Abstract
Esophageal squamous cell carcinoma (ESCC) is a deadly disease that requires extensive research. In this review, we update recent progress in the research area of targeted therapy for ESCC. Sox2 and its associated proteins (e.g., ΔNp63α), which regulate lineage survival of ESCC cells, are proposed as therapeutic targets. It is believed that targeting the lineage-survival mechanism may be more effective than targeting other mechanisms. With the advent of a new era of personalized targeted therapy, there is a need to move from the tumor-centric model into an organismic model.
Keywords: esophageal squamous cell carcinoma, targeted therapy, Sox2, lineage survival
Esophageal cancer is the eighth most prevalent cancer in the world. Each year, there are more than 480,000 incipient cases and 400,000 deaths, with more than 80% occurring in developing countries.1 Esophageal squamous cell carcinoma (ESCC) is the predominant histologic type worldwide. In China alone, more than 280,000 new cases and 200,000 deaths were estimated in 2010.2 Despite many advances in diagnosis and treatment in the past decades, the 5-year survival rate for patients with esophageal cancer ranges from 15% to 20%.3 This is mainly due to late diagnosis, aggressiveness of this cancer, and a lack of effective treatment strategies.4
Surgery remains the mainstay of treatment for ESCC, although surgery alone achieves poor locoregional control and poor long-term outcome. The 5-year survival rate for non-metastatic ESCC is 10–40% if treated with surgery alone.5 Unfortunately, esophagectomy itself is a complex procedure with significant morbidity and mortality. Two percent to 25% of patients die within 30 days after surgery.5 Since 40–50% of surgical cases have stage III disease,4,6,7 most patients are given neoadjuvant chemotherapy with cisplatin/fluorouracil and carboplatin/paclitaxel. A recent meta-analysis showed a significant improvement in overall survival after neoadjuvant chemotherapy, with a 13% reduction of relative mortality risk and a 5.1% increase in 2-year survival. However, the difference was not statistically significant.7 More than 50% of patients with ESCC present with unresectable or metastatic disease at the time of diagnosis.4 Chemoradiotherapy is preferred as a nonsurgical approach if there are no contraindications. This combination approach yields a superior palliative outcome compared to radiotherapy alone and improves long-term progression-free survival,8 although its efficacy in locoregional control is inferior to surgery.9 For chemotherapy, fluorouracil and cisplatin with or without a third drug (such as epirubicin or taxane) is known as the most efficacious combination.10 Approximately 40% of patients for whom first-line treatment fails will be potential candidates for second-line therapy.11 Unfortunately, salvage choices of second-line therapy are sparse, and there is no consensus on the optimum.10 Survival of these patients is poor, with a median survival of 5–10 months.12–22
ESCC genomics: an update
Recently, tremendous progress has been made in cancer genomics and epigenomics with the advent of high-throughput techniques like next-generation sequencing. Four groups have reported the genetic landscape of human ESCC with whole-genome and exome sequencing.23–26 On the basis of these studies, we have proposed a strategy of personalized targeted therapy for ESCC.27
Several reports on genomic alterations in human ESCC have been published this year using samples from China and Japan.28–35 In addition to single-nucleotide variants, copy-number alterations, and alterations of multiple signaling pathways in human ESCC, these studies further demonstrated (1) genomic alterations in a precancerous lesion, atypical hyperplasia; (2) mutual exclusivity of NOTCH1 and PIK3CA mutations; (3) structural variations, including deletions and translocations through non-homologous end joining or alternative end-joining mechanisms and local chromosomal misarrangements through the mechanisms of chromothripsis, kataegis, and breakage–fusion bridge. Contributions of individual genes or pathways to carcinogenesis and prognosis were further analyzed in vitro and in vivo. Diagnostic, preventive, and therapeutic applications are proposed as future research directions. Targeted sequencing of a cancer gene panel was also applied to human ESCC, to improve cost-effectiveness.36
On the other hand, the outcome of targeted therapy guided by the genomic alterations remains disappointing. Using EGFR as an example, clinical trials of targeted therapy have only shown limited success in improving the overall survival of patients with ESCC.37,38 Resistance shows up in cancer cells during the treatment, through multiple mechanisms. Combinations of multiple targeting agents may offer some small advantages.39,40 Off-label use of targeting agents based on tumor molecular profiling has not been shown to improve progression-free survival.41
The lineage-survival mechanism as a target
Among the single-nucleotide variants, copy-number alterations, and alterations of multiple signaling pathways, SOX2 amplification is clearly a cancer driver leading to ESCC.26 In fact, before these studies, SOX2 was found to be an amplified oncogene at chromosome 3q26 in human ESCC by two independent groups.42,43 As a member of the Sox family of transcription factors, SOX2 plays a critical role in maintaining embryonic stem cells, as well as adult stem cells, in multiple tissues. During fetal development, SOX2 plays major roles in ectodermal, endodermal, and mesodermal development. SOX2 deficiency causes multiple human diseases, including anophthalmia–esophageal–genital syndrome. After birth, SOX2 expression remains in some adult tissues and cells. It continues to play critical roles in adult tissue homeostasis, regeneration, reprogramming, and pathogenesis.44 The functional role of SOX2 in carcinogenesis has been extensively reviewed by multiple groups.45–47
In human ESCC, SOX2 is amplified in ~15% of cases and overexpressed in ~70% of cases, suggesting that multiple mechanisms other than amplification can lead to SOX2 overexpression. SOX2 overexpression is significantly associated with higher histological grade and poorer clinical survival of ESCC patients.48,49 Functionally, transgenic SOX2 overexpression drives the complete process of squamous cell carcinogenesis in the mouse forestomach.50 On the contrary, mice with hypomorphic SOX2 exhibit an esophagus lined by a columnar epithelium instead of a keratinized stratified squamous epithelium, suggesting an essential role of SOX2 in esophageal epithelial development.51 SOX2 is indeed an amplified lineage-survival oncogene in ESCC,42 as evidenced by its crucial role in esophageal squamous epithelial cell proliferation and survival during development, its overexpression in squamous epithelial cells of ESCC, its amplification in a subset of ESCC, its essential role for ESCC survival, and its function as a transcription factor.52 This discovery provides a possibility for developing targeted therapy of ESCC, even though targeting a transcription factor is a technical challenge. However, recent technical advances have improved the druggability of transcription factors.53
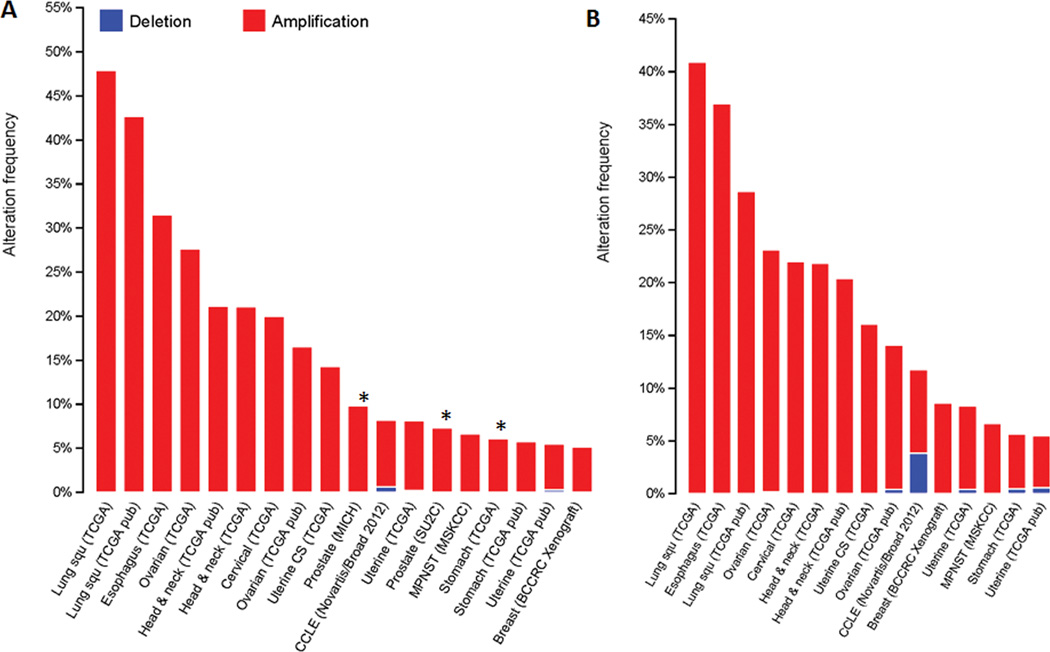
As a transcription factor, SOX2 has a DNA-binding transcription domain and functional domains that interact with other cofactors. Eighty-two SOX2-associated nuclear proteins have been identified in two human ESCC cell lines (KYSE70 and TT) using tandem affinity purification followed by liquid chromatography–tandem mass spectrometry.54 This list includes CBX3, DNMT1, HDAC2, KDM1A, KLF5, PARP1, TP63, and many other proteins. Among these, the most interesting is TP63, which is encoded by the p63 gene located ~7 Mb from Sox2. These two genes are often co-amplified in multiple cancers (Fig. 1A & 1B). It is known that SOX2 directly regulates transcription of p63 in lung cancer and lung basal cells.42,55 About 20% of ESCC cases harbor p63 gene amplification and ~60% overexpression.56–58
Figure 1.
(A) SOX2 and (B) TP63 amplification in human cancers. Data are obtained from the TCGA database (www.cbioportal.org). Only cancers with a high incidence of gene amplification (> 5%) are included. There is an obvious overlap between SOX2 and TP63 amplification in these cancers.
The predominant isoform of TP63 in the basal cells of the esophageal epithelium is known as ΔNp63α. Similar to SOX2, ΔNp63α drives carcinogenesis via multiple mechanisms.59,60 It may antagonize the transcriptional activity of TAp73 and the p73-dependent proapoptotic transcriptional program61,62 and modulate expression of its target genes through physical interactions with HDAC1/2 and the histone H2A.Z.63–65 Silencing of ΔNp63α in ESCC cells inhibited cell proliferation and colony formation via downregulation of Akt signaling.66 Overexpression of ΔNp63α in squamous epithelial cells in transgenic mice leads to increased suprabasal cRel, Ki-67, and cytokine expression, together with epidermal hyperplasia and diffuse inflammation.67 On the contrary, TP63 knockout mice exhibit an esophageal phenotype similar to that seen in SOX2 hypomorphs.68 These data suggest that ΔNp63α is also an amplified lineage-survival oncoprotein for ESCC.
In contrast to proteins directly associated with lineage survival (e.g., ΔNp63α), other SOX2-associated proteins in ESCC appear to regulate gene expression and may also be associated with lineage survival. CBX3 regulates RNA processing genome-wide, and loss of CBX3 leads to dramatic accumulation of unspliced nascent transcripts and alterations in target gene expression.69 DNMT1 contains a DNA methyltransferase domain at its C-terminus for maintenance of global CpG methylation patterns and a large N-terminal domain for protein–protein interactions.70 KLF5 regulates proliferation, apoptosis, and invasion in ESCC cells. Loss of KLF5 in the context of TP53 deletion drives invasive progression of ESCC, and restoration of KLF5 leads to apoptosis and suppresses cell survival.71,72 PARP1 interacts with and poly(ADP-ribosyl)ates SOX2 directly for degradation of SOX2 protein. As a result, PARP1 knockout enhances SOX2 expression and modifies cell differentiation.73–75 HDAC2 regulates expression of SOX2, and HDAC1/2 deficiency leads to loss of SOX2 expression and blocks proximal airway development.76 HDAC1/2 also controls the transcriptional activity of ΔNp63α.77
Genes and signaling pathways upstream or downstream of SOX2 have been extensively studied in the literature.45,47 However, cancer therapy targeting signaling pathways is known to be associated with a high rate of resistance, and resistance is predicted to appear in ESCC patients as well.27 It may be less desirable to target SOX2 upstream or downstream signaling, even though targeting the downstream Akt/mTOR or IL6/STAT3 pathways seems to be effective.43,50 The high rate of resistance to targeted therapy has prompted the cancer research community to consider combination therapy (i.e., targeting multiple targets or pathways).39,40 Alternatively, transcription factors or regulators are potentially better therapeutic targets, simply because these transcription factors define the cancer phenotype.78 Here, we argue that targeting the lineage-survival mechanism in ESCC cells is potentially more potent and less likely to produce resistance than targeting other signaling pathways. Four approaches are potentially applicable for ESCC: (1) using small interfering RNA (siRNA) to target SOX2 and/or ΔNp63α, although this approach is not feasible at this time; (2) enhancing the immune reaction against cancer cells with overexpressed SOX2 and ΔNp63α participate; and (4) targeting epigenetic modifications in which SOX2 and ΔNp63α are involved.
SOX2 itself has been targeted by vaccines79,80 and zinc finger–based artificial proteins.81,82 SOX2-associated proteins have been targeted as well. For example, specific inhibitors of KDM1A/LSD1, a SOX2-associated protein, selectively impair the growth of SOX2+ lung squamous cell carcinoma, but not that of SOX2− cells. Inactivation of KDM1A reduces SOX2 expression, promotes G1 cell cycle arrest, and induces genes for differentiation by selectively modulating the methylation states of H3K4 and H3K9.83 It would be very intriguing to target protein–protein interactions in which SOX2 and ΔNP63α are involved, with lineage survival as the readout.
There are two major concerns with regard to targeting SOX2 and/or ΔNP63α in ESCC. First, these genes are normally present in both normal tissue and cancer. Targeting the SOX2–ΔNP63α interaction may potentially alleviate the concern of side effects, because not many adult tissues coexpress these two proteins (only the larynx, bronchiole, tongue, esophagus, anus, tonsil, and exocervix).84,85 Second, there is a possibility of inducing phenotype switching of cancer cells (i.e., transdifferentiation of ESCC cells into adenosquamous cells or even adenocarcinoma cells), as loss of SOX2 and p63 is an early event in intestinal metaplasia of the esophageal squamous epithelium.86 This approach may also potentially select poorly differentiated, lineage-independent cancer cells.52
Targeting the lineage-survival mechanism requires a deep understanding of SOX2–ΔNP63α biochemistry and its interaction with other factors. The SOX2–ΔNP63α interactome in ESCC needs to be further clarified with better techniques, for example, stable isotope labeling using amino acids in cell culture. Biological replicates with forward and reverse experiments are needed to enhance confidence in protein interactions.87 The functional roles of SOX2/ΔNP63α-associated proteins in lineage survival need to be well understood. High-throughput screening has been used to screen inhibitors of protein–protein interactions. Although a few successes have been reported in the literature, classical target-based drug discovery using small molecules is challenging, in particular when the interactions involve multiple proteins.88,89 Recently, technical advances have been made in this area.90 For example, in silico modeling of protein–protein interactions has been successfully used to identify raloxifene and bazedoxifene as novel inhibitors of the IL6/GP130 interaction.91 With the well-characterized ESCC cell lines (KYSE series and TE series) and a SOX2-transgenic mouse model of ESCC,43,50 it is believed that potent agents will be developed for treatment of SOX2-overexpressing ESCC.
The organismic model in ESCC treatment and its implications
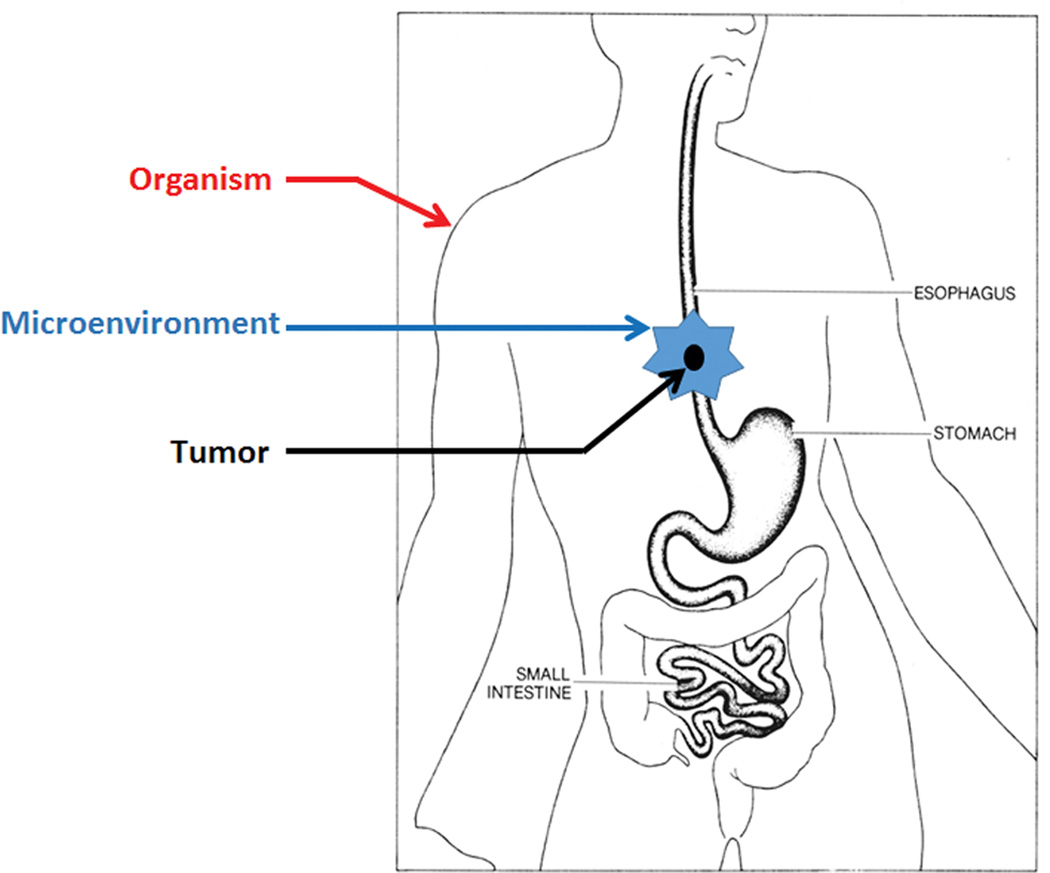
With the advent of personalized targeted therapy, there is a need to move from the tumor-centric model into an organismic model.92 According to this model, clinicians need to consider not only the tumor itself but also its microenvironment and the whole body (Fig. 2). The idea that “there is an order in cancer” suggests that homeostatic mechanisms in the tumor have to be taken into consideration in targeted therapy.93 The tumor microenvironment has been investigated for years for its capability to both promote and restrain cancer.94 However, its potential use in ESCC treatment has not been well studied (see a recent review in Ref. 95). Antiangiogenesis and immune therapy targeting the microenvironment are currently under clinical investigation. At the organismic level, it is far more complex. Intriguing questions may be asked: how the body reacts to the tumor, how the tumor reacts to the body, and how to manipulate the body against the tumor and its microenvironment.
Figure 2.
An organismic model of ESCC, as opposed to the tumor-centric model, calls for attention to the organism and the tumor microenvironment.
Nevertheless, this organismal model has multiple implications. Our therapeutic goal may turn into “keeping clinical cancer under control and living with microscopic cancer.” The mindset of “5-year survival” may give away to “life-time treatment and monitoring.” Surgical resection, which is currently the mainstay for ESCC treatment, needs to be more precise and less invasive, and may need to be repeated over the course of treatment. Monitoring disease progression will be of critical significance. Emerging techniques, such as circulating tumor cells and DNA, will be essential in monitoring therapeutic response and disease status.96,97 There will be a great need to mitigate the various side effects of targeted therapy in order to improve the quality of life. The variety of issues (e.g., financial, social, psychological, behavioral) will make decision making in ESCC treatment sophisticated.
Acknowledgments
The authors are supported by a Faculty Development Fund from the 105th Hospital of PLA and NIH/NCI U54 CA156735. The authors have not received research funding from other funding agencies or the industry for the research work discussed in this manuscript.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003–2005: A population-based study. International journal of cancer. 2014;136:1921–1930. doi: 10.1002/ijc.29227. [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 4.Enzinger PC, Mayer RJ. Esophageal cancer. The New England journal of medicine. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 5.Kaifi JT, Gusani NJ, Jiang Y, et al. Multidisciplinary management of early and locally advanced esophageal cancer. Journal of clinical gastroenterology. 2011;45:391–399. doi: 10.1097/MCG.0b013e3182049949. [DOI] [PubMed] [Google Scholar]
- 6.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. The Lancet. Oncology. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 7.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. The Lancet. Oncology. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 8.Kleinberg L, Gibson MK, Forastiere AA. Chemoradiotherapy for localized esophageal cancer: regimen selection and molecular mechanisms of radiosensitization. Nature clinical practice. Oncology. 2007;4:282–294. doi: 10.1038/ncponc0796. [DOI] [PubMed] [Google Scholar]
- 9.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. The New England journal of medicine. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 11.Thallinger CM, Raderer M, Hejna M. Esophageal cancer: a critical evaluation of systemic second-line therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4709–4714. doi: 10.1200/JCO.2011.36.7599. [DOI] [PubMed] [Google Scholar]
- 12.Conroy T, Etienne P-L, Adenis A, et al. Phase II trial of vinorelbine in metastatic squamous cell esophageal carcinoma. European Organization for Research and Treatment of Cancer Gastrointestinal Treat Cancer Cooperative Group. Journal of clinical oncology. 1996;14:164–170. doi: 10.1200/JCO.1996.14.1.164. [DOI] [PubMed] [Google Scholar]
- 13.Mafune K, Yamada K, Imamura K, et al. Docetaxel, 5-fluorouracil and nedaplatin as second-line chemotherapy for patients with esophageal cancer after esophagectomy: a pilot study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:14140. [Google Scholar]
- 14.Yoshioka T, Sakayori M, Kato S, et al. Dose escalation study of docetaxel and nedaplatin in patients with relapsed or refractory squamous cell carcinoma of the esophagus pretreated using cisplatin, 5-fluorouracil, and radiation. International journal of clinical oncology. 2006;11:454–460. doi: 10.1007/s10147-006-0610-5. [DOI] [PubMed] [Google Scholar]
- 15.Park B-B, Im Y-H, Hwang IG, et al. Salvage chemotherapy with mitomycin C, ifosfamide, and cisplatin (MIC) for previously treated metastatic or recurrent esophageal squamous cell carcinoma. Investigational new drugs. 2008;26:387–392. doi: 10.1007/s10637-008-9126-3. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima Y, Suzuki T, Haruki S, et al. A pilot trial of docetaxel and nedaplatin in cisplatin-pretreated relapsed or refractory esophageal squamous cell cancer. Hepato-gastroenterology. 2007;55:1631–1635. [PubMed] [Google Scholar]
- 17.Yamazaki K, Hironaka S, Boku N, et al. A retrospective study of second-line chemotherapy for unresectable or recurrent squamous cell carcinoma of the esophagus refractory to chemotherapy with 5-fluorouracil plus platinum. International journal of clinical oncology. 2008;13:150–155. doi: 10.1007/s10147-007-0738-y. [DOI] [PubMed] [Google Scholar]
- 18.Jin J, Xu X, Wang F, et al. Second-line combination chemotherapy with docetaxel and nedaplatin for Cisplatin-pretreated refractory metastatic/recurrent esophageal squamous cell carcinoma. Journal of Thoracic Oncology. 2009;4:1017–1021. doi: 10.1097/JTO.0b013e3181add9c7. [DOI] [PubMed] [Google Scholar]
- 19.Shim H-J, Cho S-H, Hwang J-E, et al. Phase II study of docetaxel and cisplatin chemotherapy in 5-fluorouracil/cisplatin pretreated esophageal cancer. American journal of clinical oncology. 2010;33:624–628. doi: 10.1097/COC.0b013e3181bead92. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Lin W, Wang H, et al. Phase II trial of second-line chemotherapy with docetaxel and capecitabine in advanced esophageal squamous cell carcinoma. Medical oncology (Northwood, London, England) 2013;30:746. doi: 10.1007/s12032-013-0746-x. [DOI] [PubMed] [Google Scholar]
- 21.Song Z, Zhang Y. Second-line docetaxel-based chemotherapy after failure of fluorouracil-based first-line treatment for advanced esophageal squamous cell carcinoma. OncoTargets and therapy. 2014;7:1875–1881. doi: 10.2147/OTT.S66525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee M, Jung KS, Kim HS, et al. Phase II study of a combination chemotherapy with weekly docetaxel and gemcitabine in previously treated metastatic esophageal squamous cell cancer. Ann Oncol. 2014;25:680P. [Google Scholar]
- 23.Gao YB, Chen ZL, Li JG, et al. Genetic landscape of esophageal squamous cell carcinoma. Nature genetics. 2014;46:1097–1102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 24.Lin DC, Hao JJ, Nagata Y, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nature genetics. 2014;46:467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Y, Li L, Ou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Zhou Y, Cheng C, et al. Genomic analyses reveal mutational signatures and frequently altered genes in esophageal squamous cell carcinoma. Am J Hum Genet. 2015;96:597–611. doi: 10.1016/j.ajhg.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang X, Chen K, Li Y, et al. Personalized targeted therapy for esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:7648–7658. doi: 10.3748/wjg.v21.i25.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawada G, Niida A, Uchi R, et al. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology. 2016;150:1171–1182. doi: 10.1053/j.gastro.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 29.Hu N, Kadota M, Liu H, et al. Genomic landscape of somatic alterations in esophageal squamous cell carcinoma and gastric cancer. Cancer Res. 2016;76:1714–1723. doi: 10.1158/0008-5472.CAN-15-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng C, Cui H, Zhang L, et al. Genomic analyses reveal FAM84B and the NOTCH pathway are associated with the progression of esophageal squamous cell carcinoma. Gigascience. 2016;5:1. doi: 10.1186/s13742-015-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song B, Cui H, Li Y, et al. Mutually exclusive mutations in NOTCH1 and PIK3CA associated with clinical prognosis and chemotherapy responses of esophageal squamous cell carcinoma in China. Oncotarget. 2016;7:3599–3613. doi: 10.18632/oncotarget.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng C, Zhou Y, Li H, et al. Whole-Genome Sequencing Reveals Diverse Models of Structural Variations in Esophageal Squamous Cell Carcinoma. Am J Hum Genet. 2016;98:256–274. doi: 10.1016/j.ajhg.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu N, Kadota M, Liu H, et al. Genomic Landscape of Somatic Alterations in Esophageal Squamous Cell Carcinoma and Gastric Cancer. Cancer Res. 2016;76:1714–1723. doi: 10.1158/0008-5472.CAN-15-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin HD, Liao XY, Chen YB, et al. Genomic Characterization of Esophageal Squamous Cell Carcinoma Reveals Critical Genes Underlying Tumorigenesis and Poor Prognosis. Am J Hum Genet. 2016;98:709–727. doi: 10.1016/j.ajhg.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawada G, Niida A, Uchi R, et al. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology. 2016;150:1171–1182. doi: 10.1053/j.gastro.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 36.Zheng H, Wang Y, Tang C, et al. TP53, PIK3CA, FBXW7 and KRAS Mutations in Esophageal Cancer Identified by Targeted Sequencing. Cancer Genomics Proteomics. 2016;13:231–238. [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Zheng Y, Sun X, et al. Concurrent radiotherapy with gefitinib in elderly patients with esophageal squamous cell carcinoma: Preliminary results of a phase II study. Oncotarget. 2015;6:38429–38439. doi: 10.18632/oncotarget.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. The Lancet. Oncology. 2014;15:894–904. doi: 10.1016/S1470-2045(14)70024-5. [DOI] [PubMed] [Google Scholar]
- 39.Elkabets M, Pazarentzos E, Juric D, et al. AXL mediates resistance to PI3Kalpha inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell. 2015;27:533–546. doi: 10.1016/j.ccell.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mancini M, Gaborit N, Lindzen M, et al. Combining three antibodies nullifies feedback-mediated resistance to erlotinib in lung cancer. Sci Signal. 2015;8:ra53. doi: 10.1126/scisignal.aaa0725. [DOI] [PubMed] [Google Scholar]
- 41.Le Tourneau C, Delord JP, Goncalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. The Lancet. Oncology. 2015;16:1324–1334. doi: 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- 42.Bass AJ, Watanabe H, Mermel CH, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nature genetics. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gen Y, Yasui K, Nishikawa T, et al. SOX2 promotes tumor growth of esophageal squamous cell carcinoma through the AKT/mammalian target of rapamycin complex 1 signaling pathway. Cancer science. 2013;104:810–816. doi: 10.1111/cas.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weina K, Utikal J. SOX2 and cancer: current research and its implications in the clinic. Clin Transl Med. 2014;3:19. doi: 10.1186/2001-1326-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hussenet T, du Manoir S. SOX2 in squamous cell carcinoma: amplifying a pleiotropic oncogene along carcinogenesis. Cell Cycle. 2010;9:1480–1486. doi: 10.4161/cc.9.8.11203. [DOI] [PubMed] [Google Scholar]
- 47.Liu K, Lin B, Zhao M, et al. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal. 2013;25:1264–1271. doi: 10.1016/j.cellsig.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, He W, Lu C, et al. Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinoma. Anticancer Res. 2009;29:1233–1241. [PubMed] [Google Scholar]
- 49.Saigusa S, Mohri Y, Ohi M, et al. Podoplanin and SOX2 expression in esophageal squamous cell carcinoma after neoadjuvant chemo-radiotherapy. Oncol Rep. 2011;26:1069–1074. doi: 10.3892/or.2011.1408. [DOI] [PubMed] [Google Scholar]
- 50.Liu K, Jiang M, Lu Y, et al. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 2013;12:304–315. doi: 10.1016/j.stem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Que J, Okubo T, Goldenring JR, et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garraway LA, Sellers WR. Lineage dependency and lineage-survival oncogenes in human cancer. Nat Rev Cancer. 2006;6:593–602. doi: 10.1038/nrc1947. [DOI] [PubMed] [Google Scholar]
- 53.Mapp AK, Pricer R, Sturlis S. Targeting transcription is no longer a quixotic quest. Nat Chem Biol. 2015;11:891–894. doi: 10.1038/nchembio.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe H, Ma Q, Peng S, et al. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. The Journal of clinical investigation. 2014;124:1636–1645. doi: 10.1172/JCI71545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochieng JK, Schilders K, Kool H, et al. Sox2 regulates the emergence of lung basal cells by directly activating the transcription of Trp63. Am J Respir Cell Mol Biol. 2014;51:311–322. doi: 10.1165/rcmb.2013-0419OC. [DOI] [PubMed] [Google Scholar]
- 56.Taniere P, Martel-Planche G, Saurin JC, et al. TP53 mutations, amplification of P63 and expression of cell cycle proteins in squamous cell carcinoma of the oesophagus from a low incidence area in Western Europe. Br J Cancer. 2001;85:721–726. doi: 10.1054/bjoc.2001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geddert H, Kiel S, Heep HJ, et al. The role of p63 and deltaNp63 (p40) protein expression and gene amplification in esophageal carcinogenesis. Hum Pathol. 2003;34:850–856. doi: 10.1016/s0046-8177(03)00342-3. [DOI] [PubMed] [Google Scholar]
- 58.Hibi K, Nakayama H, Taguchi M, et al. AIS overexpression in advanced esophageal cancer. Clin Cancer Res. 2001;7:469–472. [PubMed] [Google Scholar]
- 59.Gallant-Behm CL, Espinosa JM. How does DeltaNp63alpha drive cancer? Epigenomics. 2013;5:5–7. doi: 10.2217/epi.12.78. [DOI] [PubMed] [Google Scholar]
- 60.Keyes WM, Pecoraro M, Aranda V, et al. DeltaNp63alpha is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell Stem Cell. 2011;8:164–176. doi: 10.1016/j.stem.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeYoung MP, Johannessen CM, Leong CO, et al. Tumor-specific p73 up-regulation mediates p63 dependence in squamous cell carcinoma. Cancer Res. 2006;66:9362–9368. doi: 10.1158/0008-5472.CAN-06-1619. [DOI] [PubMed] [Google Scholar]
- 62.Rocco JW, Leong CO, Kuperwasser N, et al. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 63.Gallant-Behm CL, Ramsey MR, Bensard CL, et al. DeltaNp63alpha represses anti-proliferative genes via H2A.Z deposition. Genes Dev. 2012;26:2325–2336. doi: 10.1101/gad.198069.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallant-Behm CL, Espinosa JM. DeltaNp63alpha utilizes multiple mechanisms to repress transcription in squamous cell carcinoma cells. Cell Cycle. 2013;12:409–416. doi: 10.4161/cc.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramsey MR, He L, Forster N, et al. Physical association of HDAC1 and HDAC2 with p63 mediates transcriptional repression and tumor maintenance in squamous cell carcinoma. Cancer Res. 2011;71:4373–4379. doi: 10.1158/0008-5472.CAN-11-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye S, Lee KB, Park MH, et al. p63 regulates growth of esophageal squamous carcinoma cells via the Akt signaling pathway. Int J Oncol. 2014;44:2153–2159. doi: 10.3892/ijo.2014.2374. [DOI] [PubMed] [Google Scholar]
- 67.Yang X, Lu H, Yan B, et al. DeltaNp63 versatilely regulates a Broad NF-kappaB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res. 2011;71:3688–3700. doi: 10.1158/0008-5472.CAN-10-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanbokhoven H, Melino G, Candi E, et al. p63, a story of mice and men. J Invest Dermatol. 2011;131:1196–1207. doi: 10.1038/jid.2011.84. [DOI] [PubMed] [Google Scholar]
- 69.Smallwood A, Hon GC, Jin F, et al. CBX3 regulates efficient RNA processing genome-wide. Genome research. 2012;22:1426–1436. doi: 10.1101/gr.124818.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Espada J. Non-catalytic functions of DNMT1. Epigenetics. 2012;7:115–118. doi: 10.4161/epi.7.2.18756. [DOI] [PubMed] [Google Scholar]
- 71.Tarapore RS, Yang Y, Katz JP. Restoring KLF5 in esophageal squamous cell cancer cells activates the JNK pathway leading to apoptosis and reduced cell survival. Neoplasia. 2013;15:472–480. doi: 10.1593/neo.122126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y, Nakagawa H, Tetreault MP, et al. Loss of transcription factor KLF5 in the context of p53 ablation drives invasive progression of human squamous cell cancer. Cancer Res. 2011;71:6475–6484. doi: 10.1158/0008-5472.CAN-11-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lai YS, Chang CW, Pawlik KM, et al. SRY (sex determining region Y)-box2 (Sox2)/poly ADP-ribose polymerase 1 (Parp1) complexes regulate pluripotency. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3772–3777. doi: 10.1073/pnas.1108595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao F, Kwon SW, Zhao Y, et al. PARP1 poly(ADP-ribosyl)ates Sox2 to control Sox2 protein levels and FGF4 expression during embryonic stem cell differentiation. The Journal of biological chemistry. 2009;284:22263–22273. doi: 10.1074/jbc.M109.033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Plane JM, Grossenbacher SK, Deng W. PARP-1 deletion promotes subventricular zone neural stem cells toward a glial fate. Journal of neuroscience research. 2012;90:1489–1506. doi: 10.1002/jnr.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Tian Y, Morley MP, et al. Development and regeneration of Sox2+ endoderm progenitors are regulated by a Hdac1/2-Bmp4/Rb1 regulatory pathway. Developmental cell. 2013;24:345–358. doi: 10.1016/j.devcel.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.LeBoeuf M, Terrell A, Trivedi S, et al. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Developmental cell. 2010;19:807–818. doi: 10.1016/j.devcel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonda TJ, Ramsay RG. Directly targeting transcriptional dysregulation in cancer. Nat Rev Cancer. 2015;15:686–694. doi: 10.1038/nrc4018. [DOI] [PubMed] [Google Scholar]
- 79.Polakova I, Duskova M, Smahel M. Antitumor DNA vaccination against the Sox2 transcription factor. Int J Oncol. 2014;45:139–146. doi: 10.3892/ijo.2014.2402. [DOI] [PubMed] [Google Scholar]
- 80.Favaro R, Appolloni I, Pellegatta S, et al. Sox2 is required to maintain cancer stem cells in a mouse model of high-grade oligodendroglioma. Cancer Res. 2014;74:1833–1844. doi: 10.1158/0008-5472.CAN-13-1942. [DOI] [PubMed] [Google Scholar]
- 81.Stolzenburg S, Beltran AS, Swift-Scanlan T, et al. Stable oncogenic silencing in vivo by programmable and targeted de novo DNA methylation in breast cancer. Oncogene. 2015;34:5427–5435. doi: 10.1038/onc.2014.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stolzenburg S, Rots MG, Beltran AS, et al. Targeted silencing of the oncogenic transcription factor SOX2 in breast cancer. Nucleic Acids Res. 2012;40:6725–6740. doi: 10.1093/nar/gks360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X, Lu F, Wang J, et al. Pluripotent stem cell protein Sox2 confers sensitivity to LSD1 inhibition in cancer cells. Cell Rep. 2013;5:445–457. doi: 10.1016/j.celrep.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arnold K, Sarkar A, Yram MA, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Como CJ, Urist MJ, Babayan I, et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8:494–501. [PubMed] [Google Scholar]
- 86.Chen X, Qin R, Liu B, et al. Multilayered epithelium in a rat model and human Barrett's esophagus: similar expression patterns of transcription factors and differentiation markers. BMC Gastroenterol. 2008;8:1. doi: 10.1186/1471-230X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spruijt CG, Baymaz HI, Vermeulen M. Identifying specific protein-DNA interactions using SILAC-based quantitative proteomics. Methods in molecular biology. 2013;977:137–157. doi: 10.1007/978-1-62703-284-1_11. [DOI] [PubMed] [Google Scholar]
- 88.Jin L, Wang W, Fang G. Targeting protein-protein interaction by small molecules. Annual review of pharmacology and toxicology. 2014;54:435–456. doi: 10.1146/annurev-pharmtox-011613-140028. [DOI] [PubMed] [Google Scholar]
- 89.Cesa LC, Patury S, Komiyama T, et al. Inhibitors of difficult protein-protein interactions identified by high-throughput screening of multiprotein complexes. ACS chemical biology. 2013;8:1988–1997. doi: 10.1021/cb400356m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Higueruelo AP, Jubb H, Blundell TL. Protein-protein interactions as druggable targets: recent technological advances. Current opinion in pharmacology. 2013;13:791–796. doi: 10.1016/j.coph.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 91.Li H, Xiao H, Lin L, et al. Drug design targeting protein-protein interactions (PPIs) using multiple ligand simultaneous docking (MLSD) and drug repositioning: discovery of raloxifene and bazedoxifene as novel inhibitors of IL-6/GP130 interface. Journal of medicinal chemistry. 2014;57:632–641. doi: 10.1021/jm401144z. [DOI] [PubMed] [Google Scholar]
- 92.Burke HB. An organismic view of cancer. Journal of the National Cancer Institute. 2013;105:1003–1004. doi: 10.1093/jnci/djt159. [DOI] [PubMed] [Google Scholar]
- 93.Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin EW, Karakasheva TA, Hicks PD, et al. The tumor microenvironment in esophageal cancer. Oncogene. 2016 doi: 10.1038/onc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ignatiadis M, Lee M, Jeffrey SS. Circulating Tumor Cells and Circulating Tumor DNA: Challenges and Opportunities on the Path to Clinical Utility. Clin Cancer Res. 2015;21:4786–4800. doi: 10.1158/1078-0432.CCR-14-1190. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7:302ra133. doi: 10.1126/scitranslmed.aab0021. [DOI] [PubMed] [Google Scholar]