Abstract
Heat shock proteins (Hsps) are cellular repair agents that counter the effects of protein misfolding that is a characteristic feature of neurodegenerative diseases. HSPA1A (Hsp70–1) is a widely studied member of the HSPA (Hsp70) family. The little-studied HSPA6 (Hsp70B’) is present in the human genome and absent in mouse and rat; hence, it is missing in current animal models of neurodegenerative diseases. Differentiated human neuronal SH-SY5Y cells were employed to compare the dynamics of the association of YFP-tagged HSPA6 and HSPA1A with stress-sensitive cytoplasmic and nuclear structures. Following thermal stress, live-imaging confocal microscopy and Fluorescence Recovery After Photobleaching (FRAP) demonstrated that HSPA6 displayed a prolonged and more dynamic association, compared to HSPA1A, with centrioles that play critical roles in neuronal polarity and migration. HSPA6 and HSPA1A also targeted nuclear speckles, rich in RNA splicing factors, and the granular component of the nucleolus that is involved in rRNA processing and ribosomal subunit assembly. HSPA6 and HSPA1A displayed similar FRAP kinetics in their interaction with nuclear speckles and the nucleolus. Subsequently, during the recovery from neuronal stress, HSPA6, but not HSPA1A, localized with the periphery of nuclear speckles (perispeckles) that have been characterized as transcription sites. The stress-induced association of HSPA6 with perispeckles displayed the greatest dynamism compared to the interaction of HSPA6 or HSPA1A with other stress-sensitive cytoplasmic and nuclear structures. This suggests involvement of HSPA6 in transcriptional recovery of human neurons from cellular stress that is not apparent for HSPA1A.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-016-0724-2) contains supplementary material, which is available to authorized users.
Keywords: HSPA6 (Hsp70B’), HSPA1A (Hsp70–1), FRAP, Live imaging, SH-SY5Y
Introduction
Protein misfolding is a characteristic feature of human neurodegenerative diseases that disrupts normal neural function leading to premature cell death (Muchowski and Wacker 2005; Westerheide and Morimoto 2005; Asea and Brown 2008; Richter et al. 2010). Heat shock proteins (Hsps) are cellular repair agents that counter the effects of protein misfolding, and their upregulation has been proposed as a potential strategy to counter neurodegenerative disorders (Muchowski and Wacker 2005; Asea and Brown 2008; Pratt et al. 2015). The HSPA (Hsp70) family has been widely studied, particularly HSPA1A (Hsp70–1), however HSPA6 (Hsp70B’) has received comparatively little attention (Chow and Brown 2007; Noonan et al. 2007a; Noonan et al. 2007b; Noonan et al. 2008a; Noonan et al. 2008b; Chow et al. 2010; Ramirez et al. 2015; Deane and Brown 2016). Interestingly, HSPA6 is present in the human genome and absent in the genomes of mouse and rat. Hence, HSPA6 is missing in current animal models of human neurodegenerative diseases. At present, few effective therapies for human neurodegenerative diseases have been identified despite numerous clinical trials (Dunkel et al. 2012; Huang and Mucke 2012; Pratt et al. 2015). Therapeutic compounds that have been identified and appeared promising in animal models of neurodegenerative diseases have repeatedly failed to translate to effective treatments in human clinical settings. This has led to concerns about deficiencies in current animal models of human neurodegenerative diseases.
In a recent report, we created stable cell lines of human SH-SY5Y neuronal cells expressing YFP-HSPA6 and YFP-HSPA1A (Khalouei et al. 2014a) in order to investigate the targeting of these HSPA family members to cytoplasmic and nuclear sites in differentiated human neurons following thermal stress using fixed-cell preparation (Khalouei et al. 2014a; Khalouei et al. 2014b). Live imaging has now facilitated the visualization of the time course of the cellular stress response in differentiated human neuronal cells and the targeting of the little-studied HSPA6 to stress-sensitive cytoplasmic and nuclear sites in living human neurons.
In addition, Fluorescence Recovery After Photobleaching (FRAP) demonstrated that, following thermal stress, HSPA6 exhibited a prolonged and more dynamic association, compared to HSPA1A, with centrioles that play critical roles in polarity determination and migration patterns in neurons. Later in the recovery from neuronal stress, HSPA6, but not HSPA1A, localized to the periphery of nuclear speckles (perispeckles) that have been characterized as transcription sites (Brown et al. 2008; Spector and Lamond 2011; Rieder et al. 2012; Rieder et al. 2014). FRAP revealed that the stress-induced interaction of HSPA6 with perispeckles displayed the greatest dynamism compared to the association of HSPA6 and HSPA1A with other stress-sensitive cytoplasmic and nuclear structures. These results suggest involvement of HSPA6 in transcription recovery of human neuronal cells from cellular stress that is not apparent for HSPA1A. The association of HSPA6 with perispeckles is missing in current animal models of neurodegenerative diseases which do not include HSPA6.
Materials and methods
Cell culture, transfection, differentiation, and thermal stress
Human neuronal SH-SY5Y cells (American Type Culture Collection, VA, USA) were grown in Dulbecco’s modified Eagle medium (DMEM; Wisent, QC, Canada) supplemented with 10 % fetal bovine serum (FBS; Wisent) at 37 °C and 5 % CO2 humidified atmosphere. Preparation of plasmids, transfection, and selection of SH-SY5Y cells stably expressing enhanced YFP-HSPA6 or YFP-HSPA1A were carried out as previously described (Khalouei et al. 2014a). For live imaging, immunocytochemistry, and FRAP experiments, 3.0 × 105 neuronal cells were plated onto 35-mm glass-bottom dishes (MatTek Corporation, MA, USA) and differentiated in serum-free DMEM supplemented with 10 μM all-trans-retinoic acid for 72 h at 37 °C. Thermal stress was performed by submerging culture dishes in a water bath equipped with a thermal immersion circulator (Thermo Fisher, MA, USA) set to 43 °C (± 0.5 °C) for 20 min.
Immunocytochemistry
To stain for cytoplasmic and nuclear structures, differentiated human neuronal cells grown on glass-bottom dishes were fixed in 4 % paraformaldehyde in phosphate-buffered saline (PBS) for 20 min. Cells were then permeabilized for 20 min with 0.1 % Triton X-100 in PBS with 100 mM glycine, blocked in 5 % FBS, followed by incubation with primary antibodies and subsequently fluorescent secondary antibodies, each for 1 h. Primary antibodies specific for (i) centrioles (γ-tubulin, Product #11–543, Exbio, Prague, Czech Republic), (ii) nuclear speckles (SON, HPA023535, Sigma), and (iii) the granular component of the nucleolus (nucleophosmin, ab10530, Abcam, Cambridge, UK) were utilized at 1:1000 dilution. Fluorescently conjugated cy3 and cy5 donkey anti-rabbit and donkey anti-mouse secondary antibodies (Jackson ImmunoResearch Laboratories Inc., PA, USA) were used at a 1:2000 dilution.
Confocal microscopy
A WaveFX-X1 spinning disk confocal system (Quorum Technologies, ON, Canada) attached to an inverted fluorescence microscope (DMI6000B, Leica) was employed to capture images with a Plan-APO ×63/1.4 NA oil-objective on an electron-multiplying charge-coupled device camera (Hamamatsu Photonics, Japan). Visualization of YFP, cy3, and cy5 was achieved by diode laser excitation/emission at 491/520, 561/620, and 644/692 nm, respectively, at 5 % laser intensity. Photobleaching was performed using a Mosaic system (Andor Technology, Belfast, UK) attached to the spinning disk setup. The Mosaic system employed a digital micromirror device controlling the spatial distribution of a high power 405-nm diode laser into a pre-determined region of interest (ROI). For live and FRAP imaging, thermally stressed cells were placed on a XYZ motorized, piezo-controlled (Applied Scientific Instrumentation, OR, USA) stage-top incubation system (Chamlide; Live Cell Instrument, Seoul, Korea) at 37 °C and 5 % CO2.
Live imaging
Laser intensity, photobleaching, and time-lapse parameters for live and FRAP imaging were determined using MetaMorph acquisition software (Molecular Devices, CA, USA). Live and FRAP imaging parameters were optimized by adjusting the pinhole and detector gain to minimize overall photobleaching and avoid pixel saturation (Snapp et al. 2003). For fluorescence intensity (FI) measurements, pre-thermal stress images were acquired and following 20 min of thermal stress, imaging continued at 10 min intervals for a duration of 300 min. In order to capture intracellular localization events of YFP-tagged HSPA6 and HSPA1A, acquisition was performed in 3D stacks of 9.5 μm with a step size of 0.5 μm.
Movies were processed using Volocity analysis software (PerkinElmer, MA, USA). To perform FI measurements, all movies had their volumes split in order to select for the clearest and flattest plane of each stress-sensitive structure of interest. Movies were corrected for background noise using the arithmetic mean of a dark image captured with the aforementioned z-stack parameters. Background-corrected images were subsequently corrected for photobleach-induced fluorescence decay. A circular ROI was used to outline the structure of centrioles, nuclear speckles, the nucleolus, and the periphery of nuclear speckles. To measure the mean fold-change FI at centrioles and the nucleolus, all post-thermal stress images were normalized relative to the mean FI of putative centriole and nucleolar sites prior to thermal stress (verified by immunocytochemistry). For mean fold-change in FI at nuclear speckles and YFP-HSPA6 localization to the periphery of nuclear speckles, the mean FI of a free-hand drawn area covering the flattest plane of the nucleus was obtained, which excluded the nucleolus, as a reference for the nucleoplasm FI. As these intra-nuclear domains are not delineable under control live imaging conditions, it was reasoned that the homogenous signal observed in the nucleoplasm reflected the extent of YFP-tagged HSPA6 and HSPA1A within these compartments.
Fluorescence recovery after photobleaching (FRAP)
An empirically determined circular ROI was created for each stress-sensitive structure analyzed by FRAP. ROI size was determined based on the average circular diameter that each structure occupied. For centrioles and nuclear speckles, an area of 2.75 μm2 was used, whereas, for nucleoli and HSPA6-specific localization to the periphery of nuclear speckles, an area of 4.37 and 1.34 μm2 was used, respectively. For FRAP acquisition of centrioles, nuclear speckles and the nucleolus, 10 pre-bleach images were acquired in 1-s intervals, followed by a photobleach pulse duration of 1.5 s. Initial fluorescence recovery was acquired for a duration of 49.5 s at 0.5-s intervals. Plateau of fluorescence recovery was acquired for a duration of 30 s at 2-s intervals. For FRAP acquisition of YFP-HSPA6 localization to the periphery of nuclear speckles, 5 pre-bleach images were acquired in 1-s intervals, followed by a 1.5-s photobleach pulse, and subsequent fluorescence recovery acquired in 0.5-s intervals for a duration of 22.5 s. All FRAP images were captured in a single focal plane.
FRAP analysis was performed on Volocity. For each photobleached target, a circular ROI tool with equal dimension as that created for photobleaching was used to demarcate the structure of interest for FRAP analysis. The half-time rate of fluorescence recovery (τ1/2), mobile and immobile fractions were software generated after background- and photobleach-corrected FRAP data was fitted to the single exponential function f(t) = y + Ae-kt (Sprague and McNally 2005). FRAP trials demonstrating focus or cellular drift were discarded and not included in the analysis. FRAP graphical representations display the pooled mean FI obtained at each time point after raw FI data was normalized relative to pre- and post-bleach images to equal 1 and 0, respectively.
Live-cell, fixed-cell, and FRAP image processing
Prior to preparation of figures, all live-cell, fixed-cell, and FRAP images were processed on Volocity. Live- and fixed-cell imaging panels are displayed as single stack images to best illustrate each stress-sensitive structure in its plane of focus. Green pseudo-coloring was used for YFP-tagged HSPA6 and HSPA1A. Red pseudo-coloring was used for display of γ-tubulin, SON, and nucleophosmin. Contrast and brightness adjustments for live-cell, fixed-cell, and FRAP panels were identical for both YFP-tagged HSPA6 and HSPA1A as well as between control (i.e., pre-thermal stress and pre-bleach) and experimental images (i.e., post-thermal stress and 0-s post-bleach). No gamma adjustments were made to any images, and quantification of FI and FRAP at each stress-sensitive structure was obtained from raw and unprocessed images. FI and FRAP measurements were graphed using Microsoft Excel (Microsoft) and Prism (GraphPad). Preparation of figures and movies were performed using Adobe Illustrator and Premiere Pro (Adobe Systems), respectively.
Results
Live imaging of the stress response in differentiated human neurons
The effect of heat shock on the localization of YFP-tagged HSPA6 (Hsp70B’) and HSPA1A (Hsp70–1) proteins in post-mitotic, differentiated human neuronal SH-SY5Y cells was investigated by live imaging using spinning disk confocal microscopy. Following heat shock at 43 °C for 20 min (Fig. 1a) and subsequent time course recovery at 37 °C (Fig. 1b), YFP-HSPA6 and YFP-HSPA1A associated with cytoplasmic structures resembling centrioles (boxed areas) as confirmed in Fig. 1c with the centriole-specific marker protein γ-tubulin (Brito et al. 2012). A more prolonged association of YFP-HSPA6 with centrioles was observed compared to YFP-HSPA1A (Fig. 1b).
Fig. 1.

YFP-tagged HSPA6 and HSPA1A were recruited to and dynamically associated with centrioles after thermal stress in differentiated human neuronal cells. a Live imaging demonstrated that YFP-HSPA6 and YFP-HSPA1A localized to putative centrioles (indicated by boxed areas) in the cytoplasm of differentiated human neuronal SH-SY5Y cells following heat shock at 43 °C for 20 min. 0 min = prior to heat shock. 20 min = after 20 min heat shock. Scale bar = 10 μm. b Time course of the localization of YFP-HSPA6 and YFP-HSPA1A with putative centrioles by live imaging. FI fluorescence intensity. Scale bar = 1.5 μm. c The centriole-specific marker protein, γ-tubulin, confirmed that the YFP-tagged HSPA6- and HSPA1A-positive cytoplasmic structures were centrioles following immunocytochemistry on fixed-cell preparations. Scale bars = 10 and 1.5 μm. d Upper panel Live imaging of the time course of photobleaching recovery of YFP-HSPA6 and YFP-HSPA1A after FRAP of individual centrioles. Pre-B pre-bleached. Scale bar = 1.5 μm; Lower panel FRAP data of YFP-tagged HSPA6 and HSPA1A at centrioles (n = 25) from multiple photobleaching experiments
FRAP was employed to investigate dynamic aspects of the association of YFP-tagged HSPA6 and HSPA1A with centrioles. As shown in Fig. 1d (upper panel), the photobleaching recovery sequence at the level of individual centrioles was dynamic and, 15 s after bleaching, the surrounding pool of YFP-tagged HSPA6 and HSPA1A molecules exhibited repopulation of the centriole that was specifically targeted for bleaching.
Quantitative assessment, compiling data from multiple centriole photobleachings (Fig. 1d, lower panel), revealed a τ1/2 (time of 50 % maximal recovery) of 6.70 (± 0.37 SEM) seconds for YFP-HSPA6 and 12.46 (± 0.57 SEM) seconds for YFP-HSPA1A. The faster τ1/2 for YFP-HSPA6 suggested that it exhibited a faster rate of exchange with centriole-specific sites compared to YFP-HSPA1A. The plateau recovery levels obtained from Fig. 1d, lower panel, when compared to the initial unbleached fluorescence, permitted determination of the immobile fraction, a measure of the centriole-bound YFP-tagged molecules unable to participate in exchange with surrounding unbleached YFP-tagged molecules. The reduced immobile fraction observed for YFP-HSPA6 (0.14 ± 0.02 SEM), compared to YFP-HSPA1A (0.26 ± 0.02 SEM), indicated that a greater proportion of centriole sites occupied by HSPA6 are in flux with surrounding HSPA6 compared to HSPA1A.
Overall, the data suggested that YFP-tagged HSPA6 and HSPA1A participated in cycles of binding and release with centriole targets as neurons recovered from thermal stress, with HSPA6 demonstrating a faster rate of exchange and fewer HSPA6 molecules irreversibly bound to centriole components compared to HSPA1A.
Kinetics of the association of YFP-HSPA6 and HSPA1A with nuclear speckles and the nucleolus of stressed human neurons
Live imaging demonstrated that YFP-tagged HSPA6 and HSPA1A associated with nuclear components after heat shock in differentiated human neuronal cells as shown in Fig. 2a, b. These YFP-associated nuclear components colocalized with SON, a marker protein for nuclear speckles (dashed arrows) that are rich in RNA splicing factors (Fig. 2c) (Sharma et al. 2010; Spector and Lamond 2011). FRAP recovery profiles for nuclear speckle-associated YFP-HSPA6 and YFP-HSPA1A (shown in Fig. 2d), demonstrated τ1/2 values of 7.48 (± 0.41 SEM) seconds and 10.33 (± 0.57 SEM) seconds, indicating a faster exchange of HSPA6 with nuclear speckle targets compared to HSPA1A. However, the magnitude of the difference was not as great as that observed for centrioles in Fig. 1d. The immobile fraction values of 0.14 (± 0.02 SEM) and 0.17 (± 0.02 SEM), suggested a similar degree of irreversible binding of HSPA6 and HSPA1A to nuclear speckle components.
Fig. 2.

Targeting and dynamic interaction of YFP-tagged HSPA6 and HSPA1A with nuclear speckles following heat shock. a YFP-HSPA6 and YFP-HSPA1A rapidly localized to nuclear components (indicated by dashed arrows) in human neuronal cells after thermal stress. 20 min = after 20 min heat shock. Scale bar = 10 μm. b Time course of the association of YFP-tagged HSPA6 and HSPA1A with nuclear components. Scale bar = 1.5 μm. c Nuclear YFP-HSPA6 and YFP-HSPA1A colocalized with the nuclear speckle marker, SON. Scale bar = 10 and 1.5 μm. d Upper panel FRAP recovery profiles of an individual nuclear speckle after photobleaching. Scale bar = 1.5 μm. Lower panel FRAP data of YFP-tagged HSPA6 and HSPA1A at nuclear speckles (n = 25)
Subsequently, YFP-HSPA6 and YFP-HSPA1A associated with donut-shaped nuclear components (Fig. 3a, b) that colocalized (Fig. 3c) with nucleophosmin (NP), a marker protein for the granular component (GC) of the nucleolus (solid arrows), which is involved in rRNA processing and ribosomal subunit assembly (Sirri et al. 2008; Hernandez-Verdun et al. 2010). FRAP recovery profiles for nucleolar-associated YFP-HSPA6 and YFP-HSPA1A (shown in Fig. 3d) revealed τ1/2 values of 7.02 (± 0.42 SEM) and 8.60 (± 0.65 SEM) seconds, respectively. The immobile fraction values of 0.37 (± 0.02 SEM) and 0.43 (± 0.01 SEM) indicated that a greater portion of the HSPA6 and HSPA1A molecules are irreversibly bound to nucleolar-specific sites compared to centriole- or nuclear speckle-specific sites.
Fig. 3.
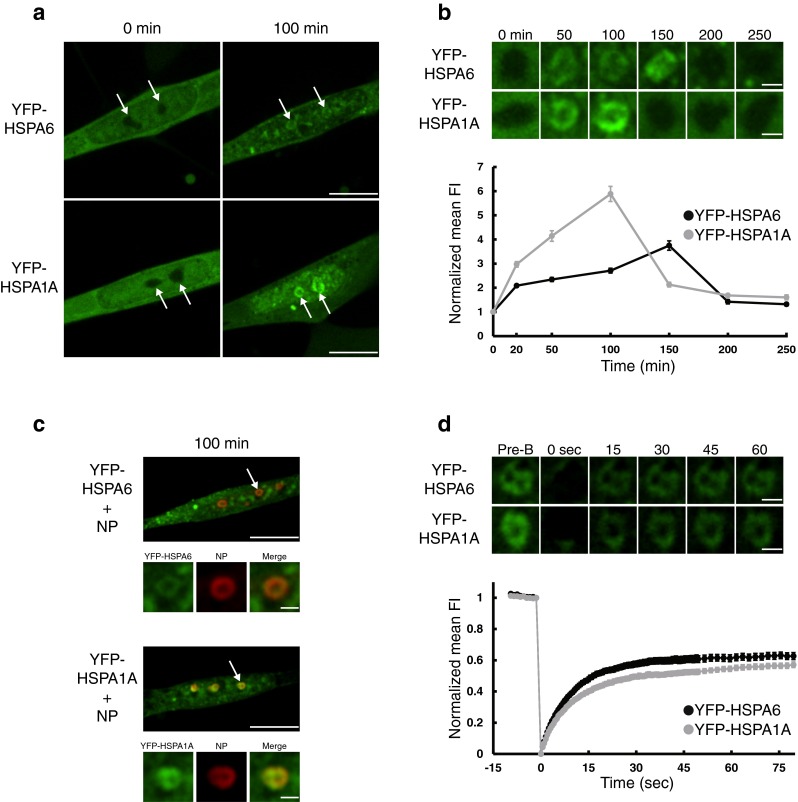
Localization of YFP-tagged HSPA6 and HSPA1A to the nucleolus in human neuronal cells after thermal stress. a YFP-HSPA6 and YFP-HSPA1A subsequently localized to donut-shaped components in neuronal nuclei (indicated by solid arrows). 100 min = 100 min after start of heat shock. Scale bar = 10 μm. b Association of YFP-tagged HSPA6 and HSPA1A with donut-shaped nuclear structures. Scale bar = 1.5 μm. c nucleophosmin (NP), a marker protein for the granular component of the nucleolus, colocalized with the donut-shaped nuclear structures that are YFP-tagged HSPA6 and HSPA1A positive. Scale bar = 10 μm and 1.5 μm. d Upper panel Photobleaching recovery profiles of individual YFP-HSPA6 and YFP-HSPA1A positive nucleoli. Scale bar = 1.5 μm. Lower panel FRAP data of YFP-tagged HSPA6 and HSPA1A at the nucleolus (n = 25)
HSPA6 exhibited dynamism in its association with the periphery of nuclear speckles following neuronal stress
After the stress-induced association of YFP-HSPA6 and YFP-HSPA1A with the granular component of the nucleolus (Fig. 3), YFP-HSPA6, but not YFP-HSPA1A, exhibited bright star-like foci (arrowheads) in the nucleus of differentiated human neurons (Fig. 4a, b) that localized around the periphery of SON-positive nuclear speckles (Fig. 4c). These perispeckles are sites of active messenger RNA (mRNA) transcription that have been termed “transcription factories” (Brown et al. 2008; Spector and Lamond 2011; Rieder et al. 2012; Rieder et al. 2014). The unique localization of HSPA6, but not HSPA1A, at the periphery of nuclear speckles suggested that this little studied member of the HSPA family, which is not present in current animal models of neurodegenerative diseases, is associated with the recovery of transcription in neuronal cells after stressful stimuli.
Fig. 4.
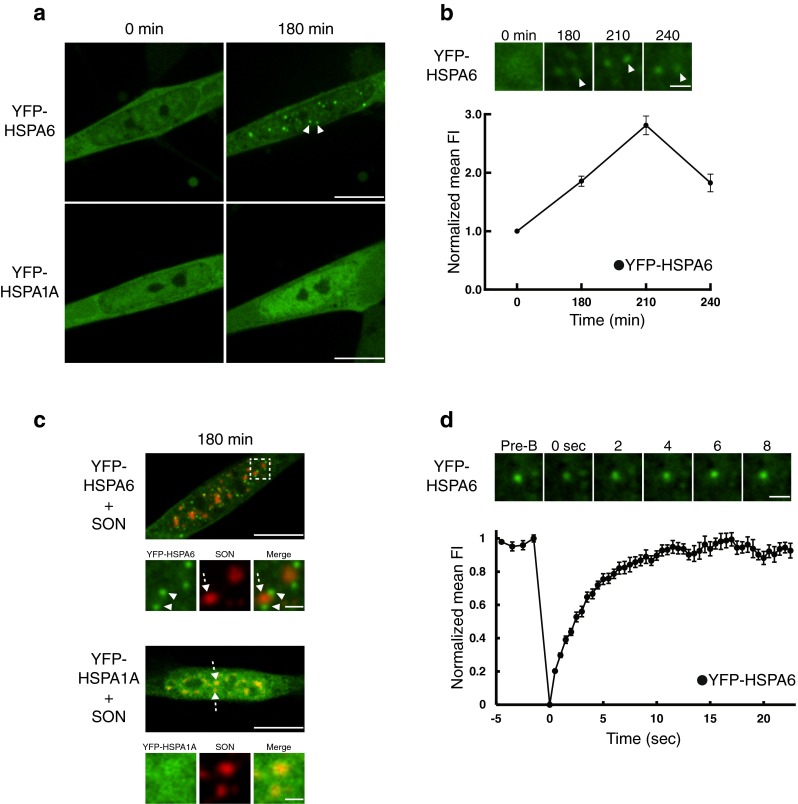
Following neuronal stress, YFP-HSPA6, but not YFP-HSPA1A, demonstrated a highly dynamic association with the periphery of nuclear speckles (perispeckles). a Following association with nucleolar components, YFP-HSPA6 localized to bright star-like foci (indicated by arrowheads) in the nucleus of neuronal cells, whereas YFP-HSPA1A did not. 180 min = 180 min after start of heat shock. Scale bar = 10 μm. b Localization of YFP-HSPA6 to bright star-like nuclear foci. Scale bar = 1.5 μm. c Bright star-like nuclear foci (termed “perispeckles”) that are YFP-HSPA6 positive (green signal indicated by arrowheads) are located at the periphery of SON-positive nuclear speckles (red signal indicated by dotted arrows). Scale bars = 10 and 1.5 μm. d Upper panel Recovery profile after photobleaching of an individual YFP-HSPA6 positive perispeckle. Scale bar = 1.5 μm. Lower panel FRAP data of YFP-tagged HSPA6 and HSPA1A at perispeckles (n = 15).
As shown in Fig. 4d (upper panel), YFP-HSPA6 recovery after photobleaching of individual perispeckles was more rapid (maximal recovery by 8 s) compared to that observed for YFP-HSPA6 photobleaching recovery for centrioles, nuclear speckles or the nucleolus (60 s in Figs. 1d, 2d, and 3d, upper panel). This was confirmed in Fig. 4d, lower panel, by quantitative assessment compiling data from multiple perispeckle photobleachings. The analysis revealed a τ1/2 of 2.36 (± 0.16 SEM) seconds, indicating that YFP-HSPA6 demonstrated a faster rate of exchange with perispeckle-specific sites compared to centrioles, nuclear speckles, and the nucleolus which exhibited τ1/2 values of 6.70, 7.48, and 7.02 s, respectively, for YFP-HSPA6.
The FRAP recovery profile (Fig. 4d, lower panel) indicated that the association of YFP-HSPA6 with perispeckles demonstrated a very low immobile fraction value of 0.02 (± 0.02 SEM) compared to YFP-HSPA6 and YFP-HSPA1A immobile values associated with centrioles (0.14 and 0.26), nuclear speckles (0.14 and 0.17) and the nucleolus (0.37 and 0.43), respectively. The data shown in Fig. 4d indicated that HSPA6 exhibited very rapid exchange with perispeckles and that comparatively few HSPA6 molecules are irreversibly bound to perispeckles, which are sites of transcription factories. HSPA6, but not HSPA1A, associates with perispeckles at the time of transcriptional recovery from stress-induced inhibition. The FRAP data suggested that HSPA6 exhibited a highly dynamic flux with transcription machinery as it recovers from stress-induced inhibition in differentiated human neurons.
Live imaging time sequence of the effect of thermal stress on the intracellular localization of YFP-HSPA6 and YFP-HSPA1A in differentiated human neurons
The time sequence of the effect of thermal stress on the intracellular localization of YFP-HSPA6 in differentiated human neurons is presented by confocal live imaging in Fig. S1 (Supplementary Movie 1). Immediately after 20 min of heat shock at 43 °C, YFP-HSPA6 localized to centrioles in the cytoplasm (boxed areas in Fig. S1 and Fig. 1) and to nuclear speckles (dashed arrows in Fig. S1 and Fig. 2). YFP-HSPA6 next associated with the granule component of the nucleolus (solid arrows in Fig. S1 and Fig. 3). Subsequently, as localization to nuclear speckles and the nucleolus diminished, live imaging revealed the transient localization of YFP-HSPA6 to multiple bright star-like foci in the nucleus (arrowheads in Fig. S1 and characterized as perispeckles in Fig. 4).
The corresponding live imaging time sequence of the effect of heat shock on neuronal intracellular localization of YFP-HSPA1A is shown in Fig. S2 (Supplementary Movie 2). Heat shock induced YFP-HSPA1A to localize to centrioles (boxed areas), nuclear speckles (dashed arrows), and the nucleolus (solid arrows) as was observed for YFP-HSPA6 (characterized in Figs. 1, 2, and 3). However, as association of YFP-HSPA1A to these structures diminished, YFP-HSPA1A did not localize to the multiple bright star-like foci that were observed throughout the nucleus for YFP-HSPA6 and termed perispeckles (characterized in Fig. 4) that are clustered around the periphery of nuclear speckles. Hence, the live imaging time sequence presented in Fig. S1 and Fig. S2 (Supplementary Movies 1 and 2) confirmed the results shown in Fig. 4 that the localization of YFP-HSPA6 to perispeckles is specific and not apparent for HSPA1A.
Discussion
In the present study, live imaging and FRAP were utilized to further knowledge of HSPA6 (Hsp70B’), a little studied member of the HSPA (Hsp70) multigene family that is present in the human genome but absent in the genomes of mouse and rat (Chow and Brown 2007; Noonan et al. 2007a; Noonan et al. 2007b; Noonan et al. 2008a; Noonan et al. 2008b; Chow et al. 2010; Ramirez et al. 2015; Deane and Brown 2016). Following thermal stress, changes in the intracellular localization of HSPA6, and the more widely studied HSPA1A (Hsp70–1), were visualized in living differentiated human neuronal SH-SY5Y cells, with FRAP employed to compare the dynamics of the exchange of these two Hsps with stress-sensitive cytoplasmic and nuclear structures.
Human neuronal SH-SY5Y cells have been used previously as a model neuronal system for neurodegenerative diseases (Cheung et al. 2009; Agholme et al. 2010; Lopes et al. 2010). Our present study was carried out on SH-SY5Y cells that have been differentiated with retinoic acid to exhibit neuronal features including cessation of cell division, development of long bipolar neuronal-like cellular processes and display of biochemical markers of neuronal differentiation (Pahlman et al. 1984; Lopez-Carballo et al. 2002; El Andaloussi-Lilja et al. 2009). Retinoic acid, which we employ to trigger neuronal differentiation in SH-SY5Y cells, is an endogenous signaling molecule of neuronal differentiation during in vivo development of the nervous system (Jacobs et al. 2006; Maden 2007; Goodman et al. 2012).
Live imaging demonstrated that following thermal stress, both YFP-HSPA6 and YFP-HSPA1A rapidly localized to centrioles in the cytoplasm of differentiated neurons. FRAP revealed that HSPA6 exhibited a faster exchange and fewer irreversibly bound molecules with centriole components compared to HSPA1A (summarized in Table 1).
Table 1.
Summary of FRAP data on YFP-HSPA6 and YFP-HSPA1A
| Stress-sensitive structure | FRAP | YFP-HSPA6 | YFP-HSPA1A | FRAP | YFP-HSPA6 | YFP-HSPA1A |
|---|---|---|---|---|---|---|
| Centrioles | Halftime recovery (τ1/2) seconds | 6.70 | 12.46 | Immobile fraction | 0.14 | 0.26 |
| Nuclear speckles | Halftime recovery (τ1/2) seconds | 7.48 | 10.33 | Immobile fraction | 0.14 | 0.17 |
| Nucleolus (GC) | Halftime recovery (τ1/2) seconds | 7.02 | 8.60 | Immobile fraction | 0.37 | 0.43 |
| Perispeckles | Halftime recovery (τ1/2) seconds | 2.36 | – | Immobile fraction | 0.02 | – |
Centrioles play important roles in controlling the polarity and migration of neuronal cells (de Anda et al. 2005; de Anda et al. 2010; Ge et al. 2010; Lizarraga et al. 2010; Kuijpers and Hoogenraad 2011). During evolution of the human brain, the neocortex, which is involved in higher cognitive function, has expanded greatly compared to other mammals (Borrell and Reillo 2012). This expansion of the neocortex involves (i) enhanced production and polarity determination of the large number of neurons that are destined for the human neocortex (Florio et al. 2015) and (ii) greater neuronal migratory distances to reach their functional destinations in the brain (Taverna et al. 2014). Hence, human neurons experience longer time periods during which cell polarity and cell migration could be distorted by cellular stress. Stress-inducible HSPA6 is present in the human genome, however, it is absent in the genomes of rat and mouse (Noonan et al. 2008b). HSPA6 exhibits a faster exchange and fewer irreversibly bound molecules with stress-sensitive centriole components compared to the widely studied HSPA1A. These features could reflect that HSPA6 evolved to confer enhanced stress repair potential to centrioles in human neurons to buffer against stress-induced distortion of cellular polarity and migration that are critical to the expansion of the neocortex that has occurred during the evolution of the human brain (Taverna et al. 2014; Florio et al. 2015).
Nuclear speckles, rich in RNA splicing factors (Hall et al. 2006), and the nucleolus, particularly the granular component involved in rRNA processing and ribosomal subunit assembly (Hernandez-Verdun et al. 2010), were also targeted by YFP-tagged HSPA6 and HSPA1A, suggesting they are stress-sensitive nuclear sites. FRAP revealed that differences in τ1/2 values and the extent of molecules bound irreversibly to nuclear speckle and nucleolar components were not as pronounced for HSPA6 vs. HSPA1A, compared to the FRAP observations for the more dynamic association of HSPA6 with centriole components (summarized in Table 1). Nuclear speckles and the nucleolus play basic roles in the metabolism of all cells (Boisvert et al. 2007; Spector and Lamond 2011). Cell polarity and cell migration in human neuronal cells, in which centrioles play a key role (de Anda et al. 2005; de Anda et al. 2010; Ge et al. 2010; Lizarraga et al. 2010; Kuijpers and Hoogenraad 2011), have facilitated the unique expansion of the neocortex during evolution of the human brain (Taverna et al. 2014; Florio et al. 2015).
Interestingly, our data revealed new information on the little studied HSPA6 that is found in the human genome and not in mouse and rat and hence is not present in current animal models of neurodegenerative diseases. HSPA6, but not HSPA1A, associated with bright star-like foci located at the periphery of nuclear speckles following thermal stress. These perispeckles are sites of active mRNA transcription that have been characterized as “transcription factories” (Brown et al. 2008; Spector and Lamond 2011; Rieder et al. 2012; Rieder et al. 2014). The unique localization of HSPA6, but not HSPA1A, to perispeckles, suggests that HSPA6 may be associated with the recovery of transcription in human neuronal cells after inhibition by stressful stimuli (Allen et al. 2004; Hieda et al. 2005; Espinoza et al. 2007; Yakovchuk et al. 2009). Our on-going experiments indicate that localization of HSPA6 to perispeckles is disrupted by application of a transcriptional inhibitor during the recovery phase. The FRAP data revealed that recovery of YFP-HSPA6 from photobleaching was more rapid in perispeckles compared to centrioles, nuclear speckles or the nucleolus (8 s compared to 60 s), and this was confirmed quantitatively by τ1/2 values summarized in Table 1. In addition, the association of YFP-HSPA6 with perispeckles demonstrated the lowest immobile fraction compared to either HSPA6 or HSPA1A associated with centriole, nuclear speckle or nucleolar components (Table 1).
The FRAP data suggests that HSPA6 exhibits a unique feature not observed for HSPA1A, namely a rapid and highly dynamic exchange with perispeckles, which have been characterized as transcription sites (Brown et al. 2008; Spector and Lamond 2011; Rieder et al. 2012; Rieder et al. 2014). The presence of HSPA6 in the human genome could provide human neuronal cells with a highly dynamic mechanism for transcriptional recovery after stressful stimuli. This is particularly critical for the human brain that requires rapid recovery of neurons involved in higher cognitive function in the greatly expanded human neocortex compared to instinctive neural functions that are operative in mouse and rat (Lui et al. 2011; Geschwind and Rakic 2013; Taverna et al. 2014; Florio et al. 2015).
Electronic supplementary material
Supplementary Movie 1: YFP-HSPA6. Live imaging time sequence of the intracellular localization of YFP-tagged HSPA6 in differentiated human neuronal cells following a 20 min heat shock at 43 °C. The same conventions used in Figs. 1, 2, 3, 4 were employed in the movie: boxed areas = centrioles; dashed arrows = nuclear speckles; solid arrows = granular component of nucleolus; arrowheads = perispeckles. (AVI 88007 kb)
Supplementary Movie 2: YFP-HSPA1A. Live imaging time sequence of YFP-tagged HSPA1A intracellular localization following heat shock of differentiated human neuronal cells. The same conventions used in Figs. 1, 2, 3, 4 were employed in the movie: boxed areas = centrioles; dashed arrows = nuclear speckles; solid arrows = granular component of nucleolus. (AVI 92712 kb)
Acknowledgments
This study is supported by grants from NSERC to I.R.B.
References
- Agholme L, Lindstrom T, Kagedal K, Marcusson J, Hallbeck M. An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis. 2010;20:1069–1082. doi: 10.3233/JAD-2010-091363. [DOI] [PubMed] [Google Scholar]
- Allen TA, Von Kaenel S, Goodrich JA, Kugel JF. The SINE-encoded mouse B2 RNA represses mRNA transcription in response to heat shock. Nat Struct Mol Biol. 2004;11:816–821. doi: 10.1038/nsmb813. [DOI] [PubMed] [Google Scholar]
- Asea AA, Brown IR (eds) (2008) Heat shock proteins and the brain: implications for neurodegenerative diseases and neuroprotection. Springer Science + Business Media B.V.
- Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- Borrell V, Reillo I. Emerging roles of neural stem cells in cerebral cortex development and evolution. Dev Neurobiol. 2012;72:955–971. doi: 10.1002/dneu.22013. [DOI] [PubMed] [Google Scholar]
- Brito DA, Gouveia SM, Bettencourt-Dias M. Deconstructing the centriole: structure and number control. Curr Opin Cell Biol. 2012;24:4–13. doi: 10.1016/j.ceb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Brown JM, et al. Association between active genes occurs at nuclear speckles and is modulated by chromatin environment. J Cell Biol. 2008;182:1083–1097. doi: 10.1083/jcb.200803174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YT, Lau WK, Yu MS, Lai CS, Yeung SC, So KF, Chang RC. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009;30:127–135. doi: 10.1016/j.neuro.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Chow AM, Brown IR. Induction of heat shock proteins in differentiated human and rodent neurons by celastrol. Cell Stress Chaperones. 2007;12:237–244. doi: 10.1379/CSC-269.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AM, Mok P, Xiao D, Khalouei S, Brown IR. Heteromeric complexes of heat shock protein 70 (HSP70) family members, including Hsp70B’, in differentiated human neuronal cells. Cell Stress Chaperones. 2010;15:545–553. doi: 10.1007/s12192-009-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Anda FC, Meletis K, Ge X, Rei D, Tsai LH. Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci. 2010;30:10391–10406. doi: 10.1523/JNEUROSCI.0381-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature. 2005;436:704–708. doi: 10.1038/nature03811. [DOI] [PubMed] [Google Scholar]
- Deane CA, Brown IR. Induction of heat shock proteins in differentiated human neuronal cells following co-application of celastrol and arimoclomol. Cell Stress Chaperones. 2016 doi: 10.1007/s12192-016-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel P, Chai CL, Sperlagh B, Huleatt PB, Matyus P. Clinical utility of neuroprotective agents in neurodegenerative diseases: current status of drug development for Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis. Expert Opin Investig Drugs. 2012;21:1267–1308. doi: 10.1517/13543784.2012.703178. [DOI] [PubMed] [Google Scholar]
- El Andaloussi-Lilja J, Lundqvist J, Forsby A. TRPV1 expression and activity during retinoic acid-induced neuronal differentiation. Neurochem Int. 2009;55:768–774. doi: 10.1016/j.neuint.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Espinoza CA, Goodrich JA, Kugel JF. Characterization of the structure, function, and mechanism of B2 RNA, an ncRNA repressor of RNA polymerase II transcription. RNA. 2007;13:583–596. doi: 10.1261/rna.310307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio M, et al. Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science. 2015;347:1465–1470. doi: 10.1126/science.aaa1975. [DOI] [PubMed] [Google Scholar]
- Ge X, Frank CL, Calderon de Anda F, Tsai LH. Hook3 interacts with PCM1 to regulate pericentriolar material assembly and the timing of neurogenesis. Neuron. 2010;65:191–203. doi: 10.1016/j.neuron.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T, Crandall JE, Nanescu SE, Quadro L, Shearer K, Ross A, McCaffery P. Patterning of retinoic acid signaling and cell proliferation in the hippocampus. Hippocampus. 2012;22:2171–2183. doi: 10.1002/hipo.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LL, Smith KP, Byron M, Lawrence JB. Molecular anatomy of a speckle. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:664–675. doi: 10.1002/ar.a.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Verdun D, Roussel P, Thiry M, Sirri V, Lafontaine DL. The nucleolus: structure/function relationship in RNA metabolism. Wiley Interdiscip Rev RNA. 2010;1:415–431. doi: 10.1002/wrna.39. [DOI] [PubMed] [Google Scholar]
- Hieda M, Winstanley H, Maini P, Iborra FJ, Cook PR. Different populations of RNA polymerase II in living mammalian cells. Chromosom Res. 2005;13:135–144. doi: 10.1007/s10577-005-7720-1. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalouei S, Chow AM, Brown IR. Stress-induced localization of HSPA6 (HSP70B’) and HSPA1A (HSP70-1) proteins to centrioles in human neuronal cells. Cell Stress Chaperones. 2014;19:321–327. doi: 10.1007/s12192-013-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalouei S, Chow AM, Brown IR. Localization of heat shock protein HSPA6 (HSP70B’) to sites of transcription in cultured differentiated human neuronal cells following thermal stress. J Neurochem. 2014;131:743–754. doi: 10.1111/jnc.12970. [DOI] [PubMed] [Google Scholar]
- Kuijpers M, Hoogenraad CC. Centrosomes, microtubules and neuronal development. Mol Cell Neurosci. 2011;48:349–358. doi: 10.1016/j.mcn.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Lizarraga SB, et al. Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development. 2010;137:1907–1917. doi: 10.1242/dev.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes FM, et al. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 2010;1337:85–94. doi: 10.1016/j.brainres.2010.03.102. [DOI] [PubMed] [Google Scholar]
- Lopez-Carballo G, Moreno L, Masia S, Perez P, Barettino D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem. 2002;277:25297–25304. doi: 10.1074/jbc.M201869200. [DOI] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Noonan E, Giardina C, Hightower L. Hsp70B’ and Hsp72 form a complex in stressed human colon cells and each contributes to cytoprotection. Exp Cell Res. 2008;314:2468–2476. doi: 10.1016/j.yexcr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Noonan EJ, Fournier G, Hightower LE. Surface expression of Hsp70B’ in response to proteasome inhibition in human colon cells. Cell Stress Chaperones. 2008;13:105–110. doi: 10.1007/s12192-007-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Giardina C, Hightower LE. Hsp70B’ regulation and function. Cell Stress Chaperones. 2007;12:393–402. doi: 10.1379/CSC-278e.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Rasoulpour RJ, Giardina C, Hightower LE. Cell number-dependent regulation of Hsp70B’ expression: evidence of an extracellular regulator. J Cell Physiol. 2007;210:201–211. doi: 10.1002/jcp.20875. [DOI] [PubMed] [Google Scholar]
- Pahlman S, Ruusala AI, Abrahamsson L, Mattsson ME, Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984;14:135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2015;55:353–371. doi: 10.1146/annurev-pharmtox-010814-124332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez VP, Stamatis M, Shmukler A, Aneskievich BJ. Basal and stress-inducible expression of HSPA6 in human keratinocytes is regulated by negative and positive promoter regions. Cell Stress Chaperones. 2015;20:95–107. doi: 10.1007/s12192-014-0529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Rieder D, et al. Co-expressed genes prepositioned in spatial neighborhoods stochastically associate with SC35 speckles and RNA polymerase II factories. Cell Mol Life Sci. 2014;71:1741–1759. doi: 10.1007/s00018-013-1465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder D, Trajanoski Z, McNally JG. Transcription factories. Front Genet. 2012;3:221. doi: 10.3389/fgene.2012.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Takata H, Shibahara K, Bubulya A, Bubulya PA. Son is essential for nuclear speckle organization and cell cycle progression. Mol Biol Cell. 2010;21:650–663. doi: 10.1091/mbc.E09-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirri V, Urcuqui-Inchima S, Roussel P, Hernandez-Verdun D. Nucleolus: the fascinating nuclear body. Histochem Cell Biol. 2008;129:13–31. doi: 10.1007/s00418-007-0359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp EL, Altan N, Lippincott-Schwartz J. Measuring protein mobility by photobleaching GFP chimeras in living cells. Curr Protoc Cell Biol. 2003;Chapter 21:Unit 21.1. doi: 10.1002/0471143030.cb2101s19. [DOI] [PubMed] [Google Scholar]
- Spector DL, Lamond AI (2011) Nuclear speckles. Cold Spring Harb Perspect Biol 3. doi:10.1101/cshperspect.a000646 [DOI] [PMC free article] [PubMed]
- Sprague BL, McNally JG. FRAP analysis of binding: proper and fitting. Trends Cell Biol. 2005;15:84–91. doi: 10.1016/j.tcb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Taverna E, Gotz M, Huttner WB. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- Yakovchuk P, Goodrich JA, Kugel JF. B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Proc Natl Acad Sci U S A. 2009;106:5569–5574. doi: 10.1073/pnas.0810738106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie 1: YFP-HSPA6. Live imaging time sequence of the intracellular localization of YFP-tagged HSPA6 in differentiated human neuronal cells following a 20 min heat shock at 43 °C. The same conventions used in Figs. 1, 2, 3, 4 were employed in the movie: boxed areas = centrioles; dashed arrows = nuclear speckles; solid arrows = granular component of nucleolus; arrowheads = perispeckles. (AVI 88007 kb)
Supplementary Movie 2: YFP-HSPA1A. Live imaging time sequence of YFP-tagged HSPA1A intracellular localization following heat shock of differentiated human neuronal cells. The same conventions used in Figs. 1, 2, 3, 4 were employed in the movie: boxed areas = centrioles; dashed arrows = nuclear speckles; solid arrows = granular component of nucleolus. (AVI 92712 kb)


