Abstract
Objective
B cell depletion therapy is widely used for treatment of cancers and autoimmune diseases. B cells are abundant in abdominal aortic aneurysms (AAA), however, it is unknown whether B cell depletion therapy affects AAA growth. Using experimental models of murine AAA, we aim to examine the effect of B cell depletion on AAA formation.
Approach and Results
Wild-type or Apolipoprotein E knockout mice were treated with mouse monoclonal anti-CD20 or control antibodies and subjected to an elastase perfusion or angiotensin II-infusion model to induce AAA, respectively. Anti-CD20 antibody treatment significantly depleted B1 and B2 cells, and strikingly suppressed AAA growth in both models. B cell depletion resulted in lower circulating IgM levels, but did not affect the levels of IgG or cytokine/chemokine levels. Although the total number of leukocyte remained unchanged in elastase perfused aortas following anti-CD20 antibody treatment, the number of B cell subtypes was significantly lower. Interestingly, plasmacytoid dendritic cells (pDCs) expressing the immunomodulatory enzyme indole 2,3-dioxygenase (IDO) were detected in the aortas of B cell depleted mice. In accordance with an increase in IDO+ pDCs, the number of regulatory T cells was higher while the expression of pro-inflammatory genes was lower in aortas of B cell depleted mice. In a coculture model, presence of B cells significantly lowered the number of IDO+ pDCs without affecting total pDC number.
Conclusions
The present results demonstrate that B cell depletion protects mice from experimental AAA formation and promotes emergence of an immunosuppressive environment in aorta.
Keywords: Abdominal aortic aneurysm, B1 and B2 cells, anti-CD20 treatment, regulatory T cell and plasmacytoid dendritic cells
Introduction
One of the most commonly clinically utilized B cell depletion drugs is Rituximab, a chimeric murine/human monoclonal IgG targeting cells expressing CD20. It was first approved for the treatment of non-Hodgkin’s lymphoma and has demonstrated efficacy in the treatment of multiple CD20 positive B cell malignancies including chronic lymphocytic leukemia. Following its approval for the treatment of rheumatoid arthritis, clinical use Rituximab has been increased1. Rituximab specifically depletes pre- and mature B cells but not the pro-B cells, plasma B cells or other cell immune types2.
Afflicting primarily elderly men and smokers, abdominal aortic aneurysms (AAA) carry significant morbidity and mortality remaining the 15th leading cause of death in the United States3. Approximately 200,000 patients are diagnosed each year with AAA and nearly 15,000 are in danger of rupture. Infiltration of immune cells has been strongly correlated with AAA formation and rupture4. Importantly, quantitative analysis of human AAA tissue demonstrated that 41% of infiltrating mononuclear cells are B cells5. We have previously reported infiltration of both B1 and B2 subtypes in murine experimental AAA6. The muMT mice, which are genetically deficient in B cells, develop AAA similar to the wild-type mice while adoptive transfer of B2 cells to muMT mice suppressed aneurysm formation6. B cells have also been reported to promote mobilization of monocytes from spleen to the AAA in response to Angiotensin II (AngII) in apolipoprotein E–knockout (ApoE KO) mice7. However, it is still unknown if B cell depletion is protective or deleterious for experimental murine AAA.
Based on our results obtained from muMT mice, we hypothesized that B cell depleted mice were prone to AAA formation. To test the hypothesis, we used anti-CD20 antibody-mediated B cell depletion strategy in both elastase perfusion and AngII-infusion models of mouse AAA. We used flow cytometry to quantify B cell depletion and characterize of immune cells in AAA, spleen, blood, thymus and bone marrow; multiplex assay to quantify circulating cytokines/chemokines, and real time RT-PCR to quantify inflammatory gene expression in aorta.
Materials and Methods
The detailed Materials and Methods section is available in the online Supplemental Material.
Results
Murine anti-CD20 antibody significantly depletes both B1 and B2 cells, and protects mice from AAA formation
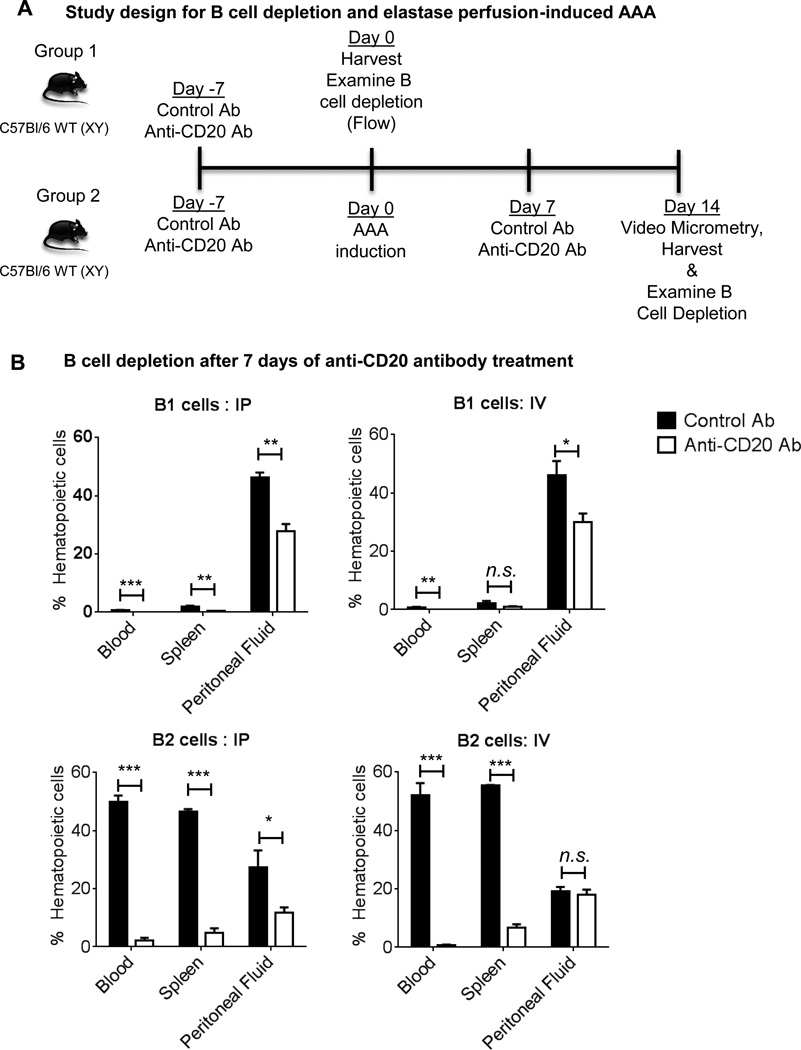
To examine effectiveness of the B cell depletion antibody, wild-type (WT) mice were injected intraperitoneally (IP) or intravenously (IV) with anti-CD20 or control antibodies, and following 7 days, B1 and B2 cells were examined from blood, spleen and peritoneal fluid using flow cytometry (Figure 1A). B1 cells were identified as CD19hiB220lo and B2 cells as CD19loB220hi6, 8. B1 cells are known to be predominantly located in the peritoneal cavity in mice9, 10 and our results confirmed these findings (Figure 1B). Notably, both IP and IV injection of anti-CD20 antibody resulted in a significant decrease in B1 cells. B2 cells were more prominent in spleen and blood and 85–95% of B2 cells were depleted at day 7 following both IP and IV treatments (Figure 1B). However, IP treatment depleted B2 cells in peritoneal fluid more efficiently compared to IV treatment. Altogether, these results demonstrate anti-CD20 treatment significantly depleted B cells in WT mice following 7 days of treatment.
Figure 1. Both IP and IV treatments of anti-CD20 antibody are effective in depleting B cells.
A, Schematic representation of study design for B cell depletion and induction of experimental elastase perfusion model of murine AAA. B, The proportion of B1 and B2 cells are expressed as percentage of CD45+ hematopoietic cells in WT mice at 7 days following administration of control or anti-CD20 antibody via IP or IV. Following one-way ANOVA, parametric unpaired t-test was applied to determine significant differences between the groups. Values are expressed as means ± SEM (n=3) and “*”, “**” and “***” indicate p<0.05, 0.01 and 0.001, respectively. ‘n.s.’ represents not significantly different (p>0.05).
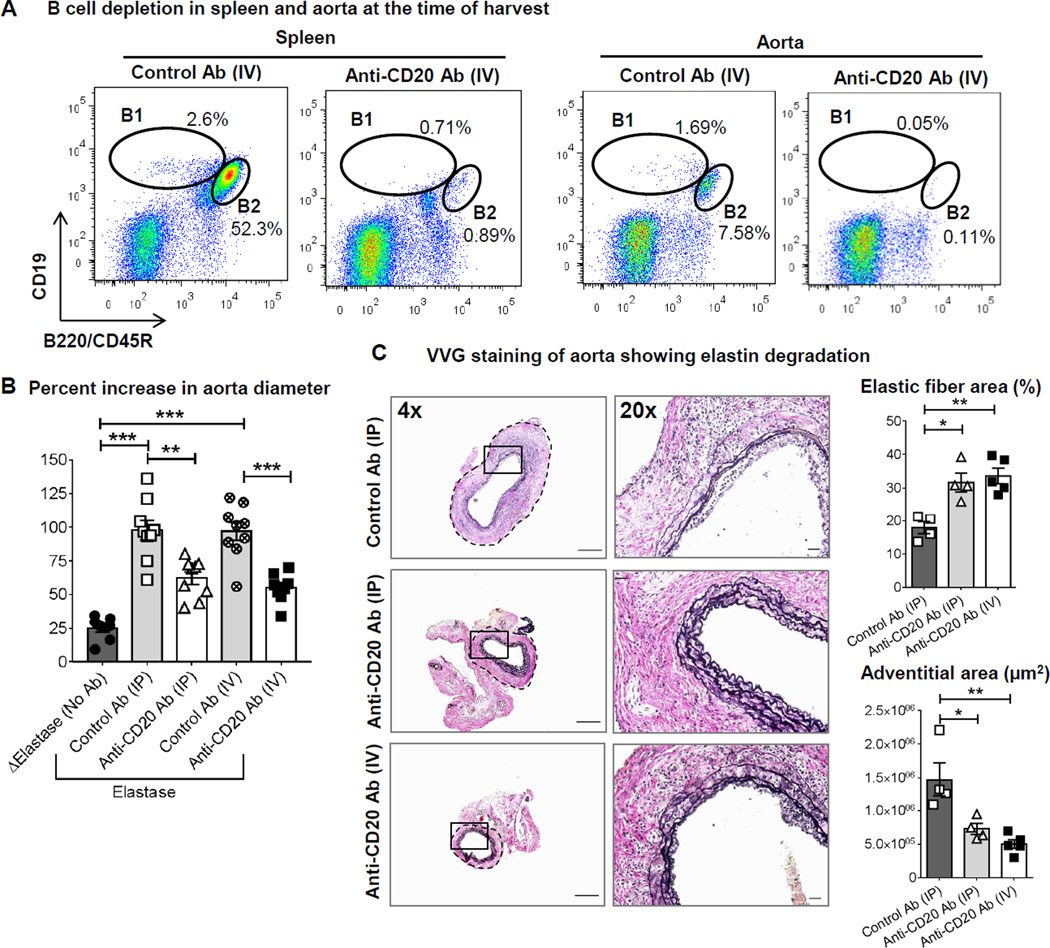
To examine if B cell depletion affects AAA formation, an elastase perfusion model of AAA was first used. A single dose of anti-CD20 treatment maintains depletion of B cells for 3 to 8 weeks11, 12. However, to prevent reappearance of B cell subtypes, we followed previously published anti-CD20-mediated B cell depletion strategy in mice13 in which, the WT mice were given two doses of anti-CD20 or control antibodies, the first on day 7 before elastase perfusion and the second on day 7 following elastase perfusion of abdominal aorta (Figure 1A). As a negative control, abdominal aortas were perfused with heat inactivated elastase. At day 14 following elastase perfusion, mice were anesthetized, aortic diameters were measured, and the perfused aorta, blood, peritoneal fluid, bone marrow, thymus and spleen were collected for analysis of B cell depletion. Compared to single dosing, two doses anti-CD20 treatment (IP or IV) depleted >95% of both B1 and B2 cells in various tissues including spleen and the elastase perfused aortas (Figure 2A and Supplemental Figure I). This method also depleted B1a, B1b and B2 cells in peritoneal fluid as determined by two methods of B cell characterization (Supplemental Figure II). Only a midCD19+B220+ population was preserved in bone marrow of anti-CD20 treated animals representing previously described pro-B or long-lived plasma cells that do not express CD202 (Supplemental figure I). Aortic perfusion with active elastase induced a significant increase in aortic diameter i.e. AAA formation compared to perfusion of heat inactivated elastase (Figure 2B). AAA formation was similar in control antibody treated IP and IV groups. In contrast to our hypothesis, B cell depletion strikingly suppressed AAA formation (IP: control vs anti-CD20, 97.9±7.4 vs 62.2±4.7%, p<0.01; IV: control vs anti-CD20, 97±6.6 vs 55±3.8%, p<0.05; n=8–9) (Figure 2B). In accordance with protection from AAA, elastase perfused aortas of both IP and IV anti-CD20 antibody treated groups displayed marked preservation of elastin layers and smaller adventitial area (Figure 2C). Altogether, these results suggest that anti-CD20 antibody treatment significantly depleted B cells in various tissues independent of delivery method of anti-CD20 antibody and protected mice from AAA formation.
Figure 2. Both IP and IV treatments of anti-CD20 antibody suppress elastase perfusion-induced AAA formation with marked depletion of B cells and preservation of elastin layers.
A, Representative Flow cytometry plots showing depletion of B cells in spleen and aorta of WT mice administered two doses of control or anti-CD20 antibody via IV. The plots have been gated for singlets, live CD45+ cells. B, Increase in aorta diameter in WT mice treated with two doses of control or anti-CD20 antibody via IP or IV. ‘Δ Elastase’ and ‘Elastase’ indicate aortic perfusion with heat-inactivated elastase and elastase, respectively (n=8–9). Following one-way ANOVA, parametric unpaired t-test was applied to determine significant differences between the groups. C, Representative images showing AAA sections stained for Verhoeff-Van Gieson or VVG (elastic fibers, black). A small segment of images acquired in 4× is shown in 20× magnification. Scale bar in 4× images is 500 µm and in 20× images is 50 µm. Elastic fiber area (presented as percentage of total aortic area) and adventitial area were determined using ImageJ. Following one-way ANOVA, non-parametric t-test (Mann-Whitney test) was applied to determine significant differences between the groups. Values are expressed as means ± SEM, “*”, “**” and “***” indicate p<0.05, 0.01 and 0.001 respectively.
To confirm this protective effect of B cell depletion in another model, we utilized an AngII infusion model of murine AAA14 in a small group of male ApoE KO mice (n=5–6). The mice were treated with anti-CD20 or control antibodies 7 days before the AngII infusion (at 1,000 ng/kg/min) via subcutaneous osmotic pumps (Supplemental figure III A). Repeat doses of antibodies were given on day 7 and 21 following pump implantation. As a negative control, a group of ApoE KO mice were infused with saline via osmotic pumps and were given control antibodies. The mice were sacrificed on day 28 following AngII infusion and aortas were harvested. Stages of aneurysm were determined as described by Daugherty et al.14. No aortic aneurysm pathology was observed in any saline infused mice (Supplemental figure III 3B and C). AngII infusion in control antibody treated mice resulted in mixed aortic aneurysm such as no aneurysm (n=1), thoracic aortic aneurysm (n=1, stage I), abdominal aortic aneurysm (stage II, n=1; and stage III, n=2), and ruptured thoracic aorta (n=1, stage IV). However, anti-CD20 treated mice were completely protected (n=5) (Supplemental figure III 3B and C). Successful infusion of AngII was confirmed via elevated level of plasma aldosterone (Supplemental figure III 3D). Moreover, the anti-CD20 treated mice demonstrated marked preservation of elastin layers and smaller adventitial area in suprarenal aortas compared to AngII-infused control antibody treated mice (Supplemental figure III 3E). Collectively, our results demonstrate that B cell depletion protects mice from AAA formation via using two unique models of experimental murine AAA.
Circulating factors in the B cell depleted mice are not responsible for the protection from AAA
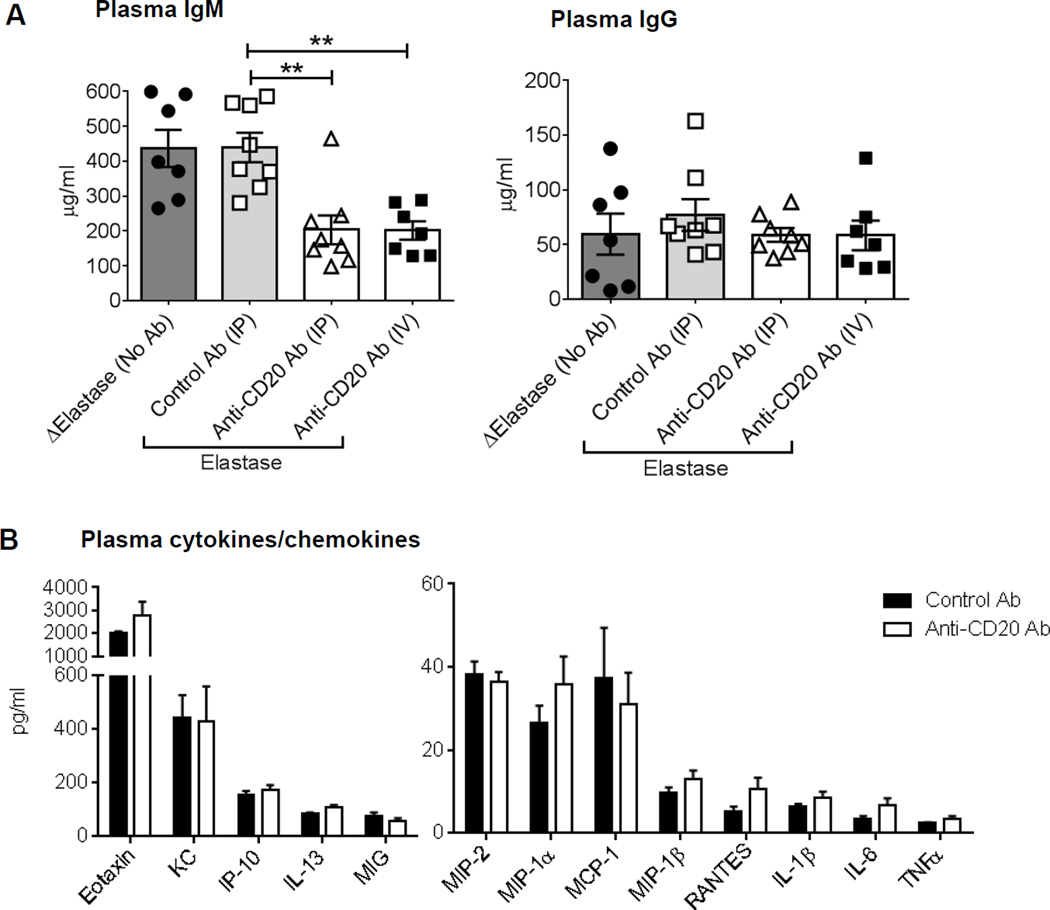
B cells regulate inflammation via secreting immunoglobulins (Igs), cytokines and chemokines or by directly contacting other immune cells. Importantly, IgM antibodies have been reported to be anti-inflammatory and protective in the settings of atherosclerosis9, whereas, B cell secreted IL-2, IL-4, TNFα, IL-6, IFNγ, IL-12 and TNFα regulates type 1 and type 2 immune responses15. We speculated that, B cell depletion would preserve the IgM level or increase the level of anti-inflammatory cytokines. However, in our study, IgM levels were significantly lower (p<0.01) while, as described before by DiLillo et al.16, IgG levels were similar in the B cell-depleted mice compared to the control mice (Figures 3A). Moreover, no significant differences were found in the plasma levels of pro- or anti-inflammatory factors such as IL-1β, IL-6, TNFα, RANTES, MIP-1α, MIP-1β, IP-10, IL-13, Eotaxin, KC, MIP-1, MIP-2 and MIG, whereas, the levels of IL-2, IL-4, IL-5, IL-10, IL-17, IL12-p70 and IFNγ were below the detection in the control and B cell depleted mice (Figures 3B). Altogether, these results suggest that changes in circulating levels of immunoglobulins, cytokines or chemokines are not the mechanism for attenuation of AAA in the B cell depleted mice.
Figure 3. Circulating factors in B cell depleted mice may not be responsible for protection against AAA.
A, Quantification of IgM and IgG levels in plasma collected from WT mice treated with control or anti-CD20 antibody (n=7–8). ‘Δ Elastase’ and ‘Elastase’ indicate aortic perfusion with heat-inactivated elastase and elastase, respectively. Following one-way ANOVA, non-parametric t-test (Mann-Whitney test) was applied to determine significant differences between the groups. B, Quantification of cytokines and chemokines in plasma of WT mice treated with control or anti-CD20 antibody via IV (n=7–8). Parametric unpaired t-test was applied to determine significant differences between the groups, except for MIP-1α (non-parametric Mann-Whitney test was applied) which was based on the D'Agostino-Pearson normality test. Values are expressed as means ± SEM, “**” indicates p<0.01.
Anti-CD20 antibody treatment does not abrogate aortic infiltration of leukocytes
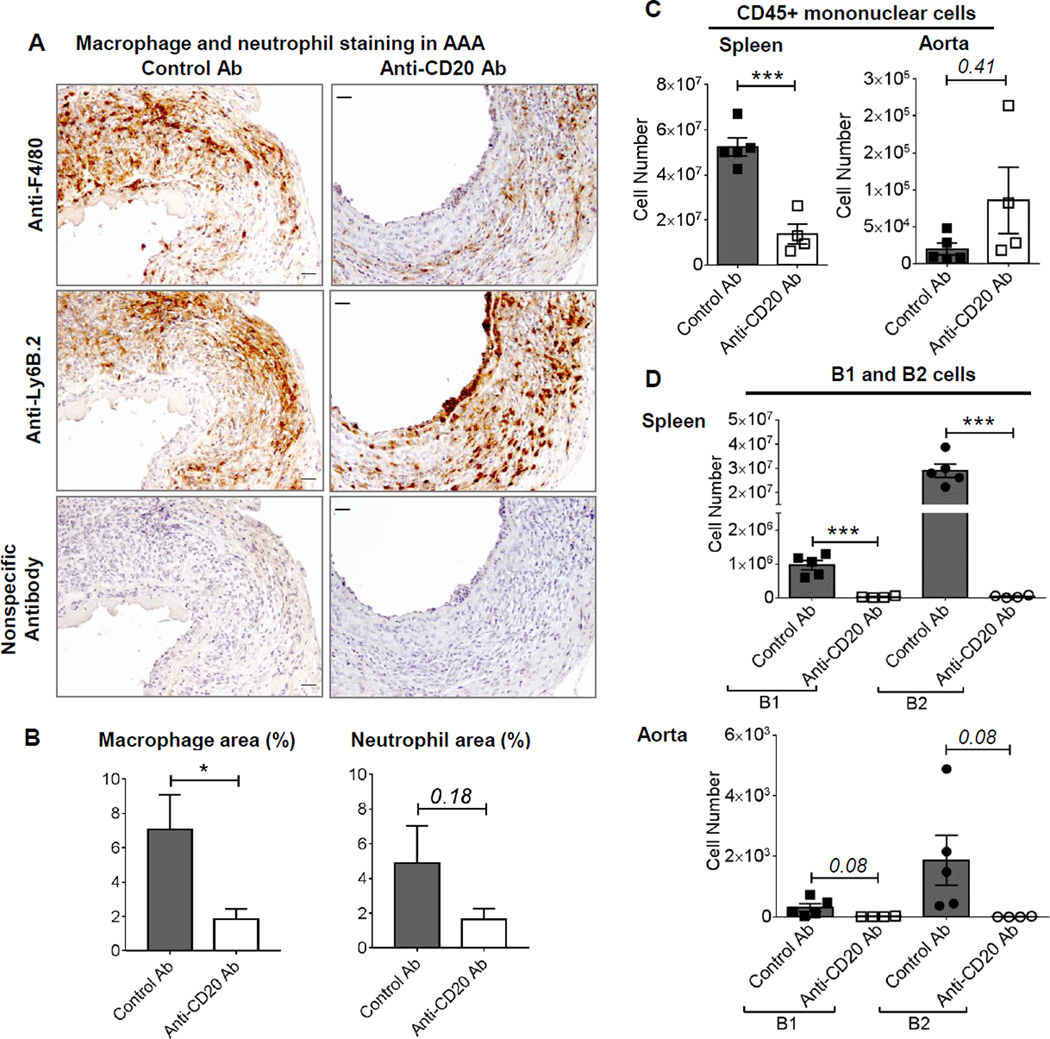
Aortic infiltration of immune cells has been strongly associated with AAA formation17; therefore, we examined the histopathology of aortic cross-sections. AAA from B cell depleted mice demonstrated infiltration of immune cells such as macrophages (F 4/80) and neutrophils (Ly6B.2) (Figure 4A). Quantification of immunostaining revealed significantly less macrophage content and a trend towards lower neutrophil content in the aortas of B cell depleted mice compared to the control antibody treated mice (Figure 4B). Next, we utilized flow cytometry to define and quantify the immune cells in AAA tissues. For cell counting, specifically the elastase perfused portion of abdominal aorta was harvested (Supplemental figure IV A), perfused with heparin-PBS solution ex vivo. Each isolated aorta was digested separately with an enzymatic cocktail, stained with fluorescent-conjugated antibodies and counting beads were added before running on a flow cytometer. As a control, the number of immune cells in spleen was counted. The gating strategy used for phenotyping immune cells is shown in Supplemental Figure IV B. The results revealed that the number of mononuclear leukocytes (CD45+ cells) was significantly lower in the spleen of anti-CD20 treated mice, however, no significant difference was observed in the aortas (Figure 4C). Furthermore, the spleen and aorta from control antibody-treated mice demonstrated the presence of B1 and B2 cells, as well as IgM+IgD+ B1 and B2 cells, the numbers of which were significantly lower or absent in anti-CD20 treated mice (Figure 4D, Supplemental Figure V). These results together suggest that immune cells accumulating in aorta may be creating an immunosuppressive microenvironment protecting the B cell depleted mice from AAA.
Figure 4. Infiltration of immune cells in the aortas of control and anti-CD20 antibody treated mice.
A, Representative images showing AAA sections stained for F 4/80 (macrophages, brown), Ly-6B.2 (neutrophils, brown) and nuclei (hematoxylin), scale bar 50 µm. B, The immunostained area in A is determined using ImageJ and expressed as percentage of total aortic area. C, D and E, Flow cytometry quantification of CD45+ immune cells, and B1 and B2 cells in spleen and AAA of control or anti-CD20 antibody treated WT mice via IV, (n=4–5). Non-parametric t-test (Mann-Whitney test) was applied to determine significant differences between the groups. Values are expressed as means ± SEM, “*” and “***” indicate p<0.05 and 0.001, respectively. ‘p’ values >0.05 are indicated on the top of the bars.
B cell depletion increases aortic infiltration of immunosuppressive cells
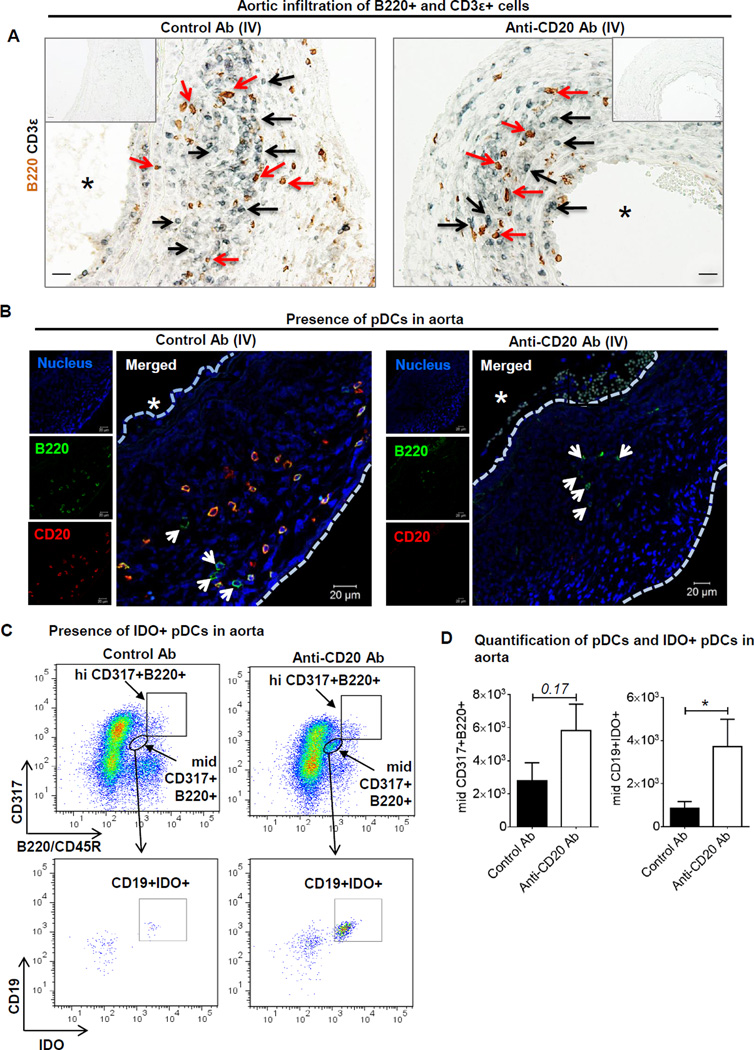
Since the number of infiltrated leukocytes in the aorta was not affected following B cell depletion and B cell depleted mice are protected from AAA formation, we speculated that the absence of B cells created an immunosuppressive environment in aorta. To investigate the presence of immunosuppressive cells, we first stained aortic cross-sections from control and anti-CD20 antibody treated mice for the presence of T and B cells using anti-CD3ε and anti-B220 antibodies, respectively (Figure 5A). CD3ε+ and B220+ cells stained in proximity to each other in AAAs from control antibody treated mice, which was in accordance with our previously published literature6. CD3ε+ and, unexpectedly, B220+ cells were found in the AAAs of B cell depleted mice (Figure 5A). To confirm if these B220+ cells were B cells, the aortic cross-sections were stained with anti-B220 and anti-CD20 antibodies. Interestingly, two types of B220+ cells i.e. B220+CD20+ and B220+CD20− were identified in AAA of control antibody treated mice, whereas, only B220+CD20− cells, though lesser in number, were present in the aorta of anti-CD20 antibody treated mice (Figure 5B). To further characterize and quantify these B220+CD20− cells, we performed flow cytometry. The number of B220+ cells was significantly lower in the aortas of anti-CD20 treated mice compared to control antibody treated mice (not shown). Furthermore, these B220+ cells in B cell depleted mice were identified as plasmacytoid dendritic cells (pDCs) expressing both CD317/PDCA-1 (Plasmacytoid Dendritic Cell Antigen-1) and B220. The pDCs were populated as hiCD317+B220+ and midCD317+B220+ in aorta (Figure 5C) and also in blood (Supplemental Figure VI A) and spleen (not shown). In blood, the midCD317+B220+ population was a well-defined, however, in aorta, a large portion of the cell population (40.2±1.6% of mononuclear live CD45+CD4− cells, n=7) was CD317+ irrespective of control or anti-CD20 antibody treatment. A small population of pDC is known to express surface CD19 and intracellular immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) and plays a strong immunosuppressive role18–20. Therefore, we included CD19 and IDO antibodies in our flow cytometry antibody panel. Specifically, in the entire population of CD45+CD317+B220+ cells, only the mid population was CD19+ positive and strongly expressed IDO. CD19+IDO+ cells were undetected in hiCD317+B220+ and the number of midCD19+IDO+ cells was significantly higher in AAA (Figure 5D) and blood (Supplemental Figure VI B) of anti-CD20 antibody treated mice. These results suggest that B cell depletion increased aortic content of IDO expressing pDCs.
Figure 5. pDCs are present in mouse AAAs.
A, Representative images acquired on a light microscope showing AAA sections stained for B220+ (brown) cells and CD3ε+ (black) cells, scale bar 10 µm. Black arrows indicate CD3+ cells and red arrows B220+ cells. ‘*’ indicates lumen. B, Immunofluorescent images acquired on a confocal microscope showing colocalized B220 (green) and CD20 (red) staining indicating B cells, and only B220 staining indicating dendritic cells (indicated by arrows) in AAA of control or anti-CD20 antibody treated WT mice. ‘*’ indicates lumen and ‘----’ indicates luminal and exterior margins of aorta. C, Representative Flow cytometry plots showing gating and increase in concentration of mid B220+CD317+CD19+IDO in AAA following control or anti-CD20 antibody via IV. The cells have been gated for singlets, live, CD45+ and CD4× mononuclear cells. D, Quantification of total pDCs and IDO+ pDCs in aorta (n=3–5). Non-parametric t-test (Mann-Whitney test) was applied to determine significant differences between the groups. Values are expressed as means ± SEM, “*” indicates p<0.05. ‘p’ values >0.05 are indicated on the top of the bars.
Since the number of circulating IDO+ pDC was increased, we hypothesized that Tryptophan catabolism would be increased systemically, leading to global immunosuppression following B cell depletion. However, we did not find significant differences in the level of Tryptophan and its metabolite Kynurenine, or in the Kynurenin-Tryptophan ratio in circulation (Supplemental Figure VI C) suggesting a recessive role of IDO expressing pDCs in circulation.
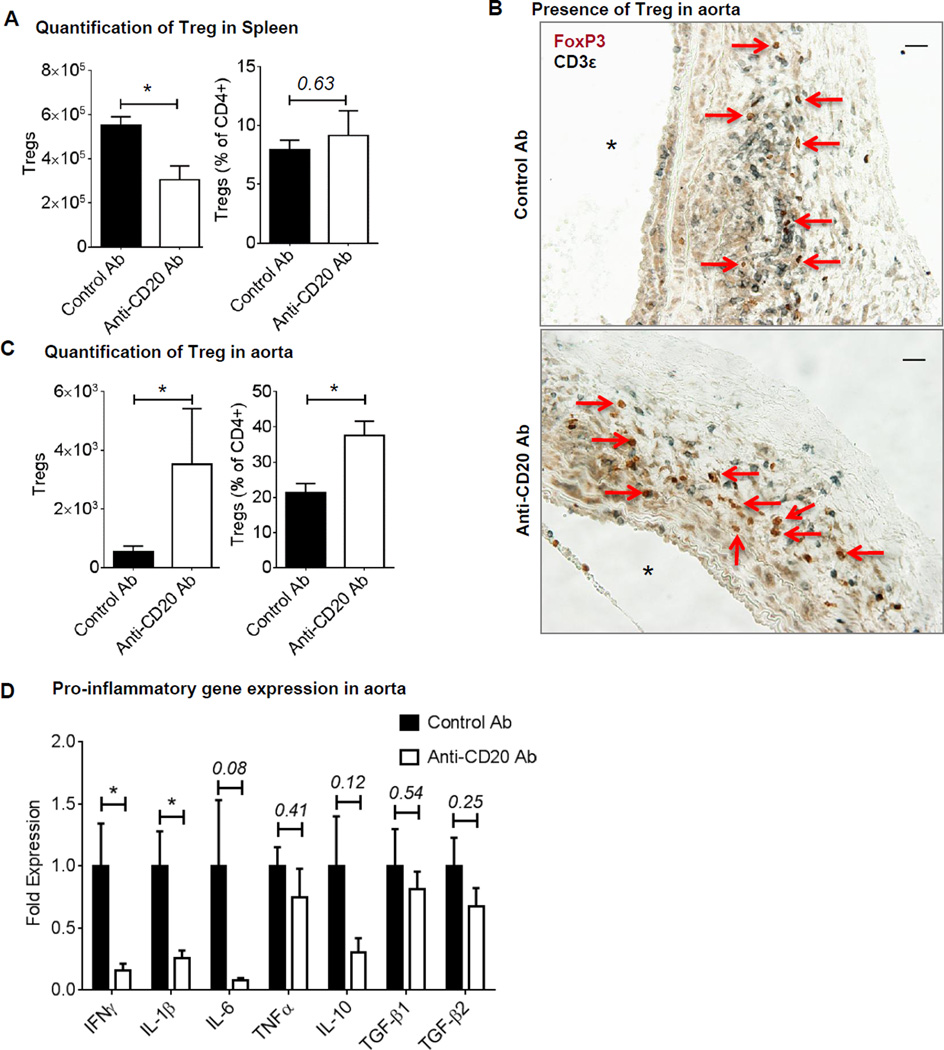
IDO expressing pDCs maintain the immunosuppressive environment by promoting Treg generation in tissues21. Therefore, we quantified regulatory T cells (Tregs) in aorta and spleen using flow cytometry. As reported before22, absolute Treg number, but not the proportion of Treg (expressed as percentage of CD4+ T cells) was significantly lower in spleen following anti-CD20-mediated B cell depletion (Figure 6A). Staining of aortic cross-sections with CD3ε and FoxP3 antibodies demonstrated presence of Tregs in aortas of control antibody and anti-CD20 antibody treated mice (Figure 6B). Furthermore, flow cytometry revealed significantly higher Treg number and percentage (% of CD4+ T cells) in the aortas of anti-CD20 treated mice (Figure 6C). Altogether, these results suggest that B cell depletion created an immunosuppressive microenvironment in aorta.
Figure 6. Increase in aortic Treg content and decrease in pro-inflammatory gene expression following B cell depletion.
Treg number and percentage in the spleen (A) and in AAA (C) of WT mice treated with control or anti-CD20 antibodies via IV, n= 3–5. Non-parametric t-test (Mann-Whitney test) was applied to determine significant differences between the groups. B, Representative images showing AAA sections stained for FoxP3 (brown) and CD3ε (black). Arrows indicate Tregs, ‘*’ indicates lumen and scale bar, 50 µm. D, Expression of pro-inflammatory genes in the AAA of WT treated with control or anti-CD20 antibody via IV, n= 6. Parametric unpaired t-test was applied to determine significant differences between the groups for individual genes. Values are expressed as means ± SEM and “*” indicates p<0.05. ‘p’ values >0.05 are indicated.
Next, to confirm presence of an immunosuppressive environment, we examined local inflammatory gene expression in aortas using real time RT-PCR. The results demonstrated significant decrease in the expression of IFNγ, IL-1β and a trend in the reductions IL-6 and IL-10 in anti-CD20 treated mice, however, TGFβ expression was similar to the control antibody treated mice (Figure 6D) confirming emergence of an immunosuppressive environment in aorta following B cell depletion.
Presence of B cells lowers the number of IDO+ pDCs
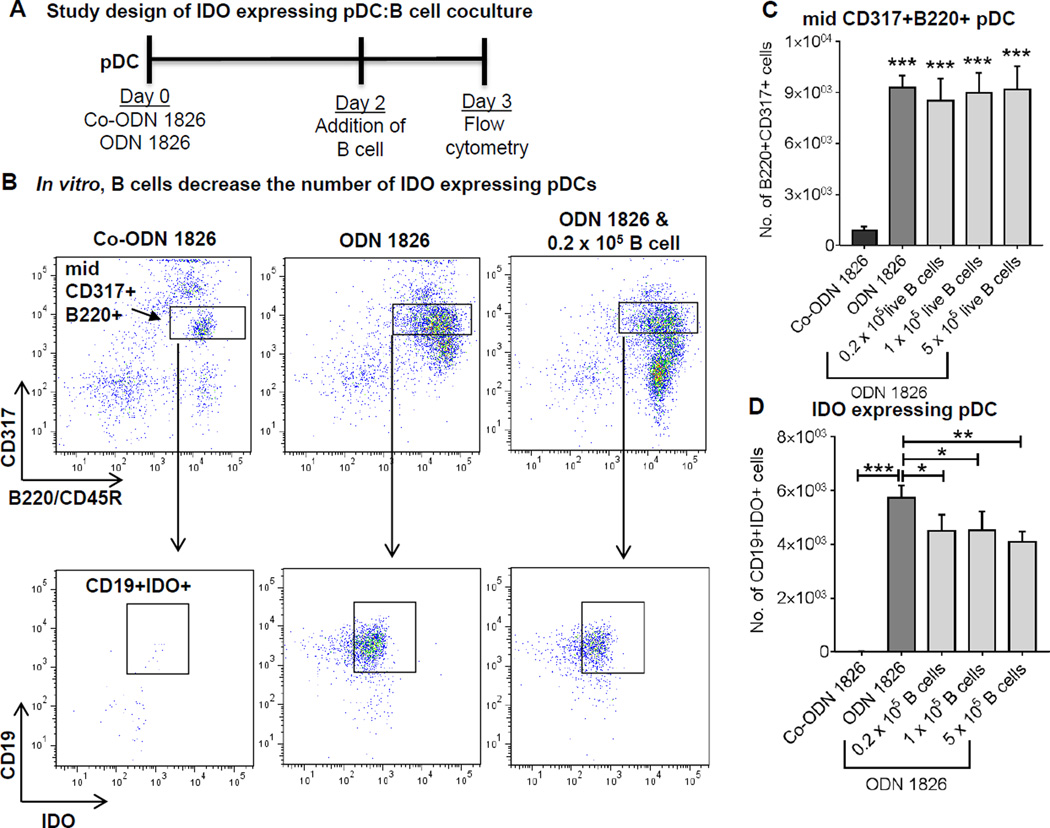
Chen et al.21 and Baban et al.23 have reported that the immunosuppressive IDO+ pDCs differentiate naive CD4 T cells to Tregs. However, it is unknown if the presence of B cells affect the number of IDO+ pDCs. Since the number of IDO+ pDC is increased in the B cell depleted mice, we hypothesized that B cells suppress IDO+ pDCs. To test this, we developed a coculture model. We used synthetic oligonucleotides containing unmethylated CpG oligodeoxynucleotide (ODN) motifs to induce IDO expression in pDCs21, 23. First, the pDC-enriched cell fraction was collected from the spleen of WT mice using MACS column and IDO expression was induced via treatment with class B CpG ODN1826 in a 96-well culture plate for 48 hours (detail experimental method is described online Supplemental Material). As a control, pDCs were treated with control ODN1826. The expression of pDC markers CD317 and B220 was found to be significantly increased in ODN1826 treated pDCs (Figure 7A, B and C). Again, only the midCD317+B220+ population was dominated with a high number of immunosuppressive CD19+IDO+ cells. Next, the entire B cell population was isolated (via MACS column) from WT spleen and various concentrations of B cells were cultured with IDO+ PDCs for 24 hours. Addition of increasing concentrations of B cells did not affect the number of pDCs, however, it significantly decreased the number of IDO+ pDC (Figure 7D). These results suggest that presence of B cells suppresses the emergence of immunosuppressive IDO+ pDCs.
Figure 7. Presence of B cells affects the number of IDO+ pDCs.
A, Schematic representation of study design of IDO expressing pDC:B cell coculture. pDCs isolated from spleen of WT mice were treated with 0.2 µM of ODN 1826 or control ODN (Co-ODN 1826) for 2 days. Subsequently, the culture medium was removed and medium containing indicated concentrations of isolated splenic B cells were added. The coculture was continued for 24 hours and expression of B220, CD317, CD19 and IDO was determined via flow cytometry. B, Representative flow plots showing mid CD317+B220+ cells, which are further gated from CD19+IDO+ population. C, Quantification of mid CD317+B220+ cells (significant difference between co-ODN and each of the ODN-treated group is shown) and D, quantification of CD19+IDO+ pDCs. Parametric unpaired t-test was applied to determine significant differences between the groups. Values are expressed as means ± SD, “*”, “**” and “***” indicate p<0.05, 0.01 and 0.001 respectively.
Discussion
Herein, we demonstrate that anti-CD20 treatment, whether injected by IP or IV, strongly suppressed AAA formation in wild-type mice. The rationale for selecting two methods of delivery is that, B1 cells are predominant in the peritoneal cavity, whereas B2 cells are in the spleen and blood of mouse. Patients, however, receive anti-CD20 treatment intravenously. We hypothesized that IV treatment would deplete more B2 cells in the spleen sparing the B1 cells in the peritoneal cavity, whereas, IP treatment would deplete more B1 cells in the peritoneal cavity sparing B2 cells in the spleen. Following 7 days of anti-CD20 treatment, a significant depletion of circulating and splenic B1 and B2 cells was achieved; however, peritoneal B1 and B2 cells were resistant to depletion. Hamaguchi et al. reported a similar finding using a different clone of monoclonal anti-CD20 antibody24. Interestingly, following two doses of anti-CD20 treatment, B1 and B2 cells were almost depleted in peritoneal cavity, spleen, blood and bone marrow. In the same line, IgM level was significantly lower in the B cell depleted mice, which is primarily produced by B1 cells. We did not find a decrease in IgG levels, potentially because long-lived plasma B cells, which do not express CD20, are preserved in bone marrow following B cell depletion and maintain circulating IgG levels16. In fact, our results demonstrated presence of a midCD19+B220+ population in bone marrow following two doses of anti-CD20 antibody treatment. Although, further flow cytometry experiments are required for confirmation, the midCD19+B220+ population are most likely long-lived plasma cells.
pDCs are known to recognize viral and bacterial derived products and induce synthesis of Type I interferon genes such as IFN-α and IFN-β, and promote activation of a pro-inflammatory response via activating effector T cell, cytotoxic T cells and natural killer cells25. On the contrary, tolerogenic pDCs express IDO and have potent immunomodulatory properties including induction of Treg differentiation from naïve CD4+ T cells23, 26, 27. Such tolerogenic pDCs appear to be representing a subset of pDCs and are tissue specific28, 29. Increased expression of IDO degrades tryptophan promoting immune tolerance or suppressing immune activation of neighboring cells30. Daissormont et al. have reported that pDCs are scarcely present in atherosclerosis, and blocking IDO activity in pDCs exacerbates atherosclerosis31. Our results provide the first evidence of emergence of IDO producing pDCs following depletion of B cell in aorta using anti-CD20 antibody. In the context of aortic aneurysms, Tregs have been shown to suppress experimental murine AAA32. Importantly, patients with AAAs do not have functional circulating Tregs33. In our study, depletion of B cells increased the number of Tregs and IDO expressing pDCs in aorta. In aortas of B cell depleted mice, the IDO expressing pDCs may create an immunosuppressive environment leading to a decrease pro-inflammatory gene expression. It is unknown if IDO expressing pDCs would differentiate naïve CD4 T cells to Treg or recruit Treg to the aorta following elastase perfusion. We found a significant increase in circulating IDO+ pDCs following B cell depletion; however, this was not correlated with an increase in Tryptophan degradation or increase in Kynurenine synthesis. Although other Tryptophan metabolites, such as anthranilic acid were not measured, it is likely that, the concentration of Tryptophan metabolites is higher in AAA tissues compared to the circulating level underscoring tissue specific role of tolerogenic pDCs. Moreover, possible role of IL-10 producing dendritic cell or T cell in the protection cannot be ruled out. On the other hand, our IDO+ pDC:B cell coculture demonstrated that presence of B cells significantly lowered the number of CD19+IDO+ pDCs. Our data is in consistent with a model that B cells control immunosuppressive effects of pDC by limiting IDO+ pDC population and regulate inflammation and AAA as shown the graphic abstract. In support of this model, recently, Yun et al. demonstrate that aortic pDCs expressing IDO protects against atherosclerosis via generation of Tregs34.
We have previously reported that the muMT mice, genetically deficient in B cells, develop AAA similar to the WT mice, and adoptive transfer of B2 cells suppresses AAA formation in muMT mice, suggesting AAA formation is dependent on B cell subsets. In the current study, we created B cell deficiency in mature WT mice by using anti-CD20 antibody which affected the homeostasis of T cells, primarily by reducing T cell number including Tregs in spleen as demonstrated by us and also reported previously by Lykken et al.22 Interestingly, IDO expressing pDC number was significantly increased in various tissues of B cell depleted mice. Moreover, B cell depletion has also been shown to improve endothelial function and reduce systemic inflammation in patients with rheumatoid arthritis35. Therefore, further studies are needed to understand the paradox immune response in muMT and anti-CD20 antibody-mediated B cell depleted mice.
In our study, the B cells were depleted prior to induction of AAA. It is unknown if B cell depletion would suppress established experimental AAA in mice. It is also unknown if AAA formation will still be suppressed following a long-term depletion of B cells. In support of our finding, Mellak et al. have reported that angiotensin II mobilizes monocytes from spleen to aorta in a B cell-dependent manner and promote AAA formation in the Apolipoprotein E KO mice7. As far as atherosclerosis is concerned, there are reports supporting34, 36 and contradicting37 protective role of IDO expressing pDCs. Therefore, generation of pDC-specific IDO knock-out mouse is required to confirm that IDO expressing pDCs protect B cell depleted mice from AAA.
In conclusion, using two experimental models of murine AAA, we demonstrated that anti-CD20 antibody-mediated B cell depletion increased the number of IDO expressing pDCs and created an immunosuppressive environment that attenuated inflammatory gene expression and suppressed AAA growth. While further mechanistic studies are needed, these findings have the potential to lead to the development of non-surgical strategies to prevent aneurysm formation. Further, this evidence suggests that patients undergoing B cell depletion therapy may be protective from AAA formation.
Supplementary Material
Highlights.
Both intraperitoneal and intravenous treatments of anti-CD20 antibody significantly depleted B cells and protected mice from AAA.
Despite significant depletion of aortic B cell content, anti-CD20 antibody treatment did not affect the number of aortic infiltrated immune cells.
A distinct population of indole 2,3-dioxygenase (IDO) expressing plasmacytoid dendritic cells (pDC) appeared in aorta following B cell depletion.
In vivo, infiltration of IDO+ pDCs was associated with increase in the number of regulatory T cells and decrease in the expression of pro-inflammatory genes in aorta suggesting emergence of an immunosuppressive environment following B cell depletion. In vitro, presence of B cells significantly lowered the number of IDO+ pDCs
B cell depletion therapy may be beneficial for AAA patients.
Acknowledgments
We acknowledge the assistance of Flow Cytometry Core Facility, Advanced Microscopy Facility, CVRC Histology Core and Research Histology Core at University of Virginia.
Sources of Funding
Grant number and support: This work was supported by National Scientist Development Grant 14SDG20380044 from the American Heart Association (to A.K.M.), K08HL098560 from the National Heart, Lung, and Blood Institute (to G. A.), NIH R01 DK096076 and a Grant in Aid from the American Heart Association (to N.L.). V.S. was supported by an AHA predoctoral grant.
Non standard abbreviations and acronyms
- AAA
Abdominal aortic aneurysm
- AngII
Angiotensin II
- pDC
Plasmacytoid dendritic cells
- IDO
Indole 2,3-dioxygenase
- Foxp3
Forkhead box P3
- Treg
Regulatory T cell
- VVG
Verhoeff-Van Geisen
Footnotes
Disclosures
None
References
- 1.Gurcan HM, Keskin DB, Stern JN, Nitzberg MA, Shekhani H, Ahmed AR. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol. 2009;9:10–25. doi: 10.1016/j.intimp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Uchida J, Lee Y, Hasegawa M, Liang Y, Bradney A, Oliver JA, Bowen K, Steeber DA, Haas KM, Poe JC, Tedder TF. Mouse CD20 expression and function. Int Immunol. 2004;16:119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 3.Bobadilla JL, Kent KC. Screening for abdominal aortic aneurysms. Adv Surg. 2012;46:101–109. doi: 10.1016/j.yasu.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Ailawadi G, Eliason JL, Upchurch GR., Jr Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003;38:584–588. doi: 10.1016/s0741-5214(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 5.Forester ND, Cruickshank SM, Scott DJ, Carding SR. Functional characterization of T cells in abdominal aortic aneurysms. Immunology. 2005;115:262–270. doi: 10.1111/j.1365-2567.2005.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meher AK, Johnston WF, Lu G, Pope NH, Bhamidipati CM, Harmon DB, Su G, Zhao Y, McNamara CA, Upchurch GR, Jr, Ailawadi G. B2 cells suppress experimental abdominal aortic aneurysms. Am J Pathol. 2014;184:3130–3141. doi: 10.1016/j.ajpath.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellak S, Ait-Oufella H, Esposito B, Loyer X, Poirier M, Tedder TF, Tedgui A, Mallat Z, Potteaux S. Angiotensin II mobilizes spleen monocytes to promote the development of abdominal aortic aneurysm in Apoe−/− mice. Arterioscler Thromb Vasc Biol. 2015;35:378–388. doi: 10.1161/ATVBAHA.114.304389. [DOI] [PubMed] [Google Scholar]
- 8.Thomas MD, Kremer CS, Ravichandran KS, Rajewsky K, Bender TP. c-Myb is critical for B cell development and maintenance of follicular B cells. Immunity. 2005;23:275–286. doi: 10.1016/j.immuni.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A, Toh BH. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109:830–840. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, Das D, McSkimming C, Taylor AM, Tsimikas S, Bender TP, Witztum JL, McNamara CA. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ Res. 2015;117:e28–e39. doi: 10.1161/CIRCRESAHA.117.306044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamel K, Doodes P, Cao Y, Wang Y, Martinson J, Dunn R, Kehry MR, Farkas B, Finnegan A. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol. 2008;180:4994–5003. doi: 10.4049/jimmunol.180.7.4994. [DOI] [PubMed] [Google Scholar]
- 12.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest. 2011;121:4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P, Bobik A, Toh BH. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185:4410–4419. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 14.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 17.Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, Pearce WH. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990;137:1199–1213. [PMC free article] [PubMed] [Google Scholar]
- 18.Baban B, Hansen AM, Chandler PR, Manlapat A, Bingaman A, Kahler DJ, Munn DH, Mellor AL. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int Immunol. 2005;17:909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- 19.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen TN, Alfaro J, Enriquez HL, Jiang C, Loo WM, Atencio S, Bupp MR, Mailloux CM, Metzger T, Flannery S, Rozzo SJ, Kotzin BL, Rosemblatt M, Bono MR, Erickson LD. Development of murine lupus involves the combined genetic contribution of the SLAM and FcgammaR intervals within the Nba2 autoimmune susceptibility locus. J Immunol. 2010;184:775–786. doi: 10.4049/jimmunol.0901322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lykken JM, DiLillo DJ, Weimer ET, Roser-Page S, Heise MT, Grayson JM, Weitzmann MN, Tedder TF. Acute and chronic B cell depletion disrupts CD4+ and CD8+ T cell homeostasis and expansion during acute viral infection in mice. J Immunol. 2014;193:746–756. doi: 10.4049/jimmunol.1302848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamaguchi Y, Uchida J, Cain DW, Venturi GM, Poe JC, Haas KM, Tedder TF. The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. J Immunol. 2005;174:4389–4399. doi: 10.4049/jimmunol.174.7.4389. [DOI] [PubMed] [Google Scholar]
- 25.Chistiakov DA, Orekhov AN, Sobenin IA, Bobryshev YV. Plasmacytoid dendritic cells: development, functions, and role in atherosclerotic inflammation. Front Physiol. 2014;5:279. doi: 10.3389/fphys.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.del Hoyo GM, Martin P, Vargas HH, Ruiz S, Arias CF, Ardavin C. Characterization of a common precursor population for dendritic cells. Nature. 2002;415:1043–1047. doi: 10.1038/4151043a. [DOI] [PubMed] [Google Scholar]
- 28.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 29.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 31.Daissormont IT, Christ A, Temmerman L, Sampedro Millares S, Seijkens T, Manca M, Rousch M, Poggi M, Boon L, van der Loos C, Daemen M, Lutgens E, Halvorsen B, Aukrust P, Janssen E, Biessen EA. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ Res. 2011;109:1387–1395. doi: 10.1161/CIRCRESAHA.111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ait-Oufella H, Wang Y, Herbin O, Bourcier S, Potteaux S, Joffre J, Loyer X, Ponuswamy P, Esposito B, Dalloz M, Laurans L, Tedgui A, Mallat Z. Natural Regulatory T Cells Limit Angiotensin II-Induced Aneurysm Formation and Rupture in Mice. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.113.301280. [DOI] [PubMed] [Google Scholar]
- 33.Yin M, Zhang J, Wang Y, Wang S, Bockler D, Duan Z, Xin S. Deficient CD4+CD25+ T regulatory cell function in patients with abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2010;30:1825–1831. doi: 10.1161/ATVBAHA.109.200303. [DOI] [PubMed] [Google Scholar]
- 34.Yun TJ, Lee JS, Machmach K, Shim D, Choi J, Wi YJ, Jang HS, Jung IH, Kim K, Yoon WK, Miah MA, Li B, Chang J, Bego MG, Pham TN, Loschko J, Fritz JH, Krug AB, Lee SP, Keler T, Guimond JV, Haddad E, Cohen EA, Sirois MG, El-Hamamsy I, Colonna M, Oh GT, Choi JH, Cheong C. Indoleamine 2,3-Dioxygenase-Expressing Aortic Plasmacytoid Dendritic Cells Protect against Atherosclerosis by Induction of Regulatory T Cells. Cell Metab. 2016;23:852–866. doi: 10.1016/j.cmet.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Hsue PY, Scherzer R, Grunfeld C, Imboden J, Wu Y, Del Puerto G, Nitta E, Shigenaga J, Schnell Heringer A, Ganz P, Graf J. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J Am Heart Assoc. 2014;3:e001267. doi: 10.1161/JAHA.114.001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole JE, Astola N, Cribbs AP, Goddard ME, Park I, Green P, Davies AH, Williams RO, Feldmann M, Monaco C. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc Natl Acad Sci U S A. 2015;112:13033–13038. doi: 10.1073/pnas.1517820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metghalchi S, Ponnuswamy P, Simon T, Haddad Y, Laurans L, Clement M, Dalloz M, Romain M, Esposito B, Koropoulis V, Lamas B, Paul JL, Cottin Y, Kotti S, Bruneval P, Callebert J, den Ruijter H, Launay JM, Danchin N, Sokol H, Tedgui A, Taleb S, Mallat Z. Indoleamine 2,3-Dioxygenase Fine-Tunes Immune Homeostasis in Atherosclerosis and Colitis through Repression of Interleukin-10 Production. Cell Metab. 2015;22:460–471. doi: 10.1016/j.cmet.2015.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.