Abstract
Naringenin is one of the most popular flavonoids derived from citrus. It has been reported to be an effective anti-inflammatory compound. Citrus fruit may be used raw, cooked, stewed, or boiled. The present study was conducted to investigate the effect of thermal processes on naringenin in its immunomodulatory and cellular antioxidant activities. The effects of flavonoids on B and T cell proliferation were assessed on splenocytes stimulated or not with mitogens. However, their effects on cytotoxic T lymphocyte (CTL) and natural killer (NK) activities were assessed in splenocytes co-incubated with target cells. The amount of nitric oxide production and the lysosomal enzyme activity were evaluated in vitro on mouse peritoneal macrophages. Cellular antioxidant activity in splenocytes and macrophages was determined by measuring the fluorescence of the dichlorofluorescin (DCF). Our findings revealed that naringenin induces B cell proliferation and enhances NK activity. The highest concentration of native naringenin exhibits a significant proliferation of T cells, induces CTL activity, and inhibits cellular oxidation in macrophages. Conversely, it was observed that when heat-processed, naringenin improves the cellular antioxidant activity in splenocytes, increases the cytotoxic activity of NK cells, and suppresses the cytotoxicity of T cells. However, heat treatment maintains the anti-inflammatory potency of naringenin.
Keywords: Naringenin, Heated naringenin, Immunomodulation, Cellular antioxidant, Anti-inflammatory potency
Introduction
Many factors affecting immune activity, such as genetic mutations, environmental exposure, and immunomodulatory treatments, may bolster a carcinogenic environment, leading to an increase in the incidence of many autoimmune diseases and cancers (Alexandrescu et al. 2011; Franks and Slansky 2012; Grivennikov et al. 2010; Sansone and Bromberg 2011). Thus, a large number of plant extracts, used in traditional medicines, and flavonoids are being extensively explored for their potential immunomodulating activities (Krifa et al. 2013; Lopez-Posadas et al. 2008). Moreover, many polyphenols, synthesized by plants during periods of stress such as drought or sun exposure, confer stress tolerance for the animals that consume them. This phenomenon of xenohormesis is a more speculative theory, suggesting that animals and fungi are able to sense chemical signals produced by plants and other autotrophs in response to stress. Thus, plants’ secondary metabolites, such as resveratrol, quercetin, and probably naringenin produced under stressful conditions, serve as a molecular warning to the animals who eat them and who, in turn, are protected against unfavorable conditions. Moreover, the theory predicts that “xenohormetic” molecules bind to conserved domains in proteins, both as an agonist on one enzyme and as an antagonist against another. This might explain the presumed variety of underlying action mechanisms, which all serve to protect the animal consumer (Baur and Sinclair 2008; Hooper et al. 2010; Howitz and Sinclair 2008). Moreover, plants and foods rich in flavonoids are generally eaten not in the raw state but after having undergone a stress such as heating, boiling, or deep frying. Therefore, in this present work, we examined the effect of heating on the immunomodulatory properties of naringenin. Naringenin is a flavanone present in citrus fruits (Orallo et al. 2005), grape fruits (Ribeiro and Ribeiro 2008), orange juices (Silva et al. 2014), and tomato (Vallverdu-Queralt et al. 2012). Various pharmacological activities of NG, including antibacterial (Ng’uni et al. 2015), antiviral (Lulu et al. 2015; Meng et al. 2015), antidiabetic (Bhattacharya et al. 2014; Priscilla et al. 2014), cardioprotective (Kara et al. 2014), neuroprotective (Raza et al. 2013), and nephroprotective effects (Hermenean et al. 2013), were described. Previous studies have also reported that naringenin has antioxidative (Cavia-Saiz et al. 2010; Kneževiæ and Petlevski 2014), anti-inflammatory (Li et al. 2015; Wu et al. 2015), and anticancer (Lin et al. 2014) activities. The aim of this study was to investigate and to compare the immunomodulatory activity and cellular antioxidant potential of native naringenin (NG) and heated naringenin (hNG).
Materials and methods
Reagents
Native and thermally treated naringenins were provided by the Laboratory of Biomolecular Engineering, ENSAIA-INPL, University of Lorraine, Vandoeuvre-lès-Nancy.
Thermal treatment
The treatment of naringenin was conducted at 130 °C for 2 h by autoclaving at the Biomolecules Engineering Laboratory of the University of Lorraine, France. Flavonoids were diluted in dimethyl sulfoxide (DMSO, 98 %) stock solutions before being added to the cell culture medium. The final concentration of DMSO never exceeded 0.1 % (v/v).
Animals
Specific pathogen-free male BALB/c mice (20–22 g) were obtained from the Pasteur Institute (Tunis, Tunisia). The mice were housed under standard conditions of temperature, humidity, and light (12 h light/dark) in an accredited pathogen-free facility. They were fed a commercial pellet diet and water ad libitum throughout the experimental periods. All experiments were performed in accordance with the guidelines for the care and use of laboratory animals as published by the US National Institutes of Health. All experiments received the explicit approval of the Ethics Animal Committee in Tunisia.
Preparation of primary splenocytes and macrophages
Spleen mice lymphocytes were obtained as previously reported (Limem et al. 2011; Mustapha et al. 2015). After washing with phosphate-buffered saline (PBS, pH 7.4), cells were resuspended in a complete Roswell Park Memorial Institute medium (RPMI) 1640 medium (Gibco BRL) containing 10 % fetal bovine serum (FBS; Gibco) and 100 mg/ml gentamicin (Gibco BRL, Paisley, UK). The other mice were used to provide peritoneal macrophages as previously described (Kilani-Jaziri et al. 2015). Cell viability was assessed using the trypan blue exclusion technique.
Cell treatment
Splenocytes were treated with various concentrations of molecules, and optimal concentrations of lectin (5 μg/ml) and lipopolysaccharide (LPS) from Escherichia coli 0127:B8 (5 μg/ml) were added to each well separately for priming T and B cells, respectively. Macrophages (3 × 105 cells per well) were incubated with various concentrations of flavanones and with or without the addition of LPS (5 μg/ml) solubilized in the complete RPMI 1640 medium (Manosroi et al. 2003). The cells were maintained at 37 °C in a humidified 5 % CO2 atmosphere.
T and B cell proliferation assay
A lymphocyte proliferation assay was assessed by the mitochondrion-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to purple formazan (Mosmann 1983). Splenocyte suspension, in the RPMI 1640 medium (2.5 × 106 cells/ml; 100 μl aliquot/well), was pre-incubated in a 96-well plate for 24 h, before the addition of both mitogen (LPS or lectin, each at 5 μg/ml), and the tested compound was solubilized in the RPMI 1640 medium. Cells were then incubated at 37 °C in a humidified 5 % CO2 atmosphere for an additional period 48 h. Thereafter, 40 μl of MTT (5 mg/ml) was added to RPMI solution and incubated for 2 h at 37 °C. Plates were then centrifuged again, the MTT was removed from each well, and the formazan was dissolved in 100 μl of DMSO. After incubation at 37 °C for 15 min, absorbance of formazan, formed in each well, was measured at 570 nm in a microplate reader (Thermo Scientific, Vantaa, Finland). The percentage of proliferation was ultimately calculated using the following equation: Proliferation (%) = 100 × (Abs sample − Abs control) / Abs control (Manosroi et al. 2003).
Natural killer cell activity
Natural killer (NK) cell activity was measured as previously described (Sarangi et al. 2006) with minor modifications. Briefly, the spleens prepared as described above were used as the source of effector cells; isolated splenocytes were seeded into 96-well microtiter plates at 5 × 106 cells/ml. The cells were then stimulated at 37 °C by different concentrations of the tested samples, for 24 h. To activate NK cells, 100 μl of target K562 cells, at the E:T ratio of 100:1, was added to each well. The plates were then incubated for 4 h at 37 °C in 5 % CO2 atmosphere. Three kinds of controls were performed: target cell control, blank control, and effector cell control. NK cell activity was calculated as follows: NK activity (%) = 100 × (Abs T − (Abs S − Abs E)) / Abs T, where Abs T is the absorbance value of target cell control, Abs S is the absorbance value of the tested samples, and Abs E is the absorbance value of effector cell control.
Cytotoxic T lymphocyte assay
Cytotoxic T lymphocyte (CTL) assay or cell-mediated cytotoxicity was performed using MTT assay. Cytotoxicity of T lymphocyte was measured as previously described for NK cell activity, with modification of target cells. In fact, B16F10 melanoma cells (5 × 104 cells/ml; yielding a 100:1 expected effector-to-target ratio) were added to each well in 50-μl aliquots. The plates were then incubated for 24 h at 37 °C in 5 % CO2 atmosphere. CTL value was calculated as follows: CTL value = 100 % × ((Abs T − (Abs S − Abs E)) / Abs T, where Abs T is the absorbance value of target cells control, Abs S is the absorbance value of test samples, and Abs E is the absorbance value of effector cell control.
Assessment of lysosomal enzyme activity
Lysosomal enzyme activity reflected by acid phosphatase (AP) activity in macrophages was measured as previously described by Manosroi et al. (2005), with some modifications. The percentage of lysosomal enzyme activity in treated cells, relative to that in control cells, was calculated as follows: Inhibition of lysosomal enzyme activity (%) = 100 × (1 − ((Abs sample − Abs control) / Abs control)).
Measurement of nitrite production
The amount of nitric oxide (NO) released by macrophages was measured by determining the amounts of accumulated nitrite (NO− 2) in cell-free supernatants, via the Griess reaction (Green et al. 1982). Briefly, cells were incubated for 48 h in the presence of increasing concentrations of the tested samples. Nitrite amount was then measured by adding 100 μl Griess reagent (1 % sulfanilamide and 0.1 % naphthalenediamine in 5 % phosphoric acid) to 100 μl of the harvested culture supernatant. The absorbance at 570 nm was then detected with a microplate reader (Thermo Scientific, Vantaa, Finland).
Cellular antioxidant activity assay
A cellular anti-oxidant activity (CAA) assay, developed by Wolfe and Liu (2007), was employed to measure the antioxidant potential of the tested samples. Briefly, splenocytes and macrophages were seeded at a density of 5 × 105 and 6 × 104, respectively (in 100 μl PBS). Triplicate wells were then treated with 10 μl of each sample (concentrations ranging from 21.6 to 2.7 μg/ml) along with 5 μl of a 25 μM solution of 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Fluka, Steinheim, Germany). After 1 h of incubation, a 100-μl aliquot of 600 μM and 1.2 mM 2,2′-azobis(2-amidinopropane) dihydrochloride (ABAP) (Sigma-Aldrich, Steinheim, Germany) in PBS was applied to the cells. In this method, DCFH-DA is taken up by cells and deacetylated to 2′,7′-dichlorofluorescin (DCFH). Peroxyl radicals generated from ABAP lead to the oxidation of DCFH to fluorescent (dichlorofluorescin, DCF). Accordingly, cells treated with natural compounds that have any antioxidant activity should have lower fluorescence compared to untreated cells. The fluorescence of each well was measured every 5 min for 1 h using a fluorescence microplate reader (BioTek, Winooski, USA) with 538-nm emission and 485-nm excitation filters. Each plate included triplicate control wells containing cells treated with DCFH-DA and the oxidant ABAP and blank wells containing cells with PBS but without the oxidant ABAP. The fluorescence values for the blank controls and the initial fluorescence values were subtracted from the sample fluorescence values. The area under the fluorescence versus time curve was integrated at each time point to calculate the CAA units using the following equation: CAA unit = 100 − (∫SA / ∫CA) × 100, where ∫SA is the integrated area under the sample fluorescence versus time curve and ∫CA is the integrated area under the control fluorescence versus time curve.
Statistical analysis
Data are expressed as the arithmetic means ± SD of three independent experiments. The statistical significance of the results obtained from in vitro studies was evaluated by Student’s t test, with probability values p < 0.05 and p < 0.01 being considered as significant.
Results
hNG induces lymphocyte proliferation
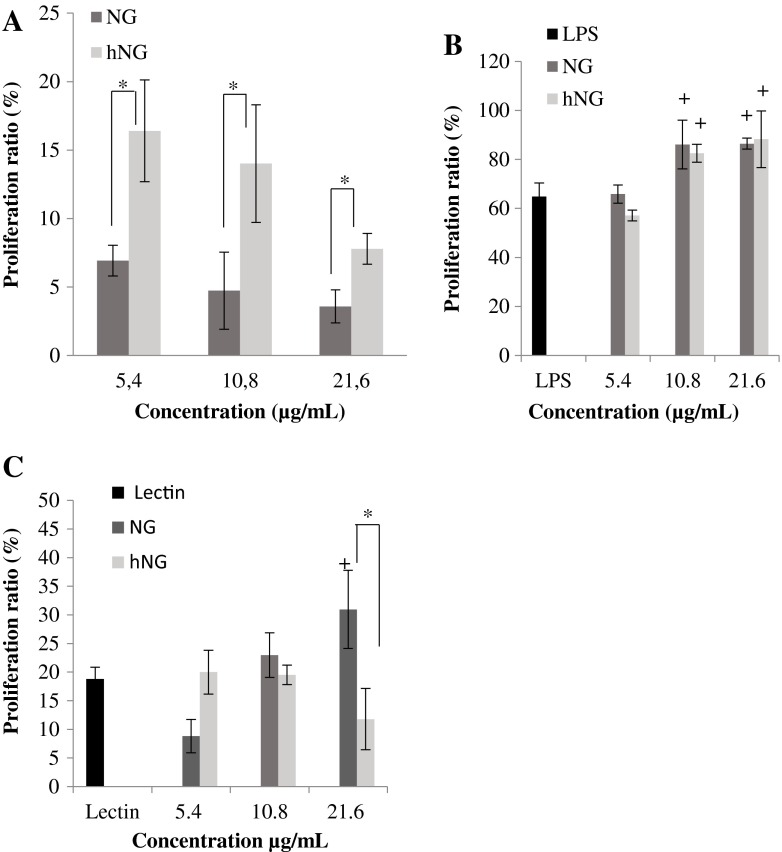
The immunomodulatory effect of both treated and native naringenins on murine splenocytes was investigated. It appears that both NG and hNG induced lymphocyte proliferation in the absence of mitogens, when compared to control cells (Fig. 1a).
Fig. 1.
Effect of native naringenin (NG) and heated naringenin (hNG) on splenocyte proliferation responses. Cells were incubated for 48 h with a increasing concentrations of samples without mitogen, b LPS (5 μg/ml) in the absence or presence of flavanones, or c lectin (5 μg/ml) in the absence or presence of flavanones. Control cells were incubated with RPMI 1640 only. Data shown are mean percentage proliferation (±SD) from three independent experiments. The statistical significance of results was evaluated by Student’s t test. + p < 0.05, value significantly different compared with control cells; *p < 0.05, value significantly different from molecularly treated cells
In contrast, in the presence of LPS, B cell proliferation was more enhanced when incubated either with NG or with hNG at the different tested concentrations, except 5.4 μg/ml, when compared to LPS-treated cells (Fig. 1b).
However, T cell proliferation was induced significantly just with 21.6 μg/ml of NG, whereas concentrations below this level do not induce any proliferation compared to lectin-tested cells (Fig. 1c). On the other hand, heated NG seems to have no inducing effect on T cell proliferation. However, hNG seems to be less efficient compared to NG in inducing a proliferation effect.
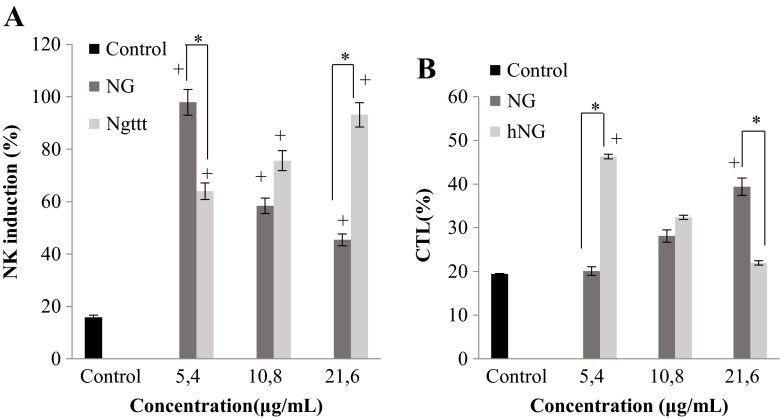
hNG enhances natural killer cell activity
NK cells are known to be important effectors in suppressing tumor growth. Thus, we measured the capacity of NG and hNG in enhancing NK activity against K562 myelogenous leukemia cells. It was noteworthy that both molecules provoked no direct cytotoxic activity against K562 cells at the tested concentrations. It is very important to note that both molecules provoked no direct cytotoxic activity against K562 cells, indicating that molecules had enhanced NK cytotoxic activity against these target cells. NG and hNG improve NK cell lysis activity at 5.4 and 21.6 μg/ml, respectively. Our results have shown that NK activity was enhanced by heated naringenin in a dose-dependent manner and by NG in an inverse dose-dependent manner (Fig. 2a).
Fig. 2.
In vitro effect of various concentrations of native naringenin (NG) and heated naringenin (hNG) on natural killer (NK) cell activity (a) and cytotoxic T cell activity (CTL) in mice. NK induction and CTL activity were measured using the MTT assay. Values shown are mean (±SD) percentage cytotoxicity from six different observations. Control was incubated with K562 target cells in NK cell activity and with B16F10 target cells in CTL activity at a 100:1 expected effector-to-target ratio. The statistical significance of results was evaluated by Student’s t test. + p < 0.05, value significantly different compared with control cells; *p < 0.05, value significantly different from molecularly treated cells
hNG improves cytotoxic T lymphocyte cell activity
To further compare the immunomodulatory effects of native and treated naringenins, we evaluated the cytotoxic activity of T lymphocytes on target cells using B16F10 as CTL-sensitive cells, as far as NG and hNG exhibited no cytotoxicity effect on B16F10 cells. Following the addition of both molecules NG and hNG, an increase in the activity of cytotoxic cells was recorded in a dose-dependent manner in the presence of naringenin and in an inverse dose-dependent manner in the presence of the treated molecule (Fig. 2b).
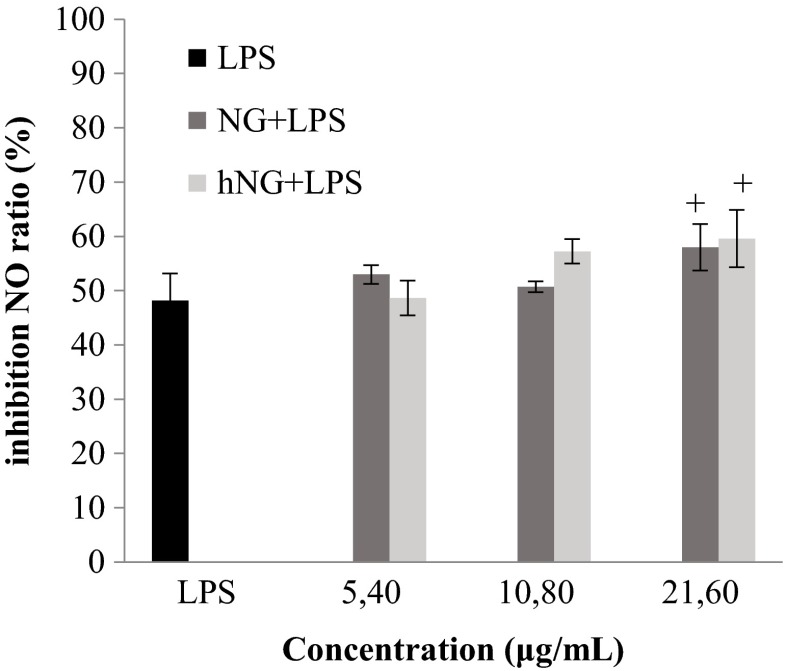
hNG inhibits moderately nitric oxide production
The ability of macrophages to induce the release of nitric oxide (NO) was measured through nitrite, which is a stable breakdown product of NO. A potent but not statistically significant increase in the inhibition of nitrite production in the culture medium was induced by both native and heated naringenins. On the other hand, at the concentration of 21.6 μg/ml, both molecules induced a significantly high NO release inhibition (p < 0.05) (Fig. 3).
Fig. 3.
The inhibition of NO production by native naringenin (NG) and heated naringenin (NG) in mouse peritoneal macrophages stimulated by LPS (5 mg/ml). Macrophages (2 × 105 cells/well) were incubated in the RPMI 1640 medium in the absence/presence of increasing concentrations of molecules for 48 h. Cells treated with 5 μg/ml LPS were used as positive control. Data shown are mean (±SD) percentage of NO production from three independent experiments. Cells incubated in the RPMI 1640 medium alone did not produce NO. The statistical significance of results was evaluated by Student’s t test. + p < 0.05, value significantly different compared with control cells
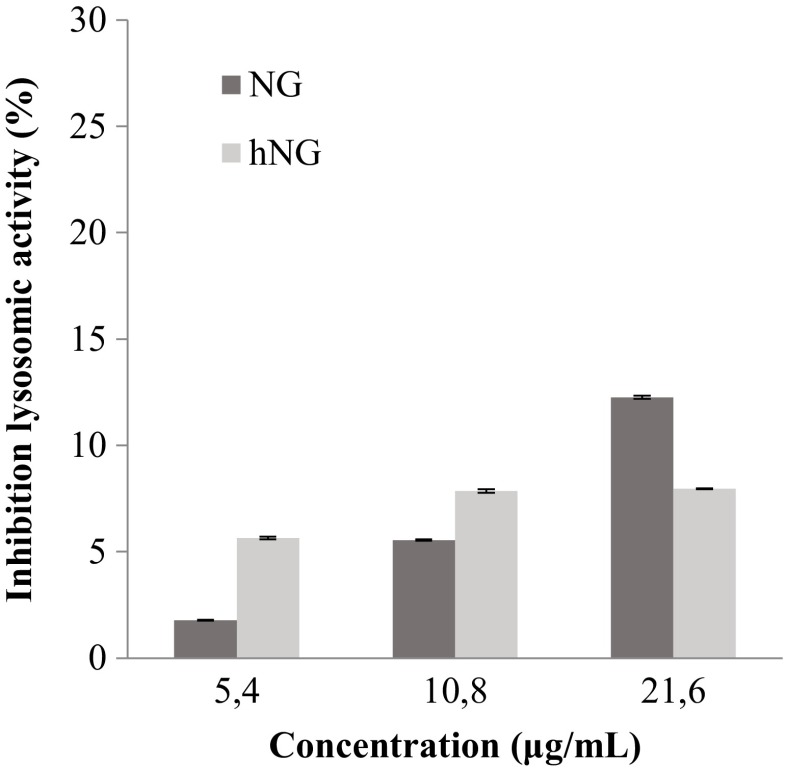
hNG inhibits the cellular lysosomal enzyme activity
The inhibition of the lysosomal enzyme activity of mouse macrophages incubated with native and heated naringenins increased in a concentration-dependent manner. Both NG and hNG showed a slight difference in the modulation of phagocytic activity, 12.25 and 7.9 %, respectively, at 21.6 μg/ml (Fig. 4).
Fig. 4.
Inhibition of mouse peritoneal macrophage lysosomal enzyme activity by native naringenin (NG) and heated naringenin (hNG). Macrophages (2 × 105 cells/well) were incubated in the presence of increasing concentrations of molecules for 48 h. Control cells were incubated with the RPMI 1640 medium only. Lysosomal enzyme activity was then assessed as indicated in the “Materials and methods.” Data shown are mean (±SD) percentages of lysosomal enzyme activity from three independent experiments
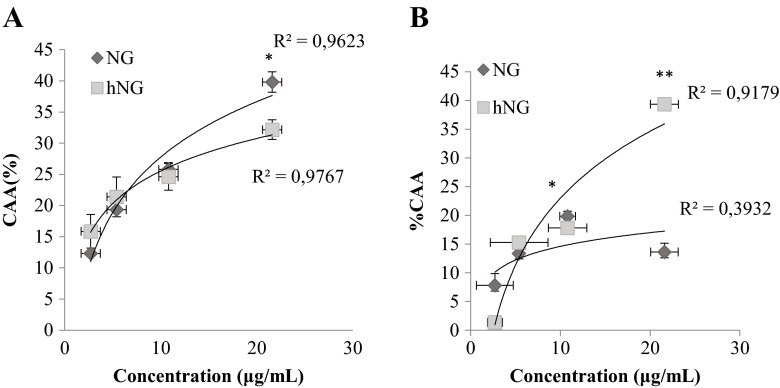
Effect of hNG on cellular antioxidant activity
In macrophages, NG significantly inhibited the oxidation of DCFH (39.8 %) in comparison with hNG 32.17 %, at the highest tested concentration (Fig. 5a). It appears that native NG exhibited stronger CAA than hNG.
Fig. 5.
Dose-response curve for oxidation of DCFH to DCF in macrophages (a) and splenocytes (b) using a cellular antioxidant activity assay in the presence of native naringenin (NG) and heated naringenin (hNG). The curves shown in each graph are from a single experiment (mean ± SD, n = 3). *p < 0.05, value significantly different from molecularly treated cells. **p< 0.01
In contrast, the oxidation of DCFH-DA by peroxyl radicals on splenocyte was significantly more inhibited by the treated naringenin, reaching 52.68 and 17.8 % at 21.6 and 10.8 μg/ml, respectively (Fig. 5b). However, NG showed a lower activity with percentage values of 13.8 and 21.3 % at 21.6 and 10.8 μg/ml, respectively, with a weak correlation (R 2) of 0.39.1.
Discussion
In response to environmental pollution, the immune system may have changes that make it harder to recognize and target abnormal cells and prevent the development of many cancers. In light of this, many researchers are interested in the use of plant products as immunomodulatory agents like extracts and flavonoids. Most of them were obtained after many treatments, such as thermal processing by cooking, boiling, frying, or roasting, in the food and dietary supplement industries. This may have an impact on flavonoid structures and therefore influence their bioavailability and bioactivities (Rohn et al. 2007). In the present study, we investigated the effect of thermal treatment on biological activities of naringenin on its immunopotency compared to the untreated naringenin. To assess splenocyte proliferation, we used the colorimetric MTT assay. It showed that naringenin did not present any cytotoxicity against the tested cells, thus confirming the results reported by Lopez-Posadas et al. (2008). Lymphocyte subpopulations were inverse dose-dependently activated, with a significant increase in the presence of inherent naringenin compared to the treated naringenin. In order to better understand flavonoid action on T and B cell populations, distinct mitogens were used. Splenocytes incubated with lipopolysaccharide (LPS), known to activate preferentially B cell response (Manosroi et al. 2005; Medzhitov 2001), present a significant proliferation at higher concentrations of the unaffected molecules. Interestingly, both NG and hNG exhibit an obvious effect on B cell activation at the concentration of 10.8 and 21.6 μg/ml. Moreover, lectin displays a considerable repertory of carbohydrate specificities. These characteristics, in addition to its ability to stimulate lymphocytes as well as other cells, have made lectin an important diagnostic and experimental tool to study the various aspects of cell growth and differentiation, taking lymphocytes as the cell type (T cells) (Ashraf and Khan 2003). At the highest concentrations, NG activates T cytotoxic cells. This finding supports previous studies which found that naringenin promotes T cell activation and increases the proportion of CD8+ T cells producing IFN-γ and IL-2 in a mice breast cancer resection model. Heat treatment increased the activity of CTL, and the enhancement of this activity may be due to the generation of novel compounds that can stimulate the secretion of cytokines by cytotoxic T lymphocytes. By activation CTL, hNG is able to reduce the proliferation of cancer cells such as melanoma cells and it may be used as a chemopreventive and/or therapeutic agent. However, these results differ from those of a published study which reported that naringenin inhibited proliferation of activated T lymphocytes, thus suppressing the immune response in contact hypersensitivity (Fang et al. 2010). In fact, cytotoxic T cells prevent the propagation of intracellular pathogens through T cell receptor (TCR) recognition of peptides. Studies of CTL target dynamics were conducted in vitro, but recently, there has been some attention on data from splenic cytotoxicity assays (Hogan et al. 2014; Regoes et al. 2007). This finding corroborates the ideas of Du et al. (2009) who mentioned that naringenin significantly increases the amount of CD8+ and CD4+ T cells, expressing the activation marker CD44 in the fibrotic lung of the bleomycin-treated mice receiving naringenin. On the other hand, the low concentration of thermally processed naringenin presents an important tumor-specific cytotoxic activity and a slight proliferation of T cells. Natural killer (NK) cells apply direct cytotoxic activity against tumor targets by cytokine production. Quiet NK cell activity is associated with an increase in the risk of carcinogenesis. Therefore, the focus of recent cancer treatment is to develop drugs that promote NK cells (Battella et al. 2015; Vivier et al. 2012). In fact, we evaluated the cytotoxic effect of NK cells on target cells by co-culturing spleens with K562 myelogenous leukemia cells as NK-sensitive cells. Naringenin improved NK cell lysis activity at the lowest tested concentration (5.4 μg/ml). In contrast, the thermally processed naringenin presented the best activity at the highest tested concentrations (21.6 μg/ml). Our findings are in accordance with a recent study conducted by Kim and Lee (2015). Macrophages perform a central role in innate and adaptive immunity through the lysosomal pathway. Lysosomes can moderate the phenotype/function of innate immune cells and can restrain the localization of internalized material (Bright et al. 2005; Luzio et al. 2007; Watts 2012). In addition, they internalize extracellular elements by endocytic or phagocytic mechanisms that lead to the formation of endolysosomal hybrids (resulting from fusions between late endosomes and lysosomes), which facilitates the exchange of intra-organelle contents. This process renders the internalized molecules disposed to degradation by the lysosomal hydrolases (Bright et al. 2005; Luzio et al. 2007). Among the enzyme secreted in this way, we found the acid phosphatase which gives a yellow color when reacting with PNPP (Alvarado et al. 2015). The tested molecules revealed inhibiting effects against the cellular lysosomal enzyme activity, suggesting that the unaffected and treated naringenins prevent the development of autoimmune responses (Alvarado et al. 2015). These results aside, macrophage phagocytosis is attended by the release of free radicals and other reactive nitrogen species (RNS, the latter comprising nitric oxide) involved in pathogen destroying (Manosroi et al. 2005). These species are also involved in many biological approaches (Xu et al. 2002). The study here showed that flavanones present an important inhibition of NO production in macrophages stimulated with LPS. This accentuates the anti-inflammatory role of native and processed naringenins and accords with other researchers who showed the immunomodulatory effect of NG (Bodet et al. 2008; Lopez-Posadas et al. 2008; Park et al. 2012; Yilma et al. 2013). On the other hand, the oxidation of DCFH-DA by peroxyl radicals was significantly inhibited by NG in macrophages and by hNG in splenocytes. As mentioned in the literature review, NG proves an important antioxidant activity during the reaction with the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) (González and Nazareno 2011). Besides, naringenin displays a strong antioxidant activity in vivo in different disease conditions. It is able to attenuate the oxidative damage induced by acetic acid and improve antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) activities (Al-Rejaie et al. 2013; Wang et al. 2012). This ability to trap free radicals may depend on the presence of hydroxyls on the A and B rings (5 and 4′–OH) (Jabbari and Jabbari 2016). The thermally processed naringenin has decreased the antioxidant activity on macrophages compared to the native molecule. Similar reports showed that thermal treatment decreases the antioxidant activity of phytochemical compounds such as rutin, quercetin, and chlorogenic acid (Buchner et al. 2006; Murakami et al. 2004).
Our results indicated that according to the target cells, some decomposition products preserve radical scavenging activity or enhance it, in splenocytes. The slight difference between the effects of native and heated naringenins on the immunomodulatory system may be ascribed to the stability of NG. Thus, heat processing of food in the industrial chain does not destroy the characteristics or reduce the qualities of the phytochemicals present in nutrients but rather improves their nutritional and/or therapeutically values.
Conclusion
This study enhances our understanding of the role of naringenin as an immunomodulatory molecule that stimulates T cells and protects an organism by activating NK cytotoxicity cells. It has demonstrated, for the first time, that native flavanone induces B cell proliferation. Moreover, heat-treated naringenin enhances significantly the cellular antioxidant activity in splenocytes, stimulates the cytotoxic activity of NK cells, and downregulates the cytotoxicity of T cells. At the same time, heat treatment maintains the anti-inflammatory potency of naringenin. The slight difference with previous findings may be explained by the high stability of NG after heat treatment.
Acknowledgments
The authors acknowledge the “Ministère Tunisien de l’Enseignement Supérieur et de la Recherche Scientifique” for its support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Alexandrescu DT, Riordan NH, Ichim TE, Kauffman CL, Kabigting F, Dutton CT, Dasanu CA. On the missing link between inflammation and cancer. Dermatol Online J. 2011;17:10. [PubMed] [Google Scholar]
- Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol. 2013;19:5633–5644. doi: 10.3748/wjg.v19.i34.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado R, O’Brien B, Tanaka A, Dalton JP, Donnelly S. A parasitic helminth-derived peptide that targets the macrophage lysosome is a novel therapeutic option for autoimmune disease. Immunobiology. 2015;220:262–269. doi: 10.1016/j.imbio.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Ashraf MT, Khan RH. Mitogenic lectins. Med Sci Monit. 2003;9:RA265–RA269. [PubMed] [Google Scholar]
- Battella S, Cox MC, Santoni A, Palmieri G. Natural killer (NK) cells and anti-tumor therapeutic mAb: unexplored interactions. J Leukoc Biol. 2015 doi: 10.1189/jlb.5VMR0415-141R. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. What is xenohormesis? Am J Pharmacol Toxicol. 2008;3:152–159. doi: 10.3844/ajptsp.2008.152.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Rasmussen MK, Christensen LP, Young JF, Kristiansen K, Oksbjerg N. Naringenin and falcarinol stimulate glucose uptake and TBC1D1 phosphorylation in porcine myotube cultures. J Biochem Pharmacol Res. 2014;2:91–98. [Google Scholar]
- Bodet C, La VD, Epifano F, Grenier D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J Periodontal Res. 2008;43:400–407. doi: 10.1111/j.1600-0765.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Bright NA, Gratian MJ, Luzio JP. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr Biol. 2005;15:360–365. doi: 10.1016/j.cub.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Buchner N, Krumbein A, Rohn S, Kroh LW. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun Mass Spectrom. 2006;20:3229–3235. doi: 10.1002/rcm.2720. [DOI] [PubMed] [Google Scholar]
- Cavia-Saiz M, Busto MD, Pilar-Izquierdo MC, Ortega N, Perez-Mateos M, Muniz P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agric. 2010;90:1238–1244. doi: 10.1002/jsfa.3959. [DOI] [PubMed] [Google Scholar]
- Du G, Jin L, Han X, Song Z, Zhang H, Liang W. Naringenin: a potential immunomodulator for inhibiting lung fibrosis and metastasis. Cancer Res. 2009;69:3205–3212. doi: 10.1158/0008-5472.CAN-08-3393. [DOI] [PubMed] [Google Scholar]
- Fang F, Tang Y, Gao Z, Xu Q. A novel regulatory mechanism of naringenin through inhibition of T lymphocyte function in contact hypersensitivity suppression. Biochem Biophys Res Commun. 2010;397:163–169. doi: 10.1016/j.bbrc.2010.05.065. [DOI] [PubMed] [Google Scholar]
- Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012;32:1119–1136. [PMC free article] [PubMed] [Google Scholar]
- González EA, Nazareno MA. Antiradical action of flavonoid–ascorbate mixtures. LWT-Food Sci Technol. 2011;44:558–564. doi: 10.1016/j.lwt.2010.09.017. [DOI] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermenean A, Ardelean A, Stan M, Herman H, Mihali C-V, Costache M, Dinischiotu A. Protective effects of naringenin on carbon tetrachloride-induced acute nephrotoxicity in mouse kidney. Chemico-Biol Interact. 2013;205:138–147. doi: 10.1016/j.cbi.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Hogan T, Kadolsky U, Tung S, Seddon B, Yates A. Spatial heterogeneity and peptide availability determine CTL killing efficiency in vivo. PLoS Comput Biol. 2014;10:e1003805. doi: 10.1371/journal.pcbi.1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL, Tytell M, Vigh L. Xenohormesis: health benefits from an eon of plant stress response evolution. Cell Stress Chaperones. 2010;15:761–770. doi: 10.1007/s12192-010-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133:387–391. doi: 10.1016/j.cell.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari M, Jabbari A. Antioxidant potential and DPPH radical scavenging kinetics of water-insoluble flavonoid naringenin in aqueous solution of micelles. Colloids Surf A Physicochem Eng Asp. 2016;489:392–399. doi: 10.1016/j.colsurfa.2015.11.022. [DOI] [Google Scholar]
- Kara S et al. (2014) Protective effect of hesperetin and naringenin against apoptosis in ischemia/reperfusion-induced retinal injury in rats. Sci World J, 2014 [DOI] [PMC free article] [PubMed]
- Kilani-Jaziri S, Mustapha N, Mokdad-Bzeouich I, El Gueder D, Ghedira K, Ghedira-Chekir L. Flavones induce immunomodulatory and anti-inflammatory effects by activating cellular anti-oxidant activity: a structure-activity relationship study. Tumour Biol. 2015 doi: 10.1007/s13277-015-4541-5. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JK. Naringenin enhances NK cell lysis activity by increasing the expression of NKG2D ligands on Burkitt’s lymphoma cells. Arch Pharm Res. 2015 doi: 10.1007/s12272-015-0624-5. [DOI] [PubMed] [Google Scholar]
- Kneževiæ T, Petlevski R (2014) Antioxidative effect of naringenin on the activity of superoxide dismutase and glutathione peroxidase in HepG2 cells under hyperglycaemic conditions. Biochemia Medica 24
- Krifa M, Bouhlel I, Ghedira-Chekir L, Ghedira K. Immunomodulatory and cellular anti-oxidant activities of an aqueous extract of Limoniastrum guyonianum gall. J Ethnopharmacol. 2013;146:243–249. doi: 10.1016/j.jep.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Li Y-R, Chen D-Y, Chu C-L, Li S, Chen Y-K, Wu C-L, Lin C-C. Naringenin inhibits dendritic cell maturation and has therapeutic effects in a murine model of collagen-induced arthritis. J Nutrit Biochem. 2015;26:1467–78. doi: 10.1016/j.jnutbio.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Limem I, Harizi H, Ghedira K, Chekir-Ghedira L. Leaf extracts from Phlomis crinita Cav. subs. mauritanica Munby affect immune cell functions in vitro. Immunopharmacol Immunotoxicol. 2011;33:309–314. doi: 10.3109/08923973.2010.504926. [DOI] [PubMed] [Google Scholar]
- Lin E-J, Zhang X, Wang D-Y, Hong S-Z, Li L-Y. Naringenin modulates the metastasis of human prostate cancer cells by down regulating the matrix metalloproteinases-2/-9 via ROS/ERK1/2 pathways. Bangladesh Journal of Pharmacology. 2014;9:419–427. [Google Scholar]
- Lopez-Posadas R, Ballester I, Abadia-Molina AC, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F. Effect of flavonoids on rat splenocytes, a structure-activity relationship study. Biochem Pharmacol. 2008;76:495–506. doi: 10.1016/j.bcp.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Lulu SS, Thabitha A, Vino S, Priya AM, Rout M. Naringenin and quercetin—potential anti-HCV agents for NS2 protease targets. Nat Prod Res. 2015;30:464–8. doi: 10.1080/14786419.2015.1020490. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- Manosroi A, Saraphanchotiwitthaya A, Manosroi J. Immunomodulatory activities of Clausena excavata Burm. f. wood extracts. J Ethnopharmacol. 2003;89:155–160. doi: 10.1016/S0378-8741(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Manosroi A, Saraphanchotiwitthaya A, Manosroi J. In vitro immunomodulatory effect of Pouteria cambodiana (Pierre ex Dubard) Baehni extract. J Ethnopharmacol. 2005;101:90–94. doi: 10.1016/j.jep.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Meng S, Wu Y, Hu X, Zhang H, Li C. Naringenin may block RSV-induced mucous hypersecretion in A549 cell via JNK/AP-1 signaling pathway. Zhonghua er ke za zhi (Chin J Pediatr) 2015;53:182–186. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murakami M, Yamaguchi T, Takamura H, Atoba T. Effects of thermal treatment on radical‐scavenging activity of single and mixed polyphenolic compounds. J Food Sci. 2004;69:FCT7–FCT10. doi: 10.1111/j.1365-2621.2004.tb17848.x. [DOI] [Google Scholar]
- Mustapha N, Mokdad-Bzeouich I, Sassi A, Abed B, Ghedira K, Hennebelle T, Chekir-Ghedira L. Immunomodulatory potencies of isolated compounds from Crataegus azarolus through their antioxidant activities. Tumour Biol. 2015 doi: 10.1007/s13277-015-4517-5. [DOI] [PubMed] [Google Scholar]
- Ng’uni T, Mothlalamme T, Daniels R, Klaasen J, Fielding BC (2015) Additive antibacterial activity of naringenin and antibiotic combinations against multidrug resistant Staphylococcus aureus
- Orallo F, Camina M, Alvarez E, Basaran H, Lugnier C. Implication of cyclic nucleotide phosphodiesterase inhibition in the vasorelaxant activity of the citrus-fruits flavonoid (+/-)-naringenin. Planta Med. 2005;71:99–107. doi: 10.1055/s-2005-837774. [DOI] [PubMed] [Google Scholar]
- Park HY, Kim GY, Choi YH. Naringenin attenuates the release of pro-inflammatory mediators from lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear factor-kappaB and inhibiting mitogen-activated protein kinases. Int J Mol Med. 2012;30:204–210. doi: 10.3892/ijmm.2012.979. [DOI] [PubMed] [Google Scholar]
- Priscilla DH, Roy D, Suresh A, Kumar V, Thirumurugan K. Naringenin inhibits alpha-glucosidase activity: a promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem Biol Interact. 2014;210:77–85. doi: 10.1016/j.cbi.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Raza SS, et al. Neuroprotective effect of naringenin is mediated through suppression of NF-kB signaling pathway in experimental stroke. Neuroscience. 2013;230:157–171. doi: 10.1016/j.neuroscience.2012.10.041. [DOI] [PubMed] [Google Scholar]
- Regoes RR, Barber DL, Ahmed R, Antia R. Estimation of the rate of killing by cytotoxic T lymphocytes in vivo. Proc Natl Acad Sci U S A. 2007;104:1599–1603. doi: 10.1073/pnas.0508830104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro IA, Ribeiro MHL. Naringin and naringenin determination and control in grapefruit juice by a validated HPLC method. Food Control. 2008;19:432–438. doi: 10.1016/j.foodcont.2007.05.007. [DOI] [Google Scholar]
- Rohn S, Buchner N, Driemel G, Rauser M, Kroh LW. Thermal degradation of onion quercetin glucosides under roasting conditions. J Agric Food Chem. 2007;55:1568–1573. doi: 10.1021/jf063221i. [DOI] [PubMed] [Google Scholar]
- Sansone P, Bromberg J. Environment, inflammation, and cancer. Curr Opin Genet Dev. 2011;21:80–85. doi: 10.1016/j.gde.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Sarangi I, Ghosh D, Bhutia SK, Mallick SK, Maiti TK. Anti-tumor and immunomodulating effects of Pleurotus ostreatus mycelia-derived proteoglycans. Int Immunopharmacol. 2006;6:1287–1297. doi: 10.1016/j.intimp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Silva LC, David JM, Borges Rdos S, Ferreira SL, David JP, Dos Reis PS, Bruns RE. Determination of flavanones in orange juices obtained from different sources by HPLC/DAD. J Anal Methods Chem. 2014;2014:296838. doi: 10.1155/2014/296838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallverdu-Queralt A, Odriozola-Serrano I, Oms-Oliu G, Lamuela-Raventos RM, Elez-Martinez P, Martin-Belloso O. Changes in the polyphenol profile of tomato juices processed by pulsed electric fields. J Agric Food Chem. 2012;60:9667–9672. doi: 10.1021/jf302791k. [DOI] [PubMed] [Google Scholar]
- Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yang Z, Lin L, Zhao Z, Liu Z, Liu X. Protective effect of naringenin against lead-induced oxidative stress in rats. Biol Trace Elem Res. 2012;146:354–359. doi: 10.1007/s12011-011-9268-6. [DOI] [PubMed] [Google Scholar]
- Watts C. The endosome-lysosome pathway and information generation in the immune system. Biochim Biophys Acta. 2012;1824:14–21. doi: 10.1016/j.bbapap.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem. 2007;55:8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- Wu LH, et al. Naringenin suppresses neuroinflammatory responses through inducing suppressor of cytokine signaling 3 expression. Mol Neurobiol. 2015 doi: 10.1007/s12035-014-9042-9. [DOI] [PubMed] [Google Scholar]
- Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12:311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- Yilma AN, Singh SR, Morici L, Dennis VA. Flavonoid naringenin: a potential immunomodulator for Chlamydia trachomatis inflammation. Mediators Inflamm. 2013;2013:102457. doi: 10.1155/2013/102457. [DOI] [PMC free article] [PubMed] [Google Scholar]