Abstract
Non-standard exceptions requests (NSERs), in which transplant centers appeal on a case-by-case basis for PELD/MELD points, have been highly utilized for pediatric liver transplant candidates. We evaluated whether NSE outcomes, are associated with waitlist and post-transplant mortality. UNOS Scientific Registry of Transplant Recipients data on pediatric liver transplant candidates listed 2009-2014 were analyzed after excluding those granted automatic UNOS exceptions. Of 2,581 pediatric waitlist candidates, 44% had an NSE request. Of the 1,134 children with NSERs, 93% were approved and 7% were denied. For children 2-18 years at listing, NSER denial increased the risk of waitlist mortality or removal for being too sick (SHR 2.99, 95% CI 1.26-7.07, p=0.01 in multivariate analysis). For children younger than 2, NSER denial did not impact waitlist mortality/removal. Children with NSER approved had reduced risk of graft loss 3 years post-transplant in univariate but not multivariable analysis (OR 0.73, 95% CI 0.53-1.01, p=06). Those with NSER denial had a higher risk of post-transplant death than those with no NSER (HR 2.43, 95% CI 0.99-5.95, p=0.05, multivariable analysis), but NSER approval did not impact post-transplant death. Further research on NSER utilization in pediatric liver transplant is needed to optimize organ allocation and outcomes for children.
Introduction
Allocating livers based on Model for End-Stage Liver Disease (MELD) and Pediatric End-Stage Liver Disease (PELD) scores is intended to minimize waitlist mortality in all candidates. (1) Since implementation of the MELD/PELD system in 2002, MELD scores have been used for allocation in pediatric liver transplant candidates 12-18 years of age, and PELD in those younger than 12. As required by the 1999 Final Rule, these scoring systems were adopted to set “priority rankings, to the extent possible, through objective and measurable medical criteria.” (2)
But even early analyses suggested that MELD/PELD scores calculated from patients’ laboratory values did not drive the majority of pediatric liver allocations. In 2003-2004, 53% of pediatric liver transplant recipients were granted “exceptions” to calculated MELD/PELD scores to increase their priority on the waiting list. (3) Regional variation in PELD scores and exception requests was also reported soon after the system's implementation. (3,4)
In addition to Status 1 listings and standardized exceptions, e.g. for hepatocellular carcinoma (HCC) or urea cycle disorders, non-standard exceptions requests (NSERs) have been highly utilized for pediatric liver transplant candidates. (3,4) NSERs are exceptions in which transplant centers appeal to Regional Review Boards (RRB) on a case-by-case basis for extra PELD/MELD points. The center provides a narrative account of the patient's illness severity with a request for a specific PELD/MELD score. The RRB reviews NSERs within 21 days of submission and votes on whether to approve the request; agreement from a majority of RRB members leads to approval. Denied NSERs can be reviewed in a conference call with the RRB, at the transplant center's request. NSERs allow centers to provide personalized detail about the patient, but they reduce the objectivity, standardization, and possibly the parity, that the PELD/MELD system was meant to provide.
Between 2002 and 2013, the incidence rate of NSERs increased five-fold; and 90% of requested pediatric exceptions were approved. (5) Perhaps most importantly, having an approved exception increased the hazard ratio (HR) for transplant, after adjustment for other factors. (5)
The impact of NSER approval and denial on pediatric waitlist mortality and post-transplant survival has not been examined. Previous analyses of pediatric liver transplant exceptions have not always separated NSERs from standard MELD/PELD exceptions (metabolic disease, HCC, etc.). (6) We hypothesized that NSER denial, although rare, might disadvantage patients—and thus be associated with increased risk of waitlist and post-transplant mortality. We used the UNOS database with data from 2009-2015 to evaluate this hypothesis.
Methods
We used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. Institutional Review Board approval from the University of California, San Francisco, was obtained prior to initiation of this analysis (CHR 14-15024).
Cohort Selection
UNOS SRTR data on pediatric liver transplant candidates listed between 1 January 2009 and 31 December 2014, ages 0-18 years at listing, were analyzed after excluding those with automatic UNOS exception points. Waitlist data was censored as of 6 March 2015.
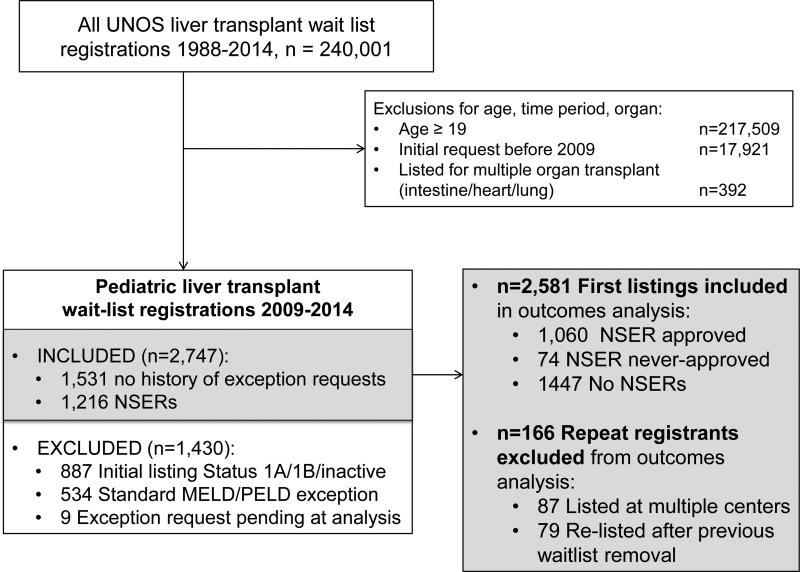
Our analysis focused on comparing waitlist candidates with NSERs to waitlist candidates with no NSERs and no other MELD/PELD exceptions, as these are the candidates that could have been eligible for NSER. We thus excluded pediatric candidates who were initially listed as Status 1a, 1b, inactive, or with a “standard” exception that earned them automatic MELD/PELD exception points by UNOS criteria. “Standard” exceptions included HCC within criteria, metabolic liver disease, hepatoblastoma, primary hyperoxaluria, hepatopulmonary syndrome or portopulmonary hypertension, and familial amyloidosis. (FIGURE 1) We classified liver transplant indication based on the categories defined by the Studies in Pediatric Liver Transplant (SPLIT) Research Group.(7)
FIGURE 1.
Creation of the study cohort using UNOS SRTR files. NSER = Non-standard exception request.
To avoid bias introduced by intra-patient correlation, only a patient's first listing within the study period was used for descriptive comparison of no NSER application, NSER approved and NSER denied groups and for analysis of waitlist mortality. (FIGURE 1) For patients with multiple listings, the listing with the earliest registration date was considered the first listing. For patients with more than one listing with the same initial date, the waitlist and exception case identification codes were used to identify earliest filing.
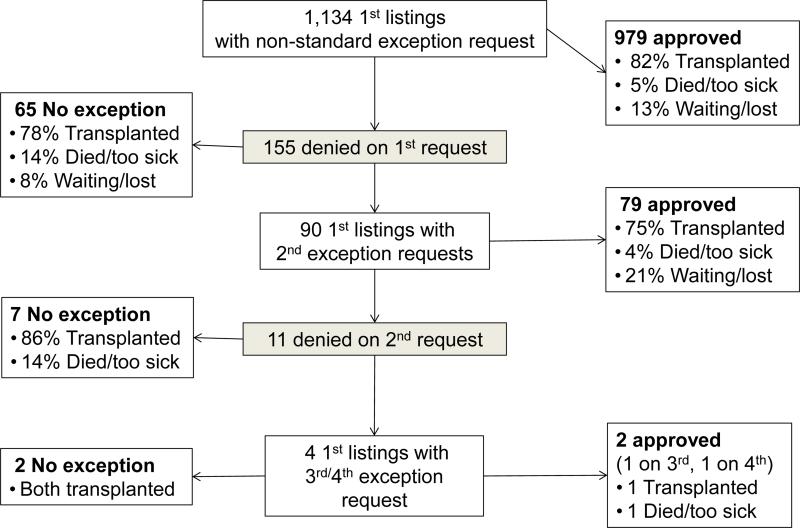
Included waitlist candidates were first compared in univariate analysis based on whether their first NSER was approved or denied, to evaluate the impact of that initial action. Patients were then compared, in univariate and multivariable analyses, based on the final outcome of their NSER cases during the first listing; they were classified as no NSER application, NSER approved, or NSER never-approved. The NSER approved subjects received an exception approval, either on the first or subsequent requests, during the waitlist registration of interest. The NSER never-approved subjects had at least one NSER submitted but never had any NSER approvals during that waitlist registration. (FIGURE 2)
FIGURE 2.
Non-standard exception request (NSER) and waitlist outcomes for children and adolescents with NSERs. Analysis cohort includes only patients’ first waitlist registration during the study period, 2009-2014.
Post-transplant survival was assessed in the subgroup of patients receiving their first liver transplant with available transplant date and follow-up data (patient status and follow-up date). Patients with a previous liver transplant or kidney-liver transplant were excluded from the post-transplant survival analysis.
Statistical Analysis
Descriptive statistics were performed with chi-squared testing for categorical variables. Median and interquartile range (IQR, 25th-75th percentile) were reported for continuous variables because of skewed distributions. Kruskal-Wallis testing for between group differences was utilized for continuous variables. Mantel-Haenszel testing was used for unidirectional trend in analyses of NSE denial by listing year.
Risk of wait list death, defined as a death or waitlist removal for being too sick to transplant, was evaluated using Fine and Gray competing risks regression.(8) Observation time was measured from the date of listing for transplant to waitlist death (event), liver transplant (competing risk), or last date on the waiting list for patients still waiting or removed for other reasons (censored). Risk of wait list death (subhazard ratios, SHR) was estimated by modeling the cumulative incidence function with competing risks regression for demographic and clinical characteristics. Factors with p<0.1 in the univariate analysis were evaluated in the multivariable model. Factors were eliminated by backward stepwise selection; NSER, as the primary predictor of interest, and all other variables that retained p<0.05, were reported in the final model.
Risk of post-transplant outcomes, patient death and graft loss (defined as re-transplant or death), within 3 years of transplant were evaluated using Cox proportional hazards regression. (9) Patient follow-up time was measured from the date of transplant to the first event, retransplant (when graft loss evaluated) or death, or last follow-up within 3 years of transplant (censored). Univariate hazard ratios (HR) estimated risk of patient death or graft loss for demographic and clinical characteristics. Factors with p<0.1 in the univariate analysis were evaluated in the multivariable model. Factors were eliminated by backward stepwise selection; NSER, as the primary predictor of interest, and all other variables that retained p<0.05 were reported in the final model.
Data analysis was completed using SAS version 9.4 (SAS Institute, Cary, NC) and Stata/IC 14 (StataCorp, College Station, TX).
Results
NSERs in all waitlist registrations
Our study cohort included 2,747 registrations on the pediatric liver transplant waitlist between 2009 and 2014 with either no NSER application or an NSER. (FIGURE 1) Of the 1,216 waitlist registrations with at least one NSER, 86% were approved on the first request, and 94% were ultimately approved. (FIGURE S1)
These 2,747 registrations included the first waitlist registration within the study period (first listing) for 2,581 pediatric patients, 87 concurrent listings at more than one transplant center, and 79 re-listings after previous waitlist removal. Figure 2 details NSERs, approvals, denials, and outcomes for those approved versus denied for a patient's first listing within the study period. Subsequent analyses include only a patient's first listing within the study period.
Factors associated with first NSER denial
Of the 1,134 children with NSERs, 86% were approved and 14% were denied on their first NSER. (FIGURE 2) Children with first NSER denied were older at listing (median 10 years, IQR 0-15) than those with first NSER approved (median 1 years, IQR 0-10, p<0.001). They were more likely to be female (63.9% vs. 53.6%, p=0.02), Caucasian (64.5% vs. 55.2%, p=0.06), and have private insurance (60.6% vs. 50.7%, p=0.02). They were less likely to have biliary atresia (30.3%, vs. 48.9% approved first NSER, p=0.001). The prevalence of first NSER denial varied widely by region, from 0% of 33 NSERs in Region 6 to 35% of 144 NSERs in Region 5. Of note, Region 6 had the smallest number of waitlist registrants in the study cohort (n=69) and Region 5 the largest (n=492). In the other 9 regions, the prevalence of first NSER denial ranged from 4% to 16% (p<0.001), with no consistent trend between region volume and first NSER denial.
Impact of first NSER on waitlist outcomes
We next examined the impact of first NSER denial on waitlist outcomes. In univariate analysis, those approved first NSER had a lower risk of waitlist mortality than those with no NSER application (HR 0.70, 95% CI 0.50-0.99, p=0.05). Denial of first NSER did not significantly impact waitlist mortality (HR 1.12, 95% CI 0.62-2.03, p=0.70). Of those denied first NSER, 5 died and 7 were removed from the waitlist for being too sick. Total waitlist time was similar for those denied first NSER (median 132 days, IQR 45-362, n=155) and those approved first NSER (median 118 days, IQR 54-249, n=888, p=0.67) but shorter for those with no NSER application (median 73 days, IQR 21-247 days, p<0.001 compared to 2 other groups).
Factors associated with denial of all NSERs
Of the 1,134 children with NSERs, 93% were ultimately approved and 7% were never-approved. (FIGURE 2) Children and adolescents never-approved for NSER were older, more likely to be Caucasian, less likely to have biliary atresia and more likely to have a tumor, and more likely to have private insurance. (TABLE 1)
Table 1.
Clinical and transplant characteristics of liver transplant recipients, by NSER status
| NSER approved | NSER submitted, never approved | No NSER applications | p | ||
|---|---|---|---|---|---|
| Number of patients | 1,060 | 74 | 1,447 | -- | |
| Female | 54.6% | 60.8% | 53.6% | 0.47 | |
| Age at listing (years)* | 2 (0-11) | 9 (0-15) | 2 (0-11) | 0.006 | |
| Previous transplant¶ | 5.3% | 5.4% | 2.8% | <0.001 | |
| Also listed for kidney or pancreas | 4.1% | 4.1% | 2.1% | <0.001 | |
| Ethnicity | 0.002 | ||||
| White | 55.6% | 67.6% | 48.1% | ||
| Black | 16.3% | 9.5% | 16.9% | ||
| Hispanic | 20.0% | 17.6% | 24.7% | ||
| Asian | 5.1% | 4.1% | 6.3% | ||
| Other | 2.9% | 1.4% | 3.9% | ||
| Indication for liver transplant† | <0.001 | ||||
| Biliary atresia | 47.1% | 35.1% | 41.4% | ||
| Cholestatic conditions | 12.9% | 12.2% | 14.3% | ||
| Metabolic liver disease | 11.1% | 12.2% | 9.1% | ||
| Tumor (outside standard criteria) | 6.0% | 9.5 | 3.1% | ||
| Acute liver failure | 2.2% | 2.7% | 8.7% | ||
| Other liver disease | 20.7% | 28.4% | 23.4% | ||
| Public Insurance | 48.6% | 37.8% | 55.7% | <0.001 | |
| Days from listing to 1st NSER (n=1,134)* | 21 (3-81) | 26 (6-70) | n/a | 0.41 | |
| Total days on waitlist* | 124 (55-263) | 66 (17-180) | 73 (21-247) | <0.001 | |
| Outcome of 1st listing during study period | ‡ | ||||
| Transplanted | 90.2% | 81.9% | 76.3% | ||
| Died/too sick | 5.3% | 13.9% | 7.8% | ||
| Other | 4.5% | 4.2% | 15.9% | ||
| Days on waitlist for transplanted (n=1,888)* | 109 (50-216) | 46 (13-149) | 45 (14-111) | 0.0001 | |
| Lab MELD/PELD at waitlist removal (n=2,545)* | 12 (4-21) | 17 (10-26) | 16 (7-26) | <0.001 | |
| Allocation MELD/PELD at waitlist removal(n=2,035)* | 30 (25-36) | 18 (10-25) | 16 (7-24) | <0.001 | |
| Status at waitlist removal, for transplanted (n=1,812) | <0.001 | ||||
| Not hospitalized | 69.5% | 58.6% | 62.6% | ||
| Hospitalized, not ICU | 21.4% | 24.1% | 19.5% | ||
| ICU | 9.1% | 17.2% | 17.9% | ||
| Transplant Type (n=1,817) | <0.001 | ||||
| Living donor | 8.1% | 10.3% | 18.7% | ||
| Cadaveric (whole) | 74.9% | 84.5% | 63.3% | ||
| Cadaveric (split) | 17.1% | 5.2% | 18.0% | ||
| Donor deceased after cardiac death (n=1,817) | 0.12% | 3.5% | 0.9% | 0.002 | |
| Donor CDC high-risk (n=1,569) | 6.2% | 7.7% | 6.6% | 0.89 | |
| Donor ≥40 years of age (n=1,816) | 5.1% | 8.6% | 6.9% | 0.21 | |
| Cold ischemia time (n=1,763)* | 6.4 (5.0-8.3) | 7.0 (5.4-8.6) | 6.2 (4.4-8.1) | 0.01 | |
Continuous variables reported as median (IQR). N in each row indicates number of patients for whom data on that variable was available in the SRTR database. Rows without n listed had no missing data for that variable.
Data on previous transplant available on n=1,817, as UNOS only includes previous transplant indicator on listings that end in liver transplant.
Cholestatic conditions include Alagille syndrome, Byler disease, progressive intrahepatic cholestatic syndromes, total parenteral nutrition cholestasis, sclerosing cholangitis, and idiopathic cholestasis. Metabolic liver disease includes alpha-1-antitrypsin deficiency, Crigler-Najjar syndrome, cystic fibrosis, glycogen storage disease, inborn errors in bile acid metabolism, neonatal hemochromatosis, primary hyperoxaluria,tyrosinemia, urea cycle defects, and Wilson's disease. Other liver disease includes congenital hepatic fibrosis, Budd-Chiari syndrome, autoimmune hepatitis cirrhosis, drug toxicity, hepatitis C cirrhosis, and unknown cirrhosis.
See results section and Table 2 for calculation of statistical significance in analysis of waitlist mortality.
The prevalence of waitlist candidates with approved NSER increased steadily, from 26% in 2009 to 44% in 2014, while the prevalence of those with no NSER application consistently decreased, from 67% in 2009 (n=466) to 50% in 2014 (n=413, p<0.001 by chi-squared). The prevalence of children with never-approved NSER remained relatively steady, at 7-9% per year. The odds of being never-approved for NSER decreased annually (OR 0.85 per one year increase, 95% CI 0.77-0.94, p=0.002), after adjusting for region.
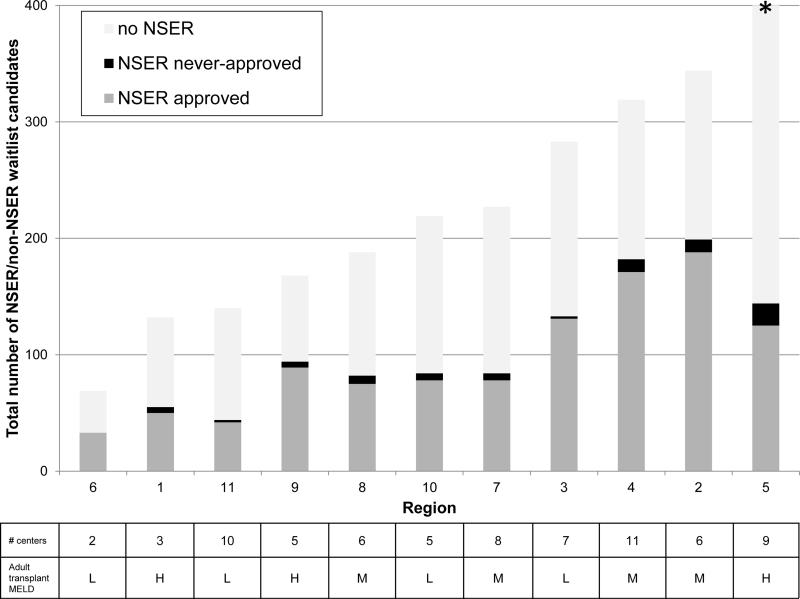
From 2009-2014, 45-49% of all registrants in Regions 2, 3, 4 and 9 had approved NSERs (n=754 listings). Region 5 had the lowest prevalence of waitlist candidates with approved NSERs (18%) and the highest prevalence of NSER never-approved (11%); it had the largest number of total listings in the cohort (n=492). Region 6 had no NSERs never-approved, and the lowest regional volume (n=69). The other regions ranged from 27-37% with approved NSERs and 0-9% with never-approved NSERs (p<0.001, chi-squared), with no consistent relationship between regional volume and never-approved prevalence within this group. (FIGURE 4)
FIGURE 4.
Prevalence of waitlist candidates with NSER approved and never-approved, by total number of NSER and non-NSER waitlist registrations during the study period 2009-2014. Table shows, for each region, number of centers submitting pediatric NSERs during the study period and median MELD at transplant for adults (H, high = ≥ 26; M, mid = 22-25; L, low = ≤ 21) (15) * Region 5 had 492 total waitlist candidates.
NSER status associated with waitlist removal for death or too sick to transplant
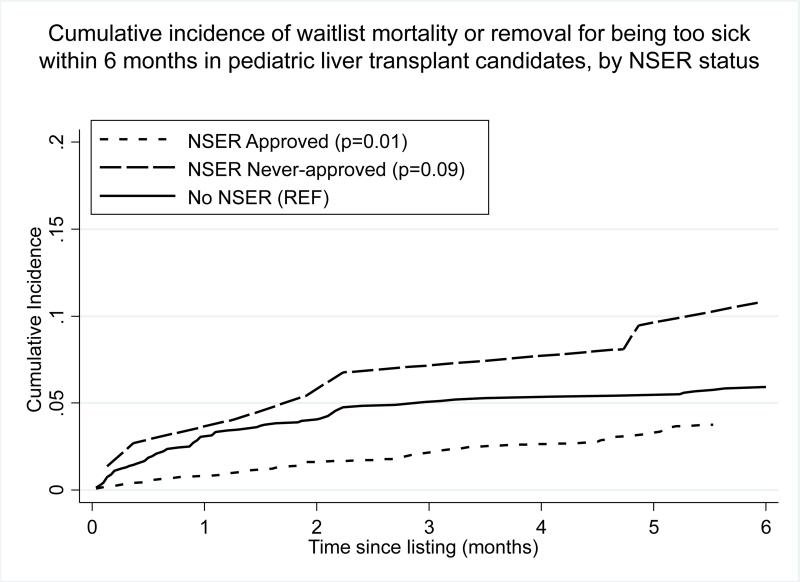
The cumulative incidence of waitlist mortality or removal for being too sick after 6 months on the waitlist was 10.8% in those never-approved for NSER (95% CI 5.1-19.0%, p=0.09), 3.8% for those with approved NSER (95% CI 2.7-5.0%, p=0.01) and 5.9% for those with no NSER application (95% CI 4.8-7.2%, reference for p-values). (FIGURE 3) In analysis of all waitlist time, patients approved for NSER had a lower risk of waitlist mortality/removal than those with no NSER (HR 0.69, 95% CI 0.49-0.97, p=0.03). Those never-approved for NSER had a higher risk of waitlist mortality/removal that did not reach statistical significance (HR 1.83, 95% CI 0.93-3.59, p=0.08).
FIGURE 3.
Cumulative incidence of waitlist mortality or removal for being too sick to transplant within 6 months of listing among pediatric liver transplant candidates, by NSER status. P-values reflect differences in waitlist mortality or removal after accounting for the competing risk of liver transplantation, compared to patients with no NSER as the reference group.
Because older children had a lower risk of waitlist mortality/removal but higher risk of having NSER denial in univariate analyses, an interaction between age at listing and NSER status was considered. (TABLE 2) Including the interaction, which was highly significant in multivariable analysis (p=0.008), revealed that for children 2-18 years at listing NSER never-approved increased the risk of waitlist mortality/removal compared to those with no NSER. NSER denial had no impact on the children younger than 2. (TABLE 2) The HR for waitlist mortality was <1 for NSER approved children in both age groups, but this possibly protective effect did not reach statistical significance.
Table 2.
Predictors of waitlist mortality or removal for being too sick to transplant
| Univariate | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| SHR | 95% CI | p | SHR | 95% CI | p | ||
| NSER status by age at listing* | |||||||
| No NSER applications | REF | REF | |||||
| 2-18 years | NSER approved | 0.70 | 0.41-1.20 | 0.19 | 1.02 | 0.57-1.82 | 0.96 |
| NSER never-approved | 2.45 | 1.04-5.74 | 0.04 | 2.99 | 1.26-7.07 | 0.01 | |
| 0-2 years | NSER approved | 0.69 | 0.44-1.07 | 0.10 | 1.17 | 0.73-1.87 | 0.52 |
| NSER never-approved | 1.39 | 0.44-4.46 | 0.57 | 1.10 | 0.29-4.14 | 0.89 | |
| <2 years of age at listing (vs. 2-18) | 1.65 | 1.09-2.50 | 0.02 | 1.88 | 1.16-3.06 | 0.01 | |
| Male | 0.85 | 0.62-1.17 | 0.32 | ||||
| Hispanic | 1.84 | 1.31-2.57 | <0.001 | 1.66 | 1.16-2.38 | 0.006 | |
| Public Insurance | 1.76 | 1.26-2.45 | 0.001 | 1.52 | 1.06-2.18 | 0.02 | |
| Calculated MELD/PELD at listing† | 1.06 | 1.04-1.07 | <0.001 | 1.05 | 1.04-1.07 | <0.001 | |
| Indication for transplant¶ | |||||||
| Biliary Atresia | REF | REF | REF | REF | REF | REF | |
| Cholestatic conditions | 1.02 | 0.58-1.78 | 0.95 | 1.31 | 0.72-2.37 | 0.38 | |
| Metabolic liver disease | 1.57 | 0.91-2.70 | 0.10 | 2.45 | 1.35-4.42 | 0.003 | |
| Tumor (outside standard criteria) | 1.61 | 0.77-3.37 | 0.21 | 4.10 | 1.83-9.16 | 0.001 | |
| Acute liver failure | 1.65 | 0.84-3.24 | 0.15 | 1.41 | 0.65-3.07 | 0.38 | |
| Other liver disease | 1.96 | 1.33-2.89 | 0.001 | 2.63 | 1.62-4.26 | <0.001 | |
| Initial Sodium† | 0.96 | 0.92-1.0 | 0.04 | ||||
| Initial INR† | 1.22 | 1.14-1.33 | <0.001 | ‡ | |||
| Initial Bilirubin† | 1.05 | 1.03-1.06 | <0.001 | ‡ | |||
| Initial Creatinine† | 1.10 | 0.97-1.25 | 0.13 | ‡ | |||
| Initial Albumin† | 0.68 | 0.55-0.84 | <0.001 | ‡ | |||
Interaction between NSER status and age category, p=0.008 in multivariate analysis.
Cholestatic conditions includes Alagille syndrome, Byler disease, progressive intrahepatic cholestatic syndromes, total parenteral nutrition cholestasis, sclerosing cholangitis, and idiopathic cholestasis. Metabolic liver disease includes alpha-1-antitrypsin deficiency, Crigler-Najjar syndrome, cystic fibrosis, glycogen storage disease, inborn errors in bile acid metabolism, neonatal hemochromatosis, primary hyperoxaluria, tyrosinemia, urea cycle defects, and Wilson's disease. Other liver disease include congenital hepatic fibrosis, Budd-Chiari syndrome, autoimmune hepatitis cirrhosis, drug toxicity, hepatitis C cirrhosis, and unknown cirrhosis.
SHR for MELD/PELD score and all laboratory values represent change for a 1 unit increase. Laboratory values reported in units of: sodium mEq/L, bilirubin mg/dL, creatinine mg/dL, albumin g/dL.
Evaluated in multivariate model sensitivity analysis with MELD/PELD score excluded. INR, bilirubin, albumin remained significant variables with no substantial changes in HR magnitude, direction or significance of other variables.
Other factors associated with increased risk of waitlist mortality/removal in univariate analysis included Hispanic ethnicity, public insurance, higher listing MELD/PELD, and lower listing serum sodium. (TABLE 2) Waitlist mortality/removal was not associated with listing year (HRs 0.81-1.36 compared to 2009, p=0.22-0.87). Compared to Region 1 as a reference, none of the regions had significantly different odds of waitlist mortality/removal (data not shown).
In a sensitivity analysis including listing bilirubin, albumin, creatinine and INR instead of MELD/PELD, total bilirubin, albumin, and creatinine remained significant in multivariable analysis (data not shown). There was no change in other variables retained in the multivariable model, and no change in HR direction or magnitude (data not shown). There was no significant interaction between NSER status and MELD/PELD score at listing.
We examined waitlist time to investigate whether patients were NSER never-approved because they died or became too sick for transplant quickly after listing. Those with approved NSER spent longer on the waiting list than those never-approved or with no NSER application. (TABLE 1) But for patients removed from the waiting list for death or too sick for transplant, there was no significant difference in waitlist time between those with NSER approved (n=50, median 100 days, IQR 48-156) and NSER never-approved (n=9, median 67 days, IQR 38-146, p=0.94). Those who died with no NSER spent a shorter amount of time on the waiting list (median 33, IQR 14-85, p=0<0.001 by Kruskal-Wallis test). For all patients that went on to transplant (n=1,888), those with NSER approved spent a longer time on the waiting list (median 109 days, IQR 50-216, n=868) than those with NSER never-approved (median 47 days, IQR 17-149 days, n=60) or those with no NSER (median 45 days, IQR 14-111, n=960; p<0.001).
Causes of waitlist mortality in those never-approved for NSER
Among the NSER never-approved, there were 5 waitlist deaths. Four occurred in adolescents; two were attributed to multi-organ system failure, one to cardiac arrest, one to respiratory failure. The death in a never-approved child < 2 years was due to variceal hemorrhage. Four candidates with NSER never-approved were removed from the waiting list for being too sick to transplant.
NSER status and liver transplant
Of the 1,888 children transplanted, those with NSER never-approved were more likely to be in the ICU at transplant than those with NSER approved. They were more likely to receive DCD donors, and their livers had longer median cold ischemia times. They were slightly more likely to get a whole liver than the other two groups. (TABLE 1)
Post-transplant graft and patient survival
For analysis of post-transplant graft and patient survival, patients were excluded if they were missing transplant date (n=92) or data on post-transplant survival time (n=5), recipients of multi-organ transplants (n=53), transplant date not specified as their first transplant (n=26), and if they had a prior transplant (n=131). After these exclusions, 1,712 patients remained for analysis, of whom 47.1% had an NSER.
NSER approval was associated with reduced risk of graft loss at 3 years post-transplant compared to those with no NSER application in univariate analysis, with borderline statistical significance in multivariable analysis. Having an NSER never-approved was not significantly associated with post-transplant graft survival compared to those with no NSER. (TABLE 3) However, having an NSER never-approved more than doubled the risk of post-transplant death in univariate and multivariable analysis. (TABLE 4) Five of the nine graft losses in NSER never-approved patients occurred at patient death (55%), compared to 46% in the NSER approved and 40% in those without NSER. There was no interaction between NSER status and age at transplant for either outcome.
Table 3.
Predictors of graft loss within 3 years after liver transplant
| Univariate | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| NSER status | |||||||
| No NSER applications | REF | REF | |||||
| NSER approved | 0.72 | 0.53-0.99 | 0.04 | 0.73 | 0.53-1.01 | 0.06 | |
| NSER never-approved | 1.53 | 0.77-3.01 | 0.22 | 1.37 | 0.69-2.73 | 0.38 | |
| Age at transplant | |||||||
| 2-18 years | REF | REF | |||||
| <2 years | 1.65 | 1.21-2.26 | 0.002 | 1.74 | 1.25-2.42 | 0.001 | |
| Male | 0.89 | 0.66-1.20 | 0.46 | ||||
| Hispanic* | 1.08 | 0.76-1.53 | 0.68 | ||||
| Public Insurance | 1.34 | 0.99-1.80 | 0.06 | ||||
| Indication for transplant | |||||||
| All other diagnoses** | REF | REF | |||||
| Tumor, <45d post-transplant | 1.09 | 0.51-2.34 | 0.83 | 1.57 | 0.72-3.43 | 0.26 | |
| Tumor, >45d post-transplant | 3.32 | 1.17-9.42 | 0.02 | 3.62 | 1.27-10.30 | 0.02 | |
| Medical condition at transplant | |||||||
| Not hospitalized | REF | REF | |||||
| Hospitalized, not ICU | 1.37 | 0.95-1.98 | 0.10 | 1.28 | 0.88-1.87 | 0.20 | |
| ICU | 2.12 | 1.47-3.07 | <0.001 | 2.01 | 1.38-2.94 | <0.001 | |
| Laboratory values at waitlist removal† | |||||||
| Calculated MELD/PELD score | 1.02 | 1.01-1.03 | 0.006 | ||||
| INR | 1.01 | 0.97-1.05 | 0.58 | ||||
| Bilirubin | 1.02 | 1.01-1.03 | 0.002 | ||||
| Creatinine | 0.98 | 0.68-1.40 | 0.90 | ||||
| Albumin | 1.08 | 0.90-1.30 | 0.42 | ||||
None of the ethnicity categories associated with graft loss, compared to Caucasian as reference group.
In univariate analysis with biliary atresia as reference group, tumor was the only diagnosis associated with significant difference in graft survival. Tumor modeled as a time-varying co-variate secondary to violation of proportional hazards assumption.
HR for MELD/PELD score and all laboratory values represent change for a 1 unit increase. Laboratory values reported in units of: sodium mEq/L, bilirubin mg/dL, creatinine mg/dL, albumin g/dL. Only MELD/PELD was included in multivariate models reported here.
Table 4.
Predictors of patient death within 3 years after liver transplant
| Univariate | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| NSER status | |||||||
| No NSER applications | |||||||
| NSER approved | 0.85 | 0.53-1.36 | 0.49 | 1.02 | 0.62-1.69 | 0.93 | |
| NSER never-approved | 2.49 | 1.06-5.84 | 0.04 | 2.43 | 0.99-5.95 | 0.05 | |
| Age at transplant | |||||||
| 2-18 years | REF | ||||||
| <2 years | 1.33 | 0.85-2.10 | 0.21 | ||||
| Male | 1.05 | 0.67-1.64 | 0.83 | ||||
| Ethnicity | |||||||
| Caucasian | REF | REF | |||||
| Asian | 2.26 | 1.18-4.35 | 0.01 | 2.43 | 1.24-4.74 | 0.01 | |
| Black | 1.43 | 0.76-2.71 | 0.27 | 1.49 | 0.78-2.83 | 0.22 | |
| Hispanic | 1.81 | 1.04-3.15 | 0.03 | 1.72 | 0.98-3.01 | 0.06 | |
| Public Insurance | 1.44 | 0.92-2.27 | 0.11 | ||||
| Indication for transplant | |||||||
| All other diagnoses* | REF | REF | |||||
| Tumor, <45d post-transplant | 1.30 | 0.40-4.19 | 0.67 | 2.59 | 0.77-8.75 | 0.13 | |
| Tumor, >45d post-transplant | 5.11 | 1.27-20.65 | 0.02 | 6.12 | 1.50-24.88 | 0.01 | |
| Medical condition at transplant | |||||||
| Not hospitalized | REF | REF | |||||
| Hospitalized, not ICU | 2.16 | 1.26-3.71 | 0.005 | 2.04 | 1.15-3.62 | 0.02 | |
| ICU | 3.53 | 2.07-6.01 | <0.001 | 2.68 | 1.45-4.93 | 0.002 | |
| Laboratory values at waitlist removal† | |||||||
| Calculated MELD/PELD score | 1.03 | 1.01-1.05 | <0.001 | 1.03 | 1.01-1.05 | 0.003 | |
| INR | 1.02 | 0.99-1.06 | 0.25 | ||||
| Bilirubin | 1.03 | 1.02-1.05 | <0.001 | ||||
| Creatinine | 0.91 | 0.49-1.70 | 0.76 | ||||
| Albumin | 0.98 | 0.73-1.30 | 0.86 | ||||
In univariate analysis with biliary atresia as a reference, tumor was the diagnosis associated with significant difference in graft survival. Tumor modeled as a time-varying co-variate secondary to violation of proportional hazards assumption.
HR for MELD/PELD score and all laboratory values represent change for a 1 unit increase. Laboratory values reported in units of: sodium mEq/L, bilirubin mg/dL, creatinine mg/dL, albumin g/dL. Only MELD/PELD was included in multivariate models reported here.
Other factors associated with post-transplant graft loss and patient death included tumor as an indication for transplant and intensive care immediately prior to transplant. Of note, tumor was the only transplant indication associated with post-transplant graft loss or death; it only increased hazard 45 days or more post-transplant. (TABLES 3, 4) Since all patients with standard exceptions were excluded, patients in this analysis all had tumors outside of standard transplant criteria. (TABLES 3, 4) Region, listing year, and type of transplant were considered but were not significant predictors of post-transplant outcomes (data not shown).
Discussion
This study is the first to examine the impact of NSER denial on waitlist mortality/removal and post-transplant outcomes in pediatric liver transplant candidates. Over the last five years, even with NSER utilization and approval rates already high, the odds of NSER denial continued to decrease. A recent analysis demonstrated that NSER approval offers pediatric liver transplant candidates an advantage: it increases their chances of being transplanted. (5) Our study showed that children aged 2-18 with an NSER that is never approved are at a life-threatening disadvantage: they are more likely to die or be removed from the waitlist for being too sick. For those that are transplanted, having an NSER that is never approved increases the risk of post-transplant mortality. These associations remained statistically significant after adjustment for other factors, despite the NSER never-approved being a small group of patients.
Our analysis adds to previous reports of demographic and regional disparities in NSER approval by exploring the impact of NSER denial on patient outcomes. (3,5,6,10) In our analyses, NSER denial was seen disproportionately in children of older age, diagnoses other than biliary atresia, Caucasian race, and those with private insurance. Some of these differences may not be flaws in the system if they accurately reflect differences in waitlist mortality risk. Older age, for example, is associated with an overall decreased risk of waitlist and post-transplant mortality. But our analysis suggests that the current system does not work perfectly to balance these risks—older children with denied NSERS had a higher risk of waitlist mortality/removal, even after adjustment for severity of illness and other factors. We found significant regional variability in the prevalence of NSER applications and NSER denial, without a clear correlation to regional waitlist volume or competitiveness. Children with public insurance had a higher risk of waitlist mortality/removal, while those with private insurance were more likely to have NSERs filed on their behalf—and more likely to have them refused, although we cannot determine here whether that reflects the NSER appropriateness.
We considered that NSER never-approved might be associated with waitlist mortality/removal because these patients died too quickly to get an NSER approved. However, our analysis of time spent on the waitlist suggests that this is not the case. There was no significant difference in waitlist times between those who died/were removed with NSER denied and those with NSER approved.
This study also shows that NSER status pre-transplant impacts outcomes post-transplant; this has not been examined previously in pediatric liver transplant candidates. NSER approval significantly decreased the risk of graft loss. NSER denial increased the risk of post-transplant death. Patients with NSERs denied had the highest prevalence of DCD donors and of hospitalization at transplant, and they had higher calculated MELD/PELD scores at transplant. These factors increase risk for post-transplant complications. In multivariable analysis, NSER status still had an impact on post-transplant outcomes—again suggesting that denial may significantly disadvantage patients.
One potential implication of our analysis is that all NSERs should be approved for pediatric candidates, to abolish the disadvantage associated with NSER denial. We discourage this, as it could further reduce the objectivity and transparency of allocation. We instead hope our analysis will motivate further research—and evidence-based discussion—on how and when transplant centers utilize NSERs, what drives their approval, and how and why NSERs impact pre- and post-transplant outcomes. We know very little about what motivates an RRB to approve or deny a particular NSER, as the approval process is not transparent. Analysis of the narratives that accompany NSERs may provide insight into these questions. Additional “objective and measurable” criteria, like serum sodium or failure to thrive severity, need to be explored as potential improvements to the PELD score. (11)
The National Review Board currently being designed should help reduce regional variability and standardize the criteria used for NSER approval (https://optn.transplant.hrsa.gov/governance/public-comment/national-liver-review-board/). But it is unlikely to erase all the disparities we have described. There will likely still be pressure to increase pediatric candidates’ MELD/PELD scores so they can compete with adults, and other children with NSERs. (4,12) Further efforts to “standardize,” or create more objective criteria for evaluating, the factors captured in NSERs but missed by MELD/PELD scores are also required to ensure fair allocation for all pediatric recipients. Pediatric waitlist candidates do have priority for donors 18 years or younger within each status and MELD/PELD category, but higher prioritization of pediatric recipients across categories—particularly for adult donors—would encourage split liver transplantation. This could further minimize waitlist mortality in children without increasing overall mortality. The full impact of Share 15 and Share 35 on allocation of pediatric donors, and of adult donors to pediatric patients, is not yet clear.
There is a paucity of research or public conversation about what motivates a transplant center to make an NSER for a given pediatric liver transplant candidate. Children listed at centers with low pediatric tranpslant volume are less likely to be transplanted; (13) whether experience with and higher utilization of NSERs in larger-volume centers may reduce their waitlist mortality, or effect their organ acceptance patterns and post-transplant outcomes is an important question for future investigations. Early reports suggested that PELD underestimated mortality risk and documented the significant morbidity experienced by children on the waiting list.(3,14) NSERs are likely used to enhance children's ability to compete for livers—with both adults and other children—particularly as MELD scores have been inflated by over-prioritization of adults with HCC. Even in 2005, UNOS data suggested that regions with high pediatric NSER utilization had higher PELD allocation scores. (4) Recent analyses suggest this cycle continues. (5)
It may also be that larger centers apply for NSERs for different indications than low volume centers because they have gained experience with the NSER approval process. What exceptions are approved by the RRB is known to the board members, who rotate on and off, and the applying centers but is opaque to patients, families and other centers. For example, an experienced center may submit a NSER for a biliary artetic at home while a less experienced center may not. This influences the volume of transplants and deaths at the centers who do not understand the RRB process or what is likely to be approved. We suggest that generic descriptions of approved cases be published by the OPTN.
One strength of our analysis is the comprehensiveness of the SRTR, which includes all U.S. pediatric liver transplants and provides longitudinal data. The dataset also lends its limitations: the study's retrospective nature and its reliance on existing data. Our cohort had a relatively small number of waitlist deaths and removals. This may have limited our power, particularly in multivariable analyses. It is possible, for example, that NSER approval would retain its potentially protective effect on waitlist mortality/removal and graft loss if a larger sample size were available. Some variables of interest—including transplant center volume, previous transplant on the full cohort, and details about the transplants—were not available in SRTR.
The high utilization of NSERs in our current system, combined with their subjective nature and potentially disparate application, make it difficult to judge whether our current system allocates organs fairly to pediatric liver transplant candidates. We have shown that NSER denial, although rare, is associated with waitlist mortality/removal for children 2 years and older, as well as post-transplant outcomes for children of all ages. Work by the pediatric liver transplant community to enhance both evidence and transparency related to NSERs is crucial to ensuring that all children on the waiting list are equally served by our allocation system.
Supplementary Material
Acknowledgments
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C (UNOS Data), the NIH-NIDDK (Dr. Perito, K23 DK0990253-A101), an American Society for Transplant Surgery-Alexion Presidential Student Mentor Grant (Ms. Braun), and the UCSF Liver Center (P30 DK026743).
Abbreviations
- CDC
Centers for Disease Control
- DCD
Deceased after cardiac death
- HCC
Hepatocellular carcinoma
- HR
Hazard ratio
- HRSA
Health Resources and Services Administration
- ICU
Intensive care unit
- INR
International normalized ratio
- IQR
Interquartile range
- MELD
Medical End-stage Liver Disease
- NSER
Non-standard exception request
- PELD
Pediatric End-stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- RRB
Regional Review Board
- SHR
Subhazard ratio
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
Footnotes
Disclaimer
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, nor does mention of trades names, commercial products, or organizations imply endorsement by the U.S. Government.
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
FIGURE S1: Non-standard exception request (NSER) and waitlist outcomes for all pediatric waitlist registrations with NSERs during the study period, 2009-2014.
REFERENCES
- 1.Freeman RB, Jr, Wiesner RH, Roberts JP, McDiarmid S, Dykstra DM, Merion RM. Improving liver allocation: MELD and PELD. Am J Transplant. 2004;4(Suppl 9):114–131. doi: 10.1111/j.1600-6135.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 2.Organ Procurement and Transplantation Network Health Resources and Services Administration, HHS. Final rule. Fed Regist. 1999 Oct 20;64(202):56650–56661. [PubMed] [Google Scholar]
- 3.Shneider BL, Suchy FJ, Emre S. National and regional analysis of exceptions to the Pediatric End-Stage Liver Disease scoring system (2003-2004). Liver Transpl. 2006 Jan;12(1):40–45. doi: 10.1002/lt.20662. [DOI] [PubMed] [Google Scholar]
- 4.Salvalaggio PR, Neighbors K, Kelly S, Emerick KM, Iyer K, Superina RA, et al. Regional variation and use of exception letters for cadaveric liver allocation in children with chronic liver disease. Am J Transplant. 2005 Aug;5(8):1868–1874. doi: 10.1111/j.1600-6143.2005.00962.x. [DOI] [PubMed] [Google Scholar]
- 5.Hsu EK, Shaffer M, Bradford M, Mayer-Hamblett N, Horslen S. Heterogeneity and disparities in the use of exception scores in pediatric liver allocation. Am J Transplant. 2015 Feb;15(2):436–444. doi: 10.1111/ajt.13089. [DOI] [PubMed] [Google Scholar]
- 6.Gish RG, Wong RJ, Honerkamp-Smith G, Xu R, Osorio RW. United Network for Organ Sharing regional variations in appeal denial rates with non-standard Model for End-Stage Liver Disease/Pediatric End-Stage Liver Disease exceptions: support for a national review board. Clin Transplant. 2015 Jun;29(6):513–522. doi: 10.1111/ctr.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng VL, Fecteau A, Shepherd R, Magee J, Bucuvalas J, Alonso E, et al. Outcomes of 5-year survivors of pediatric liver transplantation: report on 461 children from a north american multicenter registry. Pediatrics. 2008 Dec;122(6):e1128–35. doi: 10.1542/peds.2008-1363. [DOI] [PubMed] [Google Scholar]
- 8.Fine J, Gray R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496. [Google Scholar]
- 9.Cox D. Regression models and life tables. Journal of the Royal Statistical Society. 1972;B34:187–220. [Google Scholar]
- 10.Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, et al. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant. 2011 Nov;11(11):2362–2371. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugliese R, Fonseca EA, Porta G, Danesi V, Guimaraes T, Porta A, et al. Ascites and serum sodium are markers of increased waiting list mortality in children with chronic liver failure. Hepatology. 2014 May;59(5):1964–1971. doi: 10.1002/hep.26776. [DOI] [PubMed] [Google Scholar]
- 12.Shneider BL, Roberts MS, Soltys K. The HMS Birkenhead docks in Brazil: pediatric end-stage liver disease times three. Liver Transpl. 2010 Apr;16(4):415–419. doi: 10.1002/lt.22060. [DOI] [PubMed] [Google Scholar]
- 13.Rana A, Pallister Z, Halazun K, Cotton R, Guiteau J, Nalty CC, et al. Pediatrics. 2015 Jul;136(1):e99–e107. doi: 10.1542/peds.2014-3016. [DOI] [PubMed] [Google Scholar]
- 14.Shneider BL, Neimark E, Frankenberg T, Arnott L, Suchy FJ, Emre S. Critical analysis of the pediatric end-stage liver disease scoring system: a single center experience. Liver Transpl. 2005 Jul;11(7):788–795. doi: 10.1002/lt.20401. [DOI] [PubMed] [Google Scholar]
- 15.Lai JC, Roberts JP, Vittinghoff E, Terrault NA, Feng S. Patient, center and geographic characteristics of nationally placed livers. Am J Transplant. 2012 Apr;12(4):947–53. doi: 10.1111/j.1600-6143.2011.03962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.