Abstract
Objectives
To assess whether partial meniscectomy is associated with increased risk of radiographic osteoarthritis (ROA) and worsening cartilage damage in the following year.
Methods
We studied 355 knees from the Osteoarthritis Initiative that developed ROA (Kellgren-Lawrence grade ≥ 2), which were matched with control knees. The MR images were assessed using the semi-quantitative MOAKS system. Conditional logistic regression was applied to estimate risk of incident ROA. Logistic regression was used to assess the risk of worsening cartilage damage in knees with partial meniscectomy that developed ROA.
Results
In the group with incident ROA, 4.4% underwent partial meniscectomy during the year prior to the case-defining visit, compared with none of the knees that did not develop ROA. All (n=31) knees that had partial meniscectomy and 58.9% (n=165) of the knees with prevalent meniscal damage developed ROA (OR=2.51, 95% CI [1.73, 3.64]). In knees that developed ROA, partial meniscectomy was associated with an increased risk of worsening cartilage damage (OR=4.51, 95% CI [1.53, 13.33]).
Conclusions
The probability of having had partial meniscectomy was higher in knees that developed ROA. When looking only at knees that developed ROA, partial meniscectomy was associated with greater risk of worsening cartilage damage.
Keywords: Meniscus, Partial meniscectomy, Cartilage loss, MRI, Osteoarthritis
Introduction
With the introduction of arthroscopic surgical techniques and increasing awareness of the burden of osteoarthritis (OA) following total meniscectomy, partial meniscectomy became the preferred procedure to treat meniscal tears, and the concept of meniscal repair was revived and refined. This further led to the current surgical understanding of preserving as much intact meniscal tissue and function as possible [1, 2]. Although partial meniscectomy is associated less with OA than with total meniscectomy [3], controversy remains as to the best treatment option for patients with meniscal damage [4].
Five randomized clinical trials (RCTs) have been published in recent years assessing arthroscopic partial meniscectomy versus conservative treatment in relation to clinical outcome in patients with degenerative meniscus tears [5-9]. Patients included in these RCTs had mixed stages of radiographic OA, but most had no or only low-grade disease. While all of these studies focused primarily on clinical outcomes, data on the structural consequences of partial meniscectomy on knees without OA are not available [10].
Considering that loss of meniscal function is one of the greatest risk factors for incident knee OA identified to date [11], and partial meniscectomy is the most common type of orthopedic surgery performed [12], the role of surgery is of particular interest for both the patients with knee symptoms and the health professionals treating them. The Osteoarthritis Initiative (OAI) is a unique dataset that can help provide answers to some of these questions.
The primary aim of the current study, therefore, was to assess whether partial meniscectomy was associated with increased risk of incident radiographic OA. As a secondary objective, we aimed to determine whether partial meniscectomy was associated with worsening of MRI-defined cartilage damage during the year following the procedure in knees that developed ROA.
Methods
The Osteoarthritis Initiative (OAI)
The OAI is an ongoing longitudinal cohort study designed to identify biomarkers of the onset and/or progression of knee OA. Both knees of 4796 participants were studied using 3-Tesla (3T) MRI and fixed–flexion radiography at baseline and at 12, 24, 36, and 48 months of follow-up [13, 14]. The institutional review boards at each of the sites approved the study and all participants gave informed consent.
Radiography
OAI knee radiographs were acquired using the posterior–anterior–fixed flexion weight-bearing protocol [14, 15] with a plexiglass positioning frame (SynaFlexer™; Synarc Inc., San Francisco, CA) [16]. The Kellgren-Lawrence (K-L) grade was determined by central readings of serial fixed–flexion knee radiographs [15]. In brief, each film was centrally assessed by two senior musculoskeletal experts, who are not co-authors and who were blinded to each other’s reading and all other data. All radiographs were read in pairs for each participant. The weighted kappa for inter-reader agreement was 0.79 for the K-L grade. Pre-specified discrepancies were adjudicated in a consensus session with a third reader [17].
Case and control knee selection
Cases were defined as study participants who had at least one knee that developed incident radiographic OA during the 4 years of follow-up. Incident radiographic OA was defined as the first occurrence of radiographic findings compatible with OA (K-L grade of ≥ 2) during the course of study. This time point was referred to as P0, with P-1, P-2, P-3, and P-4 representing the time points 1, 2, 3, and 4 years prior to incident radiographic OA. All participants with available MRI images at the point when incidence was read (P0) or the prior time point (P-1) were included. An identical number of control knees were selected that did not develop incident radiographic OA during the study period. The controls were matched to case knees according to gender, age (within 5 years), and K-L status of both the index and contralateral knees. Each case was matched to those who were at risk at the time of case occurrence and those with available images at relevant time points, whether at 12, 24, 36, or 48 months of follow-up. Both case and control knees were either K-L 0 or 1 at baseline. Altogether, 355 case knees and 355 controls were included. A detailed overview of subject inclusion is presented as a flow chart in Fig. 1. Note that knees that showed incident OA at the first follow-up (12 months) and that were K-L grade 1 at baseline and had prevalent OA in the contralateral knee were not read or matched because of concerns that they would be too similar to knees with prevalent OA.
Fig. 1.
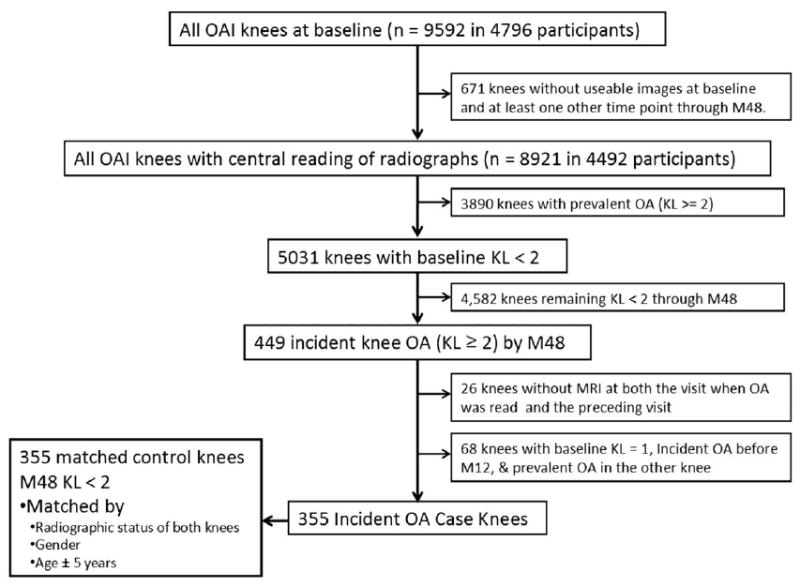
Flowchart of subject inclusion
MRI acquisition
MRI of both knees was performed on 3T systems (Siemens Trio; Siemens Healthcare, Erlangen, Germany) at the four OAI clinical sites. MRIs were acquired with a dedicated quadrature transmit/receive knee coil using a coronal intermediate-weighted (IW) two-dimensional (2D) turbo spin-echo, a sagittal three-dimensional (3D) dual-echo steady-state (DESS) sequence, and a sagittal IW fat-suppressed turbo spin-echo sequence. Additional parameters of the full OAI pulse sequence protocol and the sequence parameters were described in detail in a previous publication [13].
MRI assessment
Two musculoskeletal radiologists with 11 (FWR) and 14 (AG) years of experience in semiquantitative assessment of knee OA, and who were blinded to clinical data and case–control status, evaluated the MRIs for medial and lateral meniscal damage and for cartilage morphology at the prior time point and at the case-defining visit using the semiquantitative MRI Osteoarthritis Knee Score (MOAKS) system [18].
Cartilage was scored in 14 articular subregions, incorporating area size per subregion (from 0 to 3) and percentage of subregion that was affected by full-thickness cartilage loss (from 0 to 3). For the current analysis, only the ten tibiofemoral subregions were considered. Longitudinal cartilage loss was defined as any increase in either size or thickness of cartilage damage, or both.
Meniscal status was scored in the anterior horn, body segment, and posterior horn of the medial and lateral menisci, taking into account intrameniscal signal changes, different types of meniscal tears, and meniscal maceration, i.e., substance loss. In order to adjust for additional confounders of longitudinal cartilage loss, bone marrow lesions (BMLs) were assessed, taking into account the percentage of a subregion affected by the BML. Signal alterations in the intercondylar region of Hoffa’s fat pad were scored as a surrogate for synovial thickening, termed Hoffa-synovitis. Joint effusion (also called effusion-synovitis, as it is not possible to discern joint fluid from synovial thickening based on the sequences used in the OAI pulse sequence protocol) was graded based on the estimated maximum distention of the synovial cavity. Finally, meniscal extrusion was scored in the coronal planes [18, 19].
To determine intra-reader reliability, one radiologist (FWR) re-scored 20 randomly chosen MRIs in random order for the same features after a 4-week interval. Inter-observer reliability between the two readers was assessed using the same 20 cases.
Definition of meniscal surgery
At each visit, OAI participants were asked about meniscal surgery within the previous year. Images for all potential surgical cases were re-analyzed by an experienced musculoskeletal radiologist to confirm missing meniscal substance compared to the previous visit, indicating a partial meniscectomy. Two knees (one from a knee that developed OA) in which self-reported meniscal surgery could not be confirmed because images were not available were removed from analyses involving surgery.
Statistical analyses
Descriptive comparisons generally included all knees with the relevant available MOAKS data. All knees had baseline meniscal status, but 25 knees were lacking that information at the 1-year point prior to incidence. For odds ratios, conditional logistic regression was applied to the 328 matched case–control pairs in which both had meniscal status data to assess the risk of incident radiographic OA. In addition, the risk of worsening cartilage damage during the year prior to developing radiographic OA was assessed, using a cohort analysis approach for the incident OA cases only, by applying logistic regression adjusted for body mass index (BMI) and the matching criteria (age, gender, and KL grade). An additional analysis was performed taking into account other potential structural confounders of longitudinal cartilage loss, i.e., prevalent cartilage damage in the medial and lateral compartments, effusion-synovitis, Hoffa-synovitis, and BMLs. In the bivariate analysis, differences were observed between incident knee OA cases undergoing partial meniscectomy and other incident knee OA cases not undergoing surgery, as well as in the rest of the cohort with regard to pain and previous injury status (at any point prior to the finding of ROA). Therefore, we performed sensitivity analyses incorporating these covariates into the model.
We considered a two-tailed p value of less than 0.05 as statistically significant. Weighted kappa statistics were applied to determine inter- and intra-observer reliability. All statistical calculations were performed using Stata/IC 11.2 for Windows (StataCorp LP, College Station, TX, USA) and SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
The study sample consisted of 355 case knees and their matched controls. Participants were 60.2 years old on average (SD ± 8.6), predominantly women (66.5%), and overweight (mean BMI 28.3 SD ± 4.5). The baseline K-L grades for the matched pairs were as follows: 63 (17.8%) grade 0 in both knees, 76 (21.4%) grade 0 in one knee and grade 1 in the contralateral knee, 83 (23.4%) grade 1 in both knees, 59 (16.6%) grade 0 in one knee and grade ≥ 2 in the other, and 74 (20.9%) grade 1 in one knee and grade ≥ 2 in the contralateral knee. The case-defining visit of radiographic OA incidence was 12 months for 119 (33.5%), 24 months for 83 (23.4%), 36 months for 103 (29.0%), and 48 months for 50 (14.1%) knees. Of 710 knees, 25 case knees had missing MRI readings at the time point prior to the case-defining visit (P-1), leaving 683 knees for the time point P-1. An overview of baseline demographics is presented in Table 1.
Table 1.
Sample description
| Sample description by knee | |||||
|---|---|---|---|---|---|
|
| |||||
| All knees n=710 (%) | Case knees-incident ROA n=355 (%) | Control knees-incident ROA n=355 (%) | p for differences | ||
| Baseline K-L grade | 0 | 266 (37.46) | 133 (37.46) | 133 (37.46) | 1.000 |
| 1 | 444 (62.54) | 222(62.54) | 222(62.54) | ||
| Injury at baseline | Any | 152 (21.41) | 89 (25.07) | 63 (17.75) | 0.017 |
|
| |||||
| Sample description by person | |||||
|
| |||||
| All n=669 (%) | Cases-incident ROA n=323 (%) | Controls ROA n=346 (%) | p for cases vs. controls | ||
|
| |||||
| Women | 445 (66.52) | 213 (65.94) | 232 (67.05) | 0.762 | |
| White or Caucasian | 551 (82.36) | 260 (80.50) | 291 (84.10) | 0.221 | |
| Body mass index | 0.004 | ||||
| Normal/underweight | 168 (25.11) | 63 (19.50) | 105 (30.35) | ||
| Overweight | 268 (40.06) | 135 (41.80) | 133 (38.44) | ||
| Obese | 233 (34.83) | 125 (38.70) | 108 (31.21) | ||
| Age (±SD) | 60.16 (+/- 8.56) | 60.25 (+/- 8.69) | 60.08 (+/- 8.44) | 0.798 | |
Note: Test for differences by chi-square or t test.
Summarizing the intra- and inter-observer reliability results, all of the measures showed substantial (0.61–0.8) or almost perfect agreement (0.81–1.0) [20]. Appendix 1 gives a detailed overview of the reliability results.
Thirty-one (4.4%) of 708 knees underwent meniscal surgery in the year prior to the case-defining visit, and 238 (34.9% of 683) knees had a meniscal tear (MOAKS grades 2–5) at the time point prior to the case-defining visit. Forty-two (6.2%) knees showed partial meniscal maceration (MOAKS grade 6, i.e., substance loss, at the same time point). None showed a progressive partial or complete maceration. For five of the knees undergoing partial meniscectomy, MRI data for the year prior to the case-defining visit (P-1) were not available, leaving 26 knees with meniscal surgery for those analyses. A detailed overview of meniscal and radiographic OA status at baseline and 1 year prior to the case-defining visit, and with regard to cartilage loss in the prior year, is presented in Table 2. For both the baseline visit and the visit 1 year prior to the case-defining visit, the frequencies of meniscal tears and macerations were higher in the case group than the controls (p = 0.002 and < 0.001, respectively).
Table 2.
Frequency of meniscal damage and surgery status at enrollment and 1 year prior to the case-defining visit and outcome (incident radiographic osteoarthritis and cartilage loss)
| Outcome (incident ROA / cartilage loss) | No incident ROA (%) | Incident ROA (%) | No cartilage loss – medial (%) | Any cartilage loss – medial (%) | No cartilage loss – lateral (%) | Any cartilage loss – lateral (%) |
|---|---|---|---|---|---|---|
| Meniscal status at OAI enrolment (BL) | ||||||
| --- (N = 708) --- | --- (N = 663 with cartilage loss data) --- | |||||
|
| ||||||
| Normal or intrameniscal signal at BLa | 243 (68.5) | 209 (59.0) | 325 (63.9) | 95 (61.7) | 378 (65.0) | 44 (51.2) |
| Tear at BLb | 92 (26.0) | 129 (36.4) | 157 (30.8) | 54 (35.1) | 174 (29.9) | 37 (43.0) |
| Meniscal maceration at BLc | 19 (5.4) | 16 (4.5) | 27 (5.3) | 5 (3.3) | 30 (5.2) | 5 (5.8) |
|
| ||||||
| Meniscal status at 1 year prior to case-defining visit (P-1) | ||||||
| --- (N = 683) --- | --- (N = 663 with cartilage loss data) --- | |||||
|
| ||||||
| Normal or intrameniscal signal at P-1a | 239 (67.5) | 164 (49.9) | 309 (60.7) | 83 (53.9) | 354 (60.8) | 38 (46.9) |
| Tear at P-1b | 95 (26.8) | 143 (43.5) | 164 (32.2) | 65 (42.2) | 192 (33.0) | 37 (45.7) |
| Meniscal maceration at P-1c | 20 (5.7) | 22 (6.7) | 36 (7.1) | 6(3.9) | 36 (6.2) | 6 (7.4) |
|
| ||||||
| Meniscal surgery between P-1 and P0 | 0 (0.0) | 31 (8.8) | 8 (1.6) | 18 (11.7) | 22 (3.8) | 4 (4.7) |
MOAKS grades 0 and 1
MOAKS grades 2–5
MOAKS grades 6–8 (but only grade 6 observed)
ROA radiographic osteoarthritis, BL baseline, P-1 annual visit 1 year prior to case-defining visit, P0 annual visit at the time point when incident ROA was diagnosed (i.e., the case-defining visit)
Table 3 shows the distribution of meniscal damage for the medial and lateral compartments, collapsed into normal menisci and those exhibiting intrameniscal signal only, any tear type, and any maceration (partial or complete substance loss), which did not differ between surgery cases and other case knees. Pain and previous injury status is also shown. Knees undergoing surgery exhibited significantly higher pain scores and more previous injuries.
Table 3.
Frequency of medial and lateral meniscal damage, extrusion, injury and pain status 1 year prior to the case-defining visit (P-1)
| Meniscal status by compartment, pain status and injury status at P-1 | Control knees n=354 (%) | Case knees - incident ROA n=329 (%) | Surgery knees (all cases) n=29a (%) | p for case vs. control knees | p for surgery vs. cases |
|---|---|---|---|---|---|
| Medial meniscal morphology at P-1 (maximum grade of 3 subregions) | 0.001* | 0.236 | |||
|
| |||||
| Normal or Intrameniscal signal only | 266 (75.1) | 198 (60.2) | 16 (55.2) | ||
| Tear | 68 (19.2) | 113 (34.4) | 13 (44.8) | ||
| Radial tear | 8 (2.3) | 14 (4.3) | 2 (6.9) | ||
| Horizontal tear | 56 (15.8) | 81 (24.6) | 9 (31.0) | ||
| Vertical tear | 1 (0.3) | 6 (1.8) | 1 (3.4) | ||
| Complex tear | 3 (0.9) | 12 (3.6) | 1(3.4) | ||
| Partial maceration/substance loss | 20 (5.6) | 18 (5.5) | 0 (0.0) | ||
| Medial extrusion ≥ 2 mm at P-1 | 110 (31.1) | 144 (43.8) | 10 (38.5) | 0.001* | 0.730 |
|
| |||||
| Lateral meniscal morphology at P-1 (maximum grade of 3 subregions) | 0.056 | 0.620 | |||
| Normal or Intrameniscal signal only | 312 (88.1) | 276 (83.9) | 26 (89.7) | ||
| Tear | 42 (11.9) | 49 (14.9) | 3 (10.3) | ||
| Radial tear | 9 (2.5) | 5 (1.5) | 0 (0.00) | ||
| Horizontal tear | 31 (8.8) | 40 (12.2) | 3 (10.3) | ||
| Vertical tear | 0 (0.2) | 1 (0.3) | 0 (0.00) | ||
| Complex tear | 2 (0.6) | 3 (0.9) | 0(0.00) | ||
| Partial maceration/substance loss | 0 (0.0) | 4 (1.2) | 0 (0.0) | ||
| Lateral extrusion ≥ 2 mm at P-1 | 6(1.7) | 17 (5.2) | 1 (3.5) | 0.028* | 1.000 |
|
| |||||
| Previous injuryb | 70 (19.8) | 135 (38.1) | 20 (64.5) | < 0.001* | < 0.003* |
| KOOS pain at P-1 | 90.2 (13.5) | 84.8 (16.6) | 78.4 (20.6) | < 0.001* | 0.027* |
| WOMAC total at P-1 | 7.1 (11.1) | 11.4 (13.8) | 16.5 (18.1) | < 0.001* | 0.036* |
Two knees with partial meniscectomy had no MRI at P-1 and were excluded from these analyses
Self-reported injury any time during the course of the OAI participation or prior enrollment
Statistically significant
KOOS Knee Injury and Osteoarthritis Outcome Score, WOMAC Western Ontario and McMaster Universities Osteoarthritis Index
All 31 (100%) knees that had undergone meniscal surgery during the previous year had developed incident radiographic OA at the next follow-up visit, while 50.2% of the knees developing incident radiographic OA 1 year later had prevalent meniscal damage, compared to 32.5% of the control knees not developing radiographic OA. A more than twofold greater risk for incident radiographic OA was observed for knees exhibiting prevalent tears or maceration, which is shown in Table 4.
Table 4.
Incident radiographic osteoarthritis
| Meniscal status at P-1 or surgery case | N (%) in controls | N (%) in incident ROA knees | Odds of incident ROA*(outcome) Crude odds ratio (95% CI) |
|---|---|---|---|
| N = 354 | N = 329 | ||
|
| |||
| None/Signal | 239 (67.5%) | 164 (49.9%) | Reference |
| Tear or maceration | 115 (32.5%) | 165 (50.2%) | 2.51 (1.73, 3.64)a |
| Meniscal Surgery | |||
| No | 35 (100%) | 323 (91.2%) | Reference |
| Yes | 0 (0%) | 31 (8.8%) | N/A |
Conditional logistic regression using 328 pairs with case and control data at P-1
After adjustment for BMI: OR = 2.66 (95% CI 1.81–3.89)
CI confidence interval, ROA radiographic osteoarthritis, N number, N/A not applicable, P-1 time point/annual visit 1 year prior to case-defining visit
Among all cases and controls, 37.4% of knees with meniscal damage but not surgery and 80.8% of knees with partial meniscectomy showed cartilage loss over the same 1-year follow-up period. For cases that developed ROA, prevalent meniscal damage was not associated with worsening cartilage damage in the tibiofemoral joint (odds ratio (OR) = 0.98, 95% confidence interval (CI) [0.58, 1.67]), but partial meniscectomy was strongly associated with worsening cartilage damage (OR = 4.76, 95% CI [1.63, 13.90]) compared to knees with normal meniscal morphology as the reference. For the fully adjusted model, prevalent meniscal damage again showed no association with cartilage loss (OR = 0.88, 95% CI [0.51, 1.51]), but partial meniscectomy remained strongly associated (OR 4.51, 95% CI [1.53, 13.33]). Details of these results are presented in Table 5. Figure 2 provides an illustrative example of partial meniscectomy between P-1 and P0 and subsequent development of ROA and cartilage loss.
Table 5.
Cartilage worsening in whole knee (medial and lateral compartments), knees developing radiographic osteoarthritis only
| Meniscal status at P-1 or surgery case | No cartilage worsening N (%) | Cartilage worsening N (%) | Adjusted odds of cartilage loss (outcome) Crude odds ratio (95% CI)a | Adjusted odds of cartilage loss (outcome) Crude odds ratio (95% CI)b |
|---|---|---|---|---|
| N = 147 | N = 162 | |||
| No surgery / no meniscal damage | 74 (50.3%) | 67 (41.4%) | Reference | Reference |
| No surgery, but presence of meniscal damage (tear or maceration) | 68 (46.2%) | 74 (45.7%) | 0.98 (0.58,1.67) | 0.88 (0.51,1.51) |
|
| ||||
| Meniscal surgeryc | 5 (3.4%) | 21 (13.0) | 4.76 (1.63,13.90)* | 4.51 (1.53,13.33)* |
Logistic regression adjusted for matching criteria (radiographic OA severity defined by Kellgren-Lawrence grade of index and contralateral knee, age, gender) and BMI
Logistic regression adjusted for matching criteria (radiographic OA severity defined by Kellgren-Lawrence grade of index and contralateral knee, age, gender), BMI and prevalent MRI features (cartilage damage, effusion, synovitis, BMLs)
No data for five knees at P-1
CI confidence interval, BMI body mass index, P-1 annual visit 1 year prior to case-defining visit, BMLs bone marrow lesions
Fig. 2.
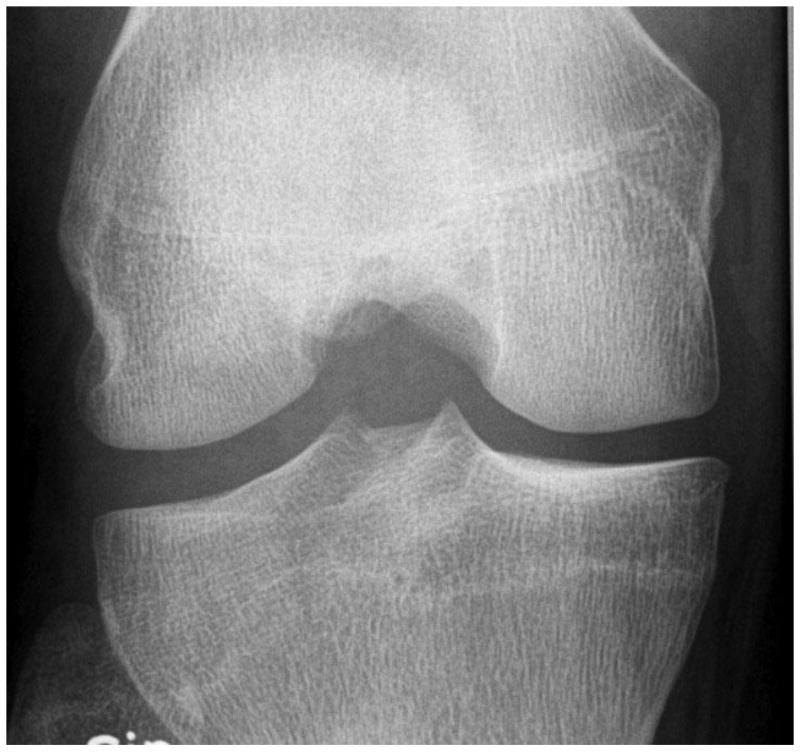
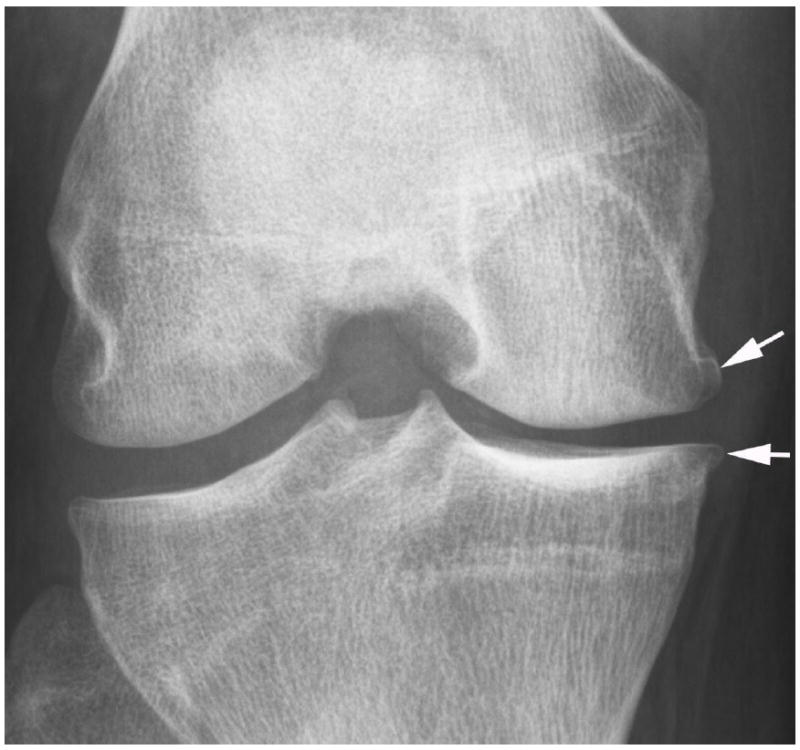
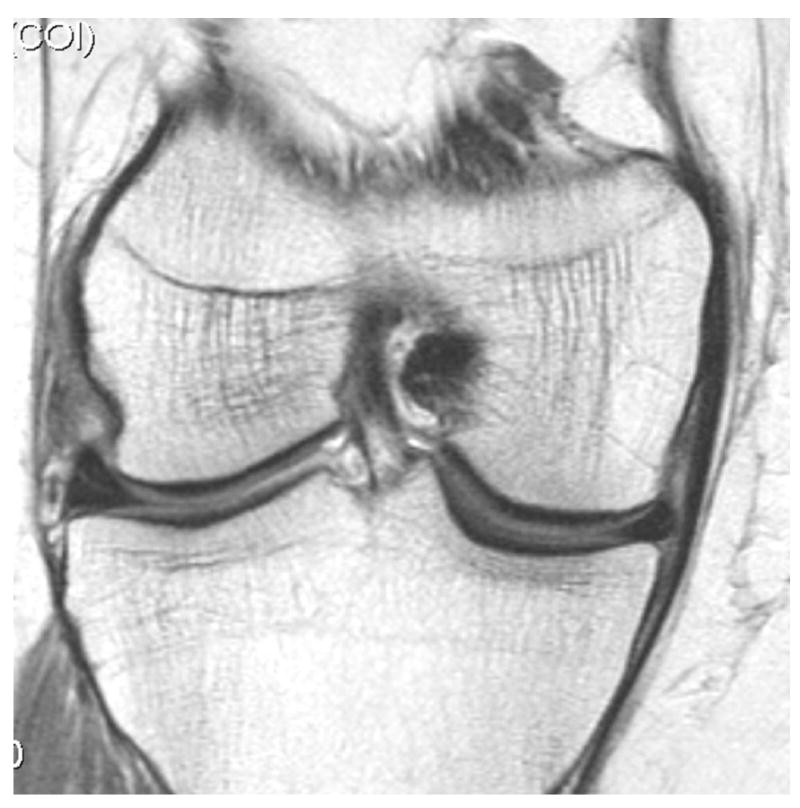
Development of radiographic osteoarthritis (OA) after partial meniscectomy. A. Baseline anterior–posterior radiograph of the knee shows physiologic joint anatomy without signs of OA. B. 12-month follow-up image shows definite radiographic OA with presence of medial tibial and femoral osteophytes at the joint margin consistent with radiographic OA Kellgren-Lawrence grade 2. C. Corresponding baseline MRI confirms absence of structural findings of OA. D. Follow-up image confirms partial meniscectomy with missing meniscal substance of the meniscal body (black arrow) and incident cartilage thinning (white arrows). An incident osteophyte is also depicted on the MRI (arrowhead).
An additional analysis incorporating the above-mentioned confounders for progression of cartilage damage plus pain status at P-1 and previous injury into the statistical model showed that the observed associations remained, although risk for cartilage loss decreased slightly, from 4.63 to 4.21. An overview of the expanded analyses incorporating pain and injury status is given in Table 6.
Table 6.
Cartilage worsening in whole knee (medial and lateral compartments), knees developing radiographic osteoarthritis only
| Meniscal status at P-1 or surgery case | Adjusted odds of cartilage loss (outcome) Crude OR (95% CI)a | Adjusted odds of cartilage loss (outcome) Crude OR (95% CI)a | Adjusted odds of cartilage loss (outcome) Crude OR (95% CI)a | Adjusted odds of cartilage loss (outcome) Crude OR (95% CI)2 | Adjusted odds of cartilage loss (outcome) Crude OR (95% CI)b | Adjusted odds of cartilage loss (outcome) Crude OR (95% CI)b |
|---|---|---|---|---|---|---|
| add KOOS to model | add injury to model | add KOOS and injury to model | add KOOS to model | add injury to model | add KOOS and injury to model | |
| Excluding adjustment for structural MRI features | Including adjustment for structural MRI features | |||||
|
| ||||||
| No surgery / no meniscal damage | Reference | Reference | Reference | Reference | Reference | Reference |
| No surgery, but presence of meniscal damage (tear or maceration) | 0.95 (0.56,1.62) | 0.96 (0.57,1.64) | 0.93 (0.54,1.59) | 0.86 (0.50,1.48) | .85 (0.50,1.48) | 0.84 (0.49,1.45) |
|
| ||||||
| Meniscal surgery3 | 4.63 (1.57, 13.59) | 4.51 (1.53,13.26) | 4.36 (1.48,12.90) | 4.52 (1.52,13.44) | 4.22 (1.41, 12.59) | 4.21 (1.41,12.63) |
Logistic regression adjusted for matching criteria (radiographic OA severity defined by Kellgren-Lawrence grade of index and contralateral knee, age, gender) and BMI
Logistic regression adjusted for matching criteria (radiographic OA severity defined by Kellgren-Lawrence grade of index and contralateral knee, age, gender), BMI and prevalent MRI features (cartilage damage, effusion, synovitis, BMLs)
No data for five knees at P-1
OR odds ratio, CI confidence interval, BMI body mass index, P-1 annual visit 1 year prior to case-defining visit, BMLs bone marrow lesions, KOOS knee osteoarthritis outcome score
Discussion
In this nested case–control study, we found a much greater probability of having had partial meniscectomy in the previous year in knees that subsequently developed radiographic OA than in control knees. Furthermore, the probability of worsening cartilage damage was much higher in knees that had developed ROA and had undergone surgery compared to knees that had just developed ROA.
We recently reported on structural risk factors with regard to the development of radiographic OA in relation to several parameters, including the severity of tissue damage and the cumulative effects of the involvement of several joint structures [11]. The strongest association with OA was observed whenever these tissue changes were present in the year prior to the case-defining visit, but none showed a comparable association with incident radiographic OA as was observed for subjects having had partial meniscectomy. However, also in that study, medial meniscus damage in particular—including meniscal tears and any kind of substance loss—strongly predicted incident ROA 1 or 2 years later [11].
Little information is available in the literature to date on subsequent risk of cartilage loss following meniscal surgery. One study of young athletes in the National Football League (NFL) in the United States who underwent MRI for various reasons reported that knees with previous meniscal surgery had a much higher prevalence of ipsi-compartmental full-thickness cartilage lesions (27% vs. 12% of those without meniscal surgery). This suggests that our findings may also be relevant for a younger active population, although reasons for meniscal surgery might differ from the indications in a non-athlete elderly patient cohort [21]. Furthermore, a repair approach, when feasible, seems to be superior to partial meniscectomy in terms of clinical outcomes [22]. In a sample at increased risk of developing radiographic OA in the Multicenter Osteoarthritis Study (MOST), for knees not undergoing surgery but with prevalent meniscal damage, the likelihood of radiographic OA within a 30-month period increased almost sixfold, which supports the importance not only of surgically induced meniscal alterations, but of meniscal integrity in general [23]. In our sample, we did find an increased risk of ROA but not of cartilage loss in knees with prevalent meniscal damage, which is likely due to the shorter follow-up period of 12 months compared to the 30 months of follow-up in the MOST study.
Our study has several limitations. One point that should be mentioned is that although the OAI is the largest longitudinal study of knee OA, only 31 knees underwent meniscal surgery within the observation period, and only 26 had MRI data available at the time point prior to the case-defining visit.
Im addition, the definition of meniscal surgery was based on participants’ answers to a question regarding prior meniscal surgery as part of a clinical assessment during the yearly OAI visits, and not on actual surgery reports. We confirmed self-reports of a partial meniscectomy by the diagnosis of missing meniscal substance on MRI at the follow-up visit, with none of the knees undergoing surgery having any meniscal maceration at the visit prior to surgery, suggesting that the confirmatory readings were valid and the surgeries correctly reported. We also did not explore potential reasons for the surgeries performed. Patients undergoing surgery had higher pain scores at the time point prior to the case-defining visit and more frequent reports of previous injury, which was reason to expand our analyses adding pain and prior knee injury to the model. Despite the odds ratios remaining markedly increased for those who had surgery compared to those who had prevalent meniscal damage only, we cannot fully rule out residual confounding by indication, i.e., those having had meniscus surgery may have had symptoms because of other features of pre-radiographic knee OA rather than symptoms from the meniscal lesion per se [8, 11, 24]. However, we found no significant differences associated with meniscal damage severity in any of the case knees or the entire cohort. Finally, we used a definition of radiographic OA only and did not take into account the clinical manifestation of OA. The meniscus is an important contributor to the normal medial and lateral tibiofemoral joint space [25], and meniscal surgery may lead to a reduction in joint space width through a rapid decrease in the overall meniscal substance, thus contributing to the diagnosis of ROA [26]. In the current analysis, we did not assess other factors associated with meniscal pathology such as previous injury, and we also did not observe any meniscal root tears, another strong risk factor of structural progression and OA incidence [27]. Although we did assess meniscal extrusion in the coronal plane, as commonly assessed using semiquantitative scoring methods, we acknowledge that change in extrusion across the entire meniscus evaluated using 3D measurements is an alternative approach that we did not include but that might yield additional information [28].
We did include the 2D spin-echo sequences and the reformatted DESS sequence of the OAI protocol for semiquantitative assessment [27]. While the additionally available T2 multi-echo spin-echo (MESE) sequence and fast low-angle shot (FLASH) sequences may add information when applying additional cartilage evaluation including 3D segmentation approaches and compositional evaluation, they do not add information when using scoring approaches [27]. As the OAI protocol was designed more than a decade ago, newer and potentially useful sequences for cartilage assessment were not included [13, 29].
Ultimately, given the data presented with regard to structural consequences and the recent clinical trials focusing on clinical outcomes, patients and their physicians should consider these findings in their clinical decision process. For the middle-aged patient with knee pain and a degenerative meniscal tear, a large body of evidence today suggests that an initial regimen of strengthening-based physical therapy should be the first step in treatment [10, 30].
In summary, the probability of having had partial meniscectomy in the previous year was higher in those who developed incident radiographic OA than in control knees. Furthermore, even when limited to knees that later developed ROA, worsening of cartilage damage within the same 1-year period was higher for knees undergoing partial meniscectomy than those with prevalent meniscal damage only. As partial meniscectomy may have deleterious effects on joint structure in knees without radiographic OA, the treatment alternatives for patients with meniscal damage and symptoms must be carefully discussed between patient and treating physician.
Supplementary Material
Key points.
Partial meniscectomy is a controversial treatment option for degenerative meniscal tears.
Partial meniscectomy is strongly associated with incident osteoarthritis within 1 year.
Partial meniscectomy is associated with increased risk of worsening cartilage damage.
Acknowledgments
The scientific guarantor of this publication is the first author, Frank Roemer. The data were presented at RSNA and at ECR 2015 as a podium presentation. The authors of this manuscript declare relationships with the following companies:
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Potential conflicts outside of this work: Dr. Guermazi has received consultancies, speaking fees, and/or honoraria from OrthoTrophix, Genzyme, Merck Serono and TissueGene, and is President and shareholder of Boston Imaging Core Lab (BICL), LLC, a company providing image assessment services. Dr. Roemer is Chief Medical Officer and shareholder of BICL, LLC. Dr. Kwoh has provided consulting services to Novartis and has received research support from AstraZeneca. Dr. Eckstein is CEO of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry. He provides consulting services to Merck Serono, Novartis, and Sanofi-Aventis, has received speaker honoraria from Merck, Glaxo-Smith-Kline, Genzyme, Medtronic, and Synthes, and has received research support from Pfizer, Eli Lilly, Merck Serono, Glaxo-Smith-Kline, Centocor R&D, Wyeth, Novartis, and Stryker. Dr. Hunter receives royalties from DJO.
The study and image acquisition was funded by the OAI, a public–private partnership comprising five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners of the OAI include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
The image analysis in this study was funded in part by a contract with the University of Pittsburgh (Pivotal OAI MRI Analyses [POMA]: NIH/NHLBI Contract No. HHSN2682010000 21C), and in part by a vendor contract from the OAI coordinating center at the University of California, San Francisco (N01-AR-2-2258).
The statistical data analysis was funded in part by a contract with the University of Pittsburgh (Pivotal OAI MRI Analyses [POMA]: NIH/NHLBI Contract No. HHSN2682010000 21C) and by the University of Pittsburgh Multidisciplinary Clinical Research Center (MCRC) for Rheumatic and Musculoskeletal Diseases (P60 AR054731).
Michael J. Hannon, Jason Grago and Robert Boudreau have significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: retrospective, case–control study, multicenter study.
Abbreviations and Acronyms
- MRI
Magnetic resonance imaging
- ROA
Radiographic osteoarthritis
- RCT
Randomized controlled trial
- OAI
Osteoarthritis Initiative
- K-L
Kellgren-Lawrence
- P0
OAI annual visit when radiographic osteoarthritis was diagnosed
- P-1
OAI annual visit 1 year prior to diagnosis of radiographic osteoarthritis
References
- 1.Lubowitz JH, Poehling GG. Save the meniscus. Arthroscopy. 2011;27:301–302. doi: 10.1016/j.arthro.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Burns TC, Giuliani JR, Svoboda SJ, Owens BD. Meniscus repair and transplantation techniques. J Knee Surg. 2011;24:167–174. doi: 10.1055/s-0031-1286051. [DOI] [PubMed] [Google Scholar]
- 3.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50:2811–2819. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 4.Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol. 2012;8:412–419. doi: 10.1038/nrrheum.2012.69. [DOI] [PubMed] [Google Scholar]
- 5.Herrlin SV, Wange PO, Lapidus G, Hallander M, Werner S, Weidenhielm L. Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five year follow-up. Knee Surg Sports Traumatol Arthrosc. 2013;21:358–364. doi: 10.1007/s00167-012-1960-3. [DOI] [PubMed] [Google Scholar]
- 6.Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675–1684. doi: 10.1056/NEJMoa1301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yim JH, Seon JK, Song EK, et al. A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. Am J Sports Med. 2013;41:1565–1570. doi: 10.1177/0363546513488518. [DOI] [PubMed] [Google Scholar]
- 8.Sihvonen R, Paavola M, Malmivaara A, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515–2524. doi: 10.1056/NEJMoa1305189. [DOI] [PubMed] [Google Scholar]
- 9.Gauffin H, Tagesson S, Meunier A, Magnusson H, Kvist J. Knee arthroscopic surgery is beneficial to middle-aged patients with meniscal symptoms: a prospective, randomised, single-blinded study. Osteoarthritis Cartilage. 2014;22:1808–1816. doi: 10.1016/j.joca.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Thorlund JB, Juhl CB, Roos EM, Lohmander LS. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747. doi: 10.1136/bmj.h2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roemer FW, Kwoh CK, Hannon MJ, et al. What comes first? Multi-tissue involvement leading to radiographic osteoarthritis: MRI-based trajectory analysis over 4 years in the Osteoarthritis Initiative. Arthritis Rheumatol. 2015;67:2085–2096. doi: 10.1002/art.39176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz JN, Martin SD. Meniscus—Friend or foe: epidemiologic observations and surgical implications. Arthritis Rheum. 2009;60:633–635. doi: 10.1002/art.24363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nevitt MC, Felson DT, Lester G. [June 23, 2015];The Osteoarthritis Initiative: protocol for the cohort study. :19–20. URL: http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf.
- 15. [June 23, 2015];Radiographic procedure manual for examinations of the knee, hand, pelvis and lower limbs. Version 2.1. 2006 Aug; URL: http://oai.epi-ucsf.org/datarelease/operationsManuals/RadiographicManual.pdf.
- 16.Kothari M, Guermazi A, von Ingersleben G, et al. Fixed–flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14:1568–1573. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 17. [June 23, 2015];Appendix A. Project 15: reader discrepancies and adjudication procedures. OAI central reading of knee x-rays for K-L grade and individual features of knee OA. 2013 :8. URL: http://oai.epi-ucsf.org/datarelease/forms/kXR_SQ_BU_Descrip.pdf?V01XRKL.
- 18.Hunter DJ, Guermazi A, Lo GH, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roemer FW, Hunter DJ, Crema MD, Kwoh CK, Ochoa-Albiztegui E, Guermazi A. An illustrative overview of semi-quantitative MRI scoring of knee osteoarthritis: lessons learned from longitudinal observational studies. Osteoarthritis Cartilage. 2015 Aug 28; doi: 10.1016/j.joca.2015.08.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 21.Nepple JJ, Wright RW, Matava MJ, Brophy RH. Full-thickness knee articular cartilage defects in national football league combine athletes undergoing magnetic resonance imaging: prevalence, location, and association with previous surgery. Arthroscopy. 2012;28:798–806. doi: 10.1016/j.arthro.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Stein T, Mehling AP, Welsch F, von Eisenhart-Rothe R, Jager A. Long-term outcome after arthroscopic meniscal repair versus arthroscopic partial meniscectomy for traumatic meniscal tears. Am J Sports Med. 2010;38:1542–1548. doi: 10.1177/0363546510364052. [DOI] [PubMed] [Google Scholar]
- 23.Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009 doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin S, LaValley MP, Guermazi A, et al. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52:3152–3159. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 26.Kim SJ, Lee SK, Kim SH, et al. Does decreased meniscal thickness affect surgical outcomes after medial meniscectomy? Am J Sports Med. 2015;43:937–944. doi: 10.1177/0363546514544677. [DOI] [PubMed] [Google Scholar]
- 27.Alizai H, Roemer FW, Hayashi D, Crema MD, Felson DT, Guermazi A. An update on risk factors for cartilage loss in knee osteoarthritis assessed using MRI-based semiquantitative grading methods. Eur Radiol. 2015;25:883–893. doi: 10.1007/s00330-014-3464-7. [DOI] [PubMed] [Google Scholar]
- 28.Bloecker K, Wirth W, Guermazi A, Hitzl W, Hunter DJ, Eckstein F. Longitudinal change in quantitative meniscus measurements in knee osteoarthritis-data from the Osteoarthritis Initiative Eur Radiol. 2015;25:2960–2968. doi: 10.1007/s00330-015-3710-7. [DOI] [PubMed] [Google Scholar]
- 29.Van Dyck P, Vanhevel F, Vanhoenacker FM, et al. Morphological MR imaging of the articular cartilage of the knee at 3 T-comparison of standard and novel 3D sequences. Insights Imaging. 2015;6:285–293. doi: 10.1007/s13244-015-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz JN, Losina E. Arthroscopic partial meniscectomy for degenerative tears: where do we stand? Osteoarthritis Cartilage. 2014;22:1749–1751. doi: 10.1016/j.joca.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


