Abstract
Natural killer cells are key components of the innate immune system. In murine cardiac transplant models, donor-specific antibodies in concert with NK cells are sufficient to inflict chronic allograft vasculopathy independently of T and B cells. In this study, we aimed to determine the effector mechanism(s) required by NK cells to trigger chronic allograft vasculopathy during antibody-mediated rejection. Specifically, we tested the relative contribution of the pro-inflammatory cytokine IFN-γ versus the contact-dependent cytotoxic mediators of perforin and the CD95/CD95L (Fas/FasL) pathway for triggering these lesions. C3H/HeJ cardiac allografts were transplanted into immune-deficient C57BL/6 rag−/−γc−/− recipients who also received monoclonal anti-MHC class I DSA. The combination of donor-specific antibodies and wild-type NK cell transfer triggered aggressive chronic allograft vasculopathy. However, transfer of IFN-γ-deficient NK cells or host IFN-γ neutralization led to amelioration of these lesions. Use of either perforin-deficient NK cells or CD95 (Fas)-deficient donors alone did not alter development of vasculopathy, but simultaneous disruption of NK cell-derived perforin and allograft Fas expression resulted in prevention of these abnormalities. Therefore, both NK cell IFN-γ production and contact-dependent cytotoxic activity are rate-limiting effector pathways that contribute to antibody-induced chronic allograft vasculopathy.
Introduction
Solid organ transplantation is an important therapy for patients with end-stage organ dysfunction. One-year adjusted graft survival rates have steadily increased within the last ten years and are now >80% for all solid organ recipients (1-5). Despite this improvement in early success rates, long-term graft outcomes have not improved significantly in the last 20 years (6, 7) and the immunological mechanisms that drive chronic allograft dysfunction remain poorly understood. Donor specific antibodies (DSA) have recently been shown to be associated with this process (6), and clinically, the de novo development of DSA is associated with decreased survival in kidney, heart, and lung transplant recipients (8-13). Using a murine heterotopic heart transplant model, Hirohashi et al. determined that natural killer (NK) cells interact with DSA through an Fc receptor-dependent mechanism to trigger chronic allograft vasculopathy (CAV) (14). Once generated, this route of CAV development occurs independently of T cells or host complement components (14). However, it is unclear which effector pathways utilized by NK cells contribute to this pathogenic process.
NK cells make up approximately 10-15% of the circulating lymphocyte population and are an important component of the innate immune system. When NK cells are “armed” with specific antibodies by binding of the antibody Fc region, they provide an antigen-specific means of directing NK cell reactivity, effectively linking the innate and adaptive immune response. Specifically, NK cell activation results in expression of key inflammatory mediators, notably IFN-γ (15). In addition, NK cell activation can trigger two independent contact-dependent cytolytic pathways: one involving secretion of the pore-forming protein perforin and the second involving CD95L (FasL) expression leading to target cell apoptosis through an interaction with CD95 (Fas) on these cells (15-18). Prior studies of acute allograft rejection mediated by CD4+ or CD8+ T cells have indicated that both IFN-γ and a combination of perforin and the Fas/FasL pathway are essential for allograft injury (19-22). In the present study, we sought to determine whether NK cells contributing to antibody-dependent chronic rejection require similar pathways to those utilized by cytotoxic T cells during acute rejection.
Materials and Methods
Mice
C3H/HeJ (C3H, H-2k), C3.MRL-Faslpr/J (C3H.lpr, H-2k), C57BL/6 (B6, H-2b), B6.129S7-Rag1tm1Mom/J (B6.rag−/−, H-2b), B6.129S7-Ifngtm1Ts/J (B6.IFNγ−/−, H-2b), and C57BL/6-Prf1tm1Sdz/J (B6.pfp−/−, H-2b) mice aged 8-10 weeks were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). B10;B6-Rag2tm1Fwa II2rgtm1Wjl (B6.rag−/−γc−/−, H-2b) were obtained from Taconic Biosciences (Hudson, NY, USA) and bred in our facility according to a material transfer agreement. All mice were maintained under pathogen-free conditions throughout the experiments and were cared for according to methods approved by the American Association for the Accreditation of Laboratory Animal Care. All live animal experiments and procedures were performed with the approval of the Institutional Animal Care and Use Committee at the University of Colorado, Anschutz Medical Campus.
Heterotopic Heart Transplantation
Cardiac allografts from C3H or C3H.lpr donor mice (10-20 weeks old) were transplanted heterotopically into either T cell / B cell-deficient B6.rag−/− or T cell / B cell / NK cell-deficient B6.rag−/−γc−/− recipients (10-20 weeks old) as previously described according to standard microsurgical techniques (23, 24). Briefly, the donor aorta and pulmonary artery were anastomosed to the recipient abdominal aorta and inferior vena cava, respectively. The transplanted hearts were removed on day 30 and the allografts were cross-sectioned into three parts (base, middle, and apex). All parts were fixed in 10% formalin and embedded in paraffin. Sections were stained using Verhoeff's method (25) for elastic fibers to evaluate and quantify the severity of coronary artery lesions.
Administration of Monoclonal Antibodies
Anti-H2k IgG2a (clone 36-7-5) monoclonal antibody (mAb) was obtained from BioXCell (Lebanon, NH, USA). Noted B6.rag−/− or B6.rag−/−γc−/− mice were given intra-peritoneal (IP) injections of mAb at a dose of 30 μg in 100 μL 0.9% normal saline beginning the day after transplantation and twice weekly (eight doses total) as previously described (14); the last dose was administered two days prior to allograft recovery (day 28). Anti-IFN-γ antibody (clone XMG1.2) mAb was harvested and quantified from in vivo hybridoma ascites production. Noted B6.rag−/− recipients were given IP injections of 1 mg in 200 μL 0.9% normal saline that were administered beginning the day of transplantation (day 0) and subsequently on days 3, 6, 9, and 15 post-transplantation (five doses total).
NK Cell Isolation and Adoptive Transfer
Splenocytes from 8-12 week old B6, B6.pfp−/−, and B6.IFN-γ−/− mice were utilized as the source of adoptively transferred NK cells. Briefly, T cells were depleted from donors in vivo by administration of anti-CD4+ (clone GK1.5, BioXCell) and anti-CD8+ (clone 2.43, BioXCell) antibodies (dose 10 mg/kg) six days before spleen harvest to minimize contamination from these cells. NK cells were then enriched from this whole splenocyte preparation by negative selection with an NK cell isolation kit (Miltenyi Biotec, San Diego, CA, USA) used according to the manufacturer's instructions. Isolation resulted in NK populations that ranged in purity from 65-85% (CD3ε- CD122+ NK1.1+) as determined by flow cytometry. This cell preparation was also analyzed for the presence of CD4+ (CD3ε+ CD45.2+ CD4+) and CD8+ T cells (CD3ε+ CD45.2+ CD8a+). Enriched NK cells (1.5 × 106) were adoptively transferred intravenously via retro-orbital injection on day 1 post-transplantation. All B6.rag−/−γc−/− recipients that received adoptively transferred cells were treated with additional doses of anti-CD4+ and anti-CD8+ mAb (dose 10 mg/kg on day 1 post-transplantation) to further ensure that any potentially contaminating T cells would not participate in a subsequent response.
Histological Techniques and Morphometric Analysis
Morphometric analysis was performed on images of coronary arteries from the three tissue sections of the explanted cardiac allografts. An image of all vessels larger than 85 μm in diameter was captured digitally by light microscopy at 10x magnification. Image processing and analysis with ImageJ software (NIH) was used to manually demarcate the borders of the lumen and the intima of the artery. The software then quantitated the luminal and intimal areas and from these area values; the neointimal index (NI) was defined as the neointimal area divided by neointimal area plus luminal area multiplied by 100 as previously described (26). This quantity was calculated for each vessel with the NI reported for each recipient representing the average of the individual values over the three cross-sections obtained per recipient.
Flow Cytometry
Flow cytometric analysis was used to assess the purity of adoptively transferred NK cells prior to transplantation. Cells obtained after NK isolation (see above) were incubated for 20 minutes at 4°C with CD3ε-PerCP/Cy5.5 (clone 145.2C11, BioLegend), CD122-FITC (clone TM- β1, BD Pharmingen), and NK1.1-APC (clone PK136, eBioscience). To detect the possible presence of CD4+ and CD8+ T cells, a separate cell preparation was also stained with CD45.2-APC (clone 104, eBioscience), CD3ε-PerCP/Cy5.5, CD4-Pacific Blue (clone RM4-5, BD Biosciences) and CD8a-PerCPCy5.5 (clone 53-6.7, BD Biosciences). The cells were then washed once with PBS, 1% BSA, and 0.1% sodium azide (FACS buffer); data was collected using a BD LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo software (Ashland, OR, USA). This same analysis was also used to detect the presence of these adoptively transferred cells in recipients post-transplantation. Here, the spleen was recovered from recipient animals on day 30 and depleted of erythrocytes via use of red blood cell lysis buffer (Sigma-Aldrich, St. Louis, MO, USA) and re-suspended in FACS buffer. Cells were incubated with the same antibodies and analyzed as described above.
Statistical Methods
Neointimal index is expressed as median ± interquartile range. The Mann-Whitney U test was performed to compare the neointimal indices between two groups. Cell population numbers post-transplantation are reported as the mean ± standard error of the mean (SEM). For cell population numbers post-transplantation, a Student's t test was performed to compare two groups and one-way analysis of variance (ANOVA) was used to compare multiple groups. A p-value of < 0.05 was considered significant. GraphPad Prism (La Jolla, CA, USA) and Microsoft Excel statistical software (Seattle, WA, USA) were used for statistical analysis.
Results
Donor specific antibody in conjunction with adoptively transferred NK cells can induce CAV
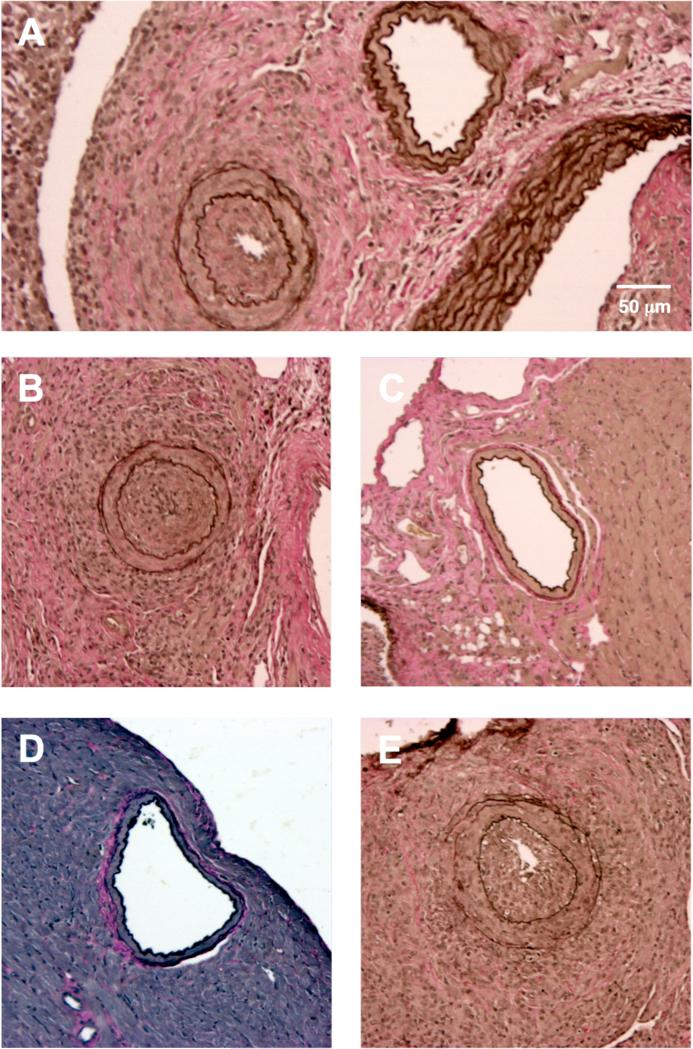
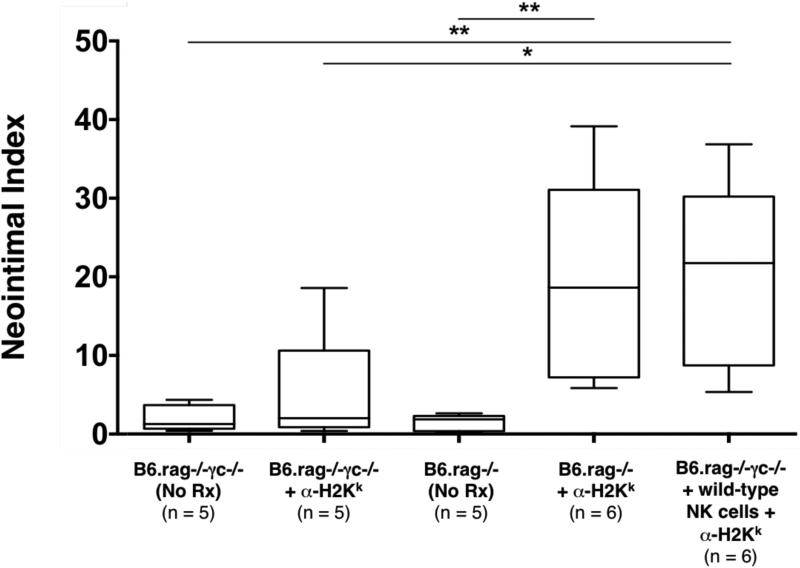
As previously described by Hirohashi et al (14), we found that administration of DSA (α-H2k IgG2a mAb) into NK cell-replete B6.rag−/− recipients bearing C3H allografts induced pronounced CAV at 30 days post-transplantation (NI 18.4% ± 18.1%) (Table 1, Figure 1A and 1B, Figure 2). These vascular lesions were heterogeneous by nature (exemplified in Figure 1A) as shown in prior studies (27). However, allografts of untreated NK cell-replete B6.rag−/− recipients showed little CAV (NI 1.86% ± 1.45%) (Figure 1C), indicating that NK cells alone are insufficient to mediate injury. In B6.rag−/−γc−/− recipients that received only DSA, there was again no significant CAV (NI 1.99% ± 1.99%), suggesting that DSA alone is insufficient to induce vascular changes. Furthermore, there was no significant difference between these latter two groups and B6.rag−/−γc−/− recipients that received neither DSA nor NK cells, who demonstrated little CAV and a low neointimal index (1.97% ± 1.71%) (Figure 1D and Figure 2).
Table 1.
Effects of DSA and NK cells (endogenous versus adoptively transferred) on the frequency of chronic allograft vasculopathy.
| Group | Donor | Recipient | Treatment | Frequency of CAV |
|---|---|---|---|---|
| 1 | C3H | B6rag−/− | No Rx | 0/5 |
| 2 | α-H2k | 5/6 | ||
| 3 | C3H | B6.rag−/−γc−/− | No Rx | 0/5 |
| 4 | α-H2k | 1/5 | ||
| 5 | α-H2k + B6 wild-type NK cells | 6/6 |
Figure 1. Representative cross-sections of coronary arteries of transplanted C3H cardiac allografts.
(A-B) Coronary arteries from B6.rag−/− recipients that received DSA (α-H2k IgG2a). Notably, severely occluded vessels were observed in close proximity to those that appeared completely normal and without neointimal thickening (A), which illustrates the heterogeneous nature of lesion formation. This is in comparison to (C), a widely patent coronary artery from a B6.rag−/− recipient that received no treatment. (D) A widely patent coronary artery from a B6.rag−/−γc−/− recipient that received no treatment in comparison to (E), a severely stenotic coronary artery from a B6.rag−/−γc−/− recipient that received DSA in addition to adoptively transferred (1.5 × 106) B6 wild-type NK cells. A-E, elastin stain, scale bar in 1A equivalent in all other images (10x magnification). DSA, donor-specific antibody.
Figure 2. Donor specific antibody in conjunction with adoptively transferred NK cells induces chronic allograft vasculopathy.
Administration of DSA (α-H2k IgG2a) into B6.rag−/− recipients bearing C3H allografts induced CAV. This effect could be duplicated in B6.rag−/−γc−/− (those lacking NK cells) by adoptive transfer of B6 wild-type NK cells (p > 0.999). In contrast, B6.rag−/−γc−/− recipients that received either no treatment or DSA alone, as well as B6.rag−/− recipients that received no treatment showed no significant CAV formation. The p values between groups were calculated with the Mann-Whitney U test; the numbers in parentheses indicate the number of allografts examined. (* p ≤ 0.05. ** p ≤ 0.01, *** p ≤ 0.001, and **** p ≤ 0.0001). CAV, chronic allograft vasculopathy; DSA, donor-specific antibody.
To test the role of specific NK cell-derived effector pathways contributing to CAV, we next developed a model utilizing NK cell adoptive transfer into B6.rag−/−γc−/− recipients, which are deficient in the common γ-chain and thus lack mature NK cells (28). B6.rag−/−γc−/− recipients bearing C3H allografts that received DSA in conjunction with adoptively transferred B6 wild-type NK cells showed marked vascular abnormalities, with 100% of animals (six out of six) demonstrating CAV (Table 1). In addition, histological examination showed prominent neointimal formation in the coronary arteries of these cardiac allografts (Figure 1E). Importantly, there was no significant difference between the degree of CAV in this group and NK cell-replete B6.rag−/− recipients that received DSA (NI 18.4% ± 18.1% vs. 21.5% ± 20.1%, respectively; p = 0.937) (Figure 2). This effectively demonstrated that CAV induced by DSA in B6.rag−/− mice could be replicated via adoptive transfer of NK cells into B6.rag−/−γc−/− mice.
NK cell-mediated CAV is IFN-γ dependent
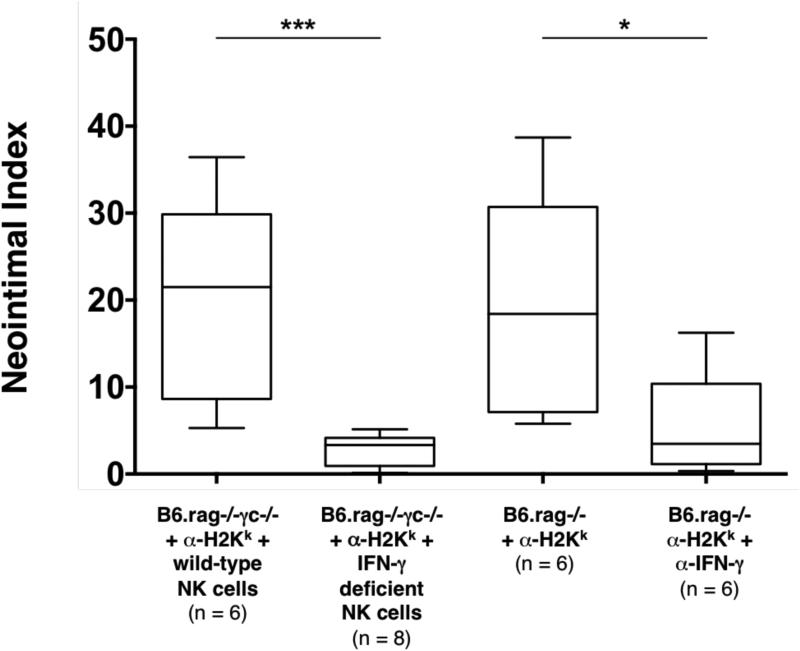
NK cell activation leads to the production of a number of inflammatory mediators, particularly INF-γ (29). Thus, we hypothesized that NK cells may mediate CAV through the secretion of this cytokine. As described above, administration of DSA and transfer of wild-type NK cells to B6.rag−/−γc−/− recipients resulted in pronounced CAV in the majority of the allografts recovered. However, administration of DSA into B6.rag−/−γc−/− recipients that instead received adoptively transferred B6 NK cells deficient in IFN-γ production resulted in complete amelioration of these lesions (NI 3.3% ± 2.9% in IFN-γ deficient NK cell recipients vs. 20.4% ± 20.1% in wild-type NK cell recipients, p = 0.0007). To determine if pharmacologic blockade of this cytokine would result in the same effect, C3H allografts were transplanted into B6.rag−/− recipients that received DSA. In addition, recipient animals were also administered anti-IFN-γ mAb. CAV severity was significantly lower in this group as compared to prior described control animals (NI 3.5% ± 4.2% in anti-IFN-γ mAb recipients vs. 18.4% ± 18.1% in control recipients, p = 0.0411). Quantitatively, there was no significant difference between the CAV lesions in the group that received pharmacologic cytokine blockade and the prior described group that utilized IFN-γ deficient NK cells (NI 3.5% ± 4.2% in anti-IFN-γ mAb recipients vs. 3.3% ± 2.9% in IFN-γ deficient NK cell recipients, p = 0.491) (Table 2 and Figure 3). Taken together, these data show that lack of NK cell-derived IFN-γ production or systemic neutralization of this cytokine significantly attenuated CAV.
Table 2.
Effect of deficiencies in IFN-γ versus cytolytic pathways on the frequency of chronic allograft vasculopathy. Control groups were re-listed for purpose of comparison.
| Group | Donor | Recipient | Treatment | Frequency of CAV |
|---|---|---|---|---|
| 1* | C3H | B6.rag−/− | α-H2k | 5/6 |
| 2 | α-H2k + α- IFN-γ | 1/6 | ||
| 3** | C3H | B6.rag−/−γc−/− | No Rx | 0/5 |
| 4*** | α-H2k + B6 wild-type NK cells | 6/6 | ||
| 5 | α-H2k + B6.IFN-γ−/− NK cells | 0/8 | ||
| 6 | α-H2k + B6.pfp−/− NK cells | 4/6 | ||
| 7 | C3H.lpr | B6.rag−/−γc−/− | α-H2k + B6 wild-type NK cells | 6/8 |
| 8 | α-H2k + B6.pfp−/− NK cells | 0/6 |
Figure 3. Effects of IFN-γ depletion on NK cell and DSA mediated chronic allograft vasculopathy.
In B6.rag−/−γc−/− recipients bearing C3H allografts that received DSA (α-H2k IgG2a) and NK cells deficient in IFN-γ production, CAV formation was virtually eliminated (p = 0.0007) in comparison to controls. To test this pathway via an alternate means, B6.rag−/− recipients bearing C3H allografts were treated with DSA in addition to IFN-γ depleting mAb. Treatment with anti-IFN-γ IgG1 also leads to a significant decrease in CAV formation in comparison to controls (p = 0.0411). The control groups as indicated on this figure are the same as depicted in Figure 2 and are provided for comparative purposes. Annotations are as in Figure 2. CAV, chronic allograft vasculopathy; DSA, donor-specific antibody.
Perforin and Fas play alternate, but obligatory roles in NK cell-mediated CAV
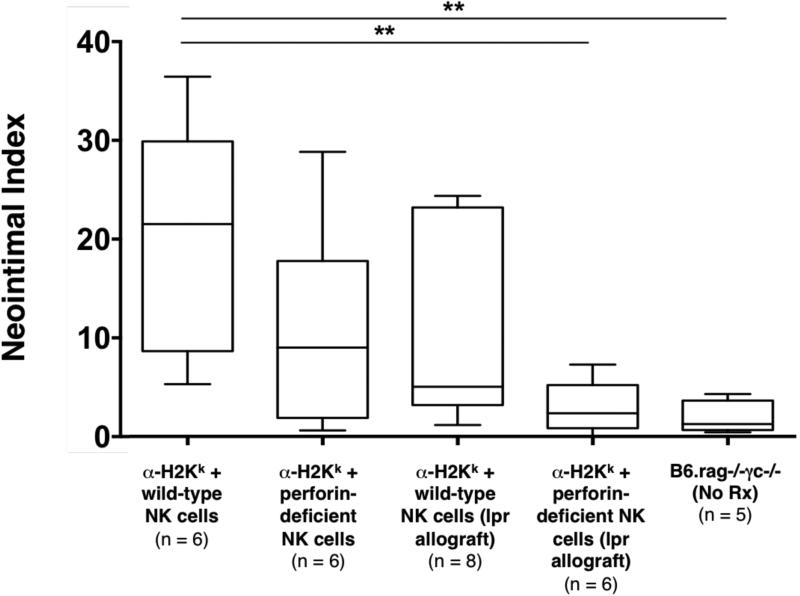
Given the additional role of NK cells as mediators of contact-dependent target cell lysis, we also examined the contribution of these pathways on CAV development. NK cells depend primarily on the perforin system to mediate target cell lysis, but they can also express FasL upon activation and can utilize this pathway as well to mediate cell death (30-33). Therefore, we hypothesized that either perforin or Fas/FasL interaction could be a key mechanism by which NK cells facilitate injury within our model. To test whether perforin deficiency would have an effect on CAV, C3H allografts were transplanted into B6.rag−/−γc−/− recipients that received DSA. Recipients also received adoptively transferred B6 NK cells deficient in perforin production. Lesions triggered by perforin-deficient NK cells appeared to be somewhat less severe than controls, but the quantitative difference on statistical analysis was not significant (NI 9.1% ± 8.6% in perforin-deficient NK cell recipients vs. 21.5% ± 20.1% in control recipients, p = 0.240) (Table 2 and Figure 4).
Figure 4. Effects of perturbations in cytolytic pathways on NK cell and DSA mediated chronic allograft vasculopathy.
In B6.rag−/−γc−/− recipients bearing C3H allografts that received DSA (α-H2k IgG2a) and perforin-deficient NK cells, CAV formation was minimally decreased, but not statistically significant in comparison to controls. Concurrently, in B6.rag−/−γc−/− recipients bearing C3H.lpr (Fas deficient) allografts that received DSA and wild-type NK cells, CAV formation again was minimally decreased, but not statistically significant in comparison to controls. Neither deficiency in perforin nor the Fas/FasL pathway alone altered CAV formation. However, a combined absence of these two pathways lead to a significant reduction in CAV (p = 0.0043) to a level that was the same as control animals that received no treatment (p = 0.662). Control groups indicated on this figure are the same as depicted in Figure 2 and are provided for comparative purposes. Annotations are as in Figure 2. CAV, chronic allograft vasculopathy; DSA, donor-specific antibody.
Alternatively, to test if Fas/FasL interaction was required for this form of chronic rejection, Fas-deficient C3H.lpr allografts were transplanted into B6.rag−/−γc−/− recipients that received DSA as described prior and adoptively transferred wild-type B6 NK cells. Due to lack of expression of Fas on cardiac allograft (target) cells, the NK cells would be unable to utilize this pathway to mediate cell lysis. On histological examination, the vascular lesions again appeared minimally reduced (with wider variability) but were not statistically significant from controls (NI 5.1% ± 8.8% in recipients bearing C3H.Ipr cardiac allografts vs. 21.5% ± 20.1% in control recipients, p = 0.081) (Table 2 and Figure 4).
Although deficiency in either pathway alone did not appreciably alter the development of CAV lesions, we then sought to test the hypothesis that perforin and Fas/FasL represent alternative, but essential mechanisms of NK cell-mediated chronic allograft rejection as found in prior studies of cytotoxic T cell-mediated acute rejection (20, 21). Therefore, we combined the transfer of perforin-deficient NK cells with the transplantation of Fas-deficient C3H.lpr allografts to achieve simultaneous disruption of perforin and Fas/FasL pathways in B6.rag−/−γc−/− recipients receiving DSA. Interestingly, none of the animals within this group (0/6) showed significant CAV lesions, demonstrating that concurrent disruption of NK cell-derived perforin and allograft Fas expression resulted in a marked reduction of CAV in comparison to controls (NI 2.4% ± 2.6 in perforin and Fas-deficient recipients vs. 21.5% ± 20.1% in control recipients, p = 0.0043) (Table 2 and Figure 4). Notably, we also confirmed that perforin-deficient NK cells retain the capacity to produce IFN-γ (Figure S1).
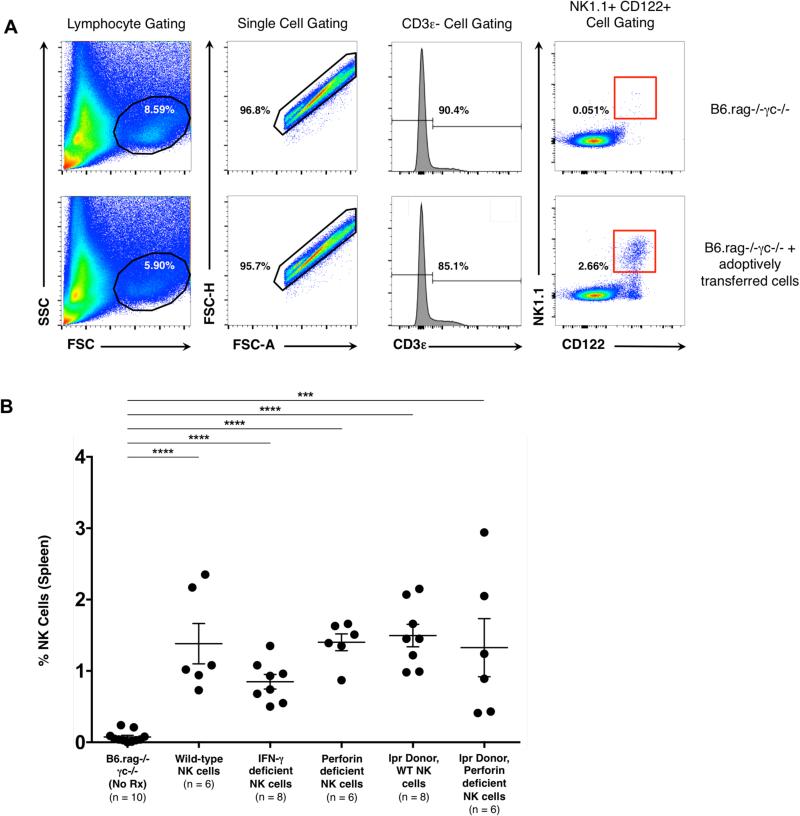
Adoptively transferred NK cells persist within recipients and are detectable post-transplantation
A key issue underlying the interpretation of these experiments is whether the NK cell phenotypes utilized impacted engraftment and survival of these cells in adoptive transfer recipients. Prior studies have shown that wild-type NK cells undergo homeostatic expansion when adoptively transferred into a lymphopenic environment; importantly, these cells are long-lived and retain their functionality (34, 35). To ensure that adoptively transferred NK cells survived in the B6.rag−/−γc−/− recipients described above, whole spleens were recovered from recipient animals at the time of allograft harvest. Analysis by flow cytometry confirmed the presence of NK cells as characterized by a CD3ε−, CD122+. NK1.1+ phenotype. This analysis also established that the adoptively transferred cells from all experimental groups survived and persisted in the recipients with no significant difference between the treatment groups (p = 0.226) (Figure 5). Recipient spleens were also analyzed for the presence of either CD4+ (CD45.2 +, CD3ε+, CD4+) or CD8+ (CD45.2 +, CD3ε+, CD8+) T cells. Neither T cell population could be detected (data not shown), further confirming that T cells were unlikely to contribute to the responses detailed above.
Figure 5. Adoptively transferred NK cells persist in B6.rag−/−γc− recipients at day 30 post-transplantation.
(A) Gating strategy to identify NK cells post-transplantation and adoptive transfer. A lymphocyte population was identified via forward / side scatter and further narrowed to single cells. Cells were then gated for those that were CD3ε-, CD122+ and NK 1.1+. Row 1 indicates representative flow plots from a B6.rag−/−γc−/− recipient that did not receive adoptively transferred cells. Row 2 indicates representative flow plots from a B6.rag−/−γc−/− that received adoptively transferred NK cells. (B) Whole spleens were obtained from recipients at the time of allograft recovery and analyzed with flow cytometry for the presence of NK cells. At baseline, B6.rag−/−γc−/− recipients have no mature NK cells. The percentage of NK cells in groups that received adoptively transferred cells was significantly higher than control animals (p < 0.001 for all groups). Error bars indicate the mean ± SEM. There was no significant difference in the percentage of cells between the treatment groups on statistical analysis using one-way ANOVA (p = 0.226).
Discussion
Despite extensive characterization of acute allograft rejection in both vascularized and nonvascularized transplant models, there is surprisingly little evidence elucidating the precise mechanism(s) that underlie the immune response in chronic allograft rejection. In addition, the exact role of donor specific antibodies and their contribution to this process also remains unclear. Prior results indicate that NK cells can play a key role in antibody-mediated chronic rejection by interacting with DSA through an Fc receptor dependent manner to trigger CAV independently of other cellular or complement components (14). NK cells are potent mediators of innate immunity and NK cell responses in solid organ transplantation have been studied in both animal models and clinical studies (36-41). However, much of the current understanding of NK cell biology is derived from basic in vitro cellular analysis and studies based on viral infectious diseases and tumor immunity. In the setting of transplantation, the role of these cells is much less clear, as NK cells have been shown to have both pathologic and regulatory effects depending on both timing of cellular interactions, as well as the nature of the allograft itself (42).
In the present study, we show that CAV inflicted by NK cells requires NK cell expression of IFN-γ. IFN-γ is considered the ‘prototypical’ pro-inflammatory cytokine and has been shown to have both pathogenic and regulatory roles in the T cell-mediated alloimmune response. On one hand, IFN-γ production correlates strongly with acute rejection; for example, it is required for efficient CD8+ T cell-mediated islet cell rejection (19) and for the rejection of MHC Class II skin allografts (43, 44). The presence of donor IFN-γ receptors is also required for CD4+ T cell-mediated acute cardiac rejection (22). Paradoxically, however, the presence of IFN-γ has also been associated with allograft prolongation. In murine skin and cardiac models of co-stimulation blockade, IFN-γ production facilitates long-term allograft survival, presumably through limiting expansion of allograft-reactive activated T cells (45). NK cells are known to be key producers of a number of cytokines, notably IFN-γ. In the context of virally infected cells, this cytokine has direct cytotoxic effects and perpetuates a continued inflammatory response since it is a potent activator of both NK cells and macrophages (46, 47). IFN-γ also induces IL-12 secretion, which further increases NK cell-mediated toxicity and promotes additional IFN-γ production (29, 48, 49). The present results indicate that NK cell IFN-γ production does not merely correlate with CAV, but is actually a rate-limiting effector pathway in this response.
NK cells are also “early responders,” to sites of inflammation and infection and a key differentiator of NK cells from conventional cytotoxic T cells is the ability to kill target cells rapidly without the requirement for prior immunization (50). Similar to cytotoxic T cells, NK cells directly induce target cell apoptosis, mainly through secretion of perforin, but also through Fas/FasL interactions (30-33). As the sensitivity to cytolytic pathways is likely dictated by the target cell (20, 51), both pathways were interrogated as potential contributors to triggering CAV. Our data shows that individual disruption of either NK cell-derived perforin or allograft Fas expression did not significantly alter the development of CAV. However, the simultaneous disruption of NK cell-perforin production and allograft Fas expression largely attenuated lesion development. This suggests that CAV is mediated by alternative, but obligate utilization of these cytolytic processes. Taken in combination, our data indicates that both IFN-γ and the alternative pathways of perforin and Fas/FasL are rate-limiting in this model of chronic rejection.
Based on current findings, we would propose the possibility of a ‘two-hit’ model of NK cell-mediated chronic allograft vasculopathy in vivo. As the first step, IFN-γ clearly plays an important role in the NK response and it is possible that this cytokine first ‘conditions’ the target cells to be more efficiently killed by a given cytolytic pathway. One possible mechanism is through up-regulation of NK cell-activating ligands or down-regulation of NK cell-inhibitory ligands. The current prevailing view of NK cells is that they distinguish between healthy and damaged cells by integrating signals from stimulatory and inhibitory receptors expressed on target cells. The resultant sum of these signals then results in either NK cell activation or quiescence (52). Both the secretion of cytokines and activation of cytolytic machinery are dependent on this interplay of surface and intracellular receptors (53). Classically, the main inhibitory ligand recognized on target cells is MHC class I, leading to the “missing-self” concept of target cell recognition [reviewed in (54)]. However, the lack of MHC class I molecules alone is not sufficient for activation of NK cells (55), suggesting that regulation of other ligands must be altered to induce a NK cell response. Another possibility is that IFN-γ is involved by making target cells more sensitive to contact-dependent cytotoxic killing. For example, expression of platelet activating factor receptor (PAFR) on tumor target cell membranes is required to render the membrane susceptible to NK cell perforin-mediated damage, and IFN-γ has been shown to induce PAFR expression on target cells (56). Therefore, it is possible that target cell susceptibility to NK cell-mediated lysis is not an inherent property of these cells, but requires the expression of appropriate cell-surface ligands to occur.
Interestingly, taken as a whole, these results are quite similar to previous studies of CD8+ T cell-mediated acute islet allograft rejection and CD4+ T cell-mediated acute cardiac allograft rejection. Diamond et al showed that IFN-γ was essential for efficient CD8+ T cell-mediated rejection of islet allografts (19). A follow-up study by Sleater et al demonstrated that individual disruption of either T cell-derived perforin or allograft Fas expression alone did not significantly delay CD8+ T cell-mediated rejection, but that simultaneous interruption of T cell-derived perforin and allograft Fas expression completely diminished CD8+ T cell-mediated rejection (21). In CD4+ T cell-mediated acute cardiac allograft rejection, expression of the IFN-γ receptors on donor cells was required (22). Furthermore, Grazia et al showed that the individual disruption of either CD4+ T cell-derived perforin or donor Fas expression had no significant impact on the tempo of rejection, but that simultaneous removal of both donor Fas expression and CD4+ T cell-derived perforin abrogated the response (20). Although our model system utilizes the additional requirement of donor specific antibody, the parallels between cytotoxic T cells and NK cells is striking. Taken together, we would postulate that these varied contact-dependent cellular pathways of acute and chronic immune-mediated injury might utilize analogous effector mechanisms to govern their response.
Limitations of these studies should be mentioned. Although NK cells require IFN-γ within this process, the exact downstream effect of this cytokine currently is unknown. The timing of this process is unclear as well; the majority of allograft injury could be initiated early during the peri-transplant period or may be a sustained response over time. Lastly, this model was specifically developed to query the mechanisms involved in NK cell-mediated chronic allograft dysfunction and thus contribution from the adaptive immune system (T cells) was intentionally eliminated. NK cells can either inhibit or enhance T cell responses [reviewed in (57)] and in a physiologic transplantation setting, the overall importance of the NK cell response in this setting remains unclear.
In conclusion, results show that NK cell-mediated chronic allograft vasculopathy requires both IFN-γ production as well as contact-dependent cytotoxic activity. These results also highlight the redundancy of the immune system and raise the possibility that both acute and chronic contact-dependent allograft rejection may have similar requirements for inflicting tissue injury, whether it is through an adaptive (CD4+ or CD8+ T cell) or an innate (NK cell) mediated immune response.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) / National Center for Advancing Translational Science Colorado CTSA Grant Number UL1 TR001082 and in part by a Fellowship Grant from the International Society for Heart and Lung Transplantation (ISHLT). Contents are the authors’ sole responsibility and do not necessarily represent official NIH or ISHLT views. The authors would like to thank Dan Koyanyagi for his expertise the preparation of all histology samples.
Abbreviations
- CAV
chronic allograft vasculopathy
- DSA
donor specific antibodies
- FasL
Fas ligand
- IFN-γ
interferon gamma
- NK
natural killer
- MHC
major histocompatibility complex
- WT
wild-type
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2013 Annual Data Report: liver. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–28. doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- 2.Valapour M, Skeans MA, Heubner BM, Smith JM, Hertz MI, Edwards LB, et al. OPTN/SRTR 2013 Annual Data Report: lung. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–28. doi: 10.1111/ajt.13200. [DOI] [PubMed] [Google Scholar]
- 3.Kandaswamy R, Skeans MA, Gustafson SK, Carrico RJ, Tyler KH, Israni AK, et al. OPTN/SRTR 2013 Annual Data Report: pancreas. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–20. doi: 10.1111/ajt.13196. [DOI] [PubMed] [Google Scholar]
- 4.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, et al. OPTN/SRTR 2013 Annual Data Report: kidney. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 5.Colvin-Adams M, Smith JM, Heubner BM, Skeans MA, Edwards LB, Waller CD, et al. OPTN/SRTR 2013 Annual Data Report: heart. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Jan;15(Suppl 2):1–28. doi: 10.1111/ajt.13199. [DOI] [PubMed] [Google Scholar]
- 6.Kwun J, Knechtle SJ. Overcoming Chronic Rejection-Can it B? Transplantation. 2009 Oct 27;88(8):955–61. doi: 10.1097/TP.0b013e3181b96646. Epub 2009/10/27. eng. [DOI] [PubMed] [Google Scholar]
- 7.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2010 Annual Data Report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: 2010. [Google Scholar]
- 8.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004 Jul;4(7):1033–41. doi: 10.1111/j.1600-6143.2004.00500.x. Epub 2004/06/16. eng. [DOI] [PubMed] [Google Scholar]
- 9.Roux A, Bendib Le Lan I, Holifanjaniaina S, Thomas KA, Hamid AM, Picard C, et al. Antibody-Mediated Rejection in Lung Transplantation: Clinical Outcomes and Donor-Specific Antibody Characteristics. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016 Feb 4; doi: 10.1111/ajt.13589. [DOI] [PubMed] [Google Scholar]
- 10.Fidler SJ, Irish AB, Lim W, Ferrari P, Witt CS, Christiansen FT. Pre-transplant donor specific anti-HLA antibody is associated with antibody-mediated rejection, progressive graft dysfunction and patient death. Transplant immunology. 2013 Jun;28(4):148–53. doi: 10.1016/j.trim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Filippone EJ, Farber JL. Humoral Immune Response and Allograft Function in Kidney Transplantation. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015 Aug;66(2):337–47. doi: 10.1053/j.ajkd.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Morrow WR, Frazier EA, Mahle WT, Harville TO, Pye SE, Knecht KR, et al. Rapid reduction in donor-specific anti-human leukocyte antigen antibodies and reversal of antibody-mediated rejection with bortezomib in pediatric heart transplant patients. Transplantation. 2012 Feb 15;93(3):319–24. doi: 10.1097/TP.0b013e31823f7eea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuadrado A, San Segundo D, Lopez-Hoyos M, Crespo J, Fabrega E. Clinical significance of donor-specific human leukocyte antigen antibodies in liver transplantation. World journal of gastroenterology. 2015 Oct 21;21(39):11016–26. doi: 10.3748/wjg.v21.i39.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Feb;12(2):313–21. doi: 10.1111/j.1600-6143.2011.03836.x. Epub 2011/11/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravetch JV, Bolland S. IgG Fc receptors. Annual review of immunology. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 16.Kagi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Lymphocyte-mediated cytotoxicity in vitro and in vivo: mechanisms and significance. Immunological reviews. 1995 Aug;146:95–115. doi: 10.1111/j.1600-065x.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 17.Kagi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annual review of immunology. 1996;14:207–32. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 18.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nature immunology. 2000 Dec;1(6):469–74. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 19.Diamond AS, Gill RG. An essential contribution by IFN-gamma to CD8+ T cell-mediated rejection of pancreatic islet allografts. J Immunol. 2000 Jul 1;165(1):247–55. doi: 10.4049/jimmunol.165.1.247. [DOI] [PubMed] [Google Scholar]
- 20.Grazia TJ, Plenter RJ, Weber SM, Lepper HM, Victorino F, Zamora MR, et al. Acute cardiac allograft rejection by directly cytotoxic CD4 T cells: parallel requirements for Fas and perforin. Transplantation. 2010 Jan 15;89(1):33–9. doi: 10.1097/TP.0b013e3181be6bc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sleater M, Diamond AS, Gill RG. Islet allograft rejection by contact-dependent CD8+ T cells: perforin and FasL play alternate but obligatory roles. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007 Aug;7(8):1927–33. doi: 10.1111/j.1600-6143.2007.01889.x. [DOI] [PubMed] [Google Scholar]
- 22.Wiseman AC, Pietra BA, Kelly BP, Rayat GR, Rizeq M, Gill RG. Donor IFN-gamma receptors are critical for acute CD4(+) T cell-mediated cardiac allograft rejection. J Immunol. 2001 Nov 1;167(9):5457–63. doi: 10.4049/jimmunol.167.9.5457. [DOI] [PubMed] [Google Scholar]
- 23.Corry RJ, Winn HJ, Russell PS. Heart transplantation in congenic strains of mice. Transplantation proceedings. 1973 Mar;5(1):733–5. [PubMed] [Google Scholar]
- 24.Niimi M. The technique for heterotopic cardiac transplantation in mice: experience of 3000 operations by one surgeon. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2001 Oct;20(10):1123–8. doi: 10.1016/s1053-2498(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 25.Pieraggi M, Nejjar I, Julian M, Bouissou H. Staining of elastic tissue by Verhoeff's iron hematoxylin. Annales de pathologie. 1986;6(1):74–7. [PubMed] [Google Scholar]
- 26.Armstrong AT, Strauch AR, Starling RC, Sedmak DD, Orosz CG. Morphometric analysis of neointimal formation in murine cardiac allografts. Transplantation. 1997 Apr 15;63(7):941–7. doi: 10.1097/00007890-199704150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong AT, Strauch AR, Starling RC, Sedmak DD, Orosz CG. Morphometric analysis of neointimal formation in murine cardiac allografts: II. Rate and location of lesion development. Transplantation. 1997 Jul 27;64(2):322–8. doi: 10.1097/00007890-199707270-00025. Epub 1997/07/27. eng. [DOI] [PubMed] [Google Scholar]
- 28.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005 Feb 1;174(3):1213–21. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 29.Paolini R, Bernardini G, Molfetta R, Santoni A. NK cells and interferons. Cytokine & growth factor reviews. 2015 Apr;26(2):113–20. doi: 10.1016/j.cytogfr.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Oshimi Y, Oda S, Honda Y, Nagata S, Miyazaki S. Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. J Immunol. 1996 Oct 1;157(7):2909–15. [PubMed] [Google Scholar]
- 31.Shresta S, Pham CT, Thomas DA, Graubert TA, Ley TJ. How do cytotoxic lymphocytes kill their targets? Current opinion in immunology. 1998 Oct;10(5):581–7. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- 32.Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, et al. Activation of NK cell cytotoxicity. Molecular immunology. 2005 Feb;42(4):501–10. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 33.Kojima Y, Kawasaki-Koyanagi A, Sueyoshi N, Kanai A, Yagita H, Okumura K. Localization of Fas ligand in cytoplasmic granules of CD8+ cytotoxic T lymphocytes and natural killer cells: participation of Fas ligand in granule exocytosis model of cytotoxicity. Biochemical and biophysical research communications. 2002 Aug 16;296(2):328–36. doi: 10.1016/s0006-291x(02)00841-0. [DOI] [PubMed] [Google Scholar]
- 34.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. The Journal of experimental medicine. 2003 Apr 21;197(8):967–76. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. The Journal of experimental medicine. 2011 Feb 14;208(2):357–68. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldwin WM, 3rd, Larsen CP, Fairchild RL. Innate immune responses to transplants: a significant variable with cadaver donors. Immunity. 2001 Apr;14(4):369–76. doi: 10.1016/s1074-7613(01)00117-0. [DOI] [PubMed] [Google Scholar]
- 37.Kitchens WH, Uehara S, Chase CM, Colvin RB, Russell PS, Madsen JC. The changing role of natural killer cells in solid organ rejection and tolerance. Transplantation. 2006 Mar 27;81(6):811–7. doi: 10.1097/01.tp.0000202844.33794.0e. [DOI] [PubMed] [Google Scholar]
- 38.Obara H, Nagasaki K, Hsieh CL, Ogura Y, Esquivel CO, Martinez OM, et al. IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005 Sep;5(9):2094–103. doi: 10.1111/j.1600-6143.2005.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersson E, Ostraat O, Ekberg H, Hansson J, Simanaitis M, Brodin T, et al. Allogeneic heart transplantation activates alloreactive NK cells. Cellular immunology. 1997 Jan 10;175(1):25–32. doi: 10.1006/cimm.1996.1031. [DOI] [PubMed] [Google Scholar]
- 40.Fildes JE, Yonan N, Tunstall K, Walker AH, Griffiths-Davies L, Bishop P, et al. Natural killer cells in peripheral blood and lung tissue are associated with chronic rejection after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2008 Feb;27(2):203–7. doi: 10.1016/j.healun.2007.11.571. Epub 2008/02/13. eng. [DOI] [PubMed] [Google Scholar]
- 41.Meehan AC, Sullivan LC, Mifsud NA, Brooks AG, Snell GI, Kotsimbos TC, et al. Natural killer cell activation in the lung allograft early posttransplantation. Transplantation. 2010 Mar 27;89(6):756–63. doi: 10.1097/TP.0b013e3181cab17f. Epub 2009/12/19. eng. [DOI] [PubMed] [Google Scholar]
- 42.Gill RG. NK cells: elusive participants in transplantation immunity and tolerance. Current opinion in immunology. 2010 Oct;22(5):649–54. doi: 10.1016/j.coi.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg AS, Finbloom DS, Maniero TG, Van der Meide PH, Singer A. Specific prolongation of MHC class II disparate skin allografts by in vivo administration of anti-IFN-gamma monoclonal antibody. J Immunol. 1990 Jun 15;144(12):4648–50. [PubMed] [Google Scholar]
- 44.Ring GH, Saleem S, Dai Z, Hassan AT, Konieczny BT, Baddoura FK, et al. Interferon-gamma is necessary for initiating the acute rejection of major histocompatibility complex class II-disparate skin allografts. Transplantation. 1999 May 27;67(10):1362–5. doi: 10.1097/00007890-199905270-00012. [DOI] [PubMed] [Google Scholar]
- 45.Konieczny BT, Dai Z, Elwood ET, Saleem S, Linsley PS, Baddoura FK, et al. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998 Mar 1;160(5):2059–64. [PubMed] [Google Scholar]
- 46.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. The Journal of experimental medicine. 1983 Sep 1;158(3):670–89. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annual review of immunology. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 48.Wang KS, Frank DA, Ritz J. Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood. 2000 May 15;95(10):3183–90. [PubMed] [Google Scholar]
- 49.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annual review of immunology. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 50.Trinchieri G. Biology of natural killer cells. Advances in immunology. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamoto Y, Guidotti LG, Pasquetto V, Schreiber RD, Chisari FV. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. J Immunol. 1997 Jun 15;158(12):5692–7. [PubMed] [Google Scholar]
- 52.Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annual review of immunology. 2001;19:291–330. doi: 10.1146/annurev.immunol.19.1.291. [DOI] [PubMed] [Google Scholar]
- 53.Poggi A, Zocchi MR. NK cell autoreactivity and autoimmune diseases. Frontiers in immunology. 2014;5:27. doi: 10.3389/fimmu.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunological reviews. 2006 Dec;214:143–54. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 55.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20-26;319(6055):675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 56.Berthou C, Bourge JF, Zhang Y, Soulie A, Geromin D, Denizot Y, et al. Interferon-gamma-induced membrane PAF-receptor expression confers tumor cell susceptibility to NK perforin-dependent lysis. Blood. 2000 Apr 1;95(7):2329–36. [PubMed] [Google Scholar]
- 57.Crouse J, Xu HC, Lang PA, Oxenius A. NK cells regulating T cell responses: mechanisms and outcome. Trends in immunology. 2015 Jan;36(1):49–58. doi: 10.1016/j.it.2014.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.