Abstract
IMPORTANCE
The relationship of prenatal diagnosis of critical congenital heart disease (CHD) with brain injury and brain development is unknown. Given limited improvement of CHD outcomes with prenatal diagnosis, the effect of prenatal diagnosis on brain health may reveal additional benefits.
OBJECTIVE
To compare the prevalence of preoperative and postoperative brain injury and the trajectory of brain development in neonates with prenatal vs postnatal diagnosis of CHD.
DESIGN, SETTING, AND PARTICIPANTS
Cohort study of term newborns with critical CHD recruited consecutively from 2001 to 2013 at the University of California, San Francisco and the University of British Columbia. Term newborns with critical CHD were studied with brain magnetic resonance imaging preoperatively and postoperatively to determine brain injury severity and microstructural brain development with diffusion tensor imaging by measuring fractional anisotropy and the apparent diffusion coefficient. Comparisons of magnetic resonance imaging findings and clinical variables were made between prenatal and postnatal diagnosis of critical CHD. A total of 153 patients with transposition of the great arteries and single ventricle physiology were included in this analysis.
MAIN OUTCOMES AND MEASURES
The presence of brain injury on the preoperative brain magnetic resonance imaging and the trajectory of postnatal brain microstructural development.
RESULTS
Among 153 patients (67% male), 96 had transposition of the great arteries and 57 had single ventricle physiology. The presence of brain injury was significantly higher in patients with postnatal diagnosis of critical CHD (41 of 86 [48%]) than in those with prenatal diagnosis (16 of 67 [24%]) (P = .003). Patients with prenatal diagnosis demonstrated faster brain development in white matter fractional anisotropy (rate of increase, 2.2%; 95% CI, 0.1%-4.2%; P = .04) and gray matter apparent diffusion coefficient (rate of decrease, 0.6%; 95%CI, 0.1%-1.2%; P = .02). Patients with prenatal diagnosis had lower birth weight (mean, 3184.5 g; 95%CI, 3050.3–3318.6) than those with postnatal diagnosis (mean, 3397.6 g; 95%CI, 3277.6–3517.6) (P = .02). Those with prenatal diagnosis had an earlier estimated gestational age at delivery (mean, 38.6 weeks; 95%CI, 38.2–38.9) than those with postnatal diagnosis (mean, 39.1 weeks; 95%CI, 38.8–39.5) (P = .03).
CONCLUSIONS AND RELEVANCE
Newborns with prenatal diagnosis of single ventricle physiology and transposition of the great arteries demonstrate less preoperative brain injury and more robust microstructural brain development than those with postnatal diagnosis. These results are likely secondary to improved cardiovascular stability. The impact of these findings on neurodevelopmental outcomes warrants further study.
Prenatal detection of congenital heart disease (CHD) has incrementally increased during the last 2 decades with improvements in ultrasonographic technology and increased rigor of screening ultrasonography in the obstetrical community.1–4 In particular, earlier detection of critical CHD requiring intervention in the newborn period has allowed for planned deliveries at or near a tertiary hospital with a congenital cardiac surgery program and intensive care units equipped to manage these neonates.5,6 Prenatal detection of critical CHD has been shown to improve the perioperative clinical condition of these neonates, with preserved preoperative hemodynamics and fewer life-threatening events for ductal-dependent lesions such as transposition of the great arteries (TGA) and hypoplastic left heart syndrome.7–11 Despite these apparent benefits, initial studies of prenatal diagnosis have not shown improved surgical outcomes and have even been associated with worse survival.12 Although the survival disadvantage may be attributable to increased detection rates of more severe defects, studies have demonstrated a potential disadvantage to prenatal diagnosis in the form of earlier gestational age at delivery and lower birth weight, both of which appear to affect morbidity and mortality.13 Given conflicting effects of prenatal diagnosis, further consideration of the effect of prenatal diagnosis on postnatal physiology, including brain health, may help to maximize potential benefits.
Multiple studies have shown that acquired brain injury is common in neonates prior to corrective surgery14 and is related to a set of clinical risk factors similar to those influenced by prenatal diagnosis.15 Despite shared clinical risk factors, few studies have assessed the relationship between prenatal diagnosis of critical CHD and preoperative brain injury.16 Similarly, brain immaturity has been identified in the preoperative period in neonates with critical CHD.17,18 However, to our knowledge, no studies have assessed the relationship between prenatal diagnosis of critical CHD and postnatal brain maturation.
The purpose of this study was to compare the prevalence of preoperative and postoperative brain injury and the trajectory of brain development in neonates with TGA and SVP with and without prenatal diagnoses. Given the improved hemodynamic state of neonates with prenatal diagnosis of critical CHD, we hypothesized that they have a lower prevalence of preoperative brain injury and improved brain maturation as compared with neonates with postnatal diagnosis for both single ventricle physiology (SVP) and TGA.
Methods
Between 2001 and 2013, newborns with critical CHD at the University of California San Francisco Benioff Children’s Hospital (UCSF) and the British Columbia Children’s Hospital in Vancouver, British Columbia, Canada (University of British Columbia [UBC]) were consecutively invited to participate in a prospective protocol studying brain development and brain injury in CHD using magnetic resonance imaging (MRI). Brain imaging findings from earlier versions of this cohort were reported previously.14,19 A total of 209 patients (132 from UCSF and 77 from UBC) provided consent to participate. Patients who were born prior to 36 weeks’ gestation, had a suspected congenital infection, had clinical evidence of a congenital malformation or syndrome, and/or had a suspected or confirmed genetic or chromosomal anomaly were excluded. Once written informed consent was received, patients underwent brain MRI before and after cardiac surgery. Institutional committees on human research at both UCSF and UBC approved the study protocol.
Key Points.
Question
Is prenatal diagnosis of critical congenital heart disease associated with less brain injury and better postnatal brain development?
Findings
This cohort study of neonates with transposition of the great arteries and single ventricle physiology with preoperative and postoperative brain magnetic resonance imaging found that those with prenatal diagnosis of critical congenital heart disease had significantly less preoperative brain injury than those with postnatal diagnosis (24% vs 48%, respectively) and a faster rate of brain development.
Meaning
Prenatal diagnosis of critical congenital heart disease appears to be protective against brain injury and associated with more robust microstructural brain development likely secondary to a better hemodynamic state.
Patients diagnosed as having TGA (n = 96) or SVP (n = 57) were included in this current study. Single ventricle physiology was defined as the presence of 1 functioning ventricle requiring a palliative surgical intervention for survival in the newborn period with either aortic or pulmonary obstruction.
MRI Study
Preoperative MRI studies were performed as soon as the baby could be safely transported to the MRI scanner as determined by the clinical team. Postoperative studies were performed after completion of perioperative care and prior to discharge from the hospital. Study methods, including brain imaging, were consistent across the duration of the study (see the eAppendix in the Supplement for detailed MRI methods). Studies at UCSF were performed with pharmacologic sedation, as needed, while studies at UBC were scanned in sleep without pharmacologic sedation. No adverse events occurred during this protocol. A neuroradiologist at each site reviewed each MRI for focal, multifocal, or global changes (A.J.B. and K.J.P.). Brain injury was characterized as stroke, white matter injury (WMI), intraventricular hemorrhage (IVH), and/or global hypoxic-ischemic injury as previously described.20 Postoperative brain injuries described in this study are limited to newly acquired lesions not evident on the preoperative scan. White matter injury was classified as mild (1–3 foci each <2 mm), moderate (>3 foci or any foci >2 mm), or severe (>5%of white matter volume). Intraventricular hemorrhage was characterized as grade I, II, III, or IV using the system of Papule et al.21 No patients were found to have IVH greater than grade II. Thus, for the analysis of overall brain injury in each cohort, brain injury was defined as the presence of WMI, stroke, or hypoxic-ischemic injury. In addition, brain injury severity (BIS) was determined for each patient as previously described.19 The BIS was assigned as follows: 0 indicates normal (no injury); 1, minimal injury (minimal WMI and IVH grade I or II); 2, stroke (all stroke); and 3, moderate to severe injury (moderate and severe WMI, IVH grade III, or global hypoxic-ischemic injury.
Diffusion Tensor Imaging
Diffusion tensor imaging was performed using a sequence optimized at each site for neonatal brain imaging to measure microstructural brain development. The diffusion tensor is an ellipsoid, the size and form of which manifest the direction and amount of free water diffusion. With increasing microstructural brain development, the magnitude of water diffusion motion decreases (apparent diffusion coefficient [ADC]) and the regional directionality of water motion increases (fractional anisotropy [FA]).22 The ADC and FA were calculated for voxels in 5 anatomical regions in the white matter (1–5) and the ADC was calculated for voxels in 4 anatomical regions in the gray matter (6–9) bilaterally using prespecified anatomical references17: (1) anterior white matter; (2) central white matter; (3) posterior white matter; (4) posterior limb of the internal capsule; (5) optic radiations; (6) caudate nucleus; (7) thalamus; (8) calcarine region; and (9) hippocampus. Correct region of interest placements were confirmed by neuroradiologists at each site (A.J.B. and K.J.P.). The values from the left and right hemispheres were averaged, and a mean value was used for analysis after log transformation.
Clinical Variables
Clinical data were prospectively collected from the medical records by a team of trained neonatal research nurses and reviewed by a pediatric intensivist (P.M.) blinded to all neuroimaging findings. Clinical variables were extracted from the patient record for each 24-hour period.
Statistical Analysis
Demographic characteristics, descriptors of brain injury, and clinical variables were compared between patients with prenatal and postnatal diagnosis using standard descriptive statistics. Linear regression models compared the average diffusivity and FA of white and gray matter voxels between patients with prenatal and postnatal diagnosis. Owing to their positive skew, these variables were log transformed. Each patient contributed multiple outcomes to the analysis from 2 scans and multiple regions of interest. Generalized estimating equations with a robust variance estimator were used to account for within-patient correlation. In these models, we adjusted for center (UCSF or UBC) and gestational age at MRI. Given the differences in the distribution of CHD diagnoses across centers and because the patients underwent imaging in different MRI scanners at each center, an interaction term for site (UCSF or UBC) by region of interest was included in all analyses of MRI diffusion data. All analyses were performed with Stata version 10.0 statistical software (StataCorp LP).
Results
A total of 153 infants were included in the study, 96 with TGA and 57 with SVP, with a slight male predominance (67%). Cardiac anatomy as well as demographic and patient characteristics are summarized in Table 1 and Table 2. Among the 86 patients with postnatal diagnosis, 80(93%) were born at hospitals outside the study centers. In the entire cohort, infants with prenatal diagnosis were born at a younger estimated gestational age than those with a postnatal diagnosis (prenatal: mean, 38.6 weeks; 95% CI, 38.2–38.9; postnatal: mean, 39.1 weeks; 95% CI, 38.8–39.5; P = .03) and had a lower birth weight (prenatal: mean 3184.5 g; 95% CI, 3050.3–3318.6; postnatal: 3397.6g; 95% CI, 3277.6–3517.6; P = .02) (Table 2).
Table 1.
Cardiac Lesions by Postnatal vs Prenatal Diagnosis of Critical Congenital Heart Disease
| Cardiac Lesion | No. (%) | ||
|---|---|---|---|
| Total (N = 153) | Postnatal Diagnosisa (n = 86) | Prenatal Diagnosisa (n = 67) | |
| TGA | 96 (63) | 68 (71) | 28 (29) |
| TGA with VSD | 41 (27) | 28 (68) | 13 (32) |
| SVP | 57 (37) | 18 (32) | 39 (68) |
| Aortic arch obstruction | 48 (31) | 17 (35) | 31 (65) |
| Pulmonary obstruction | 9 (6) | 1 (11) | 8 (99) |
Abbreviations: SVP, single ventricle physiology; TGA, transposition of the great arteries; VSD, ventricular septal defect.
The percentages for postnatal and prenatal diagnoses are calculated as the percentage of the total number for the row.
Table 2.
Demographic Characteristics by Postnatal vs Prenatal Diagnosis of Critical Congenital Heart Disease Among 153 Patients
| Characteristic | Diagnosis | ||
|---|---|---|---|
| Postnatal Diagnosis (n = 86) | Prenatal Diagnosis (n = 67) | P Value | |
| Male, No. (%) | 58 (67) | 44 (66) | .82a |
| EGA at delivery, mean (95% CI), wk | 39.1 (38.8–39.5) | 38.6 (38.2–38.9) | .03b |
| Birth weight, mean (95% CI), g | 3397.6 (3277.6–3517.6) | 3184.5 (3050.3–3318.6) | .02b |
| Cesarean delivery, No. (%) | 23 (27) | 17 (25) | .85 |
| Maternal education score, mean (SD)c | 5.0 (1.6) | 5.1 (1.6) | .83 |
Abbreviation: EGA, estimated gestational age.
Calculated by χ2 test.
Calculated by 2-sample t test.
Maternal education is represented by the Hollingshead score, with a higher score indicating a higher level of education. A mean score of 5 represents partial college completion.
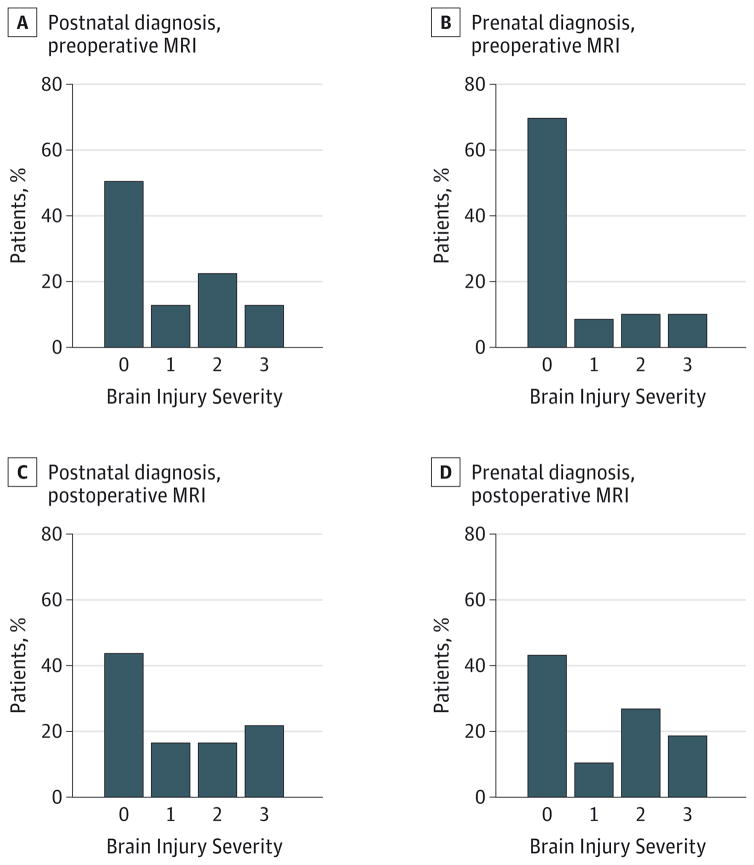
The presence of preoperative brain injury was significantly lower in patients with prenatal diagnosis (16 of 67 patients [24%]) than in those with postnatal diagnosis (41 of 86 patients [48%]) (P = .003) (Table 3). This remained true among patients with TGA and SVP. A subanalysis of patients with SVP excluding those with pulmonary outflow tract obstruction revealed the same pattern. The watershed predominant pattern of hypoxic-ischemic injury was rare in the cohort, seen in only 2 neonates, both of whom had postnatal diagnosis. The presence of postoperative brain injury was similar in the prenatal and postnatal diagnosis groups (P = .48). Atest for trends demonstrated less severe preoperative brain injury (lower BIS) in the patients with prenatal diagnosis (Figure 1). However, there was no difference in new postoperative brain injury severity (Figure 1).
Table 3.
Prevalence of Preoperative Brain Injury by Cardiac Diagnosis and Postnatal vs Prenatal Diagnosis of Critical Congenital Heart Disease
| Preoperative Brain Injury and Cardiac Diagnosis | No. With Injury/Total No. With Cardiac Diagnosis (%) | P Valuea | |
|---|---|---|---|
| Postnatal Diagnosis | Prenatal Diagnosis | ||
| Any injuryb | |||
| All patients | 41/86 (48) | 16/67 (24) | .003 |
| TGA | 31/68 (46) | 6/28 (21) | .03 |
| SVP | 10/18 (56) | 10/39 (26) | .03 |
| SVP with aortic arch obstruction | 9/17 (53) | 7/31 (23) | .02 |
| White matter injury | |||
| TGA | 17/68 (25) | 3/28 (11) | .09 |
| SVP | 8/18 (44) | 8/39 (21) | .06 |
| Stroke | |||
| TGA | 20/68 (29) | 4/28 (14) | .09 |
| SVP | 2/18 (11) | 5/39 (13) | .61 |
| Hypoxic-ischemic injuryc | |||
| TGA | 1/68 (1) | 0 | .71 |
| SVP | 1/18 (6) | 0 | .32 |
Abbreviations: SVP, single ventricle physiology; TGA, transposition of the great arteries.
Pearson χ2 test or Fisher exact test.
Any injury is defined as white matter injury and/or stroke.
Refers to watershed pattern of global hypoxic-ischemic injury.
Figure 1. Preoperative and Postoperative Brain Injury Severity by Postnatal vs Prenatal Diagnosis of Critical Congenital Heart Disease.
Brain injury severity on preoperative magnetic resonance imaging (MRI) in patients with postnatal (A) and prenatal (B) diagnosis of critical congenital heart disease as well as on postoperative MRI in patients with postnatal (C) and prenatal (D) diagnosis of critical congenital heart disease. Brain injury severity was assigned as the following: 0 indicates no injury; 1, minimal white matter injury and intraventricular hemorrhage grade I or II; 2, stroke; and 3, moderate to severe white matter injury, intraventricular hemorrhage grade III, or global hypoxic-ischemic brain injury. A test for trends demonstrates a significant trend toward less severe brain injury on preoperative MRI in the prenatal diagnosis group (P = .02). There was no meaningful difference in new postoperative brain injury severity (P = .40).
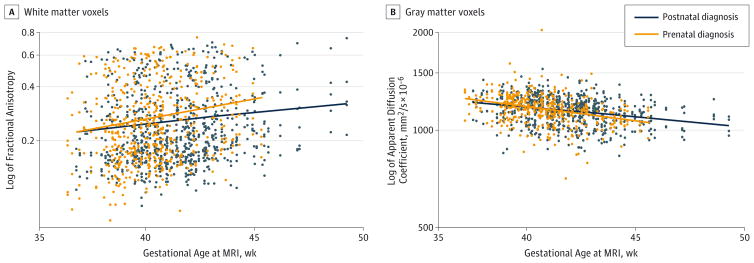
To assess postnatal brain maturation, we analyzed change in FA and ADC over time from preoperative to postoperative MRIs in the 2 groups for both white and gray matter regions, accounting for study site and region of interest on the MRI (Figure 2). As expected, FA in white matter increased significantly with increasing age in the entire cohort (3% increase in FA with each week of increase in age; P < .001). However, the prenatal diagnosis group had a faster rate of increase in FA as compared with the postnatal diagnosis group (2.2% increase in the difference with each week of increase in age; 95% CI, 0.1%-4.2%; P = .04). Similarly, ADC in gray matter decreased significantly with increasing age (1.2% decrease in ADC with each week of increase in age; P < .001), but the prenatal diagnosis group had a faster rate of decrease in ADC as compared with the postnatal diagnosis group (0.6% increase in the difference with each week of increase in age; 95% CI, 0.1%–1.2%; P = .02). The rate of decrease in ADC in white matter was not significantly different between the groups (0.3%; 95% CI, −0.7% to 1.2%; P = .55). Analysis of the data by specific lesion (TGA and SVP) demonstrated identical trends but no meaningful differences by prenatal diagnosis as a result of smaller sample size.
Figure 2. Scatterplots and Linear Regression Lines of Change in the Log of Fractional Anisotropy and the Log of Apparent Diffusion Coefficient.
Scatterplots and linear regression lines of change in the fractional anisotropy in white matter voxels (A) and apparent diffusion coefficient in gray matter voxels (B) demonstrate a faster rate of increase in fractional anisotropy (P = .04) and a faster rate of decrease in apparent diffusion coefficient (P = .02) in patients with prenatal diagnosis of critical congenital heart disease than in those with postnatal diagnosis. Time is defined as the gestational age when magnetic resonance imaging (MRI) was performed (includes both preoperative and postoperative scans).
Preoperative clinical variables for patients with TGA and SVP are summarized in eTable 1 and eTable 2 in the Supplement. Most clinical variables were similar between the prenatal and postnatal diagnosis groups. However, the group with postnatally diagnosed TGA appeared to have low preoperative oxygen saturation (mean, 58.3%). Similarly, the group with postnatally diagnosed SVP appeared to have low preoperative pH (mean, 7.22), low base excess (mean, −7.0 mEq/L [to convert to millimoles per liter, multiply by 1.0]), and 3 cases of cardiac arrest. The preoperative brain MRI was performed at an earlier age in the prenatal group for both patients with TGA and those with SVP. Patients with SVP underwent surgery at an earlier age in the prenatal diagnosis group (median, 6 days; interquartile range, 4–8 days) than in the postnatal diagnosis group (median, 9.5 days; interquartile range, 6–12.5 days) (P = .008).
Discussion
Our results demonstrate a lower prevalence of preoperative brain injury and better postnatal microstructural brain development in patients with prenatally diagnosed TGA and SVP as compared with those who had a postnatal diagnosis. Specifically, there was a 50% (gray matter voxels) to 70%(white matter voxels) increase in the rate of brain development in patients with prenatal diagnosis in this very short period from preoperative to postoperative imaging. Our findings suggest favorable preoperative clinical characteristics for infants with prenatal diagnosis, consistent with prior literature7,9–11; however, this is the first report, to our knowledge, demonstrating improved brain development with prenatal diagnosis in a large cohort of well-characterized patients across 2 centers.
Consistent with prior reports, WMI was the most frequent pattern of injury in patients with SVP.14,20,23 The mechanism of WMI is thought to be secondary to hypoxic-ischemic and inflammatory injury to susceptible immature premyelinating oligodendrocytes similar to the mechanism seen in preterm infants. 24 Indeed, brain immaturity has been reported in both fetal and neonatal imaging performed in patients with hypoplastic left heart syndrome,17,18,25 perhaps resulting in an increased susceptibility to focal and diffuse WMI. In patients with postnatally diagnosed SVP, acidosis and cardiac arrest with resulting lower oxygen delivery to the brain can explain the mechanism behind increased brain injury seen in this group of patients as compared with those with prenatal diagnosis. A similar mechanism likely holds true for WMI in patients with TGA, who have also been shown to have brain immaturity.17,19 Lower preoperative oxygen saturation and longer time to surgery have been identified as risk factors for WMI or periventricular leukomalacia.26 In our cohort, the postnatal diagnosis group had low preoperative oxygen saturations, thus explaining the possible mechanism behind increased brain injury in the postnatal group, although previously identified risk factors such as balloon atrial septostomy were not different between the 2 groups.15,27 Further imaging studies and investigation of physiological mechanisms are needed to understand this finding.
After adjusting for site and age at scan, patients with prenatal diagnosis demonstrated more robust postnatal microstructural brain development (change in FA and ADC from the preoperative to postoperative scan) in both white and gray matter as compared with patients who had postnatal diagnosis. We observed steeper increases in white matter FA and decreases in gray matter ADC. Multiple studies have demonstrated that newborns with complex CHD have immature brains, using a wide range of techniques including semiquantitative morphologic scoring (ie, total maturation score)18 and quantitative magnetic resonance measures such as diffusion tensor imaging and magnetic resonance spectroscopy.17 These differences in brain maturation persist through adolescence as reflected in reduced regional brain volumes.28 Multiple lines of evidence support the idea that brain immaturity results from reduced oxygen delivery during periods of high oxygen consumption by the brain.29–31 Fetal brain imaging studies show that delayed brain development begins in the third trimester, coincident with rapid increases in brain blood flow as a percentage of total cardiac output that occur normally to support increases in brain electrical activity.25 More recently, reduced cerebral oxygenation and impaired brain growth were observed in fetuses with CHD using novel in utero MRI approaches.31 Prenatal diagnosis likely promotes improved postnatal brain development by ensuring favorable hemodynamics to provide improved brain oxygen and substrate delivery. Even in this brief period of brain development from preoperative to postoperative scan, we detected significant differences in the rate of brain development in patients with prenatal vs postnatal diagnosis. Studies are underway to quantify ongoing brain maturity in these patients at age 6 months and beyond, which should confirm this improved trajectory of brain growth in these patients.
The relationship between brain development and injury is complex and dependent on the methods used to measure brain development. Most studies agree that brain immaturity is a risk factor for preoperative brain injury.19,32 This association has been used to explain the high prevalence of WMI similar to that observed in premature newborns. However, studies differ on the association of brain maturity with postoperative brain injury. In a prior study, no association was found between preoperative brain maturity and newly acquired postoperative brain injury.19 Similarly, in the present study, we did not find that prenatal diagnosis improved the rate of new postoperative brain injury, despite better brain development. We speculate that postoperative brain injury may be more strongly influenced by operative and postoperative risks such as cardiopulmonary by pass technique and postoperative low cardiac output syndrome or cardiac arrest.
Based on our protocol, the preoperative MRI was performed when the patient was clinically stable; therefore, a potential limitation of our findings is that the MRI was performed on average 2 days earlier in the prenatal group than in the postnatal group. This study design complicates the analysis of the relationship between time to surgery and preoperative brain injury, although earlier MRI may in part explain the lower rate of prenatal injury. However, there was also a trend toward an earlier operation in the prenatal group. Although this may influence our findings, the earlier MRI and earlier operation support our hypothesis that patients with prenatal diagnosis are “healthier,” thus having less preoperative brain injury, and are better candidates for an earlier operation. Physiologically, earlier repair may prevent further brain injury by minimizing postnatal, preoperative exposure to abnormal cerebral perfusion. In addition, earlier anatomical repair may allow for improved postnatal brain growth and development. Supporting this idea, a recent study across 2 centers16 found that among patients with prenatally diagnosed aortic arch obstruction, the center with a younger age at surgery, more intensive preoperative monitoring, and less exposure to risk factors (eg, infection) demonstrated less preoperative brain injury. Similarly, a recent single-center study of patients with hypoplastic left heart syndrome concluded that an earlier operation may be beneficial in preventing new postoperative brain injury.33
Optimizing preoperative conditions and minimizing time to surgery may be critical to realizing the potential benefits of prenatal diagnosis. Patients with a prenatal diagnosis appear to be healthier in the preoperative period, likely secondary to the early initiation of prostaglandin E1, as seen in prior studies.7,9 However, studies have repeatedly demonstrated no difference in mortality when comparing prenatal vs postnatal diagnosis of complex CHD.8,12,34–38 In addition, prenatal diagnosis often leads to earlier gestational age at birth and lower birth weight,9 both associated with poor clinical outcomes such as mortality and an increased length of stay for neonates with critical CHD.13 Similarly, early studies of prenatal diagnosis found no benefits in long-term neurodevelopmental outcome, a finding potentially explained by early preterm delivery and higher rates of preoperative intensive care therapies.39 In contrast, Calderon et al40 demonstrated improved long-term neurodevelopmental outcomes for patients with prenatally diagnosed TGA. Our findings reiterate this duality of prenatal diagnosis. Although it would seem likely that lower rates of preoperative brain injury and improved brain development outweigh negative effects of late preterm delivery, this remains to be determined by definitive studies of neurodevelopmental outcome, which are ongoing.
Conclusions
Prenatal diagnosis of SVP and TGA appears to be protective against preoperative brain injury and is associated with a faster rate of brain growth in the postnatal period. The mechanism behind these findings is likely related to a better hemodynamic state as a result of earlier use of prostaglandin E1. However, it remains to be determined how this potential protective effect relates to long-term outcomes as it is unlikely to influence other patient-specific risk factors such as genetic predispositions, intraoperative injuries, or postoperative hemodynamics.19,41–47 Further studies are needed to determine whether decreased preoperative brain injury and improved brain growth related to prenatal diagnosis translate into better long-term neurodevelopmental outcomes.
Acknowledgments
Funding/Support: This work was supported by grant MOP93780 from the Canadian Institutes of Health Research; grants R01 NS40117, R01 NS063876, P01 NS082330, and P50 NS35902 from the National Institutes of Health; grant 5-M01-RR-01271 from the National Center for Research Resources; grants 5-FY05-1231 and 6-FY2009-303 from the March of Dimes Foundation; grant 0365018Y from the American Heart Association; and grant 2002/3E from the Larry L. Hillblom Foundation. Dr Miller is the Bloorview Children’s Hospital Chair in Paediatric Neuroscience (from September 2012) and was supported by a Tier 2 Canada Research Chair in Neonatal Neuroscience from the Government of Canada and a scholar award from the Michael Smith Foundation for Health Research (to July 2012).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Drs Peyvandi and McQuillen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Peyvandi, Miller, McQuillen.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Peyvandi, McQuillen.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Peyvandi, McQuillen.
Obtained funding: Xu, Barkovich, Miller, McQuillen.
Administrative, technical, or material support: Peyvandi, De Santiago, Chakkarapani, Campbell, Xu, Barkovich, Miller, McQuillen.
Study supervision: Xu, Miller, McQuillen.
References
- 1.American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of fetal echocardiography. J Ultrasound Med. 2013;32(6):1067–1082. doi: 10.7863/ultra.32.6.1067. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho JS, Allan LD, Chaoui R, et al. International Society of Ultrasound in Obstetrics and Gynecology. ISUOG practice guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41(3):348–359. doi: 10.1002/uog.12403. [DOI] [PubMed] [Google Scholar]
- 3.Tometzki AJ, Suda K, Kohl T, Kovalchin JP, Silverman NH. Accuracy of prenatal echocardiographic diagnosis and prognosis of fetuses with conotruncal anomalies. J Am Coll Cardiol. 1999;33(6):1696–1701. doi: 10.1016/s0735-1097(99)00049-2. [DOI] [PubMed] [Google Scholar]
- 4.Allan LD, Sharland GK, Milburn A, et al. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol. 1994;23(6):1452–1458. doi: 10.1016/0735-1097(94)90391-3. [DOI] [PubMed] [Google Scholar]
- 5.Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. American Heart Association Adults With Congenital Heart Disease Joint Committee of the Council on Cardiovascular Disease in the Young and Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Council on Cardiovascular and Stroke Nursing. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129(21):2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 6.Liberman RF, Getz KD, Lin AE, et al. Delayed diagnosis of critical congenital heart defects: trends and associated factors. Pediatrics. 2014;134(2):e373–e381. doi: 10.1542/peds.2013-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tworetzky W, McElhinney DB, Reddy VM, Brook MM, Hanley FL, Silverman NH. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation. 2001;103(9):1269–1273. doi: 10.1161/01.cir.103.9.1269. [DOI] [PubMed] [Google Scholar]
- 8.Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics. 2001;107(6):1277–1282. doi: 10.1542/peds.107.6.1277. [DOI] [PubMed] [Google Scholar]
- 9.Kipps AK, Feuille C, Azakie A, et al. Prenatal diagnosis of hypoplastic left heart syndrome in current era. Am J Cardiol. 2011;108(3):421–427. doi: 10.1016/j.amjcard.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 10.Morris SA, Ethen MK, Penny DJ, et al. Prenatal diagnosis, birth location, surgical center, and neonatal mortality in infants with hypoplastic left heart syndrome. Circulation. 2014;129(3):285–292. doi: 10.1161/CIRCULATIONAHA.113.003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar RK, Newburger JW, Gauvreau K, Kamenir SA, Hornberger LK. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am J Cardiol. 1999;83(12):1649–1653. doi: 10.1016/s0002-9149(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 12.Oster ME, Kim CH, Kusano AS, et al. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol. 2014;113(6):1036–1040. doi: 10.1016/j.amjcard.2013.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costello JM, Pasquali SK, Jacobs JP, et al. Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation. 2014;129(24):2511–2517. doi: 10.1161/CIRCULATIONAHA.113.005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McQuillen PS, Barkovich AJ, Hamrick SEG, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38(2 suppl):736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 15.McQuillen PS, Hamrick SEG, Perez MJ, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113(2):280–285. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 16.Algra SO, Haas F, Poskitt KJ, et al. Minimizing the risk of preoperative brain injury in neonates with aortic arch obstruction. J Pediatr. 2014;165(6):1116–1122. e3. doi: 10.1016/j.jpeds.2014.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 18.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137(3):529–536. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimitropoulos A, McQuillen PS, Sethi V, et al. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81(3):241–248. doi: 10.1212/WNL.0b013e31829bfdcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block AJ, McQuillen PS, Chau V, et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2010;140(3):550–557. doi: 10.1016/j.jtcvs.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 22.Hüppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11(6):489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Goff DA, Shera DM, Tang S, et al. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient related. J Thorac Cardiovasc Surg. 2014;147(4):1312–1318. doi: 10.1016/j.jtcvs.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147(5):609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121(1):26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petit CJ, Rome JJ, Wernovsky G, et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119(5):709–716. doi: 10.1161/CIRCULATIONAHA.107.760819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller SP, McQuillen PS, Vigneron DB, et al. Preoperative brain injury in newborns with transposition of the great arteries. Ann Thorac Surg. 2004;77(5):1698–1706. doi: 10.1016/j.athoracsur.2003.10.084. [DOI] [PubMed] [Google Scholar]
- 28.von Rhein M, Buchmann A, Hagmann C, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137(pt 1):268–276. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- 29.Rudolph A. Congenital Diseases of the Heart. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 30.Sethi V, Tabbutt S, Dimitropoulos A, et al. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr Res. 2013;73(5):661–667. doi: 10.1038/pr.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L, Macgowan CK, Sled JG, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131(15):1313–1323. doi: 10.1161/CIRCULATIONAHA.114.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139(3):543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch JM, Buckley EM, Schwab PJ, et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2014;148(5):2181–2188. doi: 10.1016/j.jtcvs.2014.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montaña E, Khoury MJ, Cragan JD, Sharma S, Dhar P, Fyfe D. Trends and outcomes after prenatal diagnosis of congenital cardiac malformations by fetal echocardiography in a well defined birth population, Atlanta, Georgia, 1990–1994. J Am Coll Cardiol. 1996;28(7):1805–1809. doi: 10.1016/S0735-1097(96)00381-6. [DOI] [PubMed] [Google Scholar]
- 35.Yates RS. The influence of prenatal diagnosis on postnatal outcome in patients with structural congenital heart disease. Prenat Diagn. 2004;24(13):1143–1149. doi: 10.1002/pd.1072. [DOI] [PubMed] [Google Scholar]
- 36.Sivarajan V, Penny DJ, Filan P, Brizard C, Shekerdemian LS. Impact of antenatal diagnosis of hypoplastic left heart syndrome on the clinical presentation and surgical outcomes: the Australian experience. J Paediatr Child Health. 2009;45(3):112–117. doi: 10.1111/j.1440-1754.2008.01438.x. [DOI] [PubMed] [Google Scholar]
- 37.Levey A, Glickstein JS, Kleinman CS, et al. The impact of prenatal diagnosis of complex congenital heart disease on neonatal outcomes. Pediatr Cardiol. 2010;31(5):587–597. doi: 10.1007/s00246-010-9648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright LK, Ehrlich A, Stauffer N, Samai C, Kogon B, Oster ME. Relation of prenatal diagnosis with one-year survival rate for infants with congenital heart disease. Am J Cardiol. 2014;113(6):1041–1044. doi: 10.1016/j.amjcard.2013.11.065. [DOI] [PubMed] [Google Scholar]
- 39.Bartlett JM, Wypij D, Bellinger DC, et al. Effect of prenatal diagnosis on outcomes in D-transposition of the great arteries. Pediatrics. 2004;113(4):e335–e340. doi: 10.1542/peds.113.4.e335. [DOI] [PubMed] [Google Scholar]
- 40.Calderon J, Angeard N, Moutier S, Plumet M-H, Jambaqué I, Bonnet D. Impact of prenatal diagnosis on neurocognitive outcomes in children with transposition of the great arteries. J Pediatr. 2012;161(1):94–98. e1. doi: 10.1016/j.jpeds.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 41.Goff DA, Luan X, Gerdes M, et al. Younger gestational age is associated with worse neurodevelopmental outcomes after cardiac surgery in infancy. J Thorac Cardiovasc Surg. 2012;143(3):535–542. doi: 10.1016/j.jtcvs.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaynor JW, Wernovsky G, Jarvik GP, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133(5):1344–1353. e1–e3. doi: 10.1016/j.jtcvs.2006.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newburger JW, Sleeper LA, Bellinger DC, et al. Pediatric Heart Network Investigators. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the Single Ventricle Reconstruction trial. Circulation. 2012;125(17):2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellinger DC, Wypij D, Rivkin MJ, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124(12):1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galli KK, Zimmerman RA, Jarvik GP, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127(3):692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 46.Beca J, Gunn JK, Coleman L, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127(9):971–979. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- 47.Andropoulos DB, Ahmad HB, Haq T, et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr Anaesth. 2014;24(3):266–274. doi: 10.1111/pan.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]