Abstract
Objective
Compared to HIV monoinfection, HIV dual infection (DI) has been associated with decreased CD4 T-cell counts and increased viral loads. The same markers are also associated with the development of HIV-associated neurocognitive disorder (HAND), which continues to be a prevalent problem in the era of combination antiretroviral therapy (ART). We sought to determine the relationship between DI and HAND.
Methods
Participants on ART (N=38) underwent deep sequencing of four PCR-amplified HIV coding regions derived from peripheral blood mononuclear cell (PBMC) DNA samples. Phylogenetic analyses were performed to evaluate whether two distinct viral lineages, i.e. DI, were present in the same individual. All study participants underwent neurocognitive, substance use, and neuromedical assessments at each study visit.
Results
Of 38 participants, 9 (23.7%) had evidence of DI. Using clinical ratings, global neurocognitive impairment was identified in 21 (55%) participants, and multivariate analysis demonstrated a significant association between DI and impairment; OR (95%CI) = 18.3 (1.9,414.2), p = 0.028. Neurocognitive impairment was also associated with lower current (p = 0.028) and nadir (p = 0.043) CD4 T-cell counts.
Conclusions
Deep sequencing of HIV DNA populations in PBMC identified DI in nearly a quarter of HIV-infected adults receiving ART, and DI was associated with HAND. Dual infection may contribute to the development of HAND, perhaps due to increased viral diversity. Further investigation is needed to determine how DI results in worse neurocognitive performance.
Keywords: HIV dual infection, HIV co-infection, HIV superinfection, deep sequencing, HIV-associated neurocognitive impairment, HIV-associated neurocognitive disorder
Introduction
HIV-1 dual infection (DI) occurs when an individual is infected by two viral strains transmitted from different source partners [1]. DI can be further classified as HIV-1 coinfection when both viruses are present at the outset of infection, or as HIV-1 superinfection when the second infection occurs after an established immune response to the first. More than a decade has passed since the first cases of DI were described in humans [2]. Since that time, DI has been increasingly reported from several cohorts due in large part to improvements in amplification and sequencing technologies [3-5]. The incidence of superinfection has ranged between 1.44 and and 4.96 per 100 person-years, comparable to the incidence of primary HIV infection depending on the cohorts studied and their associated risk factors [3-5]. The prevalence of DI in a large cohort of men who have sex (MSM) in San Diego, California was 14.4% [3]. The importance of DI is demonstrated by its association with higher HIV viral load [6-10] and more rapid decline in the CD4 T-cell count [11, 12]. Despite these associations, few studies have investigated links between DI and end-organ damage.
A recent study of female sex workers in Kenya found that HIV-1 superinfection was associated with increased viral loads, but did not accelerate progression to AIDS or time to antiretroviral therapy (ART) initiation [10]. It remains unclear, however, if DI is associated with end-organ dysfunction, especially in the setting of ART. One organ system that can be interrogated for HIV-associated damage even among HIV-infected persons receiving ART is the central nervous system by evaluating for HIV-associated neurocognitive disorder (HAND) [13]. Increasingly sensitive neuropsychological assessments that are standardized to normative control populations have allowed characterization of the spectrum of HAND. These sensitive assessments can detect neurocognitive impairment in the absence of clinical symptoms and have identified that the prevalence of HAND remains high even in the age of current ART [14-17]. Although HAND and DI are both associated with markers of HIV disease progression and both occur more frequently, the relationship between DI and HAND has not been characterized. To address this, we applied deep sequencing to HIV DNA populations in blood samples collected from participants of the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) cohort to estimate the prevalence of DI and to compare DI to HAND.
Methods
Study Participants and Neuropsychological Assessments
We retrospectively studied data and blood samples from 38 HIV-infected adults who had previously enrolled in the NIH-funded CHARTER cohort. CHARTER is an ongoing multisite, observational cohort study of HIV-infected adults whose initial assessment occurred between 2003 and 2010 [14]. For the present study, CHARTER participants were selected who had at least two longitudinal visits and: (i) were followed for >4 years, (ii) were >50 years of age at any study visit, (iii) received ART throughout follow-up, and (iv) had no confounding issues in neurocognitive assessments, like stroke or central nervous system opportunistic infections [13]]. The age criterion was a requisite for the parent study, which investigated the virologic correlates of aging in a CHARTER subset; however, it provides the present study a sample that is representative of the aging HIV-infected U.S. population. Written informed consent was obtained from all study participants. The study was approved by Human Subjects Protection Committees at each of the participating institutions.
As part of CHARTER, all participants had neurocognitive testing to assess seven neuropsychological domains as described previously [14]: verbal fluency, executive functions, speed of information processing, learning, delayed recall, working memory, and motor skills. Based on the testing, neurocognitive impairment was defined in accordance with the Frascati criteria for HAND [13]. Comorbid conditions that may contribute to HAND were classified as incidental (conditions that may affect neurocognitive performance to a minor degree but would be unlikely to cause an individual to be classified as impaired); contributing (conditions that could cause at least mild neurocognitive impairment, but did not confound attribution of impairment to HIV); or confounding (conditions that could fully explain impairment so that HIV effects could not be reliably inferred) [13,14]. CHARTER participants with confounding comorbidities were excluded in this study.
DNA Extraction and Sequencing Methods
Blood samples were collected at each study visit and HIV-1 DNA was isolated from peripheral blood mononuclear cell (PBMC) (QIAamp DNA Mini Kit, Qiagen, Germantown, MD) and PAXgene tubes (PAXgene Blood DNA Kit, Qiagen, Germantown, MD) according to manufacturers’ instructions. Four HIV coding regions, gag p24 (HXB2 coordinates 1366-1619), pol reverse transcriptase (RT, HXB2 coordinates 2708-3242), pol protease (PR, HXB2 coordinates 2253-2550), and env C2-V3 (HXB2 coordinates 6928-7344), were amplified by PCR with region-specific primers, and sequenced on 454 GS FLX Titanium and 454 Junior instruments (Roche, Branford, CT), as described previously [9, 18].
Sequence Analysis and Dual Infection Confirmation
Sequence data were analyzed using the 454 Deep Sequencing bioinformatics pipeline, available as a part of the HyPhy software package and the Datamonkey web server, www.datamonkey.org/help/uds.php [19, 20]. Briefly, high quality reads were retained and aligned, and the maximum divergence was calculated for each coding region in sliding windows. DI was suggested when the maximum divergence exceeded previously established thresholds for each coding region [21]. Longitudinal viral sequence datasets from each participant were incorporated into a phylogeny along with a background of epidemiologically unrelated HIV sequences of the same subtype. If phylogenetic reconstruction suggested DI, sequences were checked for contamination against a database of all laboratory-generated viral sequences, and if none was found, the sample was re-sequenced to confirm DI [3]. DI was confirmed when divergent viral lineages were present in longitudinal HIV sequences isolated from the same individual.
Statistical Methods
Demographic and clinical characteristics were summarized and compared between the mono-infection and DI groups using independent samples t-test for continuous variables and Fisher's exact test for binary and categorical variables. Global neurocognitive impairment was compared between mono-infection and DI groups using Fisher's exact test in unadjusted analyses. Unadjusted and adjusted comparisons between the two groups were summarized using odds ratios and 95% confidence intervals, based on the logistic regression likelihood ratio test. For the adjusted comparison multiple logistic regression was used. Covariates that were significant predictors of neurocognitive impairment at the 0.20 level using likelihood ratio test were candidates for the multiple logistic regression model; the covariate candidates included demographic (age, gender, ethnicity, years of education) clinical (CD4 T-cell count, viral load, estimated duration of infection, hepatitis C status, neurocognitive comorbidities) and behavioral (sexually transmitted infections, intravenous drug use) factors. These covariates were then included in a backward model selection procedure, in addition to mono- versus dual infection groups, using a 0.20 threshold level. The likelihood ratio test was used for all covariates in the adjusted analysis. Two significant digits were used to report p-values ≥ 0.1 and three digits if p < 0.1.
Results
Study cohort and HIV dual infection
HIV DNA populations in blood samples collected from thirty-eight eligible study participants receiving ART underwent deep sequencing to characterize these proviral populations. Two longitudinal samples spaced at least four years apart were available for thirty-six individuals; two only had samples available from a single timepoint. The median estimated duration of infection at the first study timepoint was 10.7 years (IQR: 5.6-16.0 years) and 16.0 years (IQR: 12.4-21.2 years) at the second timepoint. The study cohort was 87% male and the main HIV risk factor was sexual exposures (95%); 21 out of 38 reported being men who had sex with other men as their main HIV risk factor (55%). The median age at the first study timepoint was 50 years (IQR: 45-53 years). Despite reporting ART use, 12 participants had a detectable plasma HIV viral load >500 copies/mL or >2.70 log10 copies/mL, with a median plasma viral load of 4.02 log10 copies/mL (IQR: 2.92-4.79 log10 copies/mL). Characteristics for the study cohort are shown in Table 1.
Table 1.
Demographic and HIV disease characteristics for monoinfection and dual infection groups.
| Monoinfection (N = 29) | Dual infection (N = 9) | p value | |
|---|---|---|---|
| Age, median (IQR) | 50 (45-53) | 52 (48-55) | 0.63 |
| Sex, No. (%) | 0.57 | ||
| Female | 3 (10) | 2 (22) | |
| Male | 26 (90) | 7 (78) | |
| Ethnicity, No. (%) | 0.47 | ||
| Non-Hispanic Black | 14 (48) | 3 (33) | |
| Non-Hispanic White | 13 (45) | 4 (44) | |
| Hispanic | 2 (7) | 2 (22) | |
| Education years, median (IQR) | 14 (12-15) | 13 (12-16) | 0.76 |
| Neurocognitive Comorbidity, No. (%) | 0.088 | ||
| Contributing | 6 (21) | 5 (56) | |
| Incidental | 23 (79) | 4 (44) | |
| Hepatitis C status, No. (%) | 1.00 | ||
| Negative | 23 (79) | 7 (78) | |
| Positive | 6 (21) | 2 (22) | |
| Estimated duration of infection (years), median (IQR) | 10.3 (5.6-14.2) | 15.5 (6.7-17.1) | 0.22 |
| Current CD4 T-cell count, median cells/μL (IQR) | 464 (327-671) | 513 (381-853) | 0.32 |
| Nadir CD4 T-cell count, median cells/μL (IQR) | 108 (15-243) | 133 (86-170) | 0.70 |
| Plasma viral load, median log10 copies/mL (IQR) | <1.7 (1.7-2.81) | <1.7 (1.7-2.07) | 0.57 |
| Detectable plasma viral load, N (%) | 0.48 | ||
| Undetectable | 15 (52) | 6 (67) | |
| Detectable | 14 (48) | 3 (33) | |
| CSF viral load, median log10 copies/mL (IQR) | <1.7 (1.7-1.7) | <1.7 (1.7-1.7) | 0.32 |
| Detectable CSF viral load, N (%) | 0.55 | ||
| Undetectable | 21 (88) | 8 (100) | |
| Detectable | 3 (12) | 0 (0) | |
| Sexually transmitted infections, No. (%)* | 0.55 | ||
| Yes | 4 (14) | 0 ( 0) | |
| No | 25 (86) | 9 (100) | |
| Overall risk factor for HIV infection, No. (%) | 0.051 | ||
| Sexual exposures | 29 (100) | 7 (78) | |
| IVDU | 0 (0) | 2 (22) | |
Sexually transmitted infections included syphilis, gonorrhea, chlamydia, and herpes simplex.
IQR: interquartile range; CSF: cerebrospinal fluid; MSM: men who have sex with men; IVDU: intravenous drug use.
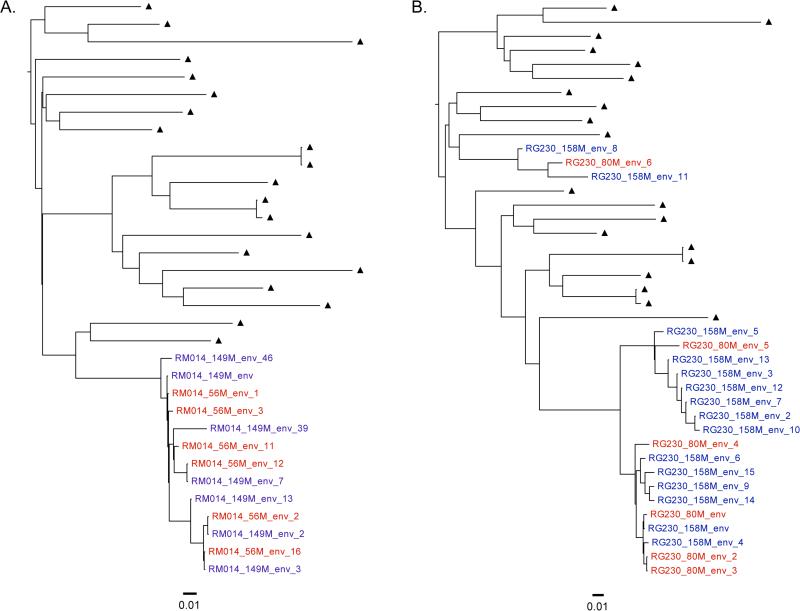
Nine study participants (24%) had two discrete viral lineages observed across longitudinal timepoints and were categorized as having DI. Illustrative cases representing mono-infection and DI are shown in Figure 1. All participant infections were with HIV-1 subtype B. There were no significant differences in terms of plasma viral loads or CD4 T-cell counts at the baseline timepoint between those with mono-infection and DI; other characteristics of the mono-infection and DI groups are displayed in Table 1. A higher frequency of intravenous drug use in the DI group was marginally significant (p = 0.051). Detectable plasma and cerebrospinal fluid (CSF) viral loads were not associated with DI.
Figure 1. Representative phylogenetic trees illustrating HIV dual infection.
Shown are maximum likelihood phylogenetic trees of longitudinal env sequences from two participants representing: A) HIV monoinfection, and B) HIV dual infection. Viral sequence labels from the first timepoint are in red, and sequences from the second timepoint are in blue. Epidemiologically unrelated subtype B background viral sequences are shown as black triangles. Phylogenetic distance is shown at the bottom of each tree for reference.
HIV-associated neurocognitive disorder and HIV dual infection
Neurocognitive impairment was assessed based on clinical ratings (global impairment) [14]. Assessment of neurocognitive impairment at the first study timepoint defined our nerocognitively impaired group. Global neurocognitive impairment was identified in twenty-one (21) participants: eleven of these also had impairment at the second timepoint. Demographic and HIV disease characteristics among these impaired and normal groups are shown in Table 2. Significantly lower CD4 T-cell counts (both current and nadir) were associated with impairment (p = 0.028 and p = 0.043, respectively). There was no association between detectable plasma or CSF viral load and presence of neurocognitive impairment. Using clinical ratings, DI was not significantly associated with impairment; however, after adjusting for variables that were significantly predictive of impairment, multivariate analysis demonstrated a significant association between DI and HAND (Table 2); OR (95%CI) = 18.30 (1.94, 414.16), p = 0.028. Of note, the CSF viral load was not included in the multivariable analysis because six participants had missing data, and the level in the remaining participants was undetectable in all but three. Important covariates including hepatitis C, intravenous drug use, sexually transmitted infections (including syphilis), were not associated with either DI or neurocognitive impairment.
Table 2.
Association of demographic and HIV disease characteristics with clinical-ratings-based global neurocognitive impairment.
| Unimpaired (N = 17) | Impaired (N = 21) | p value | |
|---|---|---|---|
| Age, median (IQR) | 50 (46-55) | 50 (45-53) | 0.18 |
| Sex, No. (%) | 0.36 | ||
| Female | 1 (6) | 4 (19) | |
| Male | 16 (94) | 17 (81) | |
| Ethnicity, No. (%) | 0.80 | ||
| Black | 8 (47) | 9 (43) | |
| Hispanic | 1 (6) | 3 (14) | |
| Caucasian | 8 (47) | 9 (43) | |
| Education years, median (IQR) | 13 (12-15) | 14 (12-16) | 0.34 |
| Neurocognitive comorbidity, No. (%) | 0.28 | ||
| Contributing | 3 (18) | 8 (38) | |
| Incidental | 14 (82) | 13 (62) | |
| Hepatitis C status, No. (%) | 0.43 | ||
| Negative | 12 (71) | 18 (86) | |
| Positive | 5 (29) | 3 (14) | |
| Estimated duration of infection (years), median (IQR) | 11.9 (10.3-15.5) | 7.3 (3.3-16.4) | 0.09 |
| Sexually transmitted infections, No. (%)* | 1.00 | ||
| Yes | 2 (12) | 2 (10) | |
| No | 15 (88) | 19 (90) | |
| Current CD4 T-cell count, median cells/μL (IQR) | 578 (424-906) | 382 (137-537) | 0.028 |
| Nadir CD4 T-cell count, median cells/μL (IQR) | 199 (19-400) | 101 (15-162) | 0.043 |
| Plasma viral load, median log10 copies/mL (IQR) | <1.7 (1.7-2.96) | <1.7 (1.7-2.76) | 0.90 |
| Detectable plasma viral load, N (%) | 0.75 | ||
| Undetectable | 10 (59) | 11 (52) | |
| Detectable | 7 (41) | 10 (48) | |
| CSF viral load, median log10 copies/mL (IQR) | <1.7 (1.7-1.7) | <1.7 (1.7-1.7) | 0.12 |
| Detectable CSF viral load, N (%) | 0.24 | ||
| Undetectable | 14 (100) | 15 (83) | |
| Detectable | 0 (0) | 3 (17) | |
| Overall risk factor for HIV infection, No. (%) | 0.49 | ||
| Sexual exposures | 17 (100) | 19 (90) | |
| IVDU | 0 (0) | 2 (10) | |
| GDS median (IQR) | 0.2 (0.0-0.4) | 0.6 (0.5-1.1) | <0.0001 |
| DI status, No. (%) | 0.15 | ||
| Monoinfection | 15 (52) | 14 (48) | |
| Dual infection | 2 (22) | 7 (78) | |
| Adjusted analysis of effect of dual infection on neurocognitive impairment | ||
|---|---|---|
| Predictor | OR (95% CI) | p value |
| Dual vs. mono-infection | 18.30 (1.94, 414.16) | 0.028 |
| Estimated duration of infection, per year | 0.87 (0.74, 0.99) | 0.17 |
| Current CD4 T-cell count, per cell | 0.997 (0.994, 0.999) | 0.017 |
Sexually transmitted infections included syphilis, gonorrhea, chlamydia, and herpes simplex.
IQR: interquartile range; GDS, global deficit score; CSF: cerebrospinal fluid; MSM: men who have sex with men; IVDU: intravenous drug use; DI: HIV dual infection; NC: neurocognitive.
Discussion
The present study used deep sequencing of HIV DNA populations in blood to screen a well-characterized cohort of individuals receiving ART for DI and found: (i) a high prevalence of DI (24%) among chronically infected individuals receiving ART, and (ii) an association between DI and HAND. These observations may provide a deeper understanding of the virologic correlates of HAND. Lentiviral DI has been described with feline immunodeficiency virus (FIV) in cats, simian immunodeficiency virus (SIV) in macaques and HIV-1 in chimpanzees [22-24], so it is not surprising that HIV-1 DI occurs in humans. A recent domestic FIV study showed DI was associated with neuroinflammation and neurodegeneration, suggesting that infection by multiple viruses might contribute to neuropathogenesis [25].
Whether the observed association between DI and HAND is causal cannot be determined by the current data. Neurocognitively impaired individuals may be more likely to become dually infected or both conditions may have a third causal condition in common. However, we did not find evidence linking both conditions with either intravenous drug use (associated with DI but not with neurocognitive impairment) or sexually transmitted infections (not associated with DI or neurocognitive impairment). The possibility that DI and neurocognitive impairment are causally linked is indirectly supported by the observations that HIV enters the CNS early in infection [26, 27] and that DI occurs most frequently in the first one to two years after primary HIV infection [3, 4]. Together, these events could lead to greater viral genetic diversity in the CNS, which might increase the risk of viral adaptation and fitness in the CNS.
The limitations of our analysis include its relatively small sample size, resulting in limited power to determine statistically significant associations with DI or HAND. While a larger sample size would have increased power, our study had adequate power to test our primary hypothesis. Of note, the number of fully characterized dually infected participants worldwide stands at less than 70.
This study demonstrated the value of PBMC DNA for deep sequencing studies of HIV DI, which is important since ART use limits DI studies using RNA from blood plasma. The current study identifies an interesting connection between DI and HAND, but the mechanisms underlying these associations remain unknown and require further investigation.
Acknowledgements
The authors would like to acknowledge the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study which was supported by awards N01 MH22005, HHSN271201000036C and HHSN271201000030C from the National Institutes of Health; as well as the principal investigators of all the CHARTER sites: Dr. Justin McArthur (Johns Hopkins University), Dr. Susan Morgello (Icahn School of Medicine at Mount Sinai), Dr. David Simpson (Icahn School of Medicine at Mount Sinai), Dr. Allen McCutchan (University of California San Diego), Dr. Benjamin Gelman (University of Texas Medical Branch at Galveston), Dr. Ann Collier (University of Washington), Dr. Christina Marra (University of Washington), and Dr. David Clifford (Washington University in St. Louis). Special thanks to Dr. Morgello for coordinating blood and Paxgene specimens, and to Dr. Marra for helpful insights with the poster on which this manuscript was based. We would also like to thank Caroline Ignacio for invaluable assistance in the laboratory, and to Demetrius Dela Cruz for his administrative support. We are particularly grateful to all the participants in the CHARTER study.
This work was supported by the U.S. Department of Veterans Affairs; the National Institutes of Health [TR001444, AI100665, AI036214, AI007384, MH062512, MH097520, AI096113, AI047745, MH22005, HHSN271201000036C and HHSN271201000030C]; the International AIDS Vaccine Initiative; and the James B. Pendleton Charitable Trust. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
These data were presented in part as a poster at the Conference on Retroviruses and Opportunistic Infections, Boston, MA; March 5, 2014 (Poster 471).
Author Contibutions
GAW helped conceive the study design, helped perform experiments, analyzed data, and wrote the manuscript; AC generated phylogenetic trees; SL performed statistical analyses; DRF acquired and mined CHARTER data and facilitated sample acquisition; GC performed deep sequencing experiments; SLKP developed the bioinformatics pipeline and contributed to manuscript editing; FV oversaw and performed statistical analyses; RKH developed the neurocognitive assessments and contributed to the manuscript; SLL edited the manuscript; IG oversaw the CHARTER cohort; DDR edited the manuscript; DMS conceived the study and edited the manuscript.
Conflicts of Interest
GAW does not have any commercial or other associations that might pose a conflict of interest. DDR has served as a consultant for BMS, Chimerix, Gilead, Antiva, HIV Immunotherapeutics Institute, and Monogram. DMS has received research support from ViiV Pharmaceuticals and has served as a consultant to Gen-Probe, ViiV Pharmaceuticals and FluxErgy. SKP has served as a consultant to Monogram Biosciences and Gen-Probe. The remaining authors do not report any conflicts of interest.
References
- 1.Smith DM, Richman DD, Little SJ. HIV superinfection. J Infect Dis. 2005;192:438–444. doi: 10.1086/431682. [DOI] [PubMed] [Google Scholar]
- 2.Ramos A, Hu DJ, Nguyen L, Phan KO, Vanichseni S, Promadej N, et al. Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users. J Virol. 2002;76:7444–7452. doi: 10.1128/JVI.76.15.7444-7452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner GA, Pacold ME, Kosakovsky Pond SL, Caballero G, Chaillon A, Rudolph AE, et al. Incidence and Prevalence of Intrasubtype HIV-1 Dual Infection in At-Risk Men in the United States. J Infect Dis. 2014;209:1032–1038. doi: 10.1093/infdis/jit633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronen K, McCoy CO, Matsen FA, Boyd DF, Emery S, Odem-Davis K, et al. HIV-1 superinfection occurs less frequently than initial infection in a cohort of high-risk Kenyan women. PLoS Pathog. 2013;9:e1003593. doi: 10.1371/journal.ppat.1003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redd AD, Mullis CE, Serwadda D, Kong X, Martens C, Ricklefs SM, et al. The Rates of HIV Superinfection and Primary HIV Incidence in a General Population in Rakai, Uganda. J Infect Dis. 2012;206:267–274. doi: 10.1093/infdis/jis325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altfeld M, Allen TM, Yu XG, Johnston MN, Agrawal D, Korber BT, et al. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature. 2002;420:434–439. doi: 10.1038/nature01200. [DOI] [PubMed] [Google Scholar]
- 7.Jost S, Bernard MC, Kaiser L, Yerly S, Hirschel B, Samri A, et al. A patient with HIV-1 superinfection. N Engl J Med. 2002;347:731–736. doi: 10.1056/NEJMoa020263. [DOI] [PubMed] [Google Scholar]
- 8.Smith DM, Wong JK, Hightower GK, Ignacio CC, Koelsch KK, Daar ES, et al. Incidence of HIV superinfection following primary infection. JAMA. 2004;292:1177–1178. doi: 10.1001/jama.292.10.1177. [DOI] [PubMed] [Google Scholar]
- 9.Pacold ME, Pond SL, Wagner GA, Delport W, Bourque DL, Richman DD, et al. Clinical, virologic, and immunologic correlates of HIV-1 intraclade B dual infection among men who have sex with men. AIDS. 2012;26:157–165. doi: 10.1097/QAD.0b013e32834dcd26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronen K, Richardson BA, Graham SM, Jaoko W, Mandaliya K, McClelland RS, et al. HIV-1 superinfection is associated with an accelerated viral load increase but has a limited impact on disease progression. AIDS. 2014;28:2281–2286. doi: 10.1097/QAD.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelissen M, Pasternak AO, Grijsen ML, Zorgdrager F, Bakker M, Blom P, et al. HIV-1 dual infection is associated with faster CD4+ T-cell decline in a cohort of men with primary HIV infection. Clin Infect Dis. 2012;54:539–547. doi: 10.1093/cid/cir849. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb GS, Nickle DC, Jensen MA, Wong KG, Grobler J, Li F, et al. Dual HIV-1 infection associated with rapid disease progression. Lancet. 2004;363:619–622. doi: 10.1016/S0140-6736(04)15596-7. [DOI] [PubMed] [Google Scholar]
- 13.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heaton RK, Cysique LA, Jin H, Shi C, Yu X, Letendre S, et al. Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural China. J Neurovirol. 2008;14:536–549. doi: 10.1080/13550280802378880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 17.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 18.Gianella S, Delport W, Pacold ME, Young JA, Choi JY, Little SJ, et al. Detection of minority resistance during early HIV-1 infection: natural variation and spurious detection rather than transmission and evolution of multiple viral variants. J Virol. 2011;85:8359–8367. doi: 10.1128/JVI.02582-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosakovsky Pond SL, Frost SDW, Muse SV. HyPhy: hypothesis testing using phylogenies. Bioinformatics. 2005;21:676–679. doi: 10.1093/bioinformatics/bti079. [DOI] [PubMed] [Google Scholar]
- 20.Kosakovsky Pond SL, Posada D, Stawiski E, Chappey C, Poon AF, Hughes G, et al. An evolutionary model-based algorithm for accurate phylogenetic breakpoint mapping and subtype prediction in HIV-1. PLoS Comput Biol. 2009;5:e1000581. doi: 10.1371/journal.pcbi.1000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacold M, Smith D, Little S, Cheng PM, Jordan P, Ignacio C, et al. Comparison of methods to detect HIV dual infection. AIDS Res Hum Retroviruses. 2010;26:1291–1298. doi: 10.1089/aid.2010.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachmann MH, Mathiason-Dubard C, Learn GH, Rodrigo AG, Sodora DL, Mazzetti P, et al. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J Virol. 1997;71:4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravell M, London WT, Hamilton RS, Stone G, Monzon M. Infection of macaque monkeys with simian immunodeficiency virus from African green monkeys: virulence and activation of latent infection. J Med Primatol. 1989;18:247–254. [PubMed] [Google Scholar]
- 24.Wei Q, Fultz PN. Extensive diversification of human immunodeficiency virus type 1 subtype B strains during dual infection of a chimpanzee that progressed to AIDS. J Virol. 1998;72:3005–3017. doi: 10.1128/jvi.72.4.3005-3017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afkhami-Goli A, Liu SH, Zhu Y, Antony JM, Arab H, Power C. Dual lentivirus infection potentiates neuroinflammation and neurodegeneration: viral copassage enhances neurovirulence. J Neurovirol. 2009;15:139–152. doi: 10.1080/13550280802534763. [DOI] [PubMed] [Google Scholar]
- 26.Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 27.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]