Abstract
Ginkgolide and bilobalide are major trilactone constituent of Ginkgo biloba leaves and have been shown to exert powerful neuroprotective properties. The aims of this study were to observe the inhibitory effects of ginkgolide and bilobalide on the activation of microglial cells induced by oxygen–glucose deprivation and reoxygenation (OGD/R) and the specific mechanisms by which these effects are mediated. For detecting whether ginkgolide and bilobalide increased cell viability in a dose-dependent manner, BV2 cells were subjected to oxygen–glucose deprivation for 4 h followed by 3 h reoxygenation with various concentrations of drugs (6.25, 12.5, 25, 50, and 100 μg/ml). The extent of apoptosis effect of OGD/R with or without ginkgolide and bilobalide treatment were also measured by Annexin V-FITC/PI staining. Similarly, the levels of pro-inflammatory cytokines TNF-α, IL-1β, IL-6, IL-8, and IL-10 were detected using a specific Bio-Plex Pro™ Reagent Kit. The effects of ginkgolide and bilobalide on protein levels of TLR2/4, MyD88, p-TAK1, p-IKKβ, p-IkBα, NF-κB p65, Bcl-2, Bax, Bak, RIP3, cleaved-Caspase-3, cleaved PARP-1 and cellular localization of NF-κB p65 were evaluated by Western blot and double-labeled immunofluorescence staining, respectively. OGD/R significantly decreased the cell viability and increased the release of IL-1β, IL-6, IL-8, IL-10, TNF-α in BV2 microglia cells; these effects were suppressed by ginkgolide and bilobalide. Meanwhile, ginkgolide and bilobalide also attenuated the OGD/R-induced increases in TLR2, TLR4, MyD88, Bak, RIP3 levels and reversed cleaved caspase-3/caspase-3, Bax/Bcl-2 and cleaved PARP-1/PARP-1 ratio. Furthermore, ginkgolide and bilobalide also downregulated p-TAK1, p-IkBα, and p-IKKβ and inhibited the OGD/R-induced transfer of NF-κB p65 from cytoplasm to nucleus in BV2 microglia cells. The results showed that ginkgolide and bilobalide can inhibit OGD/R-induced production of inflammatory factors in BV2 microglia cells by regulating the TLRs/MyD88/NF-κB signaling pathways and attenuating inflammatory response. The possible mechanism of anti-inflammatory and neuroprotective effects of ginkgolides results from the synergistic reaction among each monomer constituents.
Keywords: Ginkgolides, Bilobalide, BV2 microglia cells, TLRs, Cerebral ischemia, NF-κB, Inflammatory
Introduction
Ischemic stroke, also known as cerebral infarction, is a common and life-threatening neurological disease with substantial morbidity and mortality global, which brings a great burden to the family and society (Murray and Lopez 1997; Go et al. 2014; Jauch et al. 2013). About 87 % of stroke cases are due to sudden occlusion of a blood vessel by a thrombus or embolism (Go et al. 2014). Rapid reperfusion in ischemic area as soon as possible and rescuing dying neuron, vascular endothelial cells after cerebral ischemia is critical in the therapy of ischemic cerebrovascular incidents. However, perhaps surprisingly, the occurrence of reperfusion usually cause deterioration of brain injury and a profound inflammatory response (Eltzschig and Eckle 2011; Iadecola and Alexander 2001). It represents the most important reason in the cause of brain failure (Nagy et al. 2005; Schaller and Graf 2004). Although the mechanisms of cerebra ischemia and reperfusion (I/R) injury are complex and involve the interactions of numerous pathophysiological processes, i.e., oxygen free radical injury, excitatory amino acid neurotoxicity, intracellular calcium overload, etc., increasing evidence shows that inflammation is involved in stroke progression (Eltzschig and Eckle 2011; Iadecola and Alexander 2001, Broughton et al. 2009, Doyle et al. 2008). Mounting evidence indicates that brain ischemia triggers inflammatory responses and leads to microglia activation, which produces more cytotoxic substances including TNF, IL-1β, iNOS, and other pro-inflammatory mediators and results in more neuronal damage (Iadecola and Anrather 2011; Kaushal and Schlichter 2008). Inhibition of microglia activity in early stage of cerebral ischemia reperfusion can reduce cerebral infarction volume, thus exerting brain protection effect (Son et al. 2009). Recently, researches showed that using cell cycle inhibitor, roscovitine, can inhibit microglia proliferation and production of inflammatory cytokines such as IL-1β, MIP-1α, and NO and reduced the number of cell cycle-related proteins including cyclin A, cyclin B, and cyclin E in rat cerebral ischemia model and oxygen–glucose deprivation (OGD) of BV2 cells; these changes all contributed to neuroprotection in ischemia (Zhang et al. 2009b). There is also direct evidence of neuronal degeneration and microglia activation after transient cerebral ischemia (Moon et al. 2009). Therefore, the anti-inflammatory activities of the agents that target activated microglia cells induced by I/R may be crucial to promote the survival of neurons.
Toll-like receptors (TLRs) was first discovered in the embryos of Drosophila melanogaster in 1980. By now, 10 functional TLRs in humans and 12 in rodents have been indentified respectively (Akira 2009). Many studies demonstrated that TLR2/4-induced innate immune and inflammatory response might play an important role than other TLRs during the course of brain damage caused by ischemia/reperfusion (Winters et al. 2013; Zwagerman et al. 2010; Lehnardt et al. 2007; Tang et al. 2007; Hyakkoku et al. 2010). Moreover, TLR2 and TLR4 were mainly expressed in microglia (Takeda and Akira 2005). Cerebral ischemic/reperfusion promote TLR2/4 combined with their endogenous ligands, HSPs or HMGB1, and leads to recruitment/activation of MyD88, the interleukin-1 (IL-1) receptor-associated kinase, the tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), and the transforming growth factor beta-activated kinase 1(TAK1), thereby activating the transcription factor and increasing expression of pro-inflammatory cytokine such as TNF-α, IL-1β, and IL-6 (Vabulas et al. 2001; Park et al. 2006). Among the transcription factors that involved in the transcription of pro-inflammatory genes, NF-κB is perhaps the most important one (Kacimi et al. 2011; Wang et al. 2007). The IκBs are phosphorylated by cytokine-responsive IκB kinase (IKK) at serine residues 32 and 36 when NF-κB were activated by a variety of stimuli including oxidative stress, hypoxia, and several inflammatory mediators, which trigger its ubiquination/degradation and subsequent release of NF-κB, which then translocates to the nucleus and facilitates the transcription of several pro-inflammatory cytokines (Baeuerle and Baltimore 1996). Microglia NF-κB activation has been proposed to promote brain damage via induction of pro-inflammatory cytokines (Huang et al. 2001; Zhang et al. 2005).
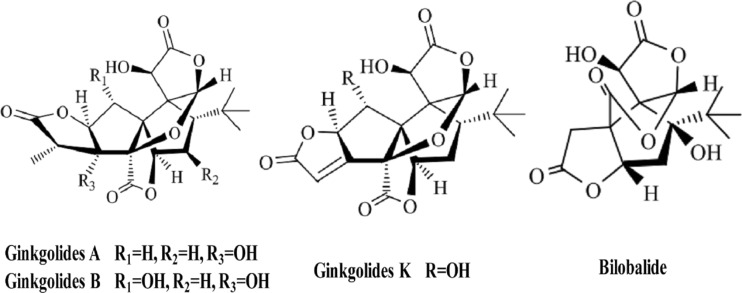
Ginkgo biloba is an ancient Chinese tree that has been cultivated and held sacred for its health-promoting properties. Substantial basic research and clinical evidence indicate that concentrated and partially purified extracts of G. biloba leaves possesses many beneficial effects against some kind of neural and vascular damage (Maclennan et al. 2002, Xia and Fang 2007, Zhu et al. 2004, Wang et al. 2004). EGb-761, a patented extract of G. biloba, has been shown to have neuroprotective effects against various cardiovascular and neurological disorders and have been wildly used to treatment ischemia, Alzheimer’s disease, and dementia (Ahlemeyer and Krieglstein 2003, Chandrasekaran et al. 2003, Andrieu et al. 2008, van Dongen et al. 2000). G. biloba extract contains two groups of bioactive constituents, the flavonoids (24 %) and the terpenoids (6 %), while ginkgolide and bilobalide are two primarily constituents of the terpenoid fraction (Jaracz et al. 2004) the main compound structure were shown in Fig. 1. Ginkglolides, including ginkgolide A, B, C, J, K, L, and M, were found to be specific and selective antagonists of platelet-activating factors (Kleijnen and Knipschild 1992, Desquand et al. 1986, Braquet 1986). Ginkgolid B is one of the major components of terpenoid fraction of G. biloba extract which has antioxidative, vascular, and neuroprotective effects (Maclennan et al. 2002). Ample data showed that ginkgolid B possesses a remarkable neuroprotective property against ischemia-induced impairments in vivo (Liu et al. 2010, Lv et al. 2011b) and in vitro (Peng et al. 2010, Wu et al. 2009) by antagonizing PAF, inhibiting thrombosis, scavenging oxygen free radicals, and inhibiting inflammation after cerebral ischemia (Xia and Fang 2007). In addition, ginkgolid A and ginkgolid B could reduce infarction volume and protect neurons in rat permanent middle cerebral artery (MCA) models (Ni et al. 2011), and the protective effects were associated with the inhibition of NF-κB signaling pathway (Wang et al. 2008), while PAF is one of the most potent mediators in many inflammatory processes via activation of the nuclear transcription factor NF-κB (De Plaen et al. 2000, Ko et al. 2006). Bilobalide has multiple mechanisms of action, including preservation of mitochondrial ATP synthesis, inhibition of apoptotic damage, and suppression of hypoxia-induced membrane deterioration against cerebral ischemia and neurodegeneration (Defeudis 2002). Recently, study showed that neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation (Jiang et al. 2014). Taking together, all of these studies clearly show that neuroprotective effects of ginkgolides are closely related to anti-inflammatory pathways, although its specific mechanisms were not well understood.
Fig. 1.
Chemical structures of ginkgolides and bilobalide
In the present study, we hypothesized that TLRs/MyD88/NF-κB pathways could be a therapeutic target of ginkgolides and bilobalide in condition of cerebral ischemia reperfusion injury. We investigated, therefore, whether the neuroprotective effects of ginkgolides and bilobalide were by regulating TLRs/MyD88/NF-κB pathways and reducing the production of pro-inflammatory in BV2 microglia after oxygen–glucose deprivation and reoxygenation (OGD/R).
Methods
Materials
BV2 mouse microglia cell line was purchased from Kunming Institute of Botany, Chinese Acasdemy of scuences (Kunming, China). Active pharmaceutical ingredients of ginkgolides (GAPIs, containing GA, GB, and GK), ginkgolide A (GA, HPLC-purity ≥ 97.4 %), ginkgolide B (GB, HPLC-purity ≥ 99.2 %), ginkgolide K (GK, HPLC-purity ≥ 98 %), and Bilobalide (BB, HPLC-purity ≥ 98 %) were separated by Kanion (Kanion Pharmaceutical, Co., China).
Cell culture and grouping
BV2 cells were maintained in high glucose DMEM medium containing 10 % FBS and 1 % penicillin–streptomycin and incubated at 37 °C in a humid atmosphere containing 95 % air and 5 % CO2. After expansion, cells after passage 3 were used for all the experiments. In order to examine whether GAPIs, GA, GB, GK, and BB increased cell viability in a dose-dependent manner, BV2 microglia were performed OGD for 4 h followed by reoxygenation for additional 3 h with various concentrations of drugs (6.25, 12.5, 25, 50, and 100 μg/ml) treatment.
Oxygen–glucose deprivation and reoxygenation and drug administration
BV2 cells at the density of 1.0 × 106/mL were seeded into 95 cm2 cell culture dish; after 24 h fusion, cells were treated differently depending on the purpose the cells were used for. Briefly, the normal cultured BV2 cells without any treatment were used as control. The cells were preformed in OGD experiment, culture medium was replaced into glucose-free DMEM, and the culture dish was placed into a sealed chamber with persistent low flow (20 L/min) of 95 % N2 and 5 % CO2 mixture to expel oxygen for 20 min; subsequently, the air inlet and the outlet of the tubes were clamped, and the chamber was placed into CO2 incubator. After 4 h OGD, oxygen–glucose deprivation was terminated by exposing cells at normal culture conditions (37 °C, 95 % air, 5 % CO2) and incubated with 50 μg/ml GAPIs, GA, GB, GK, and BB for 6 h (total protein assays) or 1 h (protein kinase assays).
Cell viability assays
Freshly collected BV2 cells were counted (>93 % viability) and dispensed into a 96-well plates at the final density of 3.0 × 104cells/100 μl/well. After a 24-h incubation to allow cell attachment, the cells without any treatment were used as control, and the others were incubated with glucose-free DMEM and executed OGD for 4 h; subsequently, GAPIs, GA, GB, GK, and BB were added into during 3 h reoxygenation. Cell viability was evaluated with the Cell Titer 96® Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI, USA) according the instruction of the manufacture. In brief, at the end of OGD/R, 20 μL of MTS was added to each well. Cell viability was determined by measuring the absorbance at 490 nm using a microplate spectrophotometer (Molecular Devices Flex Station 3, America). Six replications of each sample were analyzed in each group. The extent of apoptosis was detected by using Annexin V-FITC/PI ( propidium iodide) detection kit (Beyotime, Nantong, China) as described in the manufacturer’s instruction. Briefly, cells at the density of 2.0 × 105 cells/500 μl/well were plated into 24-well plate; after 24 h incubation, cells were performed 4 h OGD followed by 50 μg/ml ginkgolides and BB respectively for additional 3 h at 37 °C, 5 % CO2. Subsequently, all cells were harvested and carefully washed with 1× PBS for three times. After centrifugation at 1000g for 5 min, the cell pellets were resuspended in 195 μl annexin V-FITC binding buffer and gently mixed by adding another 5 μL annexin V-FITC. Thereafter, 10 μL of PI (50 mg/mL) was added, and the suspension was then incubated in the dark for 20 min at room temperature. The fluorescence of these cells was analyzed by NovoCyte D2040R flow cytometry (ACEA Biosciences, San Diego, CA, USA) using the NovoExpress 1.1.0 software. Three replications of each sample were analyzed in each group.
Measurements of secreted inflammatory cytokines in BV2 cells
Levels of TNF-α, IL-1β, IL-6, and IL-10 in each group were detected by Bio-Plex 200 System (Bio-Rad Laboratories, Hercules, CA, USA). Cell seeding and treatment of BV2 cells were the same as those described in cell viability assays. Briefly, after a 4-h OGD followed by 3-h incubation with 50 μg/ml ginkgolides and BB respectively at 37 °C, 5 % CO2, the cells were centrifuged at 4 °C, 450 g for 10 min, and 50 μl of supernatant was then collected at −80 °C for cytokines detection. Cytokines assays were preformed according to the protocol provided by the manufacturer of the commercially available Bio-Plex Pro™ Reagent Kit (Bio-Rad Laboratories, Hercules, CA, USA). Six replications of each sample were analyzed in each assay.
Western blotting analysis
The levels of TLR2/4, MyD88 (Abcam, Cambridge, UK), p-TAK1 (Thermo Fisher, USA), p-IKKβ, Bak, PARP-1, RIP3 (Cell signaling technology, CST, Co., USA), p-IkBα, Bcl-2, Bax, and Caspases-3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in the BV2 cells and NF-κB p65 (Cell Signaling Technology, CST, Co., USA) expression in nuclear and cytoplasmic fraction of microglia cells were analyzed by Western blotting. In brief, the cells were washed with cold 1× PBS and harvested under non-denaturing conditions by incubation at 4 °C with lysis buffer which contain protease inhibitor and phosphorylase inhibitor for 10 min. The cell lysates were centrifuged at 14,000g for 10 min at 4 °C to remove insoluble precipitates. The protein content in each sample was determined by the method of Bradford. Total protein (50 μg) from culture cells was denatured and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) containing 10 % acrylamide gels and subsequently transferred electrophoretically to PVDF membranes. After nonspecific binding sites were blocked 2 h with TBST (Tween Tris buffered saline) containing 5 % BSA or skimmed milk, the membrane was then incubated with corresponding primary antibodies diluted 1:1000 overnight at 4 °C. After washing with 1× TBST, the samples were subsequently incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1: 2000 dilution, Cell signaling technology, CST, Co., USA) for 2 h and visualized on Chemi Doc XRS + detection system (Bio-Rad Laboratories, Hercules, CA, USA). Histone and GAPDH were used as a loading control of nuclear and cytoplasmic fraction, respectively.
Immunofluorescent analysis
To determine the expression and localization of NF-κB p65 in BV2 cells, double-labeled immunocytochemistry was performed. In briefly, the cells were fixed with 4 % (v/v) paraformaldehyde in 1× PBS at room temperature for 15 min and washed with 1× PBS for 3 times, then blocked with 1 % (w/v) BSA in 1× PBS containing 0.3 % (v/v) Triton X-100 at room temperature for 30 min. The primary antibody against NF-κB p65 (1: 100 dilution, Cell signaling technology, CST, Co., USA) was incubated at 37 °C for 2 h. Subsequently, cells were incubated with goat anti-rabbit IgG-CFL 488 (1: 250 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 37 °C for 1 h. After washing with 1× PBS for 3 times, cell nuclei were stained using Hoechst 33258 (Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 2 μg/ml at 37 °C for additional 15 min. Fluorescent images were visualized under by a fluorescence reverse microscope (Leica DMI 4000B, Leica Co., Germany). The fluorescent intensity of nucleus and cytoplasm in each group was quantitated by Image J software; the nucleus/cytoplasm ratio was then calculated.
Statistical analysis
All data were presented as mean ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA with Tukey’ s post doc method using the software of SPSS Statistics V17.0. Differences were considered statistically significant when a P value is <0.05.
Results
GAPIs increased cell viability in BV2 microglia cells exposed to OGD/R
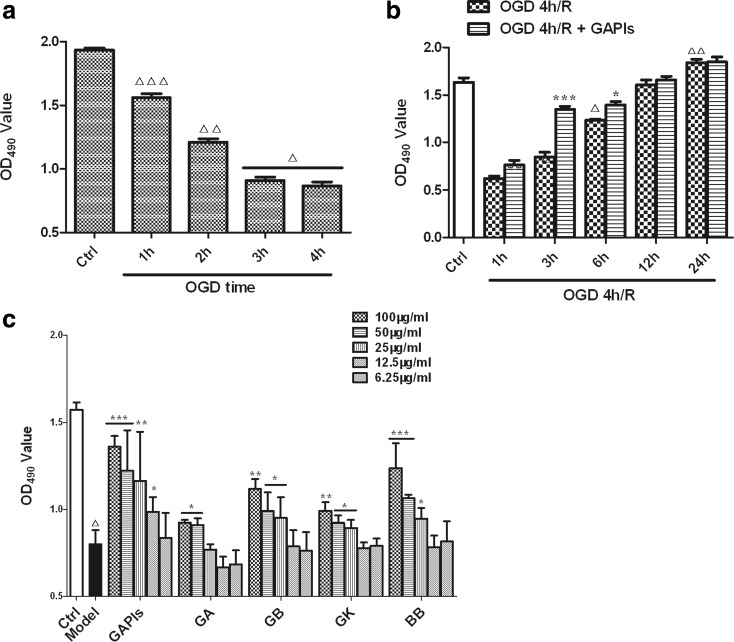
In order to ensure the consistency of results, we firstly optimized the effects of different time duration of OGD on BV2 cell viability with MTS assay. As shown in Fig. 2a, cell viability decreased to about 81, 62, 47, and 45 % after OGD 1–4 h followed by reoxgenation for 3 h respectively. Subsequently, we detected the effects of different duration of reoxygenation on BV2 cells viability. OGD 4 h/reoxgenation for 1, 3, and 6 h all reduced cell viability, but no significantly changes occurred at 12 and 24 h timepoint compared with control group. GAPIs could increase the cell viability after 4 h OGD followed by 1 and 6 h reoxygenation and augment cell viability by 10.8, 75.7, and 23.1 % respectively. The peak time of the protective effect of GAPIs was at reoxygenation 3-h timepoint and then decreased. The protective effect of GAPIs cannot be evaluated at 12 and 24 h timepoint due to the recovery of cell viability as time goes on (Fig. 2b).
Fig. 2.
Effects of ginkgolides and bilobalide on cell viability of BV2 microglia cells exposed to OGD/R. a Effects of different time duration of OGD on BV2 microglia cells. After exposure to OGD for 1, 2, 3, and 4 h, cell viabilities were tested using MTS assay by absorbance at 490 nm. b Effects of different time duration of reoxygenation on BV2 microglia cells. After exposure to OGD for 4 h, BV2 cells were reoxygenated for 1, 3, 6, 12, and 24 h with 50 μg/ml GAPIs treatment respectively; cell viabilities were tested using MTS assay by absorbance at 490 nm. c Effects of ginkgolides on cell viability of BV2 microglia cells exposed to OGD/R. BV2 cells were subjected to 4 h OGD followed by reoxygenation for additional 3 h with various concentrations of GAPIs, GA, GB, GK, and BB (6.25, 12.5, 25, 50, and 100 μg/ml) treatment; cell viabilities were tested using MTS assay by absorbance at 490 nm. All the results were expressed as MTS OD values. Each value indicates the mean ± SD and is representative of results obtained from six wells in all experiments. △ P < 0.0001, △△ P < 0.001, △△△ P < 0.05, as compared with control group; * P < 0.05, ** P < 0.01, *** P < 0.001, as compared with model group
Ginkgolides and BB exerted its neuroprotection to BV2 microglia cells in a dose-dependent manner
Based on the results of optimized OGD and reoxygenation time above, subsequently, we investigated whether the neuroprotective effects of ginkgolides and BB in a dose-dependent or dose-independent manner. MTS assay was performed in BV2 cells after subject to OGD for 4 h followed by reoxgenation for 3 h. As the results were shown in Fig. 2c, GAPIs, GB, GK, and BB significantly increased MTS OD value of OGD/R BV2 cells under the concentration of 100, 50, and 25 μg/ml respectively. In general, ginkgolides and BB may play a neuroprotective role in OGD/R BV2 microglia cell in a dose-dependent manner, while GAPIs, GK, and BB, at the dose of 12.5 and 6.25 μg/ml, have no effects on promoting cell activities compared with the model group. GAPIs, GA, GB, GK, and BB at the concentration of 100 μg/ml exhibit almost 72.5, 16, 41.0, 24.8, and 56.6 % protection rate after 4 h OGD followed by 3 h reoxgenation. Furthermore, we speculated the effective dose of GA may be more than 50 μg/ml or within the scope of 50 and 100 μg/ml because GA at the concentration of 25 μg/ml has no protective effect on cell viability (P > 0.05).
Effects of ginkgolides and bilobalide on the levels of IL-1β, IL-6, IL-8, IL-10, and TNF-α in BV2 microglia cells after OGD/R
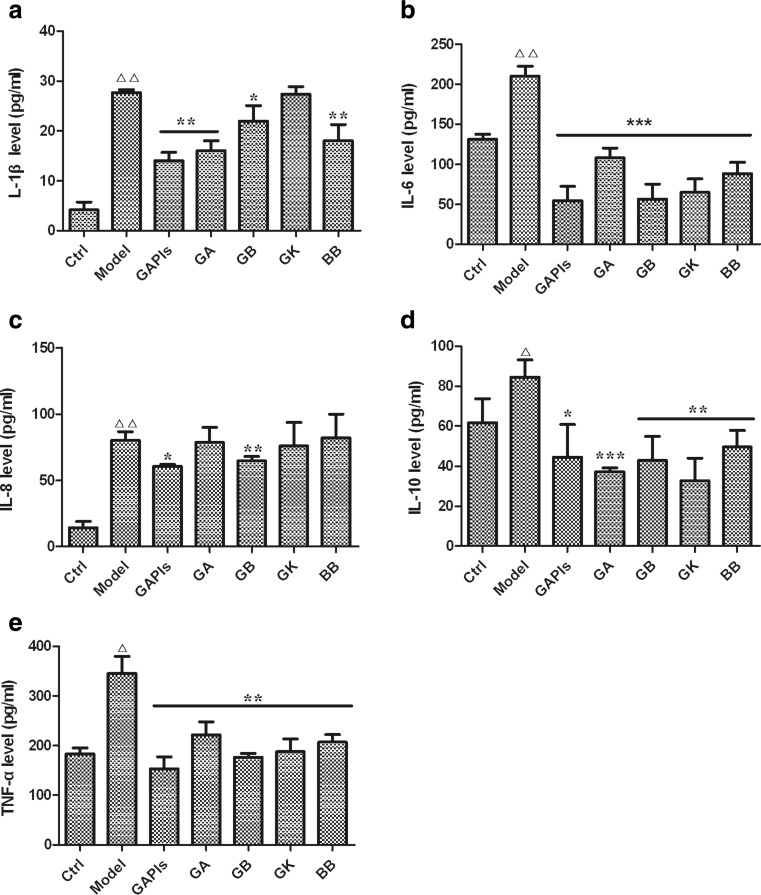
To determine further whether ginkgolides and BB treatment could associate with reduction of pro-inflammatory mediator secretion, IL-1β, IL-6, IL-8, IL-10, and TNF-α concentration was measured in OGD/R-induced BV2 microglia cells by Bio-Plex 200 System using Bio-Plex Pro™ Reagent Kit. As the results were shown in Fig. 3, in comparison with the control group, the levels of IL-1β, IL-6, IL-8, IL-10, and TNF-α significantly increased in OGD/R group. To our expectation, treatment with 50 μg/ml GAPIs, GA, GB, and BB led to an inhibition on IL-1β, IL-6, IL-10, and TNF-α secretion. While no significant reduction was observed in secretion of IL-1β in GK group, as well as IL-8 in GA, GK, and BB groups (P > 0.05) compared with the model group (Fig. 3a, c).
Fig. 3.
Effects of ginkgolides and bilobalide on the levels of inflammatory cytokines in BV2 microglia cells. After exposure to OGD for 4 h, BV2 cells were reoxygenated for additional 3 h with 50 μg/ml GAPIs, GA, GB, GK, and BB treatment respectively; subsequently, inflammatory cytokines a IL-1β, b IL-6, c IL-8, d IL-10, and e TNF-α levels in cell supernatant were detected by Bio-Plex Pro™ Reagent Kit according to manufacturer’s instructions. △ P < 0.05, △△ P < 0.001, as compared with control group; * P < 0.05, ** P < 0.01, *** P < 0.001, as compared with model group. Each value indicates the mean ± SD and is representative of results obtained from six wells in all experiments
Effects of ginkgolides and BB on the expression of TLR2, TLR4, and MyD88 in BV2 microglia cells after OGD/R
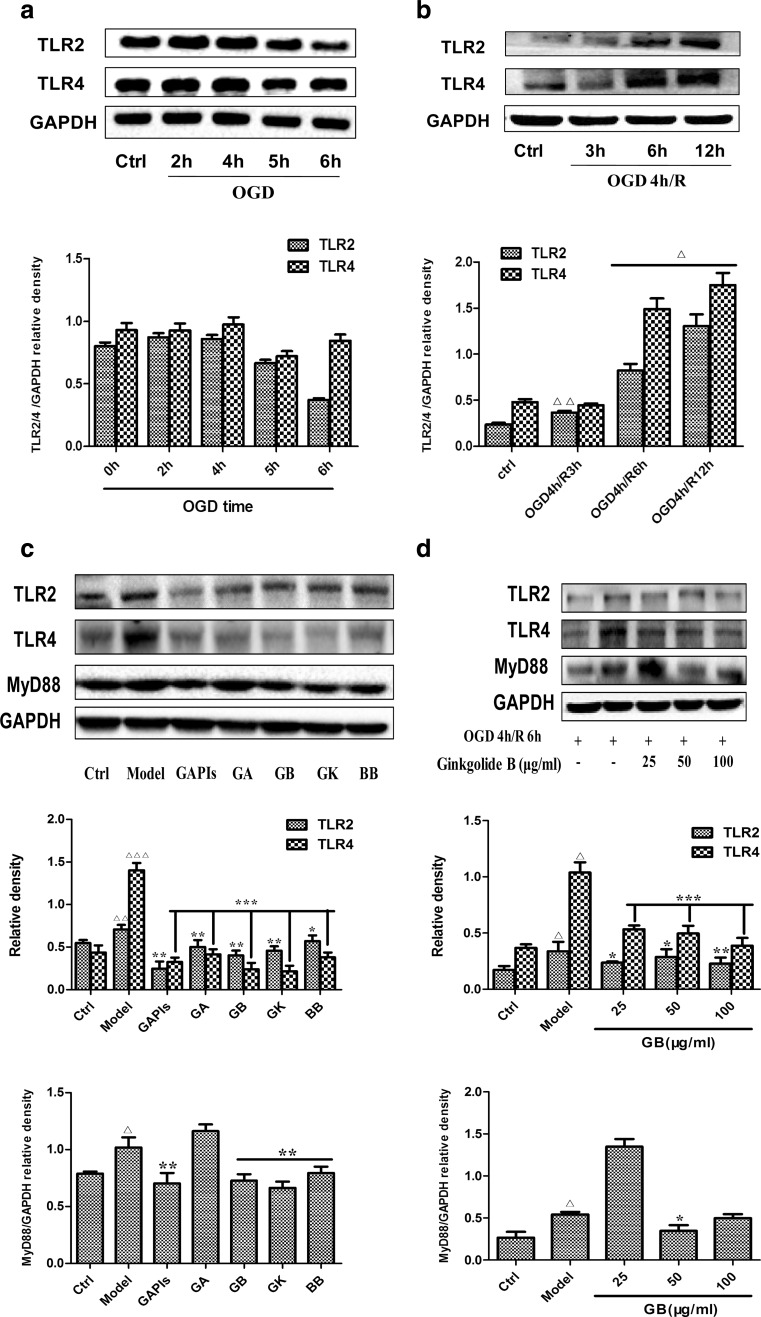
To further investigate whether ginkgolides and BB treatment would regulate the TLRs pathway induced by OGD/R in vitro, we also examined the expression of TLR2, TLR4, and MyD88 in OGD/R-induced BV2 cells by Western blot. We firstly optimized the effects of different time duration of OGD and reoxygenation on protein expression in BV2 cells. As shown in Fig. 4a, BV2 cells were exposed to OGD for 2, 4, 5, and 6 h, and TLR2 and TLR4 levels were increased and peaked at the timepoint of 4 h in the same time and then declined. Subsequently, we investigated the effects of different time of reoxygenation on protein expression. As shown in Fig. 4b, OGD 4 h/R 3, 6, and 12 h increased TLR2 and TLR4 levels in a time-dependent manner. Therefore, we choose OGD 4 h/R 6 h as the experimental condition for assessing drug efficacy in the following study. To our expectation, after the cells were exposed to 4 h OGD followed by 6 h reoxygenation, there was a significantly increased expression of TLR2, TLR4, and MyD88 in comparison with that of control group (1.29, 3.20, and 1.29-fold relative to control, respectively). In cells treated with 50 μg/ml GAPIs, GA, GB, GK, and BB during reoxygenation, there was a remarkable decrease in the levels of TLR2 and TLR4, as compared with model group. Meantime, with the exception of GA, MyD88 levels were also significantly decreased by treatment with 50 μg/ml GAPIs, GB, GK, and BB (Fig. 4c). Concentration-dependent elevation indicated that TLR2 and TLR4 levels were significantly decreased by treatment BV2 microglia cells with GB over a concentration range of 25–100 μg/ml, while low or high dose of GB could not reduce MyD88 level, with no remarkable change compared with the model group. An amount of 50 μg/ml GB significantly reduced the MyD88 level, indicating that moderate dose of GB had an inhibitory effect (Fig. 4d).
Fig. 4.
Effects of ginkgolides and bilobalide on the expression of TLR2, TLR4, and MyD88 in BV2 microglia cells after OGD/R. a, b Kinetics of OGD/R-induced increase in the levels of TLR2, TLR4 in BV2 microglia cells. c Western blot analysis of TLR2, TLR4, and MyD88 expression in BV2 microglia cells after 4 h OGD followed by reoxygenation for additional 6 h with 50 μg/ml of GAPIs, GA, GB, GK, and BB treatment. d Concentration-dependent effects of GB treatment on TLR2, TLR4, and MyD88 protein levels in BV2 microglia cells. After BV2 cells exposure to OGD for 4 h, BV2 cells were reoxygenated for either 6 or 6 h followed by treatment with 25, 50, 100 μg/ml GB respectively. GAPDH was used as the loading control. Western blot images are representative of three independent experiments. Each data point is a mean ± SD (n = 3). △ P < 0.01, △△ P < 0.05, △△△ P < 0.001, as compared with corresponding control group; * P < 0.05, ** P < 0.01, *** P < 0.001, as compared with corresponding model group
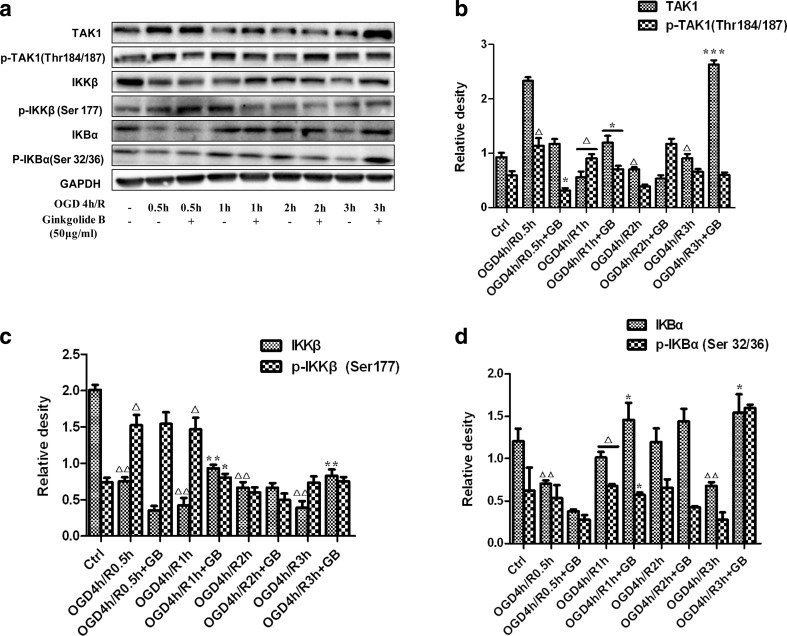
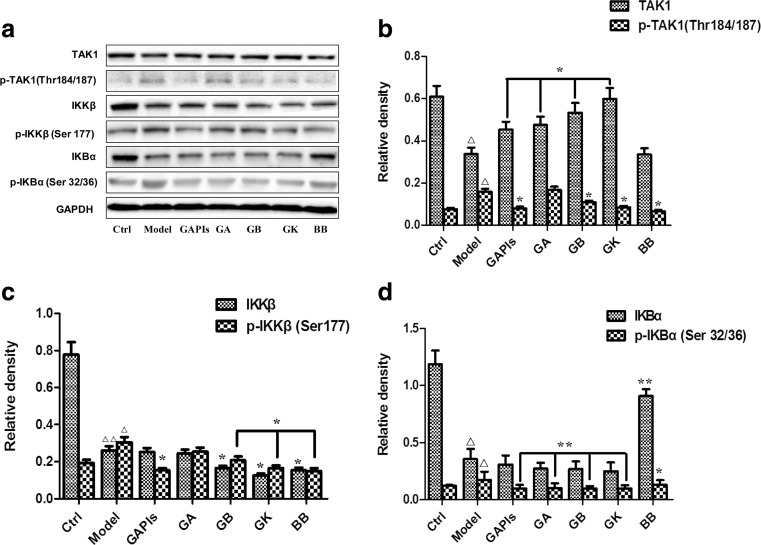
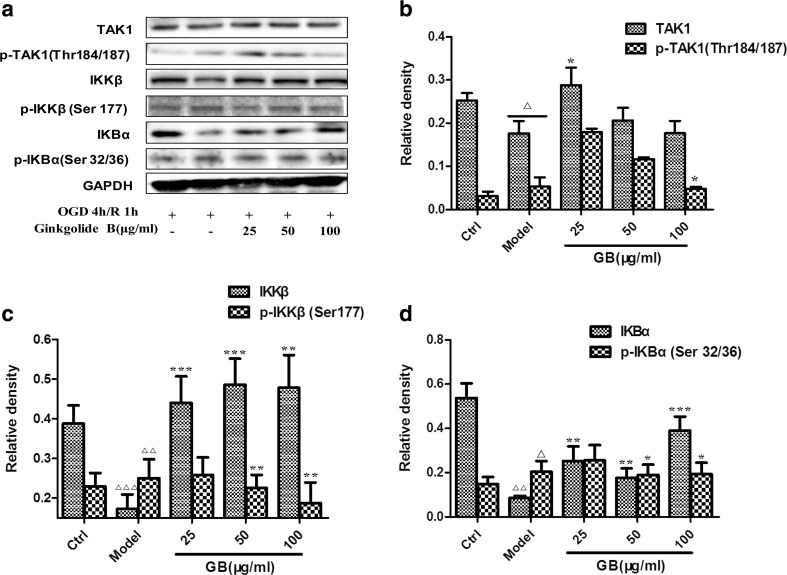
Ginkgolides and BB downregulated the levels of p-TAK1, p-IKKβ, and p-IkBα in BV2 microglia cells after OGD/R
Next, we wondered if there were any changes in the key kinase expression in TLR signaling pathways downstream. Figure 5 showed that p-TAK1, p-IKKβ, and p-IkBα expression was low in normal BV2 cells; however, their expression significantly increased and peaked at the timepoint of 1 h after 4 h OGD, with or without total protein decreasing. An amount of 50 μg/ml GB treatment significantly inhibited upregulation of kinase levels induced by OGD/R. Therefore, OGD 4 h/R 1 h might be the appropriate conditions for evaluating ginkgolides and BB efficacy. As the results were shown in Fig. 6, OGD 4 h/R 1 h induced a significant increase in p-TAK1, p-IKKβ, and p-IkBα levels compared to those in the control group (2.06, 1.57, and 1.42-fold relative to control, respectively). GAPIs, GB, GK, and BB at 50 μg/ml significantly blocked upregulation of p-TAK1, p-IKKβ, and p-IkBα levels in OGD/R-induced BV2 microglia cells, while GA, in addition to inhibition of increasing p-IkBα levels, have no effects on the expression of p-TAK1 and p-IKKβ in OGD/R-induced BV2 microglia cells. The concentration-dependent elevation was also observed for GB-treated cells over a concentration range of 25–100 μg/ml. Figure 7 showed that low dose of GB could not reduce the p-TAK1, p-IKKβ, and p-IkBα, with no significant change compared with the model group. Exposure under moderate or high dose of GB significantly reduced the p-TAK1, p-IKKβ, and p-IkBα levels in BV2 microglia cells exposed to OGD/R.
Fig. 5.
Kinetics of OGD/R-induced increase in the levels of kinases expression in BV2 microglia cells. After exposure to OGD for 4 h, BV2 cells were reoxygenated for either 0.5, 1, 2, 3 h or 0.5, 1, 2, 3 h followed by treatment with 50 μg/ml GB respectively. Representative graph a and quantitative data b p-TAK1, c p-IKKβ, and d p-IkBα levels in BV2 microglia cells. GAPDH was used as the loading control. Western blot images are representative of three independent experiments. Each data point is a mean ± SD (n = 3). △ P < 0.01, △△ P < 0.05, as compared with corresponding control group; * P < 0.05, ** P < 0.01, *** P < 0.001, as compared with corresponding OGD 4 h/R group
Fig. 6.
Effects of ginkgolides and bilobalide on the expression of p-TAK1, p-IKKβ and p-IkBα in BV2 microglia cells after OGD/R. After exposure to OGD for 4 h, BV2 cells were reoxygenated for either 1 h or 1 h followed by treatment with 50 μg/ml GAPIs, GA, GB, GK, and BB respectively. Representative graph a and quantitative data b p-TAK1, c p-IKKβ, and d p-IkBα levels in BV2 microglia cells. GAPDH was used as the loading control. Western blot images are representative of three independent experiments. Each data point is a mean ± SD (n = 3). △ P < 0.05, △△ P < 0.01, as compared with corresponding control group; * P < 0.05, ** P < 0.01, as compared with corresponding model group
Fig. 7.
Concentration-dependent effects of GB treatment on p-TAK1, p-IKKβ and p-IkBα levels in BV2 microglia cells after OGD/R. After BV2 cells exposure to OGD for 4 h, BV2 cells were reoxygenated for either 1 h or 1 h followed by treatment with 25, 50, 100 μg/ml GB respectively. Representative graph a and quantitative data b p-TAK1, c p-IKKβ and d p-IkBα levels in BV2 microglia cells. GAPDH was used as the loading control. Western blot images are representative of three independent experiments. Each data point is a mean ± SD (n = 3). △ P < 0.01, △△ P < 0.05, △△△ P < 0.001, as compared with corresponding control group; * P < 0.05, ** P < 0.01, *** P < 0.001, as compared with corresponding model group
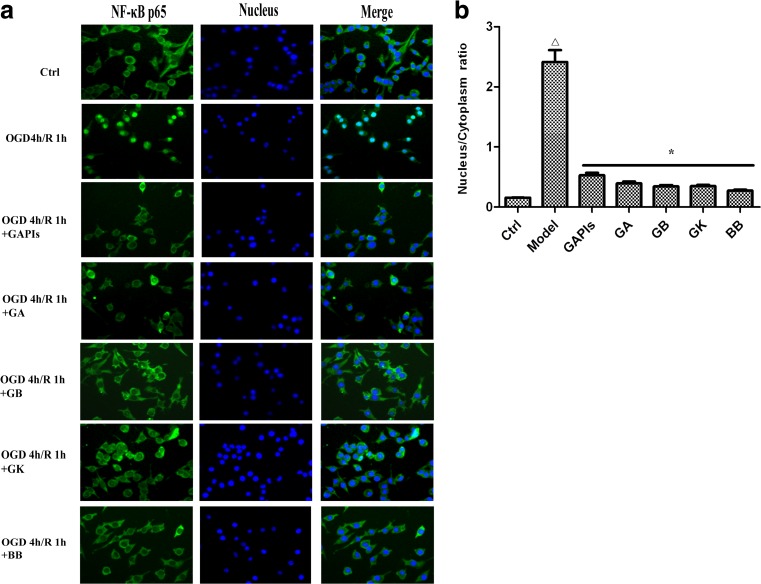
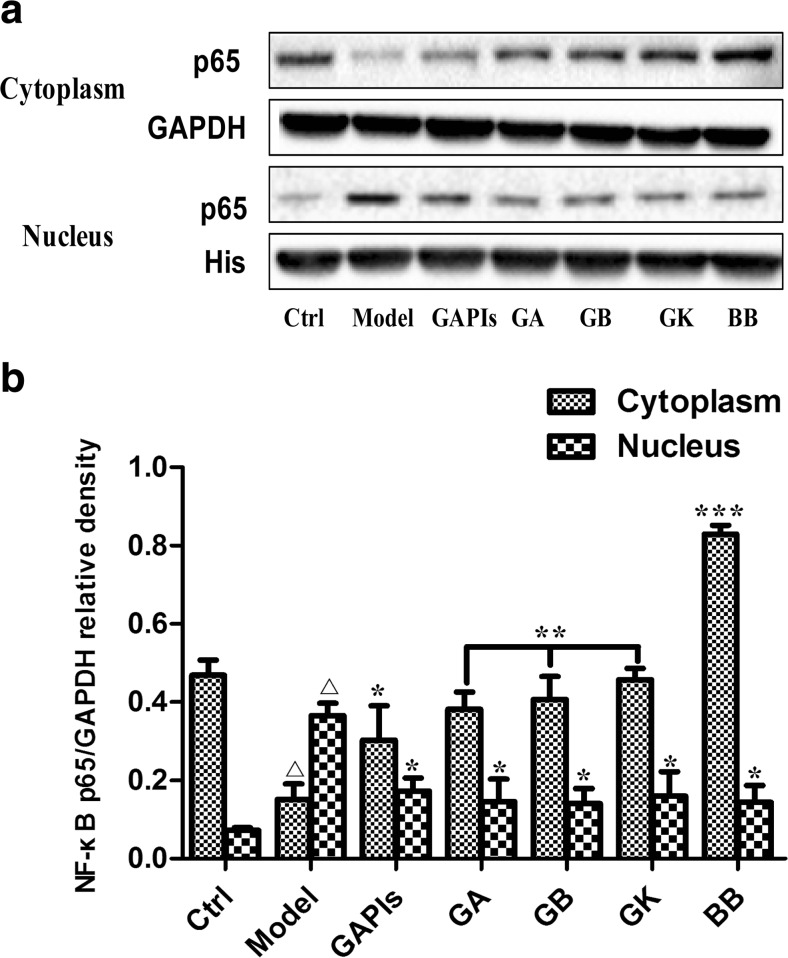
Ginkgolides and BB reduced the transfer of NF-κB p65 from cytoplasm to nucleus in BV2 microglia cells
TLR2/4 activates major signal transduction pathways through distinct adaptor proteins capable of contributing to cellular responses. Specifically, activation of NF-κB is a central event leading to inflammation, which is characterized by the translocation of p65 from cytoplasm to the nucleus. In this study, we examined the localization of NF-κB p65 in BV2 cells in different conditions by double-labeled immunofluorescent. As shown in Fig. 8, NF-κB p65 was expressed mainly in the cytoplasm (green) in control group, while translocated to the nucleus in model group (Fig. 8 OGD/R group). Merged images (blue green) indicated that OGD/R induced most of the NF-κB p65 protein transfer from cytoplasm to the nucleus. Treatment with 50 μg/ml GAPIs, GA, GB, GK, and BB could markedly reversed NF-κB p65 immunostaining to almost the basal levels and redistribute it in the nucleus and cytoplasm. GAPIs, GB, and BB exerted much stronger inhibition effect among five drugs.
Fig. 8.
Cellular location of NF-κB p65 in BV2 microglia cells exposed to OGD/R. After exposure to OGD for 4 h, BV2 cells were reoxygenated for either 1 h or 1 h followed by treatment with 50 μg/ml GAPIs, GA, GB, GK, and BB respectively. Cells were subjected to immunocytochemical analysis with an antibody directed against NF-κB p65 (green). To reveal nuclear morphology, the nuclei were stained with Hoechst 33258 (blue). The fluorescence was imaged by a fluorescence microscopy. Representative images (a) and quantitative data of nucleus/cytoplasm ratio (b) are shown, indicative of at least three independent experiments. △ P < 0.05, as compared with control group; *” P< 0.01 as compared with model group
The above results were proved by the experiment on the protein expression of NF-κB p65 nuclear and cytoplasmic fractionation of BV2 cells. As shown in Fig. 9, the expression of NF-κB p65 in nuclear and cytoplasmic fractionation in model group, respectively, increased by 5.09-fold and decreased by 3.09-fold relative to the corresponding control group. Consistent with immunofluorescent staining results, 50 μg/ml GAPIs, GA, GB, GK, and BB could significantly decrease NF-κB p65 levels in the nucleus (2.11, 1.19, 1.04, 0.88, and 1.10-fold, respectively), accompanied with increased its levels in the cytoplasma (2.00, 1.26, 1.07, 1.12, and 1.81-fold, respectively). These observations indicated NF-κB p65 translocation from cytoplasm to the nucleus were attenuated by moderate doses of GAPIs, GA, GB, GK, and BB.
Fig. 9.
Effects of ginkgolides and bilobalide on the expression of NF-κB p65 in BV2 microglia cells after OGD/R. After exposure to OGD for 4 h, BV2 cells were reoxygenated for either 6 h or 6 h followed by treatment with 50 μg/ml GAPIs, GA, GB, GK, and BB respectively. Representative graph (a) and quantitative data (b) on the expression of p65 in the cytosolic and nuclear. GAPDH and histone were used as the loading control. Western blot images are representative of three independent experiments. Each data point is a mean ± SD (n = 3). △ P < 0.05, as compared with corresponding control group; * P < 0.05, ** P < 0.01, *** P < 0.001, as compared with corresponding model group
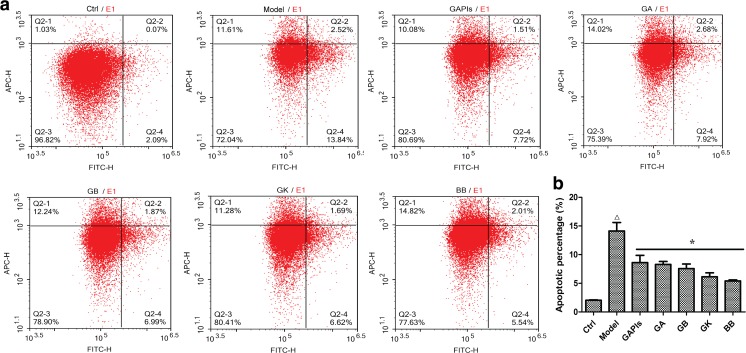
Ginkgolides and BB inhibited OGD/R induced apoptosis in BV2 microglia cells
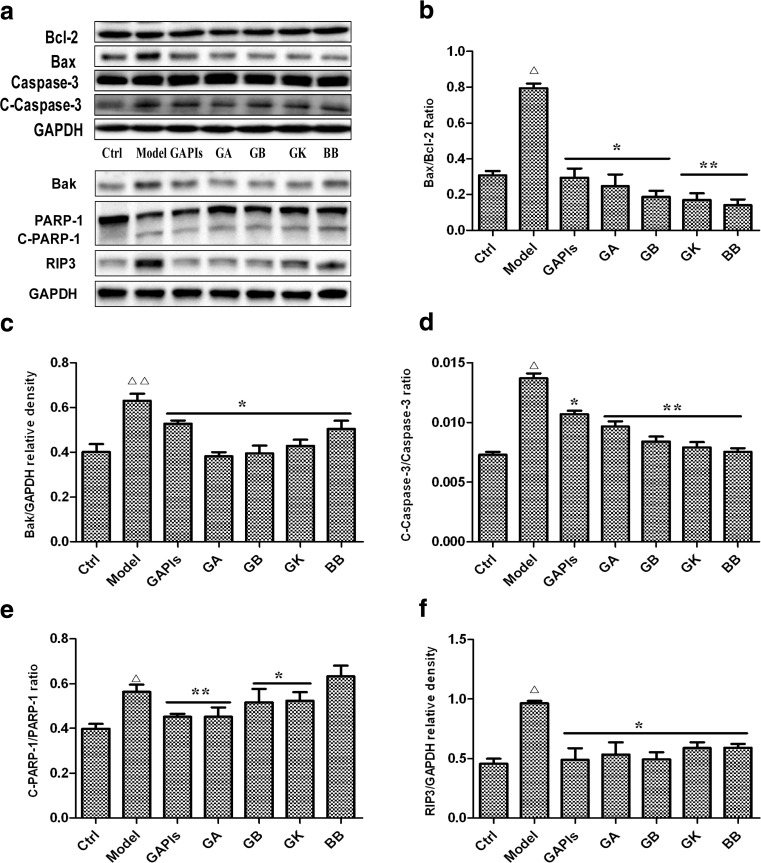
MTS assay showed that ginkgolides and BB at 25–100 μg/ml played an excellent cytoprotection after exposure to OGD 4 h/R 6 h. To quantify the extent of OGD/R-exposed apoptosis, the percentage of apoptotic cells was measured by flow cytometry at the indicated times after staining with Annexin V-FITC and PI. Flow cytometer results indicated that the percentage of apoptotic cells was increased after the cells exposed to OGD 4 h/R 6 h (14.13 ± 1.5 % in OGD /R group versus 2.04 ± 0.08 % in Control group), while the cell death percentage declined significantly in 50 μg/ml ginkgolides and BB treatment group (8.62 ± 1.27 % in GAPIs group, 8.29 ± 0.52 % in GA group, 7.56 ± 0.81 % in GB group, 6.14 ± 0.69 % in GK group, 5.41 ± 0.19 % in BB group versus 14.13 ± 1.5 % in OGD/R group respectively) (Fig. 10). To investigate whether ginkgolides and BB had protective effects against the mitochondrial apoptotic events induced by OGD/R in vitro, the protein levels of Bcl-2 family members, which included pro-apoptotic protein (Bax, Bak, cleaved PARP-1, cleaved Caspase-3) and anti-apoptotic protein (Bcl-2), were tested. Our results showed that Bak level, Bax/Bcl-2, cleaved caspase-3/caspase-3, and cleaved PARP-1/PARP-1 ratio were all significantly increased after the cells exposed to OGD4h/R6h (1.62, 2.57, 1.89, and 1.41-fold relative to control). GAPIs, GA, GB, GK, and BB at 50 μg/ml significantly decreased the Bak level, Bax/Bcl-2 ratio, cleaved caspase-3/caspase-3, and cleaved PARP-1/PARP-1 ratio (Fig. 11b–e), indicating that ginkgolides and BB inhibited apoptosis by regulating both pro- and anti-apoptotic proteins in OGD/R-exposed BV2 microglia cells. However, BB had no inhibitory effect on degradation of PARP-1 (Fig. 11e) that reveals their anti-apoptotic mechanisms are not exactly the same. Some other studies reported that endogenous receptor-interacting protein 3(RIP3) has been involved in ischemia/reperfusion injury (Yin et al. 2015; Vieira et al. 2014). RIP3 is a key molecular “switch” in tumor necrosis factor-induced apoptosis, necrosis, and necroptosis (Welz et al. 2011; Sosna et al. 2014), and we examined RIP3 expression in OGD/R-induced BV2 microglia cells and the role of ginkgolides and BB. The results showed that RIP3 level was significantly increased after OGD 4 h/R 6 h injury (2.1-fold relative to control), while elevated RIP3 expression was downregulated by 50 μg/ml ginkgolides and bilobalide in different degrees (Fig. 11f). The results suggested ginkgolides and BB might have inhibition effect on necrosis by preventing RIP3 expression.
Fig. 10.
Effect of ginkgolides and bilobalide on OGD/R-induced apoptosis in BV2 microglia. a After exposure to OGD for 4 h and reoxygenation for 6 h, BV2 cells were stained with Annexin V and PI and analyzed by ACEA NovoCyte D2040R. The apoptotic cells (the annexin V-positive and PI-negative cells) were indicated as the percentage of gated cells. b Relative percentage of the Annexin V-positive and PI-negative cells. Each data point is a mean ± SD (n = 3). △ P < 0.01, as compared with control group; * P < 0.05 as compared with model group
Fig. 11.
Effects of ginkgolides and bilobalide on the expression of Bcl-2,BAx,Bak, RIP3, Caspase 3, and PARP-1 levels in BV2 microglia cells after OGD/R. After exposure to OGD for 4 h, BV2 cells were reoxygenated for either 6 h or 6 h followed by treatment with 50 μg/ml GAPIs, GA, GB, GK, and BB respectively. Representative graph (a) and quantitative data on the ratio of Bax/Bcl-2 (b), C-Caspase-3/Caspase-3 (d), C-PARP-1/ PARP-1 (e), and expression of Bak (c) and RIP3 (f) in BV2 microglia cells. GAPDH was used as the loading control. Western blot images are representative of three independent experiments. Each data point is a mean ± SD (n = 3). △ P < 0.01, △△ P < 0.05 as compared with control group; * P < 0.05, ** P < 0.01, as compared with model group
Discussion
Stroke is the main reason of morbidity and mortality in humans which arises from occlusion or hemorrhage of blood vessels. Many drugs show the neuroprotective effects such as NMDA receptor antagonist, calcium antagonists, and pituitary adenylate cyclise-activating polypeptide (PACAP) (Neuhaus et al. 2012; Zhang et al. 2012; Qin et al. 2012). Nowadays, more and more attention have been focused on pharmacodynamic constituents from natural medicines in ischemic stroke therapy (Hou et al. 2012; Wang et al. 2012b; Luan et al. 2013). Ginkgolides and bilobalide, which are the main bioactive terpenoid fraction isolated from the G. biloba leaves, have been shown to possess various biological functions, including anti-inflammation, anti-apoptotic properties, anti-oxidation, and Ca2+ influx, thus exhibiting therapeutic potential for the treatment of ischemic stroke (Gu et al. 2012; Ma et al. 2012; Schwarzkopf et al. 2013). Due to the demonstration of its broad pharmacological effects both in vivo and in vitro, however, the effects of ginkgolides and bilobalide on microglia cells have not yet been fully reported.
It is well known that activated microglia become hypertrophic, undergo rapid proliferation, and migrate to inflammatory sites where they produce excess amounts of neurotoxic and pro-inflammatory mediators that mediate neuronal damage (Vilhardt 2005, Smith et al. 2012). Microglia activation can influence the survival of neural cells through release of pro-inflammatory and cytotoxic factors such as IL-1β, IL-6, TNF-α, MIP-1a, NO, and ROS (Dheen et al. 2007; Nishi et al. 2005). Thus, agents that inhibit the secretion of these inflammatory mediators involved in OGD/R-activated microglia cells are appealing for improving the survival of neurons. In this study, the effects of ginkgolides and bilobalide on OGD/R-induced inflammatory responses and TLRs signaling were comprehensively investigated in BV2 microglia cells. By validating the inhibitory effects of ginkgolides and bilobalide on various inflammatory factors and NF-κB signaling pathways mediated by TLRs, our results clearly clarified the pharmacological properties exhibited by ginkgolides and bilobalide in OGD/R-induced inflammatory.
In order to obtain reliable evaluation of the activity of ginkgolides and bilobalide, we firstly optimized the OGD and reoxygenation time in BV2 cells. Figure 2a, b showed that cell viability significantly decreased to 45 % and GAPIs exhibited maximal protective effect when BV2 microglia cells exposed to 4 h OGD followed by 3 h reoxygenation. No significant alteration in OD value at 12 and 24 h timepoint due to the recovery of cell viability; these were not consistent with previous studies (Qin et al. 2012; Qin et al. 2013). Therefore, OGD 4 h/R 3 h might be the optimal time to evaluate ginkgolides and bilobalide efficacy, but not reoxygenation for 12 h or longer. 100, 50, and 25 μg/ml GAPIs, GB, GK, and BB treatment under the determined optimal condition significantly improved BV2 microglia cell viability suppressed by OGD/R, thus indicating the protective effects of ginkgolides and bilobalide in this process. GAPIs, GB, and BB showed excellent protective effects among all the drugs (Fig. 2c).
Microglia-derived inflammatory factors trigger harmful downstream signaling pathways and promote harmful actions, including disruption of neuronal functions and direct neurotoxicity (Yun et al. 2011). Cell viability assays show that treatment with ginkgolides and bilobalide at 100–6.25 μg/ml significantly increased cell viability in BV2 cells subjected to OGD/R, and the dose of 100, 50, and 25 μg/ml was within the optimal effective range, so we choose the middle dosage (50 μg/ml) to investigate anti-inflammatory properties of ginkgolides and bilobalide by detecting proinflammatory cytokine and chemokine levels in culture media. It was observed that secretion of TNF-α, IL-1β, IL-6, IL-10, and IL-8 increased above basal levels to different degrees in response to OGD/R-induced damage of BV2 microglia cells. With the exception of the effect of the moderate dose of GA, GK, BB on IL-1β and/or IL-8 secretion, the rest of factors was significantly decreased by GAPIs, GA, GB, GK, and BB at 50 μg/ml (Fig. 3). Therefore, we hypothesized that the inhibition of pro-inflammatory secretion might be the synergy within each monomer composition of ginkgolides and BB, which were confirmed by our following Western blot and immunofluorescence assays.
Although the neuroprotective effects of GB, GK, and BB have been partly identified in different ways (Ma et al. 2012; Jiang et al. 2014; Gu et al. 2012), the mechanism of anti-inflammatory effects of ginkgolides and BB has not been fully elucidated, especially the major monomer of ginkgolides and BB are not been clearly understood. To explore the mechanism by which ginkgolides and BB protected microglia cells from OGD/R damage, the NF-κB mediated by TLRs was investigated in ginkgolides and BB-treated BV2 microglia cells. Various intracellular signaling molecules are involved in the modulation of NF-κB pathways and production of proinflammatory cytokines in microglia. Relevance of NF-κB pathway in general inflammatory, as well as immune response, has been indicated (Hayden and Ghosh 2008; Vallabhapurapu and Karin 2009), although little is known about the relevance of this pathway in OGD/R, which represents the actual pathological state in ischemic cerebral disease. TLR-mediated signaling plays a critical role in the induction of host innate immunity and inflammatory responses (Zhang et al. 2009a). Furthermore, TLR2/4-deficient mice exhibit a general advantage after permanent MACO and insensitive to ischemia-induced upregulation of multiple specific proinflammatory cytokines in microglia compared with wild-type (WT) mice (Pradillo et al. 2009; Weinstein et al. 2010; Ziegler et al. 2007, Lv et al. 2011a). Our Western blot results showed that OGD 4 h/R 6 h induced BV2 cells activation with upregulation of TLR2/4 protein expression. GAPIs, GA, GB, GK, and BB at the concentration of 50 μg/ml decreased TLR2/4 protein levels (Fig. 4c), with the dose-dependent manner observed in response to 25, 50, and 100 μg/ml GB (Fig. 4d). These observations indicate that activation of TLR2/4 in microglia could mediate inflammatory response, blockade of which by ginkgolides and BB could reduce microglia ischemic/hypoxic injury in the current study.
MyD88 is an essential downstream protein that integrates and transduces intracellular signals generated by the Toll-like receptor (Akira and Takeda 2004; O’Neill 2003). Once TLR is activated, MyD88 is recruited to TLR domains that link the TLRs with downstream intracellular signaling cascades (Jordan et al. 2003, O’Neill 2002). Macrophages from MyD88−/− mice are defective in many TLR4-mediated responses, such as LPS-induced secretion of IL-6, TNF-α, and IL-1β, indicating the importance of MyD88 in TLR4-mediated signaling (Kawai et al. 1999). Bolanle et al. reported that the MyD88 pathway directs the expression of neutrophil chemoattractants following cerebral ischemia (Famakin et al. 2012). In the present study, GAPIs, GB, GK, and BB treatment resulted in marked inhibition of OGD/R-induced MyD88 expression in BV2 microglia cells. Surprisingly, GAPIs, GB, and GK could inhibit TLR4 and MyD88 expression to the level lower than control (Fig. 4c).
Accumulating evidence showed that TLR2 and TLR4 are the important Toll-like receptors during cerebral ischemia/reperfusion (Winters et al. 2013; Zwagerman et al. 2010; Lehnardt et al. 2007; Tang et al. 2007; Hyakkoku et al. 2010). In downstream activation of NF-κB signaling pathways, these signaling cascades are involved in induction of proinflammatory cytokines and chemokines Muzio et al. 2013, McDermott and O’Neill 2002). NF-kB is activated in neurodegenerative disorders and in ischemic stroke (Ridder and Schwaninger 2009; Ghosh et al. 2007). Between TLR receptor and NF-κB transcription factor, TAK1 is a central target for short-term inhibition of key signaling pathways and neuroprotection in cerebral ischemia and highly expressed in the brain (Yamaguchi et al. 1995; Neubert et al. 2011). Acute inhibition of TAK1 protects against neuronal death in cerebral ischemia (Neubert et al. 2011). In addition, TAK1 is responsible for activating IKK and the canonical NF-κB signaling pathway (Shim et al. 2005; Sato et al. 2005). NF-κB is normally sequestered in an inactive form in the cytoplasm bound to IκB proteins, an interaction that regulates its activity. Multiple stimuli can activate NF-κB signaling by degradation of IκB and release of the NF-κB p65-p50 dimer, which translocates to the nucleus, binds to κB binding sites on DNA, and regulates transcriptional activation of the target genes (Wong and Tergaonkar 2009; Hayden and Ghosh 2008). Therefore, we investigated the changes of IKKβ and IκBα in the injured BV2 microglia cells and found that OGD/R enhanced IKKβ and IκBα phosphorylation in BV2 cells (Fig. 5–7), indicating release of NF-κB p65 from IκB. Meanwhile, as functional inhibitor of TLR-mediated NF-κB activation, TAK1 phosphorylation was significantly downregulated by ginkgolides and BB, as well as IKKβ and IκBα phosphorylation were also inhibited by ginkgolides and BB (Fig. 6). In support of this notion, NF-κB p65 was observed to be reduced in cytoplasmic fraction and increased in nuclear fraction by immunofluorescent and Western blot analysis (Figs. 8 and 9). Treatment with GAPIs, GA, GB, GK, and BB at 50 μg/mL attenuated NF-κB p65 expression in nuclear fraction and upregulated it in cytoplasmic fraction, suggesting that ginkgolides and BB exert its role via interference in NF-κB signaling pathway. GA, however, can inhibit IκBα phosphorylation and NF-κB p65 nuclear translocation induced by OGD/R, but has no effects on MyD88, TAK1, and IKKβ phosphorylation under the experimental dose, which represents an unusual regulatory mechanism compared with the other monomer constituents of ginkgolides (Figs. 4c and 6b, c). The NF-κB family functions as transcription factors that regulate a wide range of genes involved in inflammation, autoimmune responses, cell proliferation, and apoptosis (Karin 2006; Wang et al. 2012a; Wang and Cho 2010). Our results showed that the BV2 microglia apoptosis induced by OGD/R may depend on functional activation of TLRs and blocked by ginkgolides and BB treatment in vitro. An amount of 50 μg/mL GAPIs, GA, GB, GK, and BB significantly attenuated cell apoptosis (Fig. 10) and reduced the Bax/Bcl-2 ratio and the levels of Bak (Fig. 11b, c), along with a decrease in OGD/R-induced cleaved caspase-3 and cleaved PARP-1 activation (Fig. 11d, e), all of this contribute to the cells to improve their mitochondria function, reducing cytochrome C release and increasing their anti-apoptotic capacity to prevent OGD/R injury (Boonyarat et al. 2014). RIP3 is a key “switch” molecule in programmed necrosis, overexpression, and nuclear translocation of RIP3 that plays a critical role in the hippocampal neuronal programmed necrosis induced I/R injury (Yin et al. 2015; Xu et al. 2016; Vieira et al. 2014). These were consistent with our research that RIP3 level significantly increased after OGD/R injury, indicating OGDR-induced RIP3 activation and trigger necroptosis. Our results also demonstrated that ginkgolides and BB were effective inhibitors of RIP3 to rescue cell death and provide neuroprotective effects in certain situation (ischemic brain injury) (Fig. 11f).
In addition, it would be specially mentioned that the anti-inflammatory effects and TLR modulation among each monomer of ginkgolides were not completely consistent according to our study, and these were confirmed by proinflammatory cytokine secretion and the expression of TLR signaling pathway-related proteins. Some monomers on the regulation of inflammatory factors and protein function were very strong (such as GB and BB), while the others were relatively weak or even have no effects on some indicators (such as GA and GK). In general, the differences in cell viability, inflammatory cytokines secretion, or protein regulation between these compounds were determined by their own structure. R1–hydroxyl (the only difference between GA and GB) of GB may benefit to form hydrogen bond with water molecules or specific target of cells (such as protein, enzyme, etc.), thus increasing its water solubility and efficacy. While GK has unsaturated double bond in five-membered ring, it is easy to conjugate with adjacent carbonyl and make the electron cloud of the whole structure stronger. On one hand, it may benefit to form hydrogen bond with specific targets of cells (such as protein, enzyme, etc.), but on the other hand, it also increases its structure rigidity and thus not conducive to insert into special structure. BB have multiple hydroxyl and no unsaturated double bond, and it should have similar biological activity with GB. However, these differences do not contradict to anti-inflammatory effects and TLRs signaling regulation; on the contrary, comprehensive effects of ginkgolides result from the synergistic reaction among each monomer constituent. In summary, the results of the current study suggest that ginkgolides exhibit significant anti-inflammatory effects during OGD/R injury by inhibiting upregulation of TLR2/4 and its downstream MyD88 expression and NF-κB activation. Meanwhile, inhibition of apoptosis and proinflammatory cytokine secretion were contributed to neroprotection of ginkgolides to BV2 microglia cells. Besides, we investigated the anti-inflammatory effects of each monomer constituents of ginkgolides (GA, GB, GK) and BB for the first time and illustrated that the possible mechanisms of anti-inflammatory and neuroprotection effects may come from the cooperative action of its multicomponent. These findings point to a therapeutic potential for ginkgolides as a useful anti-inflammatory compound in cerebral ischemia reperfusion injury.
GAPIs active pharmaceutical ingredients of ginkgolides; GA, ginkgolide A; GB, ginkgolide B; GK, ginkgolide K; BB, bilobalide; DMEM, Dulbecco’s modified Eagle’s medium; FBS, fetal bovine serum; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; BSA, bull serum albumin; IL-1β, IL-6, IL-10, interleukin-1β, 6, 10; TNF-α, tumor necrosis factor α; TLRs, toll-like receptors; MyD88, myeloid differentiation protein 88, TAK1, transforming growth factor-beta-activated kinase 1; IkBα, IkappaB-α, IKKβ, IkappaB kinase-α/β; Bcl-2, B cell lymphoma-2; Bax, Bcl-2 associated X protein; Bak, Bcl-2 homologous antagonist/killer; PARP-1, poly (ADP-ribose) polymerase-1; Caspase-3, Cysteinyl aspartate specific proteinase-3; RIP3, receptor-interacting protein 3; I/R, ischemia and reperfusion; NF-κB, nuclear transcription factor κB; OGD/R, oxygen–glucose deprivation/reoxygenation; PBS, phosphate buffered saline.
Acknowledgments
This work was supported by New Drug Discovery of Ministry of Science and Technology of China: Modern Chinese medicine innovation cluster and Digital pharmaceutical technology platform (2013zx0940203).
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci: CMLS. 2003;60:1779–1792. doi: 10.1007/s00018-003-3080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S. Pathogen recognition by innate immunity and its signaling. Proc Jpn Acad Ser B, Phys Biol Sci. 2009;85:143–156. doi: 10.2183/pjab.85.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Andrieu S, Ousset PJ, Coley N, Ouzid M, Mathiex-Fortunet H, Vellas B, G. Guid Age study GuidAge study: a 5-year double blind, randomised trial of EGb 761 for the prevention of Alzheimer’s disease in elderly subjects with memory complaints. I. Rationale, design and baseline data. Curr Alzheimer Res. 2008;5:406–415. doi: 10.2174/156720508785132271. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/S0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Boonyarat C, Yenjai C, Vajragupta O, Waiwut P. Heptaphylline induces apoptosis in human colon adenocarcinoma cells through bid and Akt/NF-kappaB (p65) pathways. Asian Pac J Cancer Prev : APJCP. 2014;15:10483–10487. doi: 10.7314/APJCP.2014.15.23.10483. [DOI] [PubMed] [Google Scholar]
- Braquet P. Proofs of involvement of PAF-acether in various immune disorders using BN 52021 (ginkgolide B): a powerful PAF-acether antagonist isolated from Ginkgo biloba L. Adv Prostaglandin Thromboxane Leukot Res. 1986;16:179–198. [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke J Cereb Circ. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K, Mehrabian Z, Spinnewyn B, Chinopoulos C, Drieu K, Fiskum G. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry. 2003;36(Suppl 1):S89–S94. doi: 10.1055/s-2003-40447. [DOI] [PubMed] [Google Scholar]
- De Plaen IG, Tan XD, Chang H, Wang L, Remick DG, Hsueh W. Lipopolysaccharide activates nuclear factor kappaB in rat intestine: role of endogenous platelet-activating factor and tumour necrosis factor. Br J Pharmacol. 2000;129:307–314. doi: 10.1038/sj.bjp.0703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeudis FV. Bilobalide and neuroprotection. Pharmacol Res. 2002;46:565–568. doi: 10.1016/S1043-6618(02)00233-5. [DOI] [PubMed] [Google Scholar]
- Desquand S, Touvay C, Randon J, Lagente V, Vilain B, Maridonneau-Parini I, Etienne A, Lefort J, Braquet P, Vargaftig BB. Interference of BN 52021 (ginkgolide B) with the bronchopulmonary effects of PAF-acether in the Guinea-pig. Eur J Pharmacol. 1986;127:83–95. doi: 10.1016/0014-2999(86)90208-6. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famakin B, Mou Y, Spatz M, Lawal M, Hallenbeck J. Downstream toll-like receptor signaling mediates adaptor-specific cytokine expression following focal cerebral ischemia. J Neuroinflammation. 2012;9:174. doi: 10.1186/1742-2094-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, C. American Heart Association Statistics and S. Stroke Statistics Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JH, Ge JB, Li M, Wu F, Zhang W, Qin ZH. Inhibition of NF-kappaB activation is associated with anti-inflammatory and anti-apoptotic effects of ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm Sci: off J Eur Fed Pharm Sci. 2012;47:652–660. doi: 10.1016/j.ejps.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hou J, Wang J, Zhang P, Li D, Zhang C, Zhao H, Fu J, Wang B, Liu J. Baicalin attenuates proinflammatory cytokine production in oxygen-glucose deprived challenged rat microglial cells by inhibiting TLR4 signaling pathway. Int Immunopharmacol. 2012;14:749–757. doi: 10.1016/j.intimp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Huang CY, Fujimura M, Noshita N, Chang YY, Chan PH. SOD1 down-regulates NF-kappaB and c-Myc expression in mice after transient focal cerebral ischemia. J Cereb Blood Flow Metab : Off J Int Soc Cereb Blood Flow Metab. 2001;21:163–173. doi: 10.1097/00004647-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, Akira S, Inagaki N, Nagai H, Hara H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001;14:89–94. doi: 10.1097/00019052-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaracz S, Malik S, Nakanishi K. Isolation of ginkgolides a, B, C, J and bilobalide from G. biloba extracts. Phytochemistry. 2004;65:2897–2902. doi: 10.1016/j.phytochem.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H, C. American Heart Association Stroke, N. Council on Cardiovascular, D. Council on Peripheral Vascular and C. Council on Clinical Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke J Cereb Cir. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo C, Peng J, Li J, Yung KK, Mo Z. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J Neuroinflammation. 2014;11:167. doi: 10.1186/s12974-014-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4:110–116. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- Kacimi R, Giffard RG, Yenari MA. Endotoxin-activated microglia injure brain derived endothelial cells via NF-kappaB, JAK-STAT and JNK stress kinase pathways. J Inflamm. 2011;8:7. doi: 10.1186/1476-9255-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci: Off J Soc Neurosci. 2008;28:2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/S1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kleijnen J, Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136–1139. doi: 10.1016/0140-6736(92)93158-J. [DOI] [PubMed] [Google Scholar]
- Ko HM, Jung HH, Seo KH, Kang YR, Kim HA, Park SJ, Lee HK, Im SY. Platelet-activating factor-induced NF-kappaB activation enhances VEGF expression through a decrease in p53 activity. FEBS Lett. 2006;580:3006–3012. doi: 10.1016/j.febslet.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, Krueger C, Nitsch R, Meisel A, Weber JR. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Liu YG, Li FJ, Wang J, Wang XD. [Effects of ginkgolide B on inflammation induced by cerebral ischemia-reperfusion in rats]. Zhong yao cai Zhongyaocai. J Chin Med Mater. 2010;33:578–580. [PubMed] [Google Scholar]
- Luan H, Kan Z, Xu Y, Lv C, Jiang W. Rosmarinic acid protects against experimental diabetes with cerebral ischemia: relation to inflammation response. J Neuroinflammation. 2013;10:28. doi: 10.1186/1742-2094-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv M, Liu Y, Zhang J, Sun L, Liu Z, Zhang S, Wang B, Su D, Su Z. Roles of inflammation response in microglia cell through toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience. 2011;176:162–172. doi: 10.1016/j.neuroscience.2010.11.066. [DOI] [PubMed] [Google Scholar]
- Lv P, Fang W, Geng X, Yang Q, Li Y, Sha L. Therapeutic neuroprotective effects of ginkgolide B on cortex and basal ganglia in a rat model of transient focal ischemia. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2011;44:235–240. doi: 10.1016/j.ejps.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Ma S, Liu H, Jiao H, Wang L, Chen L, Liang J, Zhao M, Zhang X. Neuroprotective effect of ginkgolide K on glutamate-induced cytotoxicity in PC 12 cells via inhibition of ROS generation and Ca (2+) influx. Neurotoxicology. 2012;33:59–69. doi: 10.1016/j.neuro.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Maclennan KM, Darlington CL, Smith PF. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog Neurobiol. 2002;67:235–257. doi: 10.1016/S0301-0082(02)00015-1. [DOI] [PubMed] [Google Scholar]
- McDermott EP, O’Neill LA. Ras participates in the activation of p38 MAPK by interleukin-1 by associating with IRAK, IRAK2, TRAF6, and TAK-1. J Biol Chem. 2002;277:7808–7815. doi: 10.1074/jbc.M108133200. [DOI] [PubMed] [Google Scholar]
- Moon JB, Lee CH, Park CW, Cho JH, Hwang IK, Yoo KY, Choi JH, Shin HC, Won MH. Neuronal degeneration and microglial activation in the ischemic dentate gyrus of the gerbil. J Vet Med Sci Jpn Soc Vet Sci. 2009;71:1381–1386. doi: 10.1292/jvms.001381. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- Muzio M, Ni J, Feng P, Dixit VM. Pillars article: IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997. 2013;278:1612–1615. [PubMed] [Google Scholar]
- Nagy K, Domoki F, Bari F. Ischemic preconditioning in the brain. Ideggyogyaszati szemle. 2005;58:305–313. [PubMed] [Google Scholar]
- Neubert M, Ridder DA, Bargiotas P, Akira S, Schwaninger M. Acute inhibition of TAK1 protects against neuronal death in cerebral ischemia. Cell Death Differ. 2011;18:1521–1530. doi: 10.1038/cdd.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus W, Burek M, Djuzenova CS, Thal SC, Koepsell H, Roewer N, Forster CY. Addition of NMDA-receptor antagonist MK801 during oxygen/glucose deprivation moderately attenuates the upregulation of glucose uptake after subsequent reoxygenation in brain endothelial cells. Neurosci Lett. 2012;506:44–49. doi: 10.1016/j.neulet.2011.10.045. [DOI] [PubMed] [Google Scholar]
- Ni Q, Wang J, Li EQ, Zhao AB, Yu B, Wang M, Huang CR. Study on the protective effect of the mixture of Shengmai powder and Danshen decoction on the myocardium of diabetic cardiomyopathy in the rat model. Chin J Integr Med. 2011;17:116–125. doi: 10.1007/s11655-011-0639-9. [DOI] [PubMed] [Google Scholar]
- Nishi T, Maier CM, Hayashi T, Saito A, Chan PH. Superoxide dismutase 1 overexpression reduces MCP-1 and MIP-1 alpha expression after transient focal cerebral ischemia. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2005;25:1312–1324. doi: 10.1038/sj.jcbfm.9600124. [DOI] [PubMed] [Google Scholar]
- O’Neill LA. Toll-like receptor signal transduction and the tailoring of innate immunity: a role for Mal? Trends Immunol. 2002;23:296–300. doi: 10.1016/S1471-4906(02)02222-6. [DOI] [PubMed] [Google Scholar]
- O’Neill LA. The role of MyD88-like adapters in toll-like receptor signal transduction. Biochem Soc Trans. 2003;31:643–647. doi: 10.1042/bst0310643. [DOI] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple toll-like receptors. Am J Phys Cell Phys. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Peng SY, Liao WH, Nie ZG, Liu Y, Wang L, Wang F, Wang WJ. [effect of ginkgolide B on the production of NO, IL-6 and RANTES from astrocytes]. Yao xue xue bao. Acta Pharm Sin. 2010;45:1103–1108. [PubMed] [Google Scholar]
- Pradillo JM, Fernandez-Lopez D, Garcia-Yebenes I, Sobrado M, Hurtado O, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem. 2009;109:287–294. doi: 10.1111/j.1471-4159.2009.05972.x. [DOI] [PubMed] [Google Scholar]
- Qin X, Sun ZQ, Dai XJ, Mao SS, Zhang JL, Jia MX, Zhang YM. Toll-like receptor 4 signaling is involved in PACAP-induced neuroprotection in BV2 microglial cells under OGD/reoxygenation. Neurol Res. 2012;34:379–389. doi: 10.1179/1743132812Y.0000000028. [DOI] [PubMed] [Google Scholar]
- Qin X, Sun ZQ, Zhang XW, Dai XJ, Mao SS, Zhang YM. TLR4 signaling is involved in the protective effect of propofol in BV2 microglia against OGD/reoxygenation. J Physiol Biochem. 2013;69:707–718. doi: 10.1007/s13105-013-0247-6. [DOI] [PubMed] [Google Scholar]
- Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience. 2009;158:995–1006. doi: 10.1016/j.neuroscience.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Schaller B, Graf R. Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2004;24:351–371. doi: 10.1097/00004647-200404000-00001. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf TM, Koch KA, Klein J. Neurodegeneration after transient brain ischemia in aged mice: beneficial effects of bilobalide. Brain Res. 2013;1529:178–187. doi: 10.1016/j.brainres.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son HY, Han HS, Jung HW, Park YK. Panax notoginseng attenuates the infarct volume in rat ischemic brain and the inflammatory response of microglia. J Pharmacol Sci. 2009;109:368–379. doi: 10.1254/jphs.08197FP. [DOI] [PubMed] [Google Scholar]
- Sosna J, Voigt S, Mathieu S, Lange A, Thon L, Davarnia P, Herdegen T, Linkermann A, Rittger A, Chan FK, Kabelitz D, Schutze S, Adam D. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol life Sci: CMLS. 2014;71:331–348. doi: 10.1007/s00018-013-1381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- van Dongen MC, van Rossum E, Kessels AG, Sielhorst HJ, Knipschild PG. The efficacy of ginkgo for elderly people with dementia and age-associated memory impairment: new results of a randomized clinical trial. J Am Geriatr Soc. 2000;48:1183–1194. doi: 10.1111/j.1532-5415.2000.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Vieira M, Fernandes J, Carreto L, Anuncibay-Soto B, Santos M, Han J, Fernandez-Lopez A, Duarte CB, Carvalho AL, Santos AE. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous RIP3. Neurobiol Dis. 2014;68:26–36. doi: 10.1016/j.nbd.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Vilhardt F. Microglia: phagocyte and glia cell. Int J Biochem Cell Biol. 2005;37:17–21. doi: 10.1016/j.biocel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Wang H, Cho CH. Effect of NF-kappaB signaling on apoptosis in chronic inflammation-associated carcinogenesis. Curr Cancer Drug Targets. 2010;10:593–599. doi: 10.2174/156800910791859425. [DOI] [PubMed] [Google Scholar]
- Wang XX, Shang YP, Chen JZ, Zhu JH, Guo XG, Sun J. [Effects of Ginkgo biloba extract on number and activity of endothelial progenitor cells from peripheral blood] Yao xue xue bao = Acta Pharm Sin. 2004;39:656–660. [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Qin ZH, Shi H, Savitz SI, Qin AP, Jiang Y, Zhang HL. Protective effect of Ginkgolids (A + B) is associated with inhibition of NIK/IKK/IkappaB/NF-kappaB signaling pathway in a rat model of permanent focal cerebral ischemia. Brain Res. 2008;1234:8–15. doi: 10.1016/j.brainres.2008.07.102. [DOI] [PubMed] [Google Scholar]
- Wang F, Li H, Shi H, Sun B. Pro-apoptotic role of nuclear factor-kappaB in adriamycin-induced acute myocardial injury in rats. Mol Med Rep. 2012;5:400–404. doi: 10.3892/mmr.2011.636. [DOI] [PubMed] [Google Scholar]
- Wang J, Hou J, Zhang P, Li D, Zhang C, Liu J. Geniposide reduces inflammatory responses of oxygen-glucose deprived rat microglial cells via inhibition of the TLR4 signaling pathway. Neurochem Res. 2012;37:2235–2248. doi: 10.1007/s11064-012-0852-8. [DOI] [PubMed] [Google Scholar]
- Weinstein JR, Koerner IP, Moller T. Microglia in ischemic brain injury. Future Neurol. 2010;5:227–246. doi: 10.2217/fnl.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- Winters L, Winters T, Gorup D, Mitrecic D, Curlin M, Kriz J, Gajovic S. Expression analysis of genes involved in TLR2-related signaling pathway: inflammation and apoptosis after ischemic brain injury. Neuroscience. 2013;238:87–96. doi: 10.1016/j.neuroscience.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Wong ET, Tergaonkar V. Roles of NF-kappaB in health and disease: mechanisms and therapeutic potential. Clin Sci. 2009;116:451–465. doi: 10.1042/CS20080502. [DOI] [PubMed] [Google Scholar]
- Wu X, Qian Z, Ke Y, Du F, Zhu L. Ginkgolide B preconditioning protects neurons against ischaemia-induced apoptosis. J Cell Mol Med. 2009;13:4474–4483. doi: 10.1111/j.1582-4934.2008.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia SH, Fang DC. Pharmacological action and mechanisms of ginkgolide B. Chin Med J. 2007;120:922–928. [PubMed] [Google Scholar]
- Xu Y, Wang J, Song X, Wei R, He F, Peng G, Luo B. Protective mechanisms of CA074-me (other than cathepsin-B inhibition) against programmed necrosis induced by global cerebral ischemia/reperfusion injury in rats. Brain Res Bull. 2016;120:97–105. doi: 10.1016/j.brainresbull.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, K. S, Shibuya H, Irie K, Oishi I, Ueno N, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Yin B, Xu Y, Wei RL, He F, Luo BY, Wang JY. Inhibition of receptor-interacting protein 3 upregulation and nuclear translocation involved in Necrostatin-1 protection against hippocampal neuronal programmed necrosis induced by ischemia/reperfusion injury. Brain Res. 2015;1609:63–71. doi: 10.1016/j.brainres.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Yun HJ, Yoon JH, Lee JK, Noh KT, Yoon KW, Oh SP, Oh HJ, Chae JS, Hwang SG, Kim EH, Maul GG, Lim DS, Choi EJ. Daxx mediates activation-induced cell death in microglia by triggering MST1 signalling. EMBO J. 2011;30:2465–2476. doi: 10.1038/emboj.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Potrovita I, Tarabin V, Herrmann O, Beer V, Weih F, Schneider A, Schwaninger M. Neuronal activation of NF-kappaB contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2005;25:30–40. doi: 10.1038/sj.jcbfm.9600004. [DOI] [PubMed] [Google Scholar]
- Zhang P, Cox CJ, Alvarez KM, Cunningham MW. Cutting edge: cardiac myosin activates innate immune responses through TLRs. J Immunol. 2009;183:27–31. doi: 10.4049/jimmunol.0800861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chen C, Lu J, Xie M, Pan D, Luo X, Yu Z, Dong Q, Wang W. Cell cycle inhibition attenuates microglial proliferation and production of IL-1beta, MIP-1alpha, and NO after focal cerebral ischemia in the rat. Glia. 2009;57:908–920. doi: 10.1002/glia.20816. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang J, Zhang C, Jiang X, Zhou H, Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database Syst Rev. 2012;5:CD001928. doi: 10.1002/14651858.CD001928.pub2. [DOI] [PubMed] [Google Scholar]
- Zhu GY, Zhu XL, Geng QX, Zhang X, Shao J. [Change of peripheral blood monocytes derived macrophage scavenger receptors activity in patients with coronary heart disease, and the intervention effect of Ginkgo biloba extract]. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chinese journal of integrated traditional and Western medicine / Zhongguo Zhong xi yi jie he xue hui. Zhongguo Zhong yi yan jiu yuan zhu ban. 2004;24:1069–1072. [PubMed] [Google Scholar]
- Ziegler G, Harhausen D, Schepers C, Hoffmann O, Rohr C, Prinz V, Konig J, Lehrach H, Nietfeld W, Trendelenburg G. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun. 2007;359:574–579. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]
- Zwagerman N, Plumlee C, Guthikonda M, Ding Y. Toll-like receptor-4 and cytokine cascade in stroke after exercise. Neurol Res. 2010;32:123–126. doi: 10.1179/016164109X12464612122812. [DOI] [PubMed] [Google Scholar]