Abstract
Background
Prader-Willi syndrome (PWS) is a genetic disorder characterized by hyperphagia, obesity, cardiopulmonary diseases, and increased mortality. While successful weight loss improves health in PWS, few treatments cause sustained weight loss in obese patients let alone obese individuals with PWS.
Objectives
The present study uses the Magel2 knockout (KO) mouse, an animal model of PWS, to conduct a preclinical study on the efficacy of Sleeve Gastrectomy (SG) in PWS.
Setting
Academic Research Laboratory, United States.
Methods
We performed sham or SG surgeries in 24-28 week old male Magel2 KO and wild-type littermate control mice (WT) who had been maintained on a high-fat diet for 10 weeks. We monitored body weight, food intake, and fat and lean mass pre- and post-operatively. Fasting glucose, glucose tolerance, and counterregulation were measured post-operatively.
Results
Magel2 KO animals had similar recovery and mortality rates compared to WT. SG resulted in similar weight loss, by specifically loss of fat but not lean mass, in both Magel2 KO and WT mice. SG also resulted in significantly lower fasting glucose levels and a reduction in fat intake in both Magel2 KO and WT mice. We also found that Magel2 KO mice failed to increase their food intake in response to the glucoprivic agent, 2-deoxy-D-glucose (2DG) suggesting impaired glucose counterregulation, but this occurred regardless of surgical status. All results were considered significant when P < 0.05.
Conclusions
We find in this mouse model of PWS, SG is a safe, effective strategy for weight and fat loss.
Keywords: bariatric surgery, glucose regulation, obesity, sleeve gastrectomy
Introduction
Prader-Willi syndrome (PWS) is a complex genetic condition associated with intellectual and behavioral deficiencies as well as excessive hunger and progressive, life-threatening obesity in adulthood. The obesity associated with PWS leaves individuals at greater risk for mortality(1) and for obesity-associated co-morbidities(2). While dietary intervention can somewhat restrain obesity in PWS, such lifestyle interventions have limited long-term efficacy in non-PWS obese patients. Indeed, we currently have limited options for treatment of obesity in non-PWS patients, let alone patients with PWS.
Currently, the most effective treatment for obesity is bariatric surgery. Sleeve Gastrectomy (SG) is one such surgical procedure where ~80% of the stomach is removed along the greater curvature and, unlike Roux-en-Y gastric bypass (RYGB), there is no intestinal rearrangement. Yet, SG leads to significant and sustained reductions in body mass, specifically due to reduced adiposity, and improves glucose and lipid metabolism.
Interestingly, SG also reduces meal size and shifts macronutrient preference away from fat(3-5). These potent effects of bariatric surgery on feeding behavior suggest that surgery could be a viable options for patients with PWS. In the past, some studies have tested the effectiveness of bariatric surgical procedures in obese individuals with PWS, but have not demonstrated the anticipated health advantages(6). Overall, these studies concluded that bariatric surgical procedures did not lead to significant weight loss in PWS and may in fact be associated with an increased risk of complications(6). However, since these publications, bariatric surgical procedures have had great technological advancements that have contributed to greater reliability and reduced complications(7,8). Given the simplicity of the SG procedure and the reduced need for vitamin replacement but high degree of weight loss and metabolic improvements, this surgery may be an effective strategy for obese individuals with PWS.
PWS is complex syndrome in which multiple genes on chromosome 15q11-q13 are inactivated(9). Of these genes, MAGEL2 has emerged as a strong candidate for many aspects of the PWS phenotype following the discovery of children carrying mutations only in MAGEL2 with a PWS-like phenotype(10-12). Mice with a mutation in the homolog Magel2 exhibit many characteristics of PWS, including elevated adiposity, decreased physical activity, increased leptin and insulin levels, and deficits in reproduction(13-15) and metabolism(16) (17). This model offers an opportunity to determine the potential for bariatric surgery to compensate for this genetic defect and lead to a safe, effective strategy for both weight loss and improvement in the obesity-associated co-morbidities in PWS.
Materials and Methods
Animals
Female heterozygous Magel2 knockout mice (C57BL/6-Magel2tm1Stw/J, The Jackson Laboratory stock #009062) were received from Dr. Rachel Wevrick (University of Alberta) and bred in-house at the University of Cincinnati to male wild type (WT) C57Bl/6J mice. Male heterozygous Magel2 knockout mice were used for the following experiments along with their wild type littermate controls. As in human PWS, imprinting silences the maternally inherited Magel2 allele, so that heterozygous mice carrying a paternally inherited Magel2 deletion allele are effectively Magel2-null(15). At 14-18 wks old, all mice were placed on a high-fat diet (45% fat, 4.54 kcal/g, D12451 Research Diets, New Brunswick, NJ). Over the next 8 weeks, measurements of body weight, food intake, and activity were performed on a subset of mice (N = 10-12/genotype) whereas lipid analysis and all other measures were performed on all mice (N = 15-21/genotype) . Mice underwent SG (see below) at 24-28 wks of age, after 10 weeks on the high-fat diet. Post-surgery body weight, food intake, and glucose regulation was measured over the 10 wks following surgery. All mice had ad libitum access to food and water at all times, unless noted below. Mice were individually housed within temperature controlled rooms with a 12h:12H light cycle with lights on at 6 am. All studies were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of Cincinnati.
Sleeve Gastrectomy (SG)
SG and sham surgeries and postoperative care were performed as previously described(5,18). All mice remained on the high-fat diet after the surgery.
Locomotor Activity, Energy Expenditure
Animals were singly housed and placed into an automated system to measure data in increments of 5 min (Phenotyping Systems International Group, Chesterfield, MO). Activity counts were measured via horizontal beam break over the course of the 4-7 days. Energy expenditure was calculated by indirect calorimetry and expressed as average kcal/hour in 12 hr bins. Due to limitations in the number of animals we could run at one time, a subset of mice from each group (4-5/group) was chosen at random to receive activity and energy expenditure data collection.
Glucose Tolerance Test (GTT)
All animals were fasted for 4 hr prior to the glucose bolus. Dextrose (25%, 2 mg/kg) was delivered either orally (oGTT) or via ip injection (ipGTT). Blood glucose was measured by glucometer prior to glucose injection (time 0), and then 15, 30, 45, 60, and 120 min post-injection. Blood was collected from the tip of the tail by cutting a small amount of the tail and gently massaging the blood out.
Insulin Tolerance Test (ITT)
Insulin (500 mU/kg) was delivered ip. Blood glucose was measured by glucometer prior to insulin injection (time 0), and then 15, 30, 45, and 60 min post-injection. Blood was collected from the tip of the tail by cutting a small amount of the tail and gently massaging the blood out.
Lipid Analysis
Lipid analysis was performed by the Metabolic Core at the University of Cincinnati. Blood samples from the tail were taken 2 hr into the dark phase from animals during an ad libitum fed state. Immediately afterwards, animals were fasted and blood re-drawn 24 hr later to determine fasting lipid levels.
Macronutrient preference test
Food choice was assayed for five days, 13 wks after surgery, using a macronutrient selection paradigm in which the high fat diet was removed and three pure macronutrient diets were presented simultaneously in separate containers within the animal's home cage. These were pure carbohydrate (TD.02521), pure fat (TD.02522) and pure protein (TD02523) and were all purchased from all Harlan Teklad, Indianapolis, IN. The glass containers filled with the individual macronutrient diets were available ad libitum and were weighed daily to determine daily intake per macronutrient per mouse. During the macronutrient selection, animals only had access to the macronutrient diets and ad libitum water.
2-deoxyglucose (2DG)
Mice had their food removed and weighed during the early light phase (9:30am). 2DG (250mg/kg) or saline was given ip and food was immediately returned. Food was reweighed 4 hr after injection and recorded.
Statistical Analysis
Statistical analysis was performed using either GraphPad Prism or Statistica programs. Unless otherwise specified, we used 2-way ANOVA, and Repeated Measures when justified. Bonferroni's multiple comparison test was used when a significant interaction effect was found. For analysis of energy expenditure, ANCOVA was performed in accordance to(19). All statistical analysis utilized used a two-tailed design and results were considered significant when p < 0.05.
Results
The effect of a high-fat diet on body weight, fat mass, and lean mass of Magel2 mice
In preparation for the SG, mice were fed a high-fat diet to induce diet-induced obesity. After 6 wks on a high-fat diet, Magel2 knockout (referred to hereafter as Magel2) mice had similar weights (Supplementary Figure 1A; p = 0.09). While there was no significant difference in body weight, Magel2 mice had elevated fat mass compared to the WT under both chow-fed and high-fat diet conditions (Supplementary Figure 1B; main effect of genotype F (1,38) = 21.2, p < 0.0001, main effect of diet F (1,38) = 243.4, p < 0.0001). Magel2 mice also had decreased lean mass compared to WT under both chow-fed and high-fat diet conditions (Supplementary Figure 1C; main effect of genotype F (1,38) = 31.9, p < 0.0001, main effect of diet F (1,38) = 16.3, p = 0.003).
The effect of a high-fat diet on food intake, activity, and energy expenditure of Magel2 mice
While on the high-fat diet, there was no significant difference in caloric intake between Magel2 and WT mice (Supplementary Figure 2A; p = 0.07). However, Magel2 mice exhibited a reduction in locomotor activity during the active/dark phase (Supplementary Figure 2B; genotype × time F (6,120) = 9.8, p < 0.0001). Energy expenditure was analyzed as described previously(19), and found to be independent of body mass (Supplementary Figure 2C) with Magel2 mice having lower energy expenditure (main effect of genotype ANCOVA, p < 0.001). The reduced energy expenditure of the Magel2 mice was seen only during the dark/active phase (Supplementary Figure 2D; genotype × time F (6,120) = 11.9, p < 0.0001).
Lipid analysis of Magel2 and WT mice
Eight weeks after beginning the high-fat diet, lipids were analyzed in both fed (postprandial) and fasted conditions. Triglycerides decreased in the fasting state independent of genotype (Supplementary Figure 3A; F (1,68) = 22.6, p < 0.0001). Cholesterol decreased with fasting (Supplementary Figure 3B; main effect fed/fasting F (1,68) = 12.5, p = 0.0007) but was significantly elevated regardless of feeding status in Magel2 mice compared to WT (Supplementary Figure 3B; main effect genotype F (1,68) = 9.4, p = 0.003). Similarly, phospholipids decreased with fasting (Supplementary Figure 3C; main effect fed/fasting F (1,68) = 27.1, p < 0.0001) but were significantly elevated regardless of feeding status in Magel2 mice compared to WT (Supplementary Figure 3C; main effect genotype F (1,68) = 5.2, p = 0.03). Free fatty acids were not significantly different between feeding states or genotypes (Supplementary Figure 3D).
The effect of Vertical Sleeve Gastrectomy (SG) on body weight, fat mass and food intake of Magel2 mice
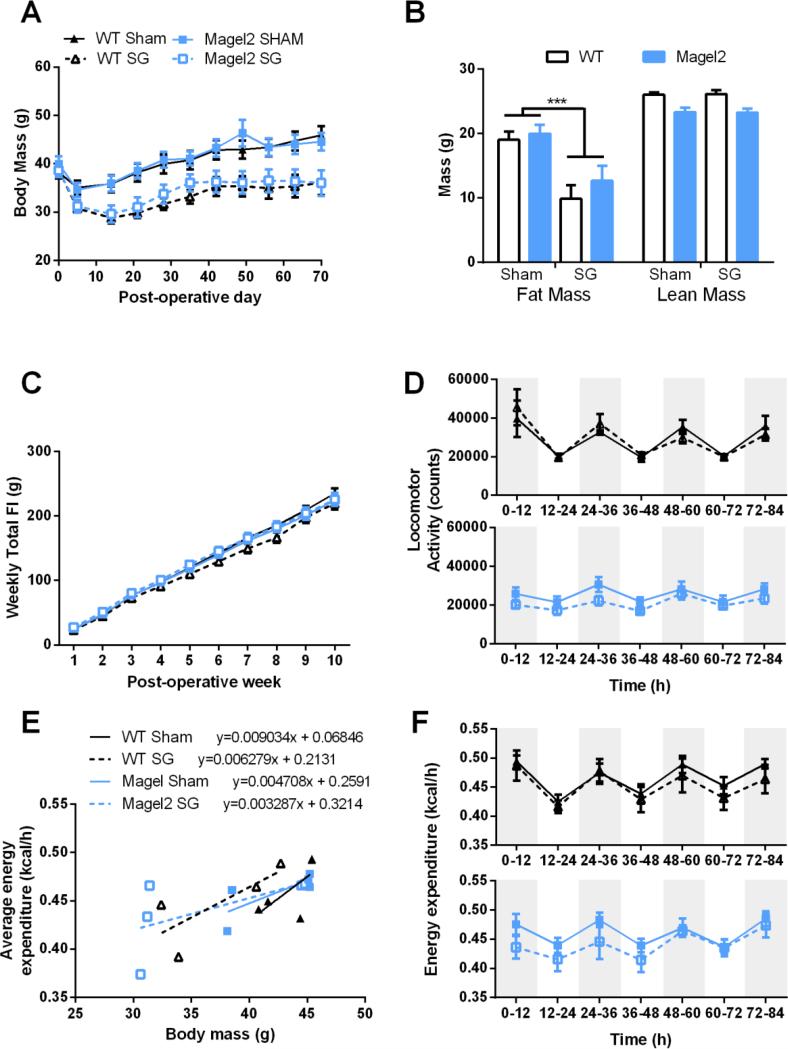
After mice became obese, Magel2 and WT mice underwent SG or Sham surgeries. SG and sham surgeries were well tolerated by all mice, with Magel2 mice having similar mortality rates as WT and comparable to our typical mortality rate in mice undergoing this surgery. One out of 8 (12.5%) WT mice receiving SG died within the first week of surgery compared to 3 out of 12 (25%) Magel2 mice receiving SG. No sham operated animals died during the study. Animals that underwent SG had reduced body weight compared to Sham operated mice for WT and Magel2 mice (Figure 1A; main effect of surgery F(6, 23) = 11.3, p = 0.00001). Weight loss, as measured 5 wks after surgery, was primarily due to loss of fat mass in both WT and Magel2 mice (Figure 1B; main effect of surgery F(1, 26) = 18.6, p = 0.0002). SG did not alter lean mass in either genotype. However, as was noted before the surgery, Magel2 mice had significantly less lean mass than WT mice (Figure 1B; main effect of genotype F(1, 27) = 20.7, p = 0.0001). SG had no influence on cumulative food intake in Magel2 or WT mice (Figure 1C; main effect of surgery F(7, 22)= 1.3, p= 0.3). Similarly, we did not observe any significant difference in weekly caloric intake (Supplementary Figure 4). Over time, locomotor activity was not significantly altered by either genotype or surgery condition (Figure 1D). However, total activity was reduced in the Magel2 compared to WT independent of surgery Supplementary Figure 5A; main effect of genotype F(1, 15) = 6.076, p = 0.03). Energy expenditure was found to be dependent on body mass (Figure 1E, F = 10.87, p = 0.005) with no statistical interaction effect or main effect of genotype or surgery on energy expenditure (Figure 1F). There was no significant difference in average total energy expenditure (Supplementary Figure 5B).
Figure 1.
Body mass, food intake, activity, and energy expenditure in Magel2 and WT mice after Sham or SG.
(A) Both Magel2 and WT mice lose weight after SG, main effect of surgery, p < 0.0001. (B) Weight loss is due to loss of fat mass in both Magel2 and WT, ***p<0.001. The SG does not affect lean mass. (C) Food intake was not different in WT or Magel2 SG mice compared to Sham. (D) Locomotor activity was not different in Magel2 SG mice compared to Sham. (E) Energy expenditure is dependent on body mass in all groups. (F) No significant main or interactions effects were detected in energy expenditure. (A-C) N=7-9/group. (D-F) N=4-5/group. WT Sham are represented as solid black lines, WT SG as dotted black lines, Magel2 Sham as solid blue lines, and Magel2 SG as dotted blue lines. Values graphed as mean ± SEM.
The effect of SG on glucose regulation in Magel2 mice
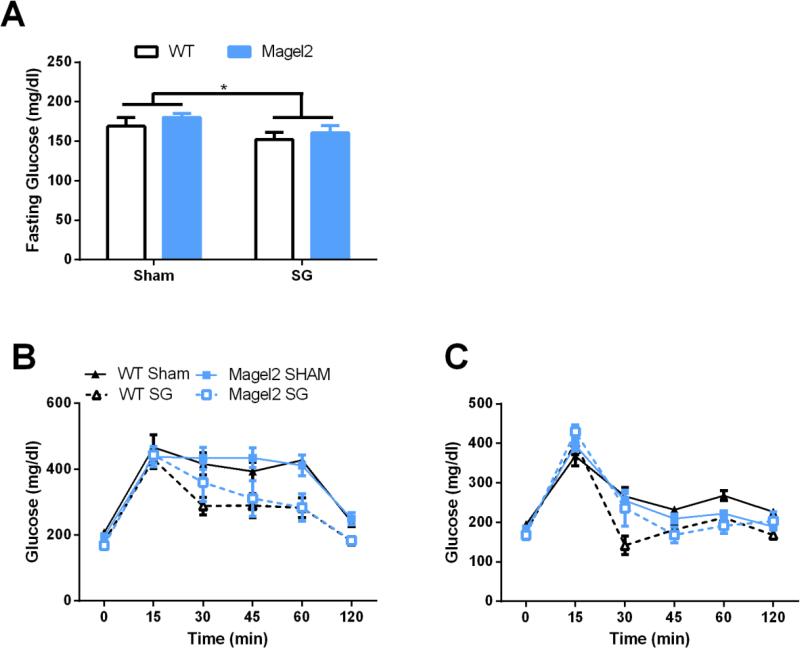
To examine glucose regulation, we performed ip and oral glucose tolerance tests. Before the glucose bolus, SG resulted in significantly lower fasting glucose levels in both WT and Magel2 mice (Figure 2A; F (1,28) = 4.5, p < 0.05). SG also significantly improved IP and oral glucose tolerance (Figure 2B,C; main effect of surgery F(6, 23) = 3.6, p = 0.01; F(6, 23) = 6.6, p = 0.0004; Repeated Measures 2-way ANOVA for IP and oral glucose tolerance tests, respectively). There was also a trend for the Magel2 mice to have greater glucose levels after the gavage compared to WT mice during the oral glucose tolerance test (main effect of genotype, F(6, 23) = 2.5, p = 0.05). We did not observe any significant differences in the Area Under the Curve (AUC) in either the ip or oral GTT (data not shown).
Figure 2.
Glucose regulation in Magel2 and WT mice after Sham or SG.
(A) SG lowers fasting glucose levels in both WT and Magel2 mice (main effect of surgery, *p < 0.05) (B) SG improves glucose tolerance in response to an ip glucose tolerance test (main effect of surgery, p < 0.05). (C) SG improves glucose tolerance in response to an oral glucose tolerance test (main effect of surgery, p < 0.05). Values graphed as mean ± SEM.
The effect of SG on macronutrient preference of Magel2 mice
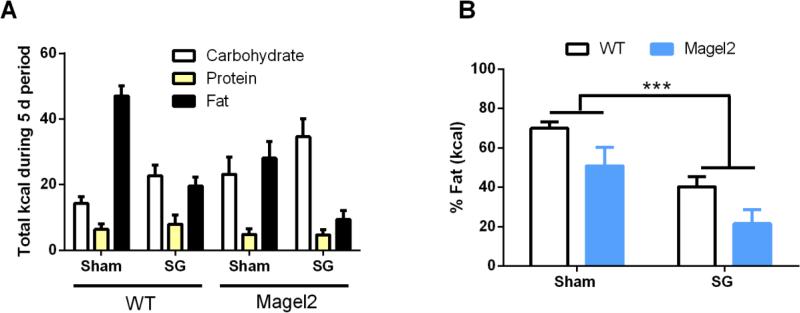
SG also changes food preference, as observed in both humans(20) and rodents(3,21). To determine if SG would alter the food preference of Magel2 mice, both Magel2 and WT mice were exposed to a macronutrient preference test for 5 days, 13 wks after surgery. During the macronutrient test, each mouse can self-select between three pure macronutrients (e.g. carbohydrate, fat, and protein). WT mice receiving SG decrease their preference for fat calories (Figure 3A, B) as previously described(3,22,23). Similar to WT SG, Magel2 SG mice also decrease their intake of fat post-operatively (Figure 3A, B; main effect of surgery F (1, 27) = 16.7, p < 0.001). Interestingly, Magel2 mice also consumed significantly fewer total calories and fewer fat calories overall (main effect of genotype F (1, 27) = 6.8, p < 0.05). However, Magel2 mice consumed more carbohydrate calories overall (main effect of genotype, F (1, 27) = 4.8, p < 0.05) and there was a main effect of the surgery to increase carbohydrate intake (F (1, 27) = 5.1, p < 0.05). There was no significant change in protein calories consumed.
Figure 3.
Macronutrient preference of Magel2 and WT mice after Sham or SG.
(A) SG decreases fat and increases carbohydrate caloric intake. Magel2 mice consume less fat and more carbohydrates compared to WT. (B) SG reduces the percentage of fat calories consumed. Magel2 mice also consume a smaller percentage of fat calories compared to WT. Values graphed as mean ± SEM.
Counterregulatory response of Magel2 mice
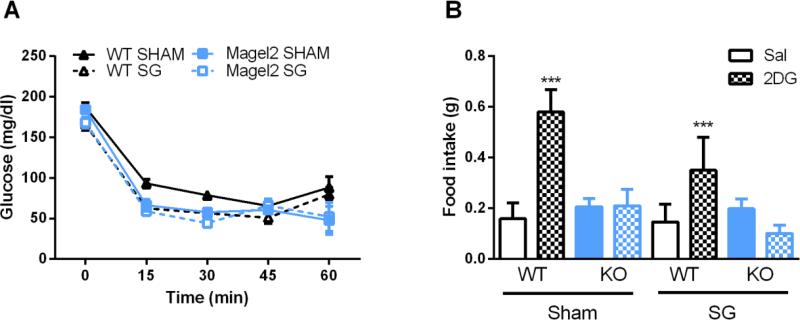
To further explore the glucose regulation of Magel2 mice after surgery, we administered an insulin tolerance test. In response to insulin (Figure 4A), glucose levels decreased significantly more in Magel2 than WT mice (main effect of genotype F(5, 22) = 4.6, p = 0.005) and in SG compared to sham (main effect of surgery F(5, 22) = 6.02, p = 0.001). 30 min after the insulin injection, 2:9 of the Magel2 SG mice dropped to 46 and 52 mg/dL glucose and become unresponsive. These 2 mice were administered an ip dose of glucose and removed from the ITT analysis. Glucose response to an insulin injection is dependent upon insulin sensitivity (0-30min) and thereafter is a combination of insulin sensitivity and the ability to mount a counterregulatory response to restore glucose levels to normal(24). In order to determine whether the differences we saw during the ITT were due to impaired counterregulation, we administered the glucoprivic agent, 2-Deoxy-D-glucose (2DG). Independent of surgical condition and unlike the WT mice, Magel2 mice fail to increase feeding for 4 hr after administration of 2DG, (Figure 4B, genotype × drug effect F(1, 50) = 15.7, p = 0.0002). Interestingly, there was a trend for the surgical condition to reduce feeding in response to 2DG independent of genotype (main effect of surgery F(1, 50) = 3.9310, p = 0.05).
Figure 4.
Response to 2DG and insulin challenge.
(A) In response to insulin, circulating glucose levels drop more in Magel2 mice (main effect of genotype p < 0.01) and after SG (main effect of surgery p < 0.01). (B) 4 hours after injection, Magel2 mice fail to increase their feeding in response to 2DG, and look similar to saline injected Magel2 mice (genotype × drug effect, ***p < 0.001). Values graphed as mean ± SEM.
Discussion
Pre-clinical studies offer a high throughput option to examine the efficacy of a variety of bariatric surgical techniques and their ability to alter adiposity, feeding, and glucose regulation for many genetic disorders that cause obesity, including PWS. In our pre-clinical genetic mouse model of PWS, SG resulted in similar weight loss in both Magel2 and WT mice, by specifically causing loss of fat but not lean mass.
While there have been several case studies and small clinical studies that have examined the impact of bariatric surgery on PWS(6), many of procedures studied are now outdated. Indeed, there have been tremendous advances in bariatric surgical techniques that greatly reduce patient complications, such as laparoscopic techniques(7). Generally, in obese patients, skilled surgeons have a complication rate of 5.2% and a surgical mortality rate of 0.05%(8). Ideally, studies that use current bariatric surgical practices will be performed to determine the current effectiveness of bariatric surgery in individuals with PWS. One recent study using current SG methods reported that children with PWS experience significant and similar weight loss after SG compared to other obese children, with no difference in surgery-related complications(25). More studies are needed to address the safety and efficacy of SG in PWS, both for weight loss and for obesity-associated co-morbidities. Our results suggest that these obese patients with PWS will have sustained improvements of both body weight and metabolic co-morbidities.
We find that high-fat fed Magel2 mice weigh similarly to WT littermate controls and consume the same amount of calories. This deviates from human PWS individuals who display hyperphagia and increased obesity. While Magel2 has been associated with many characteristics of PWS, including elevated adiposity, decreased activity, increased leptin and insulin levels, and deficits in reproduction(13-15), it is unlikely that one gene will recapitulate all characteristics of PWS. We observe that Magel2 mice have increased adiposity, reduced activity levels and reduced energy expenditure compared to WT mice.
SG leads to significant decreases in body weight and fat mass but did not lead to significant increases in activity or energy expenditure in either genotype. Similarly, there were no significant changes in cumulative food intake in either genotype. Previous research indicates that an initial reduction in caloric intake is key for the weight loss observed from bariatric surgery in rodents(4,26,27). However, in this study, we do not observe these early changes in food intake within the WT or Magel2 mouse. Unfortunately, as it may interfere with recover from surgery, we were unable to assess early post-operative changes in energy expenditure or macronutrient malabsorption that could account for the weight loss. Additional studies are required to determine exactly how weight loss is achieved in the Magel2 mouse; however these data support the conclusion that Magel2 mice are able to lose weight from SG. Independent of genotype, we also noticed small differences in energy expenditure between the pre-surgical condition and the post-surgical Sham mice. These differences may be attributed to chronic high-fat diet exposure and the increasing age of the mice.
We report here that SG significantly lowered fasting glucose levels in both WT and Magel2 mice. Similarly, both WT and Magel2 mice demonstrated improved glucose tolerance in response to both an ip glucose bolus as well as an oral glucose load. These data indicate that in addition to alleviating the obesity associated with the genetic mutation of Magel2, SG can mitigate glucose intolerance through gut-dependent (based on the oGTT) and gut-independent (based on the ipGTT) mechanisms. Furthermore, these effects on glucose tolerance are independent of food intake as the surgery did not alter total food intake in either Magel2 or WT mice. We are unable to make a conclusion about the ability of the SG to improve glucose regulation in a weight-independent manner in this study because we do not include a pair-fed, weight loss control group. In response to a high-fat diet, we found no evidence of hyperphagia in Magel2 mice, and thus cannot make conclusions about the impact of SG on hyperphagia. However, recent clinical evidence from children with PWS who underwent SG reports a postoperative improvement in hyperphagia, including patients who stop eating before finishing their prescribed meal(25). We also found that SG alters macronutrient preference, increasing a preference in carbohydrates and decreasing a preference in fat in Magel2 mice. This suggests that individuals with PWS may also experience a change in food preference after SG. Nevertheless, there is as yet insufficient clinical evidence to determine how SG might affect specific food choices or feeding patterns in individuals with PWS.
Finally, independent of surgical status, Magel2 mice failed to increase their food intake in response to the glucoprivic agent, 2-Deoxy-D-glucose (2DG) and had persistently reduced glucose levels in response to insulin suggesting impaired glucose counterregulation. This result is intriguing in light of observed hypoglycemia within individuals with PWS(28). Indeed, individuals with PWS have a greater fall in response to insulin than non-PWS, BMI-matched individuals (29). These data suggest that the Magel2 gene plays a role in regulating counterregulatory responses to falling glucose levels, a hypothesis supported by others(30). It is unclear how defective counterregulation in PWS plays in the etiology of the clearly altered feeding behavior but is an interesting future area for research. It is important to note that gastric dumping syndrome, which includes hypoglycemia associated with rapid gastric emptying, is a commonly reported problem in patients with RYGB(31), but not SG, despite the fact that both surgeries increase gastric emptying rate. Whether SG is a better alternative for individuals with PWS or whether the combination of impaired counterregulation and rapid gastric emptying will put PWS patients at increased risk for gastric dumping syndrome after SG is worthy of future study. It is also interesting to speculate that an overall defect in central nervous system sensing of both high and low nutrient levels plays a role in the hyperphagia of PWS.
Conclusions
Our data demonstrate that SG causes weight loss and improved glucose homeostasis in a genetic animal model of PWS. Further investigation of successful weight loss options within pre-clinical models of PWS can provide useful information about how humans may respond to such treatments. While additional data on the various PWS models will be crucial to move a bariatric surgery strategy forward, these data do hold promise that SG is a viable option to treat obesity and its associated co-morbidities in individuals with PWS. Moreover, continued investigation into potential defects in central nervous system nutrient sensing in Magel2 mice may provide pivotal insight into the etiology of the obesity associated with PWS.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (F32 DK097867-01, D.Arble and DK082480-01, D.Sandoval) and the Canadian Institutes of Health Research (MOP 130367) to R.Wevrick. D.Sandoval also receives research support from Sanofi, Novo Nordisk, Ethicon, and Boehringer Ingelheim.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Einfeld SL, Kavanagh SJ, Smith A, Evans EJ, Tonge BJ, Taffe J. Mortality in Prader-Willi syndrome. Am J Ment Retard. 2006;111(3):193–198. doi: 10.1352/0895-8017(2006)111[193:MIPS]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinnema M, Maaskant MA, van Schrojenstein Lantman-de Valk HM, Boer H, Curfs LM, Schrander-Stumpel CT. The use of medical care and the prevalence of serious illness in an adult Prader-Willi syndrome cohort. Eur J Med Genet. 2013;56(8):397–403. doi: 10.1016/j.ejmg.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Wilson-Perez HE, Chambers AP, Sandoval DA, et al. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes (Lond) 2013;37(2):288–295. doi: 10.1038/ijo.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefater MA, Perez-Tilve D, Chambers AP, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138(7):2426–2436. 2436, e2421–2423. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arble DM, Sandoval DA, Turek FW, Woods SC, Seeley RJ. Metabolic effects of bariatric surgery in mouse models of circadian disruption. Int J Obes (Lond) 2015;39(8):1310–1318. doi: 10.1038/ijo.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheimann AO, Butler MG, Gourash L, Cuffari C, Klish W. Critical analysis of bariatric procedures in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr. 2008;46(1):80–83. doi: 10.1097/01.mpg.0000304458.30294.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg. 2011;213(2):261–266. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Birkmeyer JD, Finks JF, O'Reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369(15):1434–1442. doi: 10.1056/NEJMsa1300625. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 10.Schaaf CP, Gonzalez-Garay ML, Xia F, et al. Truncating mutations of MAGEL2 cause Prader-Willi phenotypes and autism. Nat Genet. 2013;45(11):1405–1408. doi: 10.1038/ng.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soden SE, Saunders CJ, Willig LK, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med. 2014;6(265):265ra168. doi: 10.1126/scitranslmed.3010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mejlachowicz D, Nolent F, Maluenda J, et al. Truncating Mutations of MAGEL2, a Gene within the Prader-Willi Locus, Are Responsible for Severe Arthrogryposis. Am J Hum Genet. 2015;97(4):616–620. doi: 10.1016/j.ajhg.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozlov SV, Bogenpohl JW, Howell MP, et al. The imprinted gene Magel2 regulates normal circadian output. Nat Genet. 2007;39(10):1266–1272. doi: 10.1038/ng2114. [DOI] [PubMed] [Google Scholar]
- 14.Resnick JL, Nicholls RD, Wevrick R. Prader-Willi Syndrome Animal Models Working G. Recommendations for the investigation of animal models of Prader-Willi syndrome. Mamm Genome. 2013;24(5-6):165–178. doi: 10.1007/s00335-013-9454-2. [DOI] [PubMed] [Google Scholar]
- 15.Mercer RE, Wevrick R. Loss of magel2, a candidate gene for features of Prader-Willi syndrome, impairs reproductive function in mice. PLoS One. 2009;4(1):e4291. doi: 10.1371/journal.pone.0004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischof JM, Stewart CL, Wevrick R. Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader-Willi syndrome. Hum Mol Genet. 2007;16(22):2713–2719. doi: 10.1093/hmg/ddm225. [DOI] [PubMed] [Google Scholar]
- 17.Butler MG, Swift LL, Hill JO. Fasting Plasma Lipid, Glucose, and Insulin Levels in Prader-Willi Syndrome and Obese Individuals. Dysmorphol Clin Genet. 1990;4(1):23–26. [PMC free article] [PubMed] [Google Scholar]
- 18.Pressler JW, Haller A, Sorrell J, et al. Vertical sleeve gastrectomy restores glucose homeostasis in apolipoprotein A-IV KO mice. Diabetes. 2015;64(2):498–507. doi: 10.2337/db14-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speakman JR, Fletcher Q, Vaanholt L. The ‘39 steps’: an algorithm for performing statistical analysis of data on energy intake and expenditure. Dis Model Mech. 2013;6(2):293–301. doi: 10.1242/dmm.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning S, Pucci A, Batterham RL. Roux-en-Y gastric bypass: effects on feeding behavior and underlying mechanisms. J Clin Invest. 2015;125(3):939–948. doi: 10.1172/JCI76305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng H, Shin AC, Lenard NR, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1273–1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers AP, Wilson-Perez HE, McGrath S, et al. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am J Physiol Endocrinol Metab. 2012;303(8):E1076–1084. doi: 10.1152/ajpendo.00211.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mul JD, Begg DP, Alsters SI, et al. Effect of vertical sleeve gastrectomy in melanocortin receptor 4-deficient rats. Am J Physiol Endocrinol Metab. 2012;303(1):E103–110. doi: 10.1152/ajpendo.00159.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9-10):525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alqahtani AR, Elahmedi MO, Al Qahtani AR, Lee J, Butler MG. Laparoscopic Sleeve Gastrectomy in Children and Adolescents with Prader-Willi Syndrome: A Matched Control Study. 2015 doi: 10.1016/j.soard.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warde-Kamar J, Rogers M, Flancbaum L, Laferrere B. Calorie intake and meal patterns up to 4 years after Roux-en-Y gastric bypass surgery. Obes Surg. 2004;14(8):1070–1079. doi: 10.1381/0960892041975668. [DOI] [PubMed] [Google Scholar]
- 27.Arble DM, Sandoval DA, Seeley RJ. Mechanisms underlying weight loss and metabolic improvements in rodent models of bariatric surgery. Diabetologia. 2015;58(2):211–220. doi: 10.1007/s00125-014-3433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrington RA, Weinstein DA, Miller JL. Hypoglycemia in Prader-Willi syndrome. Am J Med Genet A. 2014;164A(5):1127–1129. doi: 10.1002/ajmg.a.36405. [DOI] [PubMed] [Google Scholar]
- 29.Bray GA, Dahms WT, Swerdloff RS, Fiser RH, Atkinson RL, Carrel RE. The Prader-Willi syndrome: a study of 40 patients and a review of the literature. Medicine (Baltimore) 1983;62(2):59–80. [PubMed] [Google Scholar]
- 30.Tennese AA, Wevrick R. Impaired hypothalamic regulation of endocrine function and delayed counterregulatory response to hypoglycemia in Magel2-null mice. Endocrinology. 2011;152(3):967–978. doi: 10.1210/en.2010-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefater MA, Kohli R, Inge TH. Advances in the surgical treatment of morbid obesity. Mol Aspects Med. 2013;34(1):84–94. doi: 10.1016/j.mam.2012.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.