Summary
We performed a retrospective study analysing the effect of sorafenib, an oral fms-Like Tyrosine Kinase 3 (FLT3/multikinase inhibitor, as post-transplant maintenance in adult patients with FLT3-internal tandem duplication (ITD) acute myeloid leukaemia (AML). We identified consecutive patients with FLT3-ITD AML diagnosed between 2008 and 2014 who received haematopoietic cell transplantation (HCT) in first complete remission (CR1). Post-HCT initiation of sorafenib (yes/no) was evaluated as a time-varying covariate in the overall survival/progression-free survival (OS/PFS) analysis and performed a landmark analysis of controls alive without relapse at the median date of sorafenib initiation. We identified 26 sorafenib patients and 55 controls. Median follow-up was 27.2 months post-HCT for sorafenib survivors, and 38.4 months for controls (p=0.021). The median time to initiating sorafenib was 68 days post-HCT; 43 controls were alive without relapse at this cut-off. Sorafenib patients had improved 2-year OS in the d+68 landmark analysis (81% vs. 62%, p=0.029). Sorafenib was associated with improved 2-year PFS (82% vs. 53%, p=0.0081) and lower 2-year cumulative incidence of relapse (8.2% vs. 37.7%, p=0.0077). In multivariate analysis, sorafenib significantly improved OS (Hazard ratio [HR] 0.26, p=0.021) and PFS (HR 0.25, p=0.016). There was no difference in 2-year non-relapse mortality (9.8% vs. 9.3%, p=0.82) or 1-year chronic graft-versus-host disease (55.5% vs. 37.2%, p=0.28). These findings suggest potential benefit of post-HCT sorafenib in FLT3-ITD AML, and support further evaluation of post-HCT FLT3 inhibition.
Keywords: Acute Myeloid Leukaemia, fms-Like Tyrosine Kinase 3, Allogeneic Transplantation, Maintenance chemotherapy, Sorafenib
Introduction
At diagnosis, approximately 25% of patients with acute myeloid leukaemia (AML) harbour an internal tandem duplication (ITD) mutation in the fms-like tyrosine kinase 3 receptor (FLT3) gene (FLT3-ITD) (Schlenk et al, 2008). The FLT3 protein is a class III receptor tyrosine kinase, similar to KIT and platelet-derived growth factor receptor (PDGFR), involved in normal haematopoietic precursor growth and differentiation (Kiyoi et al, 1998). The ITD mutation is an in-frame duplication within exon 14, found in the juxtamembrane domain of the protein (Abu-Duhier et al, 2001). Duplications may range in length from 3 base pairs, to several hundred (Schnittger et al, 2002, 3; Stirewalt et al, 2006), and result in constitutive activation of the receptor tyrosine kinase activity. With conventional induction chemotherapy regimens, patients with FLT3-ITD AML achieve remission rates similar to those with FLT3 wildtype status; however, patients with FLT3-ITD have significantly shorter remission durations and a higher rates of relapse (Röllig et al, 2011).
Given the high relapse rate, long-term survival of patients with FLT3-ITD AML treated with chemotherapy alone has been historically poor, with fewer than 30% of patients alive at 5 years after diagnosis. Several analyses have supported allogeneic transplant as a means to improve the outcomes of patients with FLT3-ITD AML, with some suggesting approximately 50% of patients remain alive at 5 years after HCT (Oran et al, 2014; Schlenk et al, 2008, 2014). Therefore, otherwise suitable patients with FLT3-ITD AML generally proceed to allogeneic haematopoietic cell transplantation (allo-HCT) in first remission (Brunet et al, 2012). The impact of transplantation may depend upon both the length of the ITD as well as the allelic burden of the FLT3-ITD subclone for a given patient (Schlenk et al, 2014; Schnittger et al, 2002). Nonetheless, while encouraging, even for patients undergoing allo-HCT, the rate of AML relapse remains unacceptably high.
Inhibition of activated FLT3 is an important developing therapeutic strategy to treat active disease, and also to prevent AML relapse during remission (Schiller et al, 2016). A number of tyrosine kinase inhibitors (TKIs) with FLT3 inhibitory activity have been studied in the up-front and relapsed/refractory setting, including midostaurin, sorafenib, quizartinib, lestaurtinib, crenolanib and gilteritinib. Sorafenib is a multikinase inhibitor, approved for the treatment of advanced hepatocellular, renal cell and thyroid cancers, possessing FLT3-ITD inhibitory activity. It can produce remissions when used as monotherapy or in combination with induction chemotherapy or hypomethylating agents to treat FLT3-ITD AML (Röllig et al, 2015; Ravandi et al, 2013).
We have recently shown sorafenib to be safely tolerated as post-HCT maintenance therapy in patients with FLT3-ITD AML (Chen et al, 2014). Although the rates of progression-free (PFS) and overall survival (OS) were superior to those seen historically, there was no comparison group in the study, and limited follow-up. Therefore, we performed a retrospective analysis to compare contemporaneously treated patients with FLT3-ITD AML in first complete remission (CR1) after standard chemotherapy and allo-HCT according to whether they received sorafenib maintenance or not. Our objectives were to determine whether sorafenib maintenance was associated with improved OS, as well as other clinical outcomes including PFS, disease relapse, incidence of graft-versus-host disease (GVHD) and non-relapse mortality.
Methods
This study was approved by the institutional review board at the Dana-Farber Harvard Cancer Center. We retrospectively identified consecutive patients treated at Massachusetts General Hospital and the Dana-Farber Cancer Institute with a new diagnosis of AML between 1 January 2008 and 31 December 2014. We included only those patients who underwent testing for the presence of a FLT3-ITD mutation at diagnosis, prior to receipt of induction chemotherapy. Patients had to receive induction chemotherapy as their initial treatment, and achieve a complete remission, according to standard criteria (Cheson et al, 2003), after one induction regimen. Patients receiving a “7+3” backbone induction regimen could be given a second course of similar chemotherapy for residual disease at a mid-treatment marrow; however, patients with chemo-refractory disease were excluded. Patients were included only if they underwent allo-HCT while in CR1; they could have received consolidation chemotherapy, including FLT3-directed TKI therapy, while in first remission prior to allo-HCT. Cytogenetic risk was assessed using the criteria proposed by the National Comprehensive Cancer Network (O’Donnell et al, 2012).
We then identified those patients who were treated with maintenance sorafenib following allo-HCT while in ongoing remission from AML. Sixteen of the 26 patients received sorafenib in the setting of a phase I clinical trial (Chen et al, 2014), while the other 10 received the drug off-label as prescribed by their treating physician. Patients given sorafenib in remission after HCT were in the “sorafenib” group, and other patients meeting the inclusion criteria above but not treated with sorafenib comprised the “control” group. Sorafenib therapy was dosed between 200 and 400 mg twice daily, and patients were assessed to initiate therapy starting approximately day +45 after transplant and onward, according to the treating physician and in a fashion similar to that previously described (NCT01398501) (Chen et al, 2014). Patients identified from the phase I study were treated according to their dose level, while patients treated outside of the trial were started at the maximum tolerated dose (MTD), 400 mg BID. The decision to start sorafenib was per the treating physician, but in general was done following haematopoietic recovery (absolute neutrophil count [ANC] > 1 × 109/l, platelet count > 50× 109/l), and in the absence of uncontrolled hypertension, bleeding, non-healing wounds or active GVHD requiring greater than 0.5 mg/kg/day of prednisone or additional therapy. Sorafenib treatment was planned for 12–24 months, but continuation or early cessation was per the discretion of the treating physician.
The maintenance effect of sorafenib was evaluated as a time-varying covariate. In order to account for the differential time post-HCT where patients would not yet start sorafenib and were at risk of early relapse, we also performed exploratory landmark analyses that included only those controls who were alive and without disease relapse at the median date of sorafenib initiation post-HCT, which was day +68, as well as a control group using a day +100 cut-off. The distribution of categorical or continuous patient characteristics was assessed between groups using Fisher’s exact test or Wilcoxon rank-sum test. OS and PFS were estimated using the Kaplan-Meier method. Patients alive without relapse were censored at the date of last contact. Log-rank tests were used to compare PFS and OS between groups, and Cox proportional hazards regression models were used to study the maintenance effect of sorafenib on PFS and OS while adjusting for other risk factors. Time to relapse and time to non-relapse mortality (NRM) were analysed using each other as competing risks. Time to chronic GVHD (cGVHD) onset was analysed using early relapse and death without GVHD as competing risks. In competing risk settings, Gray’s methods were used to compare between groups and competing risk regressions were used to test the significance of the sorafenib effect adjusting for other covariates. Other factors adjusted in regression models include age, conditioning intensity, NPM1 mutational status, donor match, cytogenetic risk classification and consolidation therapy.
Results
A total of 81 patients were identified with a new diagnosis of FLT3-ITD mutant AML undergoing allo-HCT while in CR1. Of these, 26 patients received sorafenib post-HCT (median start date +68 days, range 36 to 193) and comprised the sorafenib group. The other 55 control patients included 43 alive without relapse at day +68, the median date of sorafenib initiation, who were the control group for the day +68 landmark analysis; and 40 alive without relapse at day +100.
There were no significant differences in patient age, sex, race, performance status at diagnosis, antecedent disease, cytogenetic risk, or NPM1 mutational status between sorafenib and control patients (Table I). Two patients in the control group had concomitant FLT3- tyrosine kinase domain (TKD) mutations. At the time of transplant, there was no significant difference in performance status, conditioning intensity, donor match, or GVHD prophylaxis regimen. There was no difference in day +68 ANC (p=0.89) platelet count (p=0.80) or chimerism (all cell, p=0.50). There was longer follow-up for control patients compared to sorafenib patients; median follow-up was 27.2 months (range, 9.4–54.0) for survivors in the sorafenib group and 38.4 months (range, 15.6–93.6) in the control group (p=0.021).
Table I.
Patient Characteristics, including the entire cohort, as well as the subset of controls (n=43) included in the day +68 landmark analysis.
| Sorafenib Patients (n=26) | All Control Patients (n=55) | P-value vs. Sorafenib | Day +68 Landmark Controls (n=43) | P-value vs. Sorafenib | |
|---|---|---|---|---|---|
|
| |||||
| Age, years; median (range) | 55 (20–74) | 56 (25–73) | 0.75 | 56 (25–73) | 0.78 |
|
| |||||
| Sex, male | 12 (46%) | 19 (35%) | 0.34 | 13 (30%) | 0.21 |
|
| |||||
| Race, white | 24 (92%) | 48 (87%) | 0.99 | 37 (86%) | 0.99 |
|
| |||||
| Antecedent Disease | 0.62 | 0.64 | |||
| De novo | 22 (85%) | 43 (78%) | 34 (79%) | ||
| tAML | 3 (12%) | 6 (11%) | 4 (9%) | ||
| Prior MDS or MPN | 1 (4%) | 6 (11%) | 5 (12%) | ||
|
| |||||
| Cytogenetic Risk | 0.99 | 0.84 | |||
| Favourable | 1 (4%) | 2 (4%) | 2 (5%) | ||
| Intermediate | 23 (88%) | 47 (87%) | 38 (90%) | ||
| Adverse | 2 (8%) | 4 (8%) | 2 (5%) | ||
|
| |||||
| NPM1 mutation | 14 (56%) | 21 (76%) | 0.17 | 21 (78%) | 0.14 |
|
| |||||
| Induction Treatment | 0.71 | 0.67 | |||
| 7+3 based induction | 23 (88%) | 50 (91%) | 40 (93%) | ||
| ADE | 0 | 3 | 1 | ||
| Cytarabine/Mitoxantrone | 3 | 0 | 0 | ||
| IA | 0 | 2 | 2 | ||
|
| |||||
| Consolidation Therapy | 0.0045 | 0.016 | |||
| None | 10 | 10 | 8 | ||
| High/Int Cytarabine – 1 cycle | 14 | 19 | 15 | ||
| High/Int Cytarabine – 2+ cycles | 1 | 13 | 6 | ||
| Cytarabine + Anthracycline | 0 | 10 | 9 | ||
| Sorafenib based | 1 | 3 | 2 | ||
|
| |||||
| TKI prior to HCT | 7 (27%) | 15 (27%) | 0.99 | 11 (26%) | 0.99 |
| Midostaurin vs. Placebo | 0 | 8 | 6 | ||
| Sorafenib | 7 | 7 | 5 | ||
|
| |||||
| ECOG PS at HCT | 0.76 | 0.79 | |||
| 0 | 6 (25%) | 17 (31%) | 12 (28%) | ||
| 1 | 14 (58%) | 27 (49%) | 21 (49%) | ||
| 2 | 4 (17%) | 11 (20%) | 10 (23%) | ||
|
| |||||
| Induction Intensity prior to HCT | 0.81 | 0.99 | |||
| Myeloablative | 14 (54%) | 27 (49%) | 22 (51%) | ||
| Reduced intensity | 12 (46%) | 28 (51%) | 21 (49%) | ||
|
| |||||
| Donor Type | 0.99 | 0.74 | |||
| Matched | 21 (81%) | 45 (82%) | 37 (86%) | ||
| Mismatched | 5 (19%) | 10 (18%) | 6 (14%) | ||
|
| |||||
| aGVHD prophylaxis | 0.23 | 0.46 | |||
| CNI and Methotrexate | 17 (65%) | 27 (49%) | 24 (56%) | ||
All values given as n (%) unless otherwise indicated
ADE: cytarabine, daunorubicin; aGVHD: acute graft-versus-host disease; CNI: calcineurin inhibitor; ECOG PS: Eastern Cooperative Oncology Group performance score; HCT: haematopoietic cell transplantation; IA: idarubicin, cytarabine; MDS: myelodysplastic syndrome; MPN: myeloproliferative neoplasm; tAML: therapy-related acute myeloid leukaemia; TKI: tyrosine kinase inhibitor.
The majority of patients in either group received “7+3” based induction chemotherapy regimens; 6 control patients and 3 sorafenib patients required a second course of “5+2” or similar chemotherapy for residual disease based on a mid-treatment bone marrow. There was some difference in consolidation therapy between controls and sorafenib patients (p=0.0045; Table 1). Most patients in both arms either went directly to transplant or received one cycle of high or intermediate dose cytarabine consolidation prior to transplant. However, more patients in the control group received 2 or more cycles of cytarabine consolidation, or cytarabine+anthracycline (typically in a “5+2” fashion) for consolidation. The median time from CR to transplant was 64 days among sorafenib patients and 77 days among controls (P=0.005). A similar number of patients in each arm received pre-HCT TKI therapy (27% in each arm), although 8 of these patients, all controls, were blinded to midostaurin or placebo as part of a clinical trial (NCT00651261) (Stone et al, 2015).
Among patients treated with sorafenib, 6 started at 200 mg twice daily, 3 were treated with 400 mg in the morning and 200mg in the evening, and 17 started at 400 mg twice daily. The median number of days on sorafenib was 336.5, with a range of 19 to 1556 days. Four patients were treated for fewer than 100 days, 3 of who remained alive and in remission at the time of analysis; all 3 had decided to stop sorafenib in the setting of skin rash, and one also related to diarrhoea. One patient, who stopped sorafenib after 22 days in the setting of cytopenias and poor red blood cell engraftment, developed iron overload and died of liver cirrhosis while in continuous remission from AML, 472 days after transplant. All but 2 patients (24/26) had sorafenib therapy interrupted at some point during treatment. In total, 11 patients (42%) stopped sorafenib prior to 12 months; 2 due to the development of cGVHD, 3 due to rash, 3 due to diarrhoea and/or weight loss, 2 due to cytopenias and 1 patient in the setting of relapsed AML. The other 15 patients were able to continue at the same dose (n=8; 4/17 in the 400 mg BID group, 1/3 in the 400 mg/200 mg group, and 3/6 in the 200 mg BID group) or at a reduced dose (n=7) to complete the planned year of therapy. The last prescribed doses of sorafenib were 400 mg twice daily (n=6), 400 mg in the morning and 200mg in the evening (n=1), 200 mg twice daily (n=12), 200 mg daily (n=6) and 200 mg every other day (n=1). Sixteen of the 26 patients included in this analysis were treated as a part of the previous phase I study (Chen et al, 2014).
Patients who received sorafenib maintenance had an improved OS compared to controls when treating post-HCT sorafenib exposure as a time-varying covariate (HR for death 0.264, p=0.021), as well as an improved PFS (HR 0.25, p=0.016) (Table II), and after adjusting for patient age, conditioning intensity, NPM1 mutational status, donor match and pre-HCT consolidation in multivariate analysis. Two sorafenib patients relapsed during follow-up; at diagnosis, both had FLT3-ITD mutations and were wild-type for FLT3-TKD and NPM1 mutations. One patient had FLT3-ITD again at relapse, while the other was wild-type; neither acquired FLT3-TKD mutations.
Table II.
Multivariate predictors of OS and PFS. In this analysis, sorafenib is treated as a time-varying covariate and includes all patients (n=81). Age is divided between older/younger than age 60 years. Donor match is based comparing mismatched to matched donors. Favourable (n=3) and intermediate cytogenetic risk patients were pooled and compared to poor cytogenetic risk patients. NPM1 mutational status is compared to wildtype patients. Conditioning intensity is divided as myeloablative vs. nonmyeloablative. Receipt of consolidation therapy is compared to those without consolidation.
| Variable | OS
|
PFS
|
||||
|---|---|---|---|---|---|---|
| HR for death | 95%CI | P-value | HR for relapse or death | 95%CI | P-value | |
| Sorafenib maintenance | 0.264 | 0.09–0.82 | 0.021 | 0.250 | 0.08–0.78 | 0.016 |
| Age > 60 years | 1.209 | 0.42–3.45 | 0.722 | 1.369 | 0.50–3.75 | 0.542 |
| NPM1 mutation present | 1.49 | 0.51–4.36 | 0.470 | 1.692 | 0.59–4.83 | 0.325 |
| NPM1 testing not performed | 2.002 | 0.65–6.20 | 0.229 | 1.955 | 0.66–5.83 | 0.230 |
| Mismatched donor | 2.161 | 0.91–5.14 | 0.081 | 2.050 | 0.90–4.69 | 0.089 |
| Poor cytogenetic risk | 3.646 | 1.00–13.26 | 0.050 | 3.409 | 0.94–12.31 | 0.061 |
| Myeloablative conditioning | 0.746 | 0.26–2.14 | 0.587 | 0.766 | 0.28–2.12 | 0.608 |
| Receipt of consolidation therapy | 0.973 | 0.41–2.32 | 0.951 | 0.823 | 0.36–1.87 | 0.642 |
OS, overall survival; PFS, progression-free survival; HR, hazard ratio; 95% CI, 95% confidence interval
We performed an exploratory landmark analysis to compare sorafenib patients to those controls patients alive and without relapse at day +68, the median date of sorafenib initiation. Sorafenib maintenance was associated with improved 2-year OS (81% vs. 62%, p=0.029, Figure 1) as well as 2-year PFS (82% vs. 53%, p=0.0081, Figure 2). This corresponded with a lower 2-year cumulative incidence of relapse (8.2% vs. 37.7%, p=0.0077). Two patients on sorafenib relapsed, one at day +189, and one at day +426; neither had cGVHD requiring immunosuppression. Of the 43 control patients in the day +68 landmark analysis, 16 relapsed. The median day of relapse for these controls was day +143 (range day +83 to day +564). Of the 26 sorafenib patients, 7 developed acute GVHD (aGVHD) prior to the initiation of sorafenib, while 6 of the 43 control patients developed aGVHD prior to day +68. Of the 7 sorafenib patients with aGVHD prior to treatment, 4 had overall grade 2 and 3 patients had overall grade 1 aGVHD; all patients received treatment and started sorafenib once tapering (n=5) or off systemic steroids (n=2). New grade II–IV aGVHD was seen in 1 sorafenib patient after starting sorafenib and 6 controls after day +68. There was no difference in 2-year NRM (9.8% vs. 9.3%, p=0.82) or the cumulative incidence of cGVHD requiring systemic therapy at 1 year (55.5% vs. 37.2%, p=0.28) after HCT (Figure 3). Extending our landmark analysis to include only those controls alive without relapse at day 100 (n=40) did not significantly impact the improvement in PFS (82% vs. 57%, p=0.020), cumulative incidence of relapse (8.2% vs. 33%, p=0.022), NRM (9.8% vs 10%, p=0.75) or cGVHD requiring systemic immunosuppression (55% vs. 40%, p=0.42); at this cut-off, sorafenib patients had a trend toward improved 2-year OS (81% vs. 63%, p=0.069).
Figure 1. Kaplan Meier estimate of overall survival among sorafenib patients (dashed line) and controls (solid line).
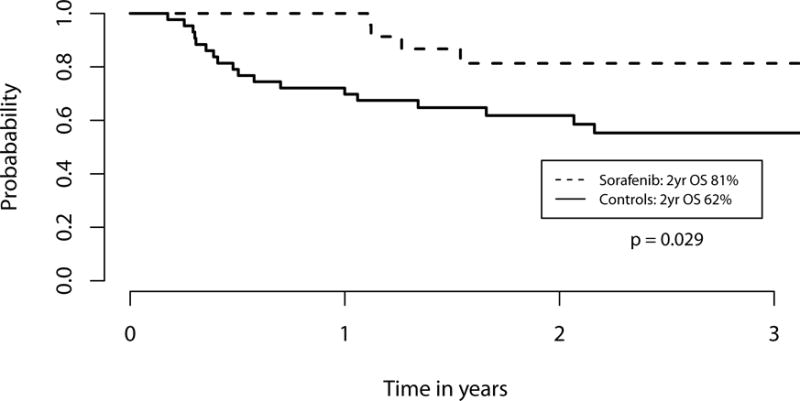
In this landmark analysis, only controls alive and without disease relapse at the median date of sorafenib initiation (+68) were included. Patients given sorafenib maintenance had a significantly higher overall survival (OS) compared to controls at 2 years (p=0.029).
Figure 2. Kaplan Meier estimate of progression-free survival among sorafenib patients (dashed line) and controls (solid line).
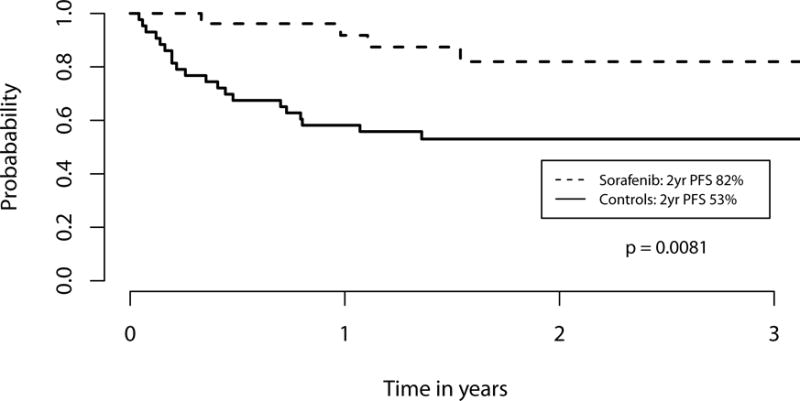
In this landmark analysis, only controls alive and without disease relapse at the median date of sorafenib initiation (+68) were included. Patients given sorafenib maintenance had a significantly higher progression-free survival (PFS) compared to controls at 2 years (p=0.0081).
Figure 3. Rates of (A) acute myeloid leukaemia (AML) relapse, (B) non-relapse mortality (NRM) and (C) chronic graft-versus-host disease (GVHD) among sorafenib patients (dashed line) and controls (solid line).
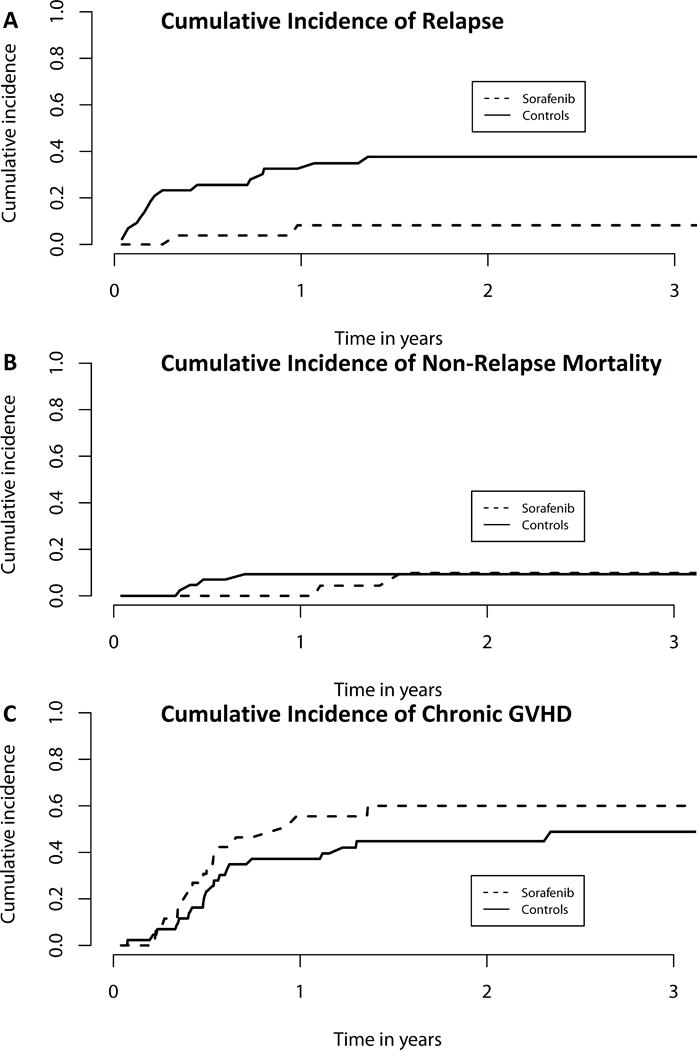
Control patients had a significantly higher cumulative incidence of relapse (A; p=0.0077). There was no significant difference in NRM (B) or chronic GVHD rates (C) between sorafenib and control patients.
Discussion
Targeted inhibition of FLT3-ITD in AML may improve the outcomes of this high-risk patient population. Previous advances have included early allogeneic transplantation in eligible patients (Oran et al, 2014; Brunet et al, 2012), and, in younger patients with FLT3 mutations, the addition of midostaurin with initial chemotherapy improved OS (Stone et al, 2015). Nonetheless, relapse rates and subsequent death remain unacceptably high. In the current analysis, we explored the efficacy of sorafenib, a multikinase inhibitor active against FLT3-ITD, as post-transplant maintenance. We identified 26 patients treated with sorafenib after allo-HCT in CR1, and compared their treatment outcomes to a contemporary cohort of control patients undergoing allo-HCT in CR1 without maintenance FLT3 inhibition. Sorafenib maintenance was associated with a significantly improved OS and PFS, driven by a decreased relapse rate, with no difference in GVHD or NRM.
Treatment with cytotoxic chemotherapy alone achieves durable remissions in approximately 30% of patients with FLT3-ITD AML; allogeneic transplantation may further improve LFS and OS (DeZern et al, 2011; Lin et al, 2013, 3; Oran et al, 2014; Brunet et al, 2012). Sorafenib and other multikinase inhibitors have been explored in various treatment settings for AML, including initial treatment for FLT3 mutant AML. In the SORAML study, sorafenib was incorporated into induction chemotherapy for younger patients regardless of FLT3 mutational status. There was improvement in LFS among all patients and a trend toward improved OS in the FLT3-ITD group (Röllig et al, 2015). The CALGB10603/RATIFY trial incorporated midostaurin into induction, consolidation, and maintenance therapy for patients with FLT3 mutations, and showed an increase in 5 year OS from 43% to 51% (Stone et al 2015). Twenty-seven per cent of these patients were treated with allo-HCT in CR1; midostaurin therapy was not given in the post-transplant setting. A separate phase II study evaluated midostaurin during induction and continued as post-HCT or post-consolidation maintenance, and reported encouragingly low relapse at 12 months (9.2%), but with limited post-HCT follow-up to date (Schlenk et al, 2015), and a current phase II randomized study in this setting is actively enrolling (NCT01883362).
Sorafenib has been studied in other settings, including post-HCT relapse and for relapsed/refractory patients prior to transplant (Metzelder et al, 2009, 2012), but there is limited experience as post-HCT maintenance. Sammons et al (2014) reported durable responses among 13 patients treated with sorafenib around HCT, 6 of whom received it in the post-HCT maintenance setting, with a wide spectrum of pre-HCT therapies and disease states at transplant. Antar et al (2015) reported encouraging results among 6 patients receiving sorafenib post-HCT, 5 as maintenance; at 16 months median follow-up, all patients were in remission. Pratz et al (2015) reported on a larger phase I study of 28 patients treated with sorafenib around the time of transplant and as maintenance; after a median follow-up of 450 days, only 5 patients had relapsed (Pratz et al, 2015). Dosing in that study was similar to our phase I sorafenib maintenance study, in which 22 patients received sorafenib after allo-HCT, 16 while in CR1 and who are included in the present analysis. Among all 22 patients in that study, 1-year PFS was 85% and OS was 95% (Chen et al, 2014). In another study, Tarlock et al (2015) reported the outcomes of 15 paediatric patients with FLT3-ITD AML who received sorafenib as maintenance (n=6) or in relapse (n=9) and showed some sustained responses. Toxicity was common, but importantly most patients received greater than the paediatric MTD of sorafenib (Tarlock et al, 2015). This contrasts with our experience; although sorafenib dose alterations were frequent, most patients were able to tolerate sustained treatment at the dosages employed (median 336.5 days). One challenge in treatment is the overlap in symptomatology between sorafenib toxicity and GVHD of the skin and gut, manifesting as rash or diarrhoea, respectively. Typically, sorafenib was held at the onset of these symptoms, and if they resolved they were attributed to drug, which could then typically be resumed but at a lower dose.
Other agents targeting FLT3-ITD have also been evaluated in the maintenance setting, including an ongoing phase I study of quizartinib (AC220) maintenance after HCT, where 13 patients were treated for up to 2 years post-HCT (Sandmaier et al, 2014). Of the 13 patients, only 1 was reported to have relapsed. Ongoing studies are currently evaluating midostaurin (NCT01883362) and crenolanib (NCT02400255) as post-HCT maintenance. Combined, these data are encouraging, but derived from retrospective or single arm designs, and do not prove definitive benefit. Moreover, access to such agents can be tenuous, and will continue to be so if appropriate prospective trials are not conducted. In our current practice, we consider starting sorafenib as maintenance after transplant, understanding the limitations of available data, and only after frankly discussing the pros and cons with individual patients. Our findings should be interpreted as strong support for a randomized trial to determine whether such a strategy should become standard of care.
How TKI therapy influences outcomes in FLT3-ITD mutant AML may relate to direct inhibition of mutated FLT3 (Zhang et al, 2008), off-target kinase inhibition, e.g. RAF or KIT (Röllig et al, 2015; Wilhelm et al, 2008), or modulation of graft-versus-leukaemia (Metzelder et al, 2012; Tschan-Plessl et al, 2015). It is also possible that sorafenib altered the kinetics of relapse, and that with longer follow-up more patients would relapse; however, with a median follow-up of over 2 years in the sorafenib group, we feel it is likely that we captured relapses after sorafenib cessation at approximately 1 year post-HCT. Maintenance strategies may be further refined to select patients most likely to benefit. Higher FLT3 allelic burden and longer ITD length have been associated with worsened outcomes (Schlenk et al, 2014; Schnittger et al, 2002); nonetheless, both high and low allelic fraction subgroups may respond to TKIs (Stone et al, 2015). During our study, standard clinical testing either did not consistently report FLT3-ITD allelic burden, or was performed elsewhere prior to HCT referral, and therefore not included in this analysis. Moreover, these patients were in CR1 at allo-HCT, and routine pre-HCT testing for FLT3-ITD minimal residual disease (MRD) was unavailable; newer techniques to sequence FLT3-ITD, such as tandem duplication polymerase chain reaction (Grunwald et al, 2014), may eventually allow for such testing. MRD assays in FLT3-ITD AML and stratification by allelic burden may improve risk assessment for maintenance therapy candidates.
In this study we identified a uniform, clinically relevant patient population receiving sorafenib maintenance post-HCT. We compared these patients to a contemporary set of controls who would have probably been eligible for consideration of sorafenib maintenance. The use of sorafenib was associated with significant improvements in PFS and OS, with lower relapse even after extending the landmark analysis to patients alive and relapse-free at 100 days post-HCT. Nonetheless, this is a small retrospective analysis, and it is unknown whether other unidentified patient characteristics would correlate with more aggressive disease or poor candidacy for sorafenib. There were unique barriers for patients treated outside of the phase I clinical trial, including sorafenib coverage for off-label use; also, some control patients were offered, but declined to take, the medication. We identified some variation in consolidation therapy between groups and prior data has suggested that patients who proceed to allo-HCT sooner after remission have improved outcomes in FLT3-ITD AML (Kayser et al, 2010). In addition, because adoption of post-HCT sorafenib increased after the phase I trial, the median year of treatment for patients in the sorafenib arm was more recent than controls. This resulted in longer follow-up among controls; it is possible that sorafenib patients may relapse with longer follow-up; however, most FLT3-ITD AML relapses occur early, which would typically be captured during the 2-year median follow-up period reported here. Other potential reasons for our findings may relate to differences in T-cell subsets in the control and sorafenib groups; while there was no overall difference, sorafenib patients had a trend toward higher T-cell chimerism prior to day +68 (p=0.0547, data not shown). We did not routinely assess bone marrow for complete remission early after transplant in control patients or in the sorafenib patients treated outside of the clinical trial. Therefore, unfortunately, relatively few patients (only 22/43 controls and 17/26 sorafenib patients) had T-cell chimerism for the analysis, making these results difficult to interpret.
In summary, these findings suggest a benefit of sorafenib in the maintenance setting following allo-HCT for FLT3-ITD AML in first remission. Compared to contemporary controls, patients receiving sorafenib maintenance had an improvement in OS and PFS mainly driven by a significant decrease in rates of relapse. These retrospective data support further evaluation of tyrosine kinase inhibition after transplant in a prospective randomized fashion. Such a trial is currently being planned through the Blood and Marrow Transplantation Clinical Trials Network.
Acknowledgments
This study was presented in part at the 2015 ASH Annual Meeting, Orlando, FL, and the 2016 ASBMT Tandem Meeting, Honolulu, HI. A.M.B. is supported in part by the National Institutes of Health (NIH) grant T32 CA 071345-18. S.L. is partially supported by National Institutes of Health (NIH) grant CA 006516.
A.M.B. designed the research, collected data, analysed the data and wrote the paper. S.L. designed the research, analysed the data and wrote the paper. Y.B.C. designed the research, contributed patients, analysed the data and wrote the paper. K.C. and C.C. collected data and wrote the paper. A.T.F., M.W., V.T.H., K.K.B., C.S.C., B.R.D., A.E.J., S.N., P.A., S.M., J.K., D.J.D., J.H.A., T.R.S., R.M.S., and R.J.S. contributed patients and wrote the paper. Y.B.C. was a consultant and received funding for clinical trials from Bayer. A.T.F. served on the advisory boards for Agios, Seattle Genetics, and Merck. The other authors report no relevant competing interests. For patients treated in the context of the clinical trial drug was supplied from Bayer Pharmaceuticals, Inc.; otherwise sorafenib was obtained for off-label use.
References
- Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Genomic structure of human FLT3: implications for mutational analysis. British Journal of Haematology. 2001;113:1076–1077. doi: 10.1046/j.1365-2141.2001.02821.x. [DOI] [PubMed] [Google Scholar]
- Antar A, Kharfan-Dabaja MA, Mahfouz R, Bazarbachi A. Sorafenib Maintenance Appears Safe and Improves Clinical Outcomes in FLT3-ITD Acute Myeloid Leukemia After Allogeneic Hematopoietic Cell Transplantation. Clinical Lymphoma Myeloma and Leukemia. 2015;15:298–302. doi: 10.1016/j.clml.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Brunet S, Labopin M, Esteve J, Cornelissen J, Socié G, Iori AP, Verdonck LF, Volin L, Gratwohl A, Sierra J, Mohty M, Rocha V. Impact of FLT3 Internal Tandem Duplication on the Outcome of Related and Unrelated Hematopoietic Transplantation for Adult Acute Myeloid Leukemia in First Remission: A Retrospective Analysis. Journal of Clinical Oncology. 2012;30:735–741. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- Chen YB, Li S, Lane AA, Connolly C, Del Rio C, Valles B, Curtis M, Ballen K, Cutler C, Dey BR, El-Jawahri A, Fathi AT, Ho VT, Joyce A, McAfee S, Rudek M, Rajkhowa T, Verselis S, Antin JH, Spitzer TR, Levis M, Soiffer R. Phase I Trial of Maintenance Sorafenib after Allogeneic Hematopoietic Stem Cell Transplantation for Fms-like Tyrosine Kinase 3 Internal Tandem Duplication Acute Myeloid Leukemia. Biology of Blood and Marrow Transplant. 2014;20:2042–2048. doi: 10.1016/j.bbmt.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of Clinical Oncology. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- DeZern AE, Sung A, Kim S, Smith BD, Karp JE, Gore SD, Jones RJ, Fuchs E, Luznik L, McDevitt M, Levis M. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: Outcomes from 133 consecutive newly-diagnosed patients from a single institution. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2011;17:1404–1409. doi: 10.1016/j.bbmt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald MR, Tseng LH, Lin MT, Pratz KW, Eshleman JR, Levis MJ, Gocke CD. Improved FLT3/ITD PCR assay predicts outcome following allogeneic transplant for AML. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2014;20:1989–1995. doi: 10.1016/j.bbmt.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser S, Döhner K, Krauter J, Casper J, Horst HA, Held G, Wilhelm S, Kobbe G, Lübbert M, Salih HR, Götze K, Rummel MJ, Fiedler W, von Lilienfeld-Toal M, Nachbaur D, Wattad M, Späth D, Erdmann P, Ganser A, Döhner H, Schlenk RF. Impact of Allogeneic Transplantation From Matched Related and Unrelated Donors on Clinical Outcome In Younger Adult AML Patients with FLT3 Internal Tandem Duplications. Blood. 2010;116:909–909. [Google Scholar]
- Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, Naoe T. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- Lin PH, Lin CC, Yang HI, Li LY, Bai LY, Chiu CF, Liao YM, Lin CY, Hsieh CY, Lin CY, Ho CM, Yang SF, Peng CT, Tsai FJ, Yeh SP. Prognostic impact of allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia patients with internal tandem duplication of FLT3. Leukemia Research. 2013;37:287–292. doi: 10.1016/j.leukres.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A, Eilers M, Enghofer E, Neubauer A, Burchert A. Compassionate use of sorafenib in FLT3-ITD–positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–6571. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Götze K, Linn YC, Kröger M, Reiter A, Salih HR, Heinicke T, Stuhlmann R, Müller L, Giagounidis A, Meyer RG, Brugger W, Vöhringer M, Dreger P, Mori M, Basara N, Schäfer-Eckart K, Schultheis B, Baldus C, Neubauer A, Burchert A. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26:2353–2359. doi: 10.1038/leu.2012.105. [DOI] [PubMed] [Google Scholar]
- O’Donnell MR, Abboud CN, Altman J, Appelbaum FR, Arber DA, Attar E, Borate U, Coutre SE, Damon LE, Goorha S, Lancet J, Maness LJ, Marcucci G, Millenson MM, Moore JO, Ravandi F, Shami PJ, Smith BD, Stone RM, Strickland SA, Tallman MS, Wang ES, Naganuma M, Gregory KM. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Acute Myeloid Leukemia. Journal of the National Comprehensive Cancer Network. 2012;10:984–1021. doi: 10.6004/jnccn.2012.0103. [DOI] [PubMed] [Google Scholar]
- Oran B, Champlin RE, Cortes JE, de Lima M, Wang X, Chen HC, Ravandi F, Ciurea SO, Kantarjian HM, Borthakur G. Allogeneic Hematopoietic Stem Cell Transplantation (HCT) in First Remission Improves Outcome Irrespective of FLT3-ITD Allelic Burden Among Patients with Acute Myeloid Leukemia and FLT3-ITD Mutation. Blood. 2014;124:2531–2531. [Google Scholar]
- Pratz KW, Gojo I, Karp JE, Luznik L, Smith BD, Jones RJ, Greer J, Gocke C, Baer MR, Duong VH, Wright JJ, Rudek MA, Emadi A, Levis MJ. Prospective Study of Peri-Transplant Use of Sorafenib As Remission Maintenance for FLT3-ITD Patients Undergoing Allogeneic Transplantation. Blood. 2015;126:3164–3164. [Google Scholar]
- Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, Pierce S, Daver N, Garcia-Manero G, Faderl S, Nazha A, Konopleva M, Borthakur G, Burger J, Kadia T, Dellasala S, Andreeff M, Cortes J, Kantarjian H, Levis M. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121:4655–4662. doi: 10.1182/blood-2013-01-480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röllig C, Bornhäuser M, Thiede C, Taube F, Kramer M, Mohr B, Aulitzky W, Bodenstein H, Tischler HJ, Stuhlmann R, Schuler U, Stölzel F, von Bonin M, Wandt H, Schäfer-Eckart K, Schaich M, Ehninger G. Long-Term Prognosis of Acute Myeloid Leukemia According to the New Genetic Risk Classification of the European LeukemiaNet Recommendations: Evaluation of the Proposed Reporting System. Journal of Clinical Oncology. 2011;29:2758–2765. doi: 10.1200/JCO.2010.32.8500. [DOI] [PubMed] [Google Scholar]
- Röllig C, Serve H, Hüttmann A, Noppeney R, Müller-Tidow C, Krug U, Baldus CD, Brandts CH, Kunzmann V, Einsele H, Krämer A, Schäfer-Eckart K, Neubauer A, Burchert A, Giagounidis A, Krause SW, Mackensen A, Aulitzky W, Herbst R, Hänel M, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. The Lancet Oncology. 2015;16:1691–1699. doi: 10.1016/S1470-2045(15)00362-9. [DOI] [PubMed] [Google Scholar]
- Sammons SL, Pratz KW, Smith BD, Karp JE, Emadi A. Sorafenib is tolerable and improves clinical outcomes in patients with FLT3-ITD acute myeloid leukemia prior to stem cell transplant and after relapse post-transplant. American journal of hematology. 2014;89:936–938. doi: 10.1002/ajh.23782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmaier BM, Khaled SK, Oran B, Gammon G, Trone D, Frankfurt O. Results of a Phase 1 Study of Quizartinib (AC220) As Maintenance Therapy in Subjects with Acute Myeloid Leukemia in Remission Following Allogeneic Hematopoietic Cell Transplantation. Blood. 2014;124:428–428. doi: 10.1002/ajh.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller GJ, Tuttle P, Desai P. Allogeneic Hematopoietic Stem Cell Transplantation in FLT3-ITD–Positive Acute Myelogenous Leukemia: The Role for FLT3 Tyrosine Kinase Inhibitors Post-Transplantation. Biology of Blood and Marrow Transplant. 2016;22:982–990. doi: 10.1016/j.bbmt.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Schlenk R, Döhner K, Salih H, Kündgen A, Fiedler W, Salwender HJ, Westermann J, Götze KS, Horst HA, Wulf G, Lübbert M, Kraemer D, Kindler T, Ringhoffer M, Brossart P, Held G, Greil R, Südhoff T, Münnich A, Weber D, Gaidzik VI, Teleanu MV, Paschka P, Theis F, Heuser M, Thol F, Benner A, Ganser A, Döhner H. Midostaurin in Combination with Intensive Induction and As Single Agent Maintenance Therapy after Consolidation Therapy with Allogeneic Hematopoietic Stem Cell Transplantation or High-Dose Cytarabine ( NCT01477606) Blood. 2015;126:322–322. [Google Scholar]
- Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, Habdank M, Späth D, Morgan M, Benner A, Schlegelberger B, Heil G, Ganser A, Döhner H. Mutations and Treatment Outcome in Cytogenetically Normal Acute Myeloid Leukemia. New England Journal of Medicine. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, Held G, Brossart P, Lübbert M, Salih HR, Kindler T, Horst HA, Wulf G, Nachbaur D, Götze K, Lamparter A, Paschka P, Gaidzik VI, Teleanu V, Späth D, Benner A, Krauter J, Ganser A, Döhner H, Döhner K. Differential impact of allelic ratio and insertion site in FLT3-ITD–positive AML with respect to allogeneic transplantation. Blood. 2014;124:3441–3449. doi: 10.1182/blood-2014-05-578070. [DOI] [PubMed] [Google Scholar]
- Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, Löffler H, Sauerland CM, Serve H, Büchner T, Haferlach T, Hiddemann W. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- Stirewalt DL, Kopecky KJ, Meshinchi S, Engel JH, Pogosova-Agadjanyan EL, Linsley J, Slovak ML, Willman CL, Radich JP. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107:3724–3726. doi: 10.1182/blood-2005-08-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RM, Mandrekar S, Sanford BL, Geyer S, Bloomfield CD, Dohner K, Thiede C, Marcucci G, Lo-Coco F, Klisovic RB, Wei A, Sierra J, Sanz MA, Brandwein JM, de Witte T, Niederwieser D, Appelbaum FR, Medeiros BC, Tallman MS, Krauter J, et al. The Multi-Kinase Inhibitor Midostaurin (M) Prolongs Survival Compared with Placebo (P) in Combination with Daunorubicin (D)/Cytarabine (C) Induction (ind), High-Dose C Consolidation (consol), and As Maintenance (maint) Therapy in Newly Diagnosed Acute Myeloid Leukemia (AML) Patients (pts) Age 18–60 with FLT3 Mutations (muts): An International Prospective Randomized (rand) P-Controlled Double-Blind Trial (CALGB 10603/RATIFY [Alliance]) Blood. 2015;126:6–6. [Google Scholar]
- Tarlock K, Chang B, Cooper T, Gross T, Gupta S, Neudorf S, Adlard K, Ho PA, McGoldrick S, Watt T, Templeman T, Sisler I, Garee A, Thomson B, Woolfrey A, Estey E, Meshinchi S, Pollard JA. Sorafenib treatment following hematopoietic stem cell transplant in pediatric FLT3/ITD acute myeloid leukemia. Pediatric Blood & Cancer. 2015;62:1048–1054. doi: 10.1002/pbc.25437. [DOI] [PubMed] [Google Scholar]
- Tschan-Plessl A, Halter JP, Heim D, Medinger M, Passweg JR, Gerull S. Synergistic effect of sorafenib and cGvHD in patients with high-risk FLT3-ITD+AML allows long-term disease control after allogeneic transplantation. Annals of Hematology. 2015;94:1899–1905. doi: 10.1007/s00277-015-2461-5. [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Molecular Cancer Therapeutics. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Konopleva M, Shi Y, McQueen T, Harris D, Ling X, Estrov Z, Quintás-Cardama A, Small D, Cortes J, Andreeff M. Mutant FLT3: A Direct Target of Sorafenib in Acute Myelogenous Leukemia. Journal of the National Cancer Institute. 2008;100:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]


