Abstract
Infrared spectral changes in bacteriorhodopsin (bR) were followed during the slow decay of the M intermediate in the temperature region 240-260 K. The decay of the M form is characterized by the disappearance of the ethylenic bands and the bands indicating the reprotonation of the Schiff base. The route of Schiff-base reprotonation completely changes between 240 K and 260 K. At 240 K reprotonation occurs from Asp-85, the group to which the proton was released during M formation, and there is no pumping. At 260 K Schiff-base reprotonation takes place through Asp-96 from the cytoplasmic side, in the normal sequence assumed for proton pumping. The dramatic change in the route of Schiff-base reprotonation is coupled to a protein conformational change characterized by the change of the ratio of the two amide I bands at 1658 cm-1 and 1669 cm-1. This conformational change is interpreted as the conformational switch crucial for proton pumping: a protein relaxation following M formation results in a local rearrangement of the group, in the vicinity of the Schiff base. The rearrangement changes the accessibility of the Schiff base and provides that its deprotonation and reprotonation occur on different sides. The conformational change has characteristics typical for relaxations in proteins. In addition, it is shown that at 260 K an equilibrium exists between the M and N forms.
Full text
PDF
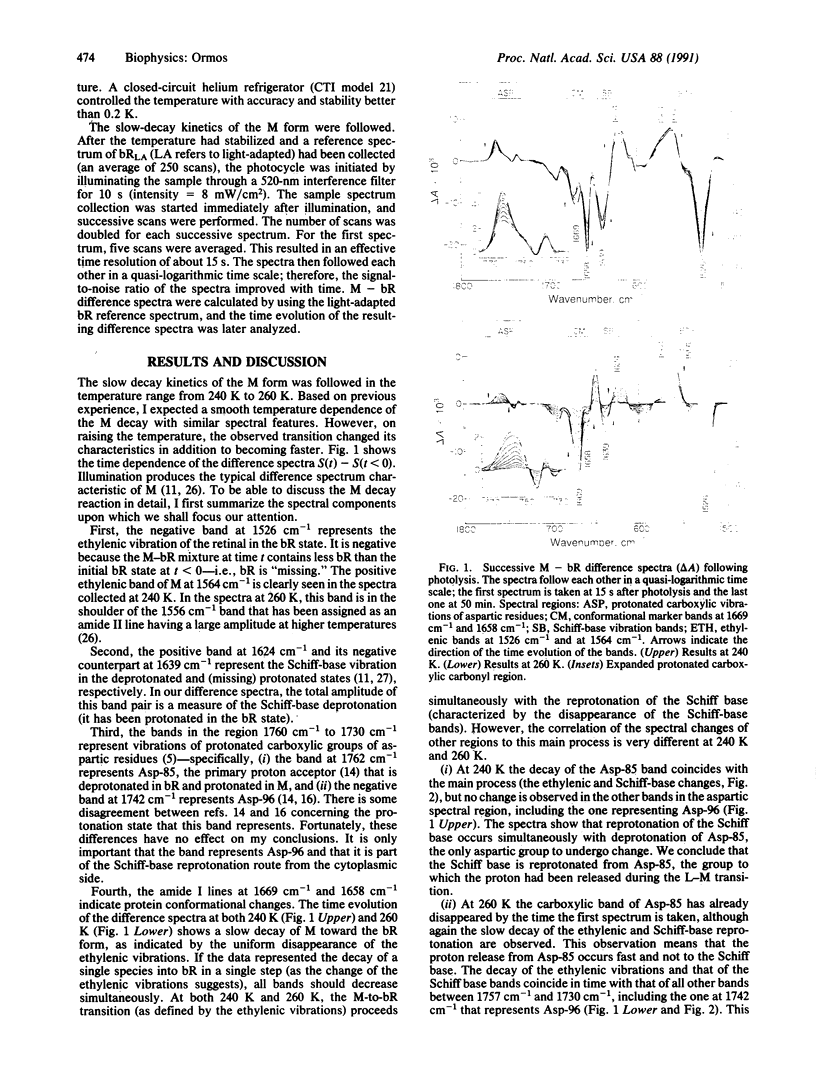

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley K., Dollinger G., Eisenstein L., Singh A. K., Zimányi L. Fourier transform infrared difference spectroscopy of bacteriorhodopsin and its photoproducts. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4972–4976. doi: 10.1073/pnas.79.16.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Ahl P. L., Rothschild K. J. Millisecond Fourier-transform infrared difference spectra of bacteriorhodopsin's M412 photoproduct. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5221–5225. doi: 10.1073/pnas.84.15.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman spectra of bacteriorhodopsin's primary photoproduct: evidence for a distorted 13-cis retinal chromophore. Proc Natl Acad Sci U S A. 1982 Jan;79(2):403–407. doi: 10.1073/pnas.79.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt H. J., Fendler K., Bamberg E., Tittor J., Oesterhelt D. Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J. 1989 Jun;8(6):1657–1663. doi: 10.1002/j.1460-2075.1989.tb03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N. A., Dresselhaus D., Zaccai G., Büldt G. Structural changes in bacteriorhodopsin during proton translocation revealed by neutron diffraction. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7876–7879. doi: 10.1073/pnas.86.20.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollinger G., Eisenstein L., Lin S. L., Nakanishi K., Odashima K., Termini J. Bacteriorhodopsin: fourier transform infrared methods for studies of protonation of carboxyl groups. Methods Enzymol. 1986;127:649–662. doi: 10.1016/0076-6879(86)27051-2. [DOI] [PubMed] [Google Scholar]
- Dollinger G., Eisenstein L., Lin S. L., Nakanishi K., Termini J. Fourier transform infrared difference spectroscopy of bacteriorhodopsin and its photoproducts regenerated with deuterated tyrosine. Biochemistry. 1986 Oct 21;25(21):6524–6533. doi: 10.1021/bi00369a028. [DOI] [PubMed] [Google Scholar]
- Drachev L. A., Kaulen A. D., Skulachev V. P. Time resolution of the intermediate steps in the bacteriorhodopsin-linked electrogenesis. FEBS Lett. 1978 Mar 1;87(1):161–167. doi: 10.1016/0014-5793(78)80157-4. [DOI] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Gerwert K., Hess B., Soppa J., Oesterhelt D. Role of aspartate-96 in proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4943–4947. doi: 10.1073/pnas.86.13.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwert K., Siebert F. Evidence for light-induced 13-cis, 14-s-cis isomerization in bacteriorhodopsin obtained by FTIR difference spectroscopy using isotopically labelled retinals. EMBO J. 1986 Apr;5(4):805–811. doi: 10.1002/j.1460-2075.1986.tb04285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iben IE, Braunstein D, Doster W, Frauenfelder H, Hong MK, Johnson JB, Luck S, Ormos P, Schulte A, Steinbach PJ. Glassy behavior of a protein. Phys Rev Lett. 1989 Apr 17;62(16):1916–1919. doi: 10.1103/PhysRevLett.62.1916. [DOI] [PubMed] [Google Scholar]
- Lin S. L., Ormos P., Eisenstein L., Govindjee R., Konno K., Nakanishi K. Deprotonation of tyrosines in bacteriorhodopsin as studied by Fourier transform infrared spectroscopy with deuterium and nitrate labeling. Biochemistry. 1987 Dec 15;26(25):8327–8331. doi: 10.1021/bi00399a045. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Lindau M., Khorana H. G., Heyn M. P. Aspartic acid-96 is the internal proton donor in the reprotonation of the Schiff base of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9228–9232. doi: 10.1073/pnas.86.23.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parak F. Correlation of protein dynamics with water mobility: Mössbauer spectroscopy and microwave absorption methods. Methods Enzymol. 1986;127:196–206. doi: 10.1016/0076-6879(86)27016-0. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., He Y. W., Gray D., Roepe P. D., Pelletier S. L., Brown R. S., Herzfeld J. Fourier transform infrared evidence for proline structural changes during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9832–9835. doi: 10.1073/pnas.86.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Roepe P., Ahl P. L., Earnest T. N., Bogomolni R. A., Das Gupta S. K., Mulliken C. M., Herzfeld J. Evidence for a tyrosine protonation change during the primary phototransition of bacteriorhodopsin at low temperature. Proc Natl Acad Sci U S A. 1986 Jan;83(2):347–351. doi: 10.1073/pnas.83.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Zagaeski M., Cantore W. A. Conformational changes of bacteriorhodopsin detected by Fourier transform infrared difference spectroscopy. Biochem Biophys Res Commun. 1981 Nov 30;103(2):483–489. doi: 10.1016/0006-291x(81)90478-2. [DOI] [PubMed] [Google Scholar]
- Schulten K., Tavan P. A mechanism for the light-driven proton pump of Halobacterium halobium. Nature. 1978 Mar 2;272(5648):85–86. doi: 10.1038/272085a0. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Lugtenburg J., Mathies R. A. Determination of retinal chromophore structure in bacteriorhodopsin with resonance Raman spectroscopy. J Membr Biol. 1985;85(2):95–109. doi: 10.1007/BF01871263. [DOI] [PubMed] [Google Scholar]
- Tittor J., Soell C., Oesterhelt D., Butt H. J., Bamberg E. A defective proton pump, point-mutated bacteriorhodopsin Asp96----Asn is fully reactivated by azide. EMBO J. 1989 Nov;8(11):3477–3482. doi: 10.1002/j.1460-2075.1989.tb08512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Keszthelyi L. Photoelectric signals from dried oriented purple membranes of Halobacterium halobium. Biophys J. 1983 Jul;43(1):47–51. doi: 10.1016/S0006-3495(83)84322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]