Abstract
Objective
To determine (1) whether delirium severity was associated with Apolipoprotein E (APOE) genotype status and occupational complexity, a measure of cognitive reserve, in individuals with delirium superimposed on dementia; and (2) whether decline in delirium severity was associated with these same factors over a post-acute care (PAC) stay.
Methods
Control group data (n=142) from a completed randomized clinical trial were used to address the aims of the study. Delirium severity was calculated by combining items from the Confusion Assessment Method and the Montreal Cognitive Assessment. APOE ε4 carriers versus non-carriers were considered. Occupational complexity, a measure of cognitive reserve, was derived from the Lifetime of Experiences Questionnaire. Covariates examined included age, gender, education, Clinical Dementia Rating Scale, and the Charlson comorbidity score. Data were nested (i.e., days nested within persons) and analyzed using multilevel models.
Results
The presence of an APOE ε4 allele and higher Clinical Dementia Rating Scale were associated with greater delirium severity at baseline. The presence of an APOE ε4 allele was also associated with greater delirium severity averaged across the PAC stay. Occupational complexity was not associated with baseline delirium severity or average daily delirium severity; however, individuals with low occupational complexity showed a significant decreased in delirium severity during the course of their PAC stay.
Conclusions
Individual differences, including genetic factors and level of cognitive reserve, contribute to the severity of delirium in older adults with dementia.
Keywords: delirium, dementia, APOE, cognitive reserve
Introduction
Delirium is an acute change in mental status that is characterized by fluctuating symptoms including inattention, level of consciousness, and cognitive disturbances (Inouye et al., 1990). Although the etiology remains poorly understood, delirium may occur in response to a variety of noxious insults including medications, infections, and surgery (Inouye et al., 2014). Individuals with dementia may be most vulnerable because of their preexisting diminished cognitive status and, thus, are at highest risk for developing delirium (Fong et al., 2015). In fact, dementia and delirium often coexist. Reports of delirium superimposed on dementia (DSD), an acute change in mental status in a person with preexisting dementia (Fick et al., 2002), describe prevalence rates of delirium as high as 89% in the hospitalized older adult (Fick et al., 2002). Evidence suggests that individuals with DSD may have a more severe course of delirium with poorer outcomes including increased length of hospitalization, poorer functional status, and higher mortality (Fick et al., 2013), but the factors underlying this are not well understood.
Delirium often persists long after hospitalization, and individuals with DSD are frequently admitted into post-acute care (Kiely et al., 2003). While there have been few studies of DSD in the post-acute care (PAC) setting, having co-occurring delirium and dementia (i.e., DSD) has been shown to be a strong predictor of worse functional outcomes and higher mortality risk compared with having either dementia or delirium alone (Morandi et al., 2014). Yet, individual factors that contribute to clinical outcomes in this highly vulnerable population have not yet been determined. While it may be difficult to prevent delirium in this population, it is important to know who is at risk for greater delirium severity and poorer prognosis.
Biological and environmental factors are thought to contribute to delirium (Kolanowski et al., 2014; Inouye et al., 2014). Biological factors such as Apolipoprotein E (APOE) genotype are non-modifiable and have been previously associated with dementia risk and accelerated cognitive decline in older adults (Liu et al., 2013; Farrer et al., 1997). For example, the APOE ε4 allele has been associated with impaired cholinergic function (Reinvang et al., 2013) and increased inflammatory response (Adamis et al., 2009) and therefore may contribute to delirium pathogenesis. However, studies examining the relationship between APOE and delirium yield mixed results. For example, Ely et al. (2007) observed that the presence of APOE ε4 was the strongest predictor for a longer delirium course in ICU patients. Conversely, in a sample of older adults undergoing elective surgery, there was no association between delirium incidence, delirium severity, or delirium duration and APOE carrier status (Vasunilashorn et al., 2015).
Environmental factors may also play a role in delirium. One of these, the concept of “reserve”, was proposed to explain the differences in clinical outcomes between individuals who sustained similar brain insults (Stern, 2002). Reserve includes elements of brain reserve (brain size and synaptic connectivity) and cognitive reserve (ability to use cognitive strategies to support brain function in the face of neuropathology). Life experiences such as education and occupation are thought to build cognitive reserve (Stern, 2002; Jones et al., 2010). Although much of the evidence related to reserve comes from individuals with dementia, there is evidence to suggest that reserve may reduce the risk for developing delirium (Jones et al., 2010). For example, Jones et al. (2006) report that higher education was associated with lower incidence of delirium in older adults. Similarly, higher scores on verbal intelligence testing were associated with lower delirium risk in older adults undergoing surgery (Saczynski et al., 2014). We previously reported a protective effect of occupational attainment in individuals with dementia (Massimo et al., 2015); therefore, we sought to incorporate this factor in the current investigation.
While the aforementioned studies examined the risk for the development of delirium, there have been few studies to together examine genetic and environmental factors associated with delirium severity in those with DSD. Therefore, the goal of this exploratory study was to extend previous work on genetic and environmental factors related to delirium in a population with dementia in a PAC setting. Our aims were to determine (1) whether delirium severity in older adults with dementia was associated with APOE status and occupational complexity, and (2) whether change in delirium severity during a PAC stay was associated with these same factors.
Methods
Data from a completed randomized clinical trial (ClinicalTrials.gov identifier: NCTO1267682) were used to address the aims of this study. The parent study tested the efficacy of cognitively stimulating activities for resolving delirium in patients with dementia during PAC rehabilitation. For this study, we utilized control group data from those who received usual care. The protocol received institutional review board approval and was published (Kolanowski et al., 2011).
Setting and sample
Subjects were recruited at admission to PAC following an inpatient hospitalization. Eight community-based skilled nursing facilities in central and northeast Pennsylvania served as recruitment sites. All subjects had a diagnosis of dementia and delirium. Dementia was established based on a score of three or greater on the Modified Blessed Dementia Rating Scale with symptoms evident for at least 6 months (Blessed et al., 1968) and a Clinical Dementia Rating (CDR) score of from 0.5 to 2.0, indicating mild to moderate dementia (Hughes et al., 1982). Presence of delirium was established by positive findings on the Confusion Assessment Method (CAM; Inouye et al., 1990), a standardized diagnostic algorithm for delirium allowing persons without formal psychiatric training to quickly and accurately identify delirium. The CAM has been validated in persons with dementia (Inouye et al., 1990). In the trial, we took a conservative approach and included subjects with full (three or more features) or subsyndromal (two features) delirium (Cole et al., 2003). All dementia and delirium diagnoses were adjudicated by a panel of three experts: neurologist, neuropsychologist, and geriatrician.
Other inclusion criteria included the following: age 65 years or older; English speaking; community-residing prior to most recent hospitalization; and having a legally authorized representative (usually a spouse or adult child) who provided medical history, education, and occupation data. These individuals meet criteria specified for knowledgeable informants, that is, monthly contact with the subject for 10 years during the subject's adult life prior to the dementia diagnosis (Ritchie and Fuhrer, 1992). Subject exclusion criteria included the following: Huntington's disease, normal pressure hydrocephalus, seizure disorder, subdural hematoma, head trauma, or known structural brain abnormalities; having a life expectancy of 6months or less; acute major depression; acute psychiatric condition; stroke; and severe hearing and vision impairment. We also excluded individuals diagnosed with Lewy body disease because of the overlap between common symptoms related to their dementia and delirium features such as inattention and fluctuating mental status.
Following written consent from the participant's legally authorized representative, demographic variables, medical history, an assessment of APOE genotype, and occupational complexity were obtained by trained research staff.
Study measures
Delirium
Delirium was assessed at admission and then daily by trained research assistants using the CAM, a reliable method for detecting the presence of delirium (Inouye et al., 1990). Briefly, the CAM assesses for an acute change in mental status or fluctuating course, inattention, and either disorganized thinking or an altered level of consciousness.
Delirium severity
Delirium severity was quantified by combining two items from the CAM and one item from the Montreal Cognitive Assessment, as previously described (Inouye et al., 1999; Kolanowski et al., 2015). The following items were summed to create a delirium severity score: CAM fluctuating course item (0=absent; 1=present); CAM level of consciousness (0=alert; 1=lethargic; 2=unarousable); and Montreal Cognitive Assessment 5-item forward digit span score reverse coded so that greater scores indicated poorer performance (0–5). Total delirium severity scores ranged from 0 to 8, with higher numbers indicating greater delirium severity. Average delirium severity was calculated by dividing each participant's daily delirium severity scores by the total number of days spent in the skilled nursing facility.
Apolipoprotein E genotype
Apolipoprotein E genotype was determined by extracting DNA from the buccal swabs using a protocol optimized by the Institute of Psychiatry, London (Freeman et al., 2003), to identify the six APOE genotypes comprising the APOE ε2, ε3, and ε4 alleles. Two single nucleotide polymorphisms, rs429358 and rs7412, which together comprise the ε site, were assayed using the TaqMan Allele Discrimination method.
Occupational complexity
The mid-life specific subscale from the Lifetime of Experiences Questionnaire, a reliable and valid instrument that assesses cognitive lifestyle, a proxy for cognitive reserve (Valenzuela and Sachdev, 2007), was used to measure occupational complexity. This subscale includes nine major occupational classifications by hierarchical level. Data were obtained by interviewing knowledgeable informants and asking them to describe the occupations held by the participant and if applicable, their supervisory or managerial experience from 30 to 65 years of age (or until retirement). Scores range from 1 (laborers) to 9 (administrators). Additional points are given for managerial capacity. Higher scores indicate higher occupational complexity.
Education
The young adulthood specific score from the Lifetime of Experiences Questionnaire was used to assess educational level. Briefly, this score includes number of years of secondary school (i.e., after grade 6) and post-secondary education (i.e., after completion of high school) and complexity of the educational experience. For example, education that is more intellectually complex (e.g., university Master's degree) is given a higher rating than technical school.
Covariates
We examined covariates associated with delirium severity including age, gender, education, CDR Scale, and the Charlson comorbidity score.
Statistical analysis
Descriptive statistics were calculated for demographic variables. Between-group differences were assessed using t-tests, chi-squared tests, or Analysis of Variance, as appropriate. Our predictor variables, APOE status and occupational complexity, were individual difference variables. That is, they did not change from day to day. Occupational complexity was derived by applying continuous values of complexity to the occupations the participant had in midlife. For each individual, APOE ε4 status was coded using a dominant model: APOE ε4 carrier versus not.
Analyses were carried out in two ways. First, we calculated an average delirium severity score across the time in study to create a more precise estimate of delirium severity for a given individual. We then examined whether APOE status and occupational complexity predicted average delirium severity using linear regression models.
Next, because data for the current study were nested (i.e., days nested within persons), we also used multilevel models (MLMs). These models allowed us to determine whether APOE status or occupational complexity moderated change in delirium severity over the course of the study. We statistically allowed for between person differences in their baseline delirium severity level. We also allowed individuals to vary in the slopes or change in delirium severity across days. A statically significant interaction of our moderators (i.e., APOE status or occupational complexity) by day would indicate that a moderator reliably influences change in delirium severity. We fit this equation twice in our analysis, we first tested the effect of APOE as a moderator, and second, we tested the effect of occupational complexity as a moderator.
For both sets of models covariates included age, comorbidity Charlson score, CDR, gender, and education. When testing the effect of APOE status on delirium severity, we controlled for occupational complexity, and similarly, we controlled for APOE status when we tested the association between occupational complexity and delirium severity. Although there is reason to believe that education and occupational complexity are strongly correlated and thus introduce multicollinearity in our regression models, correlational analysis showed only a moderate correlation (r=0.55); thus, education was retained in our models.
Results
Table 1 reports the demographics of the 142 control group participants. Consistent with the demographic breakdown of the recruitment area, 98.5% of the sample was Caucasian. Descriptive statistics are presented across levels of occupational complexity categorized by tertiles. Tertiles were used for descriptive purposes to evaluate whether there was a dose-response association of occupational complexity and delirium severity.
Table 1.
Demographic features
| Frequencies (percent) and mean (standard error) among variables of interest | ||||
|---|---|---|---|---|
| Low occupational complexity (n = 46) | Medium occupational complexity (n = 47) | High occupational complexity (n = 49) | Group differences | |
| Gender | ||||
| Male | 3 (2.13%) | 17 (12. 06%) | 27 (19.15%) | * a |
| Female | 43 (30.50%) | 31 (21.99%) | 20 (14.18%) | |
| Age | 88.20 (0.98) | 83.47 (0.96) | 85.58 (0.95) | * b |
| CDR | 1.23 (0.09) | 1.20 (0.09) | 1.24 (0.09) | |
| Charlson | 2.98 (0.28) | 2.98 (0.30) | 3.15 (0.29) | |
| APOE status | ||||
| ε4 allele not present | 29 (21.48%) | 29 (21.48%) | 28 (19.15%) | |
| ε4 allele present | 15 (11.11%) | 15 (11.11%) | 20 (14.180%) | |
| Delirium severity (Average) | 1.10 (0.17) | 1.12 (0.16) | 1.06 (0.16) | |
| Delirium severity (Baseline) | 1.22 (0.47) | 1.28 (0.54) | 1.33 (0.63) | |
| Number of study days | 21.93 (1.30) | 20.49 (1.28) | 20.75 (1.27) | |
| Education (LEQ young adult specific score) | 6.08 (4.50) | 7.57 (4.70) | 12.60 (6.59) | * b |
Note.
APOE, Apolipoprotein E; CDR, Clinical Dementia Rating; LEQ, Lifetime of Experiences Questionnaire.
p<0.05.
Chi-square test.
ANOVA.
Apolipoprotein E status and delirium severity
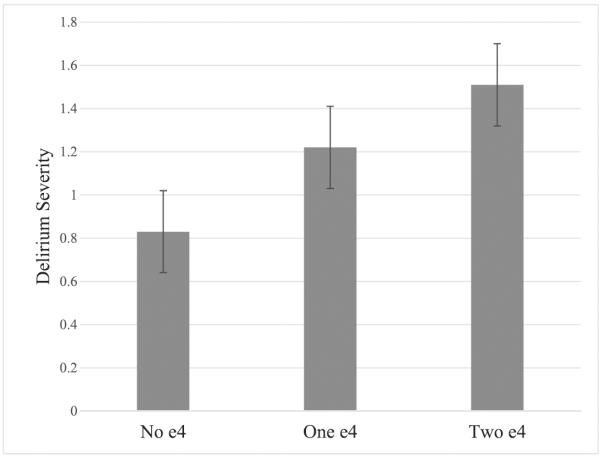
Results from our linear regression model showed that the presence of an APOE ε4 allele was associated with greater average delirium severity (B=0.42 (0.20), p=0.04), while accounting for the same covariates as the model described earlier (including occupational complexity). To increase specificity and determine if there was an additive effect of the ε4 allele, we included a code of three categories—no ε4, one ε4, and two ε4 alleles—and fit the identical model predicting average delirium severity. Results revealed a significant effect (B=0.37 (0.18), p=0.03) by APOE category. Figure 1 depicts the estimated effect of each APOE category; these estimates were all statistically significant and indicate that compared with not having an ε4 allele, the presence of one ε4 allele results in a 31% increase in delirium severity scores and an 81% increase in delirium severity with the presence of two ε4 alleles.
Figure 1.
Average delirium severity across study by ε4 status. All estimated levels statically significant (p<0.01).
Table 2 presents the results of the MLMs. Our first model examined the effect of APOE status on delirium severity. Results from this model indicate that the presence of an ε4 allele is associated with greater delirium severity at baseline (B=0.53 (0.23), p=0.03). The interaction with day in study was not statistically significant and, thus, not included in the model or presented in the table. CDR scores were also associated with greater delirium severity at baseline (B=0.59 (0.19), p=0.002).
Table 2.
Effects of APOE and occupational complexity on delirium severity
| Model 1 | Model 2 | |
|---|---|---|
|
|
||
| Estimate (SE) | Estimate (SE) | |
| Fixed effects | ||
| Intercept | 1.20 (0.19)** | 1.16 (0.19)** |
| Day | −0.089 (0.004)* | −0.006 (0.003)† |
| APOE | 0.53 (0.23)* | 0.53 (0.23)* |
| Occ. complex. | −0.01 (0.02) | −0.04 (0.03) |
| Day × occ. complex. | – | 0.001 (0.001)* |
| Age | −0.01 (0.02) | −0.01 (0.02) |
| Charlson | 0.06 (0.06) | 0.06 (0.06) |
| CDR | 0.59 (0.19)** | 0.59 (0.19)** |
| Gender (Male (0) = reference) | −0.28 (0.27) | −0.28 (0.27) |
| Education | 0.03 (0.02) | 0.03 (0.02) |
| Random Effects | ||
| Intercept | 1.97 (0.30)** | 1.95 (0.30)** |
| Day Slope | 0.001 (0.001)** | 0.0008 (0.0002)** |
Note.
Small values necessitate reporting of values up to four decimal points in some cases. Occ. Complex, Occupational Complexity; APOE, Apolipoprotein E; CDR; Clinical Dementia Rating; SE, standard error.
p<0.05.
p<0.01.
p<0.001.
Occupational complexity and delirium severity
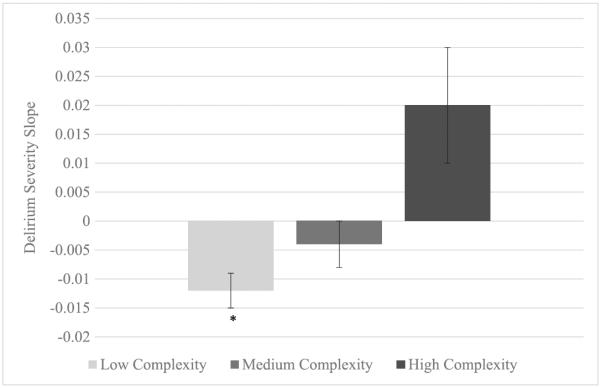
Occupational complexity did not significantly predict average delirium in a linear regression model. Table 2 shows the results for occupational complexity and delirium severity using MLMs (Model 2). Occupational complexity did not significantly predict baseline delirium severity. We did however observe a significant interaction between day in study and occupational complexity (B=0.001 (0.001), p=0.04). To explore this relationship further, we estimated the trajectories of delirium severity for scores within the lowest (low complexity), second (medium complexity), and third tertiles (high complexity), akin to those categories presented on Table 1. Figure 2 depicts the slopes for the three occupational complexity categories. Tests of significance revealed that the slope for the low occupational complexity group was the only group to show a significant decrease in delirium severity during the course of their PAC stay (B=−0.01 (0.003), p<0.01). We did not observe significant change in delirium severity in the other occupational complexity groups. Education did not predict any of our outcome variables including baseline delirium severity, average delirium severity, or change in delirium severity during the PAC stay.
Figure 2.
Slopes of delirium severity grouped by level of occupational complexity. Slope for low occupational complexity group is negative and statistically significant (p<0.001).
Discussion
This study examined the effects of genetic and environmental factors on delirium severity in older adults with DSD in PAC setting. We found that the presence of an APOE ε4 allele was associated with greater delirium severity on admission to PAC and during the course of their stay. We also found that only participants with low occupational attainment experienced a significant decrease in delirium severity during the PAC stay. These results suggest that individual differences, including genetic and cognitive reserve factors, contribute to the course of delirium in older adults with dementia.
The results of this study extend the findings of other investigations of APOE and delirium. While previous studies have yielded mixed results, it has been suggested that the observed association between APOE and delirium may be attributed to the pathologic vulnerability that occurs in individuals with preexisting dementia (Saczynski et al., 2014). For example, APOE ε4 is the most robust genetic risk factor for an accelerated cognitive decline in individuals with Alzheimer's disease (AD) (Lim et al., 2015; Carrasquillo et al., 2015), and this may be related to a heavier burden of AD pathology in ε4 carriers (Farfel et al., 2016). Therefore, it may be possible that individuals with APOE ε4 have an increased vulnerability for sustaining an insult such as delirium. The presumed pathogenesis from delirium, such as neural injury from altered neurotransmitters or cerebral ischemia (Fong et al., 2015), may lead to a more severe course of delirium in APOE ε4 positive individuals. This is consistent with our observation of greater delirium severity in individuals with an ε4 allele, and we suggest that this may be reflective of an increased vulnerability in individuals with dementia.
Environmental factors such as occupational attainment, education, and leisure activities may increase cognitive reserve, the brain's ability to actively cope with underlying neuropathology by the efficacious use of brain networks or cognitive strategies that allow the brain to preserve function (Jones et al., 2011). Historically, the cognitive reserve model has been used to explain variability in the relationship between pathologic burden and clinical expression of disease in individuals with AD (Stern, 2006). Individuals with high reserve may be able to compensate, in part, for the consequences of underlying pathology and thus slow or delay the emergence of clinical symptoms (Stern, 2009). Thus, more pathology is necessary for clinical symptoms to be expressed in high reserve individuals. Once individuals with high reserve become symptomatic, a more rapid clinical course may ensue (Stern et al., 1999). Although this model has been presented in patients with AD, there is some evidence to suggest cognitive reserve may also be important for acute cognitive impairment, such as delirium. For example, several cognitive reserve markers, such as educational attainment, physical activity participation, and verbal intelligence have been associated with a reduced risk of delirium in older adults without dementia (Saczynski et al., 2014; Jones et al., 2006; Yang et al., 2008). While previous studies suggest reserve markers are associated with reduced risk of incident delirium in older adults without dementia, we examined outcomes after delirium developed in individuals with dementia. We found that individuals with low occupational complexity show a trend towards delirium resolution compared with those with high occupational complexity whose delirium severity remained relatively unchanged during their PAC stay. Consistent with the cognitive reserve theory, we suggest that individuals with higher occupational complexity may have an increased pathological load and this may contribute to the unchanged delirium symptom severity. In contrast, individuals with low occupational complexity may have less dementia-related neuropathologic changes and, thus, are less advanced even though their clinical dementia severity (i.e., CDR) is similar to their high reserve counterparts. This model closely reflects what has been demonstrated in AD, where poorer outcomes such as a more rapid cognitive decline and higher mortality are observed in individuals with higher reserve (Stern et al., 1995; Stern et al., 1999). Our findings add to the cognitive reserve evidence that suggests individual differences in brain reserve capacity contribute to differences in cognitive outcomes.
While previous studies have found a relationship between delirium and cognitive reserve using education as a marker (Jones et al., 2006), we did not observe an association between education and measures of delirium severity. This is consistent with our previous work investigating cognitive reserve in individuals with dementia where we examined both occupation and education as proxies of cognitive reserve and found that only occupational level was associated with reserve capacity (Massimo et al., 2015). It is possible to postulate that the longer-term cognitive activity associated with work-related tasks influences reserve differently than early-life education. Another possibility is that the restriction of range in our low educational level population limited our power to detect an effect of education. Clearly, more research is needed to further elucidate the pathway by which proxies like occupational complexity impact cognitive reserve in individuals with DSD.
Several caveats should be kept in mind when interpreting our findings. First, the secondary nature of this study did not allow us to examine risk for delirium or long-term outcomes after discharge. We also observed that delirium severity was mild, consistent with a subsyndromal delirium (Cole et al., 2003), and the majority of our sample continued to have subsyndromal delirium at discharge. Thus, we were unable to evaluate complete delirium recovery. More research is needed to evaluate the long-term effects of cognitive reserve such as longitudinal cognitive and functional measures, as DSD can lead to accelerated cognitive decline (Fong et al., 2012; McCusker et al., 2003). While we examined education and occupational complexity, common proxies for cognitive reserve, future investigations also should examine the protective effects of more modifiable reserve builders such as cognitive activities, physical exercise, socialization, and diet. Lastly, data regarding the etiology of the dementia was unavailable, and our sample likely contained a mix of dementia diagnoses, including AD, which may have an enriched APOE ε4 genotype. It would be useful to independently evaluate dementia cohorts that are well characterized and allow us to determine whether APOE ε4 genotype and cognitive reserve are preferentially associated with AD or whether each modifier extends to other forms of dementia.
Conclusion
We found that genetic and environmental factors contribute to the severity of delirium in individuals with DSD. This likely reflects an increased vulnerability in individuals with APOE ε4 and cognitive reserve in individuals with high occupational complexity. A better understanding of the factors that contribute to the course of delirium is important for uncovering potential mechanisms of delirium symptoms, identifying high-risk individuals, for predicting prognosis of their delirium course, and for identifying those who are more likely to benefit from delirium interventions that target modifiable risk factors.
Key points.
The effects of delirium persist long after hospitalization and contribute to worsening global cognition in older adults with dementia, but the individual factors underlying this are not well understood.
The presence of an APOE ε4 allele was associated with greater delirium severity on admission to the PAC setting and during the course of the post-acute stay.
Low occupational complexity was associated with a significant decrease in delirium severity during the course of the PAC stay.
Individual differences including genetic factors and level of cognitive reserve contribute to the severity of delirium in older adults with dementia.
Acknowledgements
Lauren Massimo acknowledges support by the National Institute of Nursing Research (NINR) of the National Institutes of Health under Award Number F32NR014777. Ann Kolanowski and Donna Fick acknowledge partial support from NINR grant R01NR012242. We wish to thank Dr. Deborah Grove of the Penn State Genomics Core Facility for genotyping. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest None declared.
References
- Adamis D, Van Munster BC, Macdonald AJ. The genetics of deliria. Int Rev Psychiatry. 2009;21:20–29. doi: 10.1080/09540260802675510. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Carrasquillo MM, Crook JE, Pedraza O, et al. Late-onset Alzheimer's risk variants in memory decline, incident mild cognitive impairment, and Alzheimer's disease. Neurobiol Aging. 2015;36:60–67. doi: 10.1016/j.neurobiolaging.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M, McCusker J, Dendukuri N, Han L. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760. doi: 10.1046/j.1365-2389.2003.51255.x. [DOI] [PubMed] [Google Scholar]
- Ely Ew, Girard TD, Shintani Ak, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med. 2007;35:112–117. doi: 10.1097/01.CCM.0000251925.18961.CA. [DOI] [PubMed] [Google Scholar]
- Farfel JM, Yu L, De Jager PL, Schneider JA, Bennett DA. Association of APOE with tau-tangle pathology with and without beta-amyloid. Neurobiol Aging. 2016;37:19–25. doi: 10.1016/j.neurobiolaging.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50:1723–1732. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- Fick DM, Steis MR, Waller JL, Inouye SK. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013;8:500–505. doi: 10.1002/jhm.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TG, Jones RN, Marcantonio ER, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Intern Med. 2012;156:848–856. W296. doi: 10.7326/0003-4819-156-12-201206190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14:823–832. doi: 10.1016/S1474-4422(15)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, Smith N, Curtis C, et al. DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav Genet. 2003;33:67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WI, Cohen LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Yang FM, Zhang Y, et al. Does educational attainment contribute to risk for delirium? A potential role for cognitive reserve. J Gerontol A Biol Sci Med Sci. 2006;61:1307–1311. doi: 10.1093/gerona/61.12.1307. [DOI] [PubMed] [Google Scholar]
- Jones RN, Fong TG, Metzger E, et al. Aging, brain disease, and reserve: implications for delirium. Am J Geriatr Psychiatry. 2010;18:117–127. doi: 10.1097/JGP.0b013e3181b972e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Manly J, Glymour MM, et al. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17:593–601. doi: 10.1017/S1355617710001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely DK, Bergmann MA, Murphy KM, et al. Delirium among newly admitted postacute facility patients: prevalence, symptoms, and severity. J Gerontol A Biol Sci Med Sci. 2003;58:M441–M445. doi: 10.1093/gerona/58.5.m441. [DOI] [PubMed] [Google Scholar]
- Kolanowski AM, Fick DM, Litaker MS, et al. Study protocol for the recreational stimulation for elders as a vehicle to resolve delirium superimposed on dementia (Reserve For DSD) trial. Trials. 2011;12:119. doi: 10.1186/1745-6215-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanowski AM, Hill NL, Kurum E, et al. Gender differences in factors associated with delirium severity in older adults with dementia. Arch Psychiatr Nurs. 2014;28:187–192. doi: 10.1016/j.apnu.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanowski A, Mogle J, Fick DM, et al. Anticholinergic exposure during rehabilitation: cognitive and physical function outcomes in patients with delirium superimposed on dementia. Am J Geriatr Psychiatry. 2015;23:1250–1258. doi: 10.1016/j.jagp.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Villemagne VL, Pietrzak RH, et al. APOE epsilon4 moderates amyloid-related memory decline in preclinical Alzheimer's disease. Neurobiol Aging. 2015;36:1239–1244. doi: 10.1016/j.neurobiolaging.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo L, Zee J, Xie SX, et al. Occupational attainment influences survival in autopsy-confirmed frontotemporal degeneration. Neurology. 2015;84:2070–2075. doi: 10.1212/WNL.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med. 2003;18:696–704. doi: 10.1046/j.1525-1497.2003.20602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi A, Davis D, Fick DM, et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. J Am Med Dir Assoc. 2014;15:349–354. doi: 10.1016/j.jamda.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinvang I, Espeseth T, Westlye LT. APOE-related biomarker profiles in non-pathological aging and early phases of Alzheimer's disease. Neurosci Biobehav Rev. 2013;37:1322–1335. doi: 10.1016/j.neubiorev.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Fuhrer R. A comparative study of the performance of screening tests for senile dementia using receiver operating characteristics analysis. J Clin Epidemiol. 1992;45:627–637. doi: 10.1016/0895-4356(92)90135-a. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Inouye SK, Kosar CM, et al. Cognitive and brain reserve and the risk of postoperative delirium in older patients: analysis of data from a prospective observational study. Lancet Psychiatry. 2014;1:437–443. doi: 10.1016/S2215-0366(14)00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Tang MX, Denaro J, Mayeux R. Increased risk of mortality in Alzheimer's disease patients with more advanced educational and occupational attainment. Ann Neurol. 1995;37:590–595. doi: 10.1002/ana.410370508. [DOI] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Assessment of complex mental activity across the lifespan: development of the Lifetime of Experiences Questionnaire (LEQ) Psychol Med. 2007;37:1015–1025. doi: 10.1017/S003329170600938X. [DOI] [PubMed] [Google Scholar]
- Vasunilashorn SM, Ngo L, Kosar CM, et al. Does Apolipoprotein E genotype increase risk of postoperative delirium? Am J Geriatr Psychiatry. 2015;23:1029–1037. doi: 10.1016/j.jagp.2014.12.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FM, Inouye SK, Fearing MA, et al. Participation in activity and risk for incident delirium. J Am Geriatr Soc. 2008;56:1479–1484. doi: 10.1111/j.1532-5415.2008.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]