Abstract
Objective
The potential contribution of CYP4A enzymes to endothelial dysfunction in Dahl salt-sensitive (SS) rats was determined by comparison to SS-5BN consomic rats having chromosome 5 carrying CYP4A alleles from the Brown Norway (BN) rat introgressed into the SS genetic background.
Methods
The following experiments were performed in cerebral arteries from HS-fed SS and SS-5BN rats ± the SOD inhibitor DETC and/or the superoxide scavenger Tempol: 1) endothelial function was determined via video microscopy ± acute addition of the CYP4A inhibitor DDMS or Tempol; 2) vascular oxidative stress was assessed with DHE fluorescence ± acute addition of DDMS, L-NAME, or PEG-SOD; and 3) CYP4A protein levels were compared by Western blotting.
Results
In DETC-treated SS-5BN and HS-fed SS rats: 1) DDMS or Tempol ameliorated vascular dysfunction 2) DDMS reduced vascular oxidative stress to control levels; 3) Chronic Tempol treatment reduced vascular CYP4A protein expression; and 3) combined treatment with Tempol and L-NAME prevented the reduction in CYP4A protein expression in MCA of HS-fed SS rats.
Conclusion
The CYP4A pathway plays a role in vascular dysfunction in SS rats and there appears to be a direct role of reduced NO availability due to salt-induced oxidant stress in upregulating CYP4A enzyme expression.
Keywords: Endothelial Dysfunction, 20-HETE, Oxidant Stress
INTRODUCTION
Reactive oxygen species (ROS) are normal reactive byproducts of oxygen metabolism that were originally considered to be universally damaging cellular molecules capable of producing injurious effects on lipids, proteins, and DNA.[21] However, it is now known that ROS also play an integral role in normal cell function including cellular metabolism, signaling, and regulation.[26] Under physiologic conditions, ROS are produced within the vasculature in a controlled manner, producing low concentrations of these vital signaling molecules, which play a role in normal vascular smooth muscle cell contraction [9] and growth.[40,46,53] A pathophysiologic state ensues when there is either over-production of ROS, a diminished capacity to regulate ROS concentrations due to reduced antioxidant defense mechanisms,[16] or both.
20-Hydroxyeicosatetraenoic acid (20-HETE) is a vasoconstrictor metabolite of arachidonic acid that is formed endogenously in a number of vascular beds through the action of cytochrome P450 ω-hydroxylase (CYP) enzymes of the 4A and 4F family. [22–24,32,33] 20-HETE appears to play a crucial role in the regulation of blood flow, O2 sensitivity, and vascular function.[22–24,32,33] The CYP4A/20-HETE pathway can also contribute to oxidative stress in tissues because the action of the CYP4A enzyme requires the ROS-producing enzyme NADPH oxidase as a necessary co-factor [5]. 20-HETE can also directly uncouple endothelial nitric oxide synthase (eNOS) through a signaling cascade involving tyrosine kinase stimulation of the mitogen-activated protein kinase and extracellular signal-regulated kinase pathways.[8] This signaling cascade blocks the association between eNOS and heat shock protein 90 (HSP90), which is critical for the normal function of eNOS.[7,8,50] Thus, the CYP4A/20-HETE pathway is not only involved in the direct production of ROS, but also in eNOS uncoupling, leading to reduced NO production and elevated vascular levels of superoxide anion (O2−).
The Dahl salt-sensitive (SS) rat is an inbred genetic model of salt-sensitive hypertension that is susceptible to heightened oxidative stress due, at least in part, to reduced antioxidant defense mechanisms. For example, Somova et al. [42] demonstrated decreased glutathione levels and reduced glutathione peroxidase activity (indicators of antioxidant defenses) in the myocardium of Dahl SS rats. Our laboratory and others have shown that Dahl SS rats have reduced levels of antioxidant enzymes including Cu/Zn-SOD in their cerebral vasculature,[13] as well as reduced renal medullary Cu/Zn-SOD and Mn-SOD expression.[38] Collectively, those findings are consistent with the hypothesis that Dahl SS rats have reduced antioxidant defense mechanisms, leaving them vulnerable to any mechanism that may generate reactive oxygen species.
Previous studies in our laboratory have shown that cerebral arteries from SS rats exhibit a pathological upregulation of the CYP4A/20-HETE pathway resulting in 20-HETE-dependent vascular dysfunction.[35] Mesenteric resistance arteries from Sprague-Dawley rats fed a high salt diet also fail to dilate in response to reduced PO2--a response that can be restored in the presence of the CYP4A inhibitor N-methyl-sulfonyl-12,12-dibromododec-11-enamide (DDMS).[49] The salt-induced vascular dysfunction in mesenteric resistance arteries of Sprague-Dawley rats is due in part to elevated vascular CYP4A protein expression[49] and elevated vascular ROS, as evaluated via DHE fluorescence [56]. Thus, in both Dahl SS rats and HS-fed Sprague-Dawley rats, an increase in CYP4A protein expression is observed concomitant with a highly oxidant environment suggesting that either the CYP4A/20-HETE pathway is producing reactive oxygen species or, perhaps, that ROS levels are regulating the expression of the CYP4A/20-HETE pathway.
The present study tested the hypothesis that upregulation of the CYP4A/20-HETE pathway results in cerebral vascular dysfunction in the Dahl SS rat due to the production of reactive oxygen species (ROS). We also evaluated whether elevated vascular ROS levels may upregulate the expression of the CYP4A/20-HETE pathway in the vessels. This hypothesis was tested by comparing the Dahl SS rat to its close genetic counterpart, the SS-5BN consomic rat; which has chromosome 5 carrying the 20-HETE-producing CYP4A alleles from the normotensive Brown Norway (BN) rat introgressed into the SS genetic background. The SS-5BN consomic rat is protected from salt-induced hypertension,[36,51] vascular dysfunction, and vascular oxidative stress [35] that is present in the Dahl SS rat; and these protective effects of chromosomal substitution appear to be due, at least in part, to a decreased contribution of the CYP4A/20-HETE pathway.[35]
MATERIALS AND METHODS
Experimental Groups
Eight to 12 week old male SS-5BN consomic rats (SS-Chr 5BN/Mcwi strain) and Dahl SS rats were used for this study. Following weaning, Dahl SS rats were maintained on a normal salt (0.4% NaCl) diet and then switched to a high salt (HS; 4.0% NaCl; Dyets, Inc.) diet for 3 days immediately prior to the experiments, with water to drink ad libitum. A subset of HS-fed Dahl SS rats were given drinking water with either the superoxide scavenger Tempol (1 mM) alone or Tempol (1mM) and the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (L-NAME; 40mg/kg/day) for 7 days. Previous studies have shown that this duration and dosage of Tempol does not significantly alter blood pressure, regardless of salt intake [55].
The SS-5BN consomic rats were divided into three treatment groups: 1) switched to a high salt diet for 3 days; 2) HS-fed SS-5BN consomic rats receiving an i.v. infusion of the superoxide dismutase inhibitor, diethyldithiocarbamate (DETC) at a low-dose (16 mg/kg/day) for 7 days followed by three days of high dose DETC (200 mg/kg/day); or 3) the same conditions as Group 2 with Tempol in the drinking water for 7 days immediately prior to harvesting the vessels. The animals in Groups 2 and 3 were put on a HS diet for the final 3 days of the treatment session. The Medical College of Wisconsin IACUC approved all protocols.
Isolated Vessel Experiments
Animals were anesthetized with an intramuscular injection containing (in mg/kg): ketamine (75.0), acepromazine (2.5) and anased (10.0). Middle cerebral arteries (MCA) were isolated, cannulated with micropipettes, and perfused and superfused with bicarbonate-buffered physiological salt solution as previously described [20]. Internal diameter was measured using television microscopy, and vessels lacking active tone at rest were excluded from study.
After the control equilibration period, responses to the endothelium-dependent dilator acetylcholine (ACh; 10−10–10−5 M) were determined. The vessels were then incubated for 30 minutes in the presence of DDMS (50 μM) to inhibit CYP4A enzymes and the responses to ACh were repeated. Time control experiments showed no effect of incubation time on vascular responses. In a second series of experiments, acute Tempol (100 μM) was added to the perfusate and superfusate for the final 30 minutes of the control equilibration period and the responses of the arteries to acetylcholine were then recorded.
Vessel responses to an NO donor were not tested in the present study, because our earlier studies [35] showed that there is no difference in the response of MCA to an exogenous nitric oxide donor in Dahl SS and SS-5BN rats fed either a normal salt or high salt diet; and no effect of DDMS on vessel responses to NO donors. Because DDMS lowers vascular ROS levels similar to Tempol, we believe that vessel responses to SNP would also be unaffected by Tempol treatment, although this remains to be established.
At the end of the experiment, the maximum diameter of the artery was determined by adding hydrogen peroxide (H2O2; 1.76 mM) to the superfusate to achieve maximum dilation. Active resting tone (%) was calculated as [(Dmax−Drest)/Dmax] ×100, where Dmax is the maximum diameter in the presence of H2O2 and Drest is the resting control diameter (Table 1).
Table 1.
Middle Cerebral Artery Diameters and Resting Tone
| Treatment Groups | n | Resting Diameter (μm) | Maximum Diameter (μm) | % Active Tone |
|---|---|---|---|---|
| SS HS | 6 | 143±11 | 239±5.1 | 40±5.2 |
| SS HS + DDMS | 5 | 127±7.4 | 236±1.8 | 46±3.2 |
| SS HS + Acute Tempol | 6 | 135±8.2 | 230±2.8 | 41±3.8 |
| SS-5BN HS | 6 | 128±6.3 | 242±3.9 | 47±3.2 |
| DETC-treated SS-5BN | 5 | 132±6.8 | 242±3.5 | 45±3.1 |
| DETC-treated SS-5BN + Acute Tempol | 4 | 122±6.7 | 242±4.5 | 50±2.7 |
| DETC-treated SS-5BN + DDMS | 5 | 152±8.4 | 243±2.9 | 37±3.7 |
| DETC-treated SS-5BN + Chronic Tempol | 5 | 124±6.3 | 239±3.2 | 48±2.6 |
| DETC-treated SS-5BN + Chronic Tempol + DDMS | 4 | 131±18.6 | 239±3.9 | 45±8.4 |
Dihydroethidium Fluorescence
Vascular levels of reactive oxygen species (ROS) were assessed using dihydroethidium (DHE) fluorescence [13] in basilar arteries. Basilar arteries were used as a substitute for MCA, as both vessels demonstrate a NO-dependent dilation to ACh,[37] and the larger diameter of the basilar artery allows for improved cross sectioning. In those experiments, isolated arteries were incubated for 30 minutes in PSS heated to 37°C, followed by an additional 30 minute incubation with either PSS alone, DDMS (50 μM), PEG-SOD (100 U/mL), or L-NAME (100 μM). The vessels were then incubated with DHE (5 μmol/L) for an additional 15 minutes. The arteries were cut into 10 μm transverse sections and imaged with a Nikon Eclipse TS100 microscope (Nikon, Tokyo, Japan) equipped with a ×20 objective, a 540 nm excitation filter, a 605 nm emission filter (Chroma Technology Corp., Bellows Falls, VT) and QImaging Regiga-2000R digital camera (Surrey, British Columbia, Canada). Multiple images of each artery were quantified using ImageJ software and background fluorescence was subtracted from the fluorescence value of the arterial ring [13].
Western Blot
CYP4A protein expression in cerebral arteries was assessed by Western blot using CYP4A1/A2/A3 antibody (Santa Cruz, sc-53247), as previously described.[32,48,49] Relative intensity of the bands was quantified and normalized to a loading control (β-actin) using a computer-based densitometer system and Image-Quant software (Molecular Dynamics).
Statistical Analysis
Data were summarized as mean ± SEM. For comparisons of two groups, an unpaired Student’s t-test was used. For all concentration-response curves, differences between multiple groups at each concentration were determined using analysis of variance (ANOVA), and differences between individual means following ANOVA were evaluated using a post hoc Student-Newman-Keuls test. A probability level of P<0.05 was considered to be statistically significant.
RESULTS
Vessel Diameter and Active Tone in SS-5BN Consomic Rats and Dahl SS Rats
Maximum diameter, resting diameter, and active tone in middle cerebral arteries of SS-5BN consomic rats and SS rats are presented in Table 1. Active tone of isolated MCA from SS-5BN consomic rats was unaffected by chronic treatment with either the SOD inhibitor DETC or the SOD mimetic Tempol; and there was no effect of acute addition of either Tempol or the CYP4A inhibitor DDMS to the tissue bath. Collectively, these findings demonstrate that any differences in the magnitude of vascular relaxation responses observed in the present study were not due to initial differences in resting tone as a result of a pre-existing constriction of the artery or structural differences between treatment groups.
Responses to Acetylcholine in Middle Cerebral Arteries of Dahl SS and SS-5BN Consomic Rats
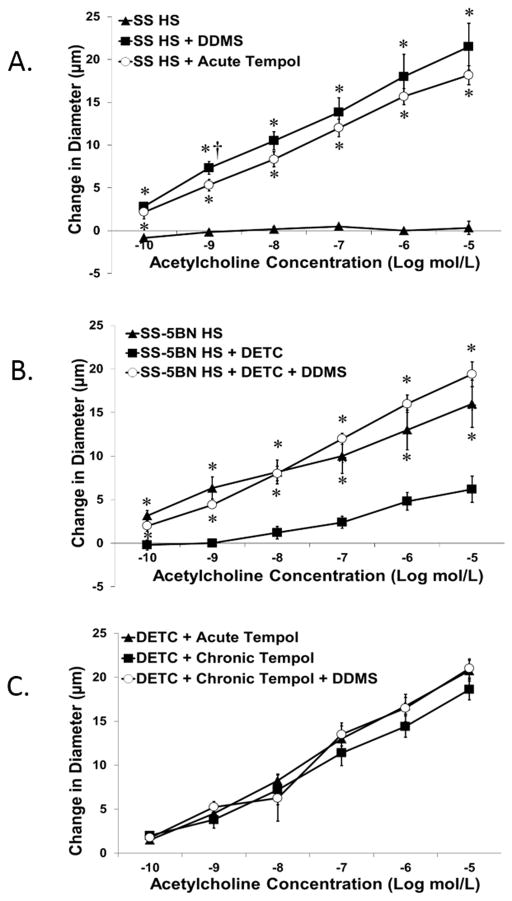
Figure 1 summarizes vascular relaxation in response to the endothelium-dependent vasodilator acetylcholine (ACh) in MCA exhibiting myogenic tone from Dahl SS and SS-5BN consomic rats. MCA from HS-fed Dahl SS rats failed to dilate to ACh in control conditions. Vascular relaxation to ACh in MCA of SS rats was restored in the presence of either the CYP4A inhibitor DDMS or the superoxide scavenger Tempol (Figure 1A).
Figure 1.
Arteries from HS-fed SS-5BN consomic rats dilated to ACh in control conditions, but ACh-induced dilation was significantly reduced when oxidative stress was artificially elevated by chronic infusion of the SOD inhibitor DETC (Figure 1B). Incubating arteries from DETC-treated SS-5BN rats with the CYP4A inhibitor DDMS restored ACh-induced dilation in those animals. Similar to HS-fed Dahl SS rats, ACh-induced relaxation of middle cerebral arteries from DETC-treated SS-5BN rats was restored either by acute incubation with Tempol, or chronic administration of Tempol in the drinking water. Addition of DDMS to the tissue bath in the presence of Tempol showed no additive effect (Figure 1C).
Vascular Oxidative Stress in Cerebral Arteries of Dahl SS Rats
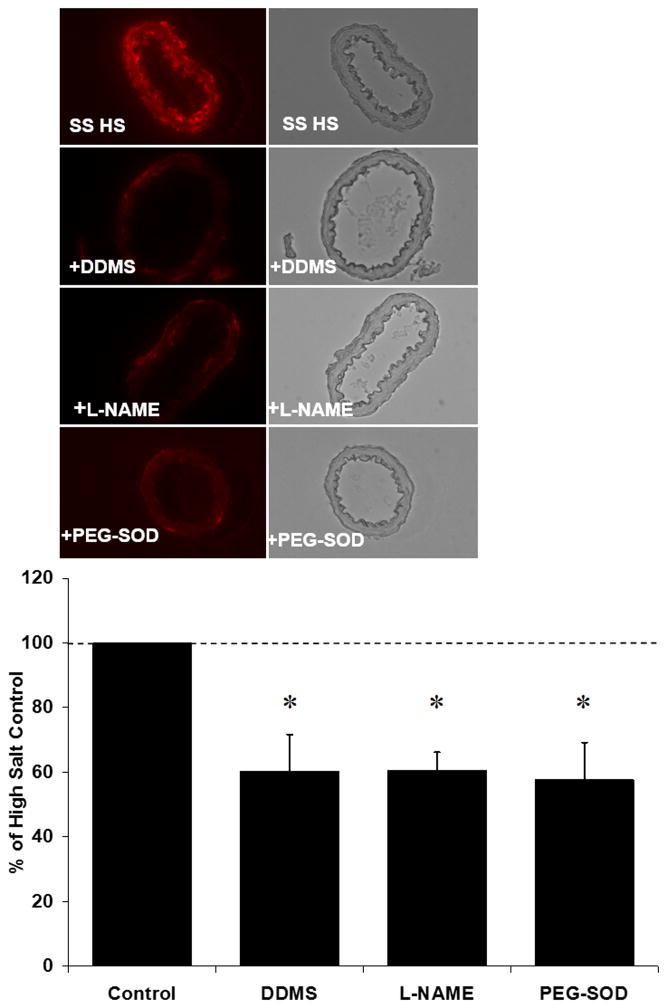
Figure 2 summarizes the ROS levels estimated semi-quantitatively via DHE fluorescence in basilar arteries from HS-fed Dahl SS rats treated with DDMS, L-NAME, or PEG-SOD. In those experiments, both DDMS and L-NAME reduced vascular ROS levels to the same degree as PEG-SOD.
Figure 2.
Vascular Oxidative Stress in Cerebral Arteries from SS-5BN Consomic Rats
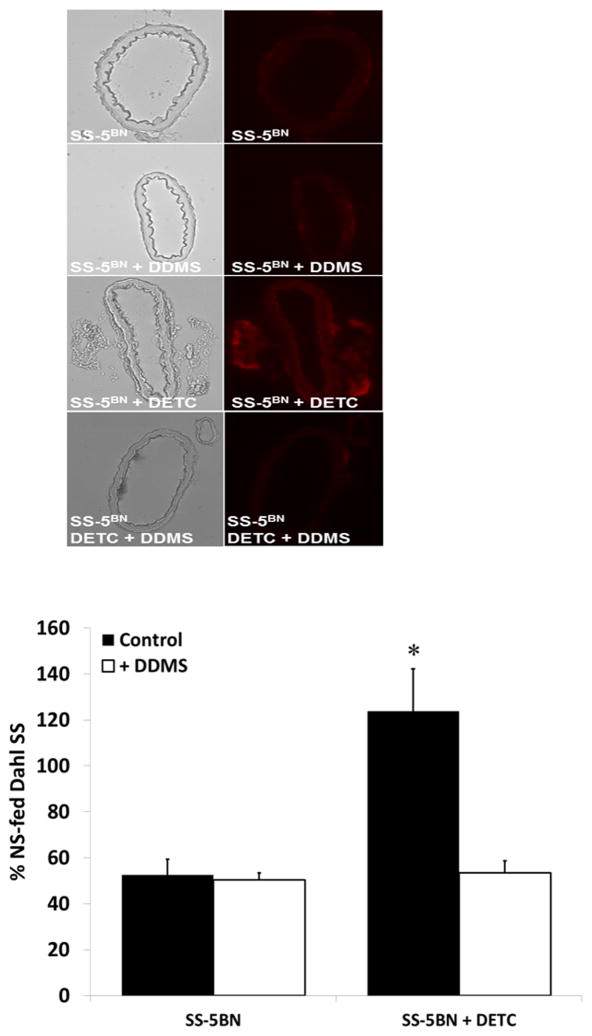
ROS levels in basilar arteries from HS-fed SS-5BN consomic rats incubated with the CYP4A inhibitor DDMS were not significantly different from those in non-treated arteries (Figure 3), confirming that levels of ROS in these arteries were not significantly affected by the CYP4A/20-HETE pathway. Chronic treatment with the SOD inhibitor DETC elevated vascular ROS levels compared to untreated SS-5BN consomic rats. Importantly, incubation of basilar arteries from the DETC-treated SS-5BN consomic rats with DDMS restored vascular ROS levels to control values.
Figure 3.
CYP4A Enzyme Expression in Cerebral Arteries from Dahl SS and SS-5BN Consomic Rats
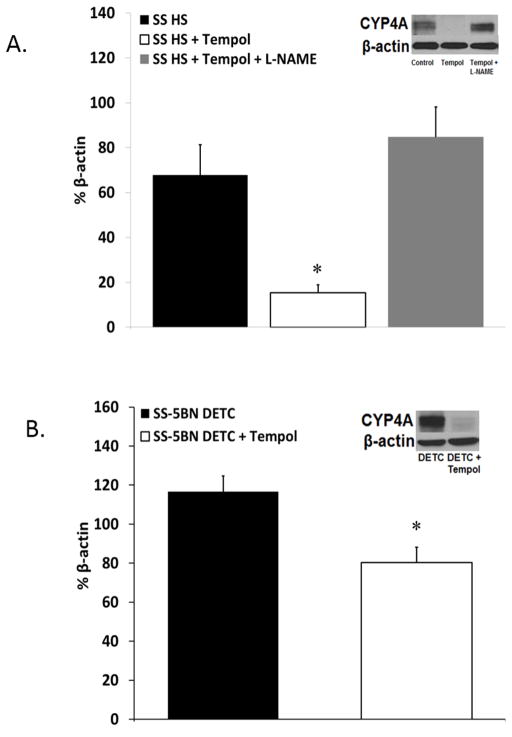
We have previously shown that CYP4A enzyme expression is lower in SS-5BN rats fed either NS or HS diet compared to SS rats on a similar diet.[35] Figure 4A summarizes the expression of CYP4A enzyme protein in cerebral arteries from HS-fed Dahl SS rats in control conditions, during chronic treatment with the SOD mimetic Tempol, or chronic co-treatment with Tempol and L-NAME. Treatment with Tempol significantly reduced CYP4A protein expression in arteries of Dahl SS rats. Co-treatment with L-NAME + Tempol prevented the reduction in CYP4A protein expression in SS rats. In DETC-treated SS-5BN consomic rats in which ROS levels were artificially elevated by the SOD inhibitor, chronic treatment with Tempol to scavenge superoxide reduced the expression of CYP4A enzyme proteins compared to animals that did not receive Tempol (Figure 4B).
Figure 4.
DISCUSSION
Reactive oxygen species and the subgroup of oxygen-derived free radicals that includes superoxide anion and hydroxyl radical, play a key role in the pathogenesis of hypertension.[2,28] Multiple animal models of salt-sensitive hypertension [Dahl SS, stroke prone spontaneously hypertensive rat, and mineralocorticoid hypertension], all share in common elevated levels of superoxide anion in either the vasculature or the kidney.[38,47] Dahl SS rats have elevated renal [38,47] and vascular [13,44] superoxide production, and also exhibit reduced renal medullary Cu/Zn-SOD and Mn-SOD expression[38] and reduced cerebral artery Cu/Zn-SOD expression.[13] Other studies [35, 36] have shown that Dahl SS rats are vulnerable to the development of vascular dysfunction due to their limited antioxidant defense mechanisms. In those studies, vascular function was restored when Cu/Zn SOD expression was upregulated in response to either chronic infusion of a low dose of angiotensin II [14] or by introgression of a normally functioning Brown Norway renin gene into the SS genetic background. [13]
A major component of the deleterious effects of ROS within the vasculature is the reaction between nitric oxide and superoxide anion, which not only reduces the bioavailability of nitric oxide, but also produces the highly reactive nitrogen species, peroxynitrite [17]. Peroxynitrite directly affects vascular tone by inhibiting calcium-activated potassium channels [3] and alters normal cellular signaling by nitration of tyrosine and tryptophan residues [1,30]. Peroxynitrite is also a significant contributor to oxidative stress via eNOS uncoupling due to oxidation of the necessary cofactor tetrahydrobiopterin (BH4) and destruction of the heme/heme center of eNOS [6].
Previous work in our laboratory has shown that acute scavenging of superoxide anions with Tempol, which restores nitric oxide bioavailability, restores vasodilator responses to ACh and reduced PO2 in cerebral arteries from SS rats. [12] Chronic treatment with Tempol in the drinking water also restores endothelium-dependent dilation to ACh in arteries of Dahl SS rats, regardless of dietary salt intake.[15] In the present study, chronic scavenging of superoxide radicals with Tempol also ameliorated the impaired relaxation in response to ACh in MCA from HS-fed SS rats, presumably by restoring NO bioavailability in the vasculature, as the protective effect of chronic Tempol treatment to restore acetylcholine-induced dilation in SS rats is eliminated by the NO synthase inhibitor L-NAME[15]. Taken together, these data are consistent with multiple reports that elevated ROS levels are a major contributor to endothelial dysfunction in the Dahl SS rat.
Previous reports have shown that basilar arteries from Dahl SS rats have elevated levels of vascular reactive oxygen species compared to SS-5BN consomic rats [35]. It is interesting to note that vessels from the SS-5BN rat, which shares ~95% genetic homology to the Dahl SS rat, exhibit no differences in expression of superoxide dismutase enzymes (Cu/Zn SOD, MnSOD, or ec-SOD) or endothelial nitric oxide synthase compared to Dahl SS rats, regardless of dietary salt intake.[35] Based on these findings, the reduced antioxidant defenses in Dahl SS rats, leaving them susceptible to vascular assault by ROS, should also be present in the SS-5BN rat. However, the SS-5BN rat demonstrates normal vascular function when fed either a normal salt (0.4% NaCl) or a high salt diet suggesting that, in addition to limited antioxidant defenses, there is an elevated production of ROS in the SS rat compared to the SS-5BN rat.
The CYP4A/20-HETE pathway appears to be a major contributor to elevated vascular oxidant stress in HS-fed SS rats, as arteries from HS-fed Dahl SS rats exhibit a significant reduction in vascular ROS levels following incubation with the CYP4A inhibitor DDMS.[35] Production of 20-HETE has the capacity to increase vascular ROS via two possible mechanisms: 1) by 20-HETE-induced NADPH oxidase activation [27,41,52,54] and 2) through the uncoupling of eNOS [7,8]. As noted above, 20-HETE has been shown to directly uncouple eNOS by interfering with the interaction of eNOS with HSP90. [7]
In the present study, basilar arteries from high salt-fed Dahl SS rats incubated with L-NAME to inhibit eNOS had reduced ROS accumulation to the same degree as vessels treated with either DDMS or the superoxide scavenger PEG-SOD. Based on the nearly identical responses of vascular ROS levels to DDMS and L-NAME treatment, it is reasonable to postulate that the upregulation of the CYP4A/20-HETE pathway that is present in cerebral arteries of Dahl SS rats [35] contributes to oxidative stress via uncoupling of eNOS, as vascular ROS levels were reduced to the same degree by treatment with either L-NAME or DDMS. Taken together, these observations suggest that 20-HETE either directly uncouples eNOS [27,41,52,54] or that the oxidative environment supported by elevated 20-HETE production leads to eNOS uncoupling by another mechanism such as peroxynitrite-induced BH4 oxidation [6].
The normal dilation of the MCA in response to ACh in arteries of HS-fed SS-5BN consomic rats was unexpected, and contrasts with the loss of endothelium-dependent dilation in arteries of HS-fed Sprague-Dawley rats, [45] and HS-fed SS-13BN consomic rats, [11] and cheek pouch arterioles of HS-fed hamsters. [39] One important difference between these findings is that HS diet leads to a significant increase in vascular ROS levels in HS-fed Sprague-Dawley rats [56] and hamster arteries, [39] but not in arteries from HS-fed SS-5BN rats (Figure 3).
In the present study, ROS levels in basilar arteries from HS-fed SS-5BN consomic rats incubated with the CYP4A inhibitor DDMS were not significantly different from those in non-treated arteries (Figure 3), confirming that levels of ROS in these arteries were not significantly affected by the CYP4A/20-HETE pathway. Chronic treatment with the SOD inhibitor DETC elevated vascular ROS levels compared to untreated SS-5BN consomic rats. Importantly, incubation of basilar arteries from the DETC-treated SS-5BN consomic rats with DDMS restored vascular ROS levels to control values, supporting the concept that the CYP4A/20-HETE pathway can contribute to vascular ROS production in the cerebral circulation. Although unlikely, another possible explanation for the latter finding could be a previously unreported effect of the CYP4A inhibitor directly on vascular ROS production. In light these observations, direct measurements of 20-HETE levels in the presence and absence of DDMS would be a valuable direction for future investigation, to exclude any unforeseen off target effects of DDMS.
Taken together, the findings of the present study support the hypothesis that the lower ROS levels in the HS-fed SS-5BN rats are crucial to maintaining the normal endothelium-dependent relaxation of the MCA in response to ACh in these animals. Specifically, with lower superoxide levels to combine with and degrade nitric oxide, there would be improved NO bioavailability to respond appropriately to stimulation by ACh in the SS-5BN vasculature. While it is tempting to speculate that the lower ROS levels and maintained endothelial function in the HS-fed SS-5BN rats are due to the presence of the Brown Norway CYP4A alleles on chromosome 5, it is important to note that the genes coding for CYP4A alleles on chromosome 5 are not the only BN genes that may differ from those in the SS rat, which could impact the findings of the present study. Therefore, a valuable target for future investigation is to develop narrowed congenic strains that contain smaller and smaller segments of the BN chromosome 5 that either include or exclude the BN CYP4A alleles, similar to previously reported studies isolating the protective effects chromosome 13 to the presence of a normally functioning BN renin allele in narrowed congenic strains derived from SS-13BN consomic rats.[15]
Chronic treatment with DETC to inhibit superoxide dismutase artificially elevates reactive oxygen species in the SS-5BN rats (Figure 3), allowing the accumulation of superoxide to go unabated by antioxidant defense mechanisms, which would result in superoxide-induced degradation of nitric oxide. Consistent with this hypothesis, MCA from DETC-treated SS-5BN consomic rats failed to dilate in response to ACh under control conditions. However, both acute and chronic treatment with Tempol to scavenge superoxide restored vascular relaxation in response to ACh in DETC-treated SS-5BN consomic rats. Collectively, these observations support the fundamental hypothesis of the present study, namely that, under conditions of reduced antioxidant defense mechanisms (i.e. in the Dahl SS rat), any oxidant-producing pathway contributes to the loss of vascular function. Therefore, inhibiting the CYP4A/20-HETE pathway and reducing vascular oxidative stress would restore NO-dependent dilation to ACh in arteries of the SS rats.
Previous studies in our laboratory [34] [35] have shown that ROS levels, assessed with DHE, are significantly lower in cerebral arteries of SS-5BN rats fed normal salt diet compared to those in SS rats fed normal salt diet. Those studies also showed that the expression of CYP4A enzyme protein, assessed by Western blotting, was also significantly higher in cerebral arteries of SS rats compared SS-5BN consomic rats fed either normal or high salt diet [34] [35].
In the present study, chronic antioxidant treatment with Tempol significantly reduced CYP4A protein levels in cerebral arteries from HS-fed Dahl SS rats and HS-fed SS-5BN rats treated with the SOD inhibitor DETC to artificially elevate superoxide levels compared to arteries from control animals. Co-treatment with L-NAME + Tempol prevented the reduction in CYP4A protein expression in SS rats, suggesting that the effect of Tempol to reduce the expression of CYP4A enzyme proteins is mediated via restoration of nitric oxide bioavailability in the presence of the superoxide scavenger. In DETC-treated SS-5BN consomic rats in which ROS levels were artificially elevated by the SOD inhibitor, chronic treatment with Tempol to scavenge superoxide also reduced the expression of CYP4A enzyme proteins in SS rats compared to animals that did not receive Tempol (Figure 4B), again supporting a role for ROS-dependent increases in vascular CYP4A protein expression. To our knowledge, this is the first demonstration of a regulatory effect of reactive oxygen species on the expression of CYP4A enzyme proteins.
The present findings are in contrast to reports from studies of isolated vascular smooth muscle cells [25] and renal microsomes,[29] showing that acute Tempol treatment has no effect on 20-HETE production and that acute addition of high concentrations of a superoxide donor reduces 20-HETE production. However, in those studies, the acute administration of either Tempol or the superoxide donor would not allow sufficient time to alter protein expression, as was observed in the present study; and, the use of isolated cells and prepared microsomes may not accurately reflect in vivo conditions. In addition, the concentration of superoxide used in those studies was likely high enough to scavenge 20-HETE via lipid peroxidation to form the more polar isoprostane [29], and to reduce the bioavailability of necessary co-factors through oxidation, which would substantially reduce 20-HETE production as well [10,26,52].
We previously reported that it is possible for reactive oxygen species to exert a regulatory influence over the CYP4A/20-HETE pathway--not directly, but as an indirect result of the effect of superoxide on nitric oxide [35]. Superoxide anion can affect basal levels of nitric oxide because O2− reacts with NO at a rate three times faster than its interaction with SOD.[18] In the Dahl SS rat, NO availability in the vasculature is reduced due to the elevated vascular ROS. [12,15] Nitric oxide inhibits CYP4A activity by forming a ferrous-nitrosyl complex at the heme binding site of the CYP4A enzyme, presumably reducing 20-HETE production.[43] Studies in other laboratories have shown that nitric oxide also has the capacity to suppress both mRNA and protein expression of cytochrome P450 enzymes in cultured hepatocytes and in liver microsomes, [19,31] The present studies support a role for NO in regulating not only the activity of the CYP4A enzymes, but also the expression of CYP4A protein in the vasculature.
In arteries of HS-fed Dahl SS rats, the inhibitory influence of NO on the CYP4A/20-HETE pathway would be absent due to the ROS-induced degradation of nitric oxide, providing a possible explanation for the elevated vascular CYP4A protein expression in arteries of Dahl SS rats compared to those of SS-5BN consomic rats.[35] Cerebral arteries from HS-fed Dahl SS rats chronically treated with a combination of Tempol to scavenge superoxide and the NOS inhibitor L-NAME to prevent the Tempol-induced restoration of NO bioavailability had elevated CYP4A protein levels similar to those of the control animals (Figure 4). These data provide compelling evidence that the ROS-induced CYP4A upregulation results indirectly from the loss of NO bioavailability and, therefore, removal of NO-induced CYP4A inhibition. This novel finding of ROS-induced regulation of CYP4A protein expression occurred in both the Dahl SS rat and in the SS-5BN strain, supporting our hypothesis that oxidative stress directly contributes to the upregulation of the CYP4A/20-HETE pathway.
A situation where reactive oxygen species stimulate a ROS-producing pathway (the CYP4A/20-HETE pathway) sets the stage for a potentially catastrophic exacerbation of ROS accumulation, especially in the presence of limited antioxidant defenses, as present in the Dahl SS rat.[13] Taking into consideration the necessity of ROS signaling for normal cell survival,[26] it is clear that a reduction in antioxidant defense mechanisms could result in the pathological oxidant environments observed in hypertensive individuals and patients with many other cardiovascular diseases[4,28]. A positive feedback loop involving the CYP4A/20-HETE pathway provides one possible explanation for the spiraling effects of increased ROS production into an advanced disease state, such as hypertension, atherosclerosis, and other cardiovascular diseases characterized by endothelial dysfunction.
Perspectives
This study used a novel consomic rat strain (the SS-5BN consomic rat) which is identical to the Dahl salt-sensitive (SS) rat genetically except for the introgression of Brown Norway chromosome 5 carrying the BN cytochrome P450-4A (CYP4A) alleles into the Dahl SS genetic background. The results of this study demonstrate not only a role for the CYP4A/20-HETE pathway in producing reactive oxygen species (ROS) (as CYP4A inhibition reduced vascular oxidative stress); but also a regulatory influence of vascular free radicals on CYP4A protein expression—most likely as a result of reduced nitric oxide bioavailability (as manipulation of local ROS levels altered CYP4A protein expression and the beneficial effect of CYP4A enzyme inhibition on vascular CYP4A enzyme expression was prevented by inhibiting NOS with L-NAME). This potential positive feedback loop between increased CYP4A enzyme expression/activity, elevated vascular ROS, reduced NO availability, and elimination of NO-mediated downregulation of the CYP4A/20-HETE system could play a major role in the exacerbation of cardiovascular diseases characterized by elevated levels of vascular oxidant stress in humans.
Acknowledgments
The authors thank Lynn Dondlinger for her outstanding technical and administrative assistance.
Funding: NIH #R01-HL065289; #R56-HL065289; R01-HL72920; and HL092026 (to JHL), and #Robert A. Welch Foundation (I-0011) and NIH #HL111392 (to JRF).
Abbreviations
- ACh
Acetylcholine
- ANOVA
Analysis of Variance
- BH4
Tetrahydrobiopterin
- BN
Brown Norway
- Cu/Zn-SOD
Copper/Zinc Superoxide Dismutase (SOD 1)
- CYP
Cytochrome P450
- CYP4A
Cytochrome P450-4A ω-Hydroxylase
- DDMS
N-methyl-sulfonyl-12,12-dibromododec-11-enamide
- DETC
Diethyldithiocarbamate
- DHE
Dihydroethidium
- eNOS
Endothelial Nitric Oxide Synthase (NOS 3)
- HSP90
Heat Shock Protein 90
- L-NAME
NG-nitro-l-arginine methyl ester
- Mn-SOD
Manganese Superoxide Dismutase (SOD 2)
- PEG-SOD
Polyethylene Glycol Superoxide Dismutase
- ROS
Reactive Oxygen Species
- SOD
Superoxide Dismutase
- SS rats
Dahl Salt-Sensitive Rats
- SS-5BN
SS-5BN Consomic Rats (SS-Chr 5BN/Mcwi strain)
- SS-13BN
SS-13BN Consomic Rats
- 20-HETE
20-Hydroxyeicosatetraenoic acid
Footnotes
Declarations of Interest: The authors have no declarations of interest to disclose.
Author Contributions:
KML: Performed experiments, analyzed data, wrote primary draft of manuscript, edited and revised the manuscript, approved final version of manuscript.
JRF: Provided conceptual guidance for CYP4A/20-HETE inhibitors and antagonists; synthesized and provided cytochrome CYP4A inhibitors and antagonists, approved final version of manuscript.
MPP: Synthesized cytochrome CYP4A inhibitors and antagonists; approved final version of manuscript.
JHL: Directed experiments, evaluated data, edited and revised the manuscript, approved final version of manuscript.
References
- 1.Alvarez B, Rubbo H, Kirk M, Barnes S, Freeman BA, Radi R. Peroxynitrite-dependent tryptophan nitration. Chemical Research in Toxicology. 1996;9:390–396. doi: 10.1021/tx950133b. [DOI] [PubMed] [Google Scholar]
- 2.Bayorh MA, Ganafa AA, Socci RR, Silvestrov N, Abukhalaf IK. The role of oxidative stress in salt-induced hypertension. American Journal of Hypertension. 2004;17:31–36. doi: 10.1016/j.amjhyper.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Brzezinska AK, Gebremedhin D, Chilian WM, Kalyanaraman B, Elliott SJ. Peroxynitrite reversibly inhibits Ca2+-activated K+ channels in rat cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;278:H1883–1890. doi: 10.1152/ajpheart.2000.278.6.H1883. [DOI] [PubMed] [Google Scholar]
- 4.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circulation Research. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 5.Capdevila J, Parkhill L, Chacos N, Okita R, Masters BS, Estabrook RW. The oxidative metabolism of arachidonic acid by purified cytochromes P-450. Biochemical and Biophysical Research Communications. 1981;101:1357–1363. doi: 10.1016/0006-291x(81)91597-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Druhan LJ, Chen CA, Hemann C, Chen YR, Berka V, Tsai AL, Zweier JL. Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: transition from reversible to irreversible enzyme inhibition. Biochemistry. 2010;49:3129–3137. doi: 10.1021/bi9016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, Schwartzman ML. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294:H1018–1026. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 8.Cheng J, Wu CC, Gotlinger KH, Zhang F, Falck JR, Narsimhaswamy D, Schwartzman ML. 20-hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IkappaB kinase-dependent endothelial nitric-oxide synthase uncoupling. The Journal of Pharmacology and Experimental Therapeutics. 2010;332:57–65. doi: 10.1124/jpet.109.159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosentino F, Sill JC, Katusic ZS. Role of superoxide anions in the mediation of endothelium-dependent contractions. Hypertension. 1994;23:229–235. doi: 10.1161/01.hyp.23.2.229. [DOI] [PubMed] [Google Scholar]
- 10.Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. The Biochemical Journal. 1997;324( Pt 1):1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drenjancevic-Peric I, Lombard JH. Introgression of chromosome 13 in Dahl salt-sensitive genetic background restores cerebral vascular relaxation. Am J Physiol Heart Circ Physiol. 2004;287:H957–962. doi: 10.1152/ajpheart.01087.2003. [DOI] [PubMed] [Google Scholar]
- 12.Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension. 2005;45:687–691. doi: 10.1161/01.HYP.0000154684.40599.03. [DOI] [PubMed] [Google Scholar]
- 13.Durand MJ, Lombard JH. Introgression of the Brown Norway renin allele onto the Dahl salt-sensitive genetic background increases Cu/Zn SOD expression in cerebral arteries. American Journal of Hypertension. 2011;24:563–568. doi: 10.1038/ajh.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durand MJ, Lombard JH. Low-dose angiotensin II infusion restores vascular function in cerebral arteries of high salt-fed rats by increasing copper/zinc superoxide dimutase expression. Am J Hypertens. 2013;26:739–747. doi: 10.1093/ajh/hpt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durand MJ, Moreno C, Greene AS, Lombard JH. Impaired relaxation of cerebral arteries in the absence of elevated salt intake in normotensive congenic rats carrying the Dahl salt-sensitive renin gene. Am J Physiol Heart Circ Physiol. 2010;299:H1865–H1874. doi: 10.1152/ajpheart.00700.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faraci FM. Oxidative stress: the curse that underlies cerebral vascular dysfunction? Stroke. 2005;36:186–188. doi: 10.1161/01.STR.0000153067.27288.8b. [DOI] [PubMed] [Google Scholar]
- 17.Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. Journal of Applied Physiology. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- 18.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari L, Peng N, Halpert JR, Morgan ET. Role of nitric oxide in down-regulation of CYP2B1 protein, but not RNA, in primary cultures of rat hepatocytes. Mol Pharmacol. 2001;60:209–216. doi: 10.1124/mol.60.1.209. [DOI] [PubMed] [Google Scholar]
- 20.Fredricks KT, Liu Y, Lombard JH. Response of extraparenchymal resistance arteries of rat skeletal muscle to reduced PO2. Am J Physiol Heart Circ Physiol. 1994;267:H706–715. doi: 10.1152/ajpheart.1994.267.2.H706. [DOI] [PubMed] [Google Scholar]
- 21.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Laboratory Investigation. 1982;47:412–426. [PubMed] [Google Scholar]
- 22.Frisbee JC, Falck JR, Lombard JH. Contribution of cytochrome P-450 omega-hydroxylase to altered arteriolar reactivity with high-salt diet and hypertension. Am J Physiol Heart Circ Physiol. 2000;278:H1517–1526. doi: 10.1152/ajpheart.2000.278.5.H1517. [DOI] [PubMed] [Google Scholar]
- 23.Frisbee JC, Roman RJ, Falck JR, Krishna UM, Lombard JH. 20-HETE contributes to myogenic activation of skeletal muscle resistance arteries in Brown Norway and Sprague-Dawley rats. Microcirculation. 2001;8:45–55. [PubMed] [Google Scholar]
- 24.Frisbee JC, Roman RJ, Krishna UM, Falck JR, Lombard JH. 20-HETE modulates myogenic response of skeletal muscle resistance arteries from hypertensive Dahl-SS rats. Am J Physiol Heart Circ Physiol. 2001;280:H1066–1074. doi: 10.1152/ajpheart.2001.280.3.H1066. [DOI] [PubMed] [Google Scholar]
- 25.Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507( Pt 3):771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 27.Hama-Tomioka K, Kinoshita H, Azma T, Nakahata K, Matsuda N, Hatakeyama N, Kikuchi H, Hatano Y. The role of 20-hydroxyeicosatetraenoic acid in cerebral arteriolar constriction and the inhibitory effect of propofol. Anesthesia and Analgesia. 2009;109:1935–1942. doi: 10.1213/ANE.0b013e3181bd1ebe. [DOI] [PubMed] [Google Scholar]
- 28.Harrison DG, Gongora MC. Oxidative stress and hypertension. The Medical Clinics of North America. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Hoagland KM, Maier KG, Roman RJ. Contributions of 20-HETE to the antihypertensive effects of Tempol in Dahl salt-sensitive rats. Hypertension. 2003;41:697–702. doi: 10.1161/01.HYP.0000047881.15426.DC. [DOI] [PubMed] [Google Scholar]
- 30.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Archives of Biochemistry and Biophysics. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 31.Khatsenko OG, Boobis AR, Gross SS. Evidence for nitric oxide participation in down-regulation of CYP2B1/2 gene expression at the pretranslational level. Toxicology Letters. 1997;90:207–216. doi: 10.1016/s0378-4274(96)03857-x. [DOI] [PubMed] [Google Scholar]
- 32.Kunert MP, Roman RJ, Alonso-Galicia M, Falck JR, Lombard JH. Cytochrome P-450 omega-hydroxylase: a potential O2 sensor in rat arterioles and skeletal muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H1840–1845. doi: 10.1152/ajpheart.2001.280.4.H1840. [DOI] [PubMed] [Google Scholar]
- 33.Kunert MP, Roman RJ, Falck JR, Lombard JH. Differential effect of cytochrome P-450 omega-hydroxylase inhibition on O2-induced constriction of arterioles in SHR with early and established hypertension. Microcirculation. 2001;8:435–443. doi: 10.1038/sj/mn/7800114. [DOI] [PubMed] [Google Scholar]
- 34.Lukaszewicz KM, Lombard JH. Role of the CYP4A/20-HETE pathway in vascular dysfunction in the Dahl salt-sensitive rat. Clinical Science. 2013;124:695–700. doi: 10.1042/CS20120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukaszewicz KM, Falck JR, Manthati VL, Lombard JH. Introgression of Brown Norway CYP4A genes on to the Dahl salt-sensitive background restores vascular function in SS-5(BN) consomic rats. Clinical Science. 2013;124:333–342. doi: 10.1042/CS20120232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW., Jr Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol. 2008;295:F837–842. doi: 10.1152/ajprenal.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayhan WG. Impairment of endothelium-dependent dilatation of basilar artery during chronic hypertension. Am J Physiol Heart Circ Physiol. 1990;259:H1455–1462. doi: 10.1152/ajpheart.1990.259.5.H1455. [DOI] [PubMed] [Google Scholar]
- 38.Meng S, Roberts LJ, 2nd, Cason GW, Curry TS, Manning RD., Jr Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am J Physiol Regulatory, Integrative and Comparative Physiology. 2002;283:R732–738. doi: 10.1152/ajpregu.00346.2001. [DOI] [PubMed] [Google Scholar]
- 39.Priestley JR, Buelow MW, McEwen ST, Weinberg BD, Delaney M, Balus SF, Hoeppner C, Dondlinger L, Lombard JH. Reduced angiotensin II levels cause generalized vascular dysfunction via oxidant stress in hamster cheek pouch arterioles. Microvasc Res. 2013;89:134–145. doi: 10.1016/j.mvr.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circulation Research. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- 41.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 42.Somova LI, Nadar A, Gregory M, Khan N. Antioxidant status of the hypertrophic heart of Dahl hypertensive rat as a model for evaluation of antioxidants. Methods and Findings in Experimental and Clinical Pharmacology. 2001;23:5–12. doi: 10.1358/mf.2001.23.1.619173. [DOI] [PubMed] [Google Scholar]
- 43.Sun CW, Alonso-Galicia M, Taheri MR, Falck JR, Harder DR, Roman RJ. Nitric oxide-20-hydroxyeicosatetraenoic acid interaction in the regulation of K+ channel activity and vascular tone in renal arterioles. Circulation Research. 1998;83:1069–1079. doi: 10.1161/01.res.83.11.1069. [DOI] [PubMed] [Google Scholar]
- 44.Swei A, Lacy F, DeLano FA, Schmid-Schonbein GW. Oxidative stress in the Dahl hypertensive rat. Hypertension. 1997;30:1628–1633. doi: 10.1161/01.hyp.30.6.1628. [DOI] [PubMed] [Google Scholar]
- 45.Sylvester FA, Stepp DW, Frisbee JC, Lombard JH. High-salt diet depresses acetylcholine reactivity proximal to NOS activation in cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283:H353–363. doi: 10.1152/ajpheart.00127.2002. [DOI] [PubMed] [Google Scholar]
- 46.Touyz RM, Schiffrin EL. Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension. 1999;34:976–982. doi: 10.1161/01.hyp.34.4.976. [DOI] [PubMed] [Google Scholar]
- 47.Trolliet MR, Rudd MA, Loscalzo J. Oxidative stress and renal dysfunction in salt-sensitive hypertension. Kidney and Blood Pressure Research. 2001;24:116–123. doi: 10.1159/000054217. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Maier KG, Roman RJ, De La Cruz L, Zhu J, Henderson L, Lombard JH. Expression of cytochrome P450-4A isoforms in the rat cremaster muscle microcirculation. Microcirculation. 2004;11:89–96. doi: 10.1080/10739680490266225. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Roman RJ, Falck JR, de la Cruz L, Lombard JH. Effects of high-salt diet on CYP450-4A omega-hydroxylase expression and active tone in mesenteric resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1557–1565. doi: 10.1152/ajpheart.00755.2004. [DOI] [PubMed] [Google Scholar]
- 50.Ward NC, Chen K, Li C, Croft KD, Keaney JF., Jr Chronic activation of AMP-activated protein kinase prevents 20-hydroxyeicosatetraenoic acid-induced endothelial dysfunction. Clinical and Experimental Pharmacology and Physiology. 2011;38:328–333. doi: 10.1111/j.1440-1681.2011.05509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams JM, Fan F, Murphy S, Schreck C, Lazar J, Jacob HJ, Roman RJ. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1209–1218. doi: 10.1152/ajpregu.00604.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolin MS. Interactions of oxidants with vascular signaling systems. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- 53.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 54.Zeng Q, Han Y, Bao Y, Li W, Li X, Shen X, Wang X, Yao F, O’Rourke ST, Sun C. 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1109–1117. doi: 10.1152/ajpheart.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol Heart Circ Physiol. 2006;291:H929–938. doi: 10.1152/ajpheart.00692.2005. [DOI] [PubMed] [Google Scholar]
- 56.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res. 2007;44:382–390. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]