA-salt treatment induced changes in passive structure of the MCA and altered mechanical properties.
Lumen diameter was reduced in MCAs from DOCA-salt rats (A). No difference in wall thickness between groups (B). Wall cross-sectional area was reduced in MCAs from DOCA-salt rats (C). Small but significant decrease in distensibility was calculated as percent increases in diameter from 3 mmHg. Data was transformed using the formula y= √y to homogenize variance (D). *p <0.05, Sham vs DOCA, two-way ANOVA. Values are mean± SEM. MCAs were mounted in a pressure myograph and kept in warm calcium free PSS.
Myogenic reactivity in the PAs
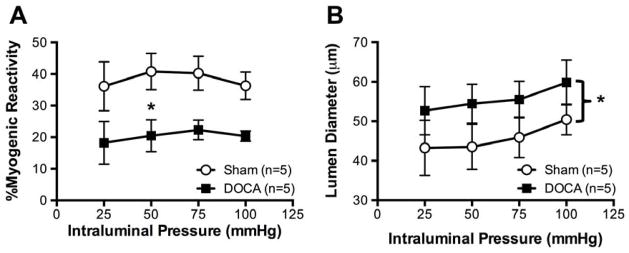
To test the hypothesis that DOCA-salt hypertension alters autoregulation, PAs were cannulated and the intraluminal pressure was increased in a stepwise manner. PAs from DOCA-salt rats showed significantly lower myogenic tone in responses to increases in intraluminal pressure when compared to PAs from Sham rats (Fig 3A). As a consequence, active lumen diameters of PAs from DOCA-salt rats were larger than Sham rats (Fig 3B).
Figure 3. DOCA-salt treatment reduced myogenic reactivity in PAs.
PAs from DOCA rats exhibited lower myogenic tone in responses to increases in intraluminal pressure when compared to PAs from Sham rats (A). Active lumen diameters of PAs from DOCA-salt rats were larger than Sham rats (B). *p <0.05, Sham vs DOCA, two-way ANOVA. Values are mean± SEM. PAs were mounted in a pressure myograph and kept in warm calcium PSS under no-flow conditions.
Endothelium-dependent dilation in the PAs
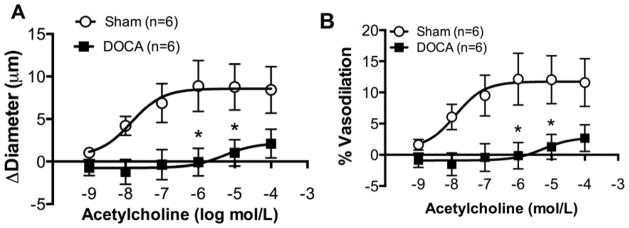
To test endothelial function, cannulated arterioles were incubated with increasing concentrations of ACh in the superfusing bath. There was a significant reduction in dilation in PAs from DOCA-salt rats when compared to Sham rats, shown as change in diameter (Fig 4A) and as % vasodilation (Fig 4B). Further, the sensitivity of PAs from DOCA-salt rats to ACh was reduced, as evidenced by the logEC50 (SHAM vs DOCA −7.62±0.45 vs −6.06±0.68, p<0.05).
Figure 4. Endothelium dependent dilation measured using ACh was impaired in DOCA-salt PAs.
Change in diameter after dilation with ACh was reduced in DOCA-salt PAs (A). Percent vasodilation was reduced in DOCA-salt PAs (B). *p <0.05, Sham vs DOCA, two-way ANOVA. Values are mean± SEM. PAs were mounted in a pressure myograph and kept in warm calcium PSS under no-flow conditions.
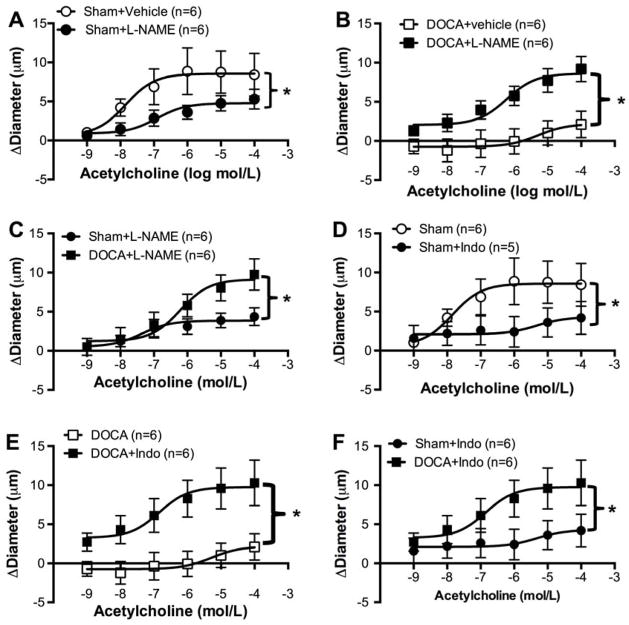
Endothelium-dependent dilation in the PAs after NOS inhibition
PAs from Sham rats pre-incubated with L-NAME showed blunted ACh-induced dilation (Fig 5A). In PAs from DOCA-salt rats, NOS inhibition improved ACh-mediated dilation (Fig 5B). Comparing the data from Sham and DOCA-salt rats showed that PAs from hypertensive rats had larger dilations than PAs from Sham rats after NOS inhibition (Fig 5C).
Figure 5. Impaired dilatory pathways involved in endothelium dysfunction in PAs from DOCA-salt.
With NO inhibition, change in diameter is reduced in Sham PAs with increasing concentration of ACh (A). NO inhibition increased change in diameter with ACh in PAs from DOCA-salt (B). With L-NAME, change in diameter in DOCA-salt PAs is increased (C). Inhibition with Indo reduced dilation in Sham PAs (D). Inhibition of COX pathway increased dilation in DOCA-salt PAs (E). With Indo DOCA-salt PAs had increased dilation compared to sham PAs (F).*p <0.05, Sham vs DOCA, two-way ANOVA. Values are mean± SEM. PAs were mounted in a pressure myograph and kept in warm calcium PSS under no-flow conditions.
Endothelium-dependent dilation in the PAs after COX inhibition
In PAs from Sham rats, pre-incubation with Indo caused a 50% reduction in the ACh mediated dilation (Fig 5D). However, in DOCA-salt treated rats Indo increased the dilatory response to ACh (Fig 5E). As a consequence, PAs from DOCA-salt animals showed larger dilations to ACh than PAs from Sham rats after COX inhibition (Fig 5F).
Endothelium-dependent dilation in the PAs with NOS and COX inhibition
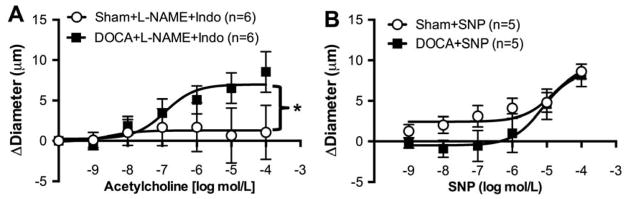
ACh-induced dilation was abolished in PAs from Sham rats after pre-incubation with L-NAME and Indo, whereas in PAs from DOCA-salt rats dilation was increased to the same degree as observed with the individual inhibitors (Fig 6A).
Figure 6. Endothelial dependent and independent dilation in PAs.
Co-inhibition of the NO and COX pathways abolished dilation in PAs from Sham rats, but increased in PAs from DOCA-salt rats (A). PAs from DOCA-salt rats showed reduced dilation to SNP, particularly at lower concentrations. At higher concentrations there was no difference in dilation between the two groups. Data was transformed using the formula y= √y to homogenize variance (B). *p <0.05, Sham vs DOCA, two-way ANOVA. Values are mean± SEM.
Endothelium-independent dilation in the PAs
To test the reactivity of PAs to an endothelium-independent dilator, PAs were incubated with increasing concentrations of the NO donor, SNP. At the lower concentrations of SNP PAs from Sham rats had increased dilation compared to PAs from DOCA-salt rats (Fig 6B). Although the two-way ANOVA showed that the two curves are significantly different (p=0.018), the Sidak correction for multiple comparisons was unable to detect differences at the individual SNP concentrations.
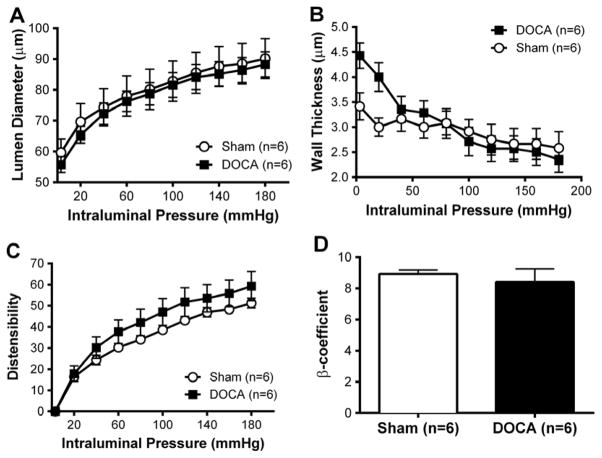
Structural and mechanical properties of the PAs
The passive structure and mechanical properties of PAs were assessed by pressure myography. DOCA-salt treatment did not alter the passive lumen diameter (Fig 7A) or wall thickness (Fig 7B) of PAs. Similarly, distensibility (Fig 7C) and stiffness (Fig 7D) were not altered by DOCA-salt hypertension.
Figure 7. Passive structure in PAs was unaltered with DOCA-salt treatment.
No difference in lumen diameter between the groups (A). No difference in wall thickness in PAs from either groups (B). PAs were mounted in a pressure myograph and kept in warm calcium free PSS under no-flow conditions.
DISCUSSION
There are several novel findings from this study. Inward remodeling was observed in MCAs but not PAs from DOCA-salt treated rats. DOCA-salt hypertension did not affect myogenic tone in the MCAs but reduced myogenic reactivity of the PAs. Endothelial dysfunction was observed in both MCAs and PAs from DOCA-salt hypertensive rats and endothelium-independent dilation was impaired in PAs from DOCA-salt rats at low concentrations of the NO donor, SNP.
Effects of DOCA-salt hypertension on artery structure
The MCAs from DOCA-salt treated rats had smaller lumen diameters, suggesting that increased vascular resistance in these arteries could subject downstream arteries and arterioles to lower perfusion pressures. MCAs from DOCA-salt treated rats also exhibited reduced wall cross-sectional area, or hypotrophy, despite the rapid increase in blood pressure observed in these rats. This surprising finding is in contrast to studies reporting that hypertension induces hypertrophic cerebral artery remodeling [47]. It is generally accepted that hypertrophic cerebral artery remodeling is an adaptive response to hypertension that reduces wall stress and protects downstream arterioles from the increased pressure [2]. Hypotrophy of MCAs in a model of chronic hypertension could increase the risk of blood brain barrier breakdown [24]. Peripheral artery hypotrophy has been observed in other models where NO production is impaired [28,29]. Chronic treatment with neuronal NOS blockers induces pronounced hypotrophy of peripheral arteries and is a model of low-renin levels [3], like the DOCA-salt rats [19]. It is possible that low-renin levels modulate a hypotrophic phenotype in the vasculature. Positive feedback regulation of renin by angiotensin (Ang) II evidenced by increases in renin expression [22,45,51] dominates over the negative feedback regulation in renal cortexes in Ang II-dependent hypertension models [45]. Moreover, Ang II increases renin activity in cultured vascular smooth muscle cells [45], implying renin involvement in the vascular effects of Ang II. Further studies elucidating a role of renin in the proliferative and hypertrophic vascular effects of Ang II could provide insight into hypotrophic remodeling of MCAs from DOCA-salt rats.
In the present study, PAs did not remodel in response to DOCA-salt hypertension. In contrast, PAs from hypertensive patients show various stages of degeneration, starting from hypertrophy to hyalinization of the vascular wall [50]. Similarly in SHRSP, a model of essential hypertension, inward hypertrophic PA remodeling is evident and dependent on mineralocorticoid receptor activation [49]. Thus, it is surprising that mineralocorticoid receptor activation with DOCA did not affect PA structure. It is possible that the duration of the hypertension is an important determinant of PA structure. The SHRSPs studied had been hypertensive for at least 10 weeks; whereas the DOCA treatment was only for 4 weeks. Other studies using genetic models of hypertension suggest that the large arteries remodel first, and that the downstream arteries only remodel after prolonged periods of hypertension [47]. A determining factor in PA remodeling could be the structure of upstream arteries. Inward remodeling of the MCAs from DOCA-salt rats, with a proposed increase in resistance could potentially reduce circumferential stress in PAs, thus negating the pro-remodeling stimuli.
Effects of DOCA-Salt hypertension on myogenic tone and reactivity
Myogenic reactivity, critical for maintaining constant blood flow despite fluctuations in blood pressure, is the ability of the artery to respond to changes in intraluminal pressure by altering its diameter. In the MCA, DOCA-salt hypertension had no effect on myogenic tone. However, PAs from DOCA-salt had reduced myogenic reactivity than PAs from Sham rats. Mechanosensors in VSMC, such as angiotensin type 1 receptors [53] or purinergic receptors [8], ultimately lead to activation of transient receptor potential channels M4, opening of voltage-dependent calcium channels and depolarization. [21]. Conversely, Ca2+ release from intracellular stores in VSMCs caused by ryanodine receptors activates large conductance Ca2+-activated K+ channels (BKCa), leading to hyperpolarization and loss of myogenic tone [31]. Importantly, expression of BKCa is increased in the aorta and mesenteric arteries of DOCA-salt hypertensive rats [61]. Thus, it is possible that BKCa expression is increased in cerebral arteriolar smooth muscle cells of DOCA-salt hypertensive rats, causing the observed reduction in myogenic reactivity. Reduced myogenic reactivity in the DOCA-salt PAs may suggest an inability to prevent cerebral edema during high perfusion levels [43] and may explain the presence of microbleeds seen during hypertension [17].
Effects of DOCA-salt hypertension of dilatory function
DOCA-salt treatment impaired ADP mediated dilation in the MCAs with no accompanying change in myogenic tone. DOCA-salt treatment also did not affect the sensitivity of the MCA to ADP. Increased O2− levels reduce levels of tetrahydrobiopterin, an essential co-factor for endothelial NOS (eNOS), and are markedly elevated during DOCA-salt hypertension [5,55,64]. It is possible that in MCAs from DOCA-salt treated rats reduced NO production impairs dilation to purinoceptor P2Y agonist ADP, that induces dilation primarily mediated by NO [63]. In cerebral arteries from SHRSPs, impaired dilation to ACh lacked any endothelium-independent component as evidenced by no differences in dilation to nitroglycerine and adenosine between SHRSPs and their normotensive controls [36]. Thus it is not inconceivable that endothelial dysfunction in MCAs from DOCA-salt is solely a consequence of impaired endothelium-dependent dilation.
While endothelial dysfunction is well studied in the MCAs in different models of hypertension [57,60], there is an urgency to study functional and structural integrity of the PAs with chronic hypertension as cerebral small vessel disease is a major contributor to cognitive impairment [38]. Thus, our studies were focused on the mechanisms by which hypertension affects the PAs. PAs from DOCA-salt rats exhibited impaired endothelium-dependent dilation. In arteries, ACh mediates dilation by release of NO, prostacylin and endothelium dependent hyperpolarizing factors (EDHFs) [14,62]. In PAs from DOCA-salt rats, inhibition of NOS enhanced dilation to ACh; similar results were observed after COX inhibition. Further, concomitant inhibition of NOS and COX further enhanced dilation in the PAs from DOCA-salt but abolished ACh-mediated dilation in PAs from Sham rats. At low concentrations of the NO donor, response to SNP appeared to be diminished in PAs from DOCA-salt treated rats. This reduced response could be due to enhanced degradation of NO, observed in mesenteric arteries from DOCA-salt rats [60]. However, there was no difference in the EC50 or in the maximum response between the two groups.
In PAs from Sham rats, dilation was abolished in the presence of L-NAME or Indo or a combination of both, suggesting that NO and prostaglandin dilatory pathways were functional and contribute to ACh-mediated dilation. In contrast, in DOCA-salt PAs NOS inhibition restored dilation to Sham levels implying dysfunction of NOS, which is frequently seen with vascular pathology [27]. This could be a consequence of eNOS uncoupling [12,30] due to increased nicotinamide adenine dinucleotide phosphate oxidase activity and reduced cofactor availability as has been observed in aortas from DOCA-salt rats [58]. Dilation was also restored in PAs from DOCA-salt rats after COX inhibition with Indo. The prostaglandin pathway produces both vasoconstrictors and vasodilators [16], thus it is possible that in PAs from DOCA-salt rats the pathway is skewed towards the production of vasoconstrictors. A similar effect of Indo has been observed in pial arteries from SHRSP [37].
Combined NOS and COX inhibition increased dilation in PAs from DOCA-salt rats, suggesting a role for EDHF mediated dilation that is predominant in arterioles [7,34,54]. Inhibition of NO and prostacyclin removes the negative feedback on EDHF production and enhances dilation [26,39], a phenomenon that is particularly evident in pathological states [20]. Thus we speculate that in PAs from DOCA-salt rats, dysfunction in NOS and COX pathways suppresses EDHF mediated dilation and contribute to impaired dilation to ACh.
The possible detrimental physiological effects of impaired dilation of the cerebral arteries and arterioles are at least twofold. It could impact the brain’s ability to respond to ischemia. Under normal conditions when a cerebral artery is occluded the surrounding arteries dilate to circumvent the occlusion. Inward artery remodeling evidenced by reduced MCA lumen diameter has been linked to increased ischemic damage in the brain [11,42] and may exacerbate the deleterious effects of impaired dilation.
PERSPECTIVES
In summary, DOCA-salt treatment impairs endothelial functions in the MCAs and the PAs and induces hypotrophic inward remodeling in the MCAs. This study adds to our knowledge of the PAs; dysfunction in which could increase the risk of cerebrovascular accidents, vascular cognitive impairment, and Alzheimer’s disease. Elucidating the mechanisms of dysfunction in PAs might provide insights into therapies that can increase cerebral perfusion and prevent or reduce the progression of cerebrovascular diseases.
Acknowledgments
Sources of Funding: American Heart Association (0840122N to AMD, 14PRE19890001 to NM and 12PRE8960019) and National Institutes of Health (P01-HL07687 to WFJ)
Abbreviations used
- PA
parenchymal artery
- DOCA
deoxycorticosterone acetate
- MCA
middle cerebral artery
- SHRSP
stroke prone spontaneously hypertensive rats
- ADP
adenosine diphosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- COX
cyclooxygenase
- EDHF
endothelium-derived hyperpolarizing factor
- ACh
acetylcholine
- VSMC
vascular smooth muscle cell
- BKCa
large conductance Ca2+-activated K+ channels
Footnotes
Author Contributions: NM, PWP, AMD conception and design of research; HG performed the animal surgeries; NM and PWP performed experiments; NM and PWP analyzed data; NM, PWP and AMD interpreted results of experiments; NM and PWP prepared figures; NM drafted manuscript; NM, PWP, WFJ and AMD edited and revised the manuscript; NM, PWP, HG, WFJ and AMD approved final version of manuscript.
DISCLOSURES
The authors have no conflict of interest to disclose.
References
- 1.Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 1993;21:816–826. doi: 10.1161/01.hyp.21.6.816. [DOI] [PubMed] [Google Scholar]
- 2.Baumbach GL, Heistad DD. Cerebral circulation in chronic arterial hypertension. Hypertension. 1988;12:89–95. doi: 10.1161/01.hyp.12.2.89. [DOI] [PubMed] [Google Scholar]
- 3.Beierwaltes WH. Macula densa stimulation of renin is reversed by selective inhibition of neuronal nitric oxide synthase. The American journal of physiology. 1997;272:R1359–1364. doi: 10.1152/ajpregu.1997.272.5.R1359. [DOI] [PubMed] [Google Scholar]
- 4.Bell DR, Webb RC, Bohr DF. Functional bases for individualities among vascular smooth muscles. J Cardiovasc Pharmacol. 1985;7(Suppl 3):S1–11. doi: 10.1097/00005344-198500073-00001. [DOI] [PubMed] [Google Scholar]
- 5.Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- 6.Bohlen HG. The microcirculation in hypertension. J Hypertens Suppl. 1989;7:S117–124. [PubMed] [Google Scholar]
- 7.Brandes RP, Schmitz-Winnenthal FH, Feletou M, Godecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci U S A. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brayden JE, Li Y, Tavares MJ. Purinergic receptors regulate myogenic tone in cerebral parenchymal arterioles. J Cereb Blood Flow Metab. 2013;33:293–299. doi: 10.1038/jcbfm.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan SL, Chapman AC, Sweet JG, Gokina NI, Cipolla MJ. Effect of PPARgamma inhibition during pregnancy on posterior cerebral artery function and structure. Front Physiol. 2010;1:130. doi: 10.3389/fphys.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalziel HH, Westfall DP. Receptors for adenine nucleotides and nucleosides: subclassification, distribution, and molecular characterization. Pharmacological reviews. 1994;46:449–466. [PubMed] [Google Scholar]
- 11.Dorrance AM, Rupp NC, Nogueira EF. Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension. 2006;47:590–595. doi: 10.1161/01.HYP.0000196945.73586.0d. [DOI] [PubMed] [Google Scholar]
- 12.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci U S A. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earley S, Gonzales AL, Garcia ZI. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Molecular pharmacology. 2010;77:612–620. doi: 10.1124/mol.109.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etgen T, Sander D, Bickel H, Forstl H. Mild cognitive impairment and dementia: the importance of modifiable risk factors. Dtsch Arztebl Int. 2011;108:743–750. doi: 10.3238/arztebl.2011.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, Hartung HP. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 18.Gasecki D, Kwarciany M, Nyka W, Narkiewicz K. Hypertension, brain damage and cognitive decline. Curr Hypertens Rep. 2013;15:547–558. doi: 10.1007/s11906-013-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavras H, Brunner HR, Laragh JH, Vaughan ED, Jr, Koss M, Cote LJ, Gavras I. Malignant hypertension resulting from deoxycorticosterone acetate and salt excess: role of renin and sodium in vascular changes. Circ Res. 1975;36:300–309. doi: 10.1161/01.res.36.2.300. [DOI] [PubMed] [Google Scholar]
- 20.Golding EM, Marrelli SP, You J, Bryan RM., Jr Endothelium-derived hyperpolarizing factor in the brain: a new regulator of cerebral blood flow? Stroke. 2002;33:661–663. [PubMed] [Google Scholar]
- 21.Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, Nelson MT, Earley S. A PLCgamma1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal. 2014;7:ra49. doi: 10.1126/scisignal.2004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez AA, Liu L, Lara LS, Bourgeois CR, Ibaceta-Gonzalez C, Salinas-Parra N, Gogulamudi VR, Seth DM, Prieto MC. PKC-alpha-dependent augmentation of cAMP and CREB phosphorylation mediates the angiotensin II stimulation of renin in the collecting duct. Am J Physiol Renal Physiol. 2015;309:F880–888. doi: 10.1152/ajprenal.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke. 2004;35:2620–2622. doi: 10.1161/01.STR.0000143318.70292.47. [DOI] [PubMed] [Google Scholar]
- 24.Hart MN, Heistad DD, Brody MJ. Effect of chronic hypertension and sympathetic denervation on wall/lumen ratio of cerebral vessels. Hypertension. 1980;2:419–423. doi: 10.1161/01.hyp.2.4.419. [DOI] [PubMed] [Google Scholar]
- 25.Henrion D, Dechaux E, Dowell FJ, Maclour J, Samuel JL, Levy BI, Michel JB. Alteration of flow-induced dilatation in mesenteric resistance arteries of L-NAME treated rats and its partial association with induction of cyclo-oxygenase-2. British journal of pharmacology. 1997;121:83–90. doi: 10.1038/sj.bjp.0701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang A, Sun D, Smith CJ, Connetta JA, Shesely EG, Koller A, Kaley G. In eNOS knockout mice skeletal muscle arteriolar dilation to acetylcholine is mediated by EDHF. Am J Physiol Heart Circ Physiol. 2000;278:H762–768. doi: 10.1152/ajpheart.2000.278.3.H762. [DOI] [PubMed] [Google Scholar]
- 27.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 28.Kristek F, Cacanyiova S, Gerova M. Hypotrophic effect of long-term neuronal NO-synthase inhibition on heart and conduit arteries of the Wistar rats. J Physiol Pharmacol. 2009;60:21–27. [PubMed] [Google Scholar]
- 29.Kristek F, Gerova M. Hypotrophy of conduit artery walls of the offspring of nitric oxide-defective rats. Braz J Med Biol Res. 2004;37:601–606. doi: 10.1590/s0100-879x2004000400018. [DOI] [PubMed] [Google Scholar]
- 30.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology. 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 32.Luo M, Hess MC, Fink GD, Olson LK, Rogers J, Kreulen DL, Dai X, Galligan JJ. Differential alterations in sympathetic neurotransmission in mesenteric arteries and veins in DOCA-salt hypertensive rats. Auton Neurosci. 2003;104:47–57. doi: 10.1016/S1566-0702(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 33.Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. 2001;124:457–467. doi: 10.1093/brain/124.3.457. [DOI] [PubMed] [Google Scholar]
- 34.Matin N, Fisher C, Jackson WF, Dorrance AM. Bilateral Common Carotid Artery Stenosis in Normotensive Rats Impairs Endothelial Dependent Dilation Of Parenchymal Arterioles. Am J Physiol Heart Circ Physiol. 2016 doi: 10.1152/ajpheart.00890.2015. ajpheart 00890 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto T, Tostes RC, Webb RC. Alterations in vasoconstrictor responses to the endothelium-derived contracting factor uridine adenosine tetraphosphate are region specific in DOCA-salt hypertensive rats. Pharmacol Res. 2012;65:81–90. doi: 10.1016/j.phrs.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayhan WG, Faraci FM, Heistad DD. Impairment of endothelium-dependent responses of cerebral arterioles in chronic hypertension. The American journal of physiology. 1987;253:H1435–1440. doi: 10.1152/ajpheart.1987.253.6.H1435. [DOI] [PubMed] [Google Scholar]
- 37.Mayhan WG, Faraci FM, Heistad DD. Responses of cerebral arterioles to adenosine 5′-diphosphate, serotonin, and the thromboxane analogue U-46619 during chronic hypertension. Hypertension. 1988;12:556–561. doi: 10.1161/01.hyp.12.6.556. [DOI] [PubMed] [Google Scholar]
- 38.Mott M, Pahigiannis K, Koroshetz W. Small blood vessels: big health problems: National Institute of Neurological Disorders and Stroke update. Stroke; a journal of cerebral circulation. 2014;45:e257–258. doi: 10.1161/STROKEAHA.114.007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol Heart Circ Physiol. 2000;279:H459–465. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura N, Rosidi NL, Iadecola C, Schaffer CB. Limitations of collateral flow after occlusion of a single cortical penetrating arteriole. J Cereb Blood Flow Metab. 2010;30:1914–1927. doi: 10.1038/jcbfm.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci U S A. 2007;104:365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osmond JM, Dorrance AM. 11beta-hydroxysteroid dehydrogenase type II inhibition causes cerebrovascular remodeling and increases infarct size after cerebral ischemia. Endocrinology. 2009;150:713–719. doi: 10.1210/en.2008-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osol G, Brekke JF, McElroy-Yaggy K, Gokina NI. Myogenic tone, reactivity, and forced dilatation: a three-phase model of in vitro arterial myogenic behavior. Am J Physiol Heart Circ Physiol. 2002;283:H2260–2267. doi: 10.1152/ajpheart.00634.2002. [DOI] [PubMed] [Google Scholar]
- 44.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circulation research. 1991;68:359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- 45.Ostermann AI, Herbers J, Willenberg I, Chen R, Hwang SH, Greite R, Morisseau C, Gueler F, Hammock BD, Schebb NH. Oral treatment of rodents with soluble epoxide hydrolase inhibitor 1-(1-propanoylpiperidin-4-yl)-3-[4-(trifluoromethoxy)phenyl]urea (TPPU): Resulting drug levels and modulation of oxylipin pattern. Prostaglandins Other Lipid Mediat. 2015;121:131–137. doi: 10.1016/j.prostaglandins.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–1614. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pires PW, Deutsch C, McClain JL, Rogers CT, Dorrance AM. Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats. Microvasc Res. 2010;80:445–452. doi: 10.1016/j.mvr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pires PW, Jackson WF, Dorrance AM. Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor. American journal of physiology Heart and circulatory physiology. 2015 doi: 10.1152/ajpheart.00168.2015. ajpheart 00168 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plesea IE, Camenita A, Georgescu CC, Enache SD, Zaharia B, Georgescu CV, Tenovici M. Study of cerebral vascular structures in hypertensive intracerebral haemorrhage. Rom J Morphol Embryol. 2005;46:249–256. [PubMed] [Google Scholar]
- 51.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res. 2007;73:198–205. doi: 10.1016/j.mvr.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schleifenbaum J, Kassmann M, Szijarto IA, Hercule HC, Tano JY, Weinert S, Heidenreich M, Pathan AR, Anistan YM, Alenina N, Rusch NJ, Bader M, Jentsch TJ, Gollasch M. Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries. Circ Res. 2014;115:263–272. doi: 10.1161/CIRCRESAHA.115.302882. [DOI] [PubMed] [Google Scholar]
- 54.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- 56.Szasz T, Davis RP, Garver HS, Burnett RJ, Fink GD, Watts SW. Long-term inhibition of xanthine oxidase by febuxostat does not decrease blood pressure in deoxycorticosterone acetate (DOCA)-salt hypertensive rats. PloS one. 2013;8:e56046. doi: 10.1371/journal.pone.0056046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tesfamariam B, Halpern W. Endothelium-dependent and endothelium-independent vasodilation in resistance arteries from hypertensive rats. Hypertension. 1988;11:440–444. doi: 10.1161/01.hyp.11.5.440. [DOI] [PubMed] [Google Scholar]
- 58.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watts SW, Fink GD, Northcott CA, Galligan JJ. Endothelin-1-induced venous contraction is maintained in DOCA-salt hypertension; studies with receptor agonists. British journal of pharmacology. 2002;137:69–79. doi: 10.1038/sj.bjp.0704831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X, Makynen H, Kahonen M, Arvola P, Porsti I. Mesenteric arterial function in vitro in three models of experimental hypertension. J Hypertens. 1996;14:365–372. doi: 10.1097/00004872-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 61.Xu H, Bian X, Watts SW, Hlavacova A. Activation of vascular BK channel by tempol in DOCA-salt hypertensive rats. Hypertension. 2005;46:1154–1162. doi: 10.1161/01.HYP.0000186278.50275.fa. [DOI] [PubMed] [Google Scholar]
- 62.You J, Golding EM, Bryan RM., Jr Arachidonic acid metabolites, hydrogen peroxide, and EDHF in cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1077–1083. doi: 10.1152/ajpheart.01046.2004. [DOI] [PubMed] [Google Scholar]
- 63.You J, Johnson TD, Childres WF, Bryan RM., Jr Endothelial-mediated dilations of rat middle cerebral arteries by ATP and ADP. The American journal of physiology. 1997;273:H1472–1477. doi: 10.1152/ajpheart.1997.273.3.H1472. [DOI] [PubMed] [Google Scholar]
- 64.Zheng JS, Yang XQ, Lookingland KJ, Fink GD, Hesslinger C, Kapatos G, Kovesdi I, Chen AF. Gene transfer of human guanosine 5′-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation. 2003;108:1238–1245. doi: 10.1161/01.CIR.0000089082.40285.C3. [DOI] [PubMed] [Google Scholar]