Abstract
Young children <2 years of age with chronic end-stage liver disease (YC2) are a uniquely vulnerable group listed for liver transplantation, characterized by a predominance of biliary atresia (BA). To investigate wait-list mortality, associated risk factors and outcomes of YC2, we evaluated UNOS registry data from April 2003-March 2013 for YC2 listed for deceased donor transplant (BA=994, other chronic liver disease, CLD=221). Overall, wait-list mortality among YC2 was 12.4% and post-transplant mortality was 8%, accounting for an overall post-listing mortality of 19.6%. YC2 demonstrated 12.2%, 18.7%, and 20.6% wait-list mortality by 90, 180, and 270 days, respectively. YC2 with CLD demonstrated significantly higher wait-list mortality compared to BA among YC2 (23.9% vs 9.8%, P<.05). Multivariable analyses revealed that listing PELD>21 (HR 3.2, 95% CI: 1.6, 6.5), lack of exception (HR 5.8, 95% CI: 2.8-11.8), listing height<60.6 cm (HR 2.1, 95% CI:1.4, 3.1), listing weight >10 kg (HR 3.8, 95% CI: 1.5, 9.2) and initial creatinine >0.5 (HR 6.8, 95% CI: 3.4-13.5) were independent risk factors for YC2 wait-list mortality (p<.005 for all). Adjusting for all variables, the risk of death among CLD patients was 2 (95% CI: 1.3, 3.1) times greater than patients with BA+surgery (presumed Kasai). Further, the risk of death in BA-surgery was 1.9 (95% CI: 1, 3.4) times greater than BA with presumed Kasai. Our data highlight unacceptably high waitlist and early post liver transplant mortality in YC2 not predicted by PELD and suggest key risk factors deserving of further study in this age group.
Keywords: Pediatrics, liver transplant, outcomes, survival, registry
Introduction
Young children <2 years of age with chronic end-stage liver disease (YC2) comprise a uniquely vulnerable cohort of children listed for liver transplant (LT), with a preponderance of biliary atresia (BA) in this age cohort 1. LT in YC2 requires special considerations relevant to donor allocation, paucity of size and age-matched donors, option of segmental transplants, and surgical issues unique to BA 2. The pediatric end-stage liver disease (PELD) score ranks and prioritizes children based on the severity of chronic liver disease (CLD) on a single liver waiting list which includes adults according to their probability of death within 90 days of listing 3,4 It has been suggested that actual PELD scores and the clinical variables used to calculate PELD often do not lead to timely allocation of livers to children and underestimates the near-term risk of death 5.
YC2 patients with BA, an isolated cholestatic liver disease with the exception of “syndromic” BA which can present with polysplenia and situs inversus 6, have recently been found to have higher waitlist and post transplant mortality than older children 7. However the impact of Kasai hepatoenterostomy in large-scale studies and outcomes in YC2 with other chronic liver diseases that may have multi-systemic involvement, and thus a theoretical risk for increased mortality have not been well studied. A recent study of Brazilian children, most whom were less than one year of age but had access to living donor transplantation 8 also suggests that up to 1 in 6 YC2 listed for transplant die while waiting. To further investigate the scale of this problem across the US, we evaluated mortality and transplant outcomes for all YC2 listed for liver transplant under the United Network of Organ Sharing/Organ Procurement and Transplantation Network (UNOS/OPTN) between 2003 and 2013, separated into eras. The primary objective of the study was to investigate wait-list and post-transplant mortality of YC2 with BA or other chronic liver disease (CLD) and explore demographic, clinical and laboratory differences between these groups. A secondary objective was to determine clinically relevant risk factors for wait-list mortality in a comprehensive survival analysis.
Methods
Data Source
Detailed data were obtained from the UNOS/OPTN database collected via an online Web application called UNetsm. This application is used to manage wait-listed transplant candidates, access and complete electronic data collection forms, and access various transplant data reports. The 26 different form types contain more than 3,500 data fields. These data stored in the UNOS/OPTN database are de-identified and available to the public.
Cohort Description
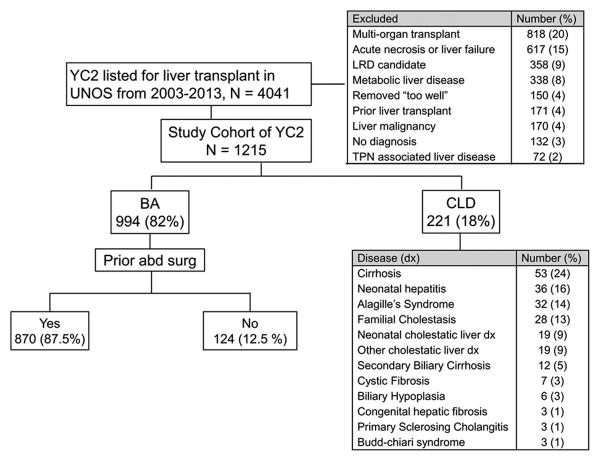
Of the 21,177 children <18 years of age listed for first liver transplant between April 2003 and March 2013 in the United States, we retrospectively studied all YC2 with chronic liver disease. We excluded: 1) children listed for multi-organ transplants, 2) acute liver failure, 3) living related donor (LRD) candidates, 4) metabolic liver disease, 5) removed because they were “too well”, 6) children with a prior liver transplant, 7) liver malignancy, 8) unknown diagnosis, or 9) TPN associated liver disease. CLD, for the purposes of this study was defined as chronic liver disease in the absence of acute liver failure or injury that included any of the following: cryptogenic cirrhosis, neonatal hepatitis, Alagille syndrome, progressive familial intrahepatic cholestasis (PFIC), other cholestatic liver disease, secondary biliary cirrhosis, cystic fibrosis, biliary hypoplasia, congenital hepatic fibrosis, Budd-Chiari, and primary sclerosing cholangitis.
Variables of Interest
Clinical, laboratory, and demographic variables were collected at time of listing, time of death, or time of transplant. Ages were reported to UNOS as <1 year or 1-2 years only. The PELD Score is calculated as follows: 0.436 (Age (<1 YR.))-0.687 × Loge (albumin g/dL) +0.480 × Loge (total bilirubin mg/dL) +1.857 × Loge (INR) + 0.667 (If patient has growth failure (<-2 Std. Deviation)). Delta PELD (ΔPELD), a measure of worsening clinical severity of disease, was defined as the change in the PELD score from the time of listing to the time of outcome. YC2 were stratified into two groups, BA or CLD based on registry verification of diagnosis at listing. BA with or without “prior abdominal surgery” (suspected to be a Kasai hepatoportoenterostomy procedure) per the UNOS registry was also analyzed to determine impact on outcome. Wait-list mortality was defined as a.) wait-list death or b.) removal from waitlist due to “too sick to transplant” (TST). Post-transplant mortality was divided into 2 sub-groups: Early Death (ED) – death ≤90 days following liver-transplant and Late Death (LD) – death >90 days following transplant.
Statistical analysis
Descriptive statistics were calculated for the total study population and compared between BA and CLD, survivors and non-survivors. Continuous variables were compared using ANOVA test and Student's t test allowing for unequal variances and categorical variables with Kruskal-Wallis test, a Chi-square or Fisher's exact test when appropriate. A p<0.05 was considered statistically significant.
Survival analysis was used to estimate the associations between independent variables and waitlist death. Time-to-death was calculated as the number of days from listing to death. Patients who did not die were censored for death at the time of transplant or the last date of follow-up. Patients who were removed from the waitlist due to TST were not considered to be censored observations. These patients were assumed to have died while on the waitlist. Kaplan-Meier curves and the log-rank test statistic were estimated and used to compare the time-to-waitlist death between groups for each of the independent variables. Continuously measured variables were initially categorized by quartiles. If the overall log-rank test was significant at the 0.1 level, then all pairwise comparisons were made between the groups. Groups that were not significant at the 0.05 level were pooled together to reduce the number of groups. Clinically relevant cutoffs were also investigated in the final Cox regression model. The multiple Cox regression model was used to estimate the hazards ratio with 95% confidence intervals. Statistical significance for the final multiple regression model was assessed at the P<.05 level. Statistical analysis was performed using SAS 9.4 (Cary, NC).
Ethical considerations
An IRB protocol for this study was submitted to the Baylor College of Medicine Institutional Review Board which provided a memorandum that this activity does not constitute human subjects research. Given that the information received from the UNOS/OPTN database is de-identified, no authorization or waiver of authorization by patients for the release of individually identifiable protected health information was required and the study does not fall under the regulation for IRB review of human subjects research found at 45 CR 46.
Results
Socio-demographic variables
The study population included 1215 YC2 with chronic liver disease who were included in the UNOS transplant database and listed for first liver transplant during the defined study period (Figure 1). Most YC2 had BA (994 (82%)) while the remainder had other forms of CLD. Within the BA group, 870 (87.5%) patients had undergone “prior abdominal surgery”, or suspected Kasai procedure. Table 1 describes their demographic and clinical characteristics at the time of listing and outcome. Overall, the study cohort was 58.6% female, and a significant female predominance was observed in the BA group when compared to the CLD group [60.1% vs. 52% (P<.001)]. The BA group at listing, had a higher proportion of children <1 year than the CLD group (85.9% vs. 70.6%, P<.001). There were no racial or ethnic differences between BA and CLD (P=0.59).
Figure 1. Young children <2 years of age with chronic end-stage liver disease (YC2) listed for liver transplant under UNOS/OPTN between 2003-2013.
YC2 – children <2 years of age with known end-stage chronic liver disease; BA – Biliary atresia, CLD – Other chronic liver disease, LRD – Living related donor
All values are reported as numbers. Percentages are rounded to whole numbers.
Table 1. Clinical characteristics and outcomes of young children <2 years of age with end-stage liver disease listed for liver transplant (YC2) with biliary atresia (BA) vs other chronic liver disease (CLD).
| BA (n=994) | CLD (n=221) | P-value | ||
|---|---|---|---|---|
| Age group at listing listing | 0-1 years | 854 (85.9%) | 156 (70.6%) | <.001 |
| 1-2 years | 140 (14.1%) | 65 (29.4%) | <.05 | |
| Female % | 597 (60.1%) | 115 (52%) | <.001 | |
| Ethnicity | Caucasian | 442 (44.5%) | 88 (39.8%) | .62 |
| African American | 207 (20.8%) | 45 (20.4%) | .86 | |
| Hispanic | 229 (23%) | 67 (30.3%) | .34 | |
| Asian | 75 (7.5%) | 8 (3.6%) | .23 | |
| Other | 41 (4.1%) | 13 (5.9%) | .83 | |
| Mean PELD Score | At Listing | 15.1 (±9.8) | 19.9 (±13.5) | <.001 |
| Outcome | 19.3 (±11.8) | 22.8 (±14.3) | <.001 | |
| Δ PELD | 4.1 (±10.2) | 2.8 (±10.9) | .12 | |
| Outcomes | Transplanted (alive) | 830 (83.5%) | 147 (66.5%) | <.001 |
| Death after removal | 37 (3.7%) | 16 (7.2%) | <.05 | |
| Death on waitlist | 61 (6.1%) | 37 (16.7%) | <.001 | |
| Early Deathπ | 43 (4.3%) | 7 (3.2%) | .43 | |
| Late Death€ | 23 (2.3%) | 14 (6.3%) | <.05 | |
| Wait time (days) | 123 (±173) | 105 (±178) | .16 | |
| Clinical Characteristics | BA (n=848) | CLD (n=174) | ||
| Total bilirubin | Listing | 11.9 (±7.4) | 15.4 (±10.2) | <.001 |
| Outcome | 14.8 (±10.6) | 16.3 (±12) | .09 | |
| Albumin | Listing | 3 (±0.7) | 3 (±0.7) | >.99 |
| Outcome | 3 (±0.7) | 3 (±0.9) | >.99 | |
| INR | Listing | 1.5 (±0.9) | 2 (±1.1) | <.001 |
| Outcome | 1.8 (±3.3) | 2.5 (±2.2) | <.05 | |
Early Death defined as death ≤90 days after transplant
Late Death defined as death >90 days after transplant
All values are reported as means ± standard deviation except for the categorical variables, which are reported as numbers.
Overall and Disease-specific Outcomes and Mortality Rates
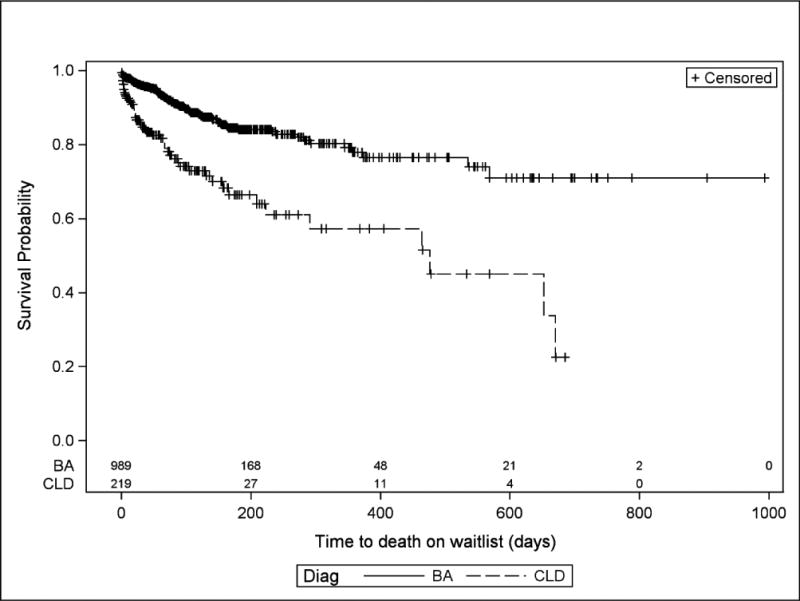
For all YC2 listed for liver transplant, wait-list mortality was 12.4% (95% CI: 10.6, 14.4) and post-transplant mortality was 8% (Table 2), accounting for an overall post-listing mortality of 19.6%. YC2 in this study demonstrated 12.2%, 18.7%, and 20.6% wait-list mortality by 90, 180, and 270 days, respectively. (Figure 2) The majority of deaths while waiting occurred while active on the wait list (64.9%) and the remainder after removal from the waitlist because they were too sick to transplant (TST). The CLD group demonstrated significantly higher overall wait-list mortality compared to BA among YC2 (23.9% vs 9.8%, P<.05) (Table 1 and Figure 3). Similarly, late deaths occurred in 6.3% of CLD while only 2.3% of the BA group experienced late deaths (P<.01). There was no difference in early death rates between BA and CLD.
Table 2. Overall Outcome and Mortality of young children <2 years of age with end-stage liver disease listed for liver transplant (YC2).
| All N = 1215 |
Post-Transplant N = 1064 |
P-value | |
|---|---|---|---|
| Death on waitlist | 98 (8.1%) | ||
| Death after removal (TST)rereremoval | 53 (4.4%) | ||
| Early Deathπ | 50 (4.1%) | 50 (4.7%) | 0.5 |
| Late Death€ | 37 (3%) | 37 (3.5%) | 0.6 |
| Transplanted (alive) | 977 (80.4%) | 977 (91.8%) | <0.001 |
Early Death is defined as death ≤90 days after transplant
Late Death is defined as death >90 days after transplant
Figure 2. Mortality risk over time of all YC2 on the waiting list under UNOS/OPTN between 2003 and 2013.
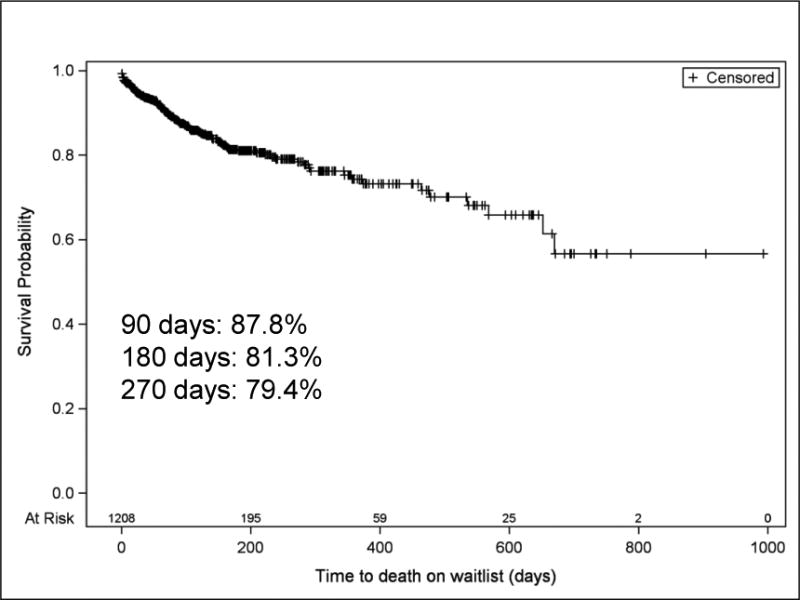
Figure 3. Comparison of Mortality risk over time of YC2 with BA and CLD on the waiting list under UNOS/OPTN between 2003 and 2013.
BA patients who died on the waitlist or early post-transplant were older (1-2 years: 42% vs. 15%, P<.001) and predominantly female (89% vs 60%, P< .001) compared to CLD. BA patients with “no abdominal surgery” (suspected no Kasai) experienced more late deaths (LD) (5.1% vs 1.9%, P<.05). However, “prior abdominal surgery” or suspected Kasai status in BA patients was no different among transplanted survivors (Kasai 84.1% vs No Kasai 79.4%, P=.16), waitlist deaths (Kasai 9.3% vs No Kasai 11.8%, P=.10), or early death (ED) post-transplant (Kasai 4.4% vs No Kasai 3.4%, P=.58 (Supplementary Table 5).
Waitlist period
No significant differences were noted in the waitlist times between BA and CLD (123 days±173 vs 105 days±178, P=.16) (Table 1). Conversely, BA patients with “prior abdominal surgery” had a longer waitlist time as compared to patients with “no abdominal surgery” (126±175 vs 101±158 days, P <.001) (Supplementary Table 5).
PELD score and biochemical parameters
At both listing and at outcome, mean PELD scores were lower in BA compared to CLD (15.1 vs 19.9, P<.001; 19.3 vs 22.8, P<.001). However, no difference was noted in ΔPELD for the 2 groups (Table 1). BA patients with “no abdominal surgery” (no suspected Kasai) had higher PELD scores at both listing and outcome than those with “prior abdominal surgery” (initial: 17 vs 14.5, outcome: 22 vs 19, all P<.001). However, CLD patients had higher initial total bilirubin (P<.001), initial INR (P<.001), and initial creatinine (P<.001), than both BA groups, though no differences in initial sodium or initial weight (Table 3.)
Table 3. PELD and non-PELD variables of YC2 BA with (+) and without (-) abdominal surgery (presumed Kasai) and CLD.
| Diagnosis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA with abd surgery (+) | BA no abd surgery (-) | CLD | ||||||||||||||
| N | Missing | Median | Min | Max | N | Missing | Median | Min | Max | N | Missing | Median | Min | Max | P-value | |
| Initial PELD | 870 | 0 | 14.5 | -9.00 | 90 | 124 | 0 | 17 | -10 | 52 | 221 | 0 | 19 | -9 | 99 | <.001 |
| Initial Height (cm) | 869 | 1 | 64 | 23.7 | 163 | 124 | 0 | 63.5 | 43.2 | 142.2 | 221 | 0 | 64.5 | 32.3 | 157.5 | .46 |
| Initial Weight (kg) | 870 | 0 | 6.6 | 2.7 | 64.1 | 124 | 0 | 6.7 | 3.6 | 15.9 | 221 | 0 | 6.8 | 2.7 | 15.8 | .5 |
| Initial Creatinine | 795 | 75 | 0.2 | 0 | 4.4 | 114 | 10 | 0.2 | 0.1 | 0.8 | 202 | 19 | 0.3 | 0.1 | 1.5 | <.001 |
| Initial Sodium | 751 | 119 | 136 | 106 | 155 | 105 | 19 | 137 | 122 | 145 | 188 | 33 | 136 | 116 | 159 | .3 |
| Outcome PELD | 870 | 0 | 19 | -11 | 94 | 124 | 0 | 22 | -10 | 52 | 221 | 0 | 22 | -10 | 71 | <.001 |
| Final Creatinine | 815 | 55 | 0.2 | 0 | 3.9 | 116 | 8 | 0.2 | 0.1 | 2.6 | 205 | 16 | 0.3 | 0 | 3 | <.001 |
| Final Sodium | 772 | 98 | 137 | 114 | 159 | 111 | 13 | 136 | 116 | 153 | 197 | 24 | 138 | 116 | 167 | .1 |
All values for continuous variables are reported as medians (min,max)
The underlying biochemical parameters of PELD score (INR, bilirubin and albumin, Table 1) in addition to creatinine and sodium (Table 3) were analyzed in all children for whom the values were documented. Total bilirubin was significantly higher in CLD at listing than their BA counterparts at 15.4 mg/dL and 11.9 mg/dL, respectively (P<.001), though not at outcome. The INR for CLD was also higher at listing than BA (2 vs. 1.5, P<.001). At the time of outcome, coagulopathy had increased in both groups, though CLD remained more severe than BA (INR 2.5 vs 1.8, P<0.01). There were no differences in serum albumin of BA vs CLD at the time of listing or outcome (Table 1).
Risk factors for wait-list mortality
The risk of death was 1.6 (95% CI: 0.92, 2.7) times greater among BA patients with “no abdominal surgery” (presumed no Kasai) compared with BA patients with “prior abdominal surgery” (presumed Kasai). The hazard ratio for CLD vs. BA with “prior abdominal surgery” was 2.98 (95% CI: 2.11, 4.22). Gender, region, initial age group, initial total bilirubin, initial INR, initial encephalopathy, exception request (data not shown) as well as initial PELD, initial height, initial creatinine, and initial weight (Table 3) were significantly different between diagnosis groups at the .05 level in the univariable analysis. A multiple Cox regression model was used to adjust for all of these variables as well as era, albumin, and sodium. Initial ascites and initial encephalopathy were excluded from this final multiple regression model because of missing data (415 and 494 missing initial ascites and encephalopathy status, respectively). BMI was also excluded from the multiple regression model because BMI was missing for all of the deceased patients except one. The final model included 973 patients. After adjusting for these variables, diagnosis group maintained a statistically significant association with wait-list mortality (P=0.003). Table 4 summarizes the adjusted hazards ratios for variables that were significantly (P<0.05) associated with waitlist death. YC2 listed with PELD>21 had a 3.2 (95% CI: 1.6-6.5) times greater risk of wait-list death than those whose PELD were <16. YC2 without petitioned exception also had a 5.8 (95% CI: 2.8-11.8, P<.001) times greater risk of wait-list mortality. Initial height (<60.6 cm, P<.001) and weight (>10 kg, P=.004) were associated with a 2.1 and 3.8 times risk of increased wait-list mortality, respectively. YC2 listed with a creatinine >0.5 also had a 6.8 (95% CI: 3.4-13.5, P<.001) times greater risk of death than those with levels <0.5. Notably 8 of 9 YC2 listed with a creatinine>0.5 died on the wait-list. Adjusting for all variables in the model, the risk of death among CLD patients was 2 (95% CI: 1.3, 3.1) times greater than patients in the BA with “prior abdominal surgery” group. Further, the risk of death in BA with “no abdominal surgery” was 1.9 (95% CI: 1, 3.4) times greater than BA patients who presumably had Kasai.
Table 4. Multivariable analysis of clinical variables associated with wait-list mortality.
| Clinical variable | Classification | Estimate | SE | Hazard Ratio | P-value | 95% CI |
|---|---|---|---|---|---|---|
| Diagnosis | BA, no abd surgery | 0.6 | 0.3 | 1.9 | .05* | 1-3.4 |
| CLD | 0.7 | 0.2 | 2 | <.001* | 1.3-3.1 | |
| Initial PELD | 16-21 | 0.9 | 0.3 | 2.5 | <.001+ | 1.5-4.4 |
| Initial PELD | >21 | 1.2 | 0.4 | 3.2 | <.001+ | 1.6-6.5 |
| Exception requested | No | 1.8 | 0.4 | 5.8 | <.001 | 2.8-11.8 |
| Initial Height | < 60.6 cm | 0.7 | 0.2 | 2.1 | <.001‡ | 1.4-3.1 |
| Initial Weight | ≥ 10 kg | 1.3 | 0.5 | 3.8 | .004€ | 1.5-9.2 |
| Initial Creatinine | >0.5 | 1.9 | 0.4 | 6.8 | <.001Δ | 3.4-13.5 |
Compared with BA with abdominal surgery
Compared with PELD < 16
Compared with height ≥ 60.6 cm
Compared with weight <10 kg
Compared with creatinine ≤ 0.5
Era Findings
By eras, namely 2003-2005, 2006-2009, and 2010-2013, 57 (21.8%), 138 (27.3%), and 143 (36.5%) YC2 with chronic liver disease received exception points at an increasing rate over time (P<.001), respectively. However, there was no difference in survival between eras based on the listing date (P=.4).
Discussion
Waitlist mortality in young children depends on the severity and duration of the liver disease (may be rather severe in a short time such as in missed BA), co-morbid extrahepatic disease, nutrition, and organ availability. We reviewed 10 years of UNOS/OPTN data and found that patient survival following listing for liver transplantation among children less than the age of 2 years was only 80.4%. In contrast, but not surprisingly, post-transplant survival was 91.8%. The high incidence of death, translating to nearly 1 in 8 among young children less than 2 years of age dying while waiting for a suitable deceased donor organ, suggest that current reliance on PELD scores to stratify organ allocation in this age group may be inadequate to predict death [6]. Systematic measures to ameliorate this unacceptable pretransplant mortality in this age group are deserving of widespread attention.
Despite UNOS allowance for regional and case-specific review of petitions for exception points based on medical need in pediatric patients and increasing utilization, 12.4% of our young study cohort died on the wait-list. These results are a divergence from earlier published data. According to a BA only study in 2005 1, using the SPLIT database, only 3% patients died awaiting transplant. Of their 755 patients, the probability of survival from the time of listing was 91% at 6 months, 89% at 1 year, and 86% at 3 years. However, our survival analysis demonstrated only 81.3% survival of YC2 at 6 months. Our review of the UNOS registry between 2003-2013 among children <2 years of age awaiting liver transplant revealed that nearly 10% of BA patients and a surprising 24% of CLD patients died while waiting on the list. While mortality rates of waitlisted children under 1 year of age have steadily declined since 2006 9, our survival analysis is concerning as we found no era differences in mortality among young children despite increases in use of exception, ranging between 30-50% as demonstrated by Hsu et al and others. 5,10 Our data reveal that higher PELD scores, particularly those with a PELD >21, correlate with a >3 times higher risk of wait-list mortality. Young children who did not receive exception points also demonstrated a nearly 6 times higher risk of wait-list mortality than those who did, highlighting again the current practice of petitioning for exception points as natural PELD often does not reflect the current morbid state of a child being listed for transplant. Interestingly, a lower initial height (<60.6 cm in this study) among young children was associated with a 2 times higher risk of death on the wait-list, suggesting that stunting may reflect poor overall nutritional status and survival. Ironically, we found that higher weight at listing (>10 kg) among children less than 2 years demonstrated a nearly 4 times higher risk of death compared to children with a lower weight. While ascites was not found to be a predictor of mortality due to high missingness, this finding of increased weight may be a surrogate of ascites given that many cholestatic children <2 years of age achieve a weight >10 kg only with enteral or total parenteral nutrition. Lastly, while the confidence intervals were high and the sample size small, the few young children listed through UNOS with an initial creatinine >0.5 had a nearly 7 times higher risk of wait-list death than those with lower values. Serum creatinine, a variable included in MELD score, is not included in PELD because it was not found to be a predictor of mortality in pediatric liver disease 3. Larger cohort studies examining the role and predictive value of an elevated creatinine are needed.
The reasons for high wait list mortality for this age group may be in part explained by our findings but we speculate that increasing competition for donor livers from those listed as Status 1A and 1B (metabolic diseases), for split adult donor livers, delayed referrals or longer waiting times of sicker patients are contributory. Allocation policies and demand have certainly changed with time, resulting in geographic inequity of donors and differences in mortality. 11 Multiple factors beyond those included in PELD, may need to be incorporated in a method to identify more ill and higher risk patients earlier, with the potential of improving outcome. Risk of waitlist mortality also reflects organ acceptance behavior, listing criteria, and access to care, all of which remain independent from the current PELD. Regional differences in outcomes likely also reflect the vast spectrum of pre-transplant medical expertise and use of variant liver surgical techniques at low and high volume pediatric transplant centers. 12
Similar to the known clinical presentation of biliary atresia, YC2 with BA who died on the waitlist are also predominantly younger and female. Our data demonstrate that a significantly larger proportion of BA patients survive post-transplant compared to those with other chronic liver diseases. Having a chronic liver disease (not BA) was associated with a 2 times increased risk of wait-list death compared to BA, even with presumed Kasai. This mortality risk may be explained by multi-organ pathophysiology leading to worse cholestasis and coagulopathy in children with non-BA chronic liver disease With the exception of “syndromic” BA (which can present with polysplenia and situs inversus), BA is an isolated cholestatic liver disease. Meanwhile, other cholestatic chronic liver diseases in this age group such as Alagille syndrome 13,14, Progressive Familial Intrahepatic Cholestasis 15, and cystic fibrosis liver disease 16 often have vascular, cardiac, renal, intestinal, pancreatic, and pulmonary involvement which may increase morbidity and mortality. Excessive missingness of “cause of death” among our CLD group precluded an analysis of co-morbid risk factors.
Multiple U.S. and European studies have shown that BA patients who have a Kasai within the first 60 days of life have an improved clinical outcome and decreased need for liver transplantation 17-19. Our study was unable to corroborate the exact timing or history of Kasai hepatoportoenterostomy with transplant outcome, given the lack of clarity within the UNOS registry database which instead utilized the term “prior abdominal surgery” and provided no associated date. However, BA patients “no abdominal surgery” were noted to have a higher initial/final PELD score (we suspect due to higher bilirubin) and shorter wait time (likely reflecting earlier cirrhotic decompensation and need for a sooner transplant), but ultimately demonstrated a 2 times higher wait-list mortality risk than those with a presumed Kasai,. Higher late death mortality in BA children was also observed in our analysis of patients with “no prior abdominal surgery”, suggesting that those without Kasai are more vulnerable even after transplantation.
Limitations of this UNOS Registry review include our inability to medically confirm that “abdominal surgery” represented a Kasai procedure among BA patients. Surgical correction of malrotation or situs inversus prior to transplant is rare, so we believe this designation to represent hepatoportoenterostomy. As with all database studies, incompleteness or inaccuracy of data or diagnosis could not be verified or refuted. Our investigation was purposefully limited to deceased donor transplantation, thus does not completely reflect overall outcomes. Living donor transplantations were excluded as they skew the “natural history” of wait list mortality and do not follow the same rules and nomenclature as those listed with PELD. We also recognize that our reported differences in PELD score among those with a poor outcome while statistically significant, are fairly small in a clinical setting.
Conclusions
Our data highlight an unfavorably high waitlist and early post liver transplant mortality in young children listed for liver transplantation not predicted by PELD, and suggests that a diagnosis of other chronic liver disease, BA without Kasai, and lack of request for exception may be key risk factors for an unfavorable outcome. Measures to ameliorate this unacceptable wait list mortality must go beyond optimizing pretransplant management (i.e. ascites, malnutrition). The data suggest that while the PELD score is useful for stratifying children in the organ allocation system and that higher scores correlate with increased mortality, it significantly underestimates the risk of death. We propose the need for pre-emptive listing for transplant, earlier petition for exception points and wider application of living donor and or split liver donation between adult and pediatric programs in all children under the age of 2 years requiring liver transplantation, but particularly in BA patients without presumed Kasai and those with other chronic liver diseases, where the waitlist mortality was 2-fold higher. The role of low height and higher weight for age as it relates to risk for waitlist mortality is not as clear but merits further consideration. A more comprehensive, multi-variable scoring system, including such factors, is deserving of future study in this age group
Supplementary Material
Supplementary Table 5: PELD and Outcomes of YC2 BA with and without “abdominal surgery” (suspected Kasai)
Acknowledgments
UNOS/OPTN
Grants and financial support related to this work: GH received grant support from NIH Training Grant (T32-DK007664).
Abbreviations
- BA
Biliary Atresia
- CLD
Chronic liver disease
- ED
early death
- LD
late death
- LRD
living related donor
- MELD
Model for end-stage liver disease
- OPTN
Organ Procurement and Transplantation Network
- PELD
Pediatric End-stage Liver disease
- PFIC
progressive familial intrahepatic cholestasis
- SPLIT
Studies of Pediatric Liver Transplantation
- TST
too sick to transplant
- UNOS
United Network for Organ Sharing
- YC2
Young children <2 years of age with chronic end-stage liver disease
Footnotes
Conflict of Interest Statement: The authors report no relevant conflicts of interest or financial affiliations.
References
- 1.Utterson EC, Shepherd RW, Sokol RJ, Bucuvalas J, Magee JC, McDiarmid SV, et al. Biliary atresia: clinical profiles, risk factors, and outcomes of 755 patients listed for liver transplantation. The Journal of pediatrics. 2005 Aug;147(2):180–185. doi: 10.1016/j.jpeds.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CD, Turmelle YP, Lowell JA, Nadler M, Millis M, Anand R, et al. The effect of recipient-specific surgical issues on outcome of liver transplantation in biliary atresia. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008 Jun;8(6):1197–1204. doi: 10.1111/j.1600-6143.2008.02223.x. [DOI] [PubMed] [Google Scholar]
- 3.Freeman RB, Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2002 Sep;8(9):851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 4.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, et al. MELD and PELD: application of survival models to liver allocation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2001 Jul;7(7):567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 5.Shneider BL, Neimark E, Frankenberg T, Arnott L, Suchy FJ, Emre S. Critical analysis of the pediatric end-stage liver disease scoring system: a single center experience. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005 Jul;11(7):788–795. doi: 10.1002/lt.20401. [DOI] [PubMed] [Google Scholar]
- 6.Bezerra JA. Potential etiologies of biliary atresia. Pediatric transplantation. 2005 Oct;9(5):646–651. doi: 10.1111/j.1399-3046.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 7.Arnon R, Annunziato RA, D'Amelio G, Chu J, Shneider BL. Liver Transplantation for Biliary Atresia: Is There a Difference in Outcome for Infants? Journal of pediatric gastroenterology and nutrition. 2016 Feb;62(2):220–225. doi: 10.1097/MPG.0000000000000986. [DOI] [PubMed] [Google Scholar]
- 8.Pugliese R, Fonseca EA, Porta G, Danesi V, Guimaraes T, Porta A, et al. Ascites and serum sodium are markers of increased waiting list mortality in children with chronic liver failure. Hepatology. 2014 May;59(5):1964–1971. doi: 10.1002/hep.26776. [DOI] [PubMed] [Google Scholar]
- 9.Kim WR, Smith JM, Skeans MA, Schladt DP, Schnitzler MA, Edwards EB, et al. OPTN/SRTR 2012 Annual Data Report: liver. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014 Jan;14(Suppl 1):69–96. doi: 10.1111/ajt.12581. [DOI] [PubMed] [Google Scholar]
- 10.Hsu EK, Shaffer M, Bradford M, Mayer-Hamblett N, Horslen S. Heterogeneity and disparities in the use of exception scores in pediatric liver allocation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015 Feb;15(2):436–444. doi: 10.1111/ajt.13089. [DOI] [PubMed] [Google Scholar]
- 11.Rana A, Riaz IB, Gruessner AC, Gruessner RW. Geographic inequity results in disparate mortality: a multivariate intent-to-treat analysis of liver transplant data. Clinical transplantation. 2015 Jun;29(6):484–491. doi: 10.1111/ctr.12499. [DOI] [PubMed] [Google Scholar]
- 12.Rana A, Pallister Z, Halazun K, Cotton R, Guiteau J, Nalty CC, et al. Pediatric Liver Transplant Center Volume and the Likelihood of Transplantation. Pediatrics. 2015 Jul;136(1):e99–e107. doi: 10.1542/peds.2014-3016. [DOI] [PubMed] [Google Scholar]
- 13.Kamath BM, Podkameni G, Hutchinson AL, Leonard LD, Gerfen J, Krantz ID, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. American journal of medical genetics Part A. 2012 Jan;158A(1):85–89. doi: 10.1002/ajmg.a.34369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamath BM, Spinner NB, Emerick KM, Chudley AE, Booth C, Piccoli DA, et al. Vascular anomalies in Alagille syndrome: a significant cause of morbidity and mortality. Circulation. 2004 Mar 23;109(11):1354–1358. doi: 10.1161/01.CIR.0000121361.01862.A4. [DOI] [PubMed] [Google Scholar]
- 15.Lykavieris P, van Mil S, Cresteil D, Fabre M, Hadchouel M, Klomp L, et al. Progressive familial intrahepatic cholestasis type 1 and extrahepatic features: no catch-up of stature growth, exacerbation of diarrhea, and appearance of liver steatosis after liver transplantation. Journal of hepatology. 2003 Sep;39(3):447–452. doi: 10.1016/s0168-8278(03)00286-1. [DOI] [PubMed] [Google Scholar]
- 16.Chryssostalis A, Hubert D, Coste J, Kanaan R, Burgel PR, Desmazes-Dufeu N, et al. Liver disease in adult patients with cystic fibrosis: a frequent and independent prognostic factor associated with death or lung transplantation. Journal of hepatology. 2011 Dec;55(6):1377–1382. doi: 10.1016/j.jhep.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Chardot C, Carton M, Spire-Bendelac N, Le Pommelet C, Golmard JL, Auvert B. Prognosis of biliary atresia in the era of liver transplantation: French national study from 1986 to 1996. Hepatology. 1999 Sep;30(3):606–611. doi: 10.1002/hep.510300330. [DOI] [PubMed] [Google Scholar]
- 18.Karrer FM, Lilly JR, Stewart BA, Hall RJ. Biliary atresia registry, 1976 to 1989. Journal of pediatric surgery. 1990 Oct;25(10):1076–1080. doi: 10.1016/0022-3468(90)90222-u. discussion 1081. [DOI] [PubMed] [Google Scholar]
- 19.Serinet MO, Wildhaber BE, Broue P, Lachaux A, Sarles J, Jacquemin E, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009 May;123(5):1280–1286. doi: 10.1542/peds.2008-1949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 5: PELD and Outcomes of YC2 BA with and without “abdominal surgery” (suspected Kasai)