Abstract
Kisspeptin controls reproduction by stimulating GnRH neurons via its receptor Kiss1r. Kiss1r is also expressed other brain areas and in peripheral tissues, suggesting additional non-reproductive roles. We recently determined that Kiss1r knockout (KO) mice develop an obese and diabetic phenotype. Here, we investigated whether Kiss1r KOs develop this metabolic phenotype due to alterations in the expression of metabolic genes involved in the appetite regulating system of the hypothalamus, including neuropeptide Y (Npy) and pro-opiomelanocortin (Pomc), as well as leptin receptor (Lepr), ghrelin receptor (Ghsr), and melanocortin receptor 3 and 4 (Mc3r, Mc4r). Body weights, leptin levels, and hypothalamic gene expression were measured in both gonad-intact and gonadectomised (GNX) mice at 8 and 20 weeks of age, fed either normal chow or a high-fat diet. We detected significant increases in Pomc expression in gonad-intact Kiss1r KOs at 8 weeks and 20 weeks, but no alterations in the other metabolic-related genes. However, the Pomc increases appeared to reflect genotype differences in circulating sex steroids, because GNX wildtype (WT) and Kiss1r KOs exhibited similar Pomc levels, along with similar Npy levels. The altered Pomc gene expression in gonad-intact Kiss1r KOs is consistent with previous reports of reduced food intake in these mice and may serve to increase the anorexigenic drive, perhaps compensating for the obese state. However, the surprising overall lack of changes in any of the hypothalamic metabolic genes in GNX KOs suggests that the aetiology of obesity in the absence of kisspeptin signalling may reflect peripheral rather than central metabolic impairments.
Keywords: Kisspeptin, Metabolism, GPR54, Kiss1, Leptin
Introduction
Kisspeptin (encoded by the Kiss1 gene) is a neuropeptide made in the hypothalamus that acts via the kisspeptin receptor, Kiss1r (originally known as Gpr54), to drive reproduction (1). Hypothalamic kisspeptin activates the reproductive axis by potently and directly stimulating gonadotropin releasing hormone (GnRH) neurons, which express Kiss1r (2–7). In humans and mice, both Kiss1 and Kiss1r mutations lead to hypogonadotrophic hypogonadism (1, 4, 8–12), highlighting the vital role for kisspeptin signalling in puberty and the maintenance of fertility.
In addition to being expressed in reproductive brain regions, Kiss1 and Kiss1r mRNA are both also expressed in ‘non-GnRH related’ areas of the brain (13–16). Moreover, in the periphery, Kiss1r is expressed in the liver, pancreas, and brown and white adipose tissue (14). This suggests kisspeptin may have roles outside of reproduction, perhaps pertaining to metabolic function or energy balance. Indeed, we recently identified a metabolic role of kisspeptin signalling (17). We demonstrated that adult Kiss1r knockout (KO) females have robustly greater body weights, increased adiposity, and elevated leptin levels compared to wildtype (WT) littermates. This obesity in Kiss1r KOs appears to reflect, at least in part, reduced metabolic rate and energy expenditure (17). Interestingly, despite their obese phenotype, Kiss1r KO mice exhibit significantly lower food intake, perhaps reflecting the actions of the elevated leptin in these mice. Importantly, the obese phenotype in Kiss1r KOs is maintained even after WTs and KOs are both gonadectomised [to equate circulating sex steroid levels, which are known to influence body weight and metabolism (18, 19)], indicating that the phenotype is not simply due to absent sex steroids in the KO’s hypogonadal state. Thus, kisspeptin signalling has a previously unappreciated metabolic role, but the underlying mechanisms causing the altered body weight, food intake, and energy expenditure in Kiss1r KO mice are currently unknown and remain to be determined.
It is possible that some aspects of the body weight or feeding phenotype in Kiss1r KO mice are due to altered hypothalamic regulation of food intake or energy expenditure. Interestingly, kisspeptin treatment has been shown to regulate both Npy and Pomc neuronal activity (20). Specifically, electrophysiological recordings in mouse brain slices demonstrated that kisspeptin administration reduced Npy neuronal firing (by indirect means) and increased Pomc neuronal firing (by direct actions). Alternatively, kisspeptin treatment increased Npy gene expression and decreased Pomc expression in ewes (21), though similar examinations have not yet been reported for rodents or other species. Therefore, it appears that kisspeptin is sufficient to alter the appetite regulating systems in the hypothalamus, but whether this actually occurs in vivo is unknown. Furthermore, whether the hypothalamic Pomc and Npy systems are perturbed in Kiss1r KO mice, perhaps contributing to their metabolic or feeding phenotype, is similarly not known.
In the present study, we hypothesised that hypothalamic metabolic gene expression in Kiss1r KO mice would be altered compared to WT mice, consistent with the former’s obese phenotype. We examined key neuropeptide genes in the appetite regulating system of the arcuate nucleus of the hypothalamus, including the primary orexigenic neuropeptide neuropeptide Y (Npy) and the main anorexigenic neuropeptide pro-opiomelanocortin (Pomc) (22). We also examined the hypothalamic expression of several key metabolic-related receptors present in Npy and/or Pomc neurons, including leptin receptor (Lepr), ghrelin receptor (Ghsr) and melanocortin 3 and melanocortin 4 receptors (Mc3r and Mc4r). All these genes were examined in mice at both 8 and 20 weeks of age, the former representing an age just prior to the emergence of the elevated body weight and the latter representing an age when the KOs are fully overweight.
Materials and Methods
Animals
Kiss1r KO mice were generated by Omeros Corporation (Seattle, WA) via retroviral mutagenesis (17). WT and KO littermates were generated from heterozygote breeder pairs. For our experiments, only WT and KO littermate offspring were used; heterozygous offspring were not studied. Experiments were performed based on the National Health and Medical Research Council/Commonwealth Scientific and Industrial Research Organisation/Australian Animal Commission Code of Practice of Care and the Use of Animals for Experimental Purposes. This study was approved by the Animal Ethics Committee from the University of Western Australia and by the Institutional Animal Care and Use Committee from the University of California San Diego.
Experimental Design
Mice were examined at two ages: 8 weeks, just prior to when the increased body weight is first starting to emerge in female Kiss1r KO mice (17), and 20 weeks old, when the obese phenotype is fully present. For each age group, both WTs and KOs were examined either gonad-intact or gonadectomised (GNX), the latter cohort to control for the effects of absent circulating gonadal sex steroids in Kiss1r KOs (which are hypogonadal). All GNXs occurred at 6 weeks of age, with mice anesthetised under isoflurane inhalation, as previously described (23). A subset of mice examined at 20 weeks were also challenged with a high fat diet (HFD) (23% fat, 4.7% crude fibre and 18.4% protein; 46% total energy from lipids; SFO4-027; Speciality Feeds, Perth, Australia. Standard chow was 4.8% fat, 4.8% crude fibre and 19.4% protein) administered for a duration of 12 weeks, from 8 to 20 weeks of age.
For tissue collection, 8 week or 20 week old mice were deeply anesthetised under isoflurane inhalation, body weight was recorded, and blood collected via cardiac puncture. Mice were then euthanised by rapid decapitation and brains were subsequently removed and frozen on dry ice. Brains and blood were stored at −80°C until further analysis.
Plasma leptin measurements
Plasma leptin levels were measured in GNX mice of both genotypes at 8 and 20 weeks. Blood samples were collected in ethylenediaminetertracetic acid (EDTA) and a protease inhibitor cocktail (Complete, Roche Applied Science, Mannheim, Germany), and the plasma immediately harvested and stored at −20 C. For the 8 week GNX mice, leptin concentrations were measured using the Milliplex Map Mouse Metabolic Hormone Magnetic Bead Panel (Millipore, Missouri, USA, cat no. MMHMAG-44K) using Luminex technology and Exponent software in accordance with manufacturer’s instructions. The minimum detectable concentration of leptin was 19 pg/mL and the intra-assay CV% was 5%. For 20 week GNX mice, leptin concentrations were determined with a mouse leptin ELISA kit (#90030; Crystal Chem Inc.) in accordance with the manufacturer’s instructions. The minimum detectable concentration of leptin was 2 pg/mL and the intra-assay CV% was <10%.
Quantitative real time PCR (qRT-PCR) for metabolic hypothalamic gene expression in the hypothalamus
For qRT-PCR analyses of metabolic genes, medial basal hypothalami were dissected from whole hypothalamic samples as described in (24). Total RNA was isolated using the phenol/chloroform method and converted to cDNA via reverse transcription (Promega, Sydney, Australia), followed by clean up using the PCR Clean Up Kit (Mio Bio Laboratories, Carlsbad, CA, USA). qRT- PCR was performed in 10µl reaction volumes and samples were tested in duplicate using a Rotorgene 3000 (Corbett Life Science, US). Reactions used either BioRad IQ SYBR Green Supermix (Biorad, Australia) or Qiagen SYBR Green PCR Kit (Qiagen, Australia). The master mix varied dependant on whether mouse Qiagen QuantiTect primers (Npy, Pomc, Ghsr, Mc3r and Mc4r) or Geneworks (leptin receptor, ObRb) primers were used (Supplementary Table 1 and 2). Samples were compared to a standard curve (10 fold dilution) and relative gene expression was normalised using a GE Norm algorithm (25) of housekeeping genes peptidylpropyl isomerase A (Ppia), succinate hydrogenase (Sdha) and TATA box binding protein (Tbp). No significant variability was noted in each housekeeping gene.
In situ hybridisation
A subset of brains from chow-fed GNX 20 week female mice were analysed for Npy or Pomc mRNA expression in the arcuate nucleus (ARC) by in situ hybridisation (ISH). Briefly, brains were cut into 5 alternating sets of 20 µm sections. For both Npy and Pomc, each group consisted of 6 brains, with one full set of slide-mounted brain sections encompassing the entire ARC region assayed using radiolabeled (33P) antisense Npy or Pomc riboprobes (0.04 pmol/mL), as previously described (26). Pomc was cloned from mouse hypothalamic RNA into pBluescript SK-plasmid and transcribed using T7 polymerase. The Pomc probe corresponds to bases 58–926 of the murine mRNA (NM_008895.4), and the Npy probe was previously described (26). ISH slides were analysed blindly with an automated grains imaging processing system (Dr Don Clifton, University of Washington, Seattle, Washington) that counted the number of silver grain clusters representing Npy or Pomc cells and the number of silver grains in each cell (a semiquantitative index of Pomc or Npy mRNA content per cell) (27).
Statistical analysis
All data are presented as mean ± SEM. For 20 week gonad intact and 8 week GNX mice, gene expression was examined in each sex separately by an unpaired t test (due to different standard curves for qRT-PCR analysis). For 8 week gonad intact and 20 week GDX mice, males and females were analysed under the same standard curve and a two-way ANOVA with a Tukey post hoc test was used, followed by student t tests. Unpaired t tests were also used to examine ISH data. For plasma leptin measurements, two-way ANOVAs with a Tukey post hoc test were performed. Body weight in 20 week GNX mice was re-examined using data in our past study (17). All statistical tests were performed with Graph Pad Prism 5 and significance was set at p < 0.05.
Results
Normal body weights but elevated plasma leptin levels in 8 week GNX Kiss1r KO mice
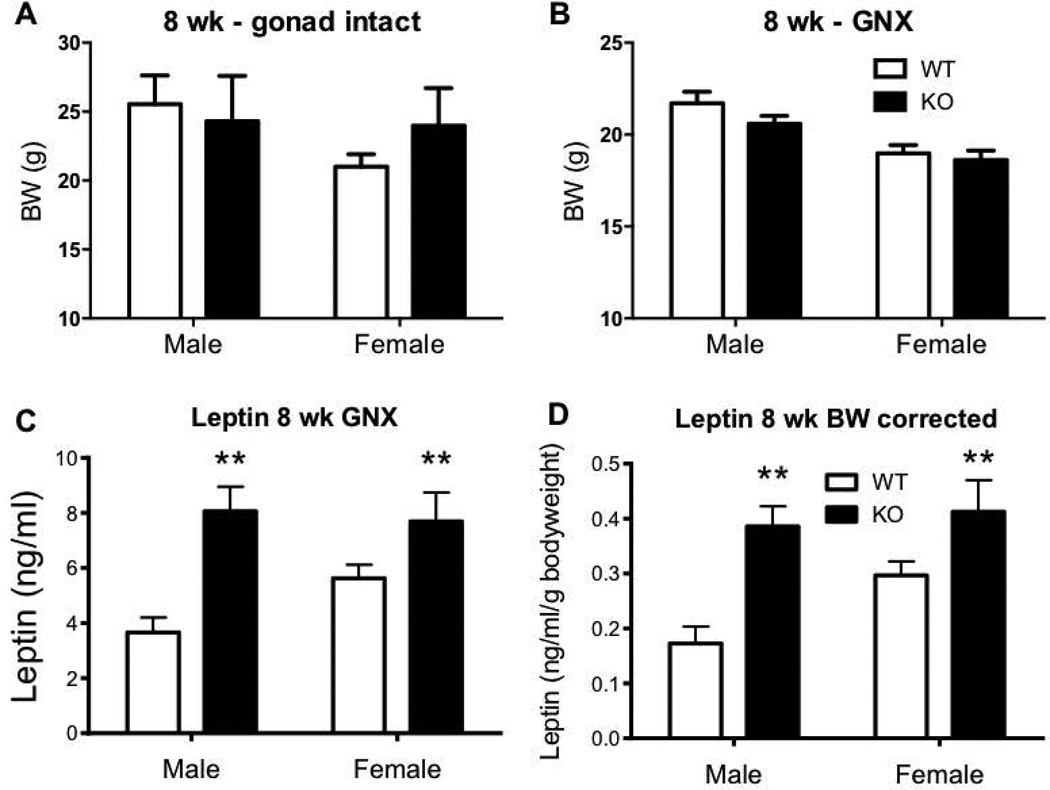
As previously reported (17), no significant difference in body weight was seen in 8 week Kiss1r KO mice of either sex compared to WTs (Figure 1A and B). However, at this age, the concentration of circulating leptin was already significantly elevated in GNX Kiss1r KO mice of both sexes: leptin levels were 120% greater in KO males and 37% greater in KO females compared to WT controls (both p<0.01, Figure 1C). When corrected for body weight, the elevated leptin levels in Kiss1r KO mice remained. Specifically, leptin per gram bodyweight was 123% higher in male KOs and 39% higher in female KOs compared to their WT counterparts (both p<0.01, Figure 1D).
Figure 1.
The effect of impaired kisspeptin signalling on body weight (BW) in 8 week (wk) intact (A) or gonadectomised (GNX) mice (B). (C, D) Leptin concentration and BW corrected leptin concentration in GNX Kiss1r KO mice. As indicated, ** p<0.01 compared to wild type (WT – shown as white bars, Kiss1r KO – black bars). Values are the mean ± SEM. n=7–9 per group.
Increased Pomc, but not Npy, gene expression in gonad intact, but not GNX, 8 week Kiss1r KO mice
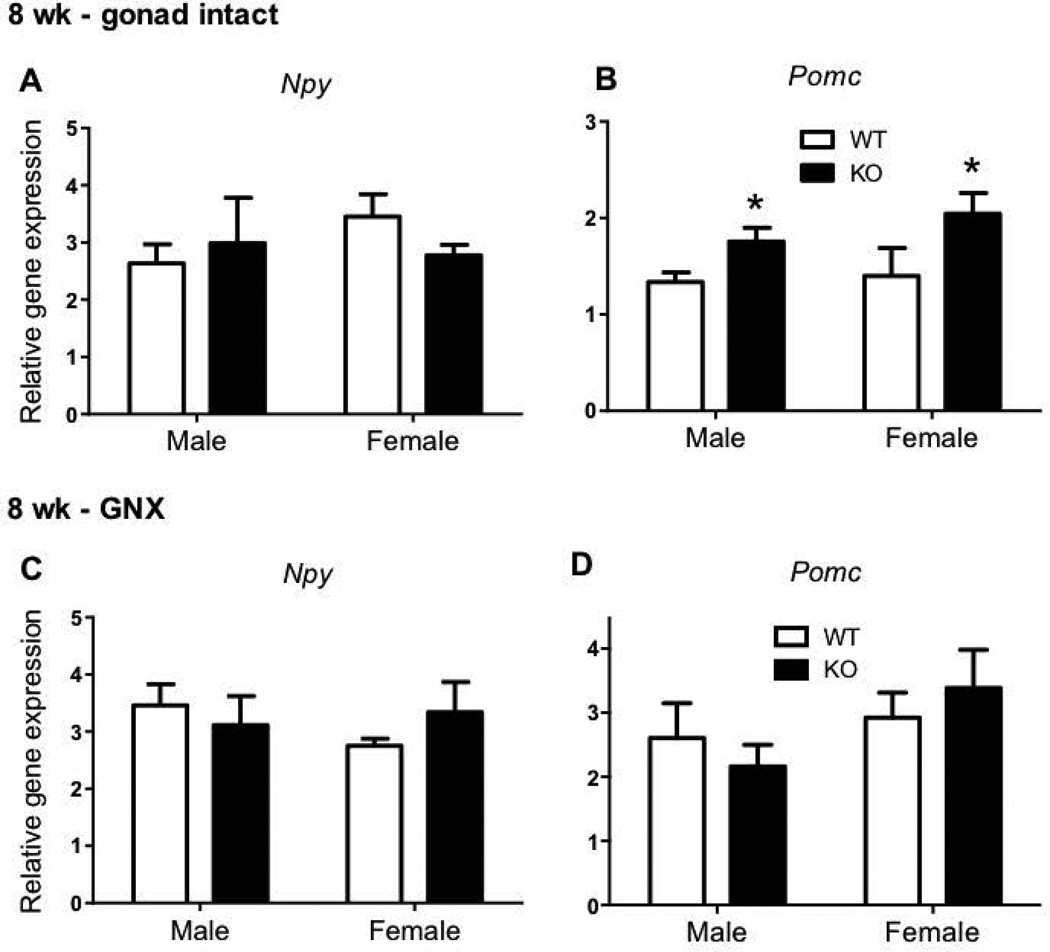
Hypothalamic Npy gene expression did not differ in 8 week old Kiss1r KO mice compared to WTs in either gonad intact (Figure 2A) or GNX conditions (Figure 2C). Hypothalamic Pomc gene expression was 31% (males) and 78% (females) higher in gonad intact Kiss1r KOs (both p<0.05) compared to WTs at 8 weeks of age (Figure 2B). However, in mice that were GNX to equate sex steroid levels, there was no longer a genotype difference in Pomc gene expression in either sex (Figure 2D).
Figure 2.
The effect of impaired kisspeptin signalling on neuropeptide Y (Npy) and pro-opiomelanocortin (Pomc) hypothalamic gene expression in 8 week (wk) gonad intact (A, B) and gonadectomised (GNX) (C, D) mice. As indicated, * p<0.05 compared to wild type (WT – shown as white bars, Kiss1r KO – black bars). Values are the mean ± SEM. n=3–9 per group (n=3 in the intact female KO group only).
No changes in hypothalamic metabolic receptor gene expression in 8-week Kiss1r KO mice
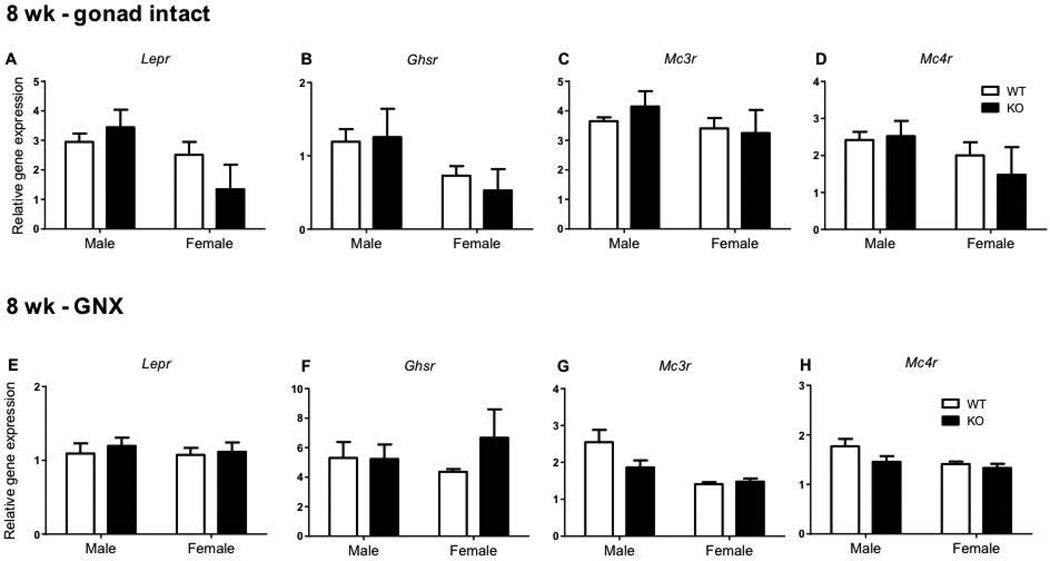
Hypothalamic expression of metabolic receptor genes was also investigated in 8 week old mice (Figure 3). There were no genotype differences in hypothalamic gene expression in leptin receptor (Lepr, Figure 3A and 3E), ghrelin receptor (Ghsr, Figure 3B and 3F), melanocortin 3 receptor (Mc3r, Figure 3C and 3G), or melanocortin 4 receptor (Mc4r, Figure 3D and 3H) in either gonad intact or GNX mice of either sex.
Figure 3.
The effect of impaired kisspeptin signalling on metabolic receptor expression in 8 week (wk) gonad intact and gonadectomised (GNX) mice. Leptin receptor (Lepr) (A, E) ghrelin receptor (Ghsr) (B, F), melanocortin 3 receptor (Mc3r) (C, G) and melanocortin 4 receptor (Mc4r) (D, H) hypothalamic gene expression in males and females (WT – shown as white bars, Kiss1r KO – black bars). Values are the mean ± SEM. n=3–9 per group (n=3 in the intact female KO group only).
Elevated body weights and higher leptin levels in 20 week Kiss1r KO mice
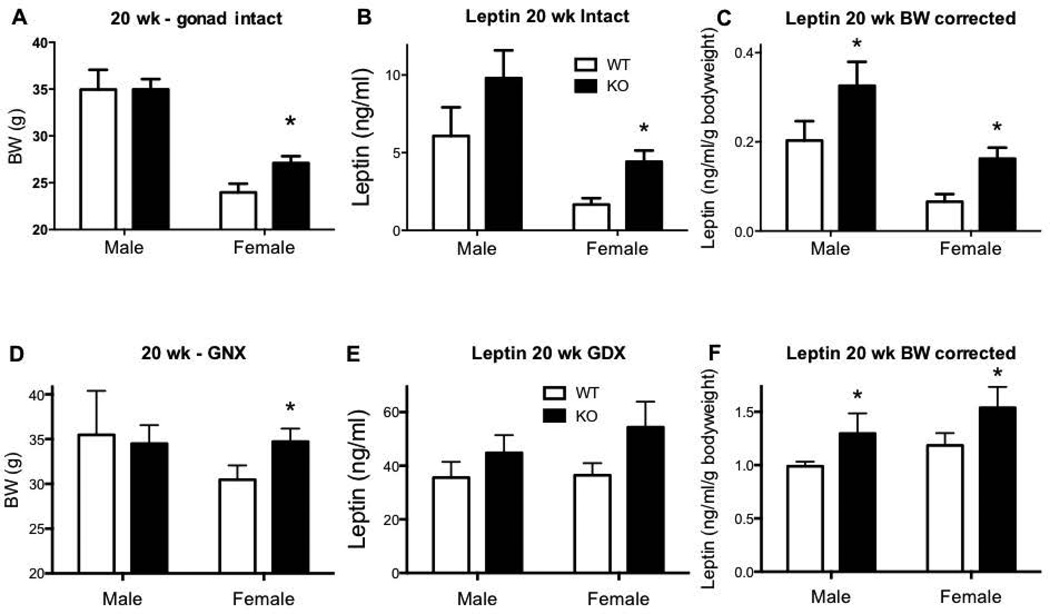
At 20 weeks of age, HFD fed gonad intact Kiss1r KO females were 13% heavier compared to WTs (p<0.05, Figure 4A). As previously reported, no difference in body weight was seen in 20 week old gonad intact males (Figure 4A). At this 20 week age, plasma leptin was 166% higher (p<0.05) in gonad intact Kiss1r KO females compared to WTs (Figure 4B), and this genotype difference remained even when correcting for bodyweight: leptin per gram bodyweight was significantly greater in gonad-intact Kiss1r KOs for both sexes (60% in males and 145% in females, P<0.05, Figure 4C). Following gonadectomy, the elevated body weight in female Kiss1r KOs remained (17% p<0.05, Figure 4D), as previously reported. While no genotype differences in overall leptin levels were seen in GNX Kiss1r KO mice of either sex at 20 weeks of age (Figure 4E), when corrected for body weight, leptin per gram bodyweight was 31% higher in Kiss1r KO males and 30% higher in Kiss1r KO females (p<0.05) compared to their WT controls (Figure 4F).
Figure 4.
The effect of impaired kisspeptin signalling on body weight (BW) in 20 week (wk) HFD fed intact (A) or gonadectomised (GNX) mice (D). (B, C) Leptin concentration and BW corrected leptin concentration in intact Kiss1r KO mice. (E, F) Leptin concentration and BW corrected leptin concentration in GNX Kiss1r KO mice. As indicated, * p<0.05 compared to wild type (WT – shown as white bars, Kiss1r KO – black bars). Values are the mean ± SEM. n=5–11 per group. Body weight in 20 week GNX Kiss1r KO mice was re-examined using data in our past study (17).
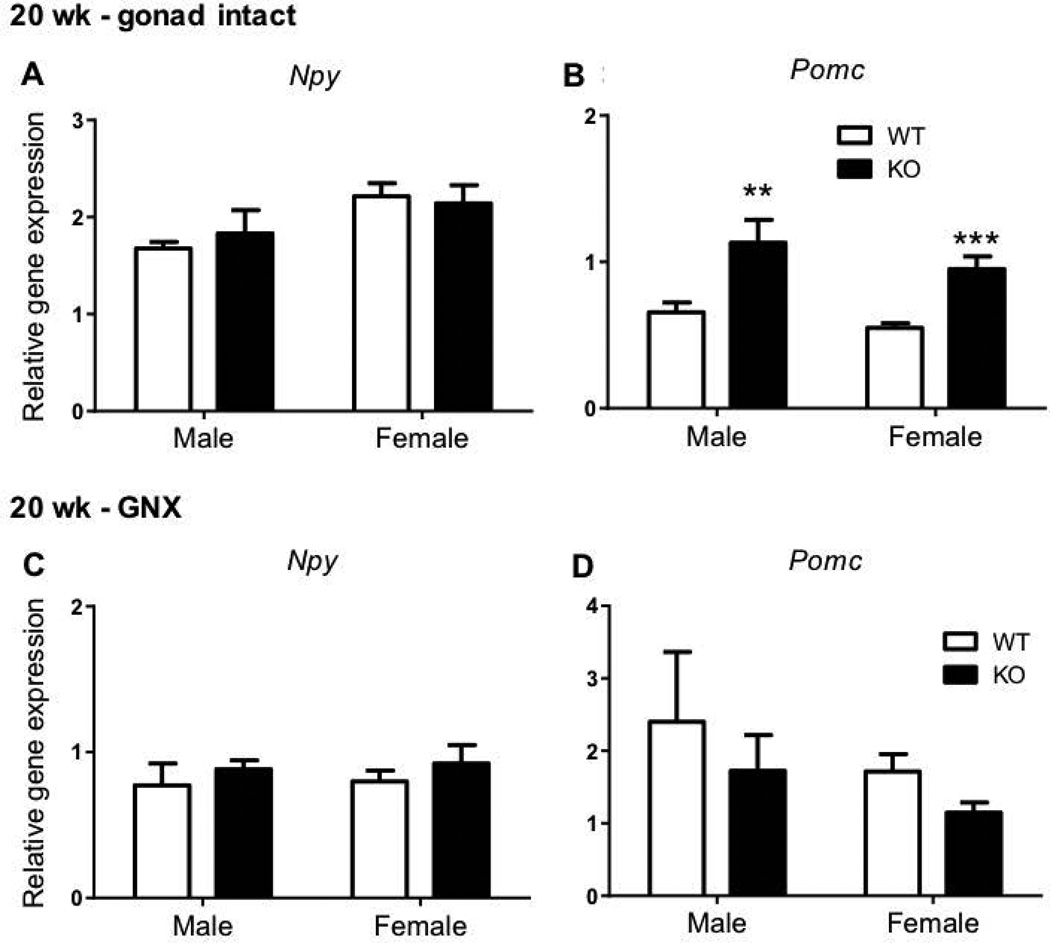
Increased Pomc gene expression in gonad intact, but not GNX, Kiss1r KO mice at 20 weeks of age
At 20 weeks of age, hypothalamic Pomc gene expression was 72% higher in gonad intact Kiss1r KO males (p<0.01) and 73% higher in gonad intact Kiss1r KO females (p<0.001, Figure 5B) compared to gonad intact WT counterparts. As with 8 week old mice, no genotype differences were evident in hypothalamic Npy gene expression at 20 weeks of age in gonad intact mice (Figure 5A). Following gonadectomy, no genotype difference was present in hypothalamic Pomc or Npy gene expression in mice of either sex (Figures 5C, 5D).
Figure 5.
The effect of impaired kisspeptin signalling on neuropeptide Y (Npy) and pro-opiomelanocortin (Pomc) hypothalamic gene expression in 20 week (wk) HFD fed gonad intact and gonadectomised (GNX) mice. As indicated, ** p<0.01 and *** p<0.001 compared to wild-type (WT – shown as white bars, Kiss1r KO – black bars). Values are the mean ± SEM. n=5–11 per group.
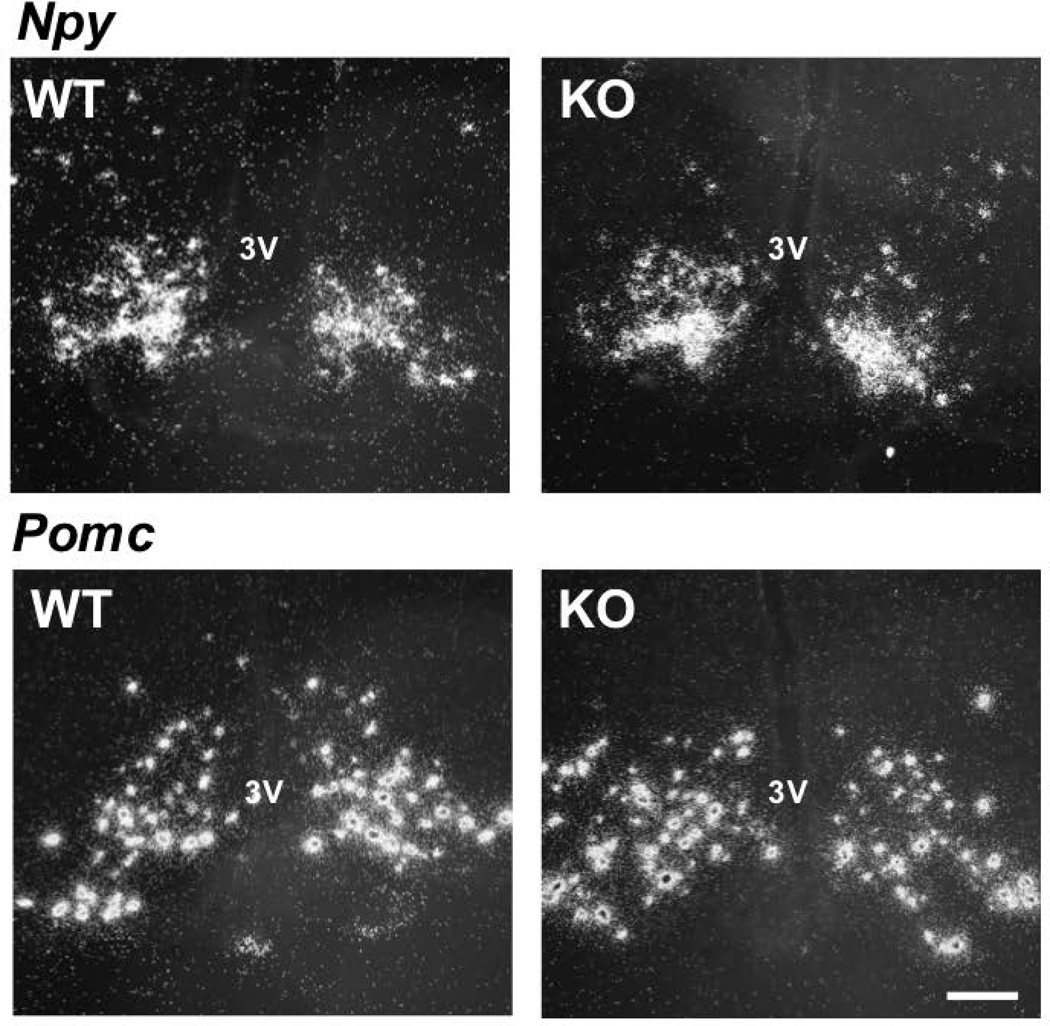
Normal ARC Npy or Pomc gene expression in GNX Kiss1r KO mice at 20 weeks of age
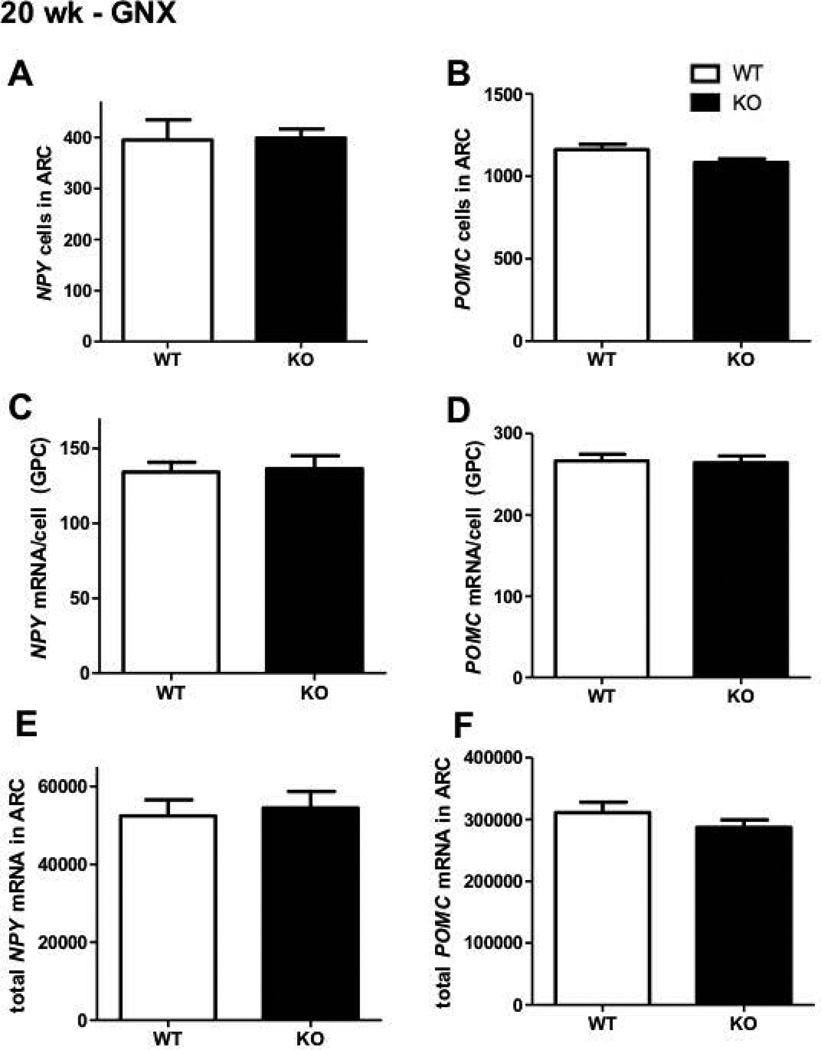
Consistent with our hypothalamic qRT-PCR data, in situ hybridisation analyses determined that Npy and Pomc gene expression in the ARC was similar between chow fed GNX female Kiss1r KOs and WTs at 20 weeks of age (Figure 6). No genotype differences were seen in either total Pomc or Npy cell number (Figure 7A and B), Pomc or Npy mRNA content per cell (Figure 7C and D), or the total Pomc or Npy mRNA content in the ARC region (Figure 7E and F).
Figure 6.
Representative dark-field photomicrographs showing Npy and Pomc mRNA-expressing cells (as reflected by the presence of white clusters of silver grains) in the ARC from 20 week old gonadectomised (GNX) chow fed female WT and Kiss1r KO mice. 3V, Third ventricle. Scale bars, 100 µm.
Figure 7.
The effect of impaired kisspeptin signalling on Npy and Pomc expression in the arcuate nucleus of 20 week old gonadectomised (GNX) chow fed female mice. No significant change was evident (WT – shown as white bars, Kiss1r KO – black bars) in either number of cells (A, B) mRNA per cell content (grains per cell, C, D) or total (combined) expression (E, F). Values are the mean ± SEM. n=6 per group.
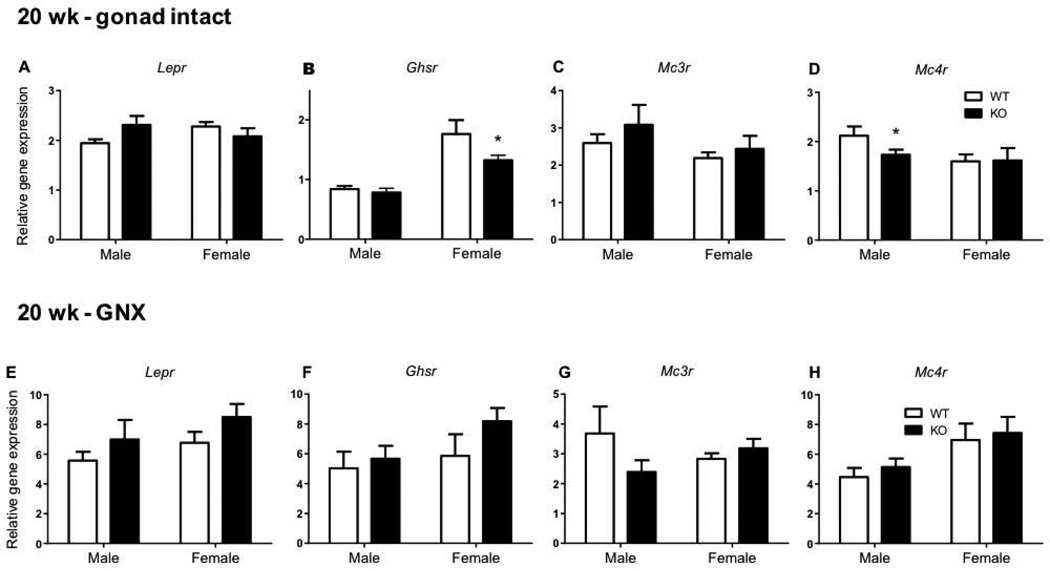
Normal hypothalamic metabolic receptor gene expression in GNX Kiss1r KO mice at 20 weeks of age
In 20 week gonad intact and GNX Kiss1r KO mice, no significant genotype differences were evident in the hypothalamic expression of Lepr (Figure 8A and 8E) and Mc3r (Figure 8C and 8G). However, in gonad intact Kiss1r KO females, hypothalamic Ghsr gene expression was 28% lower compared to WT (p<0.05, Figure 8B). Alternatively, hypothalamic Mc4r gene expression was 25% lower in gonad intact Kiss1r KO males compared to WT (p< 0.05, Figure 8D). However, as with Pomc expression, these Ghsr and Mc4r genotype differences at 20 weeks of age were no longer present following gonadectomy of both WTs and KOs (Figure 8F and H).
Figure 8.
The effect of impaired kisspeptin signalling on metabolic receptor expression in 20 week (wk) gonad intact and gonadectomised (GNX) HFD fed mice. Leptin receptor (Lepr) (A, E) ghrelin receptor (Ghsr) (B, F), melanocortin 3 receptor (Mc3r) (C, G) and melanocortin 4 receptor (Mc4r) (D, H) hypothalamic gene expression in males and females. As indicated, * p<0.05 compared to wild type (WT – shown as white bars, Kiss1r KO – black bars). Values are the mean ± SEM. n=5–11 per group.
Discussion
We previously reported that female, but not male, mice lacking kisspeptin signalling start to become obese between 8–10 weeks of age and subsequently show significantly elevated body weight, adiposity, leptin levels, and impaired glucose homeostasis and metabolic rates (17). However, given that kisspeptin and its receptor are present in multiple parts of the brain and periphery, the reason(s) why Kiss1r KO mice develop this metabolic phenotype remain unknown. Here, we examined key gene expression in the hypothalamic appetite regulating system to assess if alteration in neural feeding circuits may underlie, fully or in part, the elevated body weight and adiposity that develops in the absence of kisspeptin signalling. We first examined Kiss1r KO mice at 8 weeks of age, just before the point where the metabolic and body weight phenotype first appears, and again at 20 weeks of age, when there is a more pronounced metabolic phenotype. At both 8 and 20 weeks of age, gonad intact Kiss1r KO mice showed higher Pomc gene expression compared to WTs though few other changes in hypothalamic gene expression were evident. This genotype difference in hypothalamic Pomc gene expression appears to be sex steroid dependent, as Pomc levels were equivalent in GNX WT and KO mice at both and 8 and 20 weeks. Likewise, minor genotype differences in Ghsr and Mc4r gene expression observed in 20 week gonad intact Kiss1r KOs also appeared to be sex steroid dependent, as no genotype differences were present in these genes when mice from both genotypes were GNX. Because the hypothalamic metabolic genes were normal in GNX KOs, yet these GNX KOs still display elevated body weights, adiposity, and leptin levels (17), these data suggest that the metabolic phenotype of Kiss1r KO mice is more likely driven by peripheral metabolic mechanisms rather than major changes in central feeding circuits (albeit we did not test all possible metabolic genes).
In this study, we confirm our previous report of the sexually dimorphic metabolic phenotype of Kiss1r KO mice. Consistent with our previous data, at 20 weeks of age only female mice have increased body weight compared to WT controls (17), though we did note in the original paper greater adiposity and leptin levels in KO males (17). Despite the obese phenotype, Pomc gene expression appeared to be elevated in gonad intact Kiss1r KO mice of both sexes, which is surprising because Pomc is anorexigenic. However, this increase in Pomc gene expression in both 8 week and 20 week gonad intact Kiss1r KOs is consistent with sheep data showing reduced Pomc gene expression after treatment with kisspeptin (21), which would thereby predict an increase in Pomc gene expression in the absence of kisspeptin signalling. Clearly, higher Pomc expression does not predicate the increased body weight and adiposity observed in Kiss1r KO mice, but it may comprise part of the mechanism underling the significantly reduced food intake in gonad intact Kiss1r KO mice (17). Moreover, it is also possible that higher Pomc might indicate a compensatory mechanism in these overweight mice, reducing food intake in an attempt to defend bodyweight.
Being hypogonadal, Kiss1r KO mice have much lower circulating sex steroids compared to their WT counterparts. Because sex steroids can influence metabolic status (18, 19), we also analysed WTs and KOs that were both GNX, thereby equating the sex steroid status between genotypes. Interestingly, we found that the elevations in Pomc gene expression observed in gonad intact Kiss1r KOs were no longer apparent when mice of both genotypes were GNX to eliminate genotype differences in sex steroid levels. This suggests that the increased Pomc levels in gonad intake KOs reflects the absence of gonadal sex steroids, in particular estradiol, compared to normal sex steroid concentrations in WTs (28). The lack of any genotype differences in Pomc in GNX animals, despite these GNX animals still presenting an overweight, hyperleptinemic phenotype, suggests that this metabolic phenotype is more likely driven by peripheral metabolic factors/mechanisms rather than simple changes in central appetite neural circuitry.
Our present data suggest that the obesity and metabolic impairments in the absence of kisspeptin signalling likely arise from peripheral alterations rather than marked changes in primary hypothalamic metabolic genes. Supporting this possibility, both kisspeptin and its receptor are expressed in several peripheral tissues. Kiss1r is expressed in the liver, fat, and pancreas (14). Kiss1 is expressed in liver and adipose tissue and has been reported to be upregulated by fasting and down-regulated by either HFD or a diabetic state (29). Alternatively, Dudek and colleagues (30) reported an increase in both Kiss1 and Kiss1r mRNA in gonadal fat, pancreas, and liver of streptozotocin-induced type 1 diabetes in rats. Kisspeptin levels are also reportedly increased in both the liver and serum from humans and mice with diabetes (31). In vitro, kisspeptin has been shown to stimulate basal insulin secretion from isolated human and mouse islets (32, 33) whereas another study reported that kisspeptin suppresses glucose-stimulated insulin secretion both in vitro and in vivo in mice (31) indicative of kisspeptin as a possible regulator of beta cell function. Further exploration of the peripheral activity of kisspeptin signalling and its effects on metabolism is warranted in future studies, possibly through the experimental use of novel Kiss1r flox mouse models (34).
Our 20 week old gonad intact and GNX mice examined by qRT-PCR were challenged with a HFD for 12 weeks. This cohort was used initially to investigate whether a HFD would exacerbate the obese phenotype in our previous study (17). HFD has been used in other studies to exacerbate the phenotypic difference in KO models, including Npy KO mice, which show increased Pomc gene expression and reduced body fat compared to controls only when challenged with a HFD (35). In both our previous report and the present study, the body weight and adiposity differences are still present, though less robust, when mice were on a HFD versus normal chow, reflecting elevated body weight gain in the WTs on the former diet. Even so, body weights and leptin levels are still significantly higher in the KOs on HFD. Despite this, we did not detect any change in metabolic gene expression in GNX Kiss1r KO mice on a HFD. We further examined this age group on a chow diet using in situ hybridisation. This allowed us to both examine any change in Pomc or Npy gene expression in Kiss1r KO mice fed with normal chow, and also observe their neuroanatomical expression specifically in the ARC nucleus (Npy, for example, is expressed primarily in the ARC but also to a lesser degree in other hypothalamic areas, such as the DMN). Our findings again indicated that Npy and Pomc levels in the ARC are similar in adult GNX WT and KO mice, despite an overweight phenotype in the latter.
Unlike Pomc, there were no changes in Npy gene expression in gonad intact Kiss1r KO mice. Npy neurons receive peripheral signals from ghrelin via its receptor, Ghsr (also known as the growth hormone secretagogue receptor) (36). Ghrelin is a peptide released from the stomach that stimulates food intake by acting directly on NPY neurons (36–39). Our data demonstrate that 20 week gonad intact Kiss1r KO females had mildly reduced Ghsr gene expression, potentially suggestive for a reduction in orexigenic ghrelin drive to Npy neurons. Reduced ghrelin input may then subsequently have a potential additive effect towards driving the higher Pomc gene expression observed in these gonad intact mice.
GNX Kiss1r KO mice exhibited higher leptin levels compared to their WT controls at both 8 and 20 weeks. It should be noted that different assays were employed to examine plasma leptin at 8 and 20 weeks, so direct comparison between ages were avoided. The higher leptin in KO mice at 8 weeks occurs prior to any detectable elevation in body weight phenotype (though we have found that underlying adiposity is already moderately elevated by this age, matching the elevated leptin observed here [Tolson and Kauffman, unpublished observation]). The higher leptin levels were particularly evident when leptin concentration was corrected for body weight, indicative of the potential for partial leptin resistance (40) are consistent with an obese phenotype. Alternatively, we speculate that the high leptin from the periphery may be signalling in the ARC to induce the lower food intake previously observed in gonad intact Kiss1r KO mice, perhaps via increasing Pomc.
In conclusion, we have shown that the overweight, hyperleptinemic phenotype in Kiss1r KO mice is associated with increased hypothalamic Pomc gene expression, though this alteration in Pomc expression is no longer present when both genotypes are equalised for their sex steroid levels. Our previous study demonstrated that gonad intact Kiss1r KO females eat less (17), and increased Pomc synthesis may be at least one causative factor for that phenotype. However, our current study was unable to directly identify the cause of the elevated body weight and adiposity in Kiss1r KO mice, because no metabolic gene changes in the hypothalamus were evident in GNX mice, despite their elevated body weight and high leptin levels. Thus, these results suggest that this body weight phenotype may not simply be caused by alterations in hypothalamic feeding circuits. Rather, our findings suggest that non-central metabolic mechanisms, perhaps at the level of the liver, fat, or pancreas, all tissues where Kiss1r is also expressed, may be key causative factors for the obesity that emerges in the absence of kisspeptin signalling. Further studies are necessary to investigate impaired kisspeptin signalling outside the appetite regulating system, potentially looking to the periphery where kisspeptin and Kiss1r are both expressed.
Supplementary Material
Acknowledgments
We acknowledge Ms Dijana Tesic, Mrs Celeste Wale and Mr Greg Cozens for their technical assistance and Professor Brendan Waddell and Dr Peter Mark for the housekeeping gene primer stocks.
Funding
J.P.D.B. is supported by the Australian Postgraduate Award and the UWA Top Up Scholarship. Additional support from the Western Australian Department of Health 00910-47705300 (J.T.S.) and NSF IOS-1457226 and NIH DERC pilot grant (A.S.K.). K.P.T. was supported by NIH Grant F32 HD076606.
Footnotes
Disclosures
The authors have nothing to disclose.
Author contributions
K.P.T., A.S.K. and J.T.S. contributed to the conception and design of the research; J.P.D.B., K.P.T., C.N., A.S.K. and J.T.S. performed the experiments, analysed the data and interpreted the results of the experiments; J.P.D.B. drafted the manuscript and prepared the figures; K.P.T., A.S.K. and J.T.S. edited and revised the manuscript and figures; J.P.D.B., K.P.T., C.N., A.S.K. and J.T.S. approved the final version of the manuscript.
References
- 1.De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 3.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio SAJR. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102(5):1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pompolo S, Pereira A, Estrada KM, Clarke IJ. Colocalization of kisspeptin and gonadotropin-releasing hormone in the ovine brain. Endocrinology. 2006;147(2):804–810. doi: 10.1210/en.2005-1123. [DOI] [PubMed] [Google Scholar]
- 6.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–1012. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 7.Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150(12):5530–5538. doi: 10.1210/en.2009-0712. [DOI] [PubMed] [Google Scholar]
- 8.Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27(44):12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Wei WL, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54 −/− mice. Endocrinology. 2007;148(10):4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 10.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MBL, Crowley WF, Jr, Aparicio SAJR, Colledge WH. The GPR54 Gene as a Regulator of Puberty. N Eng J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 11.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312(4):1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 12.Kauffman AS, Jin HP, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27(33):8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The Metastasis Suppressor Gene KiSS-1 Encodes Kisspeptins, the Natural Ligands of the Orphan G Protein-coupled Receptor GPR54. J Biol Chem. 2001;276(37):34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 14.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CGC, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a Novel Human G Protein-coupled Receptor, Activated by the Peptide KiSS-1. J Biol Chem. 2001;276(31):28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 15.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411(6837):613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 16.Lehman M, Hileman S, Goodman R. Neuroanatomy of the Kisspeptin Signaling System in Mammals: Comparative and Developmental Aspects. In: Kauffman AS, Smith JT, editors. Kisspeptin Signaling in Reproductive Biology. Springer; New York: 2013. pp. 27–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolson KP, Garcia C, Yen S, Simonds S, Stefanidis A, Lawrence A, Smith JT, Kauffman AS. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest. 2014;124(7):3075–3079. doi: 10.1172/JCI71075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JA. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol. 2012;212(1):3–12. doi: 10.1530/JOE-11-0044. [DOI] [PubMed] [Google Scholar]
- 19.Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front Neuroendocrinol. 2012;33(4):342–363. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30(30):10205–10219. doi: 10.1523/JNEUROSCI.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151(5):2233–2243. doi: 10.1210/en.2009-1190. [DOI] [PubMed] [Google Scholar]
- 22.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: Perception and integration. Nat Rev Genet. 2002;3(8):589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 23.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152(4):1541–1550. doi: 10.1210/en.2010-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poling MC, Shieh MP, Munaganuru N, Luo E, Kauffman AS. Examination of the influence of leptin and acute metabolic challenge on RFRP-3 neurons of mice in development and adulthood. Neuroendocrinology. 2014;100(4):317–333. doi: 10.1159/000369276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowen JA, Argente J, Vician L, Clifton DK, Steiner RA. Pro-opiomelanocortin messenger RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinology. 1990;52(6):581–588. doi: 10.1159/000125647. [DOI] [PubMed] [Google Scholar]
- 28.Gao Y, He JR, Kapcala LP. Estrogen inhibits hypothalamic pro-opiomelanocortin gene expression in hypothalamic neuronal cultures. Brain Res Mol Brain Res. 1997;45(2):340–344. doi: 10.1016/s0169-328x(97)00028-4. [DOI] [PubMed] [Google Scholar]
- 29.Brown RE, Imran SA, Ur E, Wilkinson M. KiSS-1 mRNA in adipose tissue is regulated by sex hormones and food intake. Mol Cell Endocrinol. 2008;281(1–2):64–72. doi: 10.1016/j.mce.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Dudek M, Kołodziejski PA, Pruszyńska-Oszmałek E, Sassek M, Ziarniak K, Nowak KW, Sliwowska JH. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic-pituitary-gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides. 2016 doi: 10.1016/j.npep.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Song WJ, Mondal P, Wolfe A, Alonso LC, Stamateris R, Ong BWT, Lim OC, Yang KS, Radovick S, Novaira HJ, Farber EA, Farber CR, Turner SD, Hussain MA. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 2014;19(4):667–681. doi: 10.1016/j.cmet.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowe JE, Foot VL, Amiel SA, Huang GC, Lamb M, Lakey JRT, Jones PM, Persaud SJ. GPR54 peptide agonists stimulate insulin secretion from murine, porcine and human islets. Islets. 2012;4(1) doi: 10.4161/isl.18261. [DOI] [PubMed] [Google Scholar]
- 33.Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49(9):2131–2135. doi: 10.1007/s00125-006-0343-z. [DOI] [PubMed] [Google Scholar]
- 34.Novaira HJ, Sonko ML, Hoffman G, Koo Y, Ko C, Wolfe A, Radovick S. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol. 2014;28(2):225–238. doi: 10.1210/me.2013-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel HR, Qi Y, Hawkins EJ, Hileman SM, Elmquist JK, Imai Y, Ahima RS. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes. 2006;55(11):3091–3098. doi: 10.2337/db05-0624. [DOI] [PubMed] [Google Scholar]
- 36.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 37.Andrews ZB. Central mechanisms involved in the orexigenic actions of ghrelin. Peptides. 2011;32(11):2248–2255. doi: 10.1016/j.peptides.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Briggs DI, Andrews ZB. Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology. 2011;93(1):48–57. doi: 10.1159/000322589. [DOI] [PubMed] [Google Scholar]
- 39.Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70(5):306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 40.Asterholm IW, Rutkowski JM, Fujikawa T, Cho YR, Fukuda M, Tao C, Wang ZV, Gupta RK, Elmquist JK, Scherer PE. Elevated resistin levels induce central leptin resistance and increased atherosclerotic progression in mice. Diabetologia. 2014;57(6):1209–1218. doi: 10.1007/s00125-014-3210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.