Summary
Background
Hemophilia B (HB) is an inherited bleeding disorder caused by the absence or dysfunction of coagulation factor IX (FIX). A subset of patients who have HB develop neutralizing alloantibodies (inhibitors) against FIX following infusion therapy. HB prevalence and the proportion of patients who develop inhibitors are much lower than that of hemophilia A (HA), which makes studies of inhibitors in patients with HB challenging due to the limited availability of samples. As a result, there is a knowledge gap regarding HB inhibitors.
Objective
Evaluate the largest group of inhibitor positive HB patients studied to date to assess the relationship between anti-FIX antibody profiles and inhibitor formation.
Methods
A fluorescence immunoassay (FLI) was used to detect anti-FIX antibodies in plasma samples from 37 patients with HB.
Results
Assessments of antibody profiles showed that anti-FIX IgG1-4, IgA, and IgE were detected significantly more often in patients with a positive Nijmegen-Bethesda Assay (NBA). All NBA-positive samples were positive for IgG4. Anti-FIX IgG4 demonstrated a strong correlation with the NBA, while correlations were significant, yet more moderate, for anti-FIX IgG1-2 and IgA.
Conclusions
The anti-FIX antibody profile in HB patients who develop inhibitors is diverse and correlates well with the NBA across immunoglobulin (sub)class, and anti-FIX IgG4 is particularly relevant to functional inhibition. The anti-FIX FLI may serve as a useful tool to confirm the presence of antibodies in patients who have low positive NBA results and to more clearly define, predict, and treat alloantibody formation against FIX.
Keywords: Factor IX, Factor IX Deficiency, Hemophilia B, Inherited Blood Coagulation Disorders, Immunoassay
Introduction
Hemophilia is an X-linked inherited bleeding disorder caused by mutations resulting in deficiencies or dysfunctions in coagulation factor (F) VIII (hemophilia A (HA)) or FIX (hemophilia B (HB)). Prevention and treatment of bleeding in patients with hemophilia is accomplished via infusions of recombinant or plasma-derived factor to replace the deficient gene product. A subset of patients undergoing infusion therapy develop neutralizing alloantibodies, termed inhibitors, against the treatment product that nullify the therapeutic efficacy of factor replacement (reviewed in (1)). Inhibitor development complicates patient management (1–3) and markedly increases patient morbidity and cost of treatment (4).
Although HA and HB have similar etiologies, inhibitor formation in patients with HB includes some unique features that distinguish it from HA, which include an increased proportion of high responding inhibitors(1), the accompaniment of an anaphylactic response(5–7), decreased success of immune tolerance induction (ITI)(8;9), and a tendency for patients undergoing ITI to develop nephrotic syndrome(9;10). These unique features highlight the need for studies designed to better characterize anti-FIX alloantibody formation in patients with HB.
The gold standard method for laboratory detection of inhibitors in patients with hemophilia is the Nijmegen-Bethesda Assay (NBA), which measures the degree to which patient plasma inhibits the in vitro clotting activity of plasma from a pool of healthy donors following a two hour incubation of patient plasma with normal plasma at 37°C (11). The Bethesda Unit is defined as the dilution of patient sample required to result in 50% inactivation of factor VIII or FIX in an equivalent volume of normal plasma (e.g. 1 BU is 50% inactivation with no dilution; 100 BU is 50% inactivation following 100-fold dilution). The specificity and reliability of the original Bethesda assay was such that 1.0 BU defined the acceptable limit of positivity. However, with the Nijmegen modification of the BA (buffering the normal plasma with 0.1 M imidazole to pH 7.4) (12) and heat treating test plasmas (13) to destroy residual FVIII or FIX, an assay result of 0.5 or above for FVIII and 0.3 or above for FIX has been suggested to indicate that an inhibitor is present (13). A lack of consensus creates some ambiguity with regard to the optimal cutoff to define a positive reaction, particularly for FIX inhibitors.
In addition, the specific immune response to FIX is also controversial. Previous studies examining small patient cohorts (n=1–8) have reported that inhibitor positive patients with HB harbor anti-FIX antibodies of IgG4 subclass which, in some cases, are accompanied by other Ig subclasses (14–20). In order to address the paucity of data currently available describing the immune response to FIX, the current cross-sectional study evaluated plasmas from a large group of patients with HB using a fluorescence immunoassay (FLI) in addition to the modified NBA to investigate the relationship between anti-FIX antibody profiles and inhibitor formation.
Materials and Methods
Subjects
Characterization of the anti-FIX antibody profile in NBA-positive HB patient plasmas utilized plasma samples from patients enrolled in the Hemophilia Inhibitor Research Study (HIRS) (21). Specimens from 12 HB patients that tested ≥ 0.3 NBU were selected from the HIRS study samples. An additional 25 consecutive HIRS HB patient samples that tested < 0.3 NBU were selected as controls (Table 1). Follow-up FLIs were performed on archived samples from patients of interest identified in initial experiments. The investigational review boards of the Centers for Disease Control and participating sites approved the protocol. All participants or parents of minors gave informed consent. Control samples, which were used to establish the thresholds of positivity used in the FLI, were obtained from 50 paid healthy donors.
Table 1.
Demographics of HB subjects
| Healthy Donor | Inhibitor Negative* | Inhibitor Positive**,‡ | |
|---|---|---|---|
| Number | 50 | 25 | 12 |
| Mean age in years (range) | 39.6† (21.6–55.6) | 27.2 (2.9–65.9) | 25.0 (0.2–65.4) |
| N (%) Non-white | 46 (92) | 0 (0) | 5 (42) |
| N (%) FIX exposure days < 21 | 10 (40) | 6 (54.5) | |
| N (%) FIX exposure days 21–100 | 7 (28.0) | 2 (18.2) | |
| N (%) FIX exposure days 101–150 | 1 (4.0) | 1 (9.1) | |
| N (%) FIX exposure days >150 | 7 (28.0) | 2 (18.2) | |
| N (%) Mild HB | 6 (25.0) | 0 | |
| N (%) Moderate HB | 14 (58.3) | 0 | |
| N (%) Severe HB | 4 (16.7) | 10 (100) |
HB, Hemophilia B;
Severity data was collected for 24 of the 25 inhibitor negative patients;
Severity data was collected for 10 of 12 inhibitor positive patients;
Age data was collected for 48 of the 50 healthy donors;
Exposure data was available for 11 of the 12 NBA-positive patients
Nijmegen-Bethesda assay
Plasma was isolated from citrated blood by two rounds of centrifugation at 1600xg at 4°C for 20 minutes. Processing of blood samples was completed within 2 hours of venipuncture, and plasma samples were shipped to the CDC on cold packs. Functional inhibitor determinations were made using a modified version of the NBA as previously described (13). Developmental work established a cutoff of 0.3 NBU and above for presence of inhibitory alloantibodies (inhibitor positive) and less than 0.3 NBA for absence of inhibitory alloantibodies (inhibitor negative), based on results from 160 patients using the modified NBA (13).
Anti-FIX Fluorescence Immunoassay
The anti-FIX FLI is a modified version of our previously described method (22). Plasma samples from patients with HB or healthy donors diluted (1:10 for IgE and 1:30 for of all other immunoglobulins (Igs)) in phosphate-buffered saline (PBS) containing 1% dried milk were incubated with SeroMAP beads (Luminex Corporation, Austin, TX) coupled to recombinant FIX (BeneFIX, Wyeth Pharmaceuticals, Philadelphia, PA). Antibodies were detected using serial incubations with biotinylated anti-human Ig (anti IgG1, A-10650; anti IgG2, 05-3540; anti IgG3, MH1532; anti IgG4, MH1542; anti IgA, 62-7440; IgE, A18797; Life Technologies, Carlsbad, CA) and R-phycoerythrin-conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA) with a Bio-Plex 200 suspension array system (Bio-Rad Laboratories, Hercules, CA) measured as median fluorescence intensities (MFI). The threshold for positivity was set at two standard deviations above the mean MFI obtained for healthy donors.
Anti-FIX Fluorescence Immunoassay dilution and blocking studies
Plasma samples were diluted 1:30000, 1:3000, and 1:30, and anti-FIX IgG1-4, IgA, and IgE binding was measured as described above. Blocking studies to demonstrate the FLI’s specificity for anti-FIX antibodies were performed on 1:30 diluted samples by pre-incubating plasmas with 300μg/ml recombinant FIX or PBS alone for 1 hour prior to incubation with FIX-coupled beads.
Statistics
Statistical analyses were performed on the data presented in Figures 2 and 3. Differences in categorical data were evaluated using Fisher’s exact test. Spearman’s correlation coefficient and two-tailed P-values were generated using GraphPad Prism 6 (GraphPad Software, San Diego CA) to assess correlations between FLI and NBA results for samples positive by one or both of the assays. NBA results of 0.0 NBU were converted to 0.01 NBU to allow for plotting on the log scale used in Figure 3.
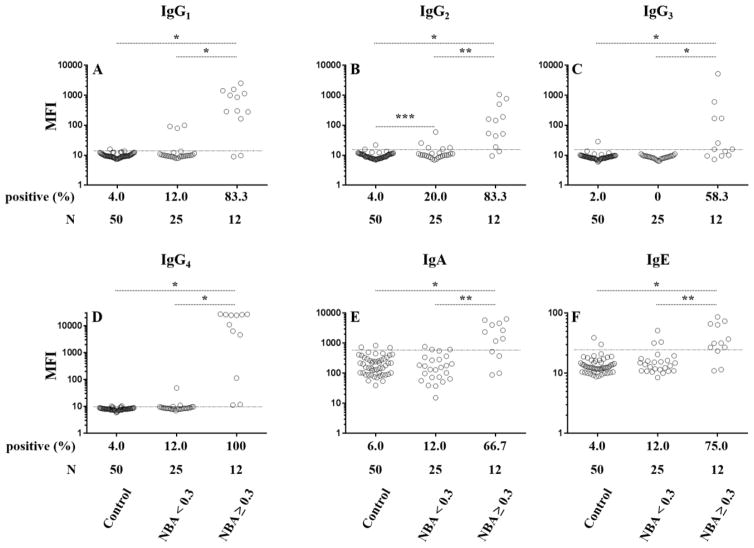
Figure 2. Fluorescence immunoassay results for anti-FIX antibodies in plasma from patients who have hemophilia B (HB) and healthy controls.
Data points represent results for individual plasma samples for IgG1 (A), IgG2 (B), IgG3 (C), IgG4 (D), IgA (E), and IgE (F). Results are displayed on a log scale for control plasmas from healthy donors and patients with hemophilia B who tested negative (< 0.3) or positive (≥ 0.3 NBU) by the Nijmegen-Bethesda assay. Thresholds for positivity, which, are set at two standard deviations above the mean MFI of control samples and represented by dashed lines are 13.6, 15.0, 15.4, 9.8, 600.5, and 24.6 for IgG1, IgG2, IgG3, IgG4, IgA, and IgE, respectively. * P ≤ 0.0001; **P ≤ 0.002; *** P = 0.0375
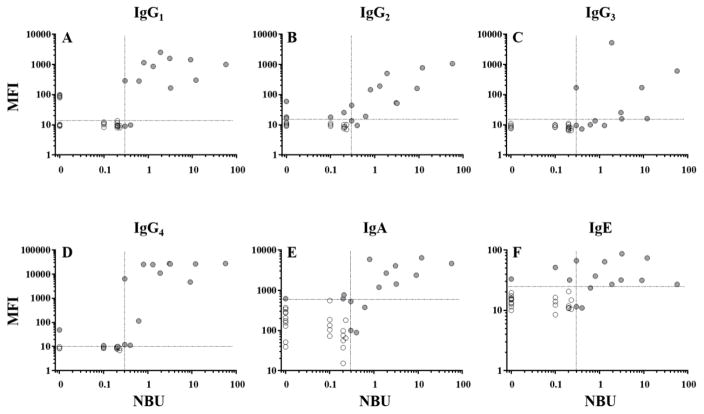
Figure 3. Correlation of anti-FIX fluorescence immunoassay and Nijmegen-Bethesda assay results for samples from patients with hemophilia B.
As shown, individual data points represent results obtained on hemophilia B patient samples using the immunoglobulin-specific FLIs (IgG1 (A), IgG2 (B), IgG3 (C), IgG4 (D), IgA (E), or IgE (F)) plotted on a log scale against NBA results for the same plasma sample. Samples positive for one or both assays (filled circles) were used to calculate linear correlations according to Spearman (r). Dashed lines represent the thresholds of positivity.
Results
Demographics of the 37 HB subjects studied are shown on Table 1. Twenty-five of the patients were negative and twelve were positive for inhibitor by NBA; 10 of the 12 patients with a positive NBA had a clinical history of an inhibitor reported by the submitting study site. Of the 12 inhibitor patients, 5 were high titer (≥5 NBU) and 7 were low titer (<5 NBU) at the time of testing. All 10 of the inhibitor positive patients with known severities have severe disease, while 25.0%, 58.3%, and 16.7% of inhibitor negative patients have mild, moderate, and severe disease, respectively. The distribution of patients in the categories for exposure days is similar in patients with and without inhibitors. Five of 12 inhibitor patients and 0 of 25 non-inhibitor patients were non-white, although enrollment into HIRS was not population-based, and results may not represent the prevalence of FIX inhibitors overall.
Anti-FIX antibody profiles
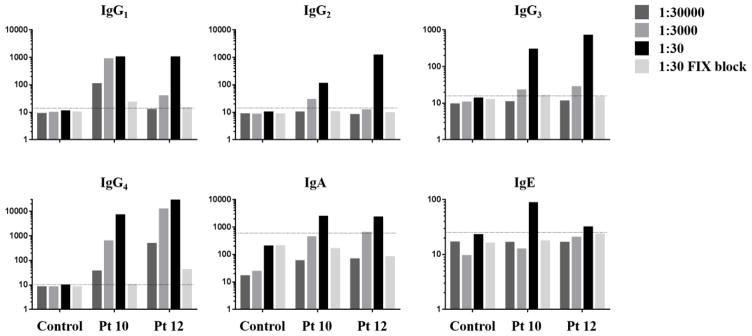
A FLI capable of detecting anti-FIX antibodies in plasma diluted up to 1:30000 in a manner that is blocked by the by the addition of excess uncoupled FIX (Figure 1), was used to characterize anti-FIX antibody profiles in plasma samples from 37 patients with HB and 50 healthy controls. Figure 2 and Table 2 report anti-FIX FLI results by Ig subclass for individual plasma samples from healthy donors (control) and from patients with HB segregated into two categories corresponding to NBA-negative (<0.3 NBU; n=25) and NBA-positive (≥ 0.3 NBU; n=12) based on a method-specific cutoff established for the modified NBA (13). A small proportion of the 25 NBA-negative samples were positive for anti-FIX IgG1, IgG4, IgA, or IgE (n= 0–3, 0–12%), but these samples demonstrated no significant difference in antibody frequency compared to that of healthy donors. A slightly higher proportion of NBA-negative samples were positive for IgG2 (n=5, 20%; P=0.0375). All twelve NBA-positive samples tested positive for anti-FIX IgG4. IgG1 and IgG2 were detected in ten (83%) of the NBA-positive samples, and IgG3, IgA, and IgE were positive in 58%, 67% and 75%, respectively. FLI rates of positivity were significantly higher on NBA-positive samples compared to both the healthy controls (P<0.0001) and NBA-negative HB samples (P<0.002) for all Igs tested.
Figure 1. Demonstration of Anti-FIX fluorescence immunoassay sensitivity and specificity.
Histograms represent median fluorescence intensities obtained on plasma samples diluted 1:30000, 1:3000, or 1:30. 1:30 dilutions were pre-incubated +/− 300μg/ml recombinant FIX. Dashed lines represent the thresholds of positivity.
Table 2.
Summary of fluorescence immunoassay results (FLI) for anti-factor IX antibodies segregated by class and IgG subclass
| Number (%) Positive for Anti-Factor IX by FLI | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n | IgG1 | IgG2 | IgG3 | IgG4 | IgA | IgE | |
| Healthy donors | 50 | 2 (4) | 2 (4) | 1 (2) | 2 (4) | 3 (6) | 2 (4) |
| HB specimens <0.3 NBA | 25 | 3 (12) | 5 (20)* | 0 | 3 (12) | 3 (12) | 3 (12) |
| HB specimens ≥0.3 NBA | 12 | 10 (83)* | 10(83)* | 7 (58)* | 12 (100)* | 8(67)* | 9 (75)* |
|
| |||||||
| Correlation of FLI and NBA r (r lower-r upper) | 0.6727 (0.23–0.88) P=0.0075 |
0.7105 (0.34–0.89) P=0.0019 |
0.5632 (−.04–.86) P=0.059 |
0.8222 (0.52–0.94) P=0.0003 |
0.7174 (0.31–0.90) P=0.0035 |
0.0895 (−0.46–0.59) P=0.7491 |
|
FLI, fluorescence immunoassay; HB, Hemophilia B; NBA, Nijmegen Bethesda Assay;
P<0.05
Correlation of anti-FIX subclass specific FLI results with NBA titers
In order to examine the link between anti-FIX antibody titers reported by the FLI and FIX-specific inhibition of in vitro clotting reported by the NBA, linear correlations were calculated according to Spearman on samples positive by one or both of the assays. FLI levels for anti-FIX IgG4 demonstrated a strong positive correlation with the NBA (r=0.8222; P=.0003; Figure 3, Table 2), while correlations were significant, yet more moderate for anti-FIX IgG1, IgG2, and IgA (Figure 3, Table 2). FLI results for IgG3 and IgE did not have significant correlations with the NBA. NBA-positive samples from patients 1 (0.3 NBU) and 3 (0.4 NBU), which had inhibitor titers close to the 0.3 NBU cut-off for positivity established in our previous study (13), were positive for anti-FIX IgG4, for which both samples tested slightly higher than the FLI’s threshold for positivity (Table 3A), but were negative for other anti-FIX Igs. In contrast, a sample from patient 2, which also tested at the threshold for positivity of the NBA (0.3 NBU), was strongly positive by FLI for anti-FIX IgG1-4. All specimens with ≥0.3 NBU were positive for IgG4.
Table 3.
Raw data for patients who tested positive for one or more anti-factor IX immunoglobulins by fluorescence immunoassay. Positive results in bold.
| Patient | Severity | History of inhibitor | F9 mutation (type) | Exposure days† | Interval days since first draw | IgG1 | IgG2 | IgG3 | IgG4 | IgA | IgE | NBU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Patients with positive Nijmegen-Bethesda assay | ||||||||||||
|
| ||||||||||||
| 1 | severe | ND | c.880C>T (nonsense) | < 21 | 0 | 9 | 13.5 | 9.5 | 12 | 100.5 | 11.5 | 0.3* |
|
| ||||||||||||
| 2 | ND | yes | c.223C>T (nonsense) | < 21 | 0 | 286.8 | 44.3 | 169.8 | 6420.8 | 527.8 | 66.5 | 0.3* |
|
| ||||||||||||
| 3 | severe | No | c.1150C>T (nonsense) | >150 | 0 | 10.3 | 14.5 | 8.8 | 15.5 | 472 | 13.5 | 0.1 |
| 384 | 12 | 15.5 | 9.3 | 13.5 | 491.3 | 9 | 0.1 | |||||
| 762 | 9.5 | 12.8 | 6.5 | 13.5 | 308.3 | 16 | 0.0 | |||||
| 1126 | 10 | 12.8 | 7.5 | 12.3 | 331.5 | 16 | 0.1 | |||||
| 1504 | 9.8 | 9.5 | 7.3 | 11.3 | 89 | 11 | 0.4* | |||||
| 1875 | 10.5 | 14 | 9.3 | 12.5 | 261.3 | 15 | 0.1 | |||||
|
| ||||||||||||
| 4 | severe | Yes | Deletion of exon 1–8 | < 21 | 0 | 281.5 | 19 | 10 | 115.3 | 375.5 | 23.5 | 0.6* |
| 281 | 2850 | 462.3 | 594.3 | 13631.3 | 2089.8 | 30 | 21.3 | |||||
| 399 | 1455 | 667.3 | 58 | 1324 | 1791.8 | 33.5 | 1.6 | |||||
|
| ||||||||||||
| 5 | severe | Yes | Deletion of exon 1–5 | 101–150 | 0 | 1582 | 53.5 | 25.5 | 27231 | 4043 | 31.8 | 3.1* |
| 462 | 195.3 | 1838.5 | 23.3 | 28648.8 | 9379.5 | 29.5 | 23.3 | |||||
|
| ||||||||||||
| 6 | severe | Yes | Deletion of exon 1–8 | >150 | 0 | 1142.5 | 145.3 | 13.5 | 25243.8 | 5862.3 | 37 | 0.8* |
|
| ||||||||||||
| 7 | severe | Yes | Deletion of exon 1–8 | < 21 | 0 | 858.3 | 191.3 | 9.5 | 24839.3 | 1182.5 | 64 | 1.3* |
|
| ||||||||||||
| 8 | severe | Yes | c.659C>G (nonsense) | < 21 | 0 | 2512.3 | 501.5 | 5219 | 11112 | 2674.3 | 27 | 1.9* |
|
| ||||||||||||
| 9 | severe | Yes | Deletion of exon 1–8 | 21–100 | 0 | 165.3 | 51.3 | 15.8 | 26332 | 1423 | 86.5 | 3.2* |
|
| ||||||||||||
| 10 | ND | Yes | c.223C>T (nonsense) | < 21 | 0 | 1431 | 160.5 | 170.5 | 4689.5 | 2364 | 31.5 | 9.1* |
|
| ||||||||||||
| 11 | severe | Yes | c.223C>T (nonsense) | 21–100 | 0 | 300 | 773.8 | 16 | 26511 | 6386.3 | 73.8 | 12.0* |
|
| ||||||||||||
| 12 | severe | Yes | c.880C>T (nonsense) | ND | 0 | 993.8 | 1056.3 | 604.3 | 27614.5 | 4610.8 | 27 | 57.2* |
|
| ||||||||||||
| B. Patients with negative Nijmegen-Bethesda assay | ||||||||||||
|
| ||||||||||||
| 13 | severe | No | c.1228G>T (missense) | >150 | 0 | 10 | 10 | 8 | 11 | 133 | 16.3 | 0.1* |
|
| ||||||||||||
| 14 | mild | No | c.1136G>A (missense) | >150 | 0 | 11.5 | 25.5 | 8.5 | 8 | 618.5 | 20.5 | 0.2* |
|
| ||||||||||||
| 15 | severe | No | c.677G>A (missense) | >150 | 0 | 9.5 | 18 | 9.5 | 8.5 | 51.3 | 13.3 | 0.0* |
| 462 | 7.8 | 8.8 | 7.5 | 8 | 61.3 | 10.3 | 0.1 | |||||
| 1870 | 7.5 | 12.5 | 8.5 | 9 | 57.3 | 12.3 | 0.0 | |||||
|
| ||||||||||||
| 16 | moderate | No | c.1328T>C (missense) | 21–100 | 0 | 9 | 10.3 | 9.5 | 8.8 | 340.5 | 33 | 0.0* |
| 323 | 8 | 11.3 | 9.5 | 9 | 357.5 | 28.5 | 0.0 | |||||
| 750 | 10.3 | 13 | 6.8 | 8.3 | 350.3 | 19.8 | 0.0 | |||||
| 1590 | 9.5 | 10.8 | 9.5 | 8.3 | 364.3 | 24 | 0.0 | |||||
|
| ||||||||||||
| 17 | mild | No | c.316G>A (missense) | 21–100 | 0 | 99.5 | 59.5 | 8 | 8.5 | 156.3 | 11.5 | 0.0* |
| 631 | 20 | 17.3 | 8.3 | 8.5 | 80 | 11 | 0.0 | |||||
|
| ||||||||||||
| 18 | moderate | No | c.1025C>T (missense) | 21–100 | 0 | 80 | 11.3 | 11 | 49 | 618.5 | 15 | 0.0* |
| 365 | 77.8 | 15 | 8.5 | 98.3 | 296 | 12.3 | 0.0 | |||||
| 771 | ND | ND | ND | ND | ND | ND | 0.1 | |||||
| 1128 | 62.3 | 9.5 | 5.5 | 41.5 | 413.8 | 12.5 | 0.0 | |||||
| 1674 | 67.3 | 9.5 | 7.5 | 40.8 | 502.5 | 14.5 | 0.0 | |||||
|
| ||||||||||||
| 19 | moderate | No | c.786T>G (missense) | 21–100 | 0 | 13 | 10.5 | 9 | 8.3 | 431 | 12 | 0.0 |
| 365 | 91.5 | 16.3 | 9.3 | 8 | 370 | 12.8 | 0.0* | |||||
| 857 | 11.8 | 14.3 | 8.5 | 9 | 462.5 | 14.3 | 0.0 | |||||
| 1256 | 10 | 11 | 10 | 9.3 | 205.8 | 11.5 | 0.0 | |||||
| 1625 | 10 | 18.3 | 11.5 | 7.8 | 310.3 | 13 | 0.0 | |||||
|
| ||||||||||||
| 20 | mild | No | c.572G>A (missense) | < 21 | 0 | 10.3 | 11.3 | 8.5 | 9.8 | 555.3 | 51.3 | 0.1* |
| 372 | 9.5 | 14 | 7.8 | 7.8 | 686.5 | 46.3 | 0.0 | |||||
| 736 | 8.3 | 13 | 8.5 | 8.5 | 417.8 | 32 | 0.0 | |||||
|
| ||||||||||||
| 21 | moderate | No | c.-35G>A (5′ UTR) | < 21 | 0 | 12.3 | 17.8 | 8.3 | 9 | 185.5 | 14 | 0.1* |
| 386 | 12.8 | 20.8 | 7 | 7.3 | 162.8 | 9.8 | 0.1 | |||||
|
| ||||||||||||
| 22 | moderate | No | c.1025C>T (missense) | < 21 | 0 | 8.5 | 9.5 | 8.5 | 10.8 | 137.8 | 9.8 | 0.1 |
| 372 | 9 | 10 | 8.5 | 10.5 | 340 | 16.3 | 0.0 | |||||
| 736 | 8.3 | 9.5 | 7.3 | 9.3 | 252.3 | 17 | 0.0 | |||||
| 1100 | 9 | 8.5 | 8.3 | 10 | 767 | 31.8 | 0.2* | |||||
| 1828 | 9.5 | 12.3 | 8.5 | 10.5 | 262.3 | 17.3 | 0.0 | |||||
| 2192 | 9.5 | 11 | 8.5 | 11.3 | 248 | 14 | 0.0 | |||||
|
| ||||||||||||
| Threshold for positivity | 13.6 | 15.0 | 15.4 | 9.8 | 600.5 | 24.6 | 0.3 | |||||
Serial Specimens
A subset of samples in the current study had detectable anti-FIX antibodies by FLI, but functional inhibition was not observed using the NBA (Table 3B). We and others have previously demonstrated that this phenomenon also occurs in a small percentage of samples from patients with HA (22–25) and may result from the presence of antibodies that are of low titer, low affinity, or directed against low-impact epitopes. In order to gain insight into the dynamics of antibody production in these patients, FLIs were performed on archived serial draws (when available) from NBA-negative patients who tested positive for one or more anti-FIX Igs in the original analysis (Table 3; NBA results for initial study samples are marked with an *). One patient (patient 15) was negative for all anti-FIX Igs tested on serial samples drawn after his original study specimen, while the other seven patients who had archived samples available for analysis, despite remaining NBA negative, had repeat positive results for one or more anti-FIX antibodies using serial draws (patients 16–22, Table 3B). FLI results for patient 18 report the presence of anti-FIX IgG1 and IgG4 in four samples taken over a period of more than 4 years. This antibody profile correlates well with functional inhibition of FIX (current study) and FVIII (22;24), yet this patient never tested positive by NBA over that time span.
As a means for comparison, Table 3A reports FLI data for all NBA-positive patient samples as well as any available archived serial samples from those patients (patients 1–12). Patient 3 is of interest due the presence of a low titer positive anti-FIX IgG4 that was detected by FLI in six study samples drawn over a period of more than 5 years (Table 3A). Notably, only one of these samples tested positive by the NBA (0.4 NBU), while the other five were negative. These results may be an indication that the patient’s low titer IgG4, which was only slightly above the level of detection by FLI, may be hovering at or just below the NBA detection threshold. Raw FLI data for patient samples that were negative by the NBA and for all subclasses of anti-FIX Igs are shown in Table 4.
Table 4.
Raw data for patients who tested negative by the Nijmegen Bethesda Assay and for all anti-factor IX immunoglobulins by the anti-Factor IX fluorescence immunoassay.
| Patient | Severity | History of inhibitor | F9 mutation (type) | Exposure days† | IgG1 | IgG2 | IgG3 | IgG4 | IgA | IgE | NBU |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 23 | moderate | No | c.881G>A (missense) | >150 | 9.5 | 11.8 | 8.8 | 8.5 | 175 | 17.5 | 0.0* |
|
| |||||||||||
| 24 | severe | No | c.1072A>G (missense) | 101–150 | 9.8 | 11.5 | 8.8 | 8.3 | 273.3 | 19.5 | 0.0* |
| 25 | moderate | No | ND | < 21 | 9.8 | 9.5 | 9.5 | 8 | 206.3 | 15.3 | 0.0* |
| 26 | moderate | No | c.1025C>T (missense) | >150 | 10.3 | 11.3 | 9 | 8 | 288 | 15.5 | 0.0* |
| 27 | moderate | No | c.1025C>T (missense) | < 21 | 10 | 9 | 9 | 9.8 | 39.3 | 10 | 0.0* |
| 28 | moderate | No | c.1328T>C (missense) | < 21 | 9 | 9.5 | 7.5 | 8 | 129.3 | 16 | 0.0* |
| 29 | moderate | No | c.1025C>T (missense) | < 21 | 8.3 | 9 | 9.5 | 9 | 103.8 | 12.3 | 0.1* |
| 30 | ND | No | ND | >150 | 11 | 10.3 | 10 | 8.5 | 72.5 | 8.5 | 0.1* |
| 31 | moderate | No | c.-35G>A (substitution in promoter) | < 21 | 9.3 | 10 | 10 | 9.3 | 98 | 11 | 0.2* |
| 32 | severe | No | c.627delC (frameshift) | 21–100 | 13.5 | 7.5 | 7 | 9.8 | 75 | 11 | 0.2* |
| 33 | moderate | No | c.1328T>C (missense) | >150 | 8 | 8.5 | 7.5 | 9 | 15.3 | 10.8 | 0.2* |
| 34 | moderate | No | c.1025C>T (missense) | < 21 | 9.8 | 7.5 | 11 | 8.5 | 37.5 | 11.8 | 0.2* |
| 35 | mild | No | c.316G>A (missense) | 21–100 | 9 | 10.3 | 6.5 | 8 | 56.5 | 10.8 | 0.2* |
| 36 | mild | No | c.1025C>T (missense) | < 21 | 9.3 | 10 | 7.8 | 7 | 64.8 | 14.8 | 0.2* |
| 37 | mild | No | c.1328T>C (missense) | 21–100 | 8 | 7 | 6.5 | 8.5 | 180 | 10.5 | 0.2* |
|
| |||||||||||
| Threshold for positivity | 13.6 | 15.0 | 15.4 | 9.8 | 600.5 | 24.6 | 0.3 | ||||
Discussion
The current study describes a method for direct detection of anti-FIX antibodies in patients who have HB. The data presented here demonstrate that the described method is capable of detecting antibodies in plasma diluted up to 1:30000, there is a strong positive correlation between plasma levels of anti-FIX IgG4 and NBA results, and samples with NBA results as low as 0.3 NBU were positive for anti-FIX IgG4.
The prevalence of inhibitors among all patients with HB and HA has been reported at 1.5% and 5–7%, respectively, a difference that is amplified when comparing patients with a severe form of their disease, in which 3.8% and 30% of HB and HA patients develop inhibitors, respectively (26–28). The lower relative frequency of neutralizing antibody formation in patients with HB compared to HA is hypothesized to result in part from three important distinguishing features of the disorders. First, there is a high degree of homology between FIX and other vitamin K-dependent clotting factors which may confer some tolerance to FIX in patients with no circulating FIX (29), a condition that is not true of FVIII. Second, there is a lower proportion of patients with HB who fall into the severe category (severe HB (30–40%) relative to patients with HA (60%) (30), and patients with undetectable plasma clotting factor are more likely to develop an inhibitory antibody response to replacement therapy. Third, patients with severe HB are three times more likely than those with severe HA to have a missense mutation (47% vs 16%) and thus a gene product (31), and the physiologic plasma concentration of FIX (0.1–0.2 micrograms/mL) far exceeds that of FVIII (3–5 micrograms/mL). These factors combine to result in patients with HB having, on average, more gene product and higher levels of circulating cross reactive material (CRM) to their treatment product relative to patients with HA who are more likely to have a null mutation (1). Notably, all of the patients in the current study who have positive NBA results have severe HB caused by nonsense or large deletion mutations, and only 2 of 14 (14.3%) patients who tested negative by the NBA and for all classes of ant-FIX Igs had severe HB. These data highlight the importance of disease severity and mutation type as indicators for risk of inhibitor development in patients who have HB.
An additional unique feature of HB is the tendency of patients to develop an allergic or anaphylactic response prior to or in conjunction with inhibitor formation(29). The mechanism responsible for anaphylaxis in patients with HB is unclear, but the high standard dose used for FIX infusions, which is required to achieve adequate plasma concentrations for hemostasis, has been hypothesized to play a role(29). Antigen exposure may lead to anaphylaxis if the dose is sufficient to overwhelm the binding capacity of IgG blocking antibodies(32;33), which share antigen specificity with IgE bound to FcεRI on mast cells, thus allowing for mast cell activation (reviewed in (34)). Alternatively, studies using mouse models suggest that IgG-immune-complex activation of FcγRIII may induce anaphylaxis via activation of macrophage, mast cells, or neutrophils (33–37).
Data reported here and previously on both HB (14–20) and HA (22;24) argue that the presence of a product-reactive IgG4 is the best predictor of a functional inhibitor. A notable exception to this rule is patient 18 in the current study, who tested positive for anti-FIX IgG4 four times over a four-years while remaining NBA-negative. The discordance of patient 18’s FLI and NBA results is potentially due to 1) the presence of an antibody pre-bound to a target that has FIX binding affinity, thus allowing for indirect capture of antibodies 2) the presence of sufficient levels of FIX CRM in the patient’s plasma to act as an anti-FIX reservoir capable of out-competing the NBA assay target, but insufficient to saturate assay targets in the FLI, a possibility supported by previous studies that reported circulating FIX immune complexes in patients with HB (38), or 3) the possibility that the composition of the patient’s anti-FIX antibody profile, despite containing significant amounts IgG1 and IgG4, may lack the affinity and/or epitope localization required to impose functional inhibition detectable by the NBA. A method-specific cutoff was established for the modified NBA used in this study based on assay results from 160 patients (13). The observation that samples in the 0.3–0.4 NBU range in the current study have detectable anti-FIX IgG4 antibodies, which are likely responsible for the trace amount of neutralizing activity reported by the NBA, highlights the need for standardization of inhibitor detection methods because many clinical laboratories place the cutoff for positivity at or above 0.5 NBU and likely miss low level inhibitors. While one or more classes of anti-FIX antibodies were present in 40% (10 of 25) of patient samples that were negative by the NBA, IgG4 was present in only 3 (12%). Studies show that inhibitor formation occurs in only 1.5% of patients with HB(27); thus, it follows that the majority of the patients who have non-neutralizing anti-FIX antibodies will not convert from NBA-negative to NBA-positive. More clinical data are needed to support this hypothesis, which differs from HA, in that a higher proportion of HA patients develop inhibitors, with evidence that anti-FVIII IgG1 may be predictive of future inhibitor development (22).
Data presented in the current study demonstrate the relevance of anti-FIX IgG4 antibodies, as determined by FLI, to functional FIX inhibitors. Given that some FIX patients have NBA results in the borderline or indeterminate range, the FLI assay can be helpful in clinical studies and potentially in clinical care of HB patients to better detect, interpret and manage patients with low titer FIX inhibitors. The current study is limited in that it is cross-sectional, not optimally age/race matched for cases and controls, and has a small sample size. Additionally, although the study provides strong empirical evidence to support the above conclusions, it is limited in that does not identify the underlying factors that contribute to IgG4 development in some patients and not others, and it remains unclear why the presence of IgG4 plays such an important role in functional inhibition of FIX. A recent study by Hofbauer et al. characterizing neutralizing versus non-neutralizing antibodies in patients with hemophilia A, showed that anti-FVIII IgG4 was found exclusively in samples from patients who were NBA positive. In addition, these authors showed that patients who had inhibitors had anti-FVIII antibodies with affinities up to 100-fold higher than patients who were inhibitor negative and that anti-FVIII IgG4 antibodies had the highest affinity of all anti-FVIII subclasses. Hofbauer et al. (25) hypothesize that these high affinity antibodies are responsible for neutralizing FVIII activity(25). A similar mechanism may be involved in inhibition of FIX in patients who have HB. Future studies designed to monitor HB patient anti-FIX antibody profiles prospectively are required to better characterize the factors that contribute to the initiation and evolution of anti-FIX antibody production and to assess the prognostic value of anti-FIX antibody profiles as they relate to FIX neutralization.
Essentials.
Studies characterizing neutralizing antibodies (inhibitors) in hemophilia B (HB) are lacking.
The current study describes anti-factor (F) IX antibody profiles in 37 patients who have HB.
Anti-FIX IgG4 levels exhibited a strong positive correlation with Nijmegen-Bethesda results.
These data will help to more clearly define, predict, and treat alloantibody formation in HB.
Acknowledgments
This work was supported by the CDC Foundation through a grant from Pfizer Pharmaceuticals. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Thank you to the patients who participated in the study and the coordinators and administrators at the study sites: F. Kelly, J. Kuhn, G. Long, P. Bryant, M. Geary, R. Lamoreaux, M. Nolte, J. Leonard, J. Thomas, B. Wilson, B. Yandell, L. Morse, N. Thukral, M. Lammer, D. Nelson, H. Davidson, M. Lemanczyk, M. Cantini, A. Khleif, C. Dekernion, J. Buehler, A. Hollatz, B. Riske, W. Mitsuyama, D. Waters, A. Riedel, M. Tomita, Y. Chong, A. Forsberg, D. Cooper-Blacketer, and R. Hauke.
Appendix. Study Group
The Hemophilia Inhibitor Research Study Investigators include the following authors:
T. C. Abshire, A. Dunn, P. L. Bockenstedt, D. B. Brettler, J. A. Di Paola, M. Radhi, S. R. Lentz, G. Massey, J. C. Barrett, A. D. Shapiro, M. Tarantino, B. M. Wicklund, C. Knoll, M. A. Escobar, M. E. Eyster, J. C. Gill, C. Leissinger, H. Yaish (USA).
Footnotes
Addendum
B. Boylan designed and performed the research, analyzed the results, and wrote the manuscript; A. S. Rice performed the research; A. T. Neff, M. J. Manco-Johnson, and C. L. Kempton provided patient samples and critically revised the manuscript; C. H. Miller analyzed results and wrote the manuscript.
Disclosure of Conflict of Interests
C. Kempton reports grants from CDC Foundation during the conduct of the study; grants and personal fees from Novo Nordisk, as well as personal fees from Baxalta, Biogen Idec, and CSL Behring outside the submitted work.
A. Neff reports grants from CDC Foundation during the conduct of the study; grants from Baxter, Novo Nordisk, and CSL Behring, as well as personal fees from Alexion outside the submitted work.
C. Miller reports grants from CDC Foundation during the conduct of the study.
A. Dunn reports grants and personal fees from Biogen, Bayer, Baxalta, and CSL Behring; grants from Octopharma, Kedrion, and Novo Nordisk outside the submitted work.
S. R. Lentz reports grants from CDC Foundation during the conduct of the study; personal fees from Novo Nordisk outside the submitted work.
M. Tarantino reports personal fees and other from Bleeding and Clotting Disorders Institute, Novo Nordisk, Baxalta, Grifols, Amgem, Pfizer, Biogen, HRSA, and CDC Foundation outside the submitted work.
A. Escobar reports grants and personal fees from Pfizer, personal fees from Baxalta, Novo Nordisk, Bayer, and CSL Behring outside the submitted work.
C. Lessinger reports grants from Baxalta, CSL Behring, and Roche; advisory board participation in Baxalta, Bayer, CSL Behring, Kedrion, Novo Nordisk, Roche, Pfizer, Biogen, and LFB.
J. C. Gill reports grants from Centers for Disease Control and Prevention during the conduct of the study; grants and personal fees from CSL Behring Shire and Bayer outside the submitted work.
B. M. Wicklund reports grants from CDC Foundation during the conduct of the study; personal fees from Novo Nordisk, Biogen, Baxalta-Shire, Bayer, and Oakstone-EBIX outside the submitted work.
Reference List
- 1.Dimichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. 2007 Aug;138:305–15. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 2.Astermark J, Morado M, Rocino A, Van Den Berg HM, von DM, Gringeri A, Mantovani L, Garrido RP, Schiavoni M, Villar A, Windyga J. Current European practice in immune tolerance induction therapy in patients with haemophilia and inhibitors. Haemophilia. 2006 Jul;12:363–71. doi: 10.1111/j.1365-2516.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 3.Benson G, Auerswald G, Elezovic I, Lambert T, Ljung R, Morfini M, Remor E, Salek SZ. Immune tolerance induction in patients with severe hemophilia with inhibitors: expert panel views and recommendations for clinical practice. Eur J Haematol. 2012 May;88:371–9. doi: 10.1111/j.1600-0609.2012.01754.x. [DOI] [PubMed] [Google Scholar]
- 4.Guh S, Grosse SD, McAlister S, Kessler CM, Soucie JM. Healthcare expenditures for males with haemophilia and employer-sponsored insurance in the United States, 2008. Haemophilia. 2012 Mar;18:268–75. doi: 10.1111/j.1365-2516.2011.02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warrier I, Ewenstein BM, Koerper MA, Shapiro A, Key N, Dimichele D, Miller RT, Pasi J, Rivard GE, Sommer SS, Katz J, Bergmann F, Ljung R, Petrini P, Lusher JM. Factor IX inhibitors and anaphylaxis in hemophilia B. J Pediatr Hematol Oncol. 1997 Jan;19:23–7. doi: 10.1097/00043426-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Dioun AF, Ewenstein BM, Geha RS, Schneider LC. IgE-mediated allergy and desensitization to factor IX in hemophilia B. J Allergy Clin Immunol. 1998 Jul;102:113–7. doi: 10.1016/s0091-6749(98)70061-2. [DOI] [PubMed] [Google Scholar]
- 7.Thorland EC, Drost JB, Lusher JM, Warrier I, Shapiro A, Koerper MA, Dimichele D, Westman J, Key NS, Sommer SS. Anaphylactic response to factor IX replacement therapy in haemophilia B patients: complete gene deletions confer the highest risk. Haemophilia. 1999 Mar;5:101–5. [PubMed] [Google Scholar]
- 8.Dimichele DM, Kroner BL. The North American Immune Tolerance Registry: practices, outcomes, outcome predictors. Thromb Haemost. 2002 Jan;87:52–7. [PubMed] [Google Scholar]
- 9.Chitlur M, Warrier I, Rajpurkar M, Lusher JM. Inhibitors in factor IX deficiency a report of the ISTH-SSC international FIX inhibitor registry (1997–2006) Haemophilia. 2009 Sep;15:1027–31. doi: 10.1111/j.1365-2516.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- 10.Ewenstein BM, Takemoto C, Warrier I, Lusher J, Saidi P, Eisele J, Ettinger LJ, Dimichele D. Nephrotic syndrome as a complication of immune tolerance in hemophilia B. Blood. 1997 Feb 1;89:1115–6. [PubMed] [Google Scholar]
- 11.Kasper CK, Aledort L, Aronson D, Counts R, Edson JR, van EJ, Fratantoni J, Green D, Hampton J, Hilgartner M, Levine P, Lazerson J, McMillan C, Penner J, Shapiro S, Shulman NR. Proceedings: A more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975 Nov 15;34:612. [PubMed] [Google Scholar]
- 12.Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost. 1995 Feb;73:247–51. [PubMed] [Google Scholar]
- 13.Miller CH, Platt SJ, Rice AS, Kelly F, Soucie JM. Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance. J Thromb Haemost. 2012 Jun;10:1055–61. doi: 10.1111/j.1538-7836.2012.04705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pike IM, Yount WJ, Puritz EM, Roberts HR. Immunochemical characterization of a monoclonal G4,lambda human antibody to factor IX. Blood. 1972 Jul;40:1–10. [PubMed] [Google Scholar]
- 15.Reisner HM, Roberts HR, Krumholz S, Yount WJ. Immunochemical characterization of a polyclonal human antibody to factor IX. Blood. 1977 Jul;50:11–9. [PubMed] [Google Scholar]
- 16.Iizuka A, Nagao T. Analysis of IgG heavy chain subclasses of alloantibodies to factor IX by crossed immunoelectrophoresis of factor IX using the intermediate gel technique. Br J Haematol. 1983 Apr;53:687–8. doi: 10.1111/j.1365-2141.1983.tb07323.x. [DOI] [PubMed] [Google Scholar]
- 17.Giddings JC, Bloom AL, Kelly MA, Spratt HC. Human factor IX inhibitors: immunochemical characteristics and treatment with activated concentrate. Clin Lab Haematol. 1983;5:165–75. doi: 10.1111/j.1365-2257.1983.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 18.Orstavik KH, Miller CH. IgG subclass identification of inhibitors to factor IX in haemophilia B patients. Br J Haematol. 1988 Apr;68:451–4. doi: 10.1111/j.1365-2141.1988.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 19.Sawamoto Y, Shima M, Yamamoto M, Kamisue S, Nakai H, Tanaka I, Hayashi K, Giddings JC, Yoshioka A. Measurement of anti-factor IX IgG subclasses in haemophilia B patients who developed inhibitors with episodes of allergic reactions to factor IX concentrates. Thromb Res. 1996 Aug 15;83:279–86. doi: 10.1016/0049-3848(96)00136-3. [DOI] [PubMed] [Google Scholar]
- 20.Christophe OD, Lenting PJ, Cherel G, Boon-Spijker M, Lavergne JM, Boertjes R, Briquel ME, de Goede-Bolder A, Goudemand J, Gaillard S, d’Oiron R, Meyer D, Mertens K. Functional mapping of anti-factor IX inhibitors developed in patients with severe hemophilia B. Blood. 2001 Sep 1;98:1416–23. doi: 10.1182/blood.v98.5.1416. [DOI] [PubMed] [Google Scholar]
- 21.Soucie JM, Miller CH, Kelly FM, Payne AB, Creary M, Bockenstedt PL, Kempton CL, Manco-Johnson MJ, Neff AT. A study of prospective surveillance for inhibitors among persons with haemophilia in the United States. Haemophilia. 2014 Mar;20:230–7. doi: 10.1111/hae.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boylan B, Rice AS, Dunn AL, Tarantino MD, Brettler DB, Barrett JC, Miller CH. Characterization of the anti-factor VIII immunoglobulin profile in patients with hemophilia A by use of a fluorescence-based immunoassay. J Thromb Haemost. 2015 Jan;13:47–53. doi: 10.1111/jth.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller CH, Rice AS, Boylan B, Shapiro AD, Lentz SR, Wicklund BM, Kelly FM, Soucie JM. Comparison of clot-based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the US Hemophilia Inhibitor Research Study. J Thromb Haemost. 2013 Jul;11:1300–9. doi: 10.1111/jth.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whelan SF, Hofbauer CJ, Horling FM, Allacher P, Wolfsegger MJ, Oldenburg J, Male C, Windyga J, Tiede A, Schwarz HP, Scheiflinger F, Reipert BM. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013 Feb 7;121:1039–48. doi: 10.1182/blood-2012-07-444877. [DOI] [PubMed] [Google Scholar]
- 25.Hofbauer CJ, Whelan SF, Hirschler M, Allacher P, Horling FM, Lawo JP, Oldenburg J, Tiede A, Male C, Windyga J, Greinacher A, Knobl PN, Schrenk G, Koehn J, Scheiflinger F, Reipert BM. Affinity of FVIII-specific antibodies reveals major differences between neutralizing and nonneutralizing antibodies in humans. Blood. 2015 Feb 12;125:1180–8. doi: 10.1182/blood-2014-09-598268. [DOI] [PubMed] [Google Scholar]
- 26.Lusher JM. Inhibitor antibodies to factor VIII and factor IX: management. Semin Thromb Hemost. 2000;26:179–88. doi: 10.1055/s-2000-9821. [DOI] [PubMed] [Google Scholar]
- 27.Katz J. Prevalence of factor IX inhibitors among patients with haemophilia B: results of a large-scale North American survey. Haemophilia. 1996;2:28–31. doi: 10.1111/j.1365-2516.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 28.Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003 Jul;9:418–35. doi: 10.1046/j.1365-2516.2003.00780.x. [DOI] [PubMed] [Google Scholar]
- 29.Warrier I. Inhibitors in Hemophilia B. In: Lee C, Berntorp E, Hoots WK, editors. Textbook of Hemophilia. Oxford: Blackwell Publishers; 2005. pp. 97–100. [Google Scholar]
- 30.High KA. Factor IX: molecular structure, epitopes, and mutations associated with inhibitor formation. Adv Exp Med Biol. 1995;386:79–86. doi: 10.1007/978-1-4613-0331-2_6. [DOI] [PubMed] [Google Scholar]
- 31.Miller CH, Benson J, Ellingsen D, Driggers J, Payne A, Kelly FM, Soucie JM, Hooper WC. F8 and F9 mutations in US haemophilia patients: correlation with history of inhibitor and race/ethnicity. Haemophilia. 2012;18:375–82. doi: 10.1111/j.1365-2516.2011.02700.x. [DOI] [PubMed] [Google Scholar]
- 32.Flicker S, Valenta R. Renaissance of the blocking antibody concept in type I allergy. Int Arch Allergy Immunol. 2003 Sep;132:13–24. doi: 10.1159/000073260. [DOI] [PubMed] [Google Scholar]
- 33.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006 Mar;116:833–41. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009 Oct;124:639–46. doi: 10.1016/j.jaci.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007 Sep;120:506–15. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 36.Khodoun MV, Strait R, Armstrong L, Yanase N, Finkelman FD. Identification of markers that distinguish IgE- from IgG-mediated anaphylaxis. Proc Natl Acad Sci U S A. 2011 Jul 26;108:12413–8. doi: 10.1073/pnas.1105695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van RN, Shimizu T, Daeron M, Bruhns P. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011 Apr;121:1484–96. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller CH, Orstavik KH, Hilgartner MW. Characterization of an occult inhibitor to factor IX in a haemophilia B patient. Br J Haematol. 1985 Oct;61:329–38. doi: 10.1111/j.1365-2141.1985.tb02833.x. [DOI] [PubMed] [Google Scholar]