Abstract
Objective
To determine the prevalence of abnormal structural findings using 3.0-T MRI in the asymptomatic knees of male and female collegiate basketball players before and after a season of high-intensity basketball.
Design
Institutional review board-approved prospective case series.
Participants
Asymptomatic knees of 24 NCAA Division I collegiate basketball players (12 male, 12 female) were imaged using a 3.0-T MRI scanner prior to and following the end of the competitive season. Three subjects did not undergo scanning after the season.
Main Outcome Measures
Images were evaluated for pre-patellar bursitis, fat pad edema, patellar and quadriceps tendinopathy, bone marrow edema, and articular cartilage and meniscal injury.
Results
Every knee imaged had at least one structural abnormality both pre- and post-season. A high pre- and post-season prevalence of fat pad edema (75% and 81%), patellar tendinopathy (83% and 90%), and quadriceps tendinopathy (75% and 90%) was seen. Intra-meniscal signal change was observed in 50% pre-season knees and 62% of post-season knees, but no discrete tears were found. Bone marrow edema was seen in 75% and 86% of knees in the pre- and post-season, respectively. Cartilage findings were observed in 71% and 81% of knees in the pre- and post-season, respectively. The cartilage injury score increased significantly in the post-season compared with the pre-season (p = 0.0009).
Conclusions
A high prevalence of abnormal knee MRI findings was observed in a population of asymptomatic young elite athletes. These preliminary data suggest high-intensity basketball may have potentially deleterious effects on articular cartilage.
Key Terms: MRI, articular cartilage, bone marrow edema, meniscus
INTRODUCTION
Magnetic resonance imaging (MRI) is an accurate tool for identifying articular cartilage lesions,1,2 meniscal tears,3–5 ligament injury,6,7 and bone marrow edema.8,9 However, previous work has shown positive MRI findings may not always be symptomatic, especially among athletic populations.10–13 Prior studies of asymptomatic knees of collegiate and professional basketball players have shown rates of one or more abnormalities in up to 89% of knees imaged, including high rates of articular cartilage lesions (41–50%), meniscal lesions (20–54%), bone marrow edema (25–41%), joint effusion (29–35%), and patellar tendinopathy (24–39%).14–16
Prior MRI studies of basketball players were performed using 0.3- and 1.5-Tesla (T) scanners. However, 3.0-T MRI is more sensitive, specific, and accurate for the assessment of articular cartilage lesions and meniscal tears.4,17,18 The higher magnetic field strength allows for increased signal-to-noise ratios (SNR), thus permitting higher spatial resolution and thinner slice thickness.19
This study utilized a 3.0-T MRI scanner to more accurately determine the prevalence of anatomic knee lesions in asymptomatic elite athletes. Moreover, previous studies have evaluated subjects at only one time point during the pre-season. In contrast, this study examined both male and female subjects before and after their competitive season in order to assess the effect of one season of high-intensity basketball. We hypothesized 3.0-T MRI of the knee in asymptomatic basketball players during the pre-season would reveal a higher prevalence of articular cartilage lesions, meniscus signal, bone marrow edema, patellar and quadriceps tendinopathy fat pad edema, and pre-patellar bursitis than previously reported with lower-field-strength scanners. Furthermore, we hypothesized these pathological findings would increase following a single season of high intensity collegiate basketball.
METHODS
Subjects
A total of 24 asymptomatic knees from 24 NCAA Division I collegiate basketball players (12 men and 12 women, age 18–22) were imaged prior to and following the competitive season. ETHICAL CONSIDERATIONS: The study was approved by the university institutional review board and all subjects provided informed written consent. Prior to scanning, subjects completed a questionnaire that assessed history of knee pain, injections, or surgeries. Athletes were included if they had at least one knee that was asymptomatic at the time of the study and no history of injury to, or surgery on, that knee.
Image Acquisition and Analysis
The asymptomatic knee of each player was imaged within 2 weeks prior to the NCAA official start of practice. If both knees were asymptomatic, the more dominant knee (used for takeoff and landing) was imaged. The same knee was re-imaged within 4 weeks of the completion of the basketball season (including any post-season tournament play). A 3.0-T MRI scanner (Signa Excite, GE Healthcare, Milwaukee, WI) was used. Pre- and post-season scans utilized identical imaging protocols (Table 1)
TABLE 1.
Magnetic resonance imaging parameters
| Parameter | Axial Proton Density (PD) | Sagittal Proton Density (PD) | Sagittal T2-weighted |
|---|---|---|---|
| TR (msec) | 4000 | 5000 | 4000 |
| TE (msec) | 30 | 30 | 68 |
| Field of view (cm) | 16 x 16 | 16 x 16 | 16 x 16 |
| Pixel Matrix | 320 x 192 | 512 x 224 | 320 x 160 |
| Slice Thickness (mm) | 3 | 3 | 3 |
| Interslice Gap (mm) | 1 | 1 | 1 |
| Fat Saturation | Yes | Yes | Yes |
TR, repetition time; TE, echo time.
All pre- and post-season scans were graded by an experienced musculoskeletal radiologist, an experienced orthopaedic sports medicine surgeon, and an orthopaedic sports medicine fellow. Any discrepancies were discussed and graded by consensus. The scans were graded for pre-patellar bursitis, fat pad edema, patellar and quadriceps tendinopathy, bone marrow edema, articular cartilage lesions, and meniscal lesions. A score of 0 was assigned for normal anatomy. Pre-patellar bursitis, fat pad edema, patellar and quadriceps tendinopathy, and bone marrow edema were rated as mild (1), moderate (2), or severe (3). The images were also evaluated for the presence or absence of joint effusion, Baker’s cysts, and Osgood-Schlatter disease.
Both articular cartilage irregularity and bone marrow edema (BME) were evaluated in the medial and lateral femoral condyle, medial and lateral tibial plateau, patella, and trochlea. Because of the large joint reaction forces within the patellofemoral compartment of basketball players, an increased prevalence of bone marrow edema and cartilage findings was expected in the patella and trochlea.12,14,16 Therefore, two distinct additive scores were formulated to compare bone marrow edema and cartilage findings. First, a patellofemoral score was constructed as the sum of the highest grade finding in the patella and the highest grade finding in the trochlea. Second, the extent of abnormal findings observed across all 3 compartments in the knee was estimated by summing the highest grade finding in all 6 regions: medial and lateral femoral condyle, medial and lateral tibial plateau, patella and trochlea. For example, a knee with moderate (grade 2) BME of the patella and trochlea and mild (grade 1) BME of the lateral femoral condyle and medial tibial plateau would have a patellofemoral bone marrow edema (BME PF) score of 4 (=2+2) and a summed bone marrow edema (BME Sum) score of 6 (=2+2+1+1).
Articular cartilage was rated using a modified-Noyes scoring system (1=signal change, 2=surface fissuring or superficial erosion involving less than 50% of the cartilage depth, 3=deep fissuring or erosion involving greater than 50%, 4=full-thickness chondral defect with exposure of underlying subchondral bone).20 Two additive cartilage scores were constructed to compare patellofemoral and total cartilage findings within each knee. The patellofemoral cartilage score (Noyes PF) was calculated from the sum of the modified-Noyes scores in the patellar and trochlear cartilage. A second composite score (Noyes SUM) was formulated to estimate the overall level of cartilage irregularity throughout the entire knee and was calculated as the sum of the modified-Noyes scores in the patella, trochlea, medial femoral condyle, lateral femoral condyle, medial tibial plateau, and lateral tibial plateau.
The medial and lateral menisci were scored based on the classification described by Crues et al.21 (1=intra-substance signal, 2=linear intra-meniscal signal not extending to meniscal surface, 3=linear signal extending to articular surface, suggesting tear). Pre- and post-season meniscus score was calculated for each knee as the sum of the medial and lateral meniscus scores (e.g., for grade 1 intra-meniscal signal present in both medial and lateral menisci, a score of 2 was assigned).
Pre- and post-season scores were compared using exact one-sided (post > pre) paired Wilcoxon tests. The number of male and female subjects was deemed too small to directly test the effect of gender; however, the effect was accounted for by stratifying according to gender. Statistical significance level was set to an unadjusted p-value < 0.05, which corresponded to a Bonferroni-adjusted p-value < 0.0056 (9 multiple comparisons). Statistical analyses were performed using R version 2.9.2 (www.r-project.org).
RESULTS
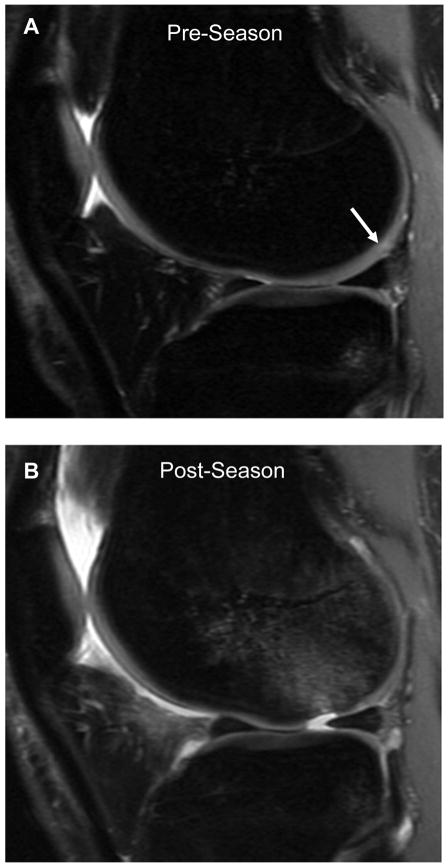
Twenty-one college basketball players completed the study, with 24 players (12 male and 12 female) participating in the pre-season scan. Three players (2 male and 1 female) were not able to complete the post-season scan, leaving 10 males and 11 females with both pre- and post-season scans. None of the athletes had any time off during the season. All players remained asymptomatic with respect to the study knee though out the season, with the exception of one female player who sustained an acute chondral injury 6 weeks after her pre-season scan. Her pre-season MRI demonstrated grade 1 and 2 changes in the articular cartilage of the lateral femoral condyle (Figure 1). While pivoting during practice, she sustained an acute, full thickness chondral injury (grade 4) to her lateral femoral condyle, near the cartilage abnormality identified on her pre-season scan.
Figure 1.
Sagittal proton density images of the lateral compartment in a 21-year-old female obtained during the pre-season (A) and post-season (B). Pre-season image (A) reveals grade 1 and 2 changes of the posterior lateral femoral condyle (white arrow). Post-season image (B) reveals full-thickness (grade 4) lesion anterior to the previous cartilage changes with subjacent bone marrow edema.
Pre-season scans revealed at least one structural abnormality in all 24 asymptomatic knees. A particularly high prevalence of patellar and quadriceps tendinopathy, fat pad and bone marrow edema, and articular cartilage changes was observed in the pre-season scans (Table 2 and Appendix A). Pre-patellar bursitis was seen in 15 of 24 knees (63%) with fat pad edema in 18 of 24 knees (75%). Patellar and quadriceps tendinopathy was demonstrated in 20 and 18 knees (83% and 75%) respectively. Bone marrow edema was observed in 18 knees (75%), 14 of which (78% of affected) involved the patellofemoral articulation. Chondral lesions were noted in 17 knees (71%), 15 of which (88% of affected) involved the patellar or trochlear cartilage (Figure 2). No complete ligament or meniscus tears were found in either the pre- or post-season scans, but 2 meniscal-capsular injuries were identified. Pre-season meniscus signal change was observed in 12 of 24 knees (50%), with grade 1 and 2 signal seen in 8 and 4 knees, respectively. Pre-season imaging also revealed small effusions in 2 knees (8%), evidence of Osgood-Schlatter disease in 2 knees (8%), and edema surrounding the iliotibial (IT) band in 11 knees (46%). Also noted were Baker’s cysts in 8 knees (33%) and cruciate ligament ganglion cysts in 2 knees (1 ACL and 1 PCL).
TABLE 2. Distribution of pre- and post-season findings.
Twenty four subjects (12 male and 12 female) completed pre-season scans and 21 subjects (10 male and 11 female) completed post-season scans.
Numbers in parentheses represent the gender breakdown (male/female) for each reported finding.
| Pre-Season | Post-Season | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 Subjects (12/12) | 21 Subjects (10/11) | |||||||||
|
| ||||||||||
| Structure/Pathology | Normal 0 | Mild 1 | Mod 2 | Severe 3 | 4 | Normal 0 | Mild 1 | Mod 2 | Severe 3 | 4 |
| Pre-Patellar Bursitis (PPB) | 9 (5/4) | 12 (7/5) | 3 (0/3) | 0 | 5 (3/2) | 14 (6/8) | 2 (1/1) | 0 | ||
|
| ||||||||||
| Fat Pad Edema (FPE) | 6 (1/5) | 10 (7/3) | 6 (4/2) | 2 (0/2) | 4 (1/3) | 11 (7/5) | 6 (2/4) | 0 | ||
|
| ||||||||||
| Patellar Tendinopathy | 4 (1/3) | 14 (7/7) | 3 (1/2) | 3 (3/0) | 2 (0/2) | 11 (5/6) | 8 (5/3) | 0 | ||
|
| ||||||||||
| Quadriceps Tendinopathy | 6 (2/4) | 14 (7/7) | 4 (3/1) | 0 | 2 (0/2) | 16 (9/7) | 3 (1/2) | 0 | ||
|
| ||||||||||
| Bone Marrow Edema (BME) | 6 (2/4) | 12 (7/5) | 6 (3/3) | 0 | 3 (2/1) | 12 (5/7) | 6 (3/3) | 0 | ||
| Patellofemoral BME | 10 (3/7) | 11 (8/3) | 3 (1/2) | 0 | 5 (3/2) | 14 (7/7) | 2 (0/2) | 0 | ||
| Tibiofemoral BME | 16 (8/8) | 5 (2/3) | 3 (2/1) | 0 | 15 (6/9) | 2 (1/1) | 4 (3/1) | 0 | ||
|
| ||||||||||
| Articular Cartilage * | 7 (4/3) | 7 (4/3) | 5 (2/3) | 5 (2/3) | 0 | 4 (3/1) | 7 (4/3) | 4 (2/2) | 5 (1/4) | 1 (0/1) |
| Patellofemoral | 9 (6/3) | 6 (2/4) | 5 (2/3) | 4 (2/2) | 0 | 6 (3/3) | 8 (5/3) | 3 (1/2) | 4 (1/3) | 0 |
| Tibiofemoral | 16 (9/7) | 6 (3/3) | 1 (0/1) | 1 (0/1) | 0 | 12 (8/4) | 4 (1/3) | 2 (1/1) | 2 (0/2) | 1 (0/1) |
|
| ||||||||||
| Meniscus | 12 (8/4) | 8 (3/5) | 4 (1/3) | 0 | 8 (5/3) | 8 (4/4) | 5 (1/4) | 0 | ||
| Medial | 13 (9/4) | 7 (2/5) | 4 (1/3) | 0 | 9 (6/3) | 7 (3/4) | 5 (1/4) | 0 | ||
| Lateral | 22 (11/11) | 2 (1/1) | 0 | 0 | 18 (8/10) | 3 (2/1) | 0 | 0 | ||
For subjects with lesions in multiple locations, the highest grade lesion is listed
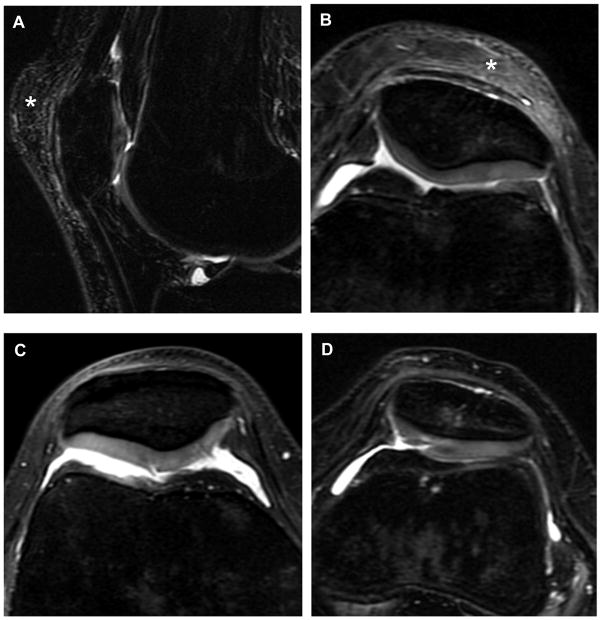
Figure 2.
Pre-season images demonstrating cartilage findings. (A) sagittal T2-weighted image that demonstrates signal heterogeneity within the cartilage of the lateral facet of the patella, representing a modified Noyes score of 1. Also note coexistent pre-patellar bursitis (asterisk). (B) Axial proton density (PD) image in the same subject also demonstrating increased signal (modified Noyes 1) within the cartilage of the lateral facet of the patella. (C) Axial PD image of another subject with a partial thickness cartilage defect (< 50%) of the central patella, representing a modified Noyes score of 2. (D) Axial PD image of a subject with a partial thickness defect (> 50%) of the patellar cartilage, representing a modified Noyes score of 3.
All 21 subjects (10 male, 11 female) scanned after the season also had one or more findings on 3.0-T MR imaging. Post-season imaging continued to demonstrate high rates of “abnormal” findings. Pre-patellar bursitis was seen in 16 of 21 knees (76%), fat pad edema in 17 knees (81%), patellar tendinopathy in 19 knees (90%), and quadriceps tendinopathy in 19 knees (90%). Bone marrow edema was evident in 18 knees (86%), 16 of which (89% of affected) involved the patellofemoral articulation. Chondral lesions were observed in 17 of 21 knees (81%) with patellofemoral chondral lesions in 15 of 21 knees (88% of affected knees). Intra-substance (grade 1) or linear (grade 2) meniscus signal was found in a total of 13 (62%) of 21 knees (Table 2). Post-season scans revealed edema surrounding the IT band in 11 of 21 knees (51%). Of the 8 Baker’s cysts noted in the pre-season scans, 2 knees were not re-imaged in the post season, 5 cysts remained essentially unchanged, and 1 cyst decreased in size.
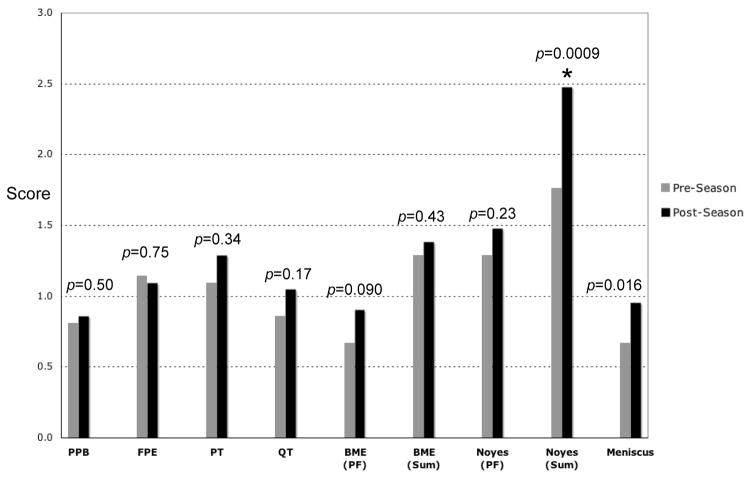
Mean scores for the 21 subjects who completed both pre- and post-season scans are seen in Figure 3. No statistically significant change between pre- and post-season was observed for pre-patellar bursitis, fat pad edema, and patellar and quadriceps tendinopathy. Patellofemoral compartment bone marrow edema (BME PF) increased after a season of play; however, this increase was not statistically significant (p=0.09). The bone marrow edema score summed over all compartments (BME SUM) was not significantly different (p=0.43) in the pre- and post-season. The mean cartilage injury score for the patellofemoral compartment (Noyes PF) increased from pre- to post-season, but was not statistically significant (p=0.23). A larger increase was observed in total cartilage injury score (Noyes Sum), which increased significantly from a pre-season mean of 1.76 to a post-season mean of 2.48 (p=0.0009). After a season of play, Noyes Sum increased in 13 of 21 subjects (62%), remained unchanged in 7 subjects (33%), and decreased in only 1 subject (5%).
Figure 3.
Comparison of mean scores for the 21 subjects with both pre- and post-season scans. Modified-Noyes cartilage scores were summed in the patellofemoral compartment (= Noyes PF) and over all 3 compartments (patellofemoral + tibiofemoral = Noyes Sum). The asterisk (*) indicates a statistically-significant increase in Noyes Sum from the pre- to post-season. PPB = pre-patellar bursitis, FPE = fat pad edema, PT = patellar tendinopathy, QT = quadriceps tendinopathy, BME = bone marrow edema.
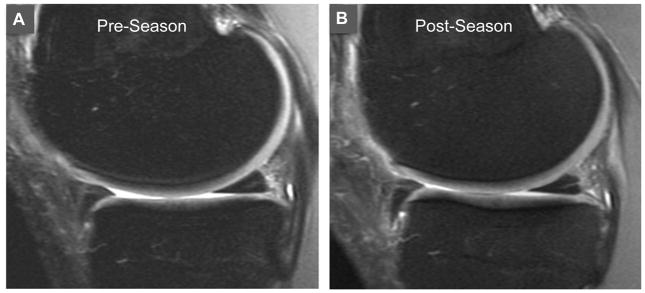
A trend towards increasing meniscus signal in the post-season was observed (Figure 4) but was not statistically significant (p=0.016). The meniscus score increased from a mean of 0.67 in the pre-season to 0.95 in the post-season. The percentage of subjects with increased meniscus signal increased from 50% (12 of 24) to 62% (13 of 21) after a season of play (Table 2). The majority of signal change was observed in the medial meniscus (46% and 57% of subjects in the pre- and post-season, respectively). Signal change in the lateral meniscus was much less common (8% and 14% of subjects in pre- and post-season, respectively). No meniscal tears (grade 3) were seen in either pre- or post-season imaging (Table 2).
Figure 4.
Sagittal proton images of the medial compartment in a female player obtained during the pre-season (A) and post-season (B). Pre-season image (A) revealed grade 1 intra-substance signal in the posterior horn of the medial meniscus, which increased to a grade 2 linear signal in the post-season scan (B).
DISCUSSION
This study demonstrated a higher prevalence of pathologic MRI findings in the knees of high-level basketball players than previously reported. In prior MR imaging studies of basketball players performed in the pre-season, Walczak et al.16 and Major and Helms15 reported patellar tendinopathy in 39% of professional and 24% of collegiate knees, respectively. Walczak et al.16 also reported the presence of quadriceps tendinopathy on MRI in 7% of 28 knees in NBA athletes. In this current study, MR changes consistent with patellar tendinopathy were seen in 83% and 90% of athletes in the pre- and post-season, respectively. High rates of quadriceps tendinopathy were also seen (75% pre- and 90% post-season). Interestingly, the degree of patellar and/or quadriceps tendinopathy improved by one grade in 2 male and 2 female subjects during the season (Appendix A); the reason for this observation is unclear, but may reflect higher intensity training during the off season by these 4 athletes. This study also found higher rates of pre-patellar bursitis (63% pre- and 76% post-season) than the 7% previously reported in NBA players.16 In contrast, fewer joint effusions were seen in this study than previously reported for NBA players (8% vs. 29%, respectively).16
Bone marrow edema and cartilage findings were observed in the majority of collegiate players, both in the pre- and the post-season. This study identified bone marrow edema in 75% of players in the pre-season and 86% at the end of the season. By comparison, Walczak et al. 16 and Major and Helms15 reported a pre-season prevalence of BME in 25% of professional and 41% of collegiate knees, respectively. In addition, previous studies have found a 41–50% prevalence of articular cartilage injuries in athletes. 14–16 Our study also identified a high rate of chondral injuries of 71% pre- and 81% post-season. The articular cartilage and bony structures of the patellofemoral joint were especially affected. Post-season scans revealed marrow edema involving the patellofemoral articulation in 76% of subjects and articular cartilage injury of the patella or trochlea in 71% of subjects. Kaplan et al.14 and Walczak et al.16 also reported a high prevalence of patellofemoral cartilage lesions, with patellar lesions noted in 35% and 44% and trochlear notch lesions noted in 25% and 26% of knees studied, respectively.14,16 These findings implicate the patellofemoral joint as a region of particularly high stress in basketball players. Patellofemoral biomechanics are strongly influenced by the applied loading 22–24 and patellar cartilage may have different mechanical properties than femoral cartilage, potentially increasing its susceptibility to fibrillation.25 These factors may play a role in the development of patellofemoral pathology as a result of the high loads generated during the repetitive, high intensity running and jumping inherent in this sport.
Importantly, this study observed a high prevalence of signal change and irregularity in articular cartilage. Moreover, the Noyes Sum cartilage score, representing overall chondral injury throughout the knee, increased significantly after a season of play (p=0.0009). Several studies have documented the short-term effects of exercise on articular cartilage, including in vivo cartilage deformation and changes in collagen structure, interstitial water content, and proteoglycan content.26,27 The long-term effects of exercise on cartilage are less well understood. In contrast to muscle and bone, cartilage has limited ability for repair following injury and is less adaptable to its mechanical environment.26,28,29 It is therefore plausible that high-intensity basketball may result in acute chondral changes, with potentially long-lasting effects. A 3.0-T MRI study of runners by Luke et al.30 did not demonstrate any gross morphologic changes in articular cartilage after running a marathon. However, T2 and T1ρ relaxation times were elevated, suggesting physiologic changes in cartilage that persisted for up to 3 months after running a marathon.30 In a recent 3.0-T MRI study of asymptomatic female collegiate athletes, Peers et al.31 measured increased T1ρ relaxation times in the radial zone of the medial femoral condyle in basketball players compared to swimmers, suggesting decreased proteoglycan content consistent with degenerative change.
The current 3.0-T MRI study revealed meniscal signal abnormality in 50% of pre-season knees and 62% of post-season knees. These results are similar to Walczak et al.16 who reported degenerative changes in the menisci of 54% of asymptomatic NBA players with a tear in one player (3.6%). Major and Helms15 found no tears in 34 knees of college basketball players. In a 1.5-T MRI study of professional basketball players, Kaplan et al.14 reported meniscal abnormalities (four grade 1, two grade 2, two grade 3) in 8 of 40 knees (20%), with 7 of these 8 lesions in the medial meniscus. Similarly in the current study, the majority of meniscal changes were localized to the medial meniscus (11 pre- and 12 post-season) as compared to the lateral meniscus (2 pre- and 3 post-season). A trend towards increasing meniscus signal was observed in the post-season. However, it was not statistically significant (p=0.016) and may represent Type II statistical error due to inadequate number of subjects. A 3.0-T MRI study of marathon runners by Stehling et al. 32 revealed a 15% prevalence of grade 1 intra-substance meniscal signal. Similar to the Luke et al.30 study of articular cartilage in marathon runners, elevated T2 and T1ρ relaxation times were measured in the menisci in post-marathon MR scans. While T2 values decreased after 3 months, T1ρ values remained elevated, indicating more persistent changes in the meniscal matrix composition after a marathon.32
The high prevalence of findings in this study is likely due to the use of 3.0-T MRI rather than 0.3- to 1.5-T MRI. As has been reported previously, the increased signal-to-noise ratio of 3.0-T may improve the diagnostic sensitivity, accuracy, and grading of cartilage abnormalities and other pathology.4,17–19 The limitations of this study include a small number of subjects and non-blinded reviewers. However, a high degree of concordance was noted between the 3 clinicians; of the few situations in which there was a discrepancy, it involved only one grade and was readily resolved by consensus. Another limitation of this study was the use of composite scoring systems for bone marrow edema and cartilage and meniscus signal that did not incorporate the size of the lesion and were not validated. Validated MRI scoring systems such as Magnetic resonance Observation of Cartilage Repair Tissue (MOCART) for cartilage repair33 and Whole-Organ Magnetic Resonance Imaging Score (WORMS) for knee osteoarthritis34 were not applicable to our study data. Finally, comparing our results to studies of male NBA players may not be appropriate as half of our subjects were female. However, a similar prevalence of structural abnormalities was seen between male and female subjects, even though a formal statistical comparison was not performed given the small number of subjects.
The high prevalence of asymptomatic MRI findings observed in this study reinforces the importance of treating the patient, not the MRI, and highlights the necessity for clinical correlation of MRI findings with patient symptoms and physical examination. Obtaining a pre-season MRI as a baseline in high-risk athletes may useful in determining whether an injury during the season represents a new finding on MRI and may help guide management of the injury.16 Further study is warranted to determine whether MRI findings in asymptomatic knees is associated with increased risk for future injury or degenerative change. In theory, morphological defects of meniscus and cartilage visualized on clinical MRI sequences are likely preceded by early degeneration of the biological matrix, including effects on proteoglycan metabolism, collagen composition, and water content.32 Physiologic cartilage MR imaging sequences such as T2 mapping, T1ρ, dGEMRIC, and sodium imaging may be useful for detecting early changes in cartilage.30,35 Suggestions for future research include (i) longitudinal studies to determine the long-term effects of competitive basketball; and (ii) studies that utilize sequential post-season MRI scans to determine the time-course for recovery of abnormal findings, which may provide insight into the amount of rest needed to prevent long-term injury.”
Supplementary Material
Summary of individual subject data (pre-season and post-season). Pre-patellar bursitis, fat pad edema, patellar tendinopathy, quadriceps tendinopathy, and bone marrow edema were graded as follows: 0=none, 1=mild, 2=moderate, 3= severe. Cartilage grade was based on a modified Noyes scale: 0 = normal, 1 = signal change, 2 = defect < 50%, 3 = defect > 50%, 4 = full thickness defect. Meniscus signal was graded as: 0 = normal, 1 intra-substance signal, 2 = linear signal not extending to surface, 3 = linear signal extending to articular surface (true tear). Abbreviations: Pat = patella, Tro = trochlea, MFC = medial femoral condyle, LFC = lateral femoral condyle, MTP = medial tibial plateau, LTP = lateral tibial plateau, MM = medial meniscus, LM = lateral meniscus.
Acknowledgments
Financial support for this research was received from National Institutes of Health (NIH grants EB002524, EB005790 to Dr. Garry Gold) and GE Healthcare. The authors thank Jarrett Rosenberg, PhD of Stanford University for assistance with the statistical analysis.
Contributor Information
George P. Pappas, South Carolina Sports Medicine & Orthopaedic Center.
Melissa A. Vogelsong, Department of Medicine, Stanford University.
Ernesto Staroswiecki, Exponent Failure Analysis.
Garry E. Gold, Department of Radiology, Stanford University.
Marc R. Safran, Department of Orthopaedic Surgery, Stanford University.
References
- 1.Bredella MA, Tirman PF, Peterfy CG, et al. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. Am J Roentgenol. 1999;172(4):1073–80. doi: 10.2214/ajr.172.4.10587150. [DOI] [PubMed] [Google Scholar]
- 2.Potter HG, Linklater JM, Allen AA, et al. Magnetic resonance imaging of articular cartilage in the knee: an evaluation with use of fast spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276–84. doi: 10.2106/00004623-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Kelly MA, Flock TJ, Kimmel JA, et al. MR imaging of the knee: clarification of its role. Arthroscopy. 1991;7(1):78–85. doi: 10.1016/0749-8063(91)90083-a. [DOI] [PubMed] [Google Scholar]
- 4.Magee T, Williams D. 3.0-T MRI of meniscal tears. Am J Roentgenol. 2006;187(2):371–5. doi: 10.2214/AJR.05.0487. [DOI] [PubMed] [Google Scholar]
- 5.Ramnath RR, Magee T, Wasudev N, et al. Accuracy of 3-T MRI using fast spin-echo technique to detect meniscal tears of the knee. Am J Roentgenol. 2006;187(1):221–5. doi: 10.2214/AJR.05.0419. [DOI] [PubMed] [Google Scholar]
- 6.Rubin DA, Kettering JM, Towers JD, et al. MR imaging of knees having isolated and combined ligament injuries. Am J Roentgenol. 1998;170(5):1207–13. doi: 10.2214/ajr.170.5.9574586. [DOI] [PubMed] [Google Scholar]
- 7.Winters K, Tregonning R. Reliability of magnetic resonance imaging of the traumatic knee as determined by arthroscopy. N Z Med J. 2005;118(1209):U1301. [PubMed] [Google Scholar]
- 8.Arndt WF, Truax AL, Barnett FM, et al. MR diagnosis of bone contusions of the knee: comparison of coronal T2-weighted fast spin-echo with fat saturation and fast spin-echo STIR images with conventional STIR images. Am J Roentgenol. 1996;166(1):119–24. doi: 10.2214/ajr.166.1.8571859. [DOI] [PubMed] [Google Scholar]
- 9.Sormaala MJ, Ruohola JP, Mattila VM, et al. Comparison of 1.5T and 3T MRI scanners in the evaluation of acute bone stress in the foot. BMC Musculoskelet Disord. 2011;12:128. doi: 10.1186/1471-2474-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunner MC, Flower SP, Evancho AM, et al. MRI of the athletic knee: findings in asymptomatic professional basketball and collegiate football players. Invest Radiol. 1989;24(1):72–5. doi: 10.1097/00004424-198901000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Connor PM, Banks DM, Tyson AB, et al. Magnetic resonance imaging of the asymptomatic shoulder of overhead athletes: a 5-year follow-up study. Am J Sports Med. 2003;31(5):724–7. doi: 10.1177/03635465030310051501. [DOI] [PubMed] [Google Scholar]
- 12.Major MN. Role of MRI in prevention of metatarsal stress fractures in collegiate basketball players. Am J Roentgenol. 2006;186(1):255–8. doi: 10.2214/AJR.04.1275. [DOI] [PubMed] [Google Scholar]
- 13.Schueller-Weidekamm C, Schueller G, Uffmann M, et al. Does marathon running cause acute lesions of the knee? Evaluation with magnetic resonance imaging. Eur Radiol. 2006;16(10):2179–85. doi: 10.1007/s00330-005-0132-y. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan LD, Schurhoff MR, Selesnick H, et al. Magnetic resonance imaging of the knee in asymptomatic professional basketball players. Arthroscopy. 2005;21(5):557–61. doi: 10.1016/j.arthro.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Major NM, Helms CA. MR imaging of the knee: findings in asymptomatic collegiate basketball players. Am J Roentgenol. 2002;179(3):641–4. doi: 10.2214/ajr.179.3.1790641. [DOI] [PubMed] [Google Scholar]
- 16.Walczak BE, McCulloch PC, Kang RW, et al. Abnormal findings on knee magnetic resonance imaging in asymptomatic NBA players. J Knee Surg. 2008;21(1):27–33. doi: 10.1055/s-0030-1247788. [DOI] [PubMed] [Google Scholar]
- 17.Kijowski R, Blankenbaker DG, Davis KW, et al. Comparison of 1.5- and 3.0-T MR imaging for evaluating the articular cartilage of the knee joint. Radiology. 2009;250(3):839–48. doi: 10.1148/radiol.2503080822. [DOI] [PubMed] [Google Scholar]
- 18.Wong S, Steinbach L, Zhao J, et al. Comparative study of imaging at 3.0 T versus 1.5 T of the knee. Skeletal Radiol. 2009;38(8):761–9. doi: 10.1007/s00256-009-0683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold GE, Suh B, Sawyer-Glover A, Beaulieu C. Musculoskeletal MRI at 3.0 T: initial clinical experience. Am J Roentgenol. 2004;183(5):1479–86. doi: 10.2214/ajr.183.5.1831479. [DOI] [PubMed] [Google Scholar]
- 20.Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505–13. doi: 10.1177/036354658901700410. [DOI] [PubMed] [Google Scholar]
- 21.Crues JV, Mink J, Levy TL, et al. Meniscal tears of the knee: accuracy of MR imaging. Radiology. 1987;164(2):445–8. doi: 10.1148/radiology.164.2.3602385. [DOI] [PubMed] [Google Scholar]
- 22.Heegaard J, Leyvraz PF, Curnier A, et al. The biomechanics of the human patella during passive knee flexion. J Biomech. 1995;28(11):1265–79. doi: 10.1016/0021-9290(95)00059-q. [DOI] [PubMed] [Google Scholar]
- 23.McWalter EJ, Hunter DJ, Wilson DR. The effect of load magnitude on three-dimensional patellar kinematics in vivo. J Biomech. 2010;43(10):1890–7. doi: 10.1016/j.jbiomech.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Simonian PT, Sussman PS, Wickiewicz TL, et al. Contact pressures at osteochondral donor sites in the knee. Am J Sports Med. 1998;26(4):491–4. doi: 10.1177/03635465980260040201. [DOI] [PubMed] [Google Scholar]
- 25.Froimson MI, Ratcliffe A, Gardner TR, et al. Differences in patellofemoral joint cartilage material properties and their significance to the etiology of cartilage surface fibrillation. Osteoarthritis Cartilage. 1997;5(6):377–86. doi: 10.1016/s1063-4584(97)80042-8. [DOI] [PubMed] [Google Scholar]
- 26.Eckstein F, Hudelmaier M, Putz R. The effects of exercise on human articular cartilage. J Anat. 2006;208:491–512. doi: 10.1111/j.1469-7580.2006.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosher TJ, Smith HE, Collins C, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234:245–249. doi: 10.1148/radiol.2341040041. [DOI] [PubMed] [Google Scholar]
- 28.Huiskies R, Ruimerman R, Van Lenthe GH, et al. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000;405:704–706. doi: 10.1038/35015116. [DOI] [PubMed] [Google Scholar]
- 29.Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10:564–572. doi: 10.1053/joca.2002.0814. [DOI] [PubMed] [Google Scholar]
- 30.Luke AC, Stehling C, Stahl R, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running. Does long-distance running lead to cartilage damage? Am J Sports Med. 2010;38(11):2273–80. doi: 10.1177/0363546510372799. [DOI] [PubMed] [Google Scholar]
- 31.Peers SC, Maerz T, Baker EA, et al. T1ρ magnetic resonance imaging for detection of early cartilage changes in knees of asymptomatic collegiate female impact and nonimpact athletes. Clin J Sport Med. 2014;24(3):218–225. doi: 10.1097/JSM.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stehling C, Luke A, Stahl R, et al. Meniscal T1rho and T2 measured with 3.0T MRI increases directly after running a marathon. Skeletal Radiol. 2011;40:725–735. doi: 10.1007/s00256-010-1058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marlovits S, Striessnig G, Resinger CT, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52:310–319. doi: 10.1016/j.ejrad.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Peterfy CG, Guermazi A, Zaim S, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Gold GE, Burstein D, Dardzinski B, et al. MRI of articular cartilage in OA: novel pulse sequences and compositional/functional markers. Osteoarthritis Cartilage. 2006;14(Suppl A):A76–86. doi: 10.1016/j.joca.2006.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of individual subject data (pre-season and post-season). Pre-patellar bursitis, fat pad edema, patellar tendinopathy, quadriceps tendinopathy, and bone marrow edema were graded as follows: 0=none, 1=mild, 2=moderate, 3= severe. Cartilage grade was based on a modified Noyes scale: 0 = normal, 1 = signal change, 2 = defect < 50%, 3 = defect > 50%, 4 = full thickness defect. Meniscus signal was graded as: 0 = normal, 1 intra-substance signal, 2 = linear signal not extending to surface, 3 = linear signal extending to articular surface (true tear). Abbreviations: Pat = patella, Tro = trochlea, MFC = medial femoral condyle, LFC = lateral femoral condyle, MTP = medial tibial plateau, LTP = lateral tibial plateau, MM = medial meniscus, LM = lateral meniscus.