Abstract
Background
Synthetic human ghrelin accelerates gastric emptying, reduces gastric accommodation, and results in numerical increases in postprandial symptom scores. The ghrelin receptor agonist, relamorelin, accelerates gastric emptying in patients with diabetic gastroparesis.
Aim
To measure pharmacological effects of relamorelin on gastric accommodation, distal antral motility, and satiation in healthy volunteers.
Methods
In a placebo-controlled, double-blind, randomized study of 16 healthy volunteers, we compared effects of 30µg subcutaneous (SQ) relamorelin to placebo on: 1) gastric volumes measured by SPECT, 2) 1-hour postprandial distal antral motility index (MI) by 15-lumen perfusion gastroduodenal manometry, and 3) satiation tested by Ensure nutrient drink test. Primary endpoints were: fasting and postprandial gastric volumes, distal antral phasic pressure activity (number of contractions, mean amplitude, and MI), and maximum tolerated volume. Results were normally distributed and the two treatment groups were compared using t-test.
Key Results
Relamorelin, 30µg SQ, significantly increased the number of contractions in the distal antrum during 0–60 minutes post-meal when compared to placebo (p=0.022); this was also observed in the first two 15minute periods (p=0.005 and 0.015 for number of contractions 0–15 and 16–30). There was borderline increase in MI0–15 (p=0.055) and numerically increased MI0–60 (p=0.139) and MI16–30 (p=0.116). The amplitude of contractions was not significantly increased. Relamorelin did not significantly alter fasting or postprandial gastric volumes, gastric accommodation, or satiation volumes and symptoms.
Conclusions & Inferences
Relamorelin increases frequency of distal antral motility contractions without significant effects on amplitude of contractions. The lack of inhibition of accommodation and absence of increase in satiation symptoms support relamorelin for the treatment of symptomatic gastroparesis (ClinicalTrials.gov NCT02466711).
Keywords: ghrelin receptor agonist, gastroparesis, gastric motility, gastric accommodation, satiation
Abbreviated abstract
In 16 healthy volunteers, we compared effects of 30µg subcutaneous (SQ) relamorelin to placebo on: 1) gastric volumes measured by SPECT, 2) 1-hour postprandial distal antral motility index (MI) by 15-lumen perfusion gastroduodenal manometry, and 3) satiation tested by Ensure nutrient drink test. Relamorelin, 30µg SQ, significantly increased the number of contractions in the distal antrum during 0–60 minutes post-meal when compared to placebo (p=0.022); this was also observed in the first two 15 minute periods (p=0.005 and 0.015 for number of contractions 0–15 and 16–30). Relamorelin increases frequency of distal antral motility contractions without significant effects on amplitude of contractions. The lack of inhibition of accommodation and absence of increase in satiation symptoms support relamorelin for the treatment of symptomatic gastroparesis.
INTRODUCTION
Disorders of gastrointestinal motility such as gastroparesis, post-operative ileus, and chronic constipation are significant sources of morbidity. Development of effective pharmacologic therapies for such disorders is desirable, as these conditions represent a significant unmet medical need.
Ghrelin is a 28-amino acid peptide produced in the stomach. It has been recognized as an endogenous ligand for the growth hormone secretagogue receptor in the oxyntic glands of the stomach (1). In rodents, centrally or peripherally administered ghrelin stimulated gastric contraction and emptying (2) and showed prokinetic effects in a post-operative ileus model in rats (3). Gastrokinetic properties of ghrelin in rats may be mediated by vagal pathways (4). Administration of ghrelin has been shown to promote gastric motility in mice, rats, dogs and humans(3, 5, 6).
Pharmacological doses of synthetic human ghrelin induced premature onset of phase III of the migrating motor complex and increased proximal gastric tone in healthy volunteers (7). On the other hand, intravenous ghrelin at doses that produced physiological levels of circulating growth hormone resulted in only a slight reduction in fasting gastric volumes and no significant effect on the gastric emptying of solids or gastric accommodation volumes (8). Several studies in the literature have documented the pharmacological stimulation of gastric emptying by ghrelin (reviewed elsewhere) (9).
More recent studies have reported acceleration of gastric emptying, but there was concomitant reduction in gastric accommodation and numerical increases in postprandial symptom scores (10). If there was increase in postprandial symptoms along with a decrease in gastric accommodation, ghrelin receptor agonists might be less effective in clinical practice. The pentapeptide ghrelin agonist, relamorelin, has similar characteristics to native ghrelin, but with improved stability, a longer plasma half-life and greater potency than ghrelin in reversing gastric ileus in animal models. Its pharmacological characteristics have been reported elsewhere (11, 12). Relamorelin accelerates gastric emptying in patients with type 1 and 2 diabetes mellitus in whom there was evidence in the prior medical records of delayed gastric emptying (13, 14). Relamorelin was also efficacious in accelerating gastric emptying in patients with diabetic gastroparesis (15). The dose of relamorelin tested in prior studies ranged from 10µg once daily to 10µg twice daily (b.i.d.) (15) to a 100µg single dose (13, 14). The clinical trial data suggested that the 20 µg total daily dose was more efficacious than the 10 µg dose. In other studies, we have shown that the 100µg dose of relamorelin causes increased frequency of colonic HAPC (16) and also increased the number of spontaneous bowel movements, and accelerated the time to first bowel movement after the first dose was given compared with placebo (17). In the current study, we elected to test a 30µg dose, which is a half-log lower than the dose that we had previously shown to significantly accelerate gastric emptying (13, 14). Our objective was to appraise a dose that would be potentially applicable in the treatment of gastroparesis. Thus, given the prior observation that the 100 µg dose causes a >50% reduction in median gastric emptying T1/2 for solids in type 1 or 2 diabetics (13, 14), and the fact that the 100 µg dose may cause bowel dysfunction, we selected 30µg relamorelin as a relevant dose to appraise the pharmacological effects of the drug, as it is likely to be close to the dose selected for clinical application. Indeed, the current multicenter clinical trial of relamorelin in the treatment for gastroparesis explores effects of three BID doses: 10, 30 and 100 µg for 12 weeks (ClinicalTrials.gov Identifier: NCT02357420; https://clinicaltrials.gov/ct2/show/NCT02357420). Since our study explored effects of relamorelin over a period of <4 hours on each of the treatment days, we were interested in understanding the acute effects of a single dose of 30 µg.
The effects of relamorelin on postprandial pressure activity of the stomach are unknown. In addition, the effects on gastric accommodation and satiation of doses that stimulate distal gastric motility are relevant to appraise the potential risk to increase postprandial symptoms; this requires clarification, given the observation that postprandial symptom scores of fullness, bloating, satiety and cramps increase at least 30% on average with doses of ghrelin that reduce gastric accommodation (10).
Our aim was to measure the pharmacological effects of relamorelin on gastric accommodation, distal antral motility, and satiation in healthy volunteers.
MATERIALS AND METHODS
Study Design
We conducted a placebo-controlled, single-dose administration, double-blinded, parallel-group design, randomized to either the study drug or matching placebo study of fasting gastric volume, gastric accommodation and distal antral motility index in 16 healthy volunteers. The 3 tests were performed in random sequence. The minimum interval to complete all 3 tests is within a 2-week time period from the day of the first test.
Participants
We studied 16 healthy, male or female participants, aged 18–65 years, with body mass index of 18–35kg/m2. All participants completed a validated bowel disease questionnaire to screen for bowel symptoms (18). Female subjects were required to have negative urine pregnancy test (unless there was record of tubal ligation, hysterectomy, or post-menopausal state) and could not be lactating prior to receiving study medication or the radiation exposure.
The study protocol was submitted to and approved by Mayo Clinic Institutional Review Board.
Exclusion criteria were: prior diagnosis of gastrointestinal diseases, structural or metabolic diseases that affect the gastrointestinal system, use of excessive alcohol or substance abuse, or use of medications that alter gastrointestinal transit including laxatives, magnesium and aluminum containing antacids, prokinetics, erythromycin, and analgesic drugs.
Study Medications – Relamorelin and Placebo
All study drug (including matching placebo) was supplied by Rhythm Pharmaceuticals (Boston, Massachusetts, USA). Relamorelin was formulated as an isotonic solution in 4.5% mannitol, USP and 0.5% phenol, USP. Relamorelin (30µg) and placebo (4.5% mannitol, USP and 0.5% phenol, USP for injection) were delivered with a consistent 300µL volume in a pre-filled syringe provided by Rhythm Pharmaceuticals. Study drug or matching placebo was administered subcutaneously to each subject once on each of three treatment days, corresponding to the studies on separate days of: 1) fasting and postprandial gastric volumes and gastric accommodation using validated single photon emission computed tomography (SPECT); 2) 1-hour distal antral motility following a 511 calorie mixed solid-liquid meal using 15-lumen perfusion gastroduodenal manometry; and 3) satiation using Ensure (Abbott Nutrition, Columbus, Ohio, USA) nutrient drink test, with measurement of volume to fullness (indicating comfortable fullness), maximum tolerated volume, and postprandial symptoms 30 minutes after maximum tolerated volume. Each of these three tests were performed independently (not in a sequence) within a 2-week time period from the day of the first test.
Gastroduodenal Manometry
Tube Placement
After an overnight (8-hour) fast, a trained technologist (DB) and staff physician (MC) performed the manometry tube placement. The tube placement was done using a transnasal approach with the participant in the sitting position. A 4-meter long Teflon guidewire was placed trans-nasally and advanced into the duodenum in order to facilitate placing the manometry tube into the distal duodenum or proximal jejunum. The position of the manometry tube was verified using fluoroscopy to ensure that at least 5 manometric sensors that are 1cm apart were located across the antropyloroduodenal junction. The 15-lumen manometric tube was custom-built for these studies by MUI Scientific, Inc. (Mississauga, Ontario, Canada) using polyvinyl chloride with an outer diameter of 4mm and inner lumen diameter of each manometric tube was 0.3mm. The multi-lumen manometric tube has 13 sensors that are 1cm apart, with two sensors that are 10 and 20cm beyond the last of the closely placed sensors.
Each of the channels of the multi-lumen manometric tube was perfused with distilled water via a pneumohydraulic pump (perfusion rate 0.5mL/minute, perfusion pressure 14psi) and attached to a strain gauge transducer (Model PX-MK099, Edwards Lifesciences, Irvine, California, USA).
Procedure
After recording fasting motility for 15 minutes, participants received the subcutaneous injection, and 15 minutes later, they ingested a 511kcal solid-liquid meal (chicken, potato, butter, pudding, and 190mL of water); motility was then monitored for 60 minutes after the meal. During the entire test, the manometric recording was monitored by a technologist to keep the manometric sensors in the antropyloroduodenal junction. At the end of the recordings, the manometric tube was withdrawn by gentle traction.
Analysis
Phasic pressure activity in the distal antrum recorded on the manometric tracing was identified in the two recordings proximal to the manometrically-identified pyloric recordings. The mean amplitude and the number of contractions were measured using computer software developed by Medical Measurement and Systems (Medical Measurement Systems B.V., Enschede, The Netherlands); in order to obtain a representative assessment of the distal antral phasic pressure activity, we analyzed statistically the average of the three most distal antral recordings; in addition, we provided the information for the immediate pre-pyloric pressure recording. Thus, most distal antral recording was identified as a recording up to three waves per minute, which was: a) just proximal (1cm) to recording of duodenal-type waves; or b) just proximal to a site exhibiting a mixture of antral-type and duodenal-type waves associated with baseline elevation (pyloric-type activity).
For each sequential 15-minute period and for the entire 1-hour postprandial period, a motility index (MI) was calculated using the formula:
MI = loge ([sum of amplitude × number of contractions] + 1).
We focused on the motility of the first postprandial hour, since this is the time during which the most prominent effects on gastric emptying were observed during the first hour in patients with type 1 or 2 diabetes (13, 14).
Single Photon Emission Computed Tomography (SPECT)
SPECT is a validated, noninvasive method to measure gastric volume (19). Intravenous injection of 99mTc sodium-pertechnetate, which is taken up by the parietal and non-parietal cells of the gastric mucosa, allows visualization of the stomach wall. Tomographic images were obtained throughout the long axis of the stomach using a SPECT gamma camera (General Electric Millennium MG, Milwaukee, Wisconsin, USA) that rotates around the body with the subject in the supine position. The duration of each scan was 8 minutes (20); we have previously published data (20 and 21) showing that two postprandial volume estimates within 20 minutes of the end of the standard meal (300mL Ensure®) were very similar (<5% difference) and, therefore, only one postprandial scan was needed, immediately after the meal was ingested. Using the AVW 3.0 (Biomedical Imaging, Mayo Foundation, Rochester, Minnesota, USA) image processing libraries, a three-dimensional rendering of the stomach was obtained and its volume (mL) calculated. Intra- and inter-individual coefficients of variation (at average 9 months) are ~12% (21). We have previously demonstrated (using barostat measurements) that the gastric accommodation response to maximal within 10 minutes of meal ingestion (22) and gastric accommodation during first and second 15minute epochs after meal ingestion are not significantly different (20).
Procedure
Fifteen minutes prior to the fasting SPECT scan, participants received a dose of relamorelin, 30µg, subcutaneous, or matching placebo, followed by a dose of i.v. 99mTc-pertechnetate a minimum of 10 minutes prior to the first fasting scan. Participants ingested 300mL Ensure meal (316kcal). A second scan was obtained during16 minutes after ingestion of the Ensure meal.
Satiation Testing
Participants received study medication. Fifteen minutes after dosing of study medication, they started to ingest Ensure nutrient drink (1kcal/mL) at a rate of 120mL every 4 minutes (30mL/min) to measure volume to fullness and maximal tolerated volume. Thirty minutes later (45 minutes after study drug injection), participants reported on the severity of their symptoms (nausea, fullness, bloating and pain) using a 100mm visual analog scale.
Endpoints for Analysis
Primary endpoints for analysis between relamorelin and placebo were: first hour postprandial distal antral motility index studied by gastroduodenal manometry (averaged over the three most distal antral recording sites); fasting gastric and accommodation volume by SPECT; volume to fullness (mL) by satiation test.
Secondary endpoints for analysis were: frequency and amplitude of antral activity during first postprandial hour; absolute postprandial gastric volume ratio (postprandial/fasting gastric volume); maximum tolerated volume; aggregate symptoms score 30 minutes after maximum tolerated volume; and individual symptom scores (nausea, bloating, fullness, pain) on satiation test.
Statistical Power and Analysis
Sample size planned for the study [11.5±0.6 (SD)] was based on the results of primary endpoints in prior studies of distal antral pressure activity in healthy volunteers in the absence of any treatment (23–25). Effect size detectable is the difference in mean distal antral motility index between the two treatment groups (shown in Table 1, with 80% power, α=0.05). Estimated effect sizes are based on unpaired t-test, with expected difference in mean of 0.9 motility index units in distal antral activity or 8% change in the antral motility index in relamorelin compared to placebo.
Table 1.
Power Calculation: Effect Size for Measured Responses
It is to be noted that this is on a logarithmic scale and, therefore, an 8% change constitutes a clinically relevant difference.
Additionally, effect size differences for relamorelin compared to placebo is 44% in the mean fasting gastric volume and 29.8% in the gastric accommodation volume, based on the coefficients of variation observed from our laboratory in prior studies, as shown in Table 1 (21).
The sample size of 8 participants receiving relamorelin was anticipated to have sufficient power to detect drug-associated differences in distal antral motility index of 0.9 units, fasting gastric volume of 98mL, and gastric accommodation of 151mL.
Unpaired student t test was used to assess associations of treatment status (relamorelin vs. placebo), as the data for both treatment groups were normally distributed. Participants, technicians, and investigators were blinded during the study until all data analyzed had been locked.
RESULTS
The participant demographics in the two treatment groups were similar (Table 2).
Table 2.
Effects of Relamorelin on Satiation, Gastric Volume and Gastric Accommodation
| Placebo | Relamorelin | p value |
|
|---|---|---|---|
| Age, years | 39.3 ± 5.4 | 40.4 ± 3.4 | ns |
| Body mass index, kg/m2 | 26.9 ± 1.4 | 26.5 ± 1.6 | ns |
| Volume to fullness, mL | 563.3 ± 111.4 | 488.6 ± 55.8 | ns |
| Maximum tolerated volume, mL | 1145.3 ± 142.5 | 1011.4 ± 100.2 | ns |
| Aggregate symptoms 30 minutes post-maximum tolerated volume |
160.4 ± 22.4 | 133.3 ± 27.0 | ns |
| Nausea, mm (on 100mm visual analog scale) | 25.4 ± 8.9 | 18.0 ± 11.1 | ns |
| Bloating, mm (on 100mm visual analog scale) | 45.3 ± 8.3 | 32.6 ± 10.9 | ns |
| Fullness, mm (on 100mm visual analog scale) | 71.1 ± 6.8 | 68.3 ± 8.7 | ns |
| Pain, mm (on 100mm visual analog scale) | 18.6 ± 8.7 | 14.4 ± 8.1 | ns |
| Fasting gastric volume, mL | 195.4 ± 15.8 | 210.5 ± 40.6 | ns |
| Postprandial gastric volume, mL | 672.4 ± 36.7 | 698.9 ± 28.1 | ns |
| Gastric accommodation ratio | 3.6 ± 0.3 | 3.9 ± 0.5 | ns |
Data are mean ± SEM; ns=not significant
Distal Antral Motility
In two participants randomized to placebo and one to relamorelin, the manometric sensors were not optimally positioned to consistently analyze the postprandial phasic pressure activity. Table 3 shows the data that was submitted to analysis statistically, that is based on the average of the three most distal antral recordings; Table 4 shows the information for the immediate pre-pyloric pressure recording.
Table 3.
Effects of Relamorelin and Placebo on Distal Antral Pressure Activity based on average of three distal antral (pre-pyloric) sensors
| Time Interval (mins) |
Placebo | Relamorelin | P value |
|---|---|---|---|
| # of contractions | |||
| 0–15 | 31.3 ± 4.0* | 44.8 ± 1.6* | 0.005* |
| 16–30 | 32.1 ± 3.7* | 44.5 ± 1.6* | 0.015* |
| 31–45 | 30.6 ± 4.3 | 36.3 ± 4.5 | 0.373 |
| 46–60 | 28.6 ± 3.6 | 36.9 ± 2.6 | 0.089 |
| 0–60 | 122.6±13.3* | 167.0 ± 9.0* | 0.022* |
| Mean Amplitude, mmHg | |||
| 0–15 | 84.1 ± 10.5 | 69.8 ± 10.1 | 0.383 |
| 16–30 | 89.1 ± 21.3 | 63.2 ± 9.0 | 0.313 |
| 31–45 | 82.7 ± 9.2 | 58.6 ± 7.0 | 0.059 |
| 46–60 | 76.6 ± 6.9 | 59.6 ± 9.5 | 0.162 |
| 0–60 | 68.3 ± 9.5 | 76.7 ± 7.8 | 0.519 |
| Motility Index post meal | |||
| 0–15 | 11.2 ± 0.2 | 11.8 ± 0.2# | 0.055 |
| 16–30 | 11.2 ± 0.2 | 11.7 ± 0.2 | 0.116 |
| 31–45 | 11.1 ± 0.4 | 11.1 ± 0.3 | 0.981 |
| 46–60 | 10.9 ± 0.3 | 11.1 ± 0.2 | 0.504 |
| 0–60 | 11.1 ± 0.2 | 11.5 ± 0.2 | 0.139 |
(data show mean ± SEM; *significant)
Table 4.
Effects of Relamorelin and Placebo on distal antral phasic pressure activity, based on the immediate pre-pyloric antral sensor (data show median and IQR)
| Time Interval (mins) | Placebo | Relamorelin |
|---|---|---|
| # of contractions | ||
| 0–15 | 28 (24–37) | 45 (37–49) |
| 16–30 | 29 (25–37) | 44 (31–46) |
| 31–45 | 30 (16–38) | 37 (25–46) |
| 46–60 | 28 (18–40) | 32 (31–39) |
| 0–60 | 105 (84–138) | 152 (126–174) |
| Mean Amplitude, mmHg | ||
| 0–15 | 61 (53–74) | 56 (39–75) |
| 16–30 | 52 (45–64) | 57 (34–69) |
| 31–45 | 67 (55–97) | 51 (41–63) |
| 46–60 | 54 (49–79) | 44 (32–63) |
| 0–60 | 60 (50–77) | 52 (45–74) |
Relamorelin, 30µg, significantly increased the number of contractions in the distal antrum during 0–60 minutes post-meal when compared to placebo (Table 3 and Figure 1; p=0.022); this was also observed in the first two 15minute periods (p=0.005 and 0.015 for number of contractions 0–15 and 16–30). There was borderline increase in MI0–15 (p=0.055) and numerical increases in MI0–60 and MI16–30. The amplitude of contractions was not significantly different between the two treatment groups (Table 3). In addition, the effect on frequency of contractions was not significant during the 30–60 minute period postprandially (Table 3).
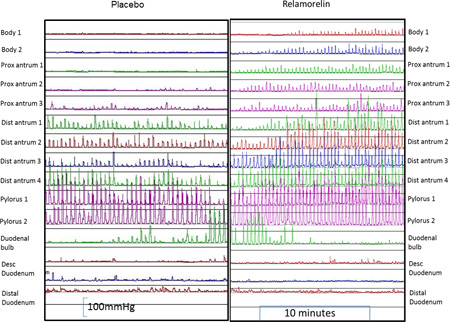
Figure 1.
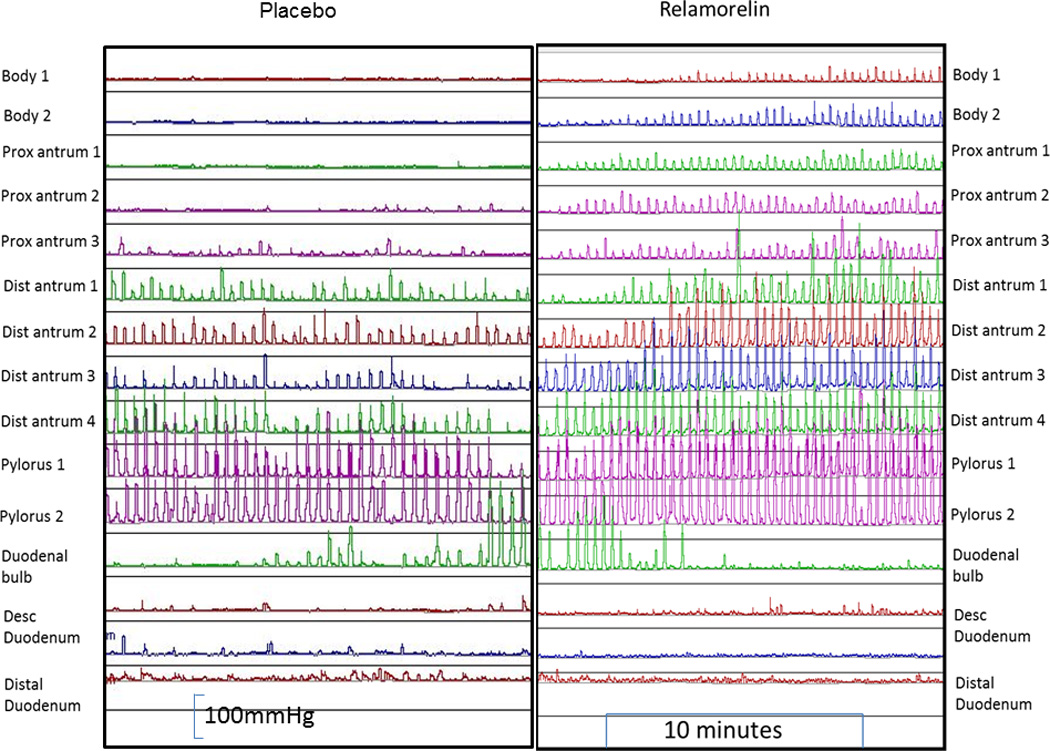
Gastroduodenal manometry in healthy subjects who were randomized to relamorelin (right panel) or placebo (left panel) showing manometric tracings in the postprandial period from the gastric body to the duodenum. Note the normal amplitude of contractions, with the marked increase in the frequency of contractions and the greater extent (relative to the pylorus) where antral contractions are recorded with relamorelin treatment.
The site of origin of detectable antral waves proximal to the pyloric antrum increased with relamorelin and showed statistical significance when compared to placebo (p=0.012) (shown in the examples in Figure 1, and analyzed for the two treatment arms for each participant in Figure 2). Relamorelin was associated with a longer distance from the manometrically-identified pylorus where gastric phasic pressure waves were recorded in the first 30 minutes postprandially.
Figure 2.
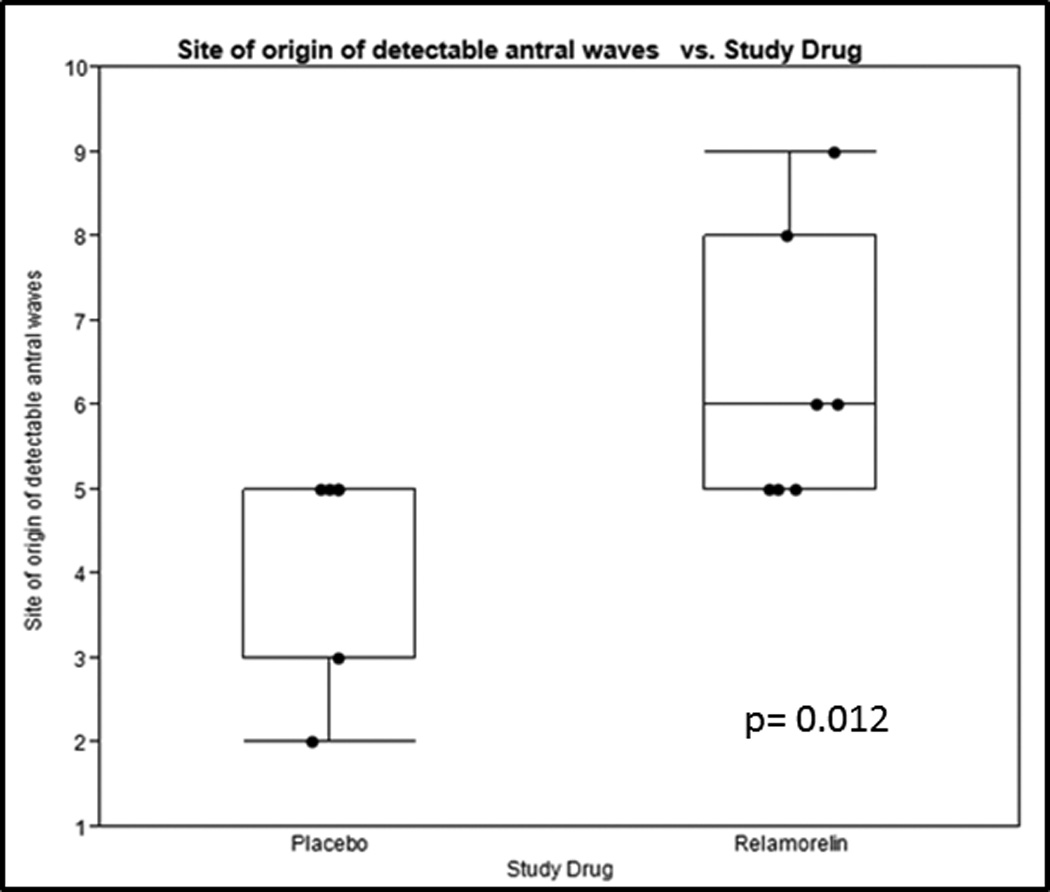
Postprandial site of origin of detectable waves proximal to the pyloric antrum for placebo and relamorelin groups.
In the duodenum, there were no specific patterns of motility identified other than typical fed patterns, irregular contractile activity. There were no phase III MMC-like activity fronts.
Gastric Accommodation and Satiation
Relamorelin did not significantly alter fasting or postprandial gastric volumes, gastric accommodation (Table 2 and Figure 3), or satiation volumes and symptoms (aggregate and nausea, fullness, bloating, pain, Table 2).
Figure 3.
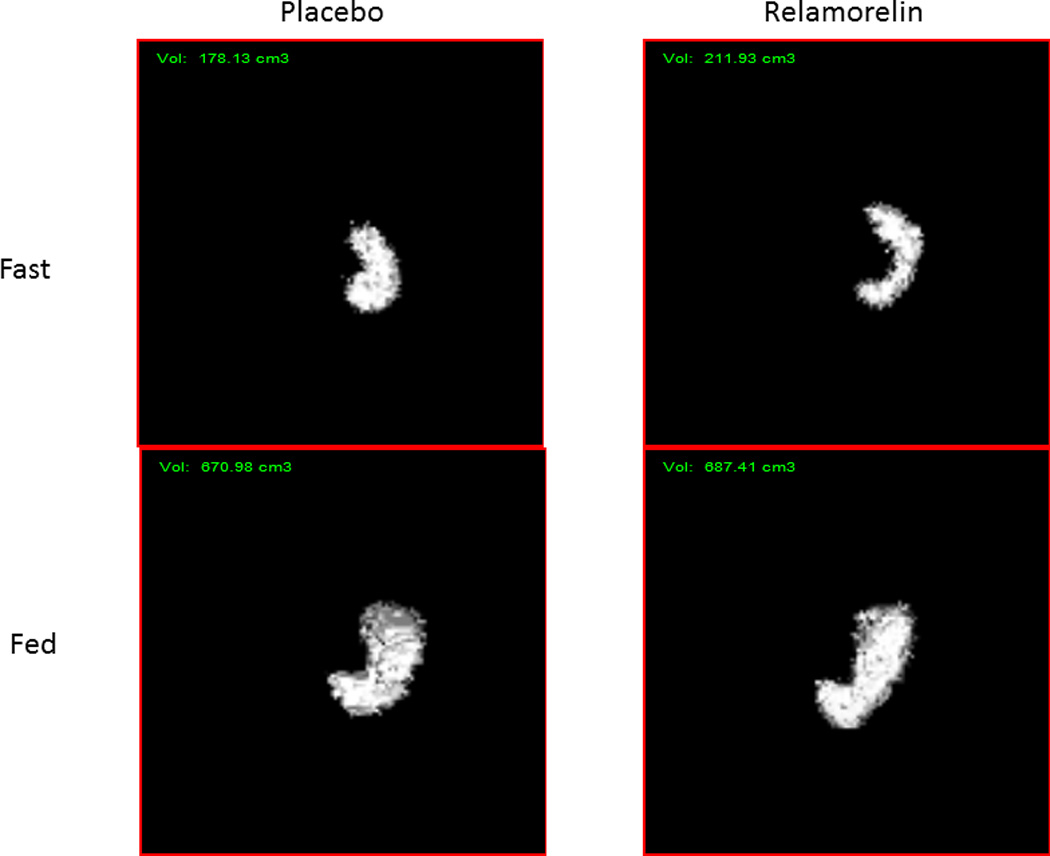
Examples of gastric volumes measured by SPECT. Note the similarity in the measured fasting and postprandial gastric volumes in the two examples treated with relamorelin or placebo.
DISCUSSION
Our study shows that relamorelin increases motor activity in the distal antrum by increasing the number of contractions and does not inhibit gastric accommodation or induce satiation, supporting the potential of relamorelin for the treatment of symptomatic patients with decreased antral motor function, such as in gastroparesis.
This investigation provides an explanation for the observed acceleration of gastric emptying in patients with diabetes mellitus(13, 14) and in those with established diabetic gastroparesis(15). In healthy subjects who have normal amplitude of antral contractions, it was not expected that the ghrelin receptor agonist would increase the amplitude of contractions. However, this is the first demonstration in humans that the ghrelin receptor agonist, relamorelin, results in an increase in the frequency of contractions during the first postprandial hour.
Ghrelin-induced gastrointestinal motility has been shown to occur through two main mechanisms (4): first, activation of vagal afferent nerve terminals and second, direct activation of ghrelin receptors in the enteric nerves of stomach and duodenum. The latter was convincingly demonstrated by the stimulation of gastric motility in response to peripherally administered intravenous ghrelin in vagally-denervated rats (26). A previous study in vagotomized patients showed that intravenous administration of ghrelin caused a significant increase in plasma growth hormone in patients, which was not significantly different from normal subjects(27). In our prior study in patients with type 1 diabetes13, relamorelin, 100µg, subcutaneous, appeared to be efficacious, even in patients with cardiovagal neuropathy [which is highly suggestive of abdominal vagal dysfunction (28)], suggesting that it may function at the stomach neuromuscular apparatus rather than through direct activation of the vagal nerve.
The dual mechanisms whereby relamorelin stimulates antral motility are potentially relevant for clinical application as therapy for gastroparesis, since they suggest that this medication would be efficacious in patients with either extrinsic vagal or intrinsic enteric neuropathy causing gastroparesis. This would simplify the management of patients with symptoms and documented retardation of gastric emptying, which remains a condition with unmet need in clinical practice (29).
Another advantage of this medication is the lack of inhibition of gastric accommodation or induction of satiation symptoms. This contrasts with the effects of the hormones, ghrelin (10) and motilin (30), which increase fasting gastric tone and reduce postprandial gastric accommodation at pharmacological doses, though infusion of synthetic human ghrelin to mimic physiological circulating levels of ghrelin did not significantly inhibit the gastric accommodation response measured by SPECT (8). Motilin definitely increases postprandial symptoms and increases satiety after meal ingestion. The effects of the hormone, ghrelin, on postprandial symptoms require further study, given observations by Ang et al. that several symptom scores increase (albeit, non-significantly) by 30–50%, such as fullness, bloating, satiety and cramps (10). This combination of effects on the function of the stomach suggests that relamorelin may be efficacious in the treatment of patients with gastroparesis, although clinical trials focused on patient response outcomes are necessary. The first trial of the effects of 10µg b.i.d. relamorelin in diabetic gastroparesis has documented significant effects on gastric emptying, as well as improvement in vomiting symptoms in patients with high baseline vomiting frequency, though not in the entire study cohort (15). The best example of the potential of such pharmacodynamic effects to predict clinical efficacy is provided by past experience with cisapride which stimulated antral motility (31) in addition to accelerating gastric emptying, enhancing gastric accommodation, (32) and reducing symptoms in patients with upper GI motility disorders. (33–35)
The sample size in our study was appropriate from a statistical power perspective for the gastric physiology and motility endpoints, for which the coefficient of variation had been thoroughly characterized in the prior literature from our laboratory. Thus, although the sample size is relatively small, the results are clinically relevant, and there is only small chance of a type II error in the observed lack of negative effects on accommodation and symptoms of satiation. The relamorelin-induced increase in the frequency of contractions of the distal antrum in the postprandal period is relevant in patients with neuropathic forms of antral hypomotility. Thus, Thumshirn et al. (36) showed an average of less than 1 contraction per minute postprandially is a simple estimate of significant neuropathic hypomotility, and our data shows that in healthy participants, relamorelin was associated with an average 2.7 contractions per minute in the first postprandial hour. Further replication studies are required in patients with demonstrated gastroparesis to appraise the prokinetics effects of relamorelin on postprandial antral contractility.
A limitation of our study is that it was conducted in healthy volunteers whose antral amplitude is presumably normal. Hence, we did not expect to observe an increase in amplitude of antral contractions, but treatment with relamorelin was associated with an increase in antral contraction frequency, particularly in the first 30 minutes. We do not know what would be observed in patients with myopathic disorders whose contraction amplitude is typically lower than 40mmHg (37). It also appears that relamorelin was associated with induction of recordable phasic pressure activity over a longer segment of the antrum and body of the stomach.
Other prokinetic agents that activate cholinergic mechanisms are also typically associated with increased frequency of antral contractions. For example, cisapride increased frequency of antral contractions in healthy beagles with electrodes implanted into the stomach wall (38), and low dose (40mg) erythromycin (acting through a cholinergic pathway) stimulated antral contractions to induce a propagated MMC-like complex during fasting (39). Unfortunately, effects of 40mg erythromycin on postprandial motility were not reported in the same study and, therefore, it is unclear whether this cholinergic activation in the healthy human stomach would induce increases in both amplitude and frequency (39).
In conclusion, these data build on the reports from our laboratory of the acceleration of gastric emptying with relamorelin in patients with type 1 or 2 diabetes (13, 14) and support the efficacy demonstrated in patients with prominent baseline vomiting frequency in a phase II clinical trial of 10µg b.i.d. relamorelin in diabetic gastroparesis (15). At present, a multi-center trial of the dose-related effects of relamorelin 10, 30 and 100µg b.i.d. is being conducted, and will provide evidence of the relative efficacy of these three doses in patients with gastroparesis. The current results demonstrating significant increases in antral contractile frequency with relamorelin suggest that relamorelin is promising for the treatment of gastroparesis.
Key Points.
-
--
In a randomized controlled study of 16 healthy volunteers, we compared effects of relamorelin to placebo on antral motor function measured with manometry.
-
--
Relamorelin increases frequency of distal antral motility contractions without significant effects on amplitude of contractions in healthy volunteers.
-
--
Relamorelin did not inhibit gastric accommodation or induce symptoms of satiation. Overall, these data support the potential of relamorelin in the treatment of gastroparesis.
Acknowledgments
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
FUNDING
Dr. Camilleri is supported by grants RO1-DK67071 and PO1-DK68055 from National Institutes of Health. This study was supported by a research grant from Rhythm Pharmaceuticals.
Footnotes
Registration: ClinicalTrials.gov #NCT02466711; this study was not a clinical trial assessing symptoms, so no CONSORT chart or checklist are needed.
DISCLOSURES
Dr. Camilleri serves on an advisory board of Rhythm Pharmaceuticals (Boston, Massachusetts, USA), with compensation to his employer, Mayo Clinic, and no personal financial remuneration. The other authors have no competing interests.
AUTHORSHIP STATEMENT
Guarantor of the article: Dr. Michael Camilleri takes responsibility for the integrity of this work, from inception to publication.
All authors have approved submission of this article.
Authors’ contributions:
A. Nelson: fellow, patient recruitment, authorship
A. Acosta: fellow, patient recruitment
M. Camilleri: Principal investigator, conceptualization, article guarantor; analysis and interpretation, authorship of manuscript
Irene Busciglio: Protocol management, regulatory
Sara Linker Nord: Study coordinator
Amy Boldingh: Study coordinator
Deborah Rhoten: Conduct of accommodation, satiation and antroduodenal motility
Michael Ryks: Conduct of accommodation, satiation
D. Burton: gastroduodenal motility placement and measurements
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Masuda Y, Tanaka T, Inomata N, et al. Ghrelin Stimulates Gastric Acid Secretion and Motility in Rats. Biochemical and Biophysical Research Communications. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 3.Trudel L, Tomasetto C, Rio MC, et al. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G948–G952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- 4.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 5.Dass NB, Munonyara M, Bassil AK, et al. Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience. 2003;120:443–453. doi: 10.1016/s0306-4522(03)00327-0. [DOI] [PubMed] [Google Scholar]
- 6.Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Alimentary Pharmacology & Therapeutics. 2005;22:847–853. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- 7.Tack J, Depoortere I, Bisschops R, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremonini F, Camilleri M, Roque MV, et al. Obesity Does Not Increase Effects of Synthetic Ghrelin on Human Gastric Motor Functions. Gastroenterology. 2006;131:1431–1439. doi: 10.1053/j.gastro.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2009;6:343–352. doi: 10.1038/nrgastro.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ang D, Nicolai H, Vos R, et al. Influence of ghrelin on the gastric accommodation reflex and on meal-induced satiety in man. Neurogastroenterology & Motility. 2009;21:e528–e529. doi: 10.1111/j.1365-2982.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, Acosta A. Emerging treatments in Neurogastroenterology: relamorelin: a novel gastrocolokinetic synthetic ghrelin agonist. Neurogastroenterology & Motility. 2015;27:324–332. doi: 10.1111/nmo.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Ploeg L, Laken H, Sharma S, et al. Preclinical gastrointestinal prokinetic efficacy and endocrine effects of the ghrelin mimetic RM-131. Life Sciences. 2014;109:20–29. doi: 10.1016/j.lfs.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Shin A, Camilleri M, Busciglio I, et al. The Ghrelin Agonist RM-131 Accelerates Gastric Emptying of Solids and Reduces Symptoms in Patients With Type 1 Diabetes Mellitus. Clinical Gastroenterology and Hepatology. 2013;11:1453–1459. e1454. doi: 10.1016/j.cgh.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin A, Camilleri M, Busciglio I, et al. Randomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamics. Diabetes Care. 2013;36:41–48. doi: 10.2337/dc12-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lembo A, Camilleri M, McCallum R, Sastre R, Breton C, Spence S, White J, Currie M, Gottesdiener K, Stoner E RM-131-004 Trial Group. Relamorelin Reduces Vomiting Frequency and Severity and Accelerates Gastric Emptying in Adults with Diabetic Gastroparesis. Gastroenterology. 2016 Apr 4; doi: 10.1053/j.gastro.2016.03.038. pii: S0016-5085(16)30051-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Acosta A, Camilleri M, Busciglio I, Boldingh A, Nelson AD, Burton D. Short-Term Effects of Relamorelin on Descending Colon Motility in Chronic Constipation: A Randomized, Controlled Trial. Dig Dis Sci. 2016;61:852–860. doi: 10.1007/s10620-015-3876-5. [DOI] [PubMed] [Google Scholar]
- 17.Acosta A, Camilleri M, Kolar G, et al. Relamorelin Relieves Constipation and Accelerates Colonic Transit in a Phase 2, Placebo-Controlled, Randomized Trial. Clinical Gastroenterology and Hepatology. 2015;13:2312–2319. e2311. doi: 10.1016/j.cgh.2015.04.184. [DOI] [PubMed] [Google Scholar]
- 18.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ., 3rd Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 19.Bouras EP, Delgado-Aros S, Camilleri M, et al. SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Gut. 2002;51:781–786. doi: 10.1136/gut.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijayvargiya P, Camilleri M, Shin A, Breen M, Burton D. Simplifying the measurement of gastric accommodation using SPECT. Neurogastroenterology & Motility. 2013;25:542–546. doi: 10.1111/nmo.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breen M, Camilleri M, Burton D, Zinsmeister AR. Performance characteristics of the measurement of gastric volume using single photon emission computed tomography. Neurogastroenterology & Motility. 2011;23:308–315. doi: 10.1111/j.1365-2982.2010.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thumshirn M, Camilleri M, Saslow SB, Williams DE, Burton DD, Hanson RB. Gastric accommodation in non-ulcer dyspepsia and the roles of Helicobacter pylori infection and vagal function. Gut. 1999;44:55–64. doi: 10.1136/gut.44.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology. 1986;91:94–99. doi: 10.1016/0016-5085(86)90444-0. [DOI] [PubMed] [Google Scholar]
- 24.Camilleri M, Malagelada JR, Brown ML, Becker G, Zinsmeister AR. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol. 1985;249:G580–G585. doi: 10.1152/ajpgi.1985.249.5.G580. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri M, Malagelada JR, Stanghellini V, Fealey RD, Sheps SG. Gastrointestinal motility disturbances in patients with orthostatic hypotension. Gastroenterology. 1985;88:1852–1859. doi: 10.1016/0016-5085(85)90010-1. [DOI] [PubMed] [Google Scholar]
- 26.Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227–240. doi: 10.1113/jphysiol.2003.040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeno R, Okimura Y, Iguchi G, et al. Intravenous administration of ghrelin stimulates growth hormone secretion in vagotomized patients as well as normal subjects. Eur J Endocrinol. 2004;151:447–450. doi: 10.1530/eje.0.1510447. [DOI] [PubMed] [Google Scholar]
- 28.Buysschaert M, Donckier J, Dive A, Ketelslegers JM, Lambert AE. Gastric acid and pancreatic polypeptide responses to sham feeding are impaired in diabetic subjects with autonomic neuropathy. Diabetes. 1985;34:1181–1185. doi: 10.2337/diab.34.11.1181. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical Guideline: Management of Gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuomo R, Vandaele P, Coulie B, et al. Influence of Motilin on Gastric Fundus Tone and on Meal-Induced Satiety in Man: Role of Cholinergic Pathways. Am J Gastroenterol. 2006;101:804–811. doi: 10.1111/j.1572-0241.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 31.Camilleri M, Malagelada JR, Abell TL, Brown ML, Hench V, Zinsmeister AR. Effect of six weeks of treatment with cisapride in gastroparesis and intestinal pseudoobstruction. Gastroenterology. 1989;96:704–712. [PubMed] [Google Scholar]
- 32.Tack, Broeckaert, Coulie, Janssens The influence of cisapride on gastric tone and the perception of gastric distension. Alimentary Pharmacology & Therapeutics. 1998;12:761–766. doi: 10.1046/j.1365-2036.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- 33.Abell TL, Camilleri M, DiMagno EP, Hench VS, Zinsmeister AR, Malagelada JR. Long-term efficacy of oral cisapride in symptomatic upper gut dysmotility. Dig Dis Sci. 1991;36:616–620. doi: 10.1007/BF01297028. [DOI] [PubMed] [Google Scholar]
- 34.Braden B, Enghofer M, Schaub M, Usadel KH, Caspary WF, Lembcke B. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Alimentary Pharmacology & Therapeutics. 2002;16:1341–1346. doi: 10.1046/j.1365-2036.2002.01257.x. [DOI] [PubMed] [Google Scholar]
- 35.Corinaldesi R, Stanghellini V, Raiti C, Rea E, Salgemini R, Barbara L. Effect of chronic administration of cisapride on gastric emptying of a solid meal and on dyspeptic symptoms in patients with idiopathic gastroparesis. Gut. 1987;28:300–305. doi: 10.1136/gut.28.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thumshirn M, Bruninga K, Camilleri M. Simplifying the evaluation of postprandial antral motor function in patients with suspected gastroparesis. Am J Gastroenterol. 1997;92:1496–1500. [PubMed] [Google Scholar]
- 37.Weston S, Thumshirn M, Wiste J, Camilleri M. Clinical and upper gastrointestinal motility features in systemic sclerosis and related disorders. Am J Gastroenterol. 1998;93:1085–1089. doi: 10.1111/j.1572-0241.1998.00334.x. [DOI] [PubMed] [Google Scholar]
- 38.Burger DM, Wiestner T, Hubler M, Binder H, Keiser M, Arnold S. Effect of anticholinergics (atropine, glycopyrrolate) and prokinetics (metoclopramide, cisapride) on gastric motility in beagles and labrador retrievers. J Vet Med A Physiol Pathol Clin Med. 2006;53:97–107. doi: 10.1111/j.1439-0442.2006.00787.x. [DOI] [PubMed] [Google Scholar]
- 39.Coulie B, Tack J, Peeters T, Janssens J. Involvement of two different pathways in the motor effects of erythromycin on the gastric antrum in humans. Gut. 1998;43:395–400. doi: 10.1136/gut.43.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]


