Abstract
Long-chain fatty acid oxidation disorders (LC-FAOD) can cause cardiac hypertrophy and cardiomyopathy, often presenting in infancy, typically leading to death or heart transplant despite ongoing treatment. Previous data on triheptanoin treatment of cardiomyopathy in LC-FAOD suggested a clinical benefit on heart function during acute failure. An additional series of LC-FAOD patients with critical emergencies associated with cardiomyopathy were treated with triheptanoin under emergency treatment or compassionate use protocols. Case reports from 10 patients (8 infants) with moderate or severe cardiomyopathy associated with LC-FAOD are summarized. The majority of these patients were detected by newborn screening, with follow up confirmatory testing, including mutation analysis; all patients were managed with standard treatment, including medium chain triglyceride (MCT) oil. While on this regimen, they presented with acute heart failure requiring hospitalization and cardiac support (ventilation, ECMO, vasopressors) and, in some cases, resuscitation. The patients discontinued MCT oil and began treatment with triheptanoin, an investigational drug. Triheptanoin is expected to provide anaplerotic metabolites, to replace deficient TCA cycle intermediates and improve effective energy metabolism. Cardiac function was measured by echocardiography and ejection fraction (EF) was assessed. EF was moderately to severely impaired prior to triheptanoin treatment, ranging from 12–45%. Improvements in EF began between 2 and 21 days following initiation of triheptanoin, and peaked at 33–71%, with 9 of 10 patients achieving EF in the normal range. Continued treatment was associated with longer-term stabilization of clinical signs of cardiomyopathy. The most common adverse event observed was gastrointestinal distress. Of the 10 patients, 7 have continued on treatment, 1 elected to discontinue due to tolerability issues, and 2 patients died from other causes. Two of the case histories illustrate that cardiomyopathy may also develop later in childhood and/or persist into adulthood. Overall, the presented cases suggest a therapeutic effect of triheptanoin in the management of acute cardiomyopathy associated with LC-FAOD.
Keywords: cardiomyopathy, metabolic disorders, fatty acid oxidation disorders, triheptanoin
INTRODUCTION
Long-chain fatty acid oxidation disorders (LC-FAODs) represent a group of autosomal recessive inborn errors of metabolism with an estimated prevalence of ~1:17,000 in the US, based on newborn screening data. LC-FAOD are caused by defects in nuclear genes that encode six mitochondrial enzymes involved in the oxidation of long chain fats for energy during times of fasting and physiologic stress. The genes and their associated diseases include carnitine palmitoyl transferase 1 (CPT-I), carnitine palmitoyl transferase 2 (CPT-II), carnitine/acylcarnitine translocase (CACT), very long-chain acyl-CoA dehydrogenase (VLCAD), long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD), and mitochondrial trifunctional protein (TFP) deficiencies. As a result of the enzymatic block, partial or incomplete oxidation of fatty acids occurs, leading to accumulation of high concentrations of potentially toxic fatty acid intermediates and an energy deficit state in many organ systems. Management of LC-FAODs includes diligent avoidance of fasting combined with the use of low fat/high carbohydrate diets, carnitine supplementation in some cases, medium chain triglyceride (MCT) oil supplementation [1, 2]. A comprehensive clinical survey over 30 years of experience and 187 cases at one center suggests LC-FAODs as a group have a mortality rate of 50% or higher when diagnosed symptomatically, despite increased awareness of the disorders and management over the last 2 decades [3]. Newborn screening and early treatment have reduced mortality, but carefully followed cohorts indicate major medical events continue to occur despite newborn screening diagnosis and management [1, 2].
Patients with LC-FAOD present at any age with acute crises of energy metabolism and severe energy deficiency, even with treatment compliance rates of >80% [1, 3]. The main presentations are characterized by involvement of the liver, skeletal muscle, or heart associated with hypoglycemia/liver dysfunction early in life, muscle weakness/rhabdomyolysis later in life, and episodic cardiomyopathy with or without arrhythmias at any age. The pattern and severity of organ involvement are generally not predictable based on the inherited defect [4–8] [9].
Cardiomyopathy in LC-FAOD
LC-FAODs display a varying degree of cardiac manifestation that likely reflects the heart’s particular reliance on fatty acid oxidation for up to 90% of its energy. Studies in mouse models support the importance of fatty acid oxidation to cardiac function; VLCAD-deficient mice develop progressive cardiac dysfunction even without the trigger of catabolic situations [10]. Cardiomyopathy secondary to LC-FAOD is more likely to present in the first year of life than in older pediatric ages [11, 12] and can present as hypertrophic or dilated cardiomyopathy (DCM); arrhythmias are also common. In a study of 18 symptomatic VLCAD patients prior to newborn screening, infantile CM was the most common presenting phenotype [8]. In general, hypertrophic cardiomyopathy carries a higher risk of death and the rate of heart transplantation is greater for those who present as infants or with inborn errors of metabolism or with mixed hypertrophic and dilated or restrictive cardiomyopathy [13]. DCM is the most common form of cardiomyopathy in LC-FAOD [14], and is characterized by dilatation and impaired systolic contractile function. Patients with DCM may be asymptomatic or demonstrate physical examination findings of congestive heart failure depending on the degree of ventricular dysfunction. A 2-dimensional echocardiogram (2D ECHO) is the primary imaging modality to establish cardiac anatomy and function. Treatment may include diuretics, angiotensin-converting enzyme (ACE) inhibition, and beta-blockers; if these fail, treatment may be escalated to intravenous inotropic therapy, respiratory ventilation, and mechanical cardiac support, including extracorporeal membrane oxygenation (ECMO) and ventricular assist devices (VAD). Patients with refractory symptomatic heart failure are candidates for transplant.
Rationale for Triheptanoin in the Treatment of Cardiomyopathy Associated with LC-FAOD
Traditional care for LC-FAOD includes supplementation with MCT oil, containing predominantly eight and ten carbon triglycerides. Since the long-chain fatty acid oxidation enzymes are not required to metabolize MCT, they can bypass the primary metabolic defect providing acetyl-CoA for the Krebs cycle. However, the Krebs cycle requires both even and odd chain intermediates to function, and thus may become secondarily impaired with this therapy, as observed directly in the heart of a murine FAOD model during heart failure [15]. The energy deficiency is accentuated by the need for aggressive gluconeogenesis with severe hypoglycemia and depletion of glycogen, which has the potential to further drain TCA cycle intermediates to feed the gluconeogenesis pathway.
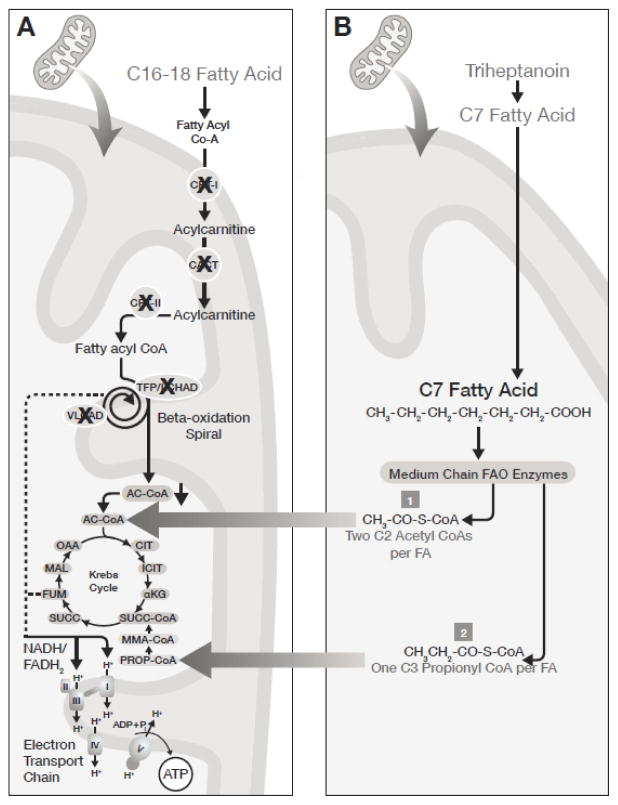
Triheptanoin is an investigational drug comprised of a highly purified, pharmaceutical-grade, synthetic medium odd chain (C7) triglyceride. It has also previously been used in a less purified food grade [15]. It is initially catabolized in the gut to free heptanoate that can diffuse across membranes to enter cells. Heptanoyl-CoA is then metabolized by medium chain fatty acid oxidation enzymes to acetyl- and propionyl-CoA, as well as 4- and 5-carbon ketone bodies (Figure 1). Propionyl-CoA is an anaplerotic molecule that restores the balance of the Krebs cycle intermediates pool via conversion to succinyl-CoA [15]. The restoration of Krebs cycle function improves flow of electrons to the mitochondrial respiratory chain recovering ATP production [16].
Figure 1. Proposed MOA of Triheptanoin in LC-FAOD.
Dual mechanism of energy substrate replacement following triheptanoin metabolism. Triheptanoin provides both acetyl-CoA and propionyl Co-A for anaplerosis, bypassing LC-FAOD blocks in fatty acid metabolism and preserving Krebs cycle function.
The potential for triheptanoin to provide both acetyl-CoA and anaplerotic substrates for the Krebs cycle as well as the known reliance of the heart on fatty acid oxidation for energy, provides a biochemical rationale to investigate treatment in pediatric cardiomyopathy including patients whose symptoms have progressed despite treatment with MCT. The first patient treated with triheptanoin, a young girl with VLCAD deficiency and chronic and recurrent cardiomyopathy (case 8 in this report), showed sustained cardiac improvement after transitioning to triheptanoin [17]. In a retrospective study, medical records from 14 patients ranging from 1.5–35 years of age with a history of cardiomyopathy who were treated with triheptanoin on a compassionate or emergency use basis were reviewed [5]. Following triheptanoin treatment, all improved or had stable ECHO parameters except one patient who required subsequent heart transplant. This manuscript reviews 10 cases (including the previously reported case 8) that further support the efficacy of triheptanoin for the treatment of severe cardiomyopathy due to LC-FAOD.
MATERIALS AND METHODS
Methodology
The reported case histories are from all patients treated with investigational triheptanoin for whom clinical data were provided by the treating physicians as of September 2015.The series includes patients with VLCAD (n=4), CACT (n=2), TFP (n=2) and LCHAD (n=2). Case 8 was previously reported after the original initiation of triheptanoin (at age 6 years)[17], and with case 10 in a retrospective chart review study [5]. The current case reports provide additional information on these patients.
Informed consent was obtained from the parent or legally authorized guardian prior to administration of triheptanoin. Triheptanoin was provided on a compassionate use basis upon request by the treating physicians, approval of the local institutional review board, and an emergency Investigational New Drug approval by the US Food and Drug Administration or enrollment in a compassionate use study. Of the 10 patients with cardiomyopathy presenting in childhood, 6 were treated under emergency INDs (US FDA) and 4 were enrolled in a compassionate use clinical program (JV).
Assessments such as ECHO were performed at multiple institutions with variable protocols according to local standards.
Treatment regimen(s)
Patients were provided with triheptanoin as a pharmaceutical-grade investigational product supplied by Ultragenyx Pharmaceutical, Inc. (Novato, CA USA), supplied as a clear and colorless to light yellow oil intended for oral administration. It is a purified, synthetic triglyceride compound manufactured by chemical synthesis from glycerol and heptanoic acid, and manufactured in accordance with Current Good Manufacturing Practice regulations, applicable International Conference on Harmonisation guidelines, and regional regulations. Historically, patients 8 and 10 were treated initially with a food-grade oil (Sasol, Germany) at the time of presentation, but were switched to pharmaceutical grade investigational product when it became available.
The dose and regimen with triheptanoin were defined based on information derived from over 13 years of clinical experience in LC-FAOD with food-grade triheptanoin [17]. These data showed an age-dependent dose related to the relatively higher energy requirements for young children versus older children and adults. The target dose was 25–35% of total calories, as tolerated, which is equivalent to approximately 2–4 g/kg in infants and young children, decreasing to 1–2 g/kg for older children and adolescents, and 1 g/kg for adults. The dose level in the cases presented ranged from 1 to 4 g/kg/day based on patient age (correlating to inherent energy requirement) and tolerability.
Individual Clinical Summaries
Case 1: VLCAD Deficiency
A female patient presented with neonatal hypoglycemia (blood glucose level of 5 mg/dL; 0.3 mmol/L) and metabolic acidosis that improved with intravenous dextrose. Newborn screening results were positive for VLCAD deficiency, later confirmed by mutation analysis. The patient was managed with MCT enriched formula. Biventricular hypertrophy was observed at age 3 weeks. At age 3 months, the patient was noted to have moderate left ventricular hypertrophy (LVH); at 7 months, she was hospitalized with severely depressed ventricular function (EF data not available), cardiogenic shock and pericardial effusion, treated surgically with a pericardial window procedure. The patient was started on triheptanoin and her condition improved rapidly. Three weeks after starting triheptanoin, echocardiography showed an EF of 63% with only mild LVH and a subsequent assessment, 3 months after starting triheptanoin, was normal. At the time of this report, the patient continues on triheptanoin treatment (4 years), cardiomyopathy has resolved, and cardiac function remains normal. No significant tolerance issues have been reported.
Case 2: VLCAD Deficiency
A male patient presented in the newborn period with hypoglycemia and cardiac dysfunction, which gradually improved on MCT-containing formula. The newborn screening result was positive for VLCAD deficiency. At age 6 months he presented with acute viral symptoms (adenovirus and rhinovirus positive) and suffered cardiac arrest requiring approximately 45 minutes of resuscitation. The patient was noted to have severe DCM and was placed on ECMO for 20 days. MCT was given only intermittently because of concern for gastrointestinal absorption. On Day 18 of the hospitalization, the patient was started on triheptanoin (EF 39%) with the dose ranging from 2.6–3.3 g/kg/day, and was weaned from ECMO 2 days later. Eight weeks after starting triheptanoin the patient was discharged. He had two further admissions for viral infections, but his cardiac status remained stable. A 2D ECHO report showed mild left ventricle dysfunction and mild-moderate cardiomyopathy. Two weeks later, triheptanoin was discontinued at the request of the parents because of ongoing gastrointestinal symptoms. A follow up 2D ECHO 1 month later showed an EF of 69%.
Case 3: TFP Deficiency
A female patient presented in the neonatal period with hypoglycemia (37 mg/dL, 2.1 mmol/L) that responded to treatment with high dextrose infusion and MCT formula. Newborn screening was positive and the diagnosis of TFP deficiency was confirmed. On routine follow up at age 8 months of age, 2D ECHO evaluation revealed LVH and an EF of 44%. The patient was started on 1.5 g/kg/d of triheptanoin at 9 months of age. At follow up 3 weeks later, the EF was 52%. At age 18 months the patient had normal motor function, speech and language development and her most recent echocardiography showed normal ventricular function. She continues on a triheptanoin (3 years) at a dose of 2g/kg/d with no significant tolerance issues reported.
Case 4: LCHAD Deficiency
A female patient was detected on newborn screening, confirmed to have LCHAD and treated with MCT containing formula. She presented at age 10 months with severe cardiomyopathy and heart failure; 2D ECHO indicated an EF of 27%. Soon afterwards, the patient developed bradyarrhythmia and cardiac arrest. Following resuscitation, the patient was started on ECMO that continued for 19 days. Additional treatment included nasogastric tube MCT with intermittent interruptions due to poor absorption, parenteral nutrition with 30% dextrose, an investigational intravenous MCT 10%/LCT 10% emulsion (Lipofundin, LB. Braun Medical Supplies, Inc.), and cardiac pressor drugs. Due to lack of improvement in cardiac status (2D ECHO EF of 22%), the patient was started on triheptanoin (4g/kg/day) on day 16 of hospitalization. Three days later the patient was weaned from ECMO and showed gradual improvement in cardiac function, with an EF of 33% at discharge. She continues on triheptanoin treatment, now for 21 months. There have been no further metabolic decompensations, and the most recent EF was 55%. There have been no adverse drug symptoms.
Case 5: CACT Deficiency
A female patient presented at 23 hours of life, with severe hypoglycemia, hypothermia, hyperammonemia, increased CK, and 2D ECHO evidence of right ventricular hypertrophy. Management consisted of standard treatment, including high dextrose infusion and MCT formula. Newborn screening ultimately was positive and mutation analysis confirmed CACT deficiency. At age 6 months, the patient had an acute decompensation with severe cardiomyopathy, managed with ECMO and MCT supplements from which she gradually recovered. At age 10 months, she again presented with severe cardiomyopathy, with heart failure and ascites, concurrent with acute viral gastroenteritis and dehydration. She developed cardio-respiratory arrest and was managed with cardiac pressors, high concentration intravenous dextrose, and G-tube infusion of MCT oil. ECMO was unable to be initiated due to inadequate vascular access. The patient was started on triheptanoin at 4g/kg/day. Pre-treatment EF was 21% and within 3 days improved to 67%. Three weeks later, the patient was successfully discharged from the hospital. She has remained stable in spite of two intercurrent illnesses, and at the time of this report (17 months), treatment with triheptanoin continues at a dose of 4 g/kg/d without significant intolerance.
Case 6: TFP Deficiency
A female patient, born at 35 weeks gestation with intrauterine growth restriction, presented with hypoglycemia (29mg/dL, 1.6 mmol/L) and elevated CK (2223 U/L, normal < 145 U/L) soon after birth. A 2D ECHO at 6 days of age showed mild biventricular dysfunction. An abnormal newborn screen suggested a diagnosis of LCHAD or TFP; follow up mutational analysis confirmed TFP deficiency likely predictive of a severe phenotype [18]. She was managed with MCT-containing formula. Other complications included severe neonatal neutropenia and intermittent hyperkalemia of unknown etiology. At 6 weeks, she developed heart failure with a decreased EF (45%). At 10 weeks of age, triheptanoin was started at a dose of 4g/kg/d. Due to worsening cardiac function the dose was increased to 5g/kg/d after 10 days. The patient developed pericardial effusions heralding a downward spiral. The patient’s condition rapidly deteriorated, with refractory cardiogenic shock and pulmonary hemorrhage that made all resuscitation efforts unsuccessful.
Case 7: VLCAD Deficiency
An abnormal newborn screen in this female led to a confirmed diagnosis of VLCAD deficiency, confirmed on plasma acylcarnitine profile. She was started on MCT-containing formula without supplementation with levocarnitine or additional MCT oil. A 2D ECHO performed at 3 weeks of age showed normal left ventricular systolic function (EF 65%) with neither ventricular hypertrophy nor dilatation. At 3 months of age, she presented with 24 hours of poor feeding, constipation, and vomiting, progressing to lethargy, loss of muscle tone, and respiratory distress. She was hypotensive, mildly hypoglycemic (51 mg/dL), with markedly elevated B-type natriuretic peptide (17,067 pg/mL, normal < 100 pg/mL), depressed EF of 35%, and hepatomegaly and extremely low free carnitine levels (2.2 umol/L, reference range 21–53). She was placed on high dextrose infusion and insulin, multiple pressors, enteral MCT (2 g/kg/day), and intravenous levocarnitine (100 mg/kg/day). EF decreased to 17%, with moderate left ventricular dilatation and increased left ventricle mass index (150 g/m2; normal < 68 g/m2). Within 12 hours of admission the patient had a bradycardia and cardiac arrest and was successfully resuscitated. She then experienced ventricular fibrillation and cardiac arrest, and was placed on ECMO emergently and taken to the cardiac catheterization lab for a balloon atrial septostomy. She required several defibrillations for persistent ventricular fibrillation. MCT oil was increased to 4 g/kg/day. Because cardiac function continued to deteriorate, she was switched to triheptanoin at 2 g/kg/day. The dose was escalated to 4 g/kg/day, then to 6 g/kg/day, in conjunction with IV nutrition. Her cardiac EF improved, reaching 42.9% and ECMO was discontinued ten days after her acute presentation. EF eventually normalized at 52% with only mild left ventricle dilatation on post-triheptanoin. She ultimately died of septic shock, necrotizing fasciitis, and multi-system failure, sequelae of her prolonged resuscitation.
Case 8: VLCAD Deficiency
This female infant (born prior to newborn screening) presented on day of life 3 with hypoglycemia and hypothermia, responsive to IV fluids [17]. She subsequently had poor feeding and poor weight gain. At 3.5 months of age, she developed acute cardiomyopathy with biventricular hypertrophy, pericardial effusion and respiratory failure. She was treated with MCT-containing formula, via gastrostomy tube, with a good response; however, she had recurrent admissions for cardiac decompensation, hepatomegaly, rhabdomyolysis and muscle weakness. At age 5, because of chronic cardiomyopathy, hepatomegaly (8 cm) below right costal margin, and muscle weakness, she was enrolled in an investigational study of triheptanoin. Within 2 weeks, her cardiac function was normal, hepatomegaly resolved and muscle weakness improved [17]. She continued on dietary triheptanoin (3.5–4.0 g/kg/d) in a compassionate use program without further metabolic decompensation, and had normal cardiac function until about age 18, when she discontinued the medication [17]. Approximately 2 years later, she developed acute metabolic decompensation with severely depressed ventricular function, cardiogenic shock, and subsequent cardiac arrest. EF prior to arrest was 20–25%). She was managed with ECMO and cardiac pressors. She restarted triheptanoin and ECMO; pressor drugs were discontinued after 8 days, with essentially normal cardiac function prior to discharge from the hospital (EF 57%). She is currently stable, on triheptanoin for 10 months.
Case 9: LCHAD Deficiency
LCHAD deficiency was diagnosed in this male patient on newborn screening following a prenatal history of maternal hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome and delivery at 31 weeks by caesarian section due to placental abruption. Preterm infant formula containing MCT was started at birth. There was no history of hypoglycemia or cardiomyopathy. He remained well, with 1–2 episodes/year of rhabdomyolysis until age 8 when he had several closely spaced admissions for severe rhabdomyolysis CK up to 50,000 U/L, (normal < 178 U/L) and persistent CK elevation between episodes. He was subsequently referred for enrollment in an expanded access program with triheptanoin. On the day of admission, he had complaints of abdominal pain and vomiting (previously noted with other rhabdomyolysis events). Lab results showed a CK of 1500 U/L. A baseline 2D ECHO showed an EF of 47%. He was managed with intravenous hydration. The following day, he had bradyarrhythmia and cardiac arrest. An acute 2D ECHO showed an EF of 12 %. He was started on triheptanoin, at a dose of 1.25 g/kg/d, titrated to 2.5 g/kg/d by 1 week of treatment, and managed with cardiac pressors and ECMO for 10 days. He was then successfully transitioned to a left ventricular assist device and was able to be successfully decannulated 28 days later. Cardiac function gradually improved and all pressor and mechanical supports were discontinued. Eight weeks after starting triheptanoin, his EF was 45%. He has continued on triheptanoin (1.4 g/kg/d) and has remained well for 10 months. His most recent EF was 62%.
Case 10: CACT Deficiency
This male patient was born at term by repeat caesarian-section prior to initiation of expanded newborn screening, following an uneventful pregnancy; he was initially observed for possible amniotic fluid aspiration. At 8 days of age, he developed hypothermia and altered level of consciousness, and underwent an evaluation for sepsis; hyperammonemia (> 1,000 mg/dL, normal < 170 mg/dL) was noted, and treated with intravenous nitrogen scavenger medication. He was also noted to have hepatomegaly and lactic acidosis. A chest x-ray showed cardiomegaly, and 2D ECHO on day of life 11 showed mild LVH and decreased left ventricle function with an EF of 41%. He was subsequently diagnosed with CACT deficiency and was started on an MCT-containing formula with additional MCT supplementation. Because of increasing cardiomyopathy and hepatomegaly, the MCT dose was increased to 4 g/kg/d. At 6 months of age he developed cardiac decompensation (no reports of acute EFs were available, one previous was 39%) and he was started on triheptanoin. At age 18 months, his triheptanoin dose was 4.8 g/kg/d. A 2D ECHO showed mild left ventricle dilatation and moderate hypertrophy with an EF of 67%. He has continued on triheptanoin, and his most recent 2D ECHO at age 5 ½ years, showed normal ventricular morphology and function. He has remained stable on triheptanoin treatment now for 6 years.
RESULTS
Baseline Characteristics
A LC-FAOD was detected via newborn screening in all patients except case 8, in whom VLCAD deficiency diagnosed at age 3.5 months of age, and case 10 in whom CACT deficiency was diagnosed during the first month of life. Both of these patients were born before the initiation of expanded newborn screening for FAODs in their respective US states. Diagnosis was confirmed by mutation analysis in all patients (Table 1). All patients were managed with standard treatment, including prior treatment with MCT or MCT-containing formula. The majority of patients (7/10) presented with clinical sequelae during the neonatal period, with neonatal hypoglycemia being the most common. Most of the patients did not present with cardiomyopathy, though cardiac dysfunction was incidentally noted in several cases. In nine of the ten cases, cardiomyopathy was diagnosed within the first year of life (Table 1). One female VLCAD deficiency patient (case 8) initially presented with acute cardiomyopathy with biventricular hypertrophy at 3.5 months of age and was successfully managed with triheptanoin continuously through childhood from age 6 years until age 18 years with normal cardiac function [17]. Following voluntary termination of triheptanoin treatment in early adulthood due to the lack of perceived need, the patient presented at age 20 years with acute cardiomyopathy, cardiogenic shock, and cardiac arrest. One male LCHAD deficiency patient (case 9) had no prior history of hypoglycemia or cardiomyopathy prior to age 8 years.
Table 1.
Baseline Characteristics of LC-FAOD Patients
| Case | Gender | LC-FAOD Diagnosis |
Genotype | Age at Diagnosis |
Initial Presentation (Age/Symptoms) |
Cardiomyopathy Presentation (Age/Symptoms) |
Age at Trihep Start |
Prior MCT Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | F | VLCAD | c.1678+3_1678+ 6 del AAGT |
NBS | Neonatal hypoglycemia, metabolic acidosis | 3 months; cardiac dysfunction | 7 months | Y |
| 2 | M | VLCAD | Homozygous c.1807dupT (p.C603LfsX2) |
NBS | Neonatal hypoglycemia, cardiac dysfunction | 6 months; cardiac arrest, severe dilated CM | 6 months | Y |
| 3 | F | TFP | c.1165A>G c.1289T>C |
NBS | Neonatal hypoglycemia | 8 months; significant left ventricular hypertrophy | 8 months | Y |
| 4 | F | LCHAD | 1528G>C 1528G>C |
NBS | 10 months; severe CM, heart failure | 10 months; severe CM, heart failure | 10 months | Y |
| 5 | F | CACT | c.84delT del+3p21.31 |
NBS | Neonatal hypoglycemia, cardiac dysfunction | 10 months; severe CM, heart failure, ascites | 10 months | Y |
| 6 | F | TFP | Homozygous c.1678C>T |
NBS | Neonatal hypoglycemia, mild biventricular dysfunction | 1.5 months; heart failure | 2.5 months | Y |
| 7 | F | VLCAD | c.1268C>T (p.S423L)/c.1913C>T (p.S638F) | NBS | 3 months; hypotensive, hypoglycemic, respiratory distress, hepatomegaly | 3 months; cardiac failure | 3 months | Y |
| 8 | F | VLCAD | c.887_888del c.1679-6G>A |
3.5 months | Neonatal hypoglycemia, hypothermia 3.5 months; acute CM w/biventricular hypertrophy, pericardial effusion, respiratory failure | 3.5 months; acute CM with biventricular hypertrophy1 | 5 years | Y |
| 20 years; acute CM, cardiogenic shock, cardiac arrest | 20 years | |||||||
| 9 | M | LCHAD | homozygous 1528 G>C |
NBS | Prior to age 8; rhabdomyolysis | 8 years; bradyarrhythmia, cardiac arrest | 8 years | Y |
| 10 | M | CACT | c.823C>T (p.R275X)/del E 5-9 | < 1 month | Hypothermia, altered consciousness, hyperammonemia | Day 11; cardiomegaly, mild LVH, decreased LV function | 6 months | Y |
Previously described in Roe et al. 2002.
CM = cardiomyopathy; LVH = left ventricular hypertrophy; MCT = medium chain triglyceride oil; NBS = newborn screening
Triheptanoin Treatment, Clinical Characteristics and Supportive Care
Patients (parents) consented to receive triheptanoin therapy under an expanded access (compassionate use) program or under emergency IND. Therapy with MCT oil was discontinued and treatment with triheptanoin (UX007) was initiated while patients were acutely ill. The majority of patients began treatment with triheptanoin as infants (aged 2.5 months–10 months; Table 1), except for case 8 (described above), and case 9 who initiated treatment at approximately 8 years of age.
The infants in this report were hospitalized with cardiac arrest, arrhythmia, pericardial effusion or heart failure requiring cardiac support. Decompensation due to secondary acute infection was reported in 4 of 10 cases (Table 2). The majority of patients received mechanical support (ventilation/ECMO) and/or vasopressors and, in some cases, resuscitation. The patients often presented with concurrent hepatic and musculoskeletal involvement. These common features of LC-FAOD included neonatal hypoglycemia, rhabdomyolysis, hepatic dysfunction, and metabolic/lactic acidosis (Table 2).
Table 2.
Clinical Characteristics and Interventions
| CASE ID | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Incidence (%) | |
| LC-FAOD | VLCAD | VLCAD | TFP | LCHAD | CACT | TFP | VLCAD | VLCAD | LCHAD | CACT | -- |
| Cardiac Dysfunction | |||||||||||
| Cardiomyopathy/LV dysfunction | X | X | X | X | X | X | X | X | X | X | 100% |
| Arrest | X | X | X | X | X | X | 60% | ||||
| Arrhythmia | X | X | X | X | 40% | ||||||
| Decompensation due to secondary acute infection | X | X | X | X | 40% | ||||||
| Heart failure | X | X | X | X | 40% | ||||||
| Pericardial effusion | X | X | X | 30% | |||||||
| Cardiogenic shock | X | X | 20% | ||||||||
| Cardiac Interventions | |||||||||||
| Mechanical Support (ECMO/Ventilation) | X | X | X | X | X | X | 60% | ||||
| Pressors | X | X | X | X | X | X | 60% | ||||
| Ventricular assist devices | X | 10% | |||||||||
| Pericardiocentesis | X | 10% | |||||||||
| Other (≥ 3 cases) | |||||||||||
| Neonatal hypoglycemia | X | X | X | X | X | X | 60% | ||||
| Rhabdomyolysis/Elevated CK | X | X | X | X | X | X | 60% | ||||
| Hepatic Dysfunction | X | X | X | X | 40% | ||||||
| Metabolic/lactic acidosis | X | X | X | X | 40% | ||||||
| Respiratory Complications | X | X | X | 30% | |||||||
| Hypothermia | X | X | X | 30% | |||||||
| Death | X | X | 20% | ||||||||
Cardiac Function and Related Biochemical Markers
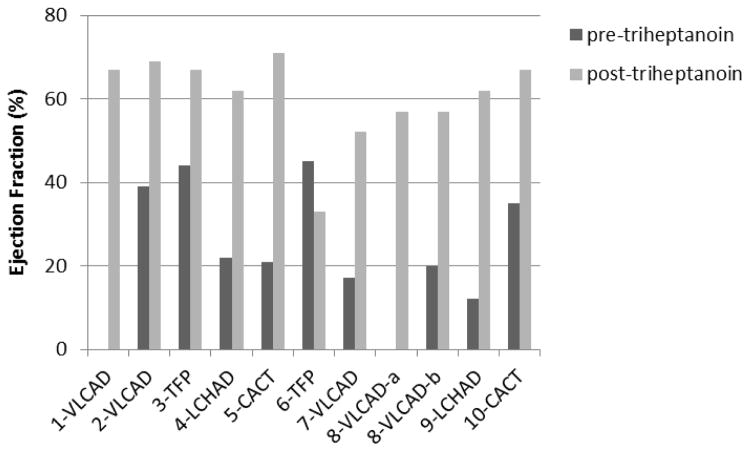
2D ECHO was performed to measure reduced EF as an indicator of systolic heart failure and deficiencies in heart muscle function. In patients with available data before and after treatment (n=9), the mean EF was 28% (range12%–45%) prior to triheptanoin treatment; half of the patients had a severely impaired baseline EF of less than 25% (Figure 2). Improvements in EF began between 2 and 21 days following initiation of triheptanoin, and peaked at mean EF of 60% (range: 33%–71%, with 8/10 patients achieving a peak EF greater than 50% (or in the normal range; Figure 2). In some cases, however, EF’s associated with acute decompensation episodes were not available. Within 2–3 days of initiating triheptanoin, some patients were weaned from ECMO (cases 2 and 4) or showed rapid improvements in EF (case 5). In others, ECMO and/or pressors could be discontinued within 8–10 days following initiation of triheptanoin (cases 7–9).
Figure 2. Peak Ejection Fraction Improvements Following Initiation of Triheptanoin.
Peak ejection fraction (EF) improvements following initiation of triheptanoin. Lines represent EF values prior to triheptanoin treatment (where available) and the peak EF achieved following initiation of triheptanoin for patients presented in Cases 1–10.
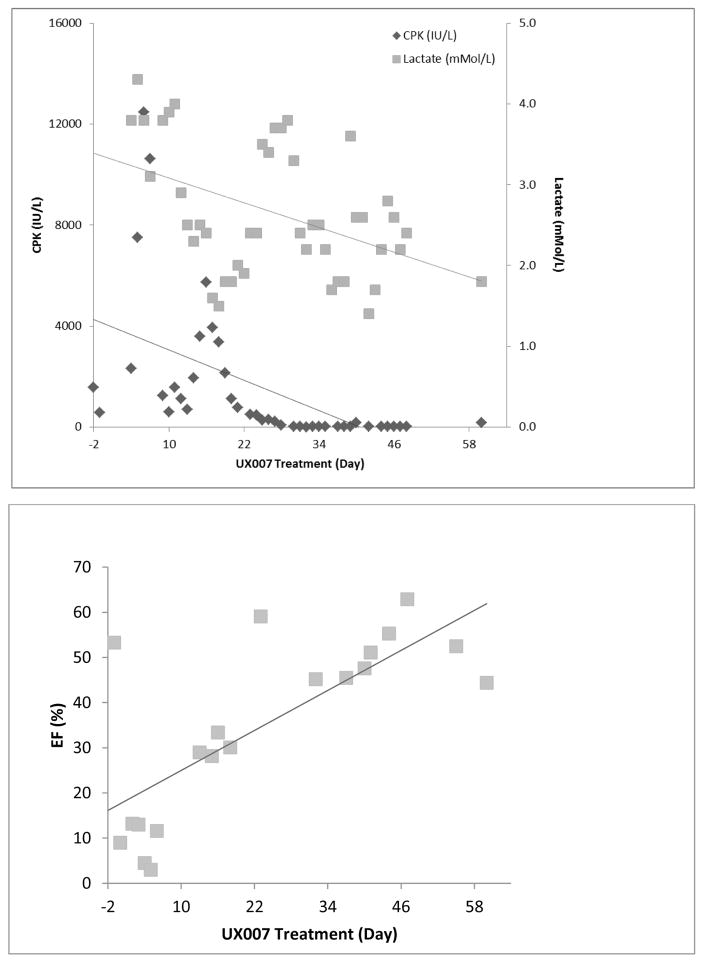
Biochemical parameters also improved coincident with increasing EF. In the 8-year old male with LCHAD (case 9), CPK and lactate were monitored nearly daily. Once the triheptanoin dose was titrated to 2.5 g/kg/d, CPK and lactate decreased along with troponin levels (data not shown), and increases in EF were observed beginning on treatment day 13 (Figure 3).
Figure 3. Changes in Serum Chemistry and ECHO Parameters following Triheptanoin Treatment in an 8 year old with LCHAD Deficiency.
Relationship between creatine phosphokinase (CPK; IU/L) and lactate (nMol/L) improvements, and increasing EF over a 60-day period prior to and following initiation of triheptanoin in Case 9. Lines represent linear regression.
Tolerability and Treatment Continuation
The most common adverse effects associated with triheptanoin treatment were gastrointestinal distress, including loose stools. The majority of these were mild and usually managed by administering triheptanoin mixed with food and divided dosing. No other significant tolerance issues or treatment-related adverse events were reported. Seven of the 10 patients continue to receive treatment with triheptanoin (up to 36 months and ongoing) and have maintained stable cardiac function despite complications from recurrent infections in some. One patient discontinued triheptanoin treatment after approximately 14 weeks due to gastrointestinal symptoms (primarily vomiting).
Two infants died from other complications. The first was a female with VLCAD deficiency (case 7) who died at approximately 3.5 months of age of sepsis/necrotizing fasciitis. A female with mitochondrial TFP deficiency (case 6) also died at approximately 3.5 months of age. She was born at 35 weeks gestation with IUGR, and presented with early hypoglycemia and mild bi-ventricular dysfunction. After 6 weeks, she developed heart failure with a decreased EF (45%), severe neonatal neutropenia, and intermittent hyperkalemia. At 10 weeks, EF declined to 37% and she was treated with triheptanoin (4–5g/kg/d). Her EF initially slightly improved to 41%. Two weeks later, she developed metabolic and lactic acidosis and respiratory distress requiring ventilation, with an EF of 33%. She developed pericardial effusions that were drained; however, re-accumulation 5 days later again required drainage. Several hours later, she developed refractory cardiogenic shock with severe pulmonary hemorrhage, and could not be resuscitated. None of these events was directly attributed to triheptanoin administration by the reporting physician, rather appearing to be related to underlying disease.
DISCUSSION
The most severe LC-FAOD phenotypes present in early infancy with severe life-threatening cardiomyopathy, arrhythmia, heart failure, hypoglycemia, hepatic dysfunction, and rhabdomyolysis [19]. Morbidity and mortality remain high despite treatment, particularly where newborn screening for LC-FAOD is not available [3]. Triheptanoin is a medium odd chain fatty acid that generates anaplerotic metabolites to increase Krebs cycle function and, ultimately, energy output. Ten patients with cardiomyopathy associated with LC-FAOD were treated with triheptanoin as part of emergency INDs or expanded access (compassionate use) programs. Treatment was associated in some cases with rapid improvements in cardiac function; continued treatment was associated with stabilization of clinical signs of cardiomyopathy, even during subsequent infections in some cases.
In the case illustrated in Figure 3, the initial peak in CPK lagged behind the acute myocardial decompensation by several days, while the troponin peak was coincident with it. While EF increased with triheptanoin treatment on ECMO, cardiac function was insufficient to sustain circulation without additional mechanical assistance, and a biventricular assist device was placed. Cardiac function improved thereafter and all mechanical assistance was discontinued after one further week. It is noteworthy that 6 of 10 patients were treated with concurrent triheptanoin and ECMO without apparent gastrointestinal difficulties. In pediatric patients with reversible causes of cardiogenic shock, ECMO has been extensively used as a bridge to recovery [20], including to bridge patients to recovery after for postcardiotomy syndrome after heart surgery, acute graft dysfunction after heart transplantation, and severe biventricular dysfunction after viral myocarditis [21–23]. Data on the use of ECMO in FAOD is scant; in this report 6 patients were successfully bridged to recovery with ECMO supporting potential myocardial recovery in these patients. Four patients in this report recovered with ECMO, consistent with other previous reports of its use in acute cardiomyopathy [17].
Assessing cardiac recovery while on ECMO is challenging and difficult to standardize since EF while on ECMO may not represent a true marker of the patient’s actual cardiac function [24]. The objective of ECMO is to support cardiac output, while the heart recovers. ECMO decompresses the heart by reducing its preload and, therefore, changes the Starling curve of the heart and makes the interpretation of EF numbers difficult. Other markers have been used to monitor recovery while on ECMO. This depends on a successful heart decompression, allowing recovery from the ischemia generated due to decreased coronary perfusion related to increased diastolic pressures secondary to the dilation. Markers of dilation such as plasma brain-type natriuretic peptide (BNP) levels have been used to monitor and predict acute decompensation in chronic heart failure and can also be used to monitor appropriate decompression while on ECMO [25]. Therefore, the use of serial plasma BNP may become an important feature not just for follow up, but also in monitoring the recovery from acute decompensation in this patient population.
Cardiac energy metabolism is significantly impaired in cardiomyopathy. The heart typically utilizes fatty acid oxidation for ~90% of energy even under fed conditions. Fatty acid oxidation provides reducing equivalents directly to the mitochondrial respiratory chain, as well as acetyl-CoA for the Krebs cycle, which in turn generates additional reducing equivalents to maintain energy homeostasis. In an otherwise normal heart, the respiratory chain is compromised in cardiac failure, leading to an increase in the NADH/NAD+ ratio and a decrease in pyruvate oxidation for gluconeogenesis [26]. This metabolic state decreases the utility of glucose as a backup energy source in the failing heart. With an accompanying LC-FAOD, utilization of stored long chain fats is also impaired. In this setting, acetyl-CoA from MCT oil can provide needed acetyl-CoA for the Krebs cycle. However, with excess reliance on this energy pathway, provision of acetyl-CoA to the exclusion of odd-chain carbon intermediates leads to depletion of the latter and secondary deficiency of the Krebs cycle. Anaplerosis refers to metabolic pathways that replenish the Krebs cycle intermediates, which are essential to energy metabolism. Animal studies have shown that depletion of the Krebs cycle (cataplerosis) may contribute to cardiac pathogenesis in LC-FAODs [27]. Since many of the factors involved in the regulation of Krebs intermediate pool sizes are known to be altered in pathophysiological states such as cardiac hypertrophy and failure, this may lead to an increased demand for anaplerotic substrates [reviewed in [28]].
The mechanism of action of triheptanoin in restoring energy metabolism in LC-FAOD is dependent on its medium chain length as well as its odd-carbon composition, providing increased availability of anaplerotic substrates, resulting in improved cardiac and skeletal muscle function. Prior reports have demonstrated the feasibility of anaplerotic interventions in LC-FAOD [5, 17]. Data from the additional patients treated with triheptanoin in this report provide additional evidence of maintained cardiac stabilization and improvement in function despite recurrent infections and secondary complications. While the majority of cardiac complications from LC-FAOD present in the first year of life, cases 8 and 9 demonstrate the cardiomyopathy may develop later in childhood, and LC-FAOD patients may still be at risk for decompensation later in life.
Two mouse models of LC-FAOD [long chain-acyl-CoA dehydrogenase (LCAD) and VLCAD] have been reported and both can develop cardiomyopathy. Metabolic studies in VLCAD deficiency mice have questioned the efficacy of treatment with MCT or triheptanoin. However, the relevance to VLCAD deficiency in humans is uncertain due to the concurrent expression of both long chain dehydrogenases in most mouse tissues including heart [29, 30].
The major concern in interpreting our experience with treatment of cardiomyopathy in LC-FAODs is that the study was neither blinded, nor placebo-controlled. However, in the life threatening situations facing clinicians treating these patients, all patients had been on MCT oil prior to the development of their cardiomyopathy, and that current therapy was failing. The success of triheptanoin in rescuing and stabilizing most patients is thus striking especially in those cases where rapid responses were observed over a few days. Overall, these results continue to identify triheptanoin as a promising treatment for LC-FAOD, and provide impetus for planned phase 3 clinical trials.
CONCLUSION
Infants with LC-FAOD and cardiomyopathy continue to suffer significant morbidity and mortality despite management with traditional treatment, including MCT oil. Substitution of MCT oil with triheptanoin, currently in clinical trials as a pharmaceutical grade medium odd-chain fatty acid, provides an alternative substrate with anaplerotic properties in these patients, restoring energy production for gluconeogenesis. The cases presented in this report demonstrate the beneficial effect of triheptanoin in the management of cardiomyopathy associated with LC-FAOD. Since this treatment is a substrate replacement therapy and not an enzyme inhibitor or other type of drug class, dosing will likely need to be individualized based on tolerability, metabolism, and energy needs. Further studies are warranted to confirm these promising findings.
Highlights.
Triheptanoin help improve cardiomyopathy in patients with cardiomyopathy already on MCT oil.
Acknowledgments
JV is funded in part by PHS grant NIH R01-DK78775. Ultragenyx Pharmaceutical, Inc. provided triheptanoin drug supply. Medical writing assistance was provided by Kimberly Denis-Mize.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spiekerkoetter U, Lindner M, Santer R, Grotzke M, Baumgartner MR, Boehles H, Das A, Haase C, Hennermann JB, Karall D, de KH, Knerr I, Koch HG, Plecko B, Roschinger W, Schwab KO, Scheible D, Wijburg FA, Zschocke J, Mayatepek E, Wendel U. Management and outcome in 75 individuals with long-chain fatty acid oxidation defects: results from a workshop. J Inherit Metab Dis. 2009;32:488–497. doi: 10.1007/s10545-009-1125-9. [DOI] [PubMed] [Google Scholar]
- 2.Lindner M, Gramer G, Haege G, Fang-Hoffmann J, Schwab KO, Tacke U, Trefz FK, Mengel E, Wendel U, Leichsenring M, Burgard P, Hoffmann GF. Efficacy and outcome of expanded newborn screening for metabolic diseases--report of 10 years from South-West Germany Orphanet. J Rare Dis. 2011;6:44. doi: 10.1186/1750-1172-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baruteau J, Sachs P, Broue P, Brivet M, Abdoul H, Vianey-Saban C, Ogier de BH. Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: a French pediatric study of 187 patients. J Inherit Metab Dis. 2012 doi: 10.1007/s10545-012-9542-6. [DOI] [PubMed] [Google Scholar]
- 4.Kelly DP, Strauss AW. Inherited cardiomyopathies. N Engl J Med. 1994;330:913–919. doi: 10.1056/NEJM199403313301308. [DOI] [PubMed] [Google Scholar]
- 5.Vockley J, Marsden D, McCracken E, DeWard S, Barone A, Hsu K, Kakkis E. Long-term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment-A retrospective chart review. Molecular genetics and metabolism. 2015 doi: 10.1016/j.ymgme.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strauss AW, Powell CK, Hale DE, Anderson MM, Ahuja A, Brackett JC, Sims HF. Molecular basis of human mitochondrial very-long-chain acyl-CoA dehydrogenase deficiency causing cardiomyopathy and sudden death in childhood. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10496–10500. doi: 10.1073/pnas.92.23.10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hale DE, Bennett MJ. Fatty acid oxidation disorders: a new class of metabolic diseases. The Journal of pediatrics. 1992;121:1–11. doi: 10.1016/s0022-3476(05)82532-6. [DOI] [PubMed] [Google Scholar]
- 8.Mathur A, Sims HF, Gopalakrishnan D, Gibson B, Rinaldo P, Vockley J, Hug G, Strauss AW. Molecular heterogeneity in very-long-chain acyl-CoA dehydrogenase deficiency causing pediatric cardiomyopathy and sudden death. Circulation. 1999;99:1337–1343. doi: 10.1161/01.cir.99.10.1337. [DOI] [PubMed] [Google Scholar]
- 9.Guertl B, Noehammer C, Hoefler G. Metabolic cardiomyopathies. Int J Exp Pathol. 2000;81:349–372. doi: 10.1046/j.1365-2613.2000.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tucci S, Flogel U, Hermann S, Sturm M, Schafers M, Spiekerkoetter U. Development and pathomechanisms of cardiomyopathy in very long-chain acyl-CoA dehydrogenase deficient (VLCAD(-/-)) mice. Biochim Biophys Acta. 2014;1842:677–685. doi: 10.1016/j.bbadis.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA : the journal of the American Medical Association. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 12.Roe CR, Brunengraber H. Anaplerotic treatment of long-chain fat oxidation disorders with triheptanoin: Review of 15years. Experience Mol Genet Metab. 2015;116:260–268. doi: 10.1016/j.ymgme.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipshultz SE, Orav EJ, Wilkinson JD, Towbin JA, Messere JE, Lowe AM, Sleeper LA, Cox GF, Hsu DT, Canter CE, Hunter JA, Colan SD. Risk stratification at diagnosis for children with hypertrophic cardiomyopathy: an analysis of data from the Pediatric Cardiomyopathy Registry. Lancet. 2013;382:1889–1897. doi: 10.1016/S0140-6736(13)61685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, Lurie PR, McCoy KL, McDonald MA, Messere JE, Colan SD. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 15.Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis. 2006;29:327–331. doi: 10.1007/s10545-006-0320-1. [DOI] [PubMed] [Google Scholar]
- 16.Kinman RP, Kasumov T, Jobbins KA, Thomas KR, Adams JE, Brunengraber LN, Kutz G, Brewer WU, Roe CR, Brunengraber H. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats. Am J Physiol Endocrinol Metab. 2006;291:E860–E866. doi: 10.1152/ajpendo.00366.2005. [DOI] [PubMed] [Google Scholar]
- 17.Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002;110:259–269. doi: 10.1172/JCI15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutron A, Acquaviva C, Vianey-Saban C, de Lonlay P, de Baulny HO, Guffon N, Dobbelaere D, Feillet F, Labarthe F, Lamireau D, Cano A, de Villemeur TB, Munnich A, Saudubray JM, Rabier D, Rigal O, Brivet M. Comprehensive cDNA study and quantitative analysis of mutant HADHA and HADHB transcripts in a French cohort of 52 patients with mitochondrial trifunctional protein deficiency. Molecular genetics and metabolism. 2011;103:341–348. doi: 10.1016/j.ymgme.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Vockley J, Bennett M, Gillingham M. Mitochondrial Fatty Acid Oxidation Disorders. In: Valle D, editor. The Metabolic and Molecular Bases of Inherited Diseases. McGraw-HIll; New York, NY: 2016. In Press. [Google Scholar]
- 20.MacLaren G, Dodge-Khatami A, Dalton HJ, MacLaren G, Dodge-Khatami A, Dalton HJ, Adachi I, Almodovar M, Annich G, Bartlett R, Bronicki R, Brown K, Butt W, Cooper D, Demuth M, D’Udekem Y, Fraser C, Guerguerian AM, Heard M, Horton S, Ichord R, Jaquiss R, Laussen P, Lequier L, Lou S, Marino B, McMullan M, Ogino M, Peek G, Pretre R, Rodefeld M, Schmidt A, Schwartz S, Shekerdemian L, Shime N, Sivarajan B, Stiller B, Thiagarajan R. Joint statement on mechanical circulatory support in children: a consensus review from the Pediatric Cardiac Intensive Care Society and Extracorporeal Life Support Organization. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14:S1–2. doi: 10.1097/PCC.0b013e318292dc09. [DOI] [PubMed] [Google Scholar]
- 21.Kumar TK, Zurakowski D, Dalton H, Talwar S, Allard-Picou A, Duebener LF, Sinha P, Moulick A. Extracorporeal membrane oxygenation in postcardiotomy patients: factors influencing outcome. The Journal of thoracic and cardiovascular surgery. 2010;140:330–336.e332. doi: 10.1016/j.jtcvs.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopal SK, Almond CS, Laussen PC, Rycus PT, Wypij D, Thiagarajan RR. Extracorporeal membrane oxygenation for the support of infants, children, and young adults with acute myocarditis: a review of the Extracorporeal Life Support Organization registry. Critical care medicine. 2010;38:382–387. doi: 10.1097/CCM.0b013e3181bc8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merrill ED, Schoeneberg L, Sandesara P, Molitor-Kirsch E, O’Brien J, Jr, Dai H, Raghuveer G. Outcomes after prolonged extracorporeal membrane oxygenation support in children with cardiac disease--Extracorporeal Life Support Organization registry study. The Journal of thoracic and cardiovascular surgery. 2014;148:582–588. doi: 10.1016/j.jtcvs.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 24.Cavarocchi NC, Pitcher HT, Yang Q, Karbowski P, Miessau J, Hastings HM, Hirose H. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. The Journal of thoracic and cardiovascular surgery. 2013;146:1474–1479. doi: 10.1016/j.jtcvs.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 25.Falkensammer CB, Heinle JS, Chang AC. Serial plasma BNP levels in assessing inadequate left ventricular decompression on ECMO. Pediatric cardiology. 2008;29:808–811. doi: 10.1007/s00246-008-9222-3. [DOI] [PubMed] [Google Scholar]
- 26.Lindroos MM, Parkka JP, Taittonen MT, Iozzo P, Karppa M, Hassinen IE, Knuuti J, Nuutila P, Majamaa K. Myocardial glucose uptake in patients with the m.3243A > G mutation in mitochondrial DNA. J Inherit Metab Dis. 2016;39:67–74. doi: 10.1007/s10545-015-9865-1. [DOI] [PubMed] [Google Scholar]
- 27.Bakermans AJ, Dodd MS, Nicolay K, Prompers JJ, Tyler DJ, Houten SM. Myocardial energy shortage and unmet anaplerotic needs in the fasted long-chain acyl-CoA dehydrogenase knockout mouse. Cardiovasc Res. 2013;100:441–449. doi: 10.1093/cvr/cvt212. [DOI] [PubMed] [Google Scholar]
- 28.Des Rosiers C, Labarthe F, Lloyd SG, Chatham JC. Cardiac anaplerosis in health and disease: food for thought. Cardiovasc Res. 2011;90:210–219. doi: 10.1093/cvr/cvr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucci S, Flogel U, Sturm M, Borsch E, Spiekerkoetter U. Disrupted fat distribution and composition due to medium-chain triglycerides in mice with a beta-oxidation defect. The American journal of clinical nutrition. 2011;94:439–449. doi: 10.3945/ajcn.111.012948. [DOI] [PubMed] [Google Scholar]
- 30.Tucci S, Behringer S, Spiekerkoetter U. De novo fatty acid biosynthesis and elongation in very long-chain acyl-CoA dehydrogenase-deficient mice supplemented with odd or even medium-chain fatty acids. FEBS J. 2015;282:4242–4253. doi: 10.1111/febs.13418. [DOI] [PubMed] [Google Scholar]