Abstract
Increased intracellular heat shock protein-72 (Hsp72) and heat shock protein-90α (Hsp90α) have been implicated as important components of acquired thermotolerance, providing cytoprotection during stress. This experiment determined the physiological responses characterising increases in Hsp72 and Hsp90α mRNA on the first and tenth day of 90-min heat acclimation (in 40.2 °C, 41.0 % relative humidity (RH)) or equivalent normothermic training (in 20 °C, 29 % RH). Pearson’s product-moment correlation and stepwise multiple regression were performed to determine relationships between physiological [e.g. (Trec, sweat rate (SR) and heart rate (HR)] and training variables (exercise duration, exercise intensity, work done), and the leukocyte Hsp72 and Hsp90α mRNA responses via reverse transcription quantitative polymerase chain reaction (RT-QPCR) (n = 15). Significant (p < 0.05) correlations existed between increased Hsp72 and Hsp90α mRNA (r = 0.879). Increased core temperature was the most important criteria for gene transcription with ΔTrec (r = 0.714), SR (r = 0.709), Trecfinal45 (r = 0.682), area under the curve where Trec ≥ 38.5 °C (AUC38.5 °C; r = 0.678), peak Trec (r = 0.661), duration Trec ≥ 38.5 °C (r = 0.650) and ΔHR (r = 0.511) each demonstrating a significant (p < 0.05) correlation with the increase in Hsp72 mRNA. The Trec AUC38.5 °C (r = 0.729), ΔTrec (r = 0.691), peak Trec (r = 0.680), Trecfinal45 (r = 0.678), SR (r = 0.660), duration Trec ≥ 38.5 °C (r = 0.629), the rate of change in Trec (r = 0.600) and ΔHR (r = 0.531) were the strongest correlate with the increase in Hsp90α mRNA. Multiple regression improved the model for Hsp90α mRNA only, when Trec AUC38.5 °C and SR were combined. Training variables showed insignificant (p > 0.05) weak (r < 0.300) relationships with Hsp72 and Hsp90α mRNA. Hsp72 and Hsp90α mRNA correlates were comparable on the first and tenth day. When transcription of the related Hsp72 and Hsp90α mRNA is important, protocols should rapidly induce large, prolonged changes in core temperature.
Keywords: Heat shock proteins, Hyperthermia, Core temperature, Heat acclimation, Thermotolerance
Introduction
Thermotolerance is an acquired cellular adaptation to heat stress (Kuennen et al. 2011) conferring cytoprotection to subsequent thermal (McClung et al. 2008) and non-thermal (Gibson et al. 2015c) stress in vitro (McClung et al. 2008) and in vivo (Lee et al. 2016). Acquired thermotolerance is reliant upon sufficient heat shock protein (HSP) gene transcription (Moran et al. 2006) and subsequent protein translation (Silver and Noble 2012). Functionally, HSPs facilitate maintenance of cellular and protein homeostasis, with regulatory roles in mitigating apoptosis, and facilitating recovery from and adaptation to stress [including exercise training (Liu et al. 1999) and/or thermal stress (Kuennen et al. 2011)] at a cellular, organ and whole body level (Henstridge et al. 2016).
The HSP70 family is present in two predominant isoforms: a constitutively expressed protein that demonstrates little basal change, HSC70 (HSP73), and a highly inducible ‘chaperone’ isoform HSP72 (HSPA1A/HSPA1B) central to cytoprotection (Kampinga et al. 2009). HSP90 also presents with a constitutively expressed isoform (HSP90β) and an inducible isoform, HSPC1 (HSP90α) (Subbarao Sreedhar et al. 2004). HSP72 provides cellular protection, notably maintaining intestinal epithelial tight junction barriers, increasing resistance to gut-associated endotoxin translocation and reducing inflammatory responses to stress (Moseley 2000; Amorim et al. 2015; Dokladny et al. 2016). In addition, HSP72 may be important in facilitating positive heat (Kuennen et al. 2011) and heat-independent adaptations (Henstridge et al. 2016). HSP90α is cytoprotective, similar to HSP72, whilst also implicit in recovery and adaptation to cellular stress, particularly the control of cellular signalling cascades (Taipale et al. 2010), recovery of global protein synthesis (Duncan 2005) and coordination of cellular repair (Erlejman et al. 2014). Increases in extracellular HSP72 (eHSP72) have been widely observed in response to acute exercise (Whitham et al. 2007; Périard et al. 2012; Gibson et al. 2014), with endogenous criteria, notably increased core temperature most important for eliciting large increases (Périard et al. 2012; Gibson et al. 2014). These eHSP72 increases are transient (Périard et al. 2012; Gibson et al. 2014) and have a proposed immunological role (Asea 2003) rather than initiating chronically beneficial (i.e. cytoprotective) HSP72 protein translation that is retained beyond the initial stressor (Marshall et al. 2007; Périard et al. 2015). Therefore, the usefulness of extracellular HSPs to characterise acquired thermotolerance (Moseley 1997; Kregel 2002), to identify cessation of the cellular stress response following adaptation in vivo (McClung et al. 2008; Kuennen et al. 2011) and ex vivo (McClung et al. 2008), or to identify functional roles in disease states (Henstridge et al. 2014a; Krause et al. 2015a) is inferior to that of the HSP gene transcript or translated protein (Lee et al. 2015). At present, the precise physiological signals for increasing Hsp72 mRNA and Hsp90α mRNA are unknown, as is whether these genes transcribe to similar stimuli and similar magnitudes during exercise/exercise-heat stress. Accordingly, similar characterisation of Hsp72 and Hsp90α gene transcription to that of eHSP72 is required given their direct relationship with thermotolerance (Lee et al. 2015).
Exercise elicits numerous cellular and molecular stressors that in isolation or combination behave as inductive stimuli for increases in HSPs (Henstridge et al. 2016). Stimuli characterising changes include, but are not limited to, whole body and local hyperthermia (Fehrenbach et al. 2001), oxidative stress/free radical formation (Khassaf et al. 2001; Taylor et al. 2010a), substrate depletion (Febbraio et al. 2002), hypoxia/ischaemia (Taylor et al. 2011), altered pH (Peart et al. 2011) and increased calcium concentration (Stary and Hogan 2016). Elevated expressions of both intracellular HSP72 (iHSP72) and intracellular HSP90α (iHSP90α) are largely dictated by their transcription factor heat shock factor 1 (HSF1), which is translocated to the nucleus where it binds to the heat shock elements (HSEs), resulting in relevant mRNA (Hsp) transcription. HSP72 and HSP90α demonstrate large changes in the net intracellular protein following acute and chronic exercise that initiates their respective gene transcripts (McClung et al. 2008; Tuttle et al. 2015). It has been demonstrated that HSP72 increases in response to thermal stress (Magalhães et al. 2010), though others have observed HSP72 protein translation as being independent of increased core and/or muscle temperature (Morton et al. 2007). At present, changes in Hsp72 mRNA, and particularly Hsp90α mRNA following heat acclimation, have not been reported relative to specific physiological stimuli either experimentally or retrospectively. As such, a dose response or minimum stimuli characterising significant transcription-translation has yet to be determined. Ambiguity in HSP response to thermal and exercise stimuli, notably during comparable heat acclimation (HA) regimes (Magalhães et al. 2010; Hom et al. 2012), suggests that a combination of/or minimum threshold for elevated endogenous stressors may be required to increase HSP protein content in vivo; such responses may well be individualised and determined by genetic, epigenetic and phenotypical factors (Horowitz 2014; Horowitz 2016). Consequently, preliminary data relative to such characterisation is required in vivo from a homogenous sample. Additionally, given the potential for epigenetic modifications in Hsp transcription (Horowitz 2016), it remains unknown whether the signals characterising increased gene expression would demonstrate equality at the onset and culmination of a HA protocol.
Inhibition of HSF1 has been proposed to increase susceptibility to acute in vivo thermal stress [i.e. heat stroke (Moran et al. 2006)], and similarly preclude procurement of optimal physiological adaptation to chronic thermal stress [i.e. heat-acclimated phenotype (Maloyan and Horowitz 2002; Kuennen et al. 2011)]. Induction of HSPs, particularly HSP72, is central to not only to the aforementioned heat adaptation (Kuennen et al. 2011), but are increasingly implicated within other positive adaptive responses to stress [i.e. promotion of mitochondrial biogenesis (Henstridge et al. 2014a)] and various disease states [e.g. type 2 diabetes mellitus (Hooper et al. 2014), cardiovascular disease (Noble and Shen 2012), and Parkinson’s disease (Erekat et al. 2014)]. Reduced iHSPs are observed in disease states such as type 2 diabetes mellitus in response to insulin-sensitive HSF1 inhibition (Kurucz et al. 2002), with heat stress-induced increases in HSP72 proving therapeutic (Gupte et al. 2011). Whilst understanding of the important role of heat shock proteins is growing, less is known of the physiological signals which facilitate the optimal transcription of the mRNA prior to protein translation (Anckar and Sistonen 2011). Characterising the signal or signals that predict Hsp72 mRNA and Hsp90α mRNA increases (and thus likely increased HSP) may enhance the efficacy of Henstridge et al. (2014b).
The aim of this experiment was to characterise the physiological stimuli (core temperature, heart rate, sweat rates) and/or training prescription markers (exercise duration, exercise intensity, exercise power and work done) that correlate most strongly with the increase in Hsp72 mRNA and Hsp90α mRNA during a 10-day HA regime or a comparable normothermic training intervention (Gibson et al. 2015c). Additionally, we sought to determine whether in a homogenous sample, experiencing equality of stress, whether the predictive criteria for Hsp72 mRNA and Hsp90α mRNA transcription would change pre- to post-HA or normothermic training. It was hypothesised that markers of thermal strain and heat storage, i.e. core temperature, would most closely predict the change in Hsp72 mRNA and Hsp90α mRNA, and these markers would demonstrate equality in predictive capacity at the beginning and end of HA/training.
Materials and methods
Participants
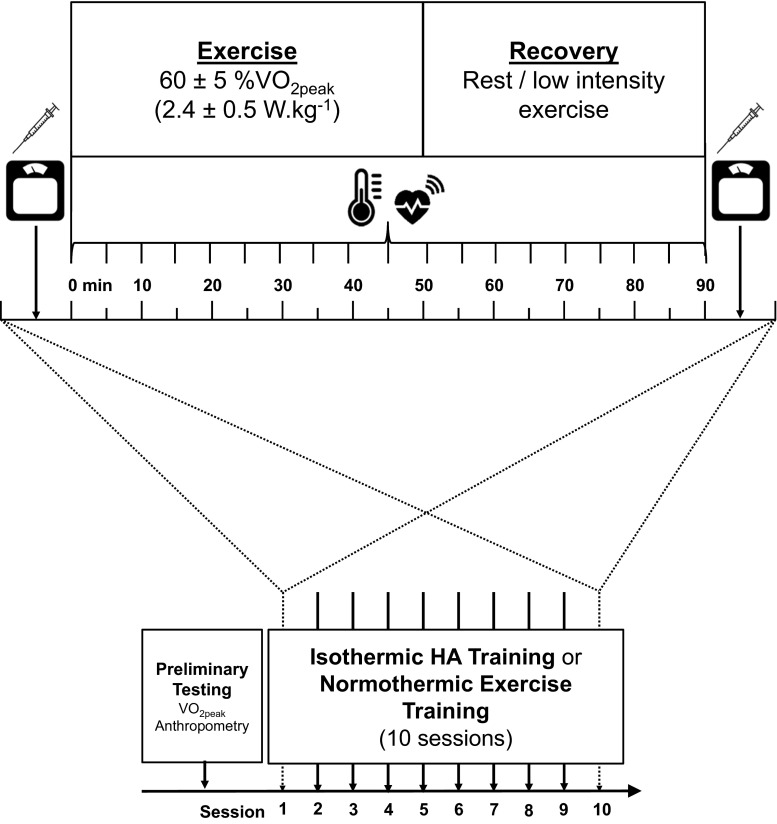
The analysis of Hsp72 mRNA and Hsp90α mRNA was performed on data collected from 15 participants who had performed ten 90-min isothermic HA sessions (n = 7; age = 23 ± 4 years, height = 183 ± 6 cm, mass = 76.4 ± 6.7 kg, body surface area = 1.98 ± 0.11 m2, body mass index = 22.9 ± 1.6 kg m2, body fat = 14.0 ± 3.1 %, O2peak = 4.16 ± 0.56 L min−1) or performed normothermic exercise training in a temperate environment (n = 8 age = 26 ± 5 years, height = 179 ± 7 cm, mass = 74.6 ± 4.8, body surface area = 1.93 ± 0.10 m2, body mass index = 23.2 ± 0.9 kg m2, body fat = 14.5 ± 2.6 %, O2peak = 4.22 ± 0.62) from one previously published experiment (Gibson et al. 2015c) (pooled descriptive characteristics in Table 1, schematic overview in Fig. 1). Given equality of training prescription [as detailed elsewhere (Gibson et al. 2015c)], both the isothermic HA and normothermic exercise training groups were pooled into one data set for each time point to increase the heterogeneity of the physiological responses and Hsp mRNA transcription. Confounding environmental (prolonged hyperthermic and/or hypoxic stress) and pharmacological variables were all controlled in line with previous work in the field (Gibson et al. 2014; Gibson et al. 2015a). Participants commenced all trials in a euhydrated state [<700 mOsm kg−1 H2O (Sawka et al. 2007)]. All protocols, procedures and methods were approved by the institutional ethics committee. Participants completed medical questionnaires and written informed consent following the principles outlined by the Declaration of Helsinki as revised in 2013 prior to commencing any preliminary or experimental sessions. In compliance with ethical approval, a testing/intervention session was terminated if a subject attained a core temperature [measured at the rectum (Trec)] of 39.7 °C (zero incidences).
Table 1.
Mean ± SD participant descriptive characteristics and correlations with Hsp72 mRNA (ALL) and Hsp90α mRNA (ALL) datasets
| Variable | Mean ± SD | Hsp72 mRNA | Hsp90α mRNA |
|---|---|---|---|
| Age (years) | 24.6 ± 4.4 | r = −0.066 | r = −0.222 |
| Height (cm) | 181 ± 6 | r = 0.084 | r = 0.419* |
| Mass (kg) | 75.4 ± 5.5 | r = −0.112 | r = 0.356 |
| Body surface area (m2) | 1.95 ± 0.10 | r = −0.066 | r = 0.412* |
| BMI (kg m2) | 23.1 ± 1.2 | r = −0.149 | r = −0.077 |
| Body fat (%) | 14.3 ± 2.7 | r = −0.102 | r = −0.085 |
| O2peak (L min−1) | 4.20 ± 0.56 | r = 0.079 | r = 0.316 |
| O2peak (mL kg min−1) | 55.6 ± 6.0 | r = 0.134 | r = 0.133 |
* denotes p < 0.05
Fig. 1.
Schematic overview of the study
Experimental design
Preliminary testing commenced with anthropometric assessment of participants, whom subsequently performed an incremental (24 W min−1) cycle test commencing at 80 W, in temperate laboratory conditions [20 °C, 40 % relative humidity (RH)] to determine peak oxygen uptake (O2peak) (Gibson et al. 2015c). Expired metabolic gas was measured at a breath by breath frequency (Metalyser 3B, Cortex, Leipzig, Germany) with O2peak defined as the highest average O2 obtained in any 30-s period. The confirmation of O2peak was made via the attainment of a heart rate (HR) within 10 b min−1 of age-predicted maximum, and respiratory exchange ratio (RER) >1.1 in all participants (Taylor et al. 1955). The data obtained during the O2peak test was subsequently used to prescribe the HA/normothermic training intervention.
Isothermic, also known as controlled hyperthermic, heat acclimation was implemented to optimise stress and adaptation throughout the regime (Taylor and Cotter 2006; Racinais et al. 2015). Each of the ten, 90-min HA sessions was performed in hot conditions (40.2 ± 0.4 °C, 41.0 ± 6.4 % RH), with participants initially exercising, at a workload corresponding to 65 % O2peak until the isothermic target Trec of ≥38.5 °C has been achieved. Upon the attainment of a Trec ≥ 38.5 °C, participants rested in a seated position on the cycle ergometer within the environmental chamber resuming exercise at a low intensity (<50 % O2peak) when their Trec fell below 38.5 °C, and continued cycling until the target Trec was re-attained (~10 min). Normothermic exercise training involved ten, 90-min sessions performed in temperate conditions (19.8 ± 0.2 °C, 28.5 ± 2.7 % RH). The normothermic exercise training participants initially cycled at an intensity corresponding to 65 % O2peak, with the workload adjusted to match the total work, and exercise intensity and duration of the isothermic HA group. Both groups exercised inside a purpose-built environmental chamber (WatFlow control system; TISS, Hampshire, UK) with temperature and humidity controlled using automated computer feedback (WatFlow control system; TISS, Hampshire, UK). Sessions were conducted at the same time of day (07:00–10:00 h) to mitigate effects of daily variation in heat shock protein expression (Taylor et al. 2010b). During each session, sweat rate (SR; L h−1) was estimated using the change in nude body mass (NBM) from the pre- to post-exercise periods (Detecto Physicians Scales; Cranlea & Co., Birmingham, UK), Trec was recorded using a thermistor (Henleys Medical Supplies Ltd., Welwyn Garden City, UK, Meter logger Model 401, Yellow Springs Instruments, Yellow Springs, MO, USA) inserted 10 cm past the anal sphincter and HR was recorded by telemetry (Polar Electro Oyo, Kempele, Finland). During each session, HR, Trec and power output (W) were recorded after 10 min of seated rest in temperate laboratory conditions, and thereafter every 5 min upon commencing exercise.
Quantification of physiological and training variables
Exercise duration (min) was the total time spent exercising during the intervention. Mean session intensity (%O2peak and W kg−1) was calculated from the relative exercise intensity during each 5-min period throughout the 90-min intervention sessions. This contrasted the mean exercise intensity (%O2peak and W kg−1), which reflected the mean relative exercise intensity only (power output >1 W), thus excluding periods of rest. Total work done (kJ) reflected the cumulative volume of worked performed on the cycle ergometer over the 90-min period.
Absolute sweat loss (L h−1) was calculated from the change in towel-dried NBM pre- to post-sessions. Mean Trec and mean HR reflected the average Trec or HR recorded throughout each intervention. Peak Trec and peak HR reflect the maximum Trec or HR recorded throughout each intervention. Change (∆) in Trec (°C) and ∆HR (b min−1) reflects the difference between resting, and peak Trec and HR, respectively. Mean Trecfinal45 (°C) quantifies the mean Trec between the 45th and 90th minutes of the intervention session to reflect the average temperature following the initial rate of increase. Trec ≥ 38.5 °C (min) represented the total number of minutes where Trec exceeded 38.5 °C during the session. The Trec area under the curve (AUC) at >38.5 °C was calculated when as the duration and magnitude where measured Trec exceeded 38.5 °C (Périard et al. 2012; Gibson et al. 2014; Périard et al. 2015), using a modification to the trapezium rule (Hubbard et al. 1977). A Trec of 38.5 °C was selected as an approximate minimum for intolerance during compensable heat stress (Sawka et al. 2001) and possible threshold for Hsp72 translation/transcription (Amorim et al. 2008). AUC for Trec > 38.5 °C was calculated as follows:
The rate of ∆Trec (°C) rate of core temperature change, typically an increase, was calculated as follows:
Note: T rec 2 and time2 are simultaneous measurements taken at any time during the intervention; and T rec 1 and time1 are resting value.
Blood sampling and RNA extraction
Venous blood samples were drawn from the antecubital vein into 6 mL EDTA tubes immediately pre- and post-first (day1) and tenth (day10) session. Using a previously validated method (Taylor et al. 2010b), venous blood (1 mL) was pipetted into 10 mL of one in ten red blood cell lysis solution (10× Red Blood Cell Lysis Solution, Miltenyi Biotech, Bisley, UK). Samples were incubated for 15 min at room temperature before isolation via 5 min centrifugation at 400 G and then washed twice in 2 mL PBS, prior to further centrifugation at 400 G for 5 min. The acid guanidinium thiocyanate-phenol-chloroform extraction (TRIzol) method (Chomczynski and Sacchi 1987) was then used to extract RNA from the leukocytes in accordance with the manufacturer instructions (Sigma-Aldrich, UK; Invitrogen, Life Technologies, Carlsbad, USA). Quantity was determined at an optical density of 260 nm, whilst quality was determined via the 260/280 and 260/230 ratios using a nanodrop spectrophotometer (Nanodrop 2000c Thermo Scientific, Waltham, MA, USA).
One-step reverse transcription quantitative polymerase chain reaction
Hsp72 and Hsp90α relative mRNA expression was quantified using reverse transcription quantitative polymerase chain reaction (RT-QPCR). Primers β2-microglobulin, Hsp72 and Hsp90α (presented in Table 2) were designed using primer design software (Primer Quest and Oligoanalyzer - Integrated DNA Technologies, Coralville, IA, USA) (Tuttle et al. 2015). During primer design, sequence homology searches were performed against the GenBank database to ensure the primers matched the gene of interest. Primers were designed to span exon-intron boundaries and avoided three or more guanine-cytosine bases within the last five bases at the 3′ end of primer to avoid non-specific binding. Further searches were performed to ensure primers did not contain secondary structures and intermolecular or intramolecular interactions (hairpins, self-dimer and cross dimers), which can inhibit product amplification. Relative Hsp mRNA expression was then quantified using RT-QPCR with reagent concentrations implemented in accordance with the manufacturer recommendations. Reactions (20.0 μL) containing 10.0 μL of SYBR Green RT-PCR Mastermix (Quantifast SYBR Green kit; Qiagen, Manchester, UK), 0.15 μL of forward primer, 0.15 μL of reverse primer, 0.20 μL of reverse transcription mix (Quantifast RT Mix, Qiagen) and 9.50 μL sample (70.0 ng RNA μL−1) were prepared using the Qiagility automated pipetting system (Qiagen). Each reaction was amplified in a thermal cycler (Rotorgene Q, Qiagen) and involved reverse transcription lasting 10 min at 50 °C and a transcriptase inactivation and initial denaturation phase lasting 5 min at 95 °C. The PCR reaction then followed with a denaturation step lasting 10 s at 95 °C and a primer annealing and extension stage lasting 30 s at 60 °C repeated for 40 cycles. Fluorescence was measured following each cycle as a result of the incorporation of SYBR Green dye into the amplified PCR product. Melt curves (50 to 95 °C; Ramp protocol, 5-s stages) were analysed for each reaction to ensure only the single gene of interest was amplified. Relative quantification of mRNA expression for each sample was assessed by determining the ratio between the cycling threshold (CT) value of the target mRNA and β2-M CT values. Fold change in relative mRNA expression was calculated using the 2−ΔΔCT method.
Table 2.
Primer sequences
| Target gene | Primer sequence (5′-3′-) | Reference sequence number | Amplicon length |
|---|---|---|---|
| B2 microglobulin | Forward: CCGTGTGAACCATGTGACT Reverse: TGCGGCATCTTCAAACCT |
NM_004048 | 91 |
| Hsp72 | Forward: CGCAACGTGCTCATCTTTGA Reverse: TCGCTTGTTCTGGCTGATGT |
NM_005345 | 198 |
| Hsp90α (variant 1 and variant 2) | Forward: AAACTGCGCTCCTGTCTTCT Reverse: TGCGTGATGTGTCGTCATCT |
NM_001017963 and NM_005348 | 180 |
Statistical analysis
Analysis was performed on data obtained on the first (day1; n = 15) and last (day10; n = 15), and the combined dataset (ALL). All data are reported as mean ± SD with two-tailed significance accepted at p < 0.05. Prior to statistical analysis, all outcome variables were checked for normality using Kolmogorov-Smirnov and sphericity using the Greenhouse-Geisser method prior to further analysis, and each was deemed plausible unless otherwise stated. Paired sample t tests (SPSS, Chicago IL, USA) were implemented to determine differences between day1 and day10 for training data and the physiological responses to training. Paired sample t tests were also used to determine differences between the magnitude of change (i.e. pre–post) in Hsp72 mRNA and the magnitude of change in Hsp90α mRNA on day1, day10 and ALL. Effect sizes [Cohen’s d (d; small = 0.20, medium = 0.50, large = 0.80)] were calculated to analyse the magnitude and trends with data. Pearson’s product correlation was performed between the physiological responses and training prescription markers (see “Quantification of physiological and training variables”), and the magnitude of change (pre–post) in Hsp72 mRNA and Hsp90α mRNA, on day1, day10 and ALL, respectively. Stepwise multiple regression was performed to determine whether a combination of markers strengthened the prediction equation for Hsp72 mRNA and Hsp90α mRNA.
Results
Physiological and Hsp72 mRNA, and Hsp90α mRNA responses
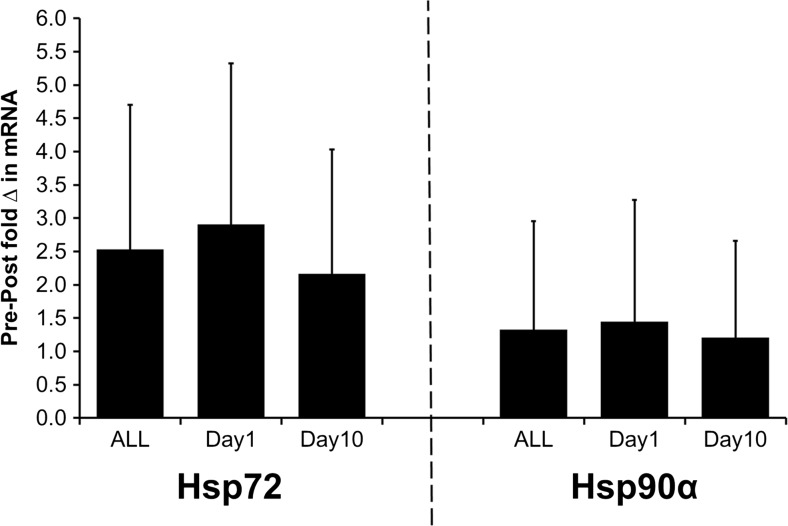
No differences in the pre–post change in Hsp72 mRNA and pre–post change in Hsp90α mRNA were observed between day1 and day10 (Hsp72 mRNA: t = 0.067; p = 0.948; d = 0.02; Hsp90α mRNA: t = 0.748; p = 0.467; d = 0.19) (Fig. 2). On day10 vs day1, participants were observed as performing exercise for longer duration (t = 5.206; p < 0.001; d < 0.01), at a greater mean intensity (t = 7.724; p < 0.001; d = 2.03) and mean power (t = 5.855; p < 0.001; d = 1.63) and producing a greater volume of work done (t = 6.424; p < 0.001; d = 1.66). Reductions in the rate of Trec increase were observed (t = 2.188; p = 0.046; d = 0.58) between day10 and day1, alongside increased SR (t = 4.123; p = 0.001; d = 0.89) (Table 3).
Fig. 2.
Mean ± SD pre- to post-session fold change in Hsp72 mRNA (left) and Hsp90α mRNA (right) for the entire dataset (ALL), and on day1 and day10 of the intervention
Table 3.
Mean ± SD training data and physiological responses for Hsp72 mRNA and Hsp90α mRNA datasets displayed for the entire data set (ALL), and day1 and day10, respectively
| Measure | ALL | Day1 | Day10 |
|---|---|---|---|
| Exercise duration (min) | 50 ± 9 | 43 ± 7 | 57 ± 5a |
| Mean intensity (%O2peak) | 33 ± 7 | 28 ± 5 | 38 ± 3a |
| Mean power (W kg−1) | 1.3 ± 0.3 | 1.1 ± 0.3 | 1.5 ± 0.3a |
| Mean exercise intensity (%O2peak) | 60 ± 5 | 60 ± 6 | 61 ± 3 |
| Mean exercise power (W kg−1) | 2.4 ± 0.5 | 2.4 ± 0.5 | 2.4 ± 0.5 |
| Total work done (kJ) | 526 ± 149 | 447 ± 111 | 606 ± 142a |
| SR (L h−1) | 0.9 ± 0.5 | 0.8 ± 0.4 | 1.0 ± 0.6a |
| Mean Trec (°C) | 37.92 ± 0.46 | 38.02 ± 0.54 | 37.83 ± 0.34 |
| Mean Trecfinal45 (°C) | 38.14 ± 0.73 | 38.19 ± 0.82 | 38.08 ± 0.65 |
| ∆Trec (°C) | 1.68 ± 0.70 | 1.64 ± 0.71 | 1.72 ± 0.71 |
| Rate of ∆Trec (°C h−1) | 2.06 ± 0.89 | 2.24 ± 1.06 | 1.87 ± 0.66a |
| Peak Trec (°C) | 38.52 ± 0.58 | 38.59 ± 0.65 | 38.44 ± 0.50 |
| Trec ≥ 38.5 °C (min) | 27 ± 28 | 29 ± 31 | 26 ± 26 |
| AUC 38.5 °C (°C min−1) | 8.0 ± 11.2 | 10.7 ± 14.0 | 5.3 ± 6.9 |
| ∆HR (b min−1) | 96 ± 24 | 93 ± 26 | 98 ± 22 |
| Mean HR (b min−1) | 129 ± 21 | 131 ± 23 | 127 ± 20 |
| Peak HR (b min−1) | 162 ± 25 | 164 ± 29 | 160 ± 21 |
aDenotes difference from day1 within gene transcript
Correlates of Hsp72 mRNA, and Hsp90α mRNA increases
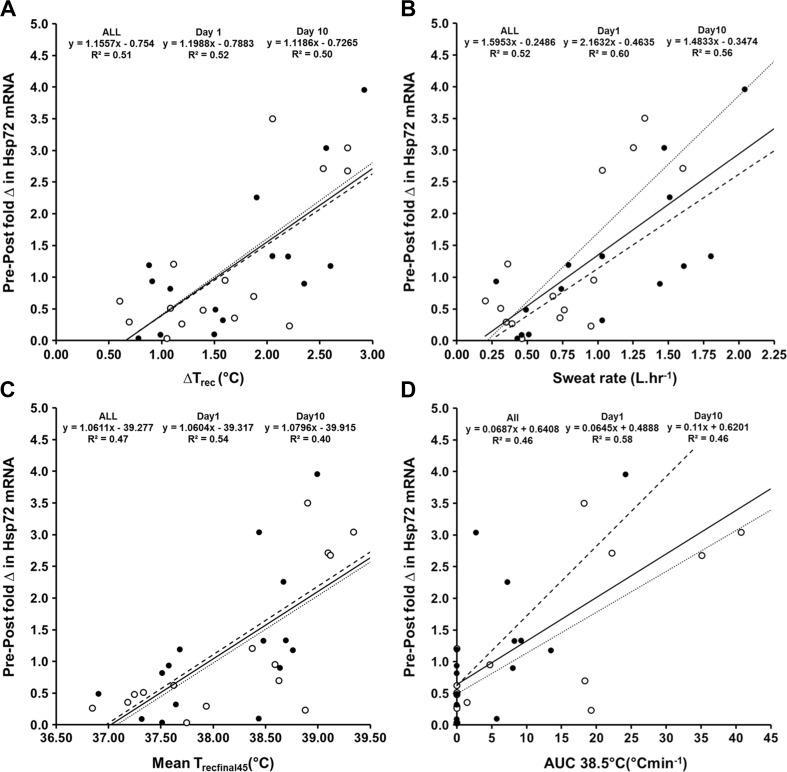
The strongest correlates of the pre–post session change in Hsp72 mRNA were the ΔTrec (ALL r = 0.714; day1 r = 0.721; day10 r = 0.709), SR (ALL r = 0.709; day1 r = 0.738; day10 r = 0.762), the mean Trecfinal45 (ALL r = 0.682; day1 r = 0.730; day10 r = 0.632), AUC at 38.5 °C (ALL r = 0.678; day1 r = 0.761; day10 r = 0.687), the peak Trec (ALL r = 0.661; day1 r = 0.688; day10 r = 0.650) and the duration Trec ≥ 38.5 °C (ALL r = 0.650; day1 r = 0.650; day10 r = 0.659) (Table 4, Fig. 3). Multiple regressions observed no improvements to the Hsp72 mRNA model with the addition of further variables for day1, day10 or ALL analyses. No participant characteristics demonstrated a significant (p > 0.05) relationship with change in Hsp72 mRNA (Table 1).
Table 4.
Ranked correlations of the pre- to post-session fold change in Hsp72 mRNA for ALL, day1 and day10
| ALL | Day1 | Day10 | |||
|---|---|---|---|---|---|
| Variable | R = | Variable | R = | Variable | R = |
| ∆Trec (°C) | 0.714** | AUC 38.5 °C (°C min−1) | 0.761** | SR (L h−1) | 0.762** |
| SR (L h−1) | 0.709** | SR (L h−1) | 0.738** | ∆Trec (°C) | 0.709** |
| Mean Trecfinal45 (°C) | 0.682** | Mean Trecfinal45 (°C) | 0.730** | AUC 38.5 °C (°C min−1) | 0.687** |
| AUC 38.5 °C (°C min−1) | 0.678** | ∆Trec (°C) | 0.721** | ∆HR (b min−1) | 0.665** |
| Peak Trec (°C) | 0.661** | Peak Trec (°C) | 0.688** | Rate of ∆Trec (°C h−1) | 0.661** |
| Trec ≥ 38.5 °C (min) | 0.650** | Mean Trec (°C) | 0.675** | Trec ≥ 38.5 °C (min) | 0.659** |
| Mean Trec (°C) | 0.565** | Trec ≥ 38.5 °C (min) | 0.650* | Peak Trec (°C) | 0.650** |
| Rate of ∆Trec (°C h−1) | 0.560** | Rate of ∆Trec (°C h−1) | 0.540 | Mean Trecfinal45 (°C) | 0.632* |
| ∆HR (b min−1) | 0.511** | ∆HR (b min−1) | 0.391 | Mean HR (b min−1) | 0.546* |
| Mean HR (b min−1) | 0.431* | Mean HR (b min−1) | 0.341 | Mean Trec (°C) | 0.454 |
| Peak HR (b min−1) | 0.374* | Peak HR (b min−1) | 0.333 | Peak HR (b min−1) | 0.448 |
| Mean exercise power (W kg−1) | 0.257 | Mean exercise power (W kg−1) | 0.231 | Mean power (W kg−1) | 0.388 |
| Total work done (kJ) | 0.209 | Exercise duration (min) | 0.223 | Total work done (kJ) | 0.371 |
| Mean power (W kg−1) | 0.173 | Mean exercise intensity (%O2peak) | 0.210 | Mean intensity (%O2peak) | 0.301 |
| Exercise duration (min) | 0.165 | Total work done (kJ) | 0.091 | Mean exercise power (W kg−1) | 0.286 |
| Mean exercise intensity (%O2peak) | 0.153 | Mean intensity (%O2peak) | −0.072 | Exercise duration (min) | 0.262 |
| Mean intensity (%O2peak) | 0.053 | Mean power (W kg−1) | −0.013 | Mean exercise intensity (%O2peak) | 0.056 |
* denotes p < 0.05; ** denotes p < 0.01
Fig. 3.
Relationship between the fold change in Hsp72 mRNA and the ΔTrec (a), SR (b), mean Trecfinal45 (c) and the AUC38.5 °C (d). Figures describe data for the entire dataset (ALL, solid line), and on day1 (open circles, dotted line) and day10 (closed circles, dashed line) of the intervention
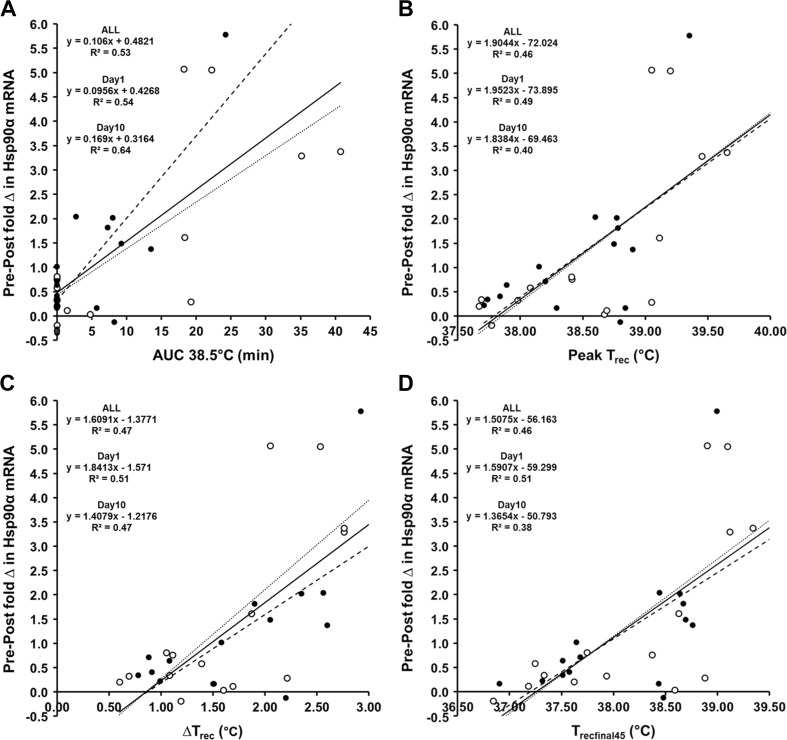
The strongest correlates of Hsp90α mRNA increase were the AUC at 38.5 °C (ALL r = 0.729; day1 r = 0.729; day10 r = 0.813), ΔTrec (ALL r = 0.691; day1 r = 0.715; day10 r = 0.690), peak Trec (ALL r = 0.680; day1 r = 0.698; day10 r = 0.645), mean Trecfinal45 (ALL r = 0.678; day1 r = 0.714; day10 r = 0.617), SR (ALL r = 0.660; day1 r = 0.760; day10 r = 0.733), duration Trec ≥ 38.5 °C (ALL r = 0.629; day1 r = 0.670; day10 r = 0.563) and rate of change in Trec (ALL r = 0.600; day1 r = 0.567; day10 r = 0.674) relating to the largest gene transcription (Table 5, Fig. 4). Multiple regression observed that the Hsp90α mRNA model for day1 and day10 was not improved by adding further variables. The entire Hsp90α mRNA dataset (ALL) was improved (r = 0.792) when AUC at 38.5 °C and SR were combined. Height (ALL r = 0.419) and BSA (ALL r = 0.412), but no other participant characteristics, demonstrated a significant (p > 0.05) relationship with change in Hsp90α mRNA (Table 1).
Table 5.
Ranked correlations of the pre- to post-session fold change in Hsp90α mRNA for ALL, day1 and day10
| ALL | Day1 | Day10 | |||
|---|---|---|---|---|---|
| Variable | R = | Variable | R = | Variable | R = |
| AUC 38.5 °C (°C min−1) | 0.729** | SR (L h−1) | 0.760** | AUC 38.5 °C (°C min−1) | 0.813** |
| ∆Trec (°C) | 0.691** | AUC 38.5 °C (°C min−1) | 0.729** | SR (L h−1) | 0.733** |
| Peak Trec (°C) | 0.680** | ∆Trec (°C) | 0.715** | ∆Trec (°C) | 0.690** |
| Mean Trecfinal45 (°C) | 0.678** | Mean Trecfinal45 (°C) | 0.714** | Rate of ∆Trec (°C h−1) | 0.674** |
| SR (L h−1) | 0.660** | Peak Trec (°C) | 0.698** | ∆HR (b min−1) | 0.655** |
| Trec ≥ 38.5 °C (min) | 0.629** | Mean Trec (°C) | 0.695** | Peak Trec (°C) | 0.645** |
| Mean Trec (°C) | 0.601** | Trec ≥ 38.5 °C (min) | 0.670** | Mean HR (b min−1) | 0.629* |
| Rate of ∆Trec (°C h−1) | 0.600** | Rate of ∆Trec (°C h−1) | 0.567* | Mean Trecfinal45 (°C) | 0.617* |
| ∆HR (b min−1) | 0.531** | ∆HR (b min−1) | 0.469 | Trec ≥ 38.5 °C (min) | 0.563* |
| Mean HR (b min−1) | 0.521** | Peak HR (b min−1) | 0.458 | Peak HR (b min−1) | 0.540* |
| Peak HR (b min−1) | 0.491** | Mean HR (b min−1) | 0.441 | Total work done (kJ) | 0.515* |
| Mean exercise power (W kg−1) | 0.289 | Mean exercise power (W kg−1) | 0.318 | Mean Trec (°C) | 0.419 |
| Total work done (kJ) | 0.223 | Mean exercise intensity (%O2peak) | 0.277 | Mean power (W kg−1) | 0.383 |
| Mean exercise intensity (%O2peak) | 0.183 | Total work done (kJ) | 0.132 | Exercise duration (min) | 0.323 |
| Mean power (W kg−1) | 0.098 | Mean intensity (%O2peak) | −0.132 | Mean intensity (%O2peak) | 0.316 |
| Exercise duration (min) | 0.075 | Exercise duration (min) | 0.127 | Mean exercise power (W kg−1) | 0.256 |
| Mean intensity (%O2peak) | −0.045 | Mean power (W kg−1) | −0.022 | Mean exercise intensity (%O2peak) | −0.025 |
* denotes p < 0.05; ** denotes p < 0.01
Fig. 4.
Relationship between the fold change in Hsp90α mRNA and the AUC38.5 °C (a), peak Trec (b), ΔTrec (c) and the mean Trecfinal45 (d). Figures describe data for the entire dataset (ALL, solid line), and on day1 (open circles, dotted line) and day10 (closed circles, dashed line) of the intervention
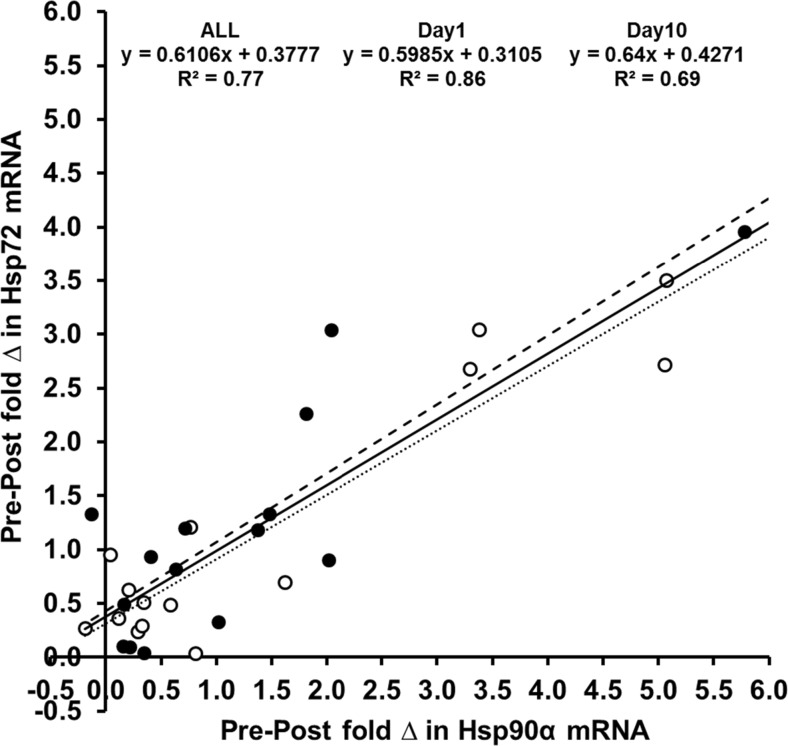
Relationship between Hsp72 and Hsp90α mRNA
A significant relationship was observed between Hsp72 mRNA and Hsp90α mRNA for ALL (r = 0.879; p < 0.001) (Fig. 5) with a significant relationship also observed on day1 (r = 0.924; p < 0.001) and day10 (r = 0.838; p < 0.001) (Fig. 5). Accordingly, no significant difference was observed between the pre- to post-session change in Hsp72 mRNA and Hsp90α mRNA on day1 (t = 1.200; p = 0.250; d = 0.32; Hsp72 mRNA = 1.2 ± 1.2-fold change; Hsp90α mRNA = 1.4 ± 1.8-fold change), day10 (t = −0.032; p = 0.975; d = 0.01; Hsp72 mRNA = 1.2 ± 1.1-fold change; Hsp90α mRNA = 1.2 ± 1.5-fold change) or ALL (t = −0.914, p = 0.368; d = 0.28; Hsp72 mRNA = 1.2 ± 1.1-fold change, Hsp90α mRNA = 1.3 ± 1.6-fold change).
Fig. 5.
Mean ± SD relationship between the fold change in Hsp72 mRNA and Hsp90α mRNA entire dataset (ALL, solid line), and on day1 (open circles, dotted line) and day10 (closed circles, dashed line) of the intervention
Discussion
In our experiment, ΔTrec, SR, the mean Trecfinal45, the Trec AUC at 38.5 °C, peak Trec and the duration Trec ≥ 38.5 °C were identified as significant predictors (R 2 ≈ 0.36–0.51; p < 0.05) of the change in Hsp72 mRNA (Table 4, Fig. 3). This is in agreement with previous observations regarding eHsp72 (Gibson et al. 2014) and is in agreement with the experimental hypothesis that endogenous markers of thermal strain and heat storage, i.e. core temperature, would most closely predict, if not directly cause, the change in Hsp72 mRNA. The experimental hypothesis also extended this observation to Hsp90α mRNA which similarly demonstrated that the AUC at 38.5 °C, ΔTrec, peak Trec, Mean Trecfinal45, SR, the duration Trec ≥ 38.5 °C and rate of change in Trec were identified as the strongest predictors (R 2 ≈ 0.42–0.53) of the increased gene expression (Table 5, Fig. 4). Similar characteristics (both physiological predictors and observed magnitude of prediction) were observed on day1 and day10 for both Hsp72 and Hsp90α mRNA; thus, the signal for transcription demonstrates equality at the onset and culmination of a 10-day HA or normothermic training intervention (Tables 4 and 5). The similarity of variables characterising increases in Hsp72 and Hsp90α mRNA was supported by the observation that a strong relationship (R 2 = 0.77) existed between the increase in Hsp72 mRNA and the increase in Hsp90α mRNA. The absolute mRNA increase was also of a comparable magnitude for both Hsp72 mRNA (~1.2 ± 1.1-fold change; +103 %) and Hsp90α mRNA (~1.3 ± 1.6-fold change; +60 %) with the lower relative increase in Hsp90α mRNA reflecting a higher basal expression (Subbarao Sreedhar et al. 2004). It is yet to be elucidated whether these variables would demonstrate a greater/lesser contribution to the magnitude of gene transcription in other tissues, i.e. skeletal muscle appears more oxidative stress dependent rather than temperature dependent compared to leukocytes, as discussed previously (Tuttle et al. 2015).
The failure for training prescription markers (exercise duration, exercise intensity, exercise power and work done) to predict the change of leukocyte Hsp72 and Hsp90α mRNA highlights the importance of designing interventions necessitating increased HSP around endogenous responses (e.g. increased core temperature), rather than exogenous variables (e.g. training prescription). SR as a predictor of increased Hsp90α mRNA is a novel observation; it is however likely that this is a secondary response, thus not causal, stemming from the required increased heat dissipation via evaporation in an attempt to acquiesce increasing heat storage (core temperature) rather than being a primary response. Manipulation of heat storage to further enhance Hsp90α mRNA transcription may be of experimental benefit within some designs and could, at least theoretically, be enhanced by facilitating greater sweating with higher humidity (e.g. >60 % RH) than those utilised within the present design (~40 % RH), thus further inhibiting evaporative heat loss (Maughan et al. 2012). Though some differences in HA adaptation have been observed by imposing dehydration (an analogue for increased sweat losses and the ensuing increases in thermal strain) (Garrett et al. 2014), the impact of this manipulation on Hsp transcription during HA remains unknown. When dehydration is imposed vs euhydration, no differences in the increases in monocyte or skeletal HSP72 have been observed during ~90 min of acute exercise-heat stress (Hillman et al. 2011) and normothermic exercise (Logan-Sprenger et al. 2015), respectively. These data reduce the appeal of increasing sweat losses as a direct method for improving the magnitude of adaptation.
A novel finding in the present study was the observation that the increases in Hsp72 and Hsp90α mRNA demonstrate a significant relationship. This provides further evidence for a common pathway towards equality of increases in HSP72 and HSP90α protein concentrations in response to ex vivo cellular stress (McClung et al. 2008). Hsp72 mRNA (Marshall et al. 2007) and Hsp90α mRNA (Tuttle et al. 2015) are responsive to thermal and physiological stimuli as supported by our data. Whilst data reporting changes in human Hsp72 mRNA is available (Febbraio and Koukoulas 2000; Marshall et al. 2007; Stary et al. 2008; Atamaniuk et al. 2008; Tuttle et al. 2015; Gibson et al. 2015a; Gibson et al. 2015c), equivalent data characterising increases in Hsp90α mRNA is limited. Our data demonstrates a novel finding and is the first in vivo experiment demonstrating that Hsp90α mRNA responds similarly to Hsp72 mRNA when core temperature is increased during exercise in the heat. It is known that exercise (Connolly et al. 2004), exercise-heat stress (Moran et al. 2006) and exercise-induced muscle damage and heat stress (Tuttle et al. 2015) provide the stimuli for Hsp72 and Hsp90α gene induction with the stimuli to increase transcription described as proportional to the change in core temperature (38.5 ± 0.2 to 39.0 ± 0.4 °C) (Tuttle et al. 2015), a finding our data supports. Our data is congruous with findings that consistent pre–post increases in Hsp72 mRNA occur at the beginning and end of heat acclimation (Gibson et al. 2015a), should the physiological signal (Trec > 38.5 °C) demonstrate equality between measurement points (Gibson et al. 2015a). In addition, we have now demonstrated that this dose response is true of Hsp90α mRNA. This observation further reinforces isothermic HA methods as optimal for ensuring signalling for HSP via the heat shock response (a key regulator of thermotolerance) and the magnitude of phenotypic heat adaptation (Kuennen et al. 2011). During the initial adaptation to heat acclimation (~3 days), Hsp72 mRNA can diminish in conjunction with reductions in mean core temperature (−0.2 to 38.7 ± 0.2 °C) and increased basal HSP72 (Marshall et al. 2007) should be the end of activity core temperature decrease. Our data now extends this signal-dependent response to Hsp90α mRNA. Our non-damaging exercise protocol (cycling) also supports the previously proposed observation that the leukocyte stress response is core temperature rather than exercise dependent (Tuttle et al. 2015).
Maintenance of increases in both Hsp72 and Hsp90α mRNA between day1 and day10 is unsurprising as no difference existed between the strongest correlates of the pre–post session change in gene expression (core temperature). This finding is concurrent with proposals that elevated core temperature as being an important component of HSF1 regulation (Gibson et al. 2015a; Gibson et al. 2015c), particularly in leukocytes. To ensure HSF1 activation in experiments such as those designed to achieve the heat-acclimated phenotype (Kuennen et al. 2011), mitigate heat stroke (Moran et al. 2006), facilitate positive adaptive responses to stress [i.e. promotion of mitochondrial biogenesis (Henstridge et al. 2014a)] or act as therapy for disease states [e.g. type 2 diabetes mellitus (Hooper et al. 2014), cardiovascular disease (Noble and Shen 2012) and Parkinson’s disease (Erekat et al. 2014)] then eliciting an increased core temperature of +1.7 °C and ensuring a minimum core temperature of 38.5 °C is maintained for ≥27 min is necessary (Table 3). However, this prescription is from a small homogenous apparently healthy population. Some of the previously detailed disease states have marked impairments regarding their ability to thermoregulate [e.g. diabetes (Kenny et al. 2016) and multiple sclerosis (Romberg et al. 2012)]. Therefore, characterisation of the Hsp mRNA response is required within such populations—particularly—whether they have a similar core temperature-mediated dose-response relationship with Hsp mRNA increases, in light of reduced basal HSP (Bruce et al. 2003). Should the disease state not directly inhibit HSP translation, it may be that HSP accumulation is more efficient in these populations due to greater increases in core temperature resulting from inhibited heat dissipation mechanisms (Davis et al. 2010; Carter et al. 2014; Kenny et al. 2016), a notion also true of those with a spinal cord injury (Price 2006). Previous work has identified that HSF1 is temperature dependent supporting our downstream observation regarding increased gene expression (Sonna et al. 2010). Similar endogenous signals to increase Hsp72 mRNA have been shown to increase eHSP72 i.e. elevated temperature (Gibson et al. 2014). The rate of rise in core temperature is also important to increase plasma concentrations (Périard et al. 2012). More relevant to acquiring thermotolerance (Kregel 2002) is the change in iHSP72 and iHSP90α (Lee et al. 2015). The accumulation of greater iHSP72 is more closely linked to the absolute (final) and change (delta) in core temperature (Magalhães et al. 2010) during HA. Mechanistically, it has been observed that the magnitude of iHSP72 expression at exhaustion or 24 h post-exhaustion is a result of the absolute temperature attained (>39.0 °C), rather than the rate of heat storage (Périard et al. 2015). During acute exercise-heat stress, equality of post-exhaustion iHSP72 did not differ in response to the rate of core temperature increase corresponding to different exercise intensities, and presumably different metabolic stressors, eliciting similar end temperatures of ~39.5 °C [(60 % O2peak = 2.2 ± 1.4 °C h−1; 75 % O2peak = 5.1 ± 1.7 °C h−1 (Périard et al. 2015)]. The present study highlights the transcription of Hsp72 mRNA and Hsp90α mRNA as having similar criteria (Trec > 38.5 °C for >27 min, Table 3) to that which predicts elevated eHSP72 and iHSP72 (Périard et al. 2012; Gibson et al. 2014; Périard et al. 2015). Given that the predictive capacity following this analysis is incomplete (Hsp72 mRNA = 47–58 % between variables, and Hsp90α mRNA = 46–66 % between variables), the present experiment does suggest that a large proportion of the signalling for gene transcription is unaccounted for by the core temperature alone. Accordingly, other endogenous signals responsible for the initial HSF1 activation should be sought (Vihervaara and Sistonen 2014); as previously discussed, this may be more important in skeletal muscle vs leukocytes. These signals likely include markers of metabolic stress/physiological strain such as oxidative stress (Sureda et al. 2005; Mestre-Alfaro et al. 2012), changes in catecholamine concentration (Tintinger et al. 2001; Giraldo et al. 2010) and increased cortisol (Ortega 2003). These signals also provide a rationale for the difference between increased Hsp72 mRNA, and HSP72 protein translation observed in heat acclimation (Marshall et al. 2007), and notable increases in monocyte HSP72 in hypoxia (Taylor et al. 2011), and following matched passive vs active hyperthermia (Morton et al. 2007) which collectively highlight the multifactorial signal cascade. Irrespective of the precise, causal signal(s), these data evidence that large, prolonged changes in core temperature are a predictor of the greatest change in Hsp72 mRNA and Hsp90α mRNA (from those endogenous and exogenous variables monitored), with this an important characteristic potentiating increased basal protein to mitigate the potential threat for subsequent thermal-mediated protein denaturation. The “absent” predictive criteria might be also explained by other unmeasured variables, and these could include factors allied to individual differences in HSP transcription perhaps due to a responder–non responder paradigm due to innate (i.e. genetic) or acquired (previous heat exposure, HA, subsequent decay, etc) phenotypical inter- and intraindividual variation (Lyashko et al. 1994).
Despite the presence of a highly homogenous sample (Table 1) and advantageous experimental protocol whereby participants did not possess the HA phenotype at the onset of the experiment and were exposed to consistent endogenous signal being obtained throughout the experiment (Table 3), ~40 % of the variability in Hsp mRNA transcription is unaccounted for. It is difficult to determine individual responses within this experiment, largely due to the differing training parameters and exercise-heat stress variables (Table 3) observed between day1 and day10 as part of the initial experimental objectives (Gibson et al. 2015c). Accordingly, bespoke experiments utilising deductive mechanistic evidence obtained in vivo and in vitro should ensure further equality of all potentiating stimuli (thermal and cellular/molecular) to facilitate a more definitive analysis of the individual response and account for the remaining variability in transcription. In spite of some absent predictive criteria, the present experiment further evidences the observation that in experiments where iHSP72 has not increased (Watkins et al. 2007; Hom et al. 2012), the mechanism for the absent protein translation is likely due to only modest and brief increases in core temperature with lower mean core temperatures reported [<38.5 °C (Amorim et al. 2008)] than others demonstrating increased iHsp72 [>39.0 °C (Périard et al. 2015)]. Whilst exercise alone increases core temperature providing a sufficient pathway to elevate iHSP72 (Fehrenbach et al. 2000; Shin et al. 2004) and Hsp72 mRNA, and HSP90α mRNA in temperate conditions (Tuttle et al. 2015), the greater heat storage when performing equivalent exercise in hot environmental conditions provides a more potent stimuli for increasing iHSP72 (Fehrenbach et al. 2001; Fehrenbach et al. 2003; Selkirk et al. 2009; Magalhães et al. 2010) via greater increases in Hsp72 mRNA. It has been observed that no difference in Hsp72 mRNA increases occurs within HA sessions performed in very hot environments when comparing isothermic methods (Gibson et al. 2015a) (whereby the magnitude of hyperthermia is controlled) with fixed intensity methods [where core temperature responds according to the individual capacity to dissipate heat (Gibson et al. 2015b)]. The present data demonstrates that in cooler conditions, it would be pertinent to implement isothermic methods with high initial workloads (and metabolic heat production) to provide the requisite stimuli (increased core temperature) for acquiring thermotolerance in addition to physiological heat adaptation (Racinais et al. 2015). The isothermic method of HA facilitates a targeted increase in core temperature (>38.5 °C) which can subsequently be prolonged (for ~30–60 min), to initiate Hsp72 mRNA and Hsp90α mRNA transcription (Gibson et al. 2015c). These guidelines are supported by data demonstrating that initial adaptations to HA (notably reduced core temperature and increased iHSP72) mitigate the increase in Hsp72 mRNA to subsequent fixed intensity work in equal environmental conditions (Marshall et al. 2007).
Future work in the field should address some of the limitations of this experiment, which are discussed within the present discussion section. To resolve the unknown threshold for Hsp72 and Hsp90α mRNA signalling, and dependence of temperature in isolation or coupled with exercise and concurrent responses, measurements of gene transcription should be made following stepwise increases in core temperature whilst using passive and exercise-heat stress models and multi-tissue analysis. This multi-tissue analysis should also be performed where experimental manipulations prolong the degree of hyperthermia for extended periods (beyond the 30–60 min of the present data) and be performed using passive heating models, e.g. hot water immersion or sauna (Krause et al. 2015b), in addition to the active exercise-induced heating implemented in the present experiment. A paucity of transcription/translation data exists for extreme core temperatures (in excess of 39.5 °C), despite attainment of these temperatures absent of pathophysiological complications within endurance runners (Byrne et al. 2006) and other competitive athletes, e.g. elite footballers (Mohr et al. 2012). Hsp72 and Hsp90α mRNA analysis should be paired with measurement of iHSP72 and iHSP90α protein concentrations across tissues to facilitate a more complete interpretation of the transcription and translational kinetics. This would subsequently facilitate more precise interventions allied to prescribing HA for increasing thermotolerance (Kuennen et al. 2011), reducing the severity of heat illness (Ruell et al. 2014) or facilitate a cellular test for heat stroke susceptibility (Amorim et al. 2008), and provide optimal prescriptions of therapeutic thermal interventions (Henstridge et al. 2014b; Henstridge et al. 2016). The time course of the mRNA transcription and subsequent HSP translation also both require further research to determine within group and interindividual responses of a heterogeneous cohort, including clinical populations. Finally, given the apparent translational differences between sexes in response to equivalent training (Morton et al. 2009), it remains to be determined whether this is a result of inhibited gene transcription or translational events attenuating changes in basal protein in females.
Conclusion
In experiments or interventions where the correlated transcription of Hsp72 and Hsp90α mRNA is important, such as those involving heat acclimation and heat illness, or therapeutic heat stress, the protocol should be designed to rapidly induce large changes (≥1.7 °C) in core temperature (>38.5 °C), which are maintained for prolonged periods (≥27 min). This may be achieved by initially implementing high intensity work, eliciting uncompensable heat stress and increased heat storage, followed by the maintenance of core temperature via lower intensity exercise or passive heat stress to elicit heat balance at the elevated temperature.
Compliance with ethical standards
All protocols, procedures and methods were approved by the institutional ethics committee. Participants completed medical questionnaires and written informed consent following the principles outlined by the Declaration of Helsinki as revised in 2013 prior to commencing any preliminary or experimental sessions.
References
- Amorim FT, Fonseca IT, Machado-Moreira CA, Magalhães FC. Insights into the role of heat shock proteins 72 to whole-body heat acclimation in humans. Temperature. 2015;2:499–505. doi: 10.1080/23328940.2015.1110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim FT, Yamada PM, Robergs RA, Schneider SM, Moseley PL. The effect of the rate of heat storage on serum heat shock protein 72 in humans. Eur J Appl Physiol. 2008;104:965–972. doi: 10.1007/s00421-008-0850-3. [DOI] [PubMed] [Google Scholar]
- Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev. 2003;9:25–33. [PMC free article] [PubMed] [Google Scholar]
- Atamaniuk J, Stuhlmeier KM, Vidotto C, Tschan H, Dossenbach-Glaninger A, Mueller MM. Effects of ultra-marathon on circulating DNA and mRNA expression of pro- and anti-apoptotic genes in mononuclear cells. Eur J Appl Physiol. 2008;104:711–717. doi: 10.1007/s00421-008-0827-2. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52:2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- Byrne C, Lee JKW, Chew SAN, Lim CL, Tan EYM. Continuous thermoregulatory responses to mass-participation distance running in heat. Med Sci Sports Exerc. 2006;38:803–810. doi: 10.1249/01.mss.0000218134.74238.6a. [DOI] [PubMed] [Google Scholar]
- Carter MR, Mcginn R, Barrera-Ramirez J, Sigal RJ, Kenny GP. Impairments in local heat loss in type 1 diabetes during exercise in the heat. Med Sci Sport Exerc. 2014;46:2224–2233. doi: 10.1249/MSS.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Connolly PH, Caiozzo VJ, Zaldivar F, Nemet D, Larson J, Hung S-P, Heck JD, Hatfield GW, Cooper DM. Effects of exercise on gene expression in human peripheral blood mononuclear cells. J Appl Physiol. 2004;97:1461–1469. doi: 10.1152/japplphysiol.00316.2004. [DOI] [PubMed] [Google Scholar]
- Davis SL, Wilson TE, White AT, Frohman EM. Thermoregulation in multiple sclerosis. J Appl Physiol. 2010;109:1531–1537. doi: 10.1152/japplphysiol.00460.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K, Zuhl MN, Moseley PL. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J Appl Physiol. 2016;120:692–701. doi: 10.1152/japplphysiol.00536.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RF. Inhibition of Hsp90 function delays and impairs recovery from heat shock. FEBS J. 2005;272:5244–5256. doi: 10.1111/j.1742-4658.2005.04921.x. [DOI] [PubMed] [Google Scholar]
- Erekat N, Al-Khatib A, Al-Jarrah M. Heat shock protein 90 is a potential therapeutic target for ameliorating skeletal muscle abnormalities in Parkinson’s disease. Neural Regen Res. 2014;9:616–621. doi: 10.4103/1673-5374.130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlejman AG, Lagadari M, Toneatto J, Piwien-Pilipuk G, Galigniana MD. Regulatory role of the 90-kDa-heat-shock protein (Hsp90) and associated factors on gene expression. Biochim Biophys Acta. 2014;1839:71–87. doi: 10.1016/j.bbagrm.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Koukoulas I. HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J Appl Physiol. 2000;89:1055–1060. doi: 10.1152/jappl.2000.89.3.1055. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Walsh R, Koukoulas I, van Hall G, Saltin B, Pedersen BK. Reduced glycogen availability is associated with an elevation in HSP72 in contracting human skeletal muscle. J Physiol. 2002;538:911–917. doi: 10.1113/jphysiol.2001.013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Schlotz E, Passek F, Dickhuth HH, Northoff H. Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J Appl Physiol. 2000;89:704–710. doi: 10.1152/jappl.2000.89.2.704. [DOI] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Veith R, Dickhuth HH, Northoff H. Changes of HSP72-expression in leukocytes are associated with adaptation to exercise under conditions of high environmental temperature. J Leukoc Biol. 2001;69:747–754. [PubMed] [Google Scholar]
- Fehrenbach E, Veith R, Schmid M, Dickhuth H-H, Northoff H, Niess AM. Inverse response of leukocyte heat shock proteins and DNA damage to exercise and heat. Free Radic Res. 2003;37:975–982. doi: 10.1080/10715760310001595748. [DOI] [PubMed] [Google Scholar]
- Garrett AT, Goosens NG, Rehrer NJ, Patterson MJ, Harrison J, Sammut I, Cotter JD. Short-term heat acclimation is effective and may be enhanced rather than impaired by dehydration. Am J Hum Biol. 2014;26:311–320. doi: 10.1002/ajhb.22509. [DOI] [PubMed] [Google Scholar]
- Gibson OR, Dennis A, Parfitt T, Taylor L, Watt PW, Maxwell NS. Extracellular Hsp72 concentration relates to a minimum endogenous criteria during acute exercise-heat exposure. Cell Stress Chaperones. 2014;19:389–400. doi: 10.1007/s12192-013-0468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson OR, Mee JA, Taylor L, Tuttle JA, Watt PW, Maxwell NS, Taylor L, Watt PW, Maxwell NS. Isothermic and fixed-intensity heat acclimation methods elicit equal increases in Hsp72 mRNA. Scand J Med Sci Sports. 2015;25:259–268. doi: 10.1111/sms.12430. [DOI] [PubMed] [Google Scholar]
- Gibson OR, Mee JA, Tuttle JA, Taylor L, Watt PW, Maxwell NS. Isothermic and fixed intensity heat acclimation methods induce similar heat adaptation following short and long-term timescales. J Therm Biol. 2015;49-50:55–65. doi: 10.1016/j.jtherbio.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Gibson OR, Turner G, Tuttle JA, Taylor L, Watt PW, Maxwell NS. Heat acclimation attenuates physiological strain and the HSP72, but not HSP90α, mRNA response to acute normobaric hypoxia. J Appl Physiol. 2015;119:889–899. doi: 10.1152/japplphysiol.00332.2015. [DOI] [PubMed] [Google Scholar]
- Giraldo E, Multhoff G, Ortega E. Noradrenaline increases the expression and release of Hsp72 by human neutrophils. Brain Behav Immun. 2010;24:672–677. doi: 10.1016/j.bbi.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol. 2011;110:451–457. doi: 10.1152/japplphysiol.00849.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, Connor T, Watt MJ, Carpenter K, Hargreaves M, McGee SL, Hevener AL, Febbraio MA. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63:1881–1894. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge DC, Febbraio MA, Hargreaves M. Heat shock proteins and exercise adaptations. Our knowledge thus far and the road still ahead. J Appl Physiol. 2016;120:683–691. doi: 10.1152/japplphysiol.00811.2015. [DOI] [PubMed] [Google Scholar]
- Henstridge DC, Whitham M, Febbraio MA. Chaperoning to the metabolic party: the emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol Metab. 2014;3:781–793. doi: 10.1016/j.molmet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman AR, Vince RV, Taylor L, McNaughton L, Mitchell N, Siegler J. Exercise-induced dehydration with and without environmental heat stress results in increased oxidative stress. Appl Physiol Nutr Metab. 2011;36:698–706. doi: 10.1139/h11-080. [DOI] [PubMed] [Google Scholar]
- Hom LL, Lee EC-H, Apicella JM, Wallace SD, Emmanuel H, Klau JF, Poh PYS, Marzano S, Armstrong LE, Casa DJ, Maresh CM. Eleven days of moderate exercise and heat exposure induces acclimation without significant HSP70 and apoptosis responses of lymphocytes in college-aged males. Cell Stress Chaperones. 2012;17:29–39. doi: 10.1007/s12192-011-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL, Balogh G, Rivas E, Kavanagh K, Vigh L. The importance of the cellular stress response in the pathogenesis and treatment of type 2 diabetes. Cell Stress Chaperones. 2014;19:447–464. doi: 10.1007/s12192-014-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M. Heat acclimation, epigenetics, and cytoprotection memory. Compr Physiol. 2014;4:199–230. doi: 10.1002/cphy.c130025. [DOI] [PubMed] [Google Scholar]
- Horowitz M. Epigenetics and cytoprotection with heat acclimation. J Appl Physiol. 2016;120:702–710. doi: 10.1152/japplphysiol.00552.2015. [DOI] [PubMed] [Google Scholar]
- Hubbard RW, Bowers WD, Matthew WT, Curtis FC, Criss RE, Sheldon GM, Ratteree JW. Rat model of acute heatstroke mortality. J Appl Physiol. 1977;42:809–816. doi: 10.1152/jappl.1977.42.6.809. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny GP, Sigal RJ, McGinn R. Body temperature regulation in diabetes. Temperature. 2016;3:119–145. doi: 10.1080/23328940.2015.1131506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol. 2001;90:1031–1035. doi: 10.1152/jappl.2001.90.3.1031. [DOI] [PubMed] [Google Scholar]
- Krause M, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, Homem De Bittencourt PI. The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat Inflamm. 2015;249205:12. doi: 10.1155/2015/249205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Ludwig MS, Heck TG, Takahashi HK. Heat shock proteins and heat therapy for type 2 diabetes: pros and cons. Curr Opin Clin Nutr Metab Care. 2015;18:374–380. doi: 10.1097/MCO.0000000000000183. [DOI] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Kuennen M, Gillum T, Dokladny K, Bedrick E, Schneider S, Moseley P. Thermotolerance and heat acclimation may share a common mechanism in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R524–R533. doi: 10.1152/ajpregu.00039.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurucz I, Morva A, Vaag A, Eriksson K-F, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–1109. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Miller A, James RS, Thake CD. Cross acclimation between heat and hypoxia: heat acclimation improves cellular tolerance and exercise performance in acute normobaric hypoxia. Front Physiol. 2016;7:78. doi: 10.3389/fphys.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Muñoz CX, McDermott BP, Beasley KN, Yamamoto LM, Hom LL, Casa DJ, Armstrong LE, Kraemer WJ, Anderson JM, Maresh CM (2015) Extracellular and cellular Hsp72 differ as biomarkers in acute exercise/environmental stress and recovery. Scand J Med Sci Sports [DOI] [PubMed]
- Liu Y, Mayr S, Opitz-Gress A, Zeller C, Lormes W, Baur S, Lehmann M, Steinacker JM. Human skeletal muscle HSP70 response to training in highly trained rowers. J Appl Physiol. 1999;86:101–104. doi: 10.1152/jappl.1999.86.1.101. [DOI] [PubMed] [Google Scholar]
- Logan-Sprenger HM, Heigenhauser GJF, Jones GL, Spriet LL (2015) The effect of dehydration on muscle metabolism and time trial performance during prolonged cycling in males. Physiol Rep 3 [DOI] [PMC free article] [PubMed]
- Lyashko VN, Vikulova VK, Chernicov VG, Ivanov VI, Ulmasov KA, Zatsepina OG, Evgen’ev MB. Comparison of the heat shock response in ethnically and ecologically different human populations. Proc Natl Acad Sci U S A. 1994;91:12492–12495. doi: 10.1073/pnas.91.26.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães FDC, Amorim FT, Passos RLF, Fonseca MA, Oliveira KPM, Lima MRM, Guimarães JB, Ferreira-Júnior JB, Martini ARP, Lima NRV, Soares DD, Oliveira EM, Rodrigues LOC. Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones. 2010;15:885–895. doi: 10.1007/s12192-010-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloyan A, Horowitz M. Beta-adrenergic signaling and thyroid hormones affect HSP72 expression during heat acclimation. J Appl Physiol. 2002;93:107–115. doi: 10.1152/japplphysiol.01122.2001. [DOI] [PubMed] [Google Scholar]
- Marshall HC, Campbell SA, Roberts CW, Nimmo MA. Human physiological and heat shock protein 72 adaptations during the initial phase of humid-heat acclimation. J Therm Biol. 2007;32:341–348. doi: 10.1016/j.jtherbio.2007.04.003. [DOI] [Google Scholar]
- Maughan RJ, Otani H, Watson P. Influence of relative humidity on prolonged exercise capacity in a warm environment. Eur J Appl Physiol. 2012;112:2313–2321. doi: 10.1007/s00421-011-2206-7. [DOI] [PubMed] [Google Scholar]
- McClung JP, Hasday JD, He J-RR, Montain SJ, Cheuvront SN, Sawka MN, Singh IS. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol. 2008;294:R185–R191. doi: 10.1152/ajpregu.00532.2007. [DOI] [PubMed] [Google Scholar]
- Mestre-Alfaro A, Ferrer MD, Banquells M, Riera J, Drobnic F, Sureda A, Tur JA, Pons A. Body temperature modulates the antioxidant and acute immune responses to exercise. Free Radic Res. 2012;46:799–808. doi: 10.3109/10715762.2012.680193. [DOI] [PubMed] [Google Scholar]
- Mohr M, Nybo L, Grantham J, Racinais S. Physiological responses and physical performance during football in the heat. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran DS, Eli-Berchoer L, Heled Y, Mendel L, Schocina M, Horowitz M. Heat intolerance: does gene transcription contribute? J Appl Physiol. 2006;100:1370–1376. doi: 10.1152/japplphysiol.01261.2005. [DOI] [PubMed] [Google Scholar]
- Morton JP, Holloway K, Woods P, Cable NT, Burniston J, Evans L, Kayani AC, McArdle A. Exercise training-induced gender-specific heat shock protein adaptations in human skeletal muscle. Muscle Nerve. 2009;39:230–233. doi: 10.1002/mus.21182. [DOI] [PubMed] [Google Scholar]
- Morton JP, Maclaren DPM, Cable NT, Campbell IT, Evans L, Bongers T, Griffiths RD, Kayani AC, McArdle A, Drust B. Elevated core and muscle temperature to levels comparable to exercise do not increase heat shock protein content of skeletal muscle of physically active men. Acta Physiol (Oxf) 2007;190:319–327. doi: 10.1111/j.1748-1716.2007.01711.x. [DOI] [PubMed] [Google Scholar]
- Moseley PL. Exercise, stress, and the immune conversation. Exerc Sport Sci Rev. 2000;28:128–132. [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol. 1997;83:1413–1417. doi: 10.1152/jappl.1997.83.5.1413. [DOI] [PubMed] [Google Scholar]
- Noble EG, Shen GX. Impact of exercise and metabolic disorders on heat shock proteins and vascular inflammation. Autoimmune Dis. 2012;2012:836519. doi: 10.1155/2012/836519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega E. Neuroendocrine mediators in the modulation of phagocytosis by exercise: physiological implications. Exerc Immunol Rev. 2003;9:70–93. [PubMed] [Google Scholar]
- Peart D, McNaughton L, Midgley A. Pre-exercise alkalosis attenuates the heat shock protein 72 response to a single-bout of anaerobic exercise. J Sci. 2011;14:435–440. doi: 10.1016/j.jsams.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Périard JD, Ruell P, Caillaud C, Thompson MW. Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperones. 2012;17:375–383. doi: 10.1007/s12192-011-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périard JD, Ruell PA, Thompson MW, Caillaud C. Moderate- and high-intensity exhaustive exercise in the heat induce a similar increase in monocyte Hsp72. Cell Stress Chaperones. 2015;20:1037–1042. doi: 10.1007/s12192-015-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MJ. Thermoregulation during exercise in individuals with spinal cord injuries. Sports Med. 2006;36:863–879. doi: 10.2165/00007256-200636100-00005. [DOI] [PubMed] [Google Scholar]
- Racinais S, Alonso JM, Coutts AJ, Flouris AD, Girard O, González-Alonso J, Hausswirth C, Jay O, Lee JKW, Mitchell N, Nassis GP, Nybo L, Pluim BM, Roelands B, Sawka MN, Wingo JE, Périard JD. Consensus recommendations on training and competing in the heat. Scand J Med Sci Sports. 2015;25:6–19. doi: 10.1111/sms.12467. [DOI] [PubMed] [Google Scholar]
- Romberg A, Ikonen A, Ruutiainen J, Virtanen A, Hämäläinen P. The effects of heat stress on physical functioning in persons with multiple sclerosis. J Neurol Sci. 2012;319:42–46. doi: 10.1016/j.jns.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Ruell PA, Simar D, Périard JD, Best S, Caillaud C, Thompson MW. Plasma and lymphocyte Hsp72 responses to exercise in athletes with prior exertional heat illness. Amino Acids. 2014;46:1491–1499. doi: 10.1007/s00726-014-1721-3. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39:377–390. doi: 10.1249/01.mss.0000272779.34140.3b. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Latzka WA, Montain SJ, Cadarette BS, Kolka MA, Kraning KK, 2nd, Gonzalez RR. Physiologic tolerance to uncompensable heat: intermittent exercise, field vs laboratory. Med Sci Sport Exerc. 2001;33:422–430. doi: 10.1097/00005768-200103000-00014. [DOI] [PubMed] [Google Scholar]
- Selkirk GA, McLellan TM, Wright HE, Rhind SG. Expression of intracellular cytokines, HSP72, and apoptosis in monocyte subsets during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol. 2009;296:R575–R586. doi: 10.1152/ajpregu.90683.2008. [DOI] [PubMed] [Google Scholar]
- Shin Y-O, Oh J-K, Sohn H-S, Bae J-S, Lee M-Y, Lee J-B, Yang H-M, Min Y-K, Song H-Y, Ko K-K, Matsumoto T. Expression of exercise-induced HSP70 in long-distance runner’s leukocytes. J Therm Biol. 2004;29:769–774. doi: 10.1016/j.jtherbio.2004.08.053. [DOI] [Google Scholar]
- Silver JT, Noble EG. Regulation of survival gene hsp70. Cell Stress Chaperones. 2012;17:1–9. doi: 10.1007/s12192-011-0290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonna LA, Hawkins L, Lissauer ME, Maldeis P, Towns M, Johnson SB, Moore R, Singh IS, Cowan MJ, Hasday JD. Core temperature correlates with expression of selected stress and immunomodulatory genes in febrile patients with sepsis and noninfectious SIRS. Cell Stress Chaperones. 2010;15:55–66. doi: 10.1007/s12192-009-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary CM, Hogan MC. Cytosolic calcium transients are a determinant of contraction-induced HSP72 transcription in single skeletal muscle fibers. J Appl Physiol. 2016 doi: 10.1152/japplphysiol.01060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary CM, Walsh BJ, Knapp AE, Brafman D, Hogan MC. Elevation in heat shock protein 72 mRNA following contractions in isolated single skeletal muscle fibers. Am J Physiol Regul Integr Comp Physiol. 2008;295:R642–R648. doi: 10.1152/ajpregu.00852.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao Sreedhar A, Kalmár É, Csermely P, Shen Y-F. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 2004;562:11–15. doi: 10.1016/S0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- Sureda A, Tauler P, Aguiló A, Cases N, Fuentespina E, Córdova A, Tur JA, Pons A. Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res. 2005;39:1317–1324. doi: 10.1080/10715760500177500. [DOI] [PubMed] [Google Scholar]
- Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. J Appl Physiol. 1955;8:73–80. doi: 10.1152/jappl.1955.8.1.73. [DOI] [PubMed] [Google Scholar]
- Taylor L, Midgley A, Chrismas B. The effect of acute hypoxia on heat shock protein 72 expression and oxidative stress in vivo. Eur J Appl Physiol. 2010;109:849–855. doi: 10.1007/s00421-010-1430-x. [DOI] [PubMed] [Google Scholar]
- Taylor L, Midgley AW, Chrismas B, Hilman AR, Madden LA, Vince RV, McNaughton LR. Daily hypoxia increases basal monocyte HSP72 expression in healthy human subjects. Amino Acids. 2011;40:393–401. doi: 10.1007/s00726-010-0644-x. [DOI] [PubMed] [Google Scholar]
- Taylor L, Midgley AW, Chrismas B, Madden LA, Vince RV, McNaughton LR. Daily quadratic trend in basal monocyte expressed HSP72 in healthy human subjects. Amino Acids. 2010;38:1483–1488. doi: 10.1007/s00726-009-0360-6. [DOI] [PubMed] [Google Scholar]
- Taylor NAS, Cotter JD. Heat adaptation: guidelines for the optimisation of human performance. Int Sport Med J. 2006;7:33–57. [Google Scholar]
- Tintinger GR, Theron AJ, Anderson R, Ker JA. The anti-inflammatory interactions of epinephrine with human neutrophils in vitro are achieved by cyclic AMP-mediated accelerated resequestration of cytosolic calcium. Biochem Pharmacol. 2001;61:1319–1328. doi: 10.1016/S0006-2952(01)00588-3. [DOI] [PubMed] [Google Scholar]
- Tuttle JA, Castle PC, Metcalfe AJ, Midgley AW, Taylor L, Lewis MP. Downhill running and exercise in hot environments increase leukocyte Hsp 72 (HSPA1A) and Hsp90α (HSPC1) gene transcripts. J Appl Physiol. 2015;118:996–1005. doi: 10.1152/japplphysiol.00387.2014. [DOI] [PubMed] [Google Scholar]
- Vihervaara A, Sistonen L. HSF1 at a glance. J Cell Sci. 2014;127:261–266. doi: 10.1242/jcs.132605. [DOI] [PubMed] [Google Scholar]
- Watkins AM, Cheek DJ, Harvey AE, Goodwin JD, Blair KE, Mitchell JB. Heat shock protein (HSP-72) levels in skeletal muscle following work in heat. Aviat Sp Environ Med. 2007;78:901–905. [PubMed] [Google Scholar]
- Whitham M, Laing SJ, Jackson A, Maassen N, Walsh NP. Effect of exercise with and without a thermal clamp on the plasma heat shock protein 72 response. J Appl Physiol. 2007;103:1251–1256. doi: 10.1152/japplphysiol.00484.2007. [DOI] [PubMed] [Google Scholar]