Abstract
Objective
The pathogenesis of immune dysfunction in chronic HIV-1 infection is unclear, and a potential role for oxidized lipids has been suggested. We hypothesize that both oxidized low- and high-density lipoproteins (HDLox, LDLox) contribute to HIV-1 related immune dysfunction.
Study
In the AIDS Clinical Trials Group (ACTG) A5260, 234 HIV-infected antiretroviral therapy (ART)-naïve participants were randomized to receive tenofovir-emtricitabine plus protease inhibitors or raltegravir and had HIV-1 RNA <50 copies/ml by week 24 and thereafter.
Methods
Associations between biomarkers of inflammation (IL-6, hs-CRP, D-Dimer), immune activation (sCD163, sCD14, sIL-2r, CD38, HLA-DR), inflammatory monocytes (CD14+CD16+), T cell senescence (CD28, CD57) and exhaustion (PD1) and HDLox, LDLox were assessed at entry and after ART (week 96) with Spearman (partial) correlations.
Results
HDLox declined and LDLox increased over 96 weeks of ART. Positive associations were observed at baseline and over time between HDLox, (but not consistently for LDLox) and most markers of inflammation and immune activation (but not senescence/exhaustion), even after adjustment for multiple comparisons, demographics, entry CD4 count and HIV-1 RNA. HDLox was positively associated with IL-6 (r=0.19–0.29, p<0.01), and sCD163 (r=0.14–0.41 p≤0.04) at all timepoints.
Conclusions
These prospective longitudinal data suggest that oxidized lipoproteins may contribute to persistent immune activation on ART.
Keywords: Oxidized lipoproteins, HIV, inflammation, immune activation
INTRODUCTION
Human Immunodeficiency Virus type 1 (HIV) infection is characterized by a chronic state of systemic inflammation and immune (T cell and macrophage/monocyte; M/M) activation that is an independent predictor of disease progression. Many of these changes do not normalize despite antiretroviral therapy (ART) [1, 2]. This residual immune activation may be largely responsible for the increased morbidity and mortality observed in HIV-infected subjects on ART but the exact mechanisms are unclear [1, 2].
Oxidative stress is involved in pathogenesis of inflammatory diseases [3] and may impair antiviral immune responses [4]. Lipids play key roles in both viral replication [5] and T cell biology [6]. During oxidative stress oxidized phospholipids acquire novel biological activities such as the ability to regulate innate [7] and adaptive immunity [8, 9] and pathogenesis of many diseases including cardiovascular disease (CVD)[10, 11]. Modified lipoproteins such as oxidized low-density lipoprotein (LDLox) carry oxidized lipids and have pleomorphic atherogenic effects [10].
While high-density lipoprotein (HDL) is generally an anti-inflammatory lipoprotein with protective effects against oxidized lipids and CVD and major immunoregulatory function [12], during systemic inflammation it can be oxidized (HDLox), becomes dysfunctional [13, 14] and may contribute to CVD in patients with inflammation [13]. We have shown that HIV-infected subjects have HDLox, demonstrated by impaired antioxidant function and increased lipid hydroperoxide content [14–17] that is associated with measures of subclinical atherosclerosis such as carotid intima media thickness (IMT) [17] and percent non-calcified coronary plaque [18]. In addition, modified lipoproteins may also directly accelerate immune dysfunction and senescence/exhaustion [19, 20] that may contribute to multiple pathologies [21] such as CVD[22]. We [23] and others [24] have shown that oxidized lipoproteins directly upregulate immune activation in vitro. However the exact role of oxidized lipoproteins in HIV pathogenesis is unclear. We hypothesize that there is a cycle of HIV-induced immune activation, inflammation, production of oxidized lipoproteins, and further immune activation and senescence. To elucidate this hypothesis the objective of this study is to investigate in vivo whether oxidized lipoproteins (HDLox, LDLox) are positively associated with markers of inflammation (hsCRP; IL-6, D- dimer), immune activation (such as sCD14, sCD163, HLA-DR, CD38 expression on CD8 + T lymphocytes) and T cell senescence (CD28, CD57) and to evaluate how these relationships change over time during successful ART.
We evaluated HDLox, LDLox in samples from the AIDS Clinical Trials Group (ACTG) A5260s, a prospective study that longitudinally evaluated the changes in biomarkers of inflammation, immunosenescence and immune activation among treatment-naïve individuals undergoing randomized ART initiation with an integrase-based regimen containing raltegravir (RAL) or a protease inhibitor-based regimen containing either atazanavir/ritonavir (ATV/r) or darunavir/ritonavir (DRV/r) [25]. This prospective study revealed an overall increase in levels of LDLox and decrease in levels of HDLox markers of inflammation, coagulation, immune activation, CD4+ T cell senescence and exhaustion, and CD8+ T cell exhaustion (but not senescence), which were similar between the different ART regimens after 96 weeks of treatment [25]. Following up on these data, we examined potential associations of plasma oxidized lipoprotein levels with markers of systemic inflammation, coagulation, and immune activation in these individuals.
METHODS
Study Design and Participants
The design of A5260 and the virologic, tolerability and metabolic outcomes of these ART regimens have been previously reported [25–27]. The parent study and substudy were approved by the Institutional Review Boards at all participating institutions, and all subjects provided written informed consent. For this analysis, the A5260s population was restricted to the subset of virologically suppressed individuals with no ART interruptions greater than seven days and who achieved HIV RNA suppression <50 copies/ml by study week 24 and thereafter.
Determination of biomarkers and plasma oxidized lipoproteins
Blood samples were drawn at study entry prior to ART initiation and at 24, 48, 96, and 144 weeks on treatment from participants who were required to fast for at least eight hours. Plasma biomarkers of systemic inflammation [IL-6, high-sensitivity C-reactive protein (hsCRP), D-dimer), M/M activation (sCD14, sCD163) and T cell activation [soluble interleukin-2 receptor (sIL-2R)], cellular markers of monocyte (CD163) and T cell (HLA-DR, CD38) activation as well as inflammatory monocyte subsets (non-classical CD14dim/CD16+ and intermediate CD14+CD16+) have previously been described in this cohort [25]. Oxidized LDL was quantified using ELISA (Mercodia) according to the manufacturer instructions. We also determined the LDLox/LDL oxidation ratio which may be a better marker of in vivo oxidation of LDL compared to total levels of LDLox [28]. Oxidized HDL was determined using a validated fluorometric cell-free biochemical assay that measures HDL lipid peroxidation (HDLox) [17]. To reduce experimental variability [17] and adjust for HDL amount, we normalized the mean fluorescence readout from quadruplicates of each sample (HDLox_sample) by the mean fluorescence readout from quadruplicates of a pooled plasma control (HDLox_control) and by concurrent HDL cholesterol concentration level (HDL-C) using the following calculation: “normalized” oxidized HDL (nHDLox) = [HDLox_sample × 40 (mg/dl)] / [HDLox_control × HDL-Csample (mg/dL)], where 40 mg/dL represents HDL-C of the pooled plasma control. This approach has been validated in clinical studies [14, 29–32]. Throughout the results section oxidized HDL is presented as normalized [nHDLox] measure to reflect the adjustment for experimental variability and HDL-C.
Statistical Analyses
Biomarkers were analyzed at study entry (baseline) and further examined at weeks 24 (cellular markers), 48 (plasma markers) and 96. Because of substantial skewness in the variables, a log transformation was used. Changes from baseline were reported as fold-changes, with the 95% confidence interval indicating a statistically significant change at the p <0.05 level. Associations of oxidized lipoproteins and markers of inflammation, immune activation, senescence were examined cross-sectionally prior to (entry) and on ART (week 96) and longitudinally (as fold-change from baseline) using Spearman (partial) correlations which adjusted for the covariates of age, sex, race, BMI, smoking status, baseline CD4 count and baseline viral load. A Spearman partial correlation is similar to its Pearson partial correlation analogue, except that all of the variables are represented as their fractional ranks instead of being in their original units. To calculate the Spearman partial correlation, the covariates (as fractional ranks) are partialed out of two variables of interest, also expressed as ranks. The Pearson correlation between the residuals for the two variables is then the Spearman partial correlation coefficient. This value represents the unique independent effects of the oxidized lipoproteins on the makers of interest. For each set of hypotheses in the multivariate analysis (i.e. each table), the false discovery rate (FDR) was controlled at alpha=0.05 using the Benjamini-Hochberg procedure [33]. Statistical hypothesis tests were two-sided with a significance threshold of 0.05 for p values. All analyses were performed with Stata, version 13.1 (Stata Corp LP, College Station, TX, USA).
RESULTS
Baseline characteristics
Baseline demographic characteristics of the 328 subjects from the A5260s study population and the 234 subjects (71%) included in the virologically suppressed population for this analysis were previously described [25, 27]. There were no differences in baseline demographic characteristics among treatment groups. Median age was 36 years, 90% of subjects were men and 48% white. Median CD4+ cell count and median HIV RNA were 338 cells/mm3 and 4.6 log10 copies/ml, respectively.
Changes over time in plasma levels of oxidized lipoproteins
Changes over time in plasma levels of lipoproteins and oxidized lipoproteins are shown in Table 1. Briefly, HDL-C and LDL-C levels increased over time in all ART treated individuals. Levels of normalized HDLox declined over week 96 of ART. Post-baseline levels of LDLox and LDLox/LDL increased over 24 weeks and remained elevated compared to baseline levels over 96 weeks of ART.
Table 1.
Lipid Panel Variables: Fold Change from Baseline over Time among On-Treatment A5260s Subjects with Viral Suppression and No ART Interruption
| Baseline (n=234) | ALL subjects (n=234) Mean Fold Change (95% CI) |
||
|---|---|---|---|
| Biomarker | Mean (95% CI) | 24 wk | 96 wk |
| Total cholesterol (mg/dl) | 154.40 (150.00, 158.80) | 1.04 (1.01, 1.07) | 1.07 (1.04, 1.10) |
| HDL (mg/dl) | 37.80 (36.30, 39.40) | 1.06 (1.02, 1.10) | 1.12 (1.08, 1.16) |
| nHDLox (normalized ratio) | 1.00 (0.96, 1.05) | 0.99 (0.95, 1.03) | 0.91 (0.87, 0.95) |
| LDL (mg/dl) | 89.70 (85.90, 93.60) | 1.03 (0.99, 1.07) | 1.05 (1.00, 1.10) |
| LDLox (U/L) | 48.13 (46.21, 50.12) | 1.11 (1.07, 1.15) | 1.11 (1.06, 1.16) |
| LDLox/LDL (U/100 ug) | 0.54 (0.52, 0.56) | 1.07 (1.03, 1.12) | 1.05 (1.00, 1.10) |
| Non-HDL (mg/dl) | 114.10 (110.20, 118.20) | 1.03 (1.00, 1.06) | 1.05 (1.01, 1.09) |
| Triglycerides (mg/dl) | 106.20 (99.40, 113.40) | 1.04 (0.98, 1.10) | 1.03 (0.97, 1.10) |
Relationship between oxidized lipoproteins with markers of inflammation, coagulation, immune activation in ART naïve individuals
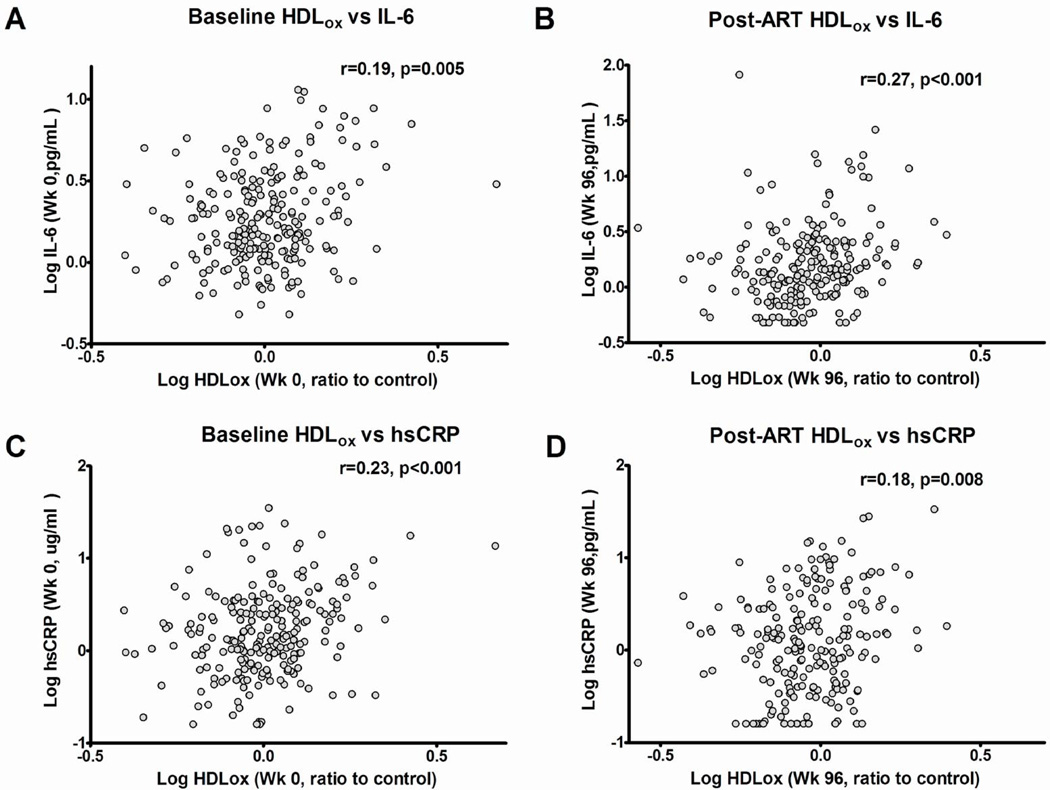
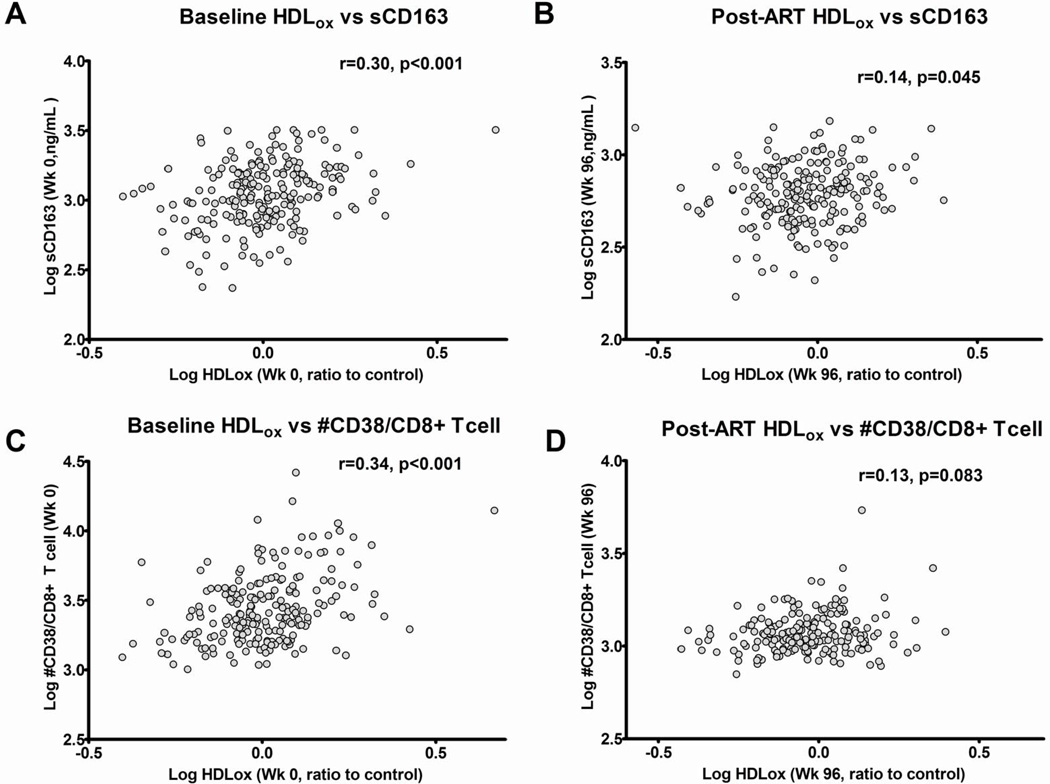
We found that nHDLox was positively associated with plasma biomarkers of inflammation (IL-6, hs-CRP) and both higher nHDLox and LDLox/LDL were associated with higher coagulation (D-dimer) at entry prior to ART (Supplemental Table 1)(Figure 1). The strongest relationships between oxidized lipoproteins and markers of inflammation/coagulation were between nHDLox and IL-6 (r=0.36, p<0.01), nHDLox and hs-CRP (r=0.27, p<0.001) and between nHDLox and D-dimer (r=0.19, p<0.01) and remained statistically significant (pFDR<0.05) even after adjusting for FDR, age, sex, race, BMI, smoking status, baseline CD4 count and viral load (Table 2). Higher nHDLox and LDLox/LDL (but not LDLox) were associated with higher levels of plasma markers of innate immune activation (sCD163 but not sCD14) and T cell activation (sIL-2r) as well as cellular markers of T cell activation such as expression of CD38 and HLA-DR (Supplemental Tables 1, 2). Similar results were observed for CD4+ and CD8+ T cells. LDLox/LDL levels correlated with sCD14 levels. We also found an inverse relationship between nHDLox and CD38-DR+ T cells, the latter having previously been correlated with favorable outcome in the MACS cohort (Supplemental Table 2)[34]. There were no relationships between levels of plasma-oxidized lipoproteins, inflammatory monocytes and cellular monocyte expression of CD163 (Supplemental Table 2). The most notable positive associations between oxidized lipoproteins and markers of immune activation at baseline were between nHDLox and CD38 expression on T cells (for both CD4+ and CD8+T cells r=0.34, p<0.001) and sCD163 (r=0.30, p<0.001) (Figure 2, Supplemental Figure 1); these associations remained statistically significant (pFDR<0.05) even after adjusting for covariates (Tables 2 and 3).
Figure 1.
Spearman correlations of nHDLox with higher levels of markers of systemic inflammation IL-6 (Figure 1 A, B), and hs-CRP (Figure 1 C, D), at baseline (A, C), week 96 after ART (B, D). Oxidized HDL represents the normalized [nHDLox] measure as described in Methods. Spearman correlation coefficients (r) are shown.
Table 2.
Partial Spearman Correlations adjusted for Age, Sex, Race, BMI, Current Smoking, Baseline CD4 and RNA (log-scale) level: HDLox, LDLox and Soluble Markers of Inflammation/Coagulation Measured Concurrently or Current Change from Baseline over Time.
| Week | 0 | 96 | Δ 96-0 | 0 | 96 | Δ 96-0 | 0 | 96 | Δ 96-0 |
|---|---|---|---|---|---|---|---|---|---|
| Soluble Markers of Inflammation/Coagulation | |||||||||
| IL-6 | hsCRP | D-dimer | |||||||
| nHDLox | 0.15* | 0.36** | 0.27** | 0.20** | 0.27** | 0.21** | 0.18** | 0.19** | 0.14 |
| LDLox | 0.04 | 0.17* | −0.01 | 0.03 | 0.19** | −0.03 | −0.05 | 0.11 | 0.01 |
| LDLox/LDL | 0.05 | 0.17* | 0.11 | 0.04 | 0.01 | −0.02 | 0.15* | 0.14 | 0.07 |
| Soluble Markers of Immune activation | |||||||||
| sCD14 | sCD163 | sIL2r | |||||||
| nHDLox | 0.08 | 0.01 | 0.24** | 0.21** | 0.13 | 0.30** | 0.12 | 0.12 | 0.17* |
| LDLox | 0.05 | 0.11 | 0.01 | 0.07 | 0.00 | 0.07 | 0.13* | 0.10 | 0.14 |
| LDLox/LDL | 0.02 | 0.02 | 0.10 | 0.18** | 0.09 | 0.09 | 0.07 | 0.01 | 0.18* |
Significant (p<0.05) correlations are shown in italics and discrepant correlations between LDLox and LDLox/LDL are shown in bold. Nominal p-values presented. P>0.05 unless noted as statistically significant:
<0.05,
<0.01.
Those with false discovery rate (FDR) < 0.05 are underlined.
Figure 2.
Spearman correlations of nHDLox with higher levels of markers of M/M activation (sCD163; Figure 2 A, B), and CD8+ T cell activation [CD38 expression on CD8+ T cells (Figure 1 C, D) at baseline (A, C), week 96 (B, D). Oxidized HDL represents the normalized [nHDLox] measure as described in Methods. Spearman correlation coefficients (r) are shown.
Table 3.
Partial Spearman Correlations adjusted for Age, Sex, Race, BMI, Current Smoking, Baseline CD4 and RNA (log-scale) level: HDLox, LDLox and Cellular Markers of immune Activation Measured Concurrently or Current Change from Baseline over Time.
| Week | 0 | 96 | Δ 96-0 | 0 | 96 | Δ 96-0 |
|---|---|---|---|---|---|---|
| Proinflammatory MNCs and Cellular markers of M/M activation | ||||||
| Parameter | % CD14dimCD16hi MNCs1 | % CD163+ MNCs2 | ||||
| nHDLox | −0.13 | −0.03 | −0.23** | 0.03 | 0.00 | −0.09 |
| LDLox | 0.00 | 0.00 | 0.01 | −0.02 | 0.01 | −0.08 |
| LDLox/LDL | −0.12 | 0.04 | −0.24** | −0.10 | 0.02 | −0.02 |
| Cellular markers of T cell activation | ||||||
| % CD38+DR+ CD8+ cells | # CD38 per CD8+ T cell3 | |||||
| nHDLox | 0.11 | 0.07 | 0.11 | 0.22** | 0.11 | 0.27** |
| LDLox | 0.08 | 0.05 | 0.09 | 0.06 | 0.05 | −0.09 |
| LDLox/LDL | 0.11 | 0.12 | 0.05 | 0.15* | 0.01 | 0.23** |
Nominal p-values presented. P>0.05 unless noted as statistically significant:
<0.05,
<0.01.
Significant (p<0.05) correlations are shown in italics and discrepant correlations between LDLox and LDLox/LDL are shown in bold. P values with false discovery rate (FDR) < 0.05 are underlined.
Similar results were observed for CD4+ and CD8+ T cells and:
CD14+CD16+MNCs;
MFI CD163 of CD163+ MNCs
% CD38+ CD8+ T cells (not shown)
Relationship between oxidized lipoproteins with markers of inflammation, coagulation, immune activation in ART-treated individuals with suppressed viremia
Consistent with the data in viremic ART-naïve individuals, we also found that LDLox, LDLox/LDL and nHDLox were positively associated with plasma biomarkers of inflammation at 96 weeks of effective ART (Supplemental Table 1). Most notably, nHDLox had positive associations with IL-6 (r=0.27, <0.001)(Figure 1) even after adjusting for covariates and applying the FDR correction (Table 2). The relationships of LDLox/LDL ratio and LDLox with hs-CRP and D-dimer were not consistent (Supplemental Table 1, Tables 2 and 3). We found that higher HDLox but not LDLox or LDLox/LDL were associated with higher levels of plasma sCD163 (r=0.14, 0.05)(at 96 weeks of effective ART (Supplemental Table 1)(Figure 2) but this association did not remain significant after covariate adjustment (Table 2).
Declines in plasma levels of oxidized HDL over 96 weeks of ART were associated with declines in markers of inflammation and immune activation
There were consistent positive associations between declines over 96 weeks in levels of nHDLox and IL-6, hs-CRP, sCD14, sCD163, inflammatory monocytes (Supplemental Table 2) that remained significant after adjusting for covariates and controlling the FDR (Tables 2,3). During this same interval LDLox and LDLox/LDL ratio increased but there were no consistent relationships between changes in LDLox, LDLox/LDL ratio and changes in markers of inflammation, coagulation and immune activation. In the subset of nHDLox values that declined most over time (first tertile), all significant associations were more notable and there was a largest decline in levels of IL-6 (data not shown).
Association between oxidized lipoproteins and markers of T cell senescence and exhaustion in ART naïve and in ART-treated individuals with suppressed viremia
We found that higher nHDLox and LDLox/LDL (but not LDLox) were associated with higher cellular markers of T cell senescence (% CD57+ of CD8+ T cells) and exhaustion (% PD1+ of CD4+ and CD8+ T cells) in ART naïve individuals (Supplemental Table 3). These associations were not present at 96 weeks of ART. Changes in levels of nHDLox and LDLox/LDL over 96 weeks were positively associated with changes in % CD28-CD57+ of CD4+ T cells, and % PD1+ of CD4+ T cells. LDLox/LDL ratio had differential relationships with markers of T cell senescence and exhaustion compared to LDLox (Supplemental Table 3). All the noted relationships were weak, not consistent across all timepoints (baseline, week 96, changes over 96 weeks), were attenuated and did not remain statistically significant after adjusting for covariates (Supplemental Table 4).
DISCUSSION
In this prospective study of ART-naïve subjects initiating RAL, ATV/r or DRV/r with TDF/FTC and successfully achieving virologic suppression, oxidized lipoproteins and primarily HDLox were associated with markers of T cell activation, as well as plasma levels of monocyte activation, inflammation and coagulation. We chose to focus our analyses on markers such as hsCRP; D- dimer, sCD14, sCD163 and CD38 expression that have been associated with serious clinical events in HIV infected persons, including CVD and mortality [35, 36]. To our knowledge, this is the most comprehensive prospective study describing changes in oxidized lipoproteins and immune activation (both M/M and lymphocyte) and inflammation after ART initiation and successful immune suppression. The data from this prospective study with in vivo oxidized lipoproteins from HIV infected subjects are consistent with our prior data [23] and data from others [24] that have shown that in vitro oxidized lipoproteins directly upregulate immune activation in vitro. Thus, we hypothesize that oxidized lipoproteins have a central role in HIV pathogenesis that both result from and contribute to systemic inflammation and immune activation of HIV infection [37].
Consistent with the proinflammatory effect of the oxidized lipoproteins, overall, we found positive associations of both HDLox and LDLox with various plasma biomarkers of inflammation and coagulation in both viremic and ART treated (at 96 weeks) HIV infected persons. HDLox showed consistent positive associations over time with IL-6 and hs-CRP, which have been associated with serious clinical events in HIV infected persons, including CVD and mortality [35, 36]. Interestingly we found that successful ART increased rather than decreased levels of LDLox. Initiation of ART within HIV-infected patients reduces markers of systemic inflammation but there is incomplete reversal of systemic inflammation [25]. HIV infected individuals receiving ART may have higher oxidative stress compared to HIV infected naïve or healthy subjects due to higher production of reactive oxygen species (ROS), and alterations in antioxidant systems [38, 39]. Thus, ART, different levels of ROS and antioxidant systems in treated compared to ART-naïve HIV infected persons, may explain the discordant relationship between LDLox and markers of systemic inflammation before and after ART initiation.
Consistent with our hypothesis that the immunostimulatory effects of oxidized lipoproteins (that carry oxidized lipids) may contribute to HIV-related immune activation, there were consistent positive associations of both HDLox and LDLox/LDL with several markers of immune activation in ART-naïve viremic persons. The immunostimulatory effects of oxidized lipoproteins have been shown in other inflammatory states [8]. Dysfunctional HDL that is known to be oxidized [14–17], has previously been shown in vitro to directly influence monocyte [40] and dendritic cell [41] function in systemic inflammatory states [40, 41]. Lipoproteins bind endotoxin which is present in viremic HIV-infected individuals, acts synergistically with LDLox [42] and may contribute to immune activation [1]. Thus, further studies are needed to elucidate whether oxidized lipoproteins, through effects on immunity and endotoxin, may contribute to immune activation in viremic HIV infected individuals.
In view of our prior data that oxidized lipoproteins directly induce T cell activation in peripheral blood mononuclear cells from HIV infected ART-treated persons [23], we hypothesized that oxidized lipoproteins may contribute to immune activation in ART-treated HIV-infected individuals. Consistent with this hypothesis we found that after 96 weeks of successful ART, HDLox was associated with several biomarkers of immune activation and importantly decline in HDLox over 96 weeks was also positively associated with decline over 96 weeks in these biomarkers of immune activation even after adjustment for multiple comparisons, demographics, entry CD4 count and HIV-1 RNA. More specifically, HDLox was associated with sCD163 and there were consistent positive associations between changes over 96 weeks in levels of HDLox with several markers of M/M (sCD14, sCD163 inflammatory monocytes) and T cell (cellular expression of CD38, HLA-DR) activation. In a prior small study of 54 HIV infected patients where 91% were receiving ART, both LDL and LDLox correlated positively with sCD14 and in vitro stimulation with LDLox, resulted in expansion of inflammatory monocytes [24]. In a randomized trial of rosuvastatin in HIV-infected subjects on ART, LDLox levels decreased 24 weeks after statin therapy, was associated with changes in markers of monocyte activation, and independently predicted changes in IMT, supporting our findings of the importance of oxidized lipids in potentially driving immune activation on ART [43]. In our study, there was also a robust positive association of HDLox with sCD163, a marker of M/M activation that has been linked to CVD in HIV disease [44]. These data are consistent with our prior data in a cohort of 102 HIV infected treated subjects, where increased HDL redox activity, a measure of HDL function, correlated positively with sCD163 [18]. Interestingly, the higher increases in baseline CD8+ T cell activation in viremic patients were also seen in subjects with the highest HDLox and sCD163. CD163 is shed in response to inflammation and is a scavenger receptor for complexes of hemoglobin/haptoglobin, which may alter HDL function [45]. The exact mechanisms that may mediate the interplay between HDLox and immune activation remain to be determined.
Senescent T cells may increase the risk of morbidity, such as CVD [46]. Modified lipoproteins have pleomorphic atherogenic effects [47] and may directly accelerate senescence [19, 20, 48] and contribute to cell exhaustion. We found weak and inconsistent relationships between HDLox and LDLox/LDL with markers of T cell senescence and exhaustion, across all timepoints, which did not remain significant after covariate adjustment. Further research is needed to determine whether oxidized lipids may contribute to HIV-related T cell dysfunction.
The differential immunoregulatory effects of HDLox versus LDLox during the first few years of ART treatment remain unknown. HDL lipids are oxidized in preference to those in LDL when human plasma is exposed to ROS [49]. Moreover, our data corroborate prior evidence that LDLox/LDL oxidation ratio may be a better marker of in vivo oxidation of LDL compared to total levels of LDLox [28]. Thus, further studies to elucidate the role of oxidized lipoproteins in HIV-related pathogenesis should focus on both HDLox and LDLox as well as the ratio of oxidized lipoproteins to their total plasma levels.
Our study has several limitations. This analysis of oxidized lipoproteins was not the primary outcome of the A5260s study and may have limited power to detect complex modifications of lipoproteins that occur in vivo in the context of measuring oxidized lipoproteins i) in a population with an overall low cardiovascular disease risk ii) in the setting of initiation of different ART regimens during a period where there may be major changes in inflammation and oxidative stress that may further increase between subject variability and may compromise the ability to detect differences in measures of oxidized lipoproteins iii) in cryopreserved rather than fresh samples [14, 17] iv) using biochemical assays that have limitations.
It is also recognized that although our in vitro data suggest that in vitro oxidized lipoproteins directly upregulate immune activation [23], oxidized lipoproteins may also reflect and result from [37] systemic inflammation and immune activation that may be driven by several other mechanisms in HIV infection. For example, ART may directly affect oxidative stress and immune activation in addition to its antiviral effects [38, 50] and these effects may lead to increased plasma levels of oxidized lipoproteins.
Despite the above limitations, this is the most comprehensive prospective study describing changes in oxidized lipoproteins with regards to markers of immune activation and inflammation after ART initiation. Our data support the hypothesis that there is a cycle of HIV-induced immune activation, inflammation, production of oxidized lipoproteins that contributes to further immune activation. Further studies are needed to confirm these findings and further elucidate the differential proinflammatory role of HDLox vs LDLox in HIV infection. A deeper understanding of the specific mechanisms causing chronic immune activation is crucial to target immune activation in HIV-infected individuals.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by NIH grants HL095132, HL095126, AI 068636, AI068634, AI69471, AI069501, and AI56933, NIH/NCATS Grant # UL1TR000124, NIH K08AI08272. The study received additional financial support from Gilead, Merck, Bristol Myers Squibb, Janssen. The project described was supported by Award Number UM1 AI068634, UM1 AI068636 and UM1 AI106701 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any of the funders.
ACTG 5260s Team Members: H. Hodis, C. Godfrey, B. Jarocki, A. Benns, K. Braun. We thank the staff, and patients from the following hospitals who participate in ACTG (in alphabetical order): Beth Israel Deaconess Medical Center, Brigham and Womens Hospital, Case University CRS, Duke University Medical Center, Harbor-UCLA Medical Center, Houston AIDS Research Team CRS, John Hopkins Adult AIDS CRS, Metrohealth, New Jersey Medical School, New York University HIV/AIDS CRS, Northwestern University, Rush University Medical Center ACTG, The Ohio State University, The Ponce De Leon Center CRS, UCLA Care Center, UCSF AIDS CRS, University of Cincinnati, University of Colorado, University of North Carolina AIDS CRS, University of Pittsburg CRS, University of Rochester ACTG AIDS Care, University of Southern California, University of Washington, Vanderbilt Therapeutics CRS, Washington University
Dr Brown has served as a consultant for BMS, GSK, Merck, Abbott, Gilead, ViiV Healthcare and has received research funding from Merck and GSK. Dr. Currier has served as a consultant for Gilead and has received research funding from Merck. Dr Stein served on a Data Safety Monitoring Board for Lilly. Dr McComsey has served as consultant or received research grants from BMS, Pfizer, Gilead, ICON, and GSK/ViiV. Dr. Dubé has served as a consultant for Gilead and Astra Zeneca, and receives research funding from Gilead, ViiV, and Merck.
Footnotes
Authors contributions: J.C, J.S., G.M., T.B, T.K. were responsible for the study concept and design. N.J, X.W, D.E carried out the statistical analyses. T.K., N.J, J.C, O.Y drafted the manuscript. T.K., O.Y, M.D, J.S., J.C., G.M. T.B. collected the data. All co-authors participated in discussions about the design of the study, interpretation of the findings, and critically reviewed the manuscript.
Potential conflicts of interest:
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Paiardini M, Muller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254:78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045:57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder C. Cholesterol-binding viral proteins in virus entry and morphogenesis. Subcell Biochem. 2010;51:77–108. doi: 10.1007/978-90-481-8622-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freigang S, Kain L, Teyton L. Transport and uptake of immunogenic lipids. Mol Immunol. 2013;55:179–181. doi: 10.1016/j.molimm.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 8.Laczik R, Szodoray P, Veres K, et al. Oxidized LDL induces in vitro lymphocyte activation in antiphospholipid syndrome. Autoimmunity. 2010;43:334–339. doi: 10.3109/08916930903540440. [DOI] [PubMed] [Google Scholar]
- 9.Graham LS, Parhami F, Tintut Y, Kitchen CM, Demer LL, Effros RB. Oxidized lipids enhance RANKL production by T lymphocytes: implications for lipid-induced bone loss. Clin Immunol. 2009;133:265–275. doi: 10.1016/j.clim.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsimikas S, Miller YI. Oxidative modification of lipoproteins: mechanisms, role in inflammation and potential clinical applications in cardiovascular disease. Curr Pharm Des. 2011;17:27–37. doi: 10.2174/138161211795049831. [DOI] [PubMed] [Google Scholar]
- 11.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaji H. High-density lipoproteins and the immune system. J Lipids. 2013;2013:684903. doi: 10.1155/2013/684903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–232. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 14.Kelesidis T, Currier JS, Huynh D, et al. A biochemical fluorometric method for assessing the oxidative properties of HDL. J Lipid Res. 2011;52:2341–2351. doi: 10.1194/jlr.D018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelesidis T, Yang OO, Currier JS, Navab K, Fogelman AM, Navab M. HIV-1 infected patients with suppressed plasma viremia on treatment have pro-inflammatory HDL. Lipids Health Dis. 2011;10:35. doi: 10.1186/1476-511X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelesidis TF M, Tseng CH, Currier JS, Yang OO. HIV-infected adults with suppressed viremia on antiretroviral therapy have dysfunctional HDL that is associated with T cell activation. Presented at ID Week 2012; October 2012; San Diego, CA. 2012. [Google Scholar]
- 17.Kelesidis T, Roberts CK, Huynh D, et al. A high throughput biochemical fluorometric method for measuring lipid peroxidation in HDL. PLoS One. 2014;9:e111716. doi: 10.1371/journal.pone.0111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanni MV, Kelesidis T, Fitzgerald ML, et al. HDL redox activity is increased in HIV-infected men in association with macrophage activation and non-calcified coronary atherosclerotic plaque. Antivir Ther. 2014;19:805–811. doi: 10.3851/IMP2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park KH, Cho KH. High-density lipoprotein (HDL) from elderly and reconstituted HDL containing glycated apolipoproteins A-I share proatherosclerotic and prosenescent properties with increased cholesterol influx. J Gerontol A Biol Sci Med Sci. 2011;66:511–520. doi: 10.1093/gerona/glr016. [DOI] [PubMed] [Google Scholar]
- 20.Park KH, Shin DG, Cho KH. Dysfunctional lipoproteins from young smokers exacerbate cellular senescence and atherogenesis with smaller particle size and severe oxidation and glycation. Toxicol Sci. 2014;140:16–25. doi: 10.1093/toxsci/kfu076. [DOI] [PubMed] [Google Scholar]
- 21.Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19:1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelesidis TC JS, Huynh D, Park S, Ng HL, Yang OO. Dysfunctional High Density Lipoprotein directly upregulates T cell activation in HIV-1 infection. Presented at Conferences on Retroviruses and Opportunistic Infections (CROI 2014); March 3–6, 2014; Boston, Massachusetts. 2014. [Abstract 258] [Google Scholar]
- 24.Zidar DA, Juchnowski S, Ferrari B, et al. Oxidized LDL Levels Are Increased in HIV Infection and May Drive Monocyte Activation. J Acquir Immune Defic Syndr. 2015;69:154–160. doi: 10.1097/QAI.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelesidis T, Tran TT, Stein JH, et al. Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. Clin Infect Dis. 2015;61:651–660. doi: 10.1093/cid/civ327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown TT, Chen Y, Currier JS, et al. Body composition, soluble markers of inflammation, and bone mineral density in antiretroviral therapy-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2013;63:323–330. doi: 10.1097/QAI.0b013e318295eb1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McComsey GA, Moser C, Currier J, et al. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Mai W, Liu D, Hao Y, Tao J, Dong Y. The oxidation ratio of LDL: a predictor for coronary artery disease. Dis Markers. 2008;24:341–349. doi: 10.1155/2008/371314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelesidis T, Roberts CK, Huynh D, et al. A high throughput biochemical fluorometric method for measuring lipid peroxidation in HDL. PLoS One. 2014;9:e111716. doi: 10.1371/journal.pone.0111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelesidis T, Yang OO, Kendall MA, Hodis HN, Currier JS. Dysfunctional HDL and progression of atherosclerosis in HIV-1-infected and -uninfected adults. Lipids Health Dis. 2013;12:23. doi: 10.1186/1476-511X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelesidis T, Reddy ST, Huynh D, et al. Effects of lipid-probe interactions in biochemical fluorometric methods that assess HDL redox activity. Lipids Health Dis. 2012;11:87. doi: 10.1186/1476-511X-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanni MV, Kelesidis T, Fitzgerald ML, et al. HDL redox activity is increased in HIV-infected men in association with macrophage activation and non-calcified coronary atherosclerotic plaque. Antivir Ther. 2014;19:805–811. doi: 10.3851/IMP2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamini YH Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300. Journal of the Royal Statistical Society. 1995:289–300. [Google Scholar]
- 34.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samson S, Mundkur L, Kakkar VV. Immune response to lipoproteins in atherosclerosis. Cholesterol. 2012;2012:571846. doi: 10.1155/2012/571846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma B. Oxidative stress in HIV patients receiving antiretroviral therapy. Curr HIV Res. 2014;12:13–21. doi: 10.2174/1570162x12666140402100959. [DOI] [PubMed] [Google Scholar]
- 39.Mandas A, Iorio EL, Congiu MG, et al. Oxidative imbalance in HIV-1 infected patients treated with antiretroviral therapy. J Biomed Biotechnol. 2009;2009:749575. doi: 10.1155/2009/749575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skaggs BJ, Hahn BH, Sahakian L, Grossman J, McMahon M. Dysfunctional, pro-inflammatory HDL directly upregulates monocyte PDGFRbeta, chemotaxis and TNFalpha production. Clin Immunol. 2010;137:147–156. doi: 10.1016/j.clim.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz D, Watson AD, Miller CS, et al. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest. 2008;118:2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hossain E, Ota A, Karnan S, et al. Lipopolysaccharide augments the uptake of oxidized LDL by up-regulating lectin-like oxidized LDL receptor-1 in macrophages. Mol Cell Biochem. 2015;400:29–40. doi: 10.1007/s11010-014-2259-0. [DOI] [PubMed] [Google Scholar]
- 43.Hileman CO, Turner R, N TF, Semba RD, McComsey GA. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS. 2016;30:65–73. doi: 10.1097/QAD.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charles-Schoeman C, Watanabe J, Lee YY, et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 2009;60:2870–2879. doi: 10.1002/art.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 47.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 48.Park KH, Jang W, Kim KY, Kim JR, Cho KH. Fructated apolipoprotein A-I showed severe structural modification and loss of beneficial functions in lipid-free and lipid-bound state with acceleration of atherosclerosis and senescence. Biochem Biophys Res Commun. 2010;392:295–300. doi: 10.1016/j.bbrc.2009.12.179. [DOI] [PubMed] [Google Scholar]
- 49.Bowry VW, Stanley KK, Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci U S A. 1992;89:10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandra S, Mondal D, Agrawal KC. HIV-1 protease inhibitor induced oxidative stress suppresses glucose stimulated insulin release: protection with thymoquinone. Exp Biol Med (Maywood) 2009;234:442–453. doi: 10.3181/0811-RM-317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.