Abstract
The human prostate gland contains extremely high zinc levels; which is due to the specialized zinc-accumulating acinar epithelial of the peripheral zone. These cells evolved for their unique capability to produce and secrete extremely levels of citrate, which is achieved by the high cellular zinc level effects on the cell metabolism. This review highlights the specific functional and metabolic alterations that result from the accumulation of the high zinc levels, especially its effects on mitochondrial citrate metabolism and terminal oxidation. The implications of zinc in the development and progression of prostate cancer are described, which is the most consistent hallmark characteristic of prostate cancer. The requirement for decreased zinc resulting from down regulation of ZIP1 to prevent zinc cytotoxicity in the malignant cells is described as an essential early event in prostate oncogenesis. This provides the basis for the concept that an agent (such as the zinc ionophore, clioquinol) that facilitates zinc uptake and accumulation in ZIP1-deficient prostate tumors cells will markedly inhibit tumor growth. In the current absence of an efficacious chemotherapy for advanced prostate cancer, and for prevention of early development of malignancy; a zinc treatment regimen is a plausible approach that should be pursued.
Keywords: Prostate, Zinc effects and cytotoxicity, Citrate metabolism, Prostate cancer, ZIP1-deficient prostate malignancy, Zinc treatment
The major functional and metabolic activity of the normal human (and other animals) prostate gland is its unique accumulation of high zinc levels, which is required for the production and secretion of extremely high levels of citrate as a major component of prostatic fluid. This is achieved by the evolution of the prostate acinar epithelial as specialized zinc-accumulating cells; which involves zinc transporter relationships, especially ZIP1 zinc uptake transporter. As such, these cells exhibit major effects on mitochondrial accumulation of zinc; and on mitochondrial citrate-related metabolism and terminal oxidation. These normal prostate epithelial cell relationships are described in this review. In addition, the implications of the zinc relationships on the development and progression of prostate malignancy; and the potential of zinc treatment for prostate cancer are described.
1. The normal human prostate gland organization and function in relation to zinc
The human prostate gland is a complex organ comprised of differing ontological, morphological and functional components defined as the peripheral zone, central zone, transition zone, and periurethral region. The peripheral zone comprises ~70%; the central zone comprises about 25%; and the transition zone/periurethral region comprise ~5% [1]. The major function of the prostate gland is its production and secretion of prostatic fluid, which then becomes an essential component of the seminal fluid. Although the specific roles of prostatic fluid in the semen are largely unknown, it is most likely required to support the maintenance, activity, and metabolism of the spermatozoa during the process of fertilization.
The peripheral zone is the major region that is involved in the production of prostatic fluid. The normal peripheral zone and prostatic fluid contain extraordinarily high concentrations of citrate and zinc. (Table 1). The peripheral zone citrate level is ~30–80 fold greater than found in other soft tissues. Even more striking is the enormous concentration of citrate in the prostatic fluid, which is ~1000-fold greater than the concentration in blood plasma.
Table 1.
Representative citrate and zinc levels in prostate.
| (nmols/gm wet wt) | Citrate | Zinc |
|---|---|---|
| Normal peripheral zone | 10,000–13000 | 2000–4000 |
| Normal central zone | 1000–3000 | 800–1000 |
| PCa peripheral zone | 500–2000 | 500–900 |
| Other soft tissues | 150–450 | 100–500 |
| Prostatic fluid | 40,000–150,000 | 7000–9000 |
| Prostatic fluid PCa | 6000–10000 | 800–1000 |
| Blood plasma | 90–110 | 13–17 |
Along with citrate, the zinc concentration in normal peripheral zone is also uniquely greater (~10–20 fold) than typically found in other soft tissues; and the zinc concentration in prostatic fluid ~500 fold greater than the concentration in blood plasma. It is also notable that both citrate and zinc are markedly decreased in prostate cancer (discussed below). These parallels between zinc and citrate exist because of their metabolic and functional linkage in the human prostate peripheral zone.
2. The zinc status in normal prostate epithelial cells: “specialized zinc-accumulating cells”
Zinc is required for the normal growth, proliferation, metabolism, and functions of all cells. All cells maintain the cellular concentration of zinc and its intracellular distribution that is optimal for their normal activities. This is achieved by cellular homeostatic processes that maintain their required zinc status; and deviations from the normal zinc range will result in dysfunctional and cytotoxic effects.
In humans, the peripheral zone acinar secretory epithelial cells are the specialized zinc-accumulating cells; which are responsible for the major prostate function of citrate production and secretion. However, the anatomy, histology, ontogeny, and physiology of the prostate gland vary widely among different animals; even including the prostate zinc and citrate relationships. To avoid confusion, we will refer to those prostate cells in humans and other animals that exhibit the specialized zinc/citrate functional and metabolic relation as the “normal prostate acinar secretory epithelial cells”.
For most mammalian cells, the total cellular zinc concentration is typically in the range of ~100–500 μM (for review [2]). In contrast, the zinc concentration in the normal specialized zinc-accumulating prostate epithelial is in the range of ~800–1500 μM. This is apparent from in situ zinc staining of peripheral zone tissue sections (Fig. 1), which reveals the prominent cellular zinc level in the normal acini epithelium; as compared to the lower zinc staining of the surrounding stromal tissues. As will be discussed below, Fig. 1 also shows that the high zinc level in the normal epithelial cells is markedly decreased in the malignant prostate cells.
Fig. 1.
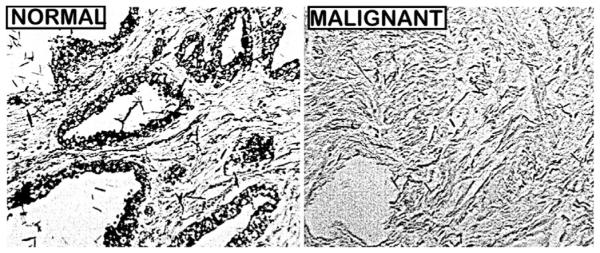
In situ zinc dithizone staining of normal and malignant peripheral zone. Note the prominent zinc staining in the normal acini epithelium compared to lower zinc in the stroma. The malignant cells exhibit low zinc staining.
Also important is the intracellular distribution of the total zinc in the normal prostate epithelial cells and other mammalian cells. Especially relevant is the localization of ~40% of the total cellular zinc in the mitochondria of most cells. Because of the much higher cellular zinc in the normal prostate epithelial cells, the mitochondrial zinc concentration is ~20-fold greater than in other cells [3]. This high mitochondrial zinc concentration is extremely important for the manifestation of the functional and metabolic effects of zinc in the normal prostate cells.
3. The alternative metabolic pathway in the normal specialized “zinc-accumulating, citrate-producing” prostate epithelial cells”
The preceding background leads to the major characterization of the normal prostate acinar epithelial cells as specialized “zinc-accumulating citrate-producing cells.” The achievement of this specialized cellular capability requires major coordinated metabolic and functional alterations that do not exist in “typical” mammalian cells. It is essential to understand the metabolic implications associated with the evolution of these specialized cells.
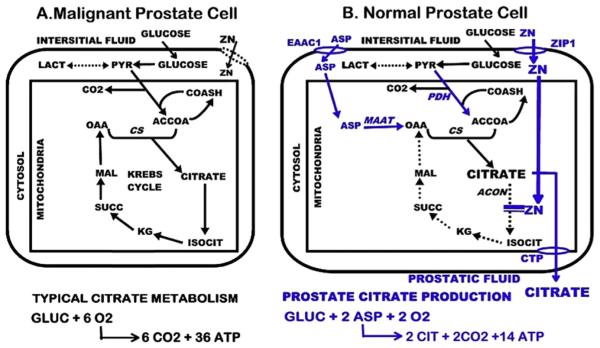
It is important to define and understand the meaning and implications of the metabolic characterization of “citrate-producing” cells; and the metabolic capability of “citrate production”. Essentially all mammalian cells “produce citrate”, i.e. “synthesize” citrate. In fact, with some exception, the major source of citrate in mammalian cells is their mitochondrial synthesis of citrate as typically shown in conjunction with the Krebs cycle (Fig. 2). The synthesized citrate is typically retained in the mitochondria; where it is utilized via its entry into the Krebs cycle. Alternatively, as in proliferating cells, the mitochondrial citrate is exported to the cytosol, where it is metabolized to acetylCoA for lipid biosynthesis.
Fig. 2.
Identification of the metabolic alterations in the specialized citrate-producing normal prostate cells compared to the typical citrate metabolism in most mammalian cells. Blue represents key transporters and enzymes required for citrate production and secretion. PDH = pyruvate dehydrogenase; MAAT = mitochondria aspartate aminotransferase; CS = citrate synthase; acon = aconitase; CTP = citrate transport protein.
In the citrate-producing prostate epithelial cells, the mitochondrial citrate is inhibited from utilization via the Krebs cycle; and is exported to the cytosol and secreted into the acini lumen during the production of prostatic fluid. This secreted citrate represents the unique cellular metabolic pathway that we refer to as “net citrate production” (Fig. 2). Although this unique metabolic capability is essential in specialized “citrate producing cells” (such as the prostate cells, and more recently in the osteoblasts [4]), this alternative citrate-producing metabolic pathway had never been identified in any cells. This became a focus of our research program since ~1975 and continued over ~25 years, leading to our current identification and understanding of the metabolic pathway of citrate production in the specialized normal prostate epithelial cells. This current status (Fig. 2) by no means represents the complete metabolic implications and requirements, which requires much more research to establish. However it does present the important metabolic transformations that we have identified as being most directly involved in prostate citrate production; and especially in relation to the required involvement of zinc.
The following highlights the major events and consequences of the transformation from the typical mammalian cell citrate-related metabolism to the citrate-producing metabolism of the specialized normal prostate epithelial cells.
Upregulation of ZIP1 zinc transporter and increased cellular/ mitochondrial accumulation of zinc.
Inhibition of m-aconitase activity and citrate oxidation (i.e. “truncated Krebs cycle”).
Export of mitochondrial citrate to cytosol and its secretion into prostatic fluid.
Loss of 6-carbon citrate which needs to be replaced by a source of acetyl CoA and OAA
Upregulation of PDH to increase production of acetylCoA.
Upregulation of aspartate transporter and uptake of aspartate as a source for mitochondrial OAA.
Upregulation of mAAT for the production of OAA.
These are coordinated and integrated genetic/metabolic events, which are regulated by testosterone and prolactin in providing the major dual hormonal regulation of prostate citrate production (described below).
It is also important to emphasize the bioenergetic implications of the metabolic pathway in the specialized zinc-accumulating citrate-producing prostate epithelial cells. The complete utilization of glucose via glycolysis/Krebs cycle oxidation, which typifies mammalian cell energy metabolism, results in the generation of ~38 ATP/glucose as shown in Fig. 2. In the prostate epithelial cells, the utilization of glucose for citrate production results in the generation of ~14 ATP/glucose. The inhibition of citrate oxidation via the Krebs cycle results in the loss of ~24 ATP/glucose utilized. Consequently, prostate epithelial cell citrate production has a significant bioenergetic cost of ~64%. To compensate for this bioenergetic consequence and to provide sufficient pyruvate → acetylCoA for continued production of citrate, these prostate epithelial cells exhibit increased aerobic glycolysis [5,6]. These relationships provide the rationale for the conclusion that citrate production and secretion represent the major function of the prostate gland.
4. The zinc connection: inhibition of m-aconitase activity in normal prostate epithelial cells
The parallels between prostate zinc and citrate levels had been known since ~1950; and had been suggestive of a likely important metabolic link between zinc accumulation and citrate levels. However, until 1997 [7], such a link or mechanism remained elusive due to the absence of a known citrate-related metabolic/ biochemical effect of zinc in mammalian cells. We had earlier identified [8] that net citrate production by normal prostate acinar epithelial cells resulted from the inhibition of citrate oxidation, and not inhibition of isocitrate oxidation, which suggested that m-aconitase might be the site of inhibition.
m-Aconitase has typically been described as an equilibrium reaction that rapidly catalyzes the conversion of citrate to isocitrate for entry into the Krebs cycle. As such, m-aconitase, unlike the “oxidation” enzymes, had not been considered to be a “regulatory enzyme” in the operation of the Krebs cycle. Fig. 3 shows the typical equilibrium reaction catalyzed by m-aconitase in mammalian cells; which generally results in a cellular citrate/isocitrate ratio ~9–10/1, regardless of the citrate and isocitrate concentrations. However our studies with prostate tissues [9] revealed a consistently higher citrate/isocitrate ratio ~30–40/1 (Table 2).
Fig. 3.

The m-aconitase reaction and its inhibition by zinc. CS = citrate synthase; IDH = isocit dehydrogenase.
Table 2.
Citrate and isocitrate in prostate tissues (nmols/gm).
| RVP | PIG | HUM | KID | |
|---|---|---|---|---|
| CIT | ~3000 | ~4000 | ~11,000 | ~275 |
| ISOCIT | ~80 | ~100 | ~400 | ~30 |
| C/I | ~37 | ~40 | ~27 | ~9 |
RVP = rat ventral prostate; KID = rat kidney.
The perplexing issue became the mechanism by which the m- aconitase reaction in the prostate cells exhibit a citrate/isocitrate ratio ~30–40/1, along with the inhibition of citrate oxidation. At that time, no known cellular metabolic/biochemical condition or agent existed to explain this phenomenon; despite the history of extensive research and interest in m-aconitase enzymology. However, in 1997 [7] we established with kinetic studies of prostate and kidney mitochondria and purified m-aconitase enzyme that increased zinc as exists in the prostate cells is a specific inhibitor of m-aconitase activity. The m-aconitase reversible equilibrium reactions (Fig. 3) present six cites of potential zinc inhibition. Zinc directly and specifically inhibits the citrate → cis-aconitate reaction; which is the initial step for citrate entry into the Krebs cycle. The equilibrium that results from this inhibition increases the citrate/isocitrate ratio to 30–40/1; which is the ratio that exists in citrate-producing prostate tissue. This provides the most specific and identifiable cellular effect of zinc; which no other cellular condition or zinc effect will mimic. Thus the combination of the increase in citrate and citrate/isocitrate ratio permits the identification and confirmation of in situ effects that are specifically due to changes in the cellular status of zinc.
5. Zinc transport into prostate mitochondria: the new understanding of zinc trafficking in mammalian cells
Because of the unique status and implications of high zinc accumulation in normal prostate cells, the issue of zinc uptake and accumulation in prostate mitochondria is an important issue. Despite decades of interest and research regarding the importance of zinc in mitochondrial metabolism and function, the mechanism or process for mitochondrial uptake of zinc from the cytosol had never been established until our reported studies in 2004 [10]. This issue becomes more relevant and consequential upon recognition of the status of zinc in mammalian cells in relation to the cellular trafficking of zinc; especially regarding mitochondrial zinc uptake from the cytosolic pool of zinc.
It must first be recognized that, as described by Vallee and Falchuk [11] “In biological systems, very little, if any, zinc is free in solution.” Outten and O'Halloran [12] estimated that the free Zn++ ion concentration in the cytosol might be in the fM range; and also reached the conclusion that it is not a physiological pool of zinc. Maret et al. [13,14] have estimated the cellular free Zn++ ion concentration to be in the range of ~5 pMe1 nM. Nevertheless, consensus exist that the free Zn++ ion concentration (fM-nM) is not a relevant pool involved in the trafficking and cellular actions of zinc.
The important issue becomes the identification of the cytosolic mobile reactive pool of Zn; which is trafficked for intracellular distribution and effects of zinc, especially as the source of mitochondrial zinc. The first consideration is the concentration of the exchangeable reactive pool of zinc; and the second consideration is the composition of the exchangeable pool of cytosolic zinc.
There are no direct measurements of the concentration of the cytosolic exchangeable reactive pool of zinc that exists in mammalian cells. Based on reasonable assumptions and available information (described in Ref. [2]), we have estimated the cytosolic concentration of exchangeable reactive zinc to be ~5–100 μM, with prostate cells being ~5–10 fold greater than other cells. We further believe that this is a reasonable estimate because it is in the range of Km values for zinc transporters and for effects of zinc on some enzyme activities that we describe below. We think it to be highly unlikely that living systems evolved and exist under conditions in which the Km values for many transporters and for effects on enzymes are >100-fold or more than the existing concentration of their substrates in their natural environment. This was corroborated by our direct studies with mitochondria [15].
Another issue relates to the composition of the cytosolic exchangeable reactive zinc pool. As we subsequently identified (described below), this pool includes relatively low molecular ligands (ZnLigands) with low to moderate zinc-binding affinities as represented by formation constant log Kf ~ 10 and lower. ZnLigands of logKf ~ 12 and greater are strong zinc chelators that do not provide any cellular exchangeable reactive zinc. Typical cytosolic zinc exchangeable ligands include ZnCit, ZnAsp, ZnHis, ZnCys, and ZnMetallothioneins; the composition of which is dependent on the cell type.
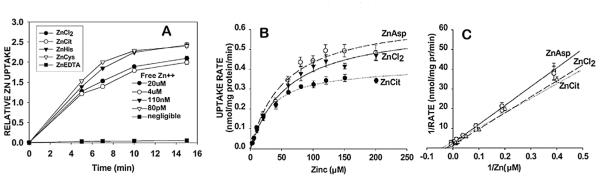
With these conditions in mind, the uptake of zinc and its kinetic properties for prostate and liver mitochondria were established [10] (Fig. 4). The studies compared zinc uptake from 20 μM ZnCl2 and 20 μM ZnCl2 containing 60 μM ligand (ZnCit, ZnHis, ZnCys). Based on the respective logKf values (5, 7, 10), the free Zn++ ion concentrations of the ZnLigands were ~4 μM, 110 nM, and 80 pM. ZnEDTA (log Kf ~ 14) was employed as a strongly bound zinc chelator containing negligible free Zn++ ion concentration. The zinc uptake rates are essentially identical for the ZnLigands with logKf ~ 10 and lower, even though the free Zn++ ion concentration varied more than 20,000-fold; and as negligible ~80 pM. Instead, the uptake rates were dependent on the total concentration of exchangeable zinc, which was 20 μM, independent of the Ligand form. With ZnEDTA and also ZnEGTA (logKf ~ 12), no zinc uptake exists.
Fig. 4.
The kinetics of normal prostate epithelial cell mitochondrial uptake of zinc from ZnLigands. A. Zinc uptake rate from ZnLigands containing 20 μM Zn, showing that the Zn uptake depends on total exchangeable zinc and is independent of the free Zn++ concentration. B. Michaelis-Menton uptake kinetics. C. Lineweaver-Burk plot of B. (Taken from Ref. [10]).
Zinc uptake rates with ZnCl2, ZnCit and ZnAsp (logKf ~ 6) exhibited Michaelis-Menton kinetics that demonstrated the existence of a transport process (Fig. 4). The Km values ranged from ~30 to 60 μM zinc, and the Vmax values ranged from ~0.4 to 0.6 nmol zinc/mg mitochondrial protein/min. Over the range of ~5–50 μM zinc, the uptake rates were identical for all three substrates. Over this range, the free Zn++ ion concentrations for ZnCl2, ZnCit, and ZnAsp preparations were ~5–50, ~1–10, and ~0.05–0.5 μM, respectively. Therefore, the kinetic uptake of zinc is independent of the concentration of free Zn++ ion concentration; but was dependent upon the total concentration of exchangeable zinc. Collectively, these results confirm the likely concentration of the cytosolic pool of exchangeable reactive zinc being in the range of ~5–50 μM, under which the zinc uptake transporter (Km value) is effectively operational.
In addition, the zinc uptake was not dependent on ATP. The kinetics were similar for mitochondrial and mitoplast preparations. Corresponding kinetic studies with liver mitochondria exhibited similar transport properties and similar mitochondrial accumulation of zinc. We subsequently showed that ZnMetallothionein (log Kf ~ 10) is also an exchangeable reactive ligand for the mitochondrial uptake of zinc. The exchange of zinc from the donor ZnLigand to the putative zinc transporter protein occurs without the entry of the Ligand into the mitochondria. Thus, these studies identified for the first time the existence of a putative facilitative zinc uptake transporter located at the inner mitochondrial membrane. The transporter protein exhibits an apparent formation constant of logKf ~ 10–11, which accepts the direct exchange of zinc from the cytosolic donor ZnLigands with logKf ~ 10 or lower. Under these conditions, the higher mitochondrial zinc concentrations that exist in prostate cells results from the higher accumulation of cellular zinc and higher cytosolic concentration of exchangeable reactive zinc that characterizes these cells.
Subsequent to these studies, Seo et al. [16] reported that ZnT2 is a putative zinc importer associated with the inner mitochondrial membrane in mammary cells. It would be important to determine if ZnT2 is the mitochondrial zinc transporter in the prostate cells.
6. Zinc inhibition of mitochondrial respiration and electron transport of normal prostate epithelial cells
The Nobel Prize laureate, Dr. George Huggins, in 1946 [5] characterized the normal human prostate citrate producing cells as “high aerobic glycolysis, low respiring cells”; which we later confirmed with rat prostate cells [5]. This caused us to initiate studies with prostate mitochondrial preparations to determine the factor(s) associated with the characterization of “low respiring cells”. We identified in 1976 [17] that the activity of prostate mitochondrial electron transport/terminal oxidation activity was ~50% lower than liver. At that time, the interrelationship of zinc and prostate metabolism was not known or suspected.
Several early studies with mammalian cells had reported that zinc inhibits mitochondrial respiration and terminal oxidation, although no consensus had been achieved regarding the mechanism and site of the zinc effects [18–23] Unfortunately, all of these studies employed uMemM concentrations of free Zn++ions; i.e. conditions that are non-existent in normal or pathological cellular environments. Since prostate mitochondria contain much higher zinc levels than other cells, it was important to determine its effect on mitochondria function. We addressed this issue in reported studies [10] with the recognition that the cytosolic concentration of free Zn++ ions is negligible and is not the physiological form of reactive zinc in the cells. We employed physiological relevant ZnLigands and concentrations as described above.
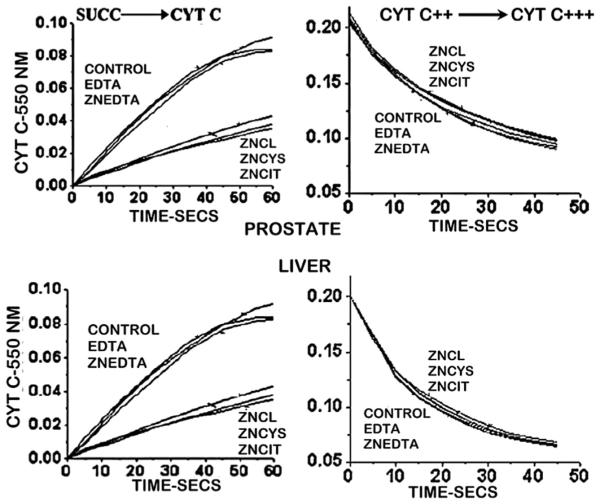
As shown in Fig. 5, exposure of prostate and liver mitochondria to ZnCl, ZnCIT, ZnCys resulted in the identical inhibition of succinate stimulated respiration, in accord with their equivalent concentration of 20 μM zinc. The concentrations of free Zn++ ions were 20 μM, ~4 μM, and ~80 pM, respectively; thereby demonstrating that the exchangeable reactive zinc and not the free Zn++ ion concentration is responsible for the zinc effect. ZnEDTA exhibits no effect on respiration due to its binding affinity (logKf ~ 14) that exceeds the logKf < 11 required for exchangeable reactive ZnLi-gands. Notably, the control and ZnEDTA respiration were identical, which demonstrates that the constitutive respiration of the prostate mitochondria is ~50% lower than liver mitochondria; but the inhibitory effect of zinc is the same.
Fig. 5.
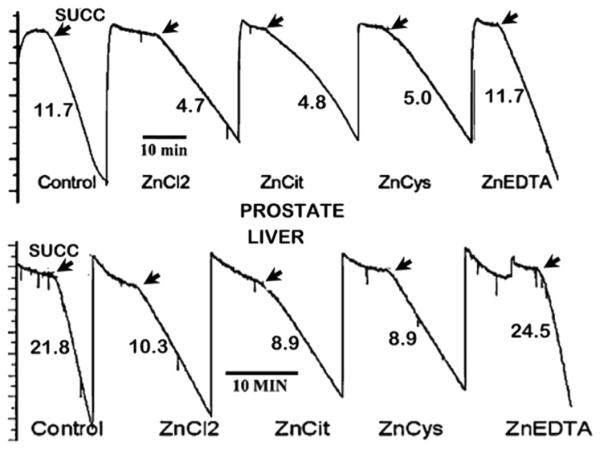
Inhibition of succinate-stimulated respiration of prostate and liver cell mitochondria by ZnLigands. All ZnLigands contained 20 μM Zn. (Taken from Ref. [10]).
The effects on respiration indicates that exposure to increased zinc levels also inhibits mitochondrial electron transport activity. Fig. 6 demonstrates that, in prostate and liver mitochondria, zinc inhibits succinate-stimulated reduction of oxidized cytochrome c; thereby likely inhibiting complex III (cytochrome c reductase). In contrast, zinc has no effect on cytochrome oxidase activity (complex IV). The inhibition is dependent on the total concentration of exchangeable reactive zinc, and independent of the concentration of free Zn++ ions. The effects are the same for prostate and liver mitochondria.
Fig. 6.
Zinc effects on electron transport of prostate and liver cell mitochondria. All ZnLigands contained 20 μM Zn (Taken from Ref. [10]).
Table 3 reveals that the constitutive prostate mitochondria respiration and terminal oxidation capacity are significantly lower (~50–80%) compared to liver mitochondria. In itself, this inherent characteristic accounts for the “low respiring” activity and classification of the specialized prostate epithelial cells. In addition, the higher cellular and mitochondrial zinc accumulation further contributes to the inhibition of terminal oxidation of the normal zinc-accumulating prostate epithelial cells. Collectively, these relationships highlight the major role of zinc in the unique and specialized metabolic and functional relationships of the prostate epithelial cells.
Table 3.
Relative terminal oxidation activities of liver and prostate mitochondria.
| Reaction | Liver | Prostate |
|---|---|---|
| O2 UPTAKE | 55.0 | 10.1 |
| NADH → UBQ | 255.5 | 114.7 |
| NADH → CYT C++ | 285.0 | 90.7 |
| SUCC → CYT C++ | 51.5 | 12.4 |
| CYT C++ → CYT+++ | 223.7 | 113.7 |
The combined transport and respiration/electron transport effects of ZnLigands provide the evidence that the trafficking, transport, and reactivity of zinc does not require the presence of a free Zn+ + ion pool; which is a negligible form of reactive zinc in cells. Instead, a direct intermolecular transfer of zinc between donor ZnLigands and recipient ZnLgands/Proteins is the process for cellular zinc relationships. In addition, these studies defined the zinc exchange properties as requiring ZnLigands with zinc binding affinity of logKf < ~11. Although such a direct intermolecular exchange without the requirement for free Zn++ ions had been suggested by others (such as [12,24–27]), these studies provided the experimental evidence that now establishes the process and conditions for zinc trafficking, transport and reactivity for prostate cells and in all cells.
7. ZIP1 (Slc39A1): the important functional zinc uptake transporter for zinc accumulation in prostate cells
Mammalian cells maintain their required zinc concentration and intracellular distribution by the expression and functional activities of zinc transporters. ZIP family transporters (Slc39A) are generally the plasma membrane transporters that import zinc from the extracellular fluid into the cells. ZnT transporters (Slc30A) are generally intracellular transporters that distribute the cytosolic zinc among the organelles. Since the total cellular zinc is first dependent on the uptake of zinc from the cell's extracellular fluid (mainly from the interstitial fluid derived from blood plasma), we initially focused on the identification of the functional ZIP transporter in prostate cells. It is important to emphasize that determination of the transporter's gene expression does not establish the functional activity of the transporter; nor does the determination of the abundance of the transporter protein by Western blot analysis. Instead, in situ immunohistochemistry is required to establish the localization of the transporter at the plasma membrane; and, when applicable, the localization at the apical and/or basolateral membrane. Moreover, the immunohistochemistry must be determined in human tissue to establish the in situ status of the transporters. Studies with cell lines should not be employed to establish the tissue in situ status of these transporters. Unfortunately, most studies have provided gene expression and Western blot analyses in the absence of immunohistochemistry; and some studies have employed cell lines to establish the in situ transporter functional relationships. Such have too often resulted in questionable or inappropriate conclusions.
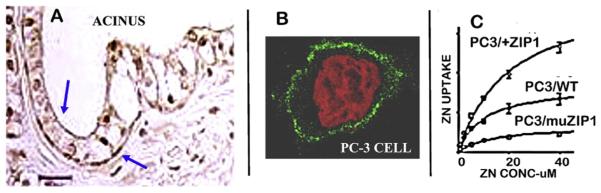
We first identified in 1999 and subsequently [28–30] that ZIP1 is a plasma membrane transporter in prostate cells that exhibits functional kinetic zinc uptake properties (Fig. 7). Zinc uptake in prostate cells is increased when ZIP1 expression and abundance are increased, and zinc uptake is markedly decreased when ZIP1 is downregulated (Fig. 7C). In situ immunohistochemistry of human prostate tissue shows that ZIP1 transporter is localized at the basolateral and apical cell membrane of the normal acini epithelium; and its specificity is demonstrated by the absence of ZIP1 in the stromal tissue (Fig. 7). Also, as described below, ZIP1 transporter coexists with the high levels of zinc in the normal epithelium; and in prostate cancer, ZIP1 transporter is essentially absent along with the decrease in zinc in prostate cancer. These collective observations provide compelling evidence that ZIP1 is the important functional transporter involved in the uptake and accumulation of zinc in normal prostate. Further support is derived from the absence of ZIP2 and ZIP3 transporter localization at the basolateral membrane of the acinar epithelium [31] thereby revealing that they are not involved in the transport and uptake of zinc from blood plasma. Others have confirmed the role of ZIP1 for zinc uptake in prostate cells [32–34]; and no other ZIP transporter has been correspondingly identified as an important functional human prostate zinc uptake transporter.
Fig. 7.
ZIP1 transporter in prostate cells and its role in zinc uptake. A. Immunohistochemistry of normal human prostate tissue section showing ZIP1 localization at the plasma membrane of the normal acinar epithelium. B. Shows that wildtype PC-3 cells exhibit plasma membrane localized ZIP1 transporter. C. Shows that the uptake of zinc by PC-3 wildtype is increased in ZIP1 over-expressed cells; and is decreased in ZIP1-mutated cells. (7A, B taken from Refs. [30];7C taken from Ref. [55].
8. Hormonal regulation of prostate zinc accumulation and citrate production
It had been known since 1965, 1966 [35,36] that prostate citrate production is regulated by testosterone; and since 1967 [37] that it is also regulated by prolactin. This redundant hormonal control underscores the importance of citrate production as a major function of the human prostate gland. To affect their respective hormonal regulation, the target of testosterone and prolactin must be the acini epithelial cells in the peripheral zone in humans. Since these are specialized zinc-accumulating cells required for citrate production, testosterone and prolactin also regulate zinc uptake and accumulation [3,28,38]. It is reasonable to suggest that a major reproductive function of prolactin in males is its regulation of prostate citrate production and secretion as a major component of seminal fluid.
The mechanisms and signaling pathways of testosterone and prolactin regulation could not be investigated until the metabolic pathway of prostate citrate production and the role of zinc were identified. This information began to evolve from our reported studies from ~1980 to 2000; which culminated in the identification of the pathway of prostate citrate production and the role of zinc as represented in Fig. 2. Consequently, we were able to investigate the regulation of this pathway by testosterone and prolactin [38].
It is noteworthy that both hormones exhibit positive regulation of the expression and abundance of ZIP1 zinc transporter; aspartate transporter (EAAC1); mitochondrial aspartate aminotransferase (mAAT); and pyruvate dehydrogenase E1a (PDH). This demonstrates that the hormonal control involves the coordinated regulation of key reactions in a metabolic sequence, which is necessary to optimize the operation of the metabolic pathway. This dual hormonal control is achieved by their regulation of the gene expression of each of these important enzymes and transporters associated with the altered specialized metabolic pathway of net citrate production.
We refer to these genes as “metabolic” genes to different them from the regulation of “cytokine” genes. This is an important distinction for reasons that we have described in Refs. [38,39]. A major difference is that these regulatory enzymes and transporters involved in metabolic pathways exist in very low concentration consistent with their cellular Vmax activities. Consequently, altered gene expression in the range of less than ~5-fold will effect significant change in the abundance and activity of the regulatory enzyme/transporter. Increased gene expression and abundance of the product that greatly exceeds its Vmax is superfluous and of no metabolic value. Thus the mechanism of gene regulation of “metabolic” genes is consistent with these requirements. However, the products of cytokine genes involve large changes in gene expression and their protein products; to the extent that changes of the magnitude of ~2-fold or less are not even considered to be relevant. This criterion should not be applied to metabolic genes.
8.1. Testosterone regulation
Our studies [38] identified that testosterone regulation is achieved by the presence of androgen response elements (ARE) located in the promoter region of these metabolic genes. Testosterone conversion to DHT (dihydrotestosterone); followed by its binding to androgen receptor results in activation of the ARE and increased gene expression. This provides a rapid androgen pathway for the regulation of the metabolic genes in the prostate cells.
8.2. Prolactin regulation
However, prolactin regulation of these metabolic genes occurs via an alternate signaling pathway from the cytokine effects of prolactin. The cytokine effects of prolactin in prostate cells are generally mediated predominantly through the hormone-receptor initiation of a tyrosine kinase-associated signaling pathway; which involves cascading activation of immediate-early, intermediate and late-acting (final effector) genes [38]. Such a gene regulation process is inconsistent with achieving the rapid and specific direct regulation of the metabolic genes. Instead our studies have shown that the metabolic genes are immediate-early final effector genes in response to prolactin. This is achieved via prolactin-receptor activation of the phospholipase-diacylglycerol pathway; which results in the direct rapid activation of PKCε, and is immediately followed by increased transcription of the metabolic gene.
It is surprising to note that, since our reported studies regarding the role and mechanism of testosterone and prolactin regulation of prostate zinc and citrate-related metabolism, this issue has received little attention and progress. This is especially unfortunate, given the importance of the issue of androgen responsive prostate cancer; and given the unsettled issue of the possible role of prolactin in the development of prostate cancer.
8.3. Summary (1–8)
It is evident that the inhibitory effects of zinc on m-aconitase activity, on respiration, and on terminal oxidation are not specific for prostate; but apply to other cells. The specificity of these effects results mainly from the uniquely higher cellular and mitochondrial zinc levels that normally exist in the zinc-accumulating specialized prostate cells as compared to other cells. This, along with the inherent low capacity of terminal oxidation, provide the metabolic conditions that result in the prostate cells being characterized as “low respiring cells”. These effects result from the important functional role of ZIP1 as a major factor in the uptake and accumulation of zinc in the prostate cells. This provides a higher cytosolic concentration of reactive exchangeable ZnLigands for increased mitochondrial transport and accumulation. The redundant regulation of zinc accumulation and prostate citrate production and the metabolic genes by testosterone and prolactin are indicative of its importance as a major function of the prostate gland in humans.
9. Decreased zinc: the “hallmark” characteristic of prostate cancer
Since 1952 [40] more than sixteen reports have consistently identified that zinc is markedly decreased (~60–80% with a standard error of <10%) in prostate cancer compared to normal and benign prostate (for reviews [41,42]). This amazing statistical consistency exists despite variables among these studies; such as different populations, differing stages of cancer, differing composition of tissue components, differing zinc assay methods, and other variables. In contrast to this established clinical zinc relationship, there exists no confirmed or corroborated report of prostate cancer in which zinc is not decreased. Moreover, it is well established by in situ zinc staining of prostate tissue sections that the decrease in the tissue zinc concentration is due to the specific decrease in the malignant cells compared to the high zinc levels found in the normal peripheral zone epithelium (Figs. 1, 7 and 8) [30,34,43]. These overwhelming data clearly establish that zinc is always decreased in prostate cancer. More importantly, the data reveal that prostate cancer in which the malignancy retains the high zinc levels of normal prostate epithelium never (or rarely) exists.
No other clinical biomarker, genomic condition, oncogenic factor, or other specific malignancy factor exhibits the global consistency among all cases of prostate cancer as the decrease in zinc (and citrate). Despite its important implications, this established clinical relationship has not received the broad recognition, attention, and interest among the clinical/biomedical community that should be expected. Thus progress over the years has been impaired in the application of the zinc relationship to important issues such as the identification of early events in the development of prostate malignancy; the identification of zinc-related biomarkers; and the potential for development of a zinc treatment approach and prevention for prostate cancer. The following presentation will highlight the role, implications, and potential application of the zinc relationship relative to these contemporary issues of prostate cancer.
10. ZIP1 downregulation: a major mechanism for decreased zinc in ZIP1-deficient prostate cancer
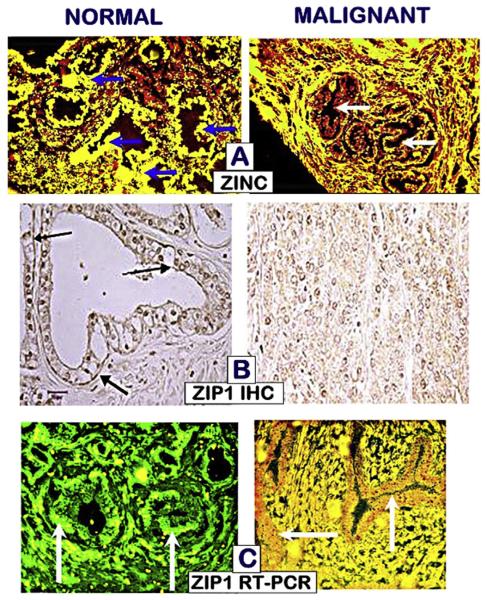
Since ZIP1 is the major zinc uptake transporter in prostate cells, we suspected that its down regulation might be involved in the decrease in zinc in the malignant cells. Notably, Rishii et al., in 2003 [44] determined by in situ RT-PCR of prostate tissue sections that ZIP1 gene expression in African-Americans was lower than the expression in matched Caucasians; which they suggested might be associated with the race-related higher incidence of prostate cancer. Our reported studies in 2005 [30] using ZIP1 immunohistochemistry, in situ RT-PCR, and in situ zinc staining established that ZIP1 gene expression, ZIP1 transporter protein abundance, and cellular zinc are prominent in normal peripheral zone acinar epithelium (Fig. 8). In contrast, ZIP1 gene expression is markedly down-regulated, and ZIP1 transporter and zinc are depleted in the adenocarcinoma glands. These concurrent changes are evident in prostate intraepithelial neoplasia (PIN), in highly-differentiated malignancy, and during its progression in the peripheral zone. Thus the absence of plasma membrane localized ZIP1 transporter in the malignant cells along with the concurrent loss of zinc is evidence that ZIP1 down regulation is the cause of decreased zinc levels in malignancy; and that this is an essential early event in the development of prostate malignancy. These observations were subsequently confirmed in similar studies reported by Johnson et al. [34].
Fig. 8.
In situ identification of the status of zinc and ZIP1 in tissue sections of normal and malignant prostate. A. Shows zinc staining identifying high zinc (yellow stain) in the normal acinar epithelium versus low zinc (red stain) in malignancy. B. Shows prominent plasma membrane localized ZIP1 in the normal epithelium; and absence of ZIP1 in malignancy. C. Shows high gene expression (green) in the normal acinar epithelium; and the absence of gene expression (red) in malignancy. (Taken from Ref. [30].
The above relationships lead to an important new clinical characterization of prostate cancer as being a “ZIP1-deficient malignancy”. This identification is essential for the future biomedical and clinical research having appropriate translational application of the implications of zinc in human prostate cancer. Prior to our 2005 report, essentially all zinc-related experimental studies and their results and conclusions involved the employment of malignant prostate cell lines (such as PC-3, LnCaP, Du-145) that were derived from human prostate cancer tissues. As such, they were presumed to represent the ZIP/zinc status that existed in the in situ malignancy in prostate cancer. The early studies of the role and effects of zinc and zinc transporters were conducted with the knowledge that these cell lines exhibited the presence ZIP1 plasma membrane localized transporter. Thus, it was presumed by us and by others that this represented the status of ZIP1 in the malignant cells in situ in prostate cancer. However, the clinical reports described above made it apparent that the in situ status of ZIP1-deficient malignant cells is not represented in the malignant cell lines under typical culture and experimental conditions. Consequently, translational application of the results and conclusions of such studies need to be re-assessed. Unfortunately, most reported zinc studies continue to employ the ZIP1-expressing cell lines for representing the in situ malignancy in prostate cancer.
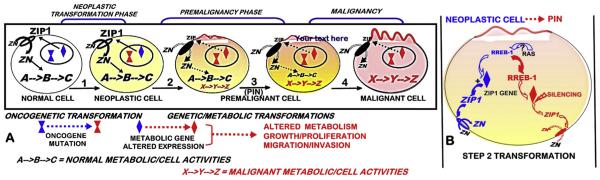
11. The ZIP1/Zn genetic/metabolic transformation during the oncogenic development of premalignancy leading to progression to malignancy
Most contemporary oncogenic concepts do not include or identify a “premalignancy phase” during transformation of a normal cell to its malignant cell. However the neoplastic cell must undergo “genetic/metabolic” transformations; so as to provide the bioenergetic/synthetic requirements for the malignant activities (such as growth, proliferation, motility, invasion). This event applies to all cancers, in recognition that the metabolism of a cell must change when the functional activity of the cell changes [45]. As represented in Fig. 9, the oncogenic initiation of the transformation of the normal cell results in the neoplastic cell that has potential capability to develop to a malignant cell. This requires genetic/metabolic alterations in the transformation of the neoplastic cell leading to premalignant cells and the progression to malignancy. Fig. 9 shows the oncogenic concept as it applies to the ZIP1/Zn transformation during the development of prostate cancer. When viewed in this context, the following important issues and relationships relating to the development of prostate cancer become evident. Since the ZIP1/Zn transformation occurs in all or nearly all cases of prostate cancer, the identification of the upstream oncogenic factors involved in the down regulation of ZIP1 becomes an important relationship for revealing the etiology of prostate malignancy. Also apparent is the potential targeting of premalignancy for a zinc treatment regimen that prevents the development and progression of prostate malignancy. Another potential is the targeting of the premalignant ZIP/Zn transformation as a histological biomarker for the early identification of questionable PSA/biopsy at-risk individuals.
Fig. 9.
The concept of the genetic/metabolic transformation in the oncogenic development of malignancy in cancer; and the implications of ZIP1/Zn down regulation in prostate cancer. A. The oncogenic initiation of the transformation of the normal cell to the neoplastic cell, and its progression to premalignancy and the development of malignancy. B. RREB-1 as a negative regulator of ZIP1gene expression, and its upregulation during the transformation of the neoplastic cell to the premalignant cell, so as to silence ZIP1 expression and prevent cytotoxic zinc accumulation in the progression to malignancy. (PIN = prostate intraepithelial neoplasia premalignant lesion).
Since ZIP1 down regulation is evident in PIN and in well-differentiated malignancy, it must be a premalignant transformation that is initiated by upstream oncogenic factors. In addressing this important issue, we recently identified [46,47] that REBB-1 transcription factor (ras responsive binding element protein-1) is a negative regulator of ZIP1 gene expression (Fig. 10); and its in situ upregulation in early development of the malignant cells results in the down regulation of ZIP (Fig. 11). This event couples the RAS initiation of prostate oncogenesis to its down-stream regulation of the RREB1/ZIP1/Zn transformation that is essential for the development of prostate malignancy (Fig. 9B). Now, it becomes important to identify the signaling pathway for RAS upregulation of RREB-1. RREB-1 is involved in either as a positive or negative regulator of respective genes in several cancers; but its regulation via RAS signaling remains poorly understood and speculative [48–51].
Fig. 10.
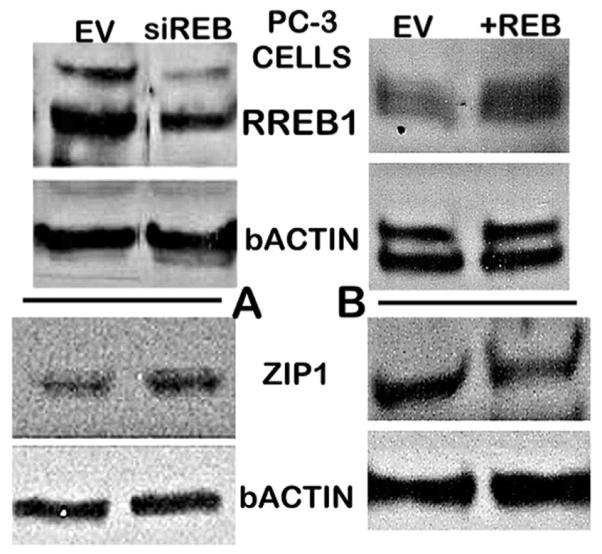
Immunohistochemistry effects of REEB-1 regulation on ZIP1 transporter abundance in PC-3 cells. A. shows the down regulation of RREB-1 increases ZIP1 abundance. B. Shows that increased RREB-1 decreases ZIP1 abundance. (Taken from Refs. [47]).
Fig. 11.
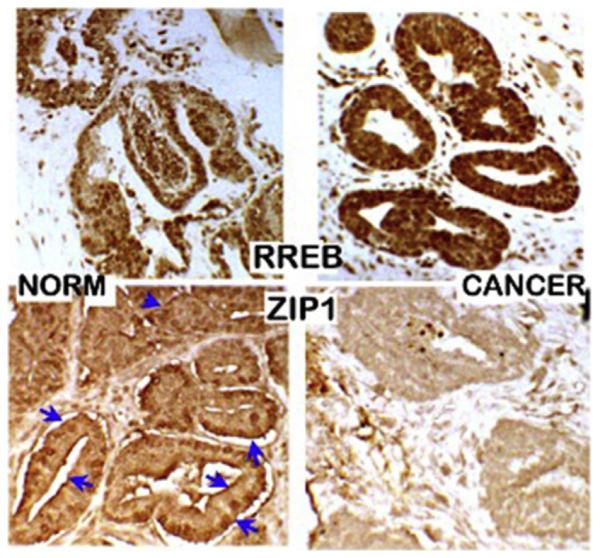
RREB-1 and ZIP1 abundance in normal versus prostate cancer tissue sections. Immunohistochemistry shows increased RREB-1 and loss of plasma membrane ZIP1 transporter in malignant acini. (Taken from Ref. [47]).
12. Zinc as a cytotoxic agent in malignant prostate cells
As described above, the normal peripheral zone prostate glandular epithelial cells evolved as unique zinc-accumulating cells; which was necessary to fulfil the major specialized prostate function of citrate production and secretion. This required that these cells possess conditions that prevent the high cellular zinc levels from imposing cytotoxic effects; while providing the normal activities of the specialized cells. However, the evolution of malignant cells involves the elimination of the highly specialized zinc-accumulating capability of the normal cells. As such, the malignant cells are susceptible to cytotoxic effects that will result from the high zinc levels that exist in the normal cells. Therefore the malignant cells evolved with conditions that silence ZIP1 expression, which results in decreased abundance of plasma membrane ZIP1 transporter. This prevents the uptake and accumulation of high cellular zinc level and its cytotoxic effects; which is why malignancy that retains the normal high levels of zinc rarely, if ever, exists.
Many reports have demonstrated that the treatment of malignant prostate cells with physiological levels of zinc that will increase the cellular accumulation of zinc results in cytotoxic effects, such as inhibition of cell proliferation, promotion of apoptosis, and inhibition of cell migration and invasion (for reviews [45–47]). It is important to note that some studies purport to demonstrate that zinc treatment promotes cell growth and prevents apoptosis of malignant cells. Such results exist when one employs experimentally zinc-depleted cells (for example TPEN-treated cells) [42,52–55]. TPEN has a logKf ~ 15, which, even at low concentrations, will irreversibly tightly bind all of the exchangeable reactive zinc, and most (or all) of the total cellular zinc. Such conditions result in dying cells, in which zinc treatment restores the zinc levels that the cells require for their survival and normal activities. Such an experimental model does not represent a cellular zinc status that exists in situ under living conditions.
13. Zinc for the treatment of ZIP1-deficient prostate cancer
13.1. The need for an efficacious chemotherapy for prostate cancer
In recent years, about 230,000 new cases and about 30,000 deaths due to prostate cancer occur annually in the USA [56]. Although the mortality rate from prostate cancer is relatively low, it is still the second leading cause of cancer deaths in males. Thus it becomes evident that the morbidity and mortality of prostate cancer continues to constitute an important health issue. The major problem is the absence of effective treatment for advanced stage malignancy and metastasis; and for the hormone-resistant cancer following androgen-deprivation treatment. Organ-confined prostate cancer is generally treatable and curable, but it requires invasive procedures often with attending side-effects. A reliable and relatively innocuous chemotherapy would be advantageous in many of these cancer cases. In addition, the suspicion of unconfirmed early malignancy or the presence of low volume and low grade malignancy is often followed by “active surveillance”; during which no treatment is employed until the appearance of malignant progression. When malignancy is confirmed, invasive treatment regimens are employed. Such conditions would also benefit from an effective chemotherapy or prophylactic regimen. For decades these issues have been the focus of intense research in search of an effective chemotherapeutic approach for prostate cancer; and, yet the problem continues to exist.
13.2. The cytotoxic effect of zinc as a basis for a zinc treatment approach for prostate cancer
We first identified zinc as an inhibitor of prostate citrate metabolism in 1981 [57]; In 1999 we identified zinc as an inhibitor of prostate malignant cell proliferation and also induced apoptosis [58], which lead us to propose that zinc treatment might provide an approach for prostate cancer. Since then many reported studies have confirmed and extended the in vitro cytotoxic effects of zinc on malignant prostate cells.
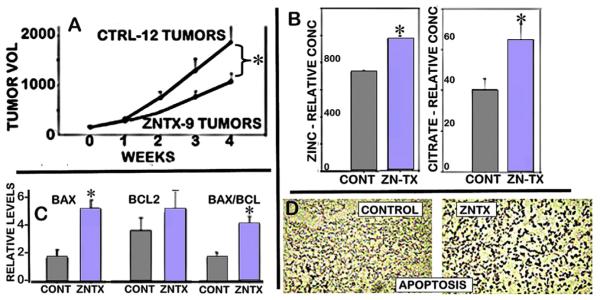
Notably, we demonstrated in 2003 [59] that the in vitro cytotoxic effects of zinc on malignant prostate cells are also manifested in vivo for zinc-treatment suppression of the growth of PC-3 tumors in the mouse xenograft model (Fig. 12). At that time, we employed wildtype PC-3 cells that exhibit ZIP1 transporter; so that the ZIP1-expressing tumor cells were capable of importing and accumulating zinc from circulation. However, the importance of that study was its demonstration that under in vivo conditions in which tumor cells accumulate zinc, tumor growth is inhibited, and apoptosis, the Bax and Bax/Bcl2 ratio, and citrate are increased; which are direct effects of zinc on malignant cells [60,61].
Fig. 12.
A. Effects of zinc treatment on PC-3 tumor growth in xenograft mice. B. Zinc and citrate levels in the resected tumor tissues. C. Bax and Bcl-2 levels in the resected tumors. D. Tunel assay for apoptosis in tumors. (Taken from Ref. [59]).
Consequently these cytotoxic effects of zinc, coupled with the clinical identification of the requirement of decreased zinc for the development and progression of prostate malignancy, strengthens the plausibility of a zinc treatment approach for prostate cancer. However, the identification of the in situ “ZIP1-deficient” status of prostate cancer malignancy imposes the issue that a treatment regimen based on increasing the concentration of plasma zinc delivered to the malignant site will not effectively increase the uptake and accumulation of zinc in the ZIP1-deficient malignant cells. Thus a zinc treatment approach must recognize and address this clinical relationship.
13.3. Zinc ionophore (clioquinol) as a model for treatment of ZIP1-deficient prostate cancer
It is now evident that a zinc treatment approach requires a mechanism or factor that will facility the uptake of zinc from the interstitial fluid and into the cytosol of the ZIP1-deficient malignant cells. In addition, the delivered intracellular zinc must be in an exchangeable reactive ZnLigand form (logKf < 11) that will manifest the cytotoxic effects of zinc in the malignant cells. One approach to achieve these requirements is the employment of an appropriate zinc ionophore as represented by clioquinol (5-chloro-7-iodo-8-hydroxyquinoline), which has a formation constant of logKf ~ 8. An additional requirement is that such an ionophore, once in circulation, will successfully compete with the other ligands for binding of the plasma zinc; so that sufficient ZnIonophore is available for delivery into the ZIP1-deficient malignant cells. Whether or not these requirements can be achieved under the complex and competing factors that exist in the in human in vivo status of the cancer had not been established until our recent studies [62,63]. However Ding et al. [64–66] have demonstrated that clioquinol administration in xenograft animals with experimental tumors exhibits tumor suppressor effects, but none had considered or involved the status of ZIP transporter deficient tumors.
To address this issue we developed a ZIP1-deficient PC-3 cell line (PC3/-ZIP1). These cells were employed to induce ZIP-1 deficient tumor development in the mouse xenograft model, in which the animals were treated with clioquinol or vehicle (described in Ref. [62]). The animals received IP administration of 30 mg clioquinol/Kg or vehicle every other day for five or more weeks after initial tumor development. This dosage regimen approximates the relatively non-toxic dosage in humans [67–69] and in mice [64,65].
Fig. 13 summarizes the combined results of two experiments reported in Refs. [62,63]; and shows that clioquinol treatment markedly suppressed the tumor growth rate by ~85%. We also determined that the citrate concentration of the tumors from CQTX animals was increased by ~110% compared to the citrate concentration of the tumors from the untreated animals. This verifies that clioquinol effectively increased the mobile reactive zinc concentration in the ZIP1-deficient tumors. This also supports the reason why ZIP1-deficient human prostate cancer rarely, if ever, exists with the high level of zinc that is in the normal epithelial cells.
Fig. 13.
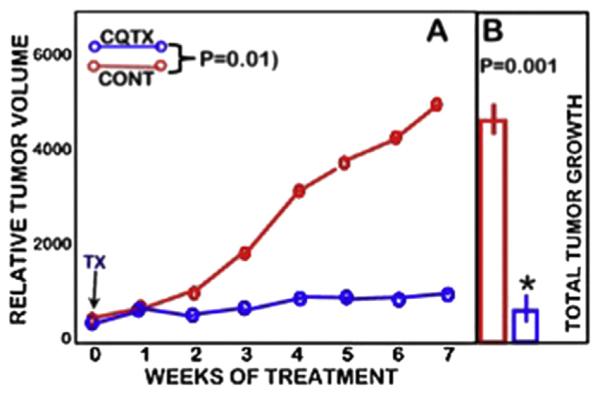
Effects of clioquinol treatment on PC-3 ZIP1-deficient tumor growth in the mouse xenograft animals. A. Shows the tumor growth rates during the treatment period. B. Shows the total tumor growth at the end of the treatment period. (Modified from Refs. [62,63]).
The strength of the existing supporting clinical and experimental evidence should lead to pursuant studies for the development of a zinc treatment regimen to address the extensive morbidity and mortality presented by prostate cancer.
13.4. Applying the zinc treatment for prostate cancer
At this time the evidence presented above of the successful suppression by zinc treatment of human prostate tumor growth has been demonstrated in the mouse xenograft model. This model demonstrates that the tumors, as representing primary site malignancy, responds to the cytotoxic effects of increased uptake and accumulation of zinc. However, the xenograft model does not permit the determination of zinc treatment effects on the development of malignancy, nor generally on metastases and metastatic cells. Studies of such effects are essential to the practicality of the application of zinc treatment for prostate cancer.
The expectation that the zinc treatment cytotoxic effects will be manifested during the initiation and early development of prostate malignancy is supported by clinical evidence. Our studies [30] and those of Johnson et al. [34] have shown that the down regulation of ZIP1 and the decrease in zinc are evident in PIN and in well-differentiated malignancy. Cortesi et al. [70] employing in situ identification of zinc levels in peripheral zone similarly described that “the zinc depletion occurs not only in the cancerous tissue segments but also … in the non-cancer components surrounding the lesion.” Horn et al. [71] with in situ MRS imaging showed that PIN lesions exhibit decreased citrate, which would be due to loss of zinc. Such clinical observations are consistent with our view that the events of down regulation of ZIP1 and loss of zinc occur in premalignancy, and prior to the histopathological identification of malignancy. Such relationships make it likely that a zinc treatment regimen could prevent the development of malignancy such as for individuals with elevated PSA and questionable biopsy confirmation of malignancy. Since the zinc treatment would likely be somewhat innocuous, its employment to abort potential early development and progression of malignancy would be advantageous over the absence of any mitigating interventions in an “active surveillance” protocol.
Although the zinc status in metastatic and advanced hormone-independent prostate cancer has not been established, some existing evidence indicates the likelihood that the loss of zinc persists in the advanced stages of malignancy. Heijmink et al. [72] showed that citrate is absent in lymph node metastasis; and this would be indicative of decreased zinc. Kim et al. [73] reported that promotion of prostate cancer invasion and metastasis occurs by decreasing intracellular zinc levels. Compelling evidence is also provided by identification of the loss of citrate, zinc, and ZIP1 in the metastatic lymph nodes in TRAMP. Based on such evidence, it is reasonable to expect that a zinc treatment approach will increase zinc accumulation and its cytotoxic effects in metastatic cells.
Until recently, no prostate cancer animal model had been identified that exhibits the zinc/ZIP1/citrate relationship as exists in human prostate cancer. Our studies, in collaboration of Kurhanewicz et al. (UCSF), identified that the human prostate ZIP1/Zn/citrate relationships exist in the development and progression of malignancy in the TRAMP animal model, including the cytotoxic effects of zinc on the TRAMP malignant cells [74]. It is extremely relevant that this identical relationship exists in both human prostate cancer and TRAMP; which re-enforces the validity that the development and progression of prostate malignancy requires the decrease in zinc in order to prevent zinc cytotoxicity; and that this is universal regardless of the genomic variations that are represented in prostate cancer. This now provides an excellent animal model to study and explore develop a zinc treatment approach for developing and progressing ZIP1-deficient prostate cancer.
Acknowledgement
Studies of LCC and RBF presented in this review were supported in part by NIH grants CA79903, DK076783 and DK42839 and AR064808.
Footnotes
This article is part of a Special Issue entitled The Cutting Edge of Zinc Biology, edited by Shinya Toyokuni, Taiho Kambe, and Toshiyuki Fukada.
References
- [1].McNeal JE. Normal histology of the prostate. Am. J. Surg. Pathol. 1988;12:619–633. doi: 10.1097/00000478-198808000-00003. [DOI] [PubMed] [Google Scholar]
- [2].Costello LC, Fenselau CC, Franklin RB. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J. Inorg. Biochem. 2011;105:589–599. doi: 10.1016/j.jinorgbio.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu Y, Franklin RB, Costello LC. Prolactin and testosterone regulation of mitochondrial zinc in prostate epithelial cells. Prostate. 1997;30:26–32. doi: 10.1002/(sici)1097-0045(19970101)30:1<26::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- [4].Franklin RB, Chellaiah M, Zou J, Reynolds MA, Costello LC. Evidence that osteoblasts are specialized citrate-producing cells that provide the citrate for Incorporation into the structure of bone. Open Bone J. 2014;6:1–7. doi: 10.2174/1876525401406010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huggins C. The prostatic secretion. Harvey Lect. 1947;42:148–193. [Google Scholar]
- [6].Costello LC, Franklin RB. Prostate epithelial cells utilize glucose and aspartate as the carbon sources for net citrate production. Prostate. 1989;15:335–342. doi: 10.1002/pros.2990150406. [DOI] [PubMed] [Google Scholar]
- [7].Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J. Biol. Chem. 1997;272:28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- [8].Costello LC, Franklin RB. Aconitase activity, citrate oxidation, and zinc inhibition in rat ventral prostate. Enzyme. 1981;26:281–287. doi: 10.1159/000459195. [DOI] [PubMed] [Google Scholar]
- [9].Costello LC, Franklin RB. Concepts of citrate production and secretion in prostate. 1.Metabolic relationships. Prostate. 1991;18:25–46. doi: 10.1002/pros.2990180104. [DOI] [PubMed] [Google Scholar]
- [10].Costello LC, Guan Z, Kukoyi B, Feng P, Franklin RB. Terminal oxidation and the effects of zinc in prostate versus liver mitochondria. Mitochondrion. 2004;4:331–338. doi: 10.1016/j.mito.2004.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- [12].Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- [13].Maret W, Li Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009;109:4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- [14].Maret W. Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals. Biometals. 2009;22:149–157. doi: 10.1007/s10534-008-9186-z. [DOI] [PubMed] [Google Scholar]
- [15].Guan Z, Kukoyi B, Feng P, Kennedy MC, Franklin RB, Costello LC. Kinetic identification of a mitochondrial zinc uptake transport process in prostate cells. J. Inorg. Biochem. 2003;97:199–206. doi: 10.1016/s0162-0134(03)00291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Seo YA, Lopez V, Kelleher SL. A histidine-rich motif mediates mitochondrial localization of ZnT2 to modulate mitochondrial function. Am. J. Physiol. Cell Physiol. 2011;300:C1479–C1489. doi: 10.1152/ajpcell.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Costello LC, Franklin RB, Stacey R. Mitochondrial isocitrate dehydrogenase and isocitrate oxidation of rat ventral prostate. Enzyme. 1976;21:495–506. doi: 10.1159/000458902. [DOI] [PubMed] [Google Scholar]
- [18].Hunter FE, Ford L. Inactivation of oxidative and phosphorylative systems in mitochondria by preincubation with phosphate and other ions. J. Biol. Chem. 1955;216:357–369. [PubMed] [Google Scholar]
- [19].Skulachev VP, Chistyakov VV, Jasaitis AA, Smirnova EG. Inhibition of the respiratory chain by zinc ions. Biochem. Biophys. Res. Commun. 1967;26:1–6. doi: 10.1016/0006-291x(67)90242-2. [DOI] [PubMed] [Google Scholar]
- [20].Nicholls P, Malviya AN. Inhibition of nonphosphorylating electron transfer by zinc. The problem of delineating interaction sites. Biochemistry. 1968;7:305–310. doi: 10.1021/bi00841a038. [DOI] [PubMed] [Google Scholar]
- [21].Kleiner D, von Jagow G. On the inhibition of mitochondrial electron transport by Zn(2+) ions. FEBS Lett. 1972;20:229–232. doi: 10.1016/0014-5793(72)80802-0. [DOI] [PubMed] [Google Scholar]
- [22].Lorusso M, Cocco T, Sardanelli AM, Minuto M, Bonomi F, Papa S. Interaction of Zn2+ with the bovine-heart mitochondrial bcl complex. Eur. J. Biochem. 1991;197:555–561. doi: 10.1111/j.1432-1033.1991.tb15944.x. [DOI] [PubMed] [Google Scholar]
- [23].Link TA, von JG. Zinc ions inhibit the QP center of bovine heart mitochondrial bc1 complex by blocking a protonatable group. J. Biol. Chem. 1995;270:25001–25006. doi: 10.1074/jbc.270.42.25001. [DOI] [PubMed] [Google Scholar]
- [24].Halthout Y, Fabrisa D, Fenselau C. Stoichiometry in zinc ion transfer from metallothionein to zinc finger peptides. Intern. J. Mass Spectrom. 2001;204:1–6. [Google Scholar]
- [25].Brierely GP, Knight VA. Ion transport by heart mitochondria. X. The uptake and release of Zn2+ and its relation to the energy-linked accumulation of magnesium. Biochemistr. 1967;6:3892–3901. doi: 10.1021/bi00864a035. [DOI] [PubMed] [Google Scholar]
- [26].Saris N-E, Niva K. Is Zn2+ transported by the mitochondrial calcium uniporter? FEBS Lett. 1994;356:195–198. doi: 10.1016/0014-5793(94)01256-3. [DOI] [PubMed] [Google Scholar]
- [27].Feng W, Cai J, Pierce WM, Franklin RB, Maret W, Benz FW, Kang YJ. Metallothionein transfers zinc to mitochondrial aconitase through a direct interaction in mouse hearts. Biochem. Biophys. Res. Comm. 2005;332:853–858. doi: 10.1016/j.bbrc.2005.04.170. [DOI] [PubMed] [Google Scholar]
- [28].Costello LC, Liu Y, Zou J, Franklin RB. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J. Biol. Chem. 1999;274:17499–17504. doi: 10.1074/jbc.274.25.17499. [DOI] [PubMed] [Google Scholar]
- [29].Franklin RB, Ma J, Zou J, Guan Z, Kukoyi BI, Feng P, Costello LC. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J. Inorg. Biochem. 2003;96:435–442. doi: 10.1016/s0162-0134(03)00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Bagasra O, Costello LC. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer. 2005:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol. Cancer. 2007;6:37. doi: 10.1186/1476-4598-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Golovine K, Makhov P, Uzzo RG, Shaw T, Kunkle D, Kolenko VM. Overexpression of the zinc uptake transporter hZIP1 inhibits nuclear factor-kappaB and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin. Cancer Res. 2008;14:5376–5384. doi: 10.1158/1078-0432.CCR-08-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Makhov P, Golovine K, Uzzo RG, Wuestefeld T, Scoll BJ, Kolenko VM. Transcriptional regulation of the major zinc uptake protein hZip1 in prostate cancer cells. Gene. 2009;431:39–46. doi: 10.1016/j.gene.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52:316–321. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- [35].Grayhack JT. Effect of testosterone-estradiol administration on citric acid and fructose content of the rat prostate. Endocrinology. 1965;77:1068–1074. doi: 10.1210/endo-77-6-1068. [DOI] [PubMed] [Google Scholar]
- [36].Farnsworth WE. Testosterone stimulation of citric acid synthesis in the rat prostate. Biochim. Biophys. Acta. 1966;117:247–254. doi: 10.1016/0304-4165(66)90172-3. [DOI] [PubMed] [Google Scholar]
- [37].Grayhack JT, Lebowitz A. Effect of prolactin on citric acid of lateral lobe of prostate of Sprague-Dawley rats. Investig. Urol. 1967;5:87–94. [Google Scholar]
- [38].Costello LC, Franklin RB. Testosterone and prolactin regulation of metabolic genes and citrate metabolism of prostate epithelial cells. Horm. Metabol. Res. 2002;34:417–424. doi: 10.1055/s-2002-33598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Costello LC, Franklin RB. Integration of molecular genetics and proteomics with cell metabolism: how to proceed; how not to proceed! Gene. 2011;486:88–93. doi: 10.1016/j.gene.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mawson CA, Fischer MI. The occurrence of zinc in the human prostate gland. Can. J. Med. Sci. 1952;30:336–339. doi: 10.1139/cjms52-043. [DOI] [PubMed] [Google Scholar]
- [41].Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7:111–117. doi: 10.1038/sj.pcan.4500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Costello LC, Franklin RB. Cytotoxic/tumor suppressor role of zinc for the treatment of cancer: an enigma and an opportunity. Exp. Rev. Anticancer Ther. 2012;12:121–128. doi: 10.1586/era.11.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Györkey F, Min KW, Huff JA, Györkey P. Zinc and magnesium in human prostate gland: normal, hyperplastic, and neoplastic. Cancer Res. 1967;27:1348–1353. [PubMed] [Google Scholar]
- [44].Rishi I, Baidouri H, Abbasi JA, Pestaner JP, Skacel M, Tubbs R, Bagasra O. Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl. Immunohistochem. Mol. Morphol. 2003;11:253–260. doi: 10.1097/00129039-200309000-00009. [DOI] [PubMed] [Google Scholar]
- [45].Costello LC, Franklin RB. The genetic/metabolic transformation concept of carcinogenesis. Cancer Metastasis Rev. 2012;31:123–130. doi: 10.1007/s10555-011-9334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Milon BC, Agyapong A, Bautista R, Costello LC, Franklin RB. Ras responsive element binding protein-1 (RREB-1) down-regulates hZIP1 expression in prostate cancer cells. Prostate. 2010;70:288–296. doi: 10.1002/pros.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zou J, Milon BC, Desouki MM, Costello LC, Franklin RB. hZIP1 zinc transporter down-regulation in prostate cancer involves the overexpression of ras responsive element binding protein-1 (RREB-1) Prostate. 2011;71:1518–1524. doi: 10.1002/pros.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Oxford G, Smith SC, Hampton G, Theodorescu D. Expression profiling of Ral-depleted bladder cancer cells identifies RREB-1 as a novel transcriptional Ral effector. Oncogene. 2007;26:7143–7152. doi: 10.1038/sj.onc.1210521. [DOI] [PubMed] [Google Scholar]
- [49].Thiagalingam A, Borges M, Jasti R, Compton D, Diamond L, Mabry M, Ball DW, Baylin SB, Nelkin BD. RREB-1, a novel zinc finger protein, is involved in the differentiation response to Ras in human medullary thyroid carcinomas. Mol. Cell. Biol. 1996;16:5335–5345. doi: 10.1128/mcb.16.10.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang S, Qian X, Redman C, Bliskovski V, Ramsay ES, Lowy DR, Mock BA. p16 INK4a gene promoter variation and differential binding of a repressor, the ras-responsive zinc-finger transcription factor, RREB. Oncogene. 2003;22:2285–2295. doi: 10.1038/sj.onc.1206257. [DOI] [PubMed] [Google Scholar]
- [51].Liu H, Hew HC, Lu ZG, Yamaguchi T, Miki Y, Yoshida K. DNA damage signalling recruits RREB-1 to the p53 tumour suppressor promoter. Biochem. J. 2009;422:543–551. doi: 10.1042/BJ20090342. [DOI] [PubMed] [Google Scholar]
- [52].Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol. Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Franklin RB, Costello LC. The important role of the apoptotic effects of zinc in the development of cancers. J. Cell. Biochem. 2009;106:750–757. doi: 10.1002/jcb.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch. Biochem. Biophys. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front. Biosci. 2005;10:2230–2239. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- [57].Costello LC, Franklin RB. Aconitase activity, citrate oxidation, and zinc inhibition in rat ventral prostate. Enzyme. 1981;26:281–287. doi: 10.1159/000459195. [DOI] [PubMed] [Google Scholar]
- [58].Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–207. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Effect of zinc on prostatic tumorgenicity in nude mice. Ann. N. Y. Acad. Sci. 2003;1010:316–320. doi: 10.1196/annals.1299.056. [DOI] [PubMed] [Google Scholar]
- [60].Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52:311–318. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Feng P, Li T, Guan Z, Franklin RB, Costello LC. The involvement of Bax in zinc-Induced mitochondrial apoptogenesis in malignant prostate cells. Mol. Cancer. 2008;7:25. doi: 10.1186/1476-4598-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Costello LC, Franklin RB, Zou J, Naslund MJ. Evidence that human prostate cancer is a ZIP1-deficient malignancy that could be effectively treated with a zinc Ionophore (Clioquinol) approach. Chemother. (Los Angel ) 2015;4:2. doi: 10.4172/2167-7700.1000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Franklin Renty B., Zou Jing, Zheng Yao, Naslund Michael J., Costello Leslie C. Zinc ionophore (clioquinol) inhibition of human ZIP1-deficient prostate tumor growth in the mouse ectopic xenograft model; A zinc approach for the efficacious treatment of prostate cancer. Int. J. Cancer Clin. Res. 2016;3:037. doi: 10.23937/2378-3419/3/1/1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE. Anticancer activity of the antibiotic clioquinol. Cancer Res. 2005;65:3389–3395. doi: 10.1158/0008-5472.CAN-04-3577. [DOI] [PubMed] [Google Scholar]
- [65].Ding WQ, Yu HJ, Lind SE. Zinc-binding compounds induce cancer cell death via distinct modes of action. Cancer Lett. 2008;271:251–259. doi: 10.1016/j.canlet.2008.06.011. [DOI] [PubMed] [Google Scholar]
- [66].Ding WQ, Lind SE. Metal ionophores e an emerging class of anticancer drugs. IUBMB Life. 2009;61:1013–1018. doi: 10.1002/iub.253. [DOI] [PubMed] [Google Scholar]
- [67].Mao X, Schimmer AD. The toxicology of Clioquinol. Toxicol. Lett. 2008;182:1–6. doi: 10.1016/j.toxlet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- [68].Regland B, Lehmann W, Abedini I, Blennow K, Jonsson M, Karlsson I, Sjogren M, Wallin A, Xilinas M, Gottfries CG. Treatment of Alzheimer's disease with clioquinol. Dement. Geriatr. Cogn. Disord. 2001;12:408–414. doi: 10.1159/000051288. [DOI] [PubMed] [Google Scholar]
- [69].Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch. Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- [70].Cortesi M, Fridman E, Volkov A, Shilstein SS, Chechik R, Breskin A, Vartsky D, Kleinman N, Kogan G, Moriel E, Gladysh V, Huszar M, Ramon J, Raviv G. Clinical assessment of the cancer diagnostic value of prostatic zinc: a comprehensive needle-biopsy study. Prostate. 2008;68:994–1006. doi: 10.1002/pros.20766. [DOI] [PubMed] [Google Scholar]
- [71].Horn JJ, Coakley FV, Simko JP, Lu Y, Qayyum A, et al. High-grade prostatic intraepithelial neoplasia in patients with prostate cancer: MR and MR spectroscopic imaging featureseinitial experience. Radiology. 2007;242:483–489. doi: 10.1148/radiol.2422051828. [DOI] [PubMed] [Google Scholar]
- [72].Heijmink SW, Scheenen TW, Futterer JJ, Klomp DW, Heesakkers RA, Hulsbergen-van de Kaa CA, van Lin EN, Heerschap A, Barentsz JO. Prostate and lymph node proton magnetic resonance (MR) spectroscopic imaging with external array coils at 3 T to detect recurrent prostate cancer after radiation therapy. Investig. Radiol. 2007;42:420–427. doi: 10.1097/01.rli.0000262759.46364.50. [DOI] [PubMed] [Google Scholar]
- [73].Kim YR, Kim IJ, Kang TW, Choi C, Kim KK, et al. HOXB13 downregulates intracellular zinc and increases NF-κB signaling to promote prostate cancer metastasis. Oncogene. 2014;33:4558–4567. doi: 10.1038/onc.2013.404. [DOI] [PubMed] [Google Scholar]
- [74].Costello LC, Franklin RB, Zou J, Feng P, Bok R, Mark GS, Kurhanewicz J. Human prostaxte cancer ZIP1/zinc/citrate genetic/metabolic relationship in the TRAMP prostate cancer animal model. Cancer Biol. Ther. 2011;12:1078–1084. doi: 10.4161/cbt.12.12.18367. [DOI] [PMC free article] [PubMed] [Google Scholar]