Abstract
Background
Recent evidence in rat neuron-free mucosa suggests the membrane bile acid receptor TGR5 decreases colonic secretion under basal and stimulated conditions. As submucosal neurons are key players in secretory processes and highly express TGR5, we investigated their role in TGR5 agonist-induced inhibition of secretion and the pathways recruited.
Methods
TGR5 expression and localization were assessed in rat proximal (pC) and distal (dC) colon by qPCR and IHC with double labeling for cholinergic neurons in whole-mount preparations. The influence of a selective (INT-777) or weak (ursodeoxycholic acid, UDCA) TGR5 agonist on colonic secretion was assessed in Ussing chambers, in dC preparation removing seromuscular ± submucosal tissues, in the presence of different inhibitors of secretion pathways.
Key Results
TGR5 mRNA is expressed in whole thickness dC and pC and immunoreactivity is located in colonocytes and pChAT positive neurons. Addition of INT-777, and less potently UDCA, decreases colonic secretion in seromuscular stripped dC by −58.17 ± 2.6%. INT-777 effect on basal secretion was reduced in neuron-free and TTX-treated mucosal-submucosal preparations. Atropine, hexamethonium, indomethacin, and L-NAME all reduced significantly INT-777 inhibitory effect while the 5-HT4 antagonist RS-39604 and lidocaine abolished it. INT-777 inhibited stimulated colonic secretion induced by nicotine, but not cisapride, carbachol or PGE2.
Conclusions and Inferences
TGR5 activation inhibits the basal and stimulated distal colonic secretion in rats by acting directly on epithelial cells and also inhibiting submucosal neurons. This could represent a counter-regulatory mechanism, at the submucosal level, of the known prosecretory effect of bile acids in the colon.
Keywords: bile acids, distal colon, secretion, submucosal neurons, TGR5, Ussing chambers
INTRODUCTION
In normal physiology, 95% of the bile acids secreted into the duodenum are actively reabsorbed in the terminal ileum through a specific transporter and return to the liver.(1) During digestion, these amphipathic molecules facilitate dietary lipids absorption by fat micellization. After deconjugation and dehydroxylation by ileal bacteria, the remaining 5% entering the colon are either passively reabsorbed through the epithelium, or excreted in the feces.(2) In the colon, this small excreted component of bile acids will act as “endogenous laxatives”. Well described in patients suffering from bile acid malabsorption,(3) this laxative effect results from the contribution of separate prokinetic and prosecretory mechanisms, which have both been demonstrated in human and rodent colons.(3)
Over the past decade, the consensus was that luminal administration of bile acids triggered secretion and inhibited water absorption in vivo and in vitro in human and rodent colonic tissue.(4-6) Of note, the concentrations of bile acids experimentally used in these studies were often supraphysiological (> mM) and found to cause chemical damage to the colonic epithelium.(6) Recently however, Keely's group challenged this consensual “prosecretory effect” by showing that prolonged exposure (3-6 h) to physiological concentrations of endogenous bile acids, at the apical (CA, DCA, TDCA) or basolateral (UDCA, TUDCA) side of epithelial cells, could reduce Ca2+ (CCh) and cAMP (FSK) dependent secretagogue-induced epithelial secretion in T84 human colonic epithelial cells and rat distal colon mucosa mounted in Ussing chambers.(7, 8) Lately, the same group demonstrated a role for the newly identified bile acid receptor TGR5 in this antisecretory effect, by showing a reduction of basal and cholinergic-stimulated colonic secretory responses in response to basolateral but not apical application of the selective TGR5 agonist, INT-777, on isolated preparations of rat colonic neuron-free mucosa.(9)
Discovered over a decade ago, TGR5 is a G-protein coupled receptor binding specifically bile acids.(10) It is ubiquitously expressed throughout the body and has been identified in the gastrointestinal tract of humans and rodents.(10, 11) Most of the rodent data, generated in mice, show an abundant expression of TGR5 protein in the submucosal and myenteric neurons and to a lesser extent in the mucosa.(12) In murine colon, nearly all submucosal neurons coexpress PGP 9.5 (pan neuronal marker) and TGR5, and 50 % of the TGR5 positive neurons coexpress the cholinergic marker, choline acyltransferase (ChAT).(12) Only one study thus far has assessed TGR5 mRNA and protein expression in the rat gastrointestinal tract where it was found expressed at the apical and basolateral sides of epithelial cells, however this report was limited to distal colon isolated crypts.(9)
In the enteric nervous system (ENS), the neurons of the submucosal plexus represent the main physiological control mechanism regulating epithelial ion transport.(13) Because TGR5 is highly expressed in mouse submucosal neurons,(12) the aim of our study was to assess the potential role and contribution of submucosal neurons in TGR5-induced inhibition of colonic secretion. In the present study, we first assessed the gene and protein expression of TGR5 in the rat distal and proximal colon using semi-quantitative PCR, immunofluorescence of whole tissue sections and whole-mount preparations of myenteric and submucosal plexus. Taking into consideration the fact that there are regional differences in the properties of submucosal neurons and in the epithelial responses between the proximal and distal colon of rodents,(14-17) we then studied how proximal and distal rat colonic preparations mounted in Ussing chambers were affected by TGR5 activation by assessing the transepithelial resistance (TEER) and electrogenic anion secretion (short-circuit current, Isc) using a selective TGR5 agonist (INT-777) and an endogenous weak agonist (UDCA). We observed that TGR5 activation in the rat distal colon decreases the Isc, which reflects the colonic transepithelial chloride secretion. To elucidate the contribution of submucosal neurons in TGR5-induced alterations of TEER and Isc, we assessed the influence of INT-777 on: 1) the basolateral side of seromuscular free distal colonic preparations containing submucosal enteric neurons, pretreated or not with neurotoxins (sodium channel blockers: TTX or lidocaine) and 2) the serosal side of neuron-free (mucosa only) preparations. Lastly, we studied the cellular and neuronal pathways modulated by TGR5 under basal and stimulated conditions using different inhibitors or stimulators.
MATERIAL AND METHODS
Animals
Adult male Sprague-Dawley rats (Harlan Laboratory, San Diego, CA, USA) weighing 250-350 g were used. Animals were kept under controlled conditions of illumination (12:12h light-dark cycle starting at 6 am), temperature (21-23°C) and humidity (30-35%) and allowed free access to water and food (Purina rat chow, USA). Animals were allowed to acclimate to the animal facility for 1 week after their arrival. Experiments followed NIH guidelines according to protocol # 9906-020 approved by the Institutional Animal Care and Use Committee (IACUC) of the VA Greater Los Angeles Healthcare System under the auspice of the Office of Laboratory Animal Welfare - Assurance of Compliance (A3002-01).
Electrophysiological measurements
Tissue preparation
Rats were anesthetized with isoflurane 5% and decapitated. Segments of rat distal colon (dC, 2–6 cm proximal to anus) and proximal colon (pC, 2-6 cm distal from caecum) were cut along the mesenteric border, and the lumen was gently washed in Krebs solution at room temperature. The colon segments were pinned on a Sylgard-plated Petri dish containing ice-cold Krebs-Ringer solution (pH 7.4, 4°C), and the seromuscular layer was peeled off using microdissection forceps. In some experiments, the submucosa was also removed to obtain neuron-free samples as previously done.(18)
Measurement of electrical parameters in vitro
The mucosa-submucosa preparations of rat dC and pC were cut in 3 to 6 cross-sectional area (depending on the quality of the dissection), mounted on 0.30 cm2 slides dedicated to Ussing chamber. The mucosal and serosal surfaces of the tissue were bathed with 5 ml (or 4 ml for the Expt 4) of oxygenated (carbogen) Krebs-Ringer solution containing (in mM) 115 NaCl, 1.2 CaCl2, 1.2 MgCl2, 4.8 KH2PO4, 48 K2HPO4 and 25 NaHCO3, and 10 glucose. The solution was bubbled with 95% O2-5% CO2 to maintain pH at 7.4 and kept at 37°C during the course of the experiments by a circulating water bath heater. Tissues were left to equilibrate [as judged by a stable basal short-circuit current (Isc) and TEER] in the chambers for 45 min before conducting the experiments. The tissues were short-circuited by a voltage clamp (VCC MC6, Physiologic Instruments, San Diego, CA, USA) at zero potential automatically with compensation for solution resistance. Isc and tissue conductance (Gt) were determined every 2 s and recorded by the DataQ system (Physiologic Instruments, San Diego, CA). Positive values for Isc indicate a negative electrical charge flux from serosal→luminal bath indicating anion secretion or cation absorption
Real-time quantitative PCR for TGR5 in the rat proximal and distal colon
Four naïve male adult SD rats were euthanized by decapitation. Both pC and dC were harvested and kept as whole thickness and processed for RNA extraction and cDNA synthesis as described previously.(19) Real-time quantitative PCR for TGR5 was performed in duplicates using StepOnePlus™ Real-Time PCR System (Applied Biosystems) in a 25 μl reaction volume. The optimized reaction contained 12.5 μl of SABiosciences™ RT2 SYBR Green ROX qPCR MasterMix (QIAGEN), 0.5 μl each of oligonucleotide primers (10 μM), 1 μl of the cDNA synthesis reaction, and 10.5 μl of H2O. Selected forward (f) and reverse (r) primers listed in Table 1 were used for rat TGR5. The housekeeping gene, rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also analyzed as internal control. Thermal conditions were as follows: 95°C for 4 min followed by 40 cycles of 95°C for 5s, 60°C for 60 s. The specificity of the amplification reaction was determined by performing a melting curve analysis of the PCR fragments. Each target gene was normalized by GAPDH and calculated using the comparative ΔΔCt method. Results were calculated by the equation 2 −ΔΔCt and expressed as relative expression.
Table 1.
Sequences of oligonucleotide primers used for real-time qPCR
| cDNA | Direction | Primer (5’-3’) | Size (bp) |
|---|---|---|---|
| rTGR5 | forward | TGCTCTTTTTGCTGTGTTGG | 226 |
| reverse | GGTCTTCCTCGAAGCACTTG | ||
| rGAPDH | forward | AGACAGCCGCATCTTCTTGT | 142 |
| reverse | TGATGGCAACAATGTCCACT |
Immunohistochemistry
Paraffin whole tissue sections (4 μm) were used. Tissue sections were preblocked in 10% normal donkey serum (Jackson ImmunoResearch, WestGrove, PA) in 0.3% Triton X-100 in PBS and incubated with the following primary antibody: goat anti-TGR5 antibody (1:200, sc-48687, Santa Cruz Biotechnology). Tissues were then washed with phosphate buffered saline (PBS) and incubated with the secondary antibody donkey anti-goat IgG conjugated to rhodamine Red-X (1:100; Jackson ImmunoResearch) for 1.5 hours at room temperature. Tissues were subsequently washed 3 times in PBS and mounted with anti-fade mounting media (Vector Laboratory, Inc, Burlingame, CA) before being visualized by standard fluorescence microscopy. Immunohistochemical controls were performed routinely following the same procedure except that the primary antibody was replaced by PBS-T. No IR was seen with the omission of the primary antibody.
Double labeling of TGR5 with pChAT
Segments of pC and dC collected from 4 naive adult male Sprague–Dawley rats were processed for longitudinal muscle myenteric plexus (LMMP) and circular muscle submucosal plexus (CMSP) whole-mount preparations as described earlier,(20) and double-stained for TGR5 and peripheral choline acetyltransferase (pChAT), a peripheral isoform of ChAT and a marker of peripheral cholinergic neurons.(21) Free floating CSMP and LMMP whole-mounts were rinsed in 0.3% Triton-x 100 in PBS and incubated in 10% normal donkey serum (Jackson ImmunoResearch Laboratories, Inc, West Grove, PA) in 0.3% Triton-X 100/PBS for 30 min at room temperature (species same as secondary antibody). Whole-mounts were then incubated in the mixture of antibodies (rabbit against pChAT: 1:4000 and Goat anti TGR5: 1:200) in 0.3% Triton-x 100 in PBS in a humidified chamber for 1 hour at room temperature then overnight at 4°C. After rinsing in 0.3% Triton-x 100 in PBS, whole-mounts were incubated in the mixture of secondary antibodies (Alexa 488-conjugated donkey anti rabbit IgG: 1:400 and Alexa 594-conjugated donkey goat IgG:1:400) in 0.3% Triton-x 100 in PBS in a dark humidified chamber for 1.5 hour at room temperature. Tissues were subsequently washed 3 times in PBS and mounted with anti-fade mounting media before being visualized by standard fluorescence microscopy. The rabbit anti-pChAT serum was a gift from Dr H. Kimura (Shiga University of Medical Science, Otsu, Japan) and the goat anti-TGR5 antibody (mouse origin) (sc-48687) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Double-labeled neurons were counted under light microscopy in 25 ganglia from each LMMP and CMSP whole-mount preparations in four rats. Neurons double-stained with TGR5/pChAT (yellow staining) were expressed in percentage of total number of pChAT-positive neurons respectively. The means of labeled neurons from each animal were used to calculate the group mean.
Experimental protocol
To target the submucosal plexus and the basolateral side of the mucosa, reagents were added on the serosal side. Concentrations of the final solutions in chamber and volume of stock solution added in the chamber for each reagent are added in parenthesis. It was confirmed that 10 μl DMSO (0.2% of bathing solution) had no effect on ion transport in rat distal colon when added on serosal side. All drugs were added after a stabilization period of 45 min. Carbachol (10 μM, 5 μl), was added at the end of the each experiment as a positive control. Doses were selected based on previously published work as indicated for each reagent.
1. Influence of TGR5 activation on basal Isc and TEER in proximal and distal colon
INT-777 (3, 10, 30, 60 and 100 μM, 10 μl) or UDCA (3, 10, 30 and 60μM, 10 μl) were added on the mucosal or serosal side of seromuscular stripped proximal and distal colonic preparations.
2. Role of submucosal neurons in TGR5 inhibition of distal colon secretion
INT-777 (100 μM, 10 μl) was added on the serosal side of a) seromuscular stripped distal colon preparations pretreated 10 minutes before with either tetrodotoxin (TTX, 1 μM, 5 μl)(22) or lidocaine HCl (1 μM, 5 μl),(23) or b) neuron-free (seromuscular and submucosa stripping) distal colon preparation.(18)
3. Pathways recruited in inhibitory effect of TGR5 on basal secretion
INT-777 (100 μM, 10 μl) was added on seromuscular stripped distal colon preparations pretreated 10 minutes before with atropine sulfate (10 μM, 5 μl),(18) hexamethonium bromide (100 μM, 5 μl),(24) RS-39604 (10 μM, 5 μl),(25) indomethacin (10 μM, 5 μl serosal/mucosal),(18) or L-NAME (200 μM, 10 μl).(26)
4. Influence of TGR5 activation on stimulated secretion
To assess the inhibitory potency of TGR5 on stimulated secretion, seromuscular stripped distal colon preparations were pretreated with a) DMSO (4 μl) or INT-777 (100 μM, 4 μl) with cisapride (100 μM, 4 μl)(18) or carbachol (10 μM, 4 μl) or nicotine (100 μM, 4 μl)(27) added 15 minutes after on the serosal side; b) indomethacin (10 μM, 4 μl serosal/mucosal)(18), followed 15 minutes after by DMSO (4 μl) or INT-777 (100 μM, 4 μl) followed 15 minutes after by PGE2 (10 μM, 4 μl, serosal)(28) and then nicotine (100 μM, 4 μl, serosal).
Chemicals
INT-777 (6a-ethyl-23(S)-methylcholic acid) was a kind gift of Intercept Pharmaceutical. Atropine sulfate (A-2057), carbachol (C-4382), DMSO (D8418), hexamethonium bromide (H-0879), 5-hydroxytryptamine hydrochloride (H-9523), indomethacin (I-7378), lidocaine HCl (L-5647), L-NAME (N-5751) and ursodeoxycholic acid (UDCA) (U5127), were obtained from Sigma-Aldrich (St Louis, LA, USA). RS-39604 was obtained from Roche Bioscience, (Palo Alto, CA, USA). Tetrodotoxin was obtained from Calbiochem (EMD Millipore, Billerica, MA, USA). INT-777, UDCA and RS-39604 were dissolved in DMSO. Carbachol, atropine sulfate and hexamethonium bromide were dissolved in distilled water. Lidocaine HCl and L-NAME were dissolved in saline. Indomethacin was dissolved in saline (Expt. 3) or ethanol (Expt. 4). TTX was dissolved in citrate buffer (0.1 M, pH 4.5).
Statistical analysis
Values are presented as means ± SEM. qPCR data were analyzed by unpaired t test. For Ussing experiments, baselines Isc and TEER were compared using unpaired Mann Whitney test or paired t test as indicated. Comparisons within multiple groups were performed using two-way ANOVA followed by a Bonferroni's Multiple Comparison post hoc test. A p value < 0.05 was considered statistically significant.
RESULTS
TGR5 is distributed in colonic crypts and enteric nerves, with higher expression in the distal vs proximal colon
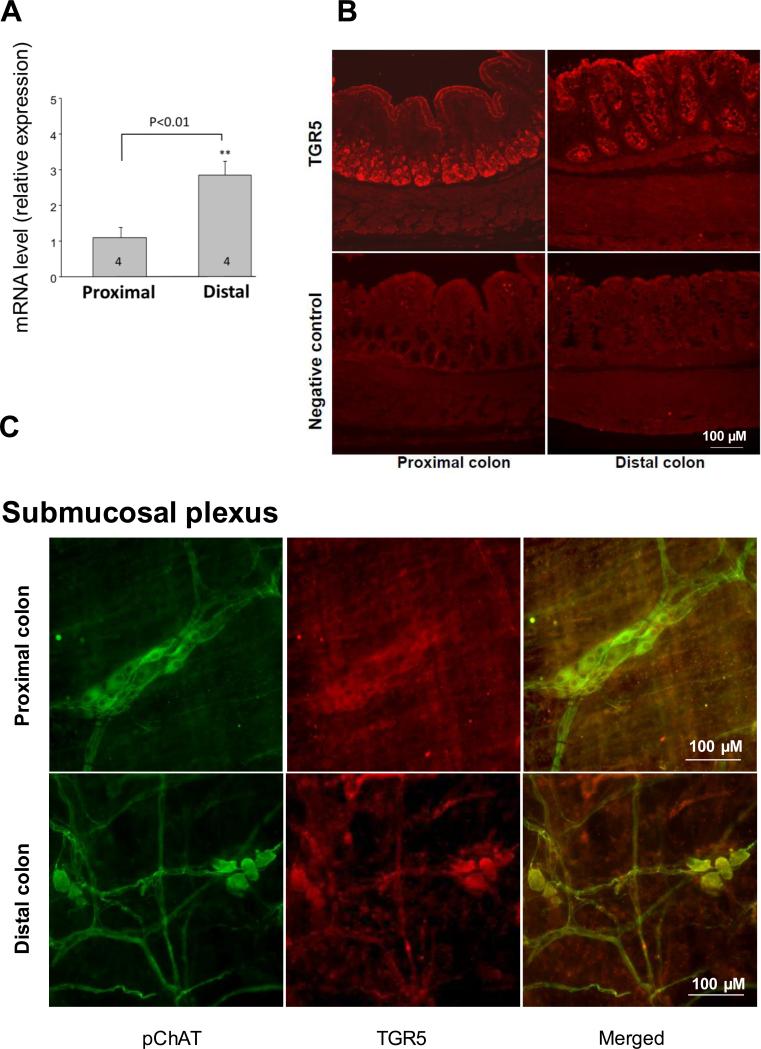
TGR5 mRNA was detected in both pC and dC by RT-PCR (Suppl Fig. 1) and qPCR (Fig. 1A). TGR5 mRNA levels were 2.8-fold higher in dC than in pC (2.842±0.763 vs 1.097±0.572, p<0.01, n=4). TGR5 immunoreactivity was located in cells of the epithelial layer, with staining visible both on the apical and basolateral sides of epithelial cells, mostly in crypts (Fig. 1B). TGR5 was also localized in the submucosal (Fig. 1C) and myenteric nervous plexus (Suppl. Fig.2) where 85.7% and 33.9% in pC and 99.3% and 31.7% in dC of pChAT immunoreactive neurons were TGR5 positive, respectively.
Figure 1.
Expression and distribution of TGR5 mRNA and protein in rat colonic tissue. (A) Comparison of TGR5 mRNA expression in distal and proximal rat colon by semi-quantitative qPCR (right). Data are means ± SEM, n as indicated in columns. **p<0.01 vs proximal colon, unpaired t test. (B) Localization of TGR5 protein expression in distal and proximal colon tissue sections by immunofluorescence. TGR5 immunofluorescence is located in cells of the epithelia, with staining visible both on the apical and basolateral sides of epithelial cells mostly in crypts and in submucosal and myenteric neurons. Scale: 100 μM. (C) Co-localization (yellow) of TGR5 (green) and pChAT (red) positive neurons in submucosal plexus of proximal (upper panel) and distal (lower panel) colon. Scale: 100 μM.
The selective TGR5 agonist, INT-777, lowers Isc and increases TEER when added on the serosal side of seromuscular stripped distal colon segments, while UDCA, a weak endogenous TGR5, agonist has limited influence
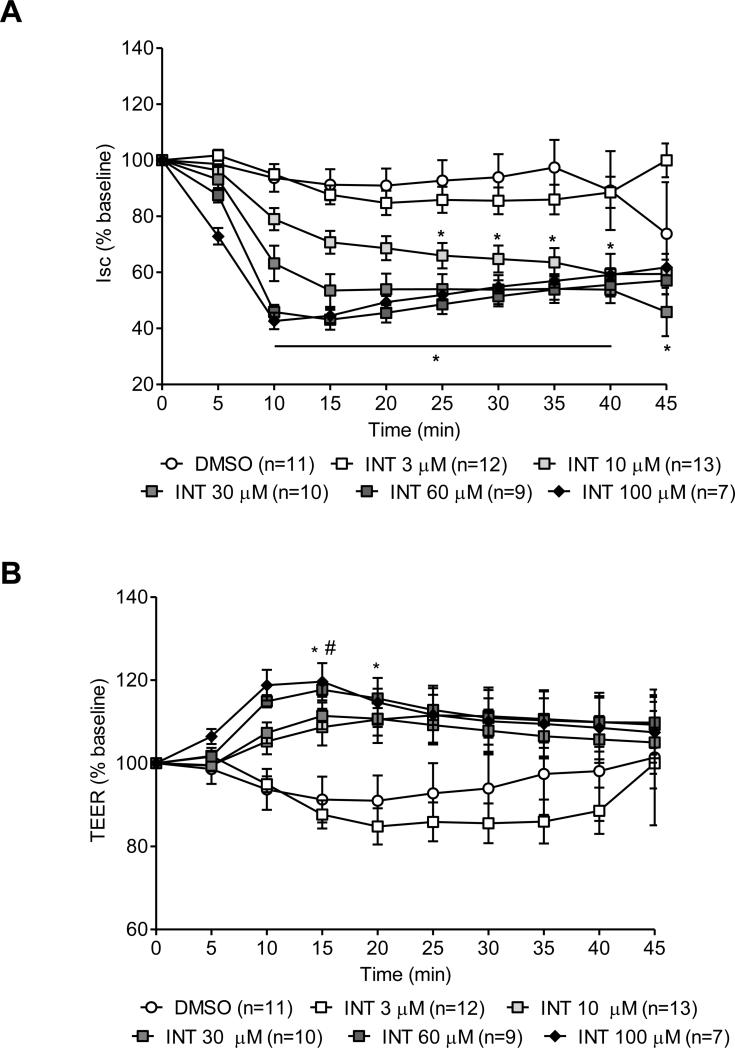
The specific TGR5 agonist INT-777 added on the serosal side of seromuscular stripped distal colon segments in Ussing chambers induced a dose-dependent decrease in Isc starting at 10 μM and plateauing at the dose of 60 μM (Fig. 2A). INT-777 (10 μM; n=13) significantly decreased the Isc at 25 min (−34.03 ± 4.53% of basal Isc, p<0.05, vs. vehicle (DMSO; n=11) with a peak change at 35 min (−36.41 ± 5.1% of basal Isc, p<0.001). This response had a shorter onset (10 min) and increased magnitude at higher concentrations of INT-777 (−36.82 ± 7.1 %, −54.15 ± 2.57% and −57.35 ± 2.95% of basal Isc at 30, 60 and 100 μM, p<0.05, p<0.0001 and p<0.0001, n=10, 9 and 7, respectively). At the lowest dose of 3 μM (n=12), INT-777 had no effect on Isc (Fig. 2A). Concomitantly, INT-777 induced a transient increase in TEER (Fig. 2B), only observed at the highest doses tested (60 or 100 μM): the TEER was increased by 17.68 ± 1.71% and 19.61 ± 1.57% compared to vehicle (p<0.05). The addition of vehicle did not modify baseline Isc or TEER. INT-777 or vehicle added on the mucosal side of the dC or serosal side of the pC at similar concentrations did not modify either Isc or TEER (data not shown).
Figure 2.
Dose-dependent effect of the selective TGR5 agonist, INT-777, on short-circuit current (Isc, in % baseline) (A) and transepithelial electrical resistance (TEER, in % baseline) (B) in seromuscular stripped preparations of rat distal colon mounted in Ussing chambers. INT-777 administered on the serosal side significantly reduced the Isc starting at the dose of 10 μM, and increased the TEER at the dose of 60 and 100 μM. Data are means ± SEM, n as indicated in parenthesis. *p<0.05 vs DMSO group, two-way ANOVA followed by Bonferroni's multiple comparison post hoc test.
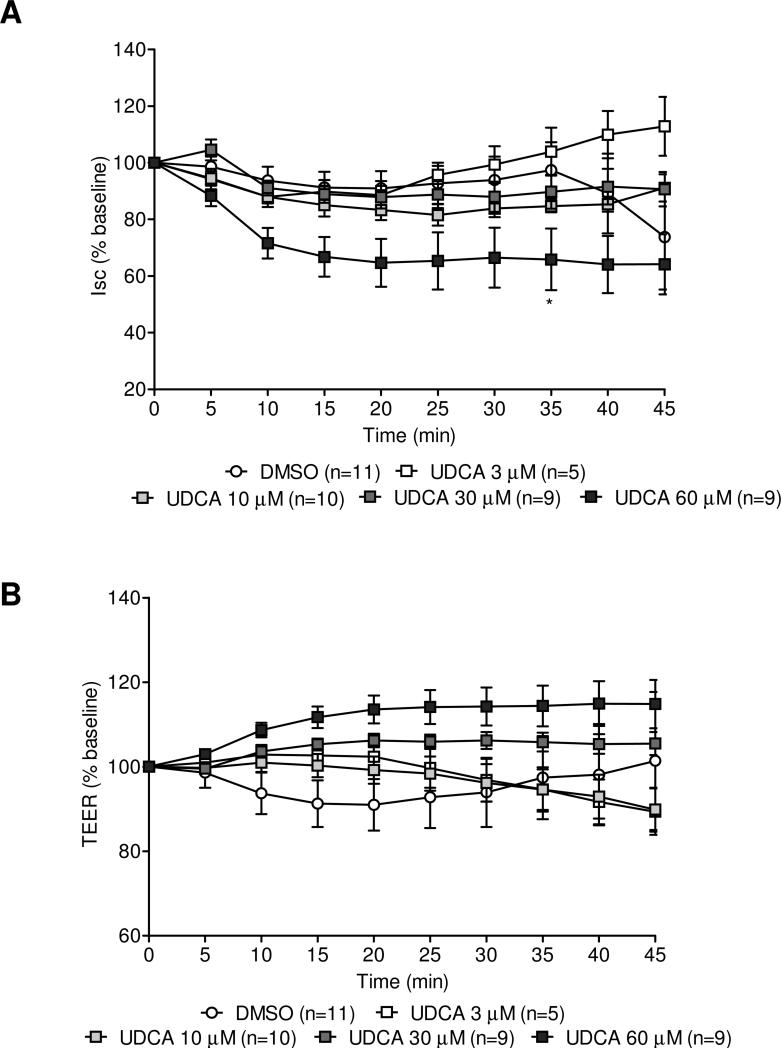
Next we assessed the effect of a weak TGR5 endogenous bile acid agonist UDCA, under the same experimental conditions, applied serosally. At 60 μM, UDCA induced a 34.1 ± 10.8% decrease of baseline Isc (p<0.01 vs. vehicle, n=9) occurring at 35 min (Fig. 3A) while having no significant effect on the TEER (Fig. 3B). None of the other doses of UDCA (3, 10 and 30 μM; n=5, 10 and 9, respectively) had any effect on Isc or TEER (Figs. 3A and 3B).
Figure 3.
Influence of a low affinity endogenous agonist for TGR5 receptor, ursodeoxycholic acid (UDCA) on short-circuit current (Isc, in % baseline) (A) and transepithelial electrical resistance (TEER, in % baseline) (B) in seromuscularly-stripped preparations of rat distal colon mounted in Ussing chambers. UDCA administered on the serosal side at 60 μM reduced significantly the Isc at 35 min (A), but had no effect on TEER (4D). Data are means ± SEM, n as indicated in parenthesis. *p<0.05 (60 μM) and #p<0.05 (100 μM) vs DMSO group, two-way ANOVA followed by Bonferroni's multiple comparison post hoc test.
Role of the submucosal plexus and epithelial cells in the inhibitory effect of TGR5 activation on rat distal colonic short circuit current
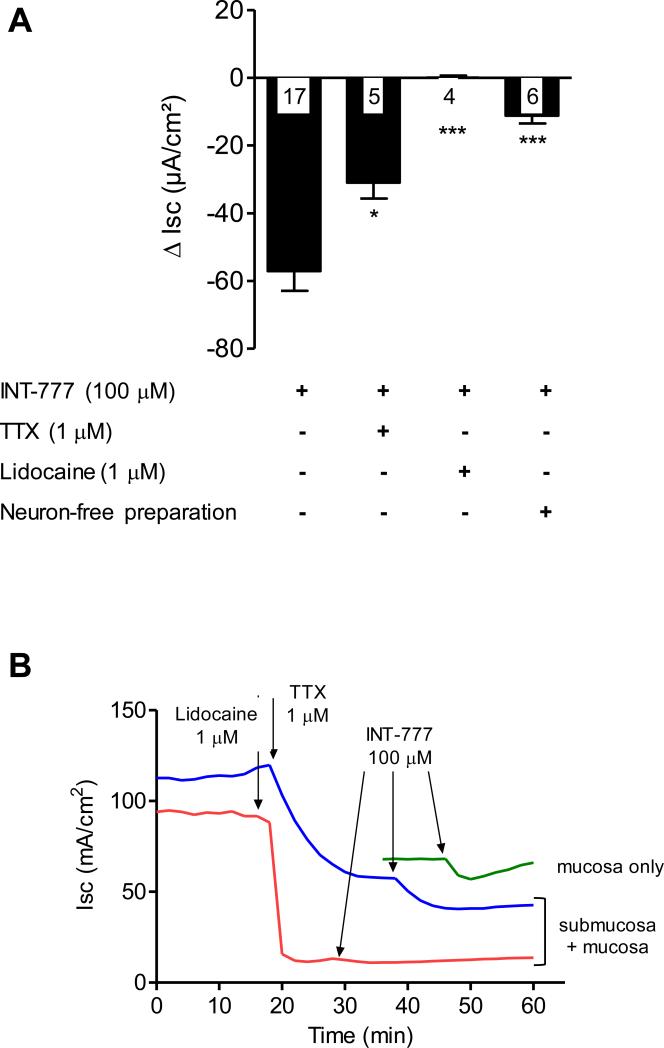
Because TGR5 is expressed in both epithelial cells and submucosal neurons, we aimed to investigate their respective contributions in INT-777 inhibitory effect on secretion. To assess the role of submucosal neurons (Fig. 4A), INT-777 (100 μM) was applied under two experimental conditions: 1) on the serosal side of seromuscular stripped distal colonic preparations pretreated or not with neurotoxins (sodium channel blockers: TTX or lidocaine) or 2) on the serosal side of neuron-free (seromuscular and submucosal stripped) preparations.
Figure 4.
Contribution of the submucosal plexus and epithelial cells in the selective TGR5 agonist (INT-777) induced inhibition of basal Isc. (A) In seromuscular stripped preparations of rat distal colon mounted in Ussing chambers INT-777-induced Isc inhibition is partially reduced by TTX pretreatment (1 μM) and abolished by pretreatment with the sodium channel blocker, lidocaine (1 μM). The inhibitory effect of INT-777 in neuron-free preparations of distal colon is significantly less than in preparations containing enteric nerves. DMSO does not affect the Isc in distal colon preparations with or without enteric neurons. Representative graphs of pooled Ussing experiments: data are means ± SEM, n as indicated in columns. *p<0.05, ***p<0.001 vs INT-777 treated group, unpaired t test. (B) Representative graphs from Isc recordings in Ussing chambers, showing the influence of INT-777 (100 μM) in neuron-containing distal colon preparations pretreated with TTX or lidocaine, and in neuron-free rat distal colonic tissue.
The seromuscular stripped tissues exhibited greater basal Isc than neuron-free tissues (99.11 ± 6.54 vs 54.25 ± 10.09 μA/cm2 p < 0.01, n=17-9). The basal TEER was not different in seromuscular stripped tissues vs neuron-free tissues (98.22 ± 3.05 vs 94.64 ± 8.84 Ω/cm2; p >0.05, n=17-9). In the presence of neurons (seromuscular stripped preparation, Fig. 5A), INT-777 induced a significant deep and plateauing decrease in Isc (−57.09 ± 5.8 μΑ/cm2, n=17) representing 58.17 ± 2.59% of basal Isc. Pretreatment with TTX (1 μM) for 10 min decreased the Isc by −57.63 ± 12.9 μΑ /cm2 (i.e. 49.9 ± 7.22 % of basal Isc; n=5) before plateauing (Fig. 4B), confirming the presence of functional enteric neurons in our preparation. After this TTX pretreatment, INT-777 was still able to produce a decrease in Isc of −17.42 ± 2.74 μΑ /cm2 (about 32.57 ± 9.26 % of basal Isc; n=5) (Fig. 4A and experimental illustration Fig. 4B). To further confirm that submucosal neurons were involved in INT-777 effect, we pretreated a seromuscular stripped distal colonic preparation with lidocaine (1 μM), a sodium channel blocker for 10 min (blocking both TTX-sensitive and TTX-insensitive sodium channels). By itself, lidocaine rapidly abolished the basal Isc, indicating that lidocaine inhibited basal ion transport under stabilized conditions (Fig. 4B) and abolished the effect of INT-777 (n=4; Fig. 4A and 4B).
Figure 5.
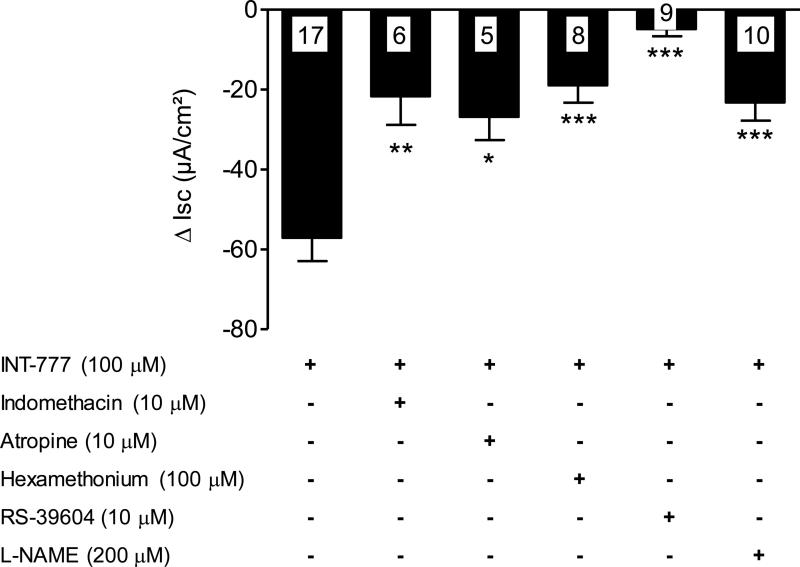
TGR5 agonist (INT-777) inhibition of basal secretion induced by several endogenously-released segretagogues in rat distal colon. Influence of INT-777 on Isc in seromuscular peeled preparations pretreated with the indomethacin (10 μM), atropine (10 μM), hexamethonium (100 μM), 5-HT4 antagonist (RS-39604, 10 μM), L-NAME (100 μM), or DMSO (10 μl). Data are means ± SEM, n as indicated in columns. *p<0.05, **p<0.01, ***p<0.001 vs INT-777 treated group, unpaired t test.
We then assessed the role of colonocytes in the reduced secretion mediated by the selective TGR5 agonist, by removing entirely the submucosal plexus in distal colon preparations. In the absence of neurons, INT-777 applied serosally induced a significant decrease in Isc that was shorter in duration and of lesser intensity (−11.18 ± 2.89 μΑ /cm2, representing 19.50 ± 2.55% of basal Isc; n=6; Fig. 4A and 4B), compared to the decrease observed when neurons were present in the preparation (32.57 ± 9.26% vs 19.50 ± 2.55% of basal Isc, respectively, p<0.05).
TGR5 activation inhibits basal distal colon secretion
Under basal conditions, pretreatment of seromuscular stripped distal colonic preparations with indomethacin (10 μM; n=12), atropine (10 μM; n=9) and hexamethonium (100 μM; n=8) partially reduced the basal Isc by 64.88 ± 1.6, 23 ± 2.09 and 23.15 ± 5.28 % and the antisecretory effect of the INT-777 (100 μM) by 62.1, 53.1 and 66.8% (n=6, n=5 and n=8) respectively. The 5-HT4 receptor antagonist, RS-39604 (10 μM), induced a significant decrease in basal Isc (−52.49 ± 5.3 %) and abolished INT-777 effect (n=9, Fig. 5). The NOS inhibitor, L-NAME (200 μM; n=12) reduced basal Isc by −17.14 ± 3.64 % and INT-777 inhibitory effect by 64.0 % (n=7).
Influence of TGR5 activation on stimulated secretion: a role for nicotinic cholinergic submucosal neurons
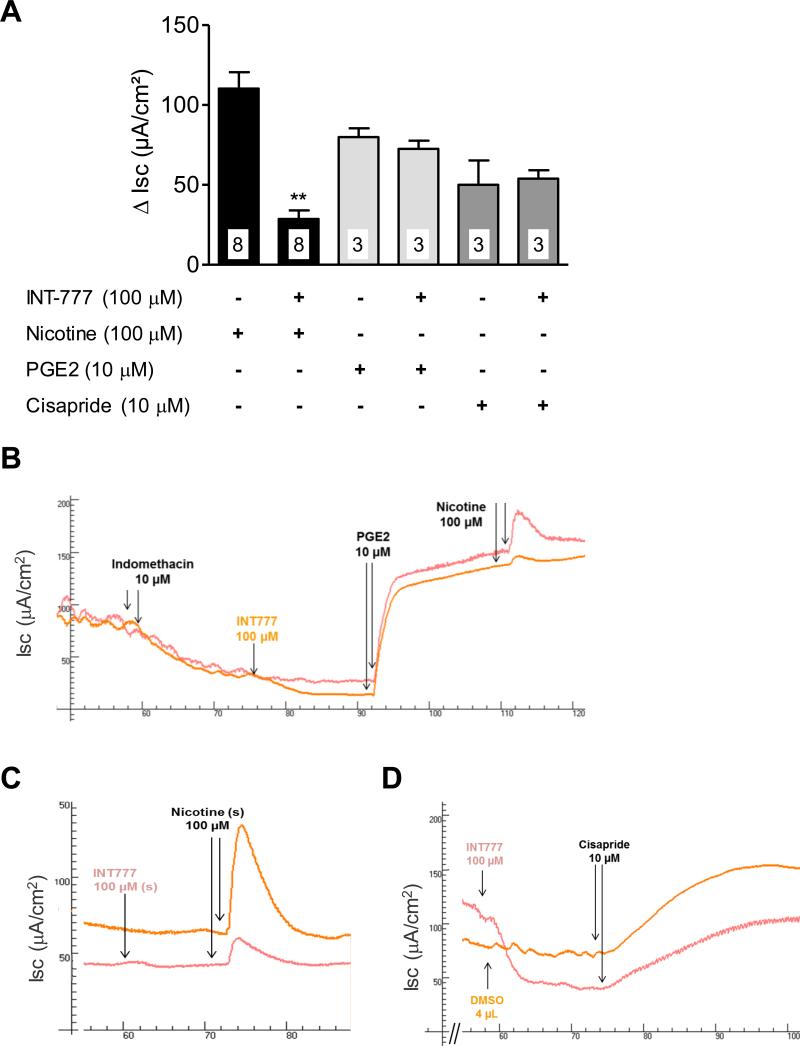
Cisapride, a 5-HT4 receptor agonist (Fig. 6A and 6D) (or carbachol - data not shown) led to a powerful enhancement of the Isc (50.1 ± 15.2 μA/cm2, n=3). This supraphysiological activation of chloride secretion was not altered by a pretreatment with INT-777 (53.9 ± 5.3 μA/cm2, n=3). In the presence of indomethacin (to inhibit mucosal production of prostaglandin that occurs after the tissue peeling), exogenous PGE2 (10 μM) induced a significant increase in Isc (80.0 ± 5.5 μA/cm2, n=3) (Fig. 6A and 6B). INT-777 pretreatment did not change the Isc in response to exogenous PGE2 (72.6 ± 5.1 μA/cm2, n=3; Fig. 6A and 6B). Interestingly, addition of nicotine (100 μM) after PGE2 induced a significant increase in Isc (110.3 ± 10.2 μA/cm2, n=8), which was significantly reduced by a pretreatment with INT-777 (28.7 ± 5.3 μA/cm2; p<0.01, n=8) (Fig. 6A and 6B). This selective inhibitory effect of INT-777 on nicotinergic pathways was verified and reproduced in a fresh preparation without indomethacin and PGE2 (Fig. 6C). The nicotine-induced increases in Isc were 111.1 ± 21.4 μA/cm2 in control vs. 31.6 ± 10.4 μA/cm2 in INT-777 (p<0.01, n=4).
Figure 6.
Influence of TGR5 activation on stimulated secretion: a role for nicotinic cholinergic submucosal neurons. Representative graphs of INT-777 inhibition of stimulated secretion. (A) INT-777 (100 μM) pretreatment reduced basal Isc, but did not affect the increase in secretion mediated by the 5-HT4 agonist, cisapride (10 μM). (B) In the presence of indomethacin (10 μM), INT-777 (100 μM) still reduced the basal Isc, but did not prevent PGE2 (10 μM)-induced secretion. In contrast, nicotine (100 μM)-induced secretion was reduced by INT-777 pretreatment. (C) Inhibition of nicotine-induced secretion by INT-777 was reproduced in a fresh preparation. Data are means ± SEM, n as indicated in columns. **p<0.01 vs nicotine-treated group, paired t test.
DISCUSSION
Over the last five years, the role of bile acids in relationship with gut transit and secretion has gained a new understanding with the discovery of the membrane receptor to bile acid, TGR5.(29) With regard to secretory processes, a series of work performed this past decade indicate that bile acids, considered as prosecretory molecules for a long time, are able to reduce both basal and stimulated secretion in human colonic epithelial cell lines or in rodent colonic preparations in vitro.(7, 8, 30, 31) This antisecretory effect has a chronic component, occurring hours after exposure, which is mediated by the nuclear bile acid receptor FXR both in vivo and in vitro.(31) More recently, Keely's group identified a fast antisecretory effect of bile acids involving activation of TGR5 receptors present on epithelial cells.(9) It was also reported that in mice colon almost all submucosal neurons expressing PGP 9.5 coexpress TGR5.(12) Because the submucosal plexus is a key effector regulating fluids and electrolytes movement across the intestinal epithelium, the aim of our work was to determine how TGR5 receptors located on submucosal neurons modulate colonic secretion in rat. First, using qPCR, we showed that TGR5 is expressed at the mRNA level in both the proximal and distal colon of rat with a more prominent (2.8-fold) expression in the distal colon. Our data confirm and extend previous report of TGR5 mRNA expression in rat neuron-free distal colonic mucosa,(9) by highlighting for the first time a regional difference in colonic TGR5 expression. We then showed, using immunofluorescence, that TGR5 is localized in the colonic mucosa of both proximal and distal rat colon. In addition, in the proximal and distal colon, we found colocalization of TGR5 immunofluorescence in a majority (85.7% and 99.3%, respectively) of cholinergic submucosal neurons and in about a third of cholinergic myenteric neurons. Although the myenteric neurons were not further characterized in this manuscript, the detection of TGR5 protein expression and its distribution in rat cholinergic submucosal and myenteric neurons confirm and extend previous reports in mice.(12) The neuronal localization of TGR5 was further supported by the detection of mRNA in primary cultures of submucosal and myenteric neurons, as assessed by qPCR (Suppl. Fig. 3).
When added to seromuscular stripped preparations of distal colon, the TGR5 agonist, INT-777, induced a dose-dependent inhibition of the basal Isc, reflecting an inhibition of basal secretion. Interestingly, this effect was only observed when INT-777 was applied on the basolateral but not apical sides of preparations, in agreement with earlier work.(9) We further observed that UDCA, a weak endogenous agonist of TGR5,(10, 32, 33) when applied serosally to seromuscular stripped rat distal colon preparations shared the anti-secretory properties of INT-777, although much less potently. This result differs from a previous report by Keely's group showing that UDCA alone at doses ranging from 50 to 1000 μM did not affect the basal secretion when applied bilaterally to neuron-free preparations such as T84 colonic cell monolayers or muscle-stripped human colon treated with TTX.(8) One likely explanation for this discrepancy could be the presence of submucosal neurons in our preparation which could be targeted by UDCA. Although UDCA is reported as a TGR5 agonist at the doses used in our experiments,(10, 32, 33) it is difficult to eliminate other effect via FXR or other bile acid receptors in the present study. Nevertheless, the timeline of effects resemble the fast acting action of the selective TGR5 agonist, INT-777, and suggest that UDCA antisecretory influence is likely mediated by direct activation of TGR5. A selective TGR5 antagonist, when available, will clarify the specificity of UDCA on TGR5 activation in our setup. Taken together, these data suggest that to exert their anti-secretory actions via TGR5 receptors in vivo, bile acids must either enter colonic cells via paracellular transport(34) or reach the basolateral sides of cells via blood circulation.
The colonic basal secretion is dependent on the spontaneous release of endogenous paracrine or neural mediators which alter ion transport in epithelial cells, and is under the control of the enteric nervous system. In the rat distal colon, most of the secretomotor neurons are contained in the submucosal plexus and are made up of vasoactive intestinal polypeptide (VIP)-containing secretomotor/vasodilator neurons, which also contain neuropeptide Y (NPY) or nitric oxide synthase (NOS) and of three other types of neurons which are choline acetyltransferase (ChAT)-immunoreactive (IR), namely cholinergic neurons.(35) Among this latter group of cholinergic submucosal neurons, one type is known as the intrinsic primary afferent neuron (IPAN), which projects into the mucosa to sense mucosal stimuli,35 and release neurotransmitters such a neurokinins(36) in response to their activation which can be direct by intraluminal stimulus (chemical or mechanical) but also indirect by the serotonin secreted by enterochromaffin cells.(37-39) The other two types of cholinergic submucosal neurons are secretomotor/vasodilator and secretomotor (nonvasodilator)(35) neurons.
Seromuscular stripping before mounting in Ussing chambers causes submucosal damage in the tissue and induces phospholipase C or A2 activity, resulting in liberation of arachidonic acid and subsequent eicosanoid generation.(40) Indomethacin, a prostaglandin synthesis inhibitor acting on cyclooxygenase 1 and 2, reduced the basal secretory response in the distal colonic preparations by 59 ± 6.49 % of baseline, confirming the release of prostaglandins from the tissue. Prostaglandins activate G protein-coupled receptors (EP1 and EP4)(41) located on epithelial cells (enterocytes, EC cells) and enteric nerves,(42) resulting in release of acetylcholine at the neuroepithelial junction to evoke a large secretory response by activation of muscarinic receptors. In the presence of indomethacin, INT-777 effect was partially reduced (62.1%), suggesting that activation of TGR5 signaling can inhibit the neuronal and/or cellular pathways recruited by prostaglandins to induce secretion. Furthermore, mechanical stimulation of the mucosal surface, such as can occur during seromuscular stripping, leads to 5-HT release, which activates neurally mediated Cl− and bicarbonate secretion.(43) The neurogenic secretory responses to 5-HT seem to be mediated primarily by 5-HT3 and 5-HT4 receptors, but 5-HT1P receptors might also be involved.(44) Serotonin released from enterochromaffin cells can also stimulate secretion through a direct paracrine action on nearby enterocytes, and this response is mediated by the activation of 5-HT2 receptors. 5-HT4 receptors are expressed in the mucosa (on the apical and basolateral sides(45)), and in the submucosal plexus of murine distal colon, where they are presynaptically located. 5-HT4 receptors are known to mediate the 5-HT-induced increase in acetylcholine and tachykinins release from submucosal IPANs, thereby promoting neurotransmission by enhancing release of excitatory neurotransmitters. The 5-HT4 antagonist, RS-39604, when added on the serosal side, reduced basal distal colon secretion and abolished INT-777 inhibitory effect, suggesting a role for cholinergic IPANs in the inhibitory effect of INT-777. Lastly, nitric oxide (NO) is known to function as a non-adrenergic non-cholinergic (NANC) neurotransmitter in response to neural serotonin receptor activation in the rat(46) and VIP/nNOS submucosal neurons represent 2/3 of neurons in the rat distal colon.(16) Blockade of NO production by L-NAME reduced the basal Isc but also significantly reduced the inhibitory response to INT-777, supporting a role for TGR5 receptors in the modulation of neural (including NANC submucosal neurons) and cellular pathways involved in serotonin-induced secretion.
TGR5 is expressed on both epithelial cells and enteric neurons, and we found that INT-777 inhibitory effect on basal secretion was small and of short duration (<5 min) in neuron-free distal colon preparations, while in the presence of submucosal neurons, the decrease of Isc was strong and maintained for over 45 min with a plateau effect, indicating a much greater inhibitory effect of TGR5 on secretion with an intact submucosal plexus. We therefore attempted to delineate the contribution of submucosal neurons to INT-777-mediated inhibition of colonic secretion. Pretreatment of seromuscular stripped distal colon preparations with the neurotoxin TTX reduced the basal secretory response, confirming the presence of submucosal neurons and a tonic neuronal prosecretory tone in our preparations. TTX also reduced the inhibitory effect of the TGR5 agonist on Isc, but INT-777's response still remained superior to that observed in neuron-free tissues (32.57 ± 9.26% vs 19.50 ± 2.55% of basal Isc, respectively, p<0.05). TTX is an established blocker of voltage-gated sodium channels,(47) and is frequently used as a “gold standard” to evaluate the contribution of neurons in a response.(48) However, TTX-resistant sodium channels also are present in enteric neurons.(49) Hence, taken together our results indicate that the antisecretory effect of the selective TGR5 agonist is mediated by recruitment of both TTX-sensitive and TTX-resistant submucosal neurons combined with a direct action on enterocytes. In support of this theory, pretreatment with lidocaine, a sodium channel blocker acting both on neuronal voltage-gated TTX-sensitive and TTX-resistant sodium channels(49-51) and on basolateral potassium channels in epithelial cells(52, 53) abolished the basal secretion and blocked the effect of INT-777. Altogether, these results suggest that INT-777 inhibits rat distal colonic secretion mainly via the recruitment of submucosal neurons (~2/3 of the response), but that a minor part of this effect is via direct action on colonic epithelial cells (~1/3 of the response).
Considering the abundant expression of TGR5 in cholinergic enteric neurons, one likely mechanism through which INT-777 could reduce basal colonic secretion is via inhibition of acetylcholine release, which has been shown to occur both in neurons and in epithelial cells.(24) We observed that blocking the cholinergic component of the secretory response by antagonizing nAChRs using hexamethonium and mAChRs using atropine before treatment with INT-777 in dC preparation, reduced the basal secretion by the same amount (about 23% each) and led to a partial and significant decrease in INT-777-induced Isc inhibition, that was slightly higher with hexamethonium than atropine (66.8% vs 53.1%). This data suggest that TGR5 may inhibit some of the pathways involved in cholinergic-mediated basal secretion, either by inhibiting the release of acetylcholine from submucosal nerves thereby reducing the cholinergic input to other submucosal neurons (IPANs or cholinergic secretomotor neurons) or to epithelial cells, or by directly inhibiting the release of acetylcholine by epithelial cells. Indeed, muscarinic acetylcholine receptors (mAChRs) type 1 and 3 are present on colonic epithelial cells(54, 55) and M1 are present on postganglionic submucosal enteric neurons.(56, 57) Nicotinic acetylcholine receptors (nAChRs) (α3β2-, α3β4-, α3β2β4- and α7-nAChR) are also present on the soma and fibers of submucosal enteric neurons(58, 59) and recently found to be expressed by colonocytes.(24)
Contrary to Ward et al.(9) who observed an inhibition of muscarinic cholinergic-induced secretion (carbachol) by INT-777 pretreatment in neuron-free tissues, in our preparations containing submucosal neurons, TGR5 activation did not affect carbachol-induced secretion, most likely too powerful to counteract. Similarly, INT-777 did not reduce PGE2 or cisapride-induced secretion but it lowered the secretion induced by nicotine. Nicotine stimulates neurons in submucosal plexus, leading to a TTX resistant anion secretion and an increase in Isc across colonic mucosa.(24) The inhibitory effect of INT-777 on nicotine supports the hypothesis that TGR5 suppresses neural activity in the submucosal plexus. Interestingly, in mice, the distal colon exhibits a significantly larger nicotinic ion transport response than the proximal colon, suggesting that the properties of murine submucosal neurons and their control of epithelial ion transport differ between colonic regions.(15) Whether the regional difference observed in our study in the rat colonic response to TGR5 is linked to similar pathway remains to be demonstrated.
These data raise a point of discussion about the physiological relevance of this unexpected “submucosal retrocontrol” of chloride secretion in the distal colon by the membrane bile acid receptor.(60, 61) Until recently, it has been widely accepted, that bile acids – mainly dihydroxy bile acids- enhance the permeability and the colonic secretion of water or electrolytes. Bajor et al(1) reviewed 4 different described mechanisms involved in this action: in animals, at high concentrations, mucosal damage(6) and increased permeability,(62) cellular activation of adenylate cyclase,(63) and in cell lines, the decrease of chloride uptake by lowering the apical Cl-/OH- exchange. These mechanisms have been described with experimental concentrations of bile acids that are toxic, rarely reached in distal colon lumen and mainly observed in pathologic condition e.g. bile acid malabsorption.(1) On the contrary, lower and more physiologic concentrations of bile acids mediate an anti-secretory effect by acting on basolateral membrane of enterocytes and submucosal neurons and stimulating FXR(31) or TGR5.(9) Our data add to the concept put forward by Keely's group that bile acids behave as colonic osmosignals and can modulate the fluidity of the colonic luminal contents.(7) We also show that this is a complex system of regulation involving not only epithelial cells, but also submucosal neurons. Our data also suggest that nicotinic cholinergic and NANC secretomotor neurons and IPANs contribute to TGR5 inhibition of colonic secretion. Intriguingly, despite the presence of TGR5 receptors in the proximal colon, INT-777 did not have any effect on Isc, suggesting that TGR5-induced inhibition of secretion might be region specific as has been shown for other secretory processes in rodent and human colon.(14, 64, 65) Together with the higher expression of TGR5 in distal versus proximal colon, these data raise the interesting possibility that TGR5 may be involved in site-specific regulation of water colonic absorption: it makes more sense to use this potent “submucosal retrocontrol” at the end of the colon, to provide a fine equilibrium of fecal water content on the final stool, than in the proximal colon, where colonic water and electrolyte are mainly and massively reabsorbed.(66)
In summary, selective serosal, but not apical, activation of TGR5 inhibited the basal and stimulated secretion in rat colon in a region-specific manner (distal but not proximal colon). In the distal colon, TGR5 activation inhibited the basal secretory tone linked to prostaglandins and serotonin release following tissue seromuscular peeling, and the stimulated secretion induced by nicotine, but not PGE2, carbachol or cisapride. This inhibitory effect was mediated by a direct effect both on epithelial cells (1/3 of the response) and on submucosal nerves (2/3 of the response). Together with the dense expression of TGR5 receptors on cholinergic submucosal neurons, our data suggest a major role for TGR5 located on nicotinic cholinergic submucosal neurons, NANC secretomotor neurons and IPANs to inhibit colonic secretion. In conclusion, these data highlight potential new venues for the development of TGR5 agonists as therapeutic tools to modulate colonic secretion in functional bowel disorders such as irritable bowel syndrome, where there is evidence of genetic variation in TGR5 in some patients.(67)
Supplementary Material
Key Points.
Bile acids (BA) stimulate colonic water and electrolyte secretion. However, in rat colonic mucosa, activation of the newly identified membrane BA receptor, TGR5, inhibits epithelial chloride secretion. We aimed to determine submucosal neurons role in TGR5-induced inhibition of colonic secretion.
TGR5 is expressed in the rat colonic epithelium and cholinergic enteric neurons. TGR5 activation reduces basal and stimulated (nicotine) chloride secretion by inhibiting colonocytes and cholinergic submucosal neurons.
Colonic TGR5 could be a potential target against secretory diarrhea-associated GI disorders.
ACKNOWLEDGEMENTS
The authors would like to thanks Pr. Alan Hofmann from UCSD, for his supporting help and advice. The authors would like to thanks Dr. Lucino Adorini from Intercept Pharmaceuticals, (450 W 15th Street, New York, NY 10011) for kindly providing the TGR5 agonist INT-777.
FUNDING
- This work was supported by the Robert Tournut Grant from the Societé Nationale Française de Gastroentérologie (HD).
- This work was supported by a research grant from the Philippe Foundation (HD).
- This work was supported by a mobility grant from Assistance-Publique Hopitaux de Paris (HD).
- This work was supported by the budget program (# 2201250) for education and training of Ukrainian students, graduate students, researchers and teaching staff in leading universities abroad.
- This work was supported by NIH K01 DK088937 (ML).
- This work was supported by NIH P50 DK064539 (YT).
Abbreviations
- CA
cholic acid
- CCh
carbachol
- dC
distal colon
- DCA
deoxycholic acid
- FSK
forskolin
- Isc
short-circuit current
- mAChRs
muscarinic acetylcholine receptors
- nAChRs
nicotinic acetylcholine receptors
- NANC
non-adrenergic non-cholinergic
- NO
nitric oxide
- NOS
nitric oxide synthase
- pC
proximal colon
- pChAT
peripheral choline acetyltransferase
- TDCA
taurodeoxycholic acid
- TEER
transepithelial electrical resistance
- TUDCA
tauro-UDCA
- UDCA
ursodeoxycholic acid
Footnotes
- HD and GT contributed equally to the manuscript
- HD, GT, ML, MB, VW, PQY and IK performed the research
- HD, GT, ML, MM, AY designed the research study
- AY, IK, JK and VW contributed essential reagents
- HD, GT, ML, PQY, VW analyzed the data
- HD, GT and ML wrote the paper.
- AY, MM and YT reviewed the manuscript.
DISCLOSURE
None of the authors have any competing interest to declare.
REFERENCES
- 1.Bajor A, Gillberg PG, Abrahamsson H. Bile acids: short and long term effects in the intestine. Scandinavian journal of gastroenterology. 2010;45:645–664. doi: 10.3109/00365521003702734. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann AF. The enterohepatic circulation of bile acids in mammals: form and functions. Frontiers in bioscience (Landmark edition) 2009;14:2584–2598. doi: 10.2741/3399. [DOI] [PubMed] [Google Scholar]
- 3.Walters JR. Bile acid diarrhoea and FGF19: new views on diagnosis, pathogenesis and therapy. Nature reviews Gastroenterology & hepatology. 2014;11:426–434. doi: 10.1038/nrgastro.2014.32. [DOI] [PubMed] [Google Scholar]
- 4.Edwards CA, Brown S, Baxter AJ, Bannister JJ, Read NW. Effect of bile acid on anorectal function in man. Gut. 1989;30:383–386. doi: 10.1136/gut.30.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder HJ, Rawlins CL. Effect of conjugated dihydroxy bile salts on electrolyte transport in rat colon. The Journal of clinical investigation. 1973;52:1460–1466. doi: 10.1172/JCI107320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camilleri M, Murphy R, Chadwick VS. Dose-related effects of chenodeoxycholic acid in the rabbit colon. Digestive diseases and sciences. 1980;25:433–438. doi: 10.1007/BF01395507. [DOI] [PubMed] [Google Scholar]
- 7.Keating N, Mroz MS, Scharl MM, Marsh C, Ferguson G, Hofmann AF, Keely SJ. Physiological concentrations of bile acids down-regulate agonist induced secretion in colonic epithelial cells. Journal of cellular and molecular medicine. 2009;13:2293–2303. doi: 10.1111/j.1582-4934.2009.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly OB, Mroz MS, Ward JB, Colliva C, Scharl M, Pellicciari R, Gilmer JF, Fallon PG, et al. Ursodeoxycholic acid attenuates colonic epithelial secretory function. The Journal of physiology. 2013;591:2307–2318. doi: 10.1113/jphysiol.2013.252544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward JB, Mroz MS, Keely SJ. The bile acid receptor, TGR5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25:708–711. doi: 10.1111/nmo.12148. [DOI] [PubMed] [Google Scholar]
- 10.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, et al. A G protein-coupled receptor responsive to bile acids. The Journal of biological chemistry. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, Miyamoto Y, Kanatani A, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. The Journal of endocrinology. 2006;191:197–205. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- 12.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22:814–825. e227–818. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke HJ. Role of the “little brain” in the gut in water and electrolyte homeostasis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1989;3:127–138. doi: 10.1096/fasebj.3.2.2464517. [DOI] [PubMed] [Google Scholar]
- 14.Klompus M, Ho W, Sharkey KA, McKay DM. Antisecretory effects of neuropeptide Y in the mouse colon are region-specific and are lost in DSS-induced colitis. Regulatory peptides. 2010;165:138–145. doi: 10.1016/j.regpep.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Foong JP, Tough IR, Cox HM, Bornstein JC. Properties of cholinergic and non cholinergic submucosal neurons along the mouse colon. The Journal of physiology. 2014;592:777–793. doi: 10.1113/jphysiol.2013.265686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chino Y, Fujimura M, Kitahama K, Fujimiya M. Colocalization of NO and VIP in neurons of the submucous plexus in the rat intestine. Peptides. 2002;23:2245–2250. doi: 10.1016/s0196-9781(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham SM, Hirai K, Mihara S, Lees GM. Electrophysiological characteristics of submucosal neurones in the proximal colon of guinea-pigs: comparisons with caecum and descending colon. Experimental physiology. 1997;82:859–870. doi: 10.1113/expphysiol.1997.sp004069. [DOI] [PubMed] [Google Scholar]
- 18.Kaji I, Akiba Y, Said H, Narimatsu K, Kaunitz JD. Luminal 5-HT stimulates colonic bicarbonate secretion in rats. British journal of pharmacology. 2015;172:4655–4670. doi: 10.1111/bph.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan PQ, Wu SV, Pothoulakis C, Tache Y. Urocortins and CRF Receptor Type 2 Variants in the Male Rat Colon: Gene Expression and Regulation by Endotoxin and Anti-inflammatory Effect. American journal of physiology Gastrointestinal and liver physiology. 2016:ajpgi.00337.02015. doi: 10.1152/ajpgi.00337.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan PQ, Million M, Wu SV, Rivier J, Tache Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2007;19:923–936. doi: 10.1111/j.1365-2982.2007.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakajima K, Tooyama I, Yasuhara O, Aimi Y, Kimura H. Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats. Journal of chemical neuroanatomy. 2000;18:31–40. doi: 10.1016/s0891-0618(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 22.Schulzke JD, Fromm M, Hegel U, Riecken EO. Ion transport and enteric nervous system (ENS) in rat rectal colon: mechanical stretch causes electrogenic Cl-secretion via plexus Meissner and amiloride-sensitive electrogenic Na-absorption is not affected by intramural neurons. Pflugers Archiv : European journal of physiology. 1989;414:216–221. doi: 10.1007/BF00580966. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Navarro R, Martinez-Augustin O, Ballester I, Zarzuelo A, Sanchez de Medina F. Experimental inflammation of the rat distal colon inhibits ion secretion in the proximal colon by affecting the enteric nervous system. Naunyn-Schmiedeberg's archives of pharmacology. 2005;371:114–121. doi: 10.1007/s00210-005-1023-0. [DOI] [PubMed] [Google Scholar]
- 24.Bader S, Diener M. Novel aspects of cholinergic regulation of colonic ion transport. Pharmacology research & perspectives. 2015;3:e00139. doi: 10.1002/prp2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozarov A, Wang YZ, Yu JG, Wunderlich J, Hassanain HH, Alhaj M, Cooke HJ, Grants I, et al. Activation of adenosine low-affinity A3 receptors inhibits the enteric short interplexus neural circuit triggered by histamine. American journal of physiology Gastrointestinal and liver physiology. 2009;297:G1147–1162. doi: 10.1152/ajpgi.00295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berni Canani R, Cirillo P, Mallardo G, Buccigrossi V, Passariello A, Ruotolo S, De Marco G, Porcaro F, et al. Growth hormone regulates intestinal ion transport through a modulation of the constitutive nitric oxide synthase-nitric oxide-cAMP pathway. World journal of gastroenterology. 2006;12:4710–4715. doi: 10.3748/wjg.v12.i29.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaji I, Akiba Y, Konno K, Watanabe M, Kimura S, Iwanaga T, Kuri A, Iwamoto KI, et al. Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon. The Journal of physiology. 2016 doi: 10.1113/JP271441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaji I, Karaki S, Fukami Y, Terasaki M, Kuwahara A. Secretory effects of a luminal bitter tastant and expressions of bitter taste receptors, T2Rs, in the human and rat large intestine. American journal of physiology Gastrointestinal and liver physiology. 2009;296:G971–981. doi: 10.1152/ajpgi.90514.2008. [DOI] [PubMed] [Google Scholar]
- 29.Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keely SJ, Scharl MM, Bertelsen LS, Hagey LR, Barrett KE, Hofmann AF. Bile acid- induced secretion in polarized monolayers of T84 colonic epithelial cells: Structure-activity relationships. American journal of physiology Gastrointestinal and liver physiology. 2007;292:G290–297. doi: 10.1152/ajpgi.00076.2006. [DOI] [PubMed] [Google Scholar]
- 31.Mroz MS, Keating N, Ward JB, Sarker R, Amu S, Aviello G, Donowitz M, Fallon PG, et al. Farnesoid X receptor agonists attenuate colonic epithelial secretory function and prevent experimental diarrhoea in vivo. Gut. 2014;63:808–817. doi: 10.1136/gutjnl-2013-305088. [DOI] [PubMed] [Google Scholar]
- 32.Iguchi Y, Nishimaki-Mogami T, Yamaguchi M, Teraoka F, Kaneko T, Une M. Effects of chemical modification of ursodeoxycholic acid on TGR5 activation. Biological & pharmaceutical bulletin. 2011;34:1–7. doi: 10.1248/bpb.34.1. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochemical and biophysical research communications. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 34.Krag E, Phillips SF. Active and passive bile acid absorption in man. Perfusion studies of the ileum and jejunum. The Journal of clinical investigation. 1974;53:1686–1694. doi: 10.1172/JCI107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bornstein JC, Gwynne R, Sjövall H. Enteric Neural Regulation of Mucosal Secretion. In: Johnson LR, Ghishan FK, Kaunitz JD, Merchant JL, M. SH, Wood JD, editors. Physiology of the Gastrointestinal Tract (Fifth Edition) Elsevier; 2012. pp. 769–790. [Google Scholar]
- 36.Mitsui R. Immunohistochemical characteristics of submucosal Dogiel type II neurons in rat colon. Cell and tissue research. 2010;340:257–265. doi: 10.1007/s00441-010-0954-z. [DOI] [PubMed] [Google Scholar]
- 37.Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. The American journal of physiology. 1997;273:G422–435. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- 39.Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:3295–3309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bern MJ, Sturbaum CW, Karayalcin SS, Berschneider HM, Wachsman JT, Powell DW. Immune system control of rat and rabbit colonic electrolyte transport. Role of prostaglandins and enteric nervous system. The Journal of clinical investigation. 1989;83:1810–1820. doi: 10.1172/JCI114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakab RL, Collaco AM, Ameen NA. Lubiprostone targets prostanoid signaling and promotes ion transporter trafficking, mucus exocytosis, and contractility. Digestive diseases and sciences. 2012;57:2826–2845. doi: 10.1007/s10620-012-2352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding M, Kinoshita Y, Kishi K, Nakata H, Hassan S, Kawanami C, Sugimoto Y, Katsuyama M, et al. Distribution of prostaglandin E receptors in the rat gastrointestinal tract. Prostaglandins. 1997;53:199–216. doi: 10.1016/s0090-6980(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 43.Stoner MC, Scherr AM, Lee JA, Wolfe LG, Kellum JM. Nitric oxide is a neurotransmitter in the chloride secretory response to serotonin in rat colon. Surgery. 2000;128:240–245. doi: 10.1067/msy.2000.107608. [DOI] [PubMed] [Google Scholar]
- 44.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nature reviews Gastroenterology & hepatology. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854. e844. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King BN, Haque SM, Stoner MC, Ellis ZM, Kellum JM. Inhibition of neural nitric oxide synthase attenuates the chloride secretory response to stroking in human jejunum. Surgery. 2003;134:255–259. doi: 10.1067/msy.2003.230. [DOI] [PubMed] [Google Scholar]
- 47.Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, et al. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 48.Narahashi T, Moore JW, Scott WR. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. The Journal of general physiology. 1964;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida S. Tetrodotoxin-resistant sodium channels. Cellular and molecular neurobiology. 1994;14:227–244. doi: 10.1007/BF02088322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padilla F, Couble ML, Coste B, Maingret F, Clerc N, Crest M, Ritter AM, Magloire H, et al. Expression and localization of the Nav1.9 sodium channel in enteric neurons and in trigeminal sensory endings: implication for intestinal reflex function and orofacial pain. Molecular and cellular neurosciences. 2007;35:138–152. doi: 10.1016/j.mcn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Scholz A, Kuboyama N, Hempelmann G, Vogel W. Complex blockade of TTX-resistant Na+ currents by lidocaine and bupivacaine reduce firing frequency in DRG neurons. Journal of neurophysiology. 1998;79:1746–1754. doi: 10.1152/jn.1998.79.4.1746. [DOI] [PubMed] [Google Scholar]
- 52.Richards NW, Dawson DC. Single potassium channels blocked by lidocaine and quinidine in isolated turtle colon epithelial cells. The American journal of physiology. 1986;251:C85–89. doi: 10.1152/ajpcell.1986.251.1.C85. [DOI] [PubMed] [Google Scholar]
- 53.Laffon M, Jayr C, Barbry P, Wang Y, Folkesson HG, Pittet JF, Clerici C, Matthay MA. Lidocaine induces a reversible decrease in alveolar epithelial fluid clearance in rats. Anesthesiology. 2002;96:392–399. doi: 10.1097/00000542-200202000-00026. [DOI] [PubMed] [Google Scholar]
- 54.Haberberger R, Schultheiss G, Diener M. Epithelial muscarinic M1 receptors contribute to carbachol-induced ion secretion in mouse colon. European journal of pharmacology. 2006;530:229–233. doi: 10.1016/j.ejphar.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 55.O'Malley KE, Farrell CB, O'Boyle KM, Baird AW. Cholinergic activation of Cl- secretion in rat colonic epithelia. European journal of pharmacology. 1995;275:83–89. doi: 10.1016/0014-2999(94)00758-y. [DOI] [PubMed] [Google Scholar]
- 56.Harrington AM, Hutson JM, Southwell BR. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Progress in histochemistry and cytochemistry. 2010;44:173–202. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Harrington AM, Peck CJ, Liu L, Burcher E, Hutson JM, Southwell BR. Localization of muscarinic receptors M1R, M2R and M3R in the human colon. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22:999–1008. e1262–1003. doi: 10.1111/j.1365-2982.2009.01456.x. [DOI] [PubMed] [Google Scholar]
- 58.Glushakov AV, Voytenko LP, Skok MV, Skok V. Distribution of neuronal nicotinic acetylcholine receptors containing different alpha-subunits in the submucosal plexus of the guinea-pig. Autonomic neuroscience : basic & clinical. 2004;110:19–26. doi: 10.1016/j.autneu.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Obaid AL, Nelson ME, Lindstrom J, Salzberg BM. Optical studies of nicotinic acetylcholine receptor subtypes in the guinea-pig enteric nervous system. The Journal of experimental biology. 2005;208:2981–3001. doi: 10.1242/jeb.01732. [DOI] [PubMed] [Google Scholar]
- 60.Bunnett NW. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. The Journal of physiology. 2014;592:2943–2950. doi: 10.1113/jphysiol.2014.271155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camilleri M, Gores GJ. Therapeutic targeting of bile acids. American journal of physiology Gastrointestinal and liver physiology. 2015;309:G209–215. doi: 10.1152/ajpgi.00121.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. The Journal of laboratory and clinical medicine. 1979;94:661–674. [PubMed] [Google Scholar]
- 63.Conley DR, Coyne MJ, Bonorris GG, Chung A, Schoenfield LJ. Bile acid stimulation of colonic adenylate cyclase and secretion in the rabbit. The American journal of digestive diseases. 1976;21:453–458. doi: 10.1007/BF01072128. [DOI] [PubMed] [Google Scholar]
- 64.Krueger D, Michel K, Zeller F, Demir IE, Ceyhan GO, Slotta-Huspenina J, Schemann M. Neural influences on human intestinal epithelium in vitro. The Journal of physiology. 2016;594:357–372. doi: 10.1113/JP271493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Przyborski SA, Levin RJ. Cholinergic modulation of electrogenic ion transport in different regions of the rat small intestine. The Journal of pharmacy and pharmacology. 1997;49:691–697. doi: 10.1111/j.2042-7158.1997.tb06094.x. [DOI] [PubMed] [Google Scholar]
- 66.Szmulowicz UM, Hull TL. Colonic Physiology. In: Beck DE, Wexner SD, Hull TL, et al., editors. The ASCRS Manual of Colon and Rectal Surgery. Springer; New York: 2014. pp. 27–48. [Google Scholar]
- 67.Camilleri M, Shin A, Busciglio I, Carlson P, Acosta A, Bharucha AE, Burton D, Lamsam J, et al. Genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. American journal of physiology Gastrointestinal and liver physiology. 2014;307:G508–516. doi: 10.1152/ajpgi.00178.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.