Abstract
Introduction
Mandibular reconstruction has changed significantly over the years and continues to evolve with the introduction of newer technologies and techniques.
Purpose
This article reviews the history of oromandibular reconstruction, biomechanics of mandible, summarizes the reconstruction options available for mandible with defect classification, goals in reconstruction, the various donor sites, current reconstructive options, dental rehabilitation and persistent associated problems.
Summary
Oromandibular reconstruction, although a challenge for the head and neck reconstructive surgeon, is now reliable and highly successful with excellent long-term functional and aesthetic outcomes with the use of autogenous bone grafts and current reconstructive options. The ideal reconstruction would provide a solid arch to articulate with the upper jaw, restoring swallowing speech, mastication, and esthetics. Autogenous vascularized bone grafts in combination with microsurgical techniques have revolutionized mandibular reconstruction in oral cancer surgery. Current trends in mandibular reconstruction aim to achieve reestablishment of a viable mandible of proper form and maxillary mandibular relationship while decreasing the need for invasive autogenous graft procurement. However the optimal reconstruction of mandibular defects is still controversial in regards to reconstructive options which include the donor site selection, timing of surgery and method of reconstruction.
Keywords: Mandibular reconstruction, Vascularized and non vascularized bone grafts, Bone substitutes, Implants
Introduction
Oromandibular reconstruction resulting from resection of benign tumour, malignant tumor, and osteomyelitis or osteoradionecrotic mandible remains a challenge for the surgeon today. Mandibular defects following ablative surgery for malignant tumours of the head and neck region impact both form and function and require a multidisciplinary approach to optimize functional and cosmetic outcomes. Comprehensive reconstructive strategies require the restoration of facial dimensions, including width, height, and projection. To achieve optimal functional and aesthetic results, reconstructive surgeons must be able to replace the skeletal buttresses, restore the external/internal soft tissue envelope, eliminate fistulas, and provide a foundation for dental rehabilitation. The geometric design of the inferior border of the mandible defines the aesthetic contour of the lower third of the face. This horizontal buttress or mandibular plane defines a soft tissue cephalometric parameter formed by a line that connects menton to gonion. Replacement of the dentoalveolar segment allows for ideal placement of endosteal implants and eventual rehabilitation with an implant-bone prosthesis at the level of the occlusal plane. The U-shaped mandible serves as the arch of the oral cavity, supporting the tongue and muscles of the mouth floor, thus permitting mastication, articulation, deglutition, and respiration. In addition, the functional deficit associated with loss of sensation in the reconstructed oral cavity is readily apparent when oral function is critically assessed. These days tumour surgery of maxillofacial region demands not just a radical removal of a tumour with primary or secondary reconstruction. Furthermore, the patient requires the full function and dental rehabilitation. To be a normal member of the society is sometimes more important for the patient than the success of radical tumorectomy and the possibility of the recurrences.
History
Bardenheur and Skyoff described the use of free autologous bone grafts for mandibular reconstruction [1, 2]. Martin described the immediate restoration of a resected segment of mandible with a prosthetic appliance [3]. Partsch used metal band to restore the continuity of mandible [4]. Berndt recommended the use of celluloid material. White [5] favoured silver wire. Scudder et al used hard rubber [6]. Konig used ivory. Metals have also been used; Vitallium, Stainless steel [7] and Titanium. World War I saw the increased use in bone grafting for jaw defects. During World War II, Mowlen emphasized the importance of cancellous bone. Blocker and Stout published an extensive review of free bone grafts used for mandible reconstruction taken from tibia, rib and iliac crest [8]. Castermans et al. [9] reported results of mandibular reconstruction using a threaded Kirschner (K) wire in 47 patients. Bowerman reported the use of a titanium plate (Bowerman-Conroy implant) to reconstruct the mandible in 17 patients [10]. Leuke and Rappaport, Schwartz and Albert and associates used Dacron urethane mesh for holding the cancellous chips [11]. Wersal et al. [12] addressed the use of split-rib grafts for reconstruction of the mandible. Taylor, as well as Sanders and Mayou described the deep circumflex iliac artery and vein (DCIA/V) as a reliable and easily utilizable vascular pedicle to transfer iliac bone and the overlying skin as a free tissue transfer [13]. In 1986, Swartz et al. [14] introduced the scapular osteocutaneous free flap (SOFF) for use in head and neck reconstruction. In 1989, Hidalgo [15] became the first to report the transfer of fibular bone to reconstruct a segmental defect of the mandible. Bradley [16] in 1978 and 1982 reported a two-stage procedure for reimplantation of ‘autogenous freeze treated mandibular bone’. Wang et al observed the ultrastructure of the frozen section and found that freezing procedure could effectively destroy tumor cells. Dong et al. [17] reported a large series of mandibular reconstruction using ‘autogenous freeze treated mandibular bone’ for tumours of the mandible and floor of the mouth. Most recently, in 2010, Kuo et al. [18] combined partial soleus muscle with fibula osteoseptocutaneous flap for dead space obliteration.
Etiology
Acquired segmental defects of the mandible are most commonly secondary to ablative tumor therapy or avulsive traumatic injury. Other less common causes include inflammatory or infectious conditions that result in devitalisation of the mandibular bone requiring its debridement. Segmental defects secondary to tumour therapy may result from the management of aggressive benign tumours arising within the mandible such as ameloblastoma or myxoma or from malignancies carcinomas/sarcomas) arising in the associated soft tissue envelope that invade or extend to the mandible. Management of oral squamous cell carcinoma is the most common malignancy resulting in acquired segmental defects of the mandible. Avulsive segmental wounds most commonly arise from high-velocity injuries such as firearms, industrial accidents, and occasionally motor vehicle collisions. Fortunately, most traumatic injuries to the jaws do not result in segmental defects because of the lower kinetic energies associated with the injury. Kinetic energy (KE = mv2) increases dramatically as the speed of the missile or the speed of impact increases, resulting in comminution of bone, destruction of the soft tissue envelope, and devitalisation of large areas of bone and soft tissue. The extent of devitalisation from high-velocity injuries may not be completely apparent at the time of presentation. The astute clinician will recognize the potential for extensive tissue loss in these patients and utilize wound care and temporary stabilization of the wound until all tissue loss has had opportunity to declare itself prior to definitive reconstruction.
Goals of Reconstruction
The goals of mandibular reconstruction are to re-establish the form of the lower third of the face and to restore the patient’s ability to eat in public, be intelligible to both trained and untrained listeners, and to maintain an unencumbered airway that allows the freedom to perform all activities. The greater the loss of tongue volume, the greater the negative impact on the patient’s prognosis for recovery of oral function. Thus, the approach to the reconstruction should start by addressing the impact of the surgery on the patient’s tongue. In most cases, optimizing tongue bulk and mobility is more critical to the post-operative functional recovery than management of the bony defect. Loss of mucosa from the floor of mouth is critical in the assessment of whether to restore this component of the defect with non-native tissue. Preventing the tongue from becoming tethered to the neomandible is vital to preservation of mobility [19].
Restoring tongue bulk and preserving mobility allow for palatoglossal contact which is critical for improving articulation during speech and bolus manipulation during deglutition. Oral reconstruction must also address lower lip function by attempting to achieve oral competence while preserving the expressive motion of the lips that is so important to normal facial movement. The goals and the criteria for a successful mandibular reconstruction are to [20]
Establish continuity
Establish alveolar height
Establish arch form
Establish arch width
Maintain bones
Improve facial contours
Biomechanics of Mandible
Factors that influence the biomechanics of the mandible include the integrity of the temporomandibular joint (TMJ), the bone stock distribution, and the forces associated with scar contracture. The preservation of the joint biomechanics is ideal during reconstruction of the mandible. Because the complex movements of the TMJ are difficult to reconstruct, the integrity of the joint should be preserved whenever possible. The mandible itself is uniquely designed with increased bone stock along the inferior border and anterior aspect of the ascending ramus in response to the forces of mastication. When approaching a mandibular reconstruction, it is ideal to recreate these structures with the biomechanics in mind. The contour of the mandible is important to facial symmetry, and the continuity of the mandible is important to oral function. The mandibular arch, for example, serves to anchor the suprahyoid muscles, whereas the mandibular body anchors the mylohyoid muscle, which supports the position of the tongue. When either the body or the arch of the mandible is disrupted, the ability to raise the larynx during swallowing or the position of the tongue may be greatly affected, leading to a disturbance in swallowing, speech, and articulation. According to Misch et al. [21] the anterior mandible has a greater trabecular bone density, which correlates with its greater elastic modulus and compressive strength than other regions. The presence of the cortical plate increases the elastic modulus of the trabecular bone in all regions, with the anterior mandible having the highest values. When cortical bone was present, the elastic modulus ranged from 24.9 to 240 MPa with a mean value of 96.2 MPa. When the cortical bone was absent, the elastic modulus ranged from 3.5 to 125.6 MPa. The ultimate compressive strength of the trabecular bone ranged from 0.22 to 10.44 MPa (mean 3.9 MPa). These data show that the cortical bone plays a major role in dissipating occlusal loads. The trabecular bone of the mandible also has anisotropic properties [22].
Type of Defect and Approach to Reconstruction
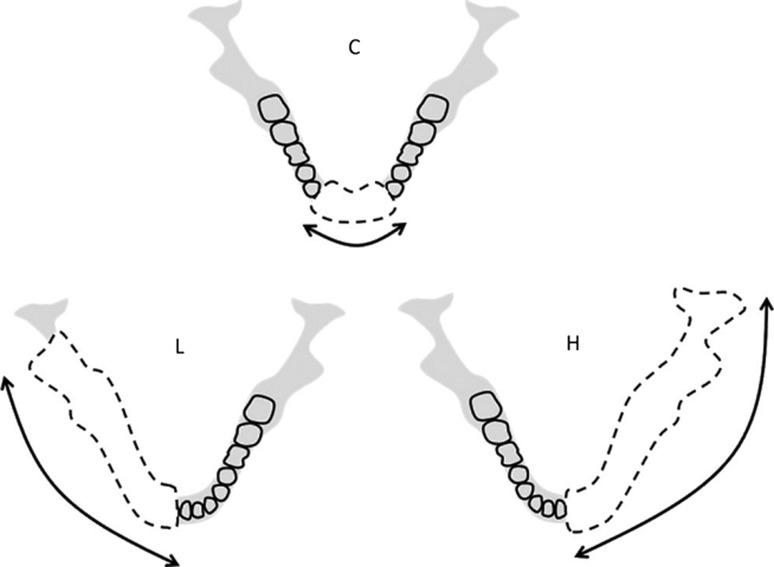
Mandibular defects can generally be considered by their location and extent and can be divided into defects involving the anterior mandible, lateral mandible, and ramus/condyle. Jewer et al. [23] used a classification system designed to reflect the complexity of the reconstruction. The Jewer classification provides an aid in classifying mandibular defects and reflects the complexity of the reconstructive problem. Central defects including both canines are designated “C”, and lateral segments that exclude the condyle are designated “L”. When the condyle is resected together with the lateral mandible, the defect is designated “H”, or hemi mandibular. Eight permutations of these capital letters—C, L, H, LC, HC, LCL, HCL, and HH—are encountered for mandibular defects. The significance of this is that a lateral defect can be reconstructed with a straight segment of bone, whereas a central defect would require osteotomies. The classification was modified to include a soft tissue description as well, with “t” representing a significant tongue defect, “m” a mucosal defect, and “s” an external skin defect. Boyd et al. [24] improved upon this classification system by taking into account the mucosal and/or soft tissue component of the defect. He added lower-case characters o, m, and s to specify if the defect was osseous only, involved mucosa, and/or external skin, respectively. Urken et al. classification is based on functional considerations caused by detachment of different muscle groups and difficulties with cosmetic restoration. C—Condyle, R—ramus, B—body, S—total symphysis, SH—hemisymphysis (Fig. 1) [38].
Fig. 1.
Basis of the HCL method for classifying mandibular defects
Timing of Reconstruction
The ideal timing of mandibular reconstruction has been widely debated, especially in patients with malignant disease. Historically, proponents of a delayed or staged approach advocated a period of observation to monitor the patient for development of recurrent disease or to establish histologically clear bony margins prior to reconstruction. Today, however, it is widely accepted that immediate reconstruction may be performed without risk for a delayed diagnosis of recurrent disease [25]. Prior to microsurgical techniques, delayed reconstruction was critical to allow maturation of the wound bed for nonvascular bone grafting. Lawson et al. [26] reported their results with immediate and delayed reconstruction utilizing corticocancellous grafts. They reported a 90 % success rate with delayed reconstruction, compared to 46 % with immediate reconstruction when using nonvascularized bone grafts. Subsequent reports have demonstrated successful use of delayed, nonvascular bone grafts in select patients, and indeed nonvascular grafts are still frequently utilized today [27–29]. Immediate reconstruction has several other advantages over delayed reconstruction for mandibular reconstruction. Health-related quality of life (QOL) studies have demonstrated that immediate reconstruction significantly improves QOL and that most patients prefer immediate reconstruction [30–34]. Furthermore, Boyd reported that patients who underwent reconstruction with vascularized bone flaps experienced an average of 4 days life lost for secondary procedures, compared to 35 days for patients who underwent plate and soft tissue flaps [35].
Cardinal Prerequisites of Successful Bone Grafting [36]
Bone transplantation into healthy tissues.
Wide contact between adjacent bone and the graft.
Recipient area with adequate blood supply.
Positive fixation.
Reconstructive Options Used for Reconstruction of Mandible
Modern mandibular reconstruction requires the surgeons to have many options at their expertise. Many reconstruction modalities have been attempted and reported. These modalities include reconstruction bars with or without pedicled myocutaneous flaps, alloplasts, free grafts including particulate or cortical bone, pedicled osteomyocutaneous flaps, and a variety of free vascularized bone flaps. No one method of reconstruction deals with all the variables affecting each patient with a mandibular defect. It is important to mention that one option is no reconstruction of the mandible [37, 38]. Komisar concluded that mandibular continuity did not enhance the functional rehabilitation in the majority of patients he studied with oropharyngeal malignancies [39]. Peeled et al. proposed a protocol for functional reconstruction of mandibular discontinuity defects, which consisted of several differentiated steps: (1) resection of the tumor with free margins, together with reconstruction of the newly generated mandibular defect by means of the fibular vascularized osteocutaneous flap; (2) performance of a skin flap vestibuloplasty to provide an adequate amount of keratinized mucosa 6 months after the resection of the mandible and reconstruction with fibular flap; (3) insertion of screw-type dental implants 4 months following the skin graft; (4) over denture placement, performed 4 months following insertion of implants [40]. The fibular osteocutaneous free flap (FOFF) is the workhorse donor site for mandibular reconstruction. Multiple studies demonstrate a greater than 95 % flap survival rate with skin paddle viability in over 90 % of cases [41]. Vascularized bone is historically reserved for more difficult secondary reconstructions where a large defect existed, where soft tissue is inadequate, or where the recipient bed has been compromised by radiation, chronic infection, or previous surgery. Even in those adverse situations the success rate has been reported to be very high. Later, this method was also successfully applied in cases of primary reconstruction in aggressive odontogenic tumours [42].
Reconstruction Plates
Mandibular reconstruction plates and screws are the most widely used alloplastic devices for mandibular reconstruction. The most common metals used in the fabrication of these plates are stainless steel, vitallium, and titanium. Vitallium is an alloy of cobalt, chromium, and molybdenum. This type of plate initially seemed to be ideal, however, the low malleability can make application difficult. AO stainless steel and AO titanium reconstruction plates were developed in an attempt to find a mandibular reconstructive option that was fast, single-staged, and reliable while maintaining oral function and form. These plates have been used with varying rates of success. The titanium hollow osseointegrated reconstruction plate (THORP) reconstruction plate system uses a perforated hollow titanium screw that allows bone ingrowth and osseointegration which, in theory, increases the stability of the bone-screw interface [43]. The development of the THORP was an attempt to address the failures of the older plating systems. This plate has a hollow screw made of titanium with perforations along the screw body which allow bone ingrowth and result in increased plate stability at the bone-screw interface. An expansion bolt within the screw head allows the plate to be anchored to the interosseous screw instead of being compressed to the underlying mandible. This prevents pressure necrosis of the underlying bone decreasing the potential for plate failure at the screw-bone interface. There are several large series describing the use of reconstruction plates for mandible reconstruction after tumor resection [44, 45].
Placement of mandibular reconstruction plates does not contraindicate the use of post-operative radiation therapy. In 1991, Gullane [37] reported an analysis of 64 cases evaluating the interface radiation dose using both stainless steel and titanium plates with a parallel beam radiation technique. He noted that the radiation dose at the plate-bone interface increased only 15 % at the 6-mV level with the excess tissue dose scatter extending only 1.1 mm to the surrounding soft tissue.
Techniques for Autogenous Bone Replacement
Non-vascularized bone grafts
Vascularized free flaps
Out of the two, the technique to be used is determined by the following factors:
Quality of the soft tissue environment (the history of radiation, previous graft failure and infection produces lots of scarring comprising the soft tissue bed)
Adequacy of the soft tissue (associated lining and cover requirement)
Size and contour of defect.
Experience of the surgeon.
Non vascularized Bone Grafting
This is done for small defects of mandible with little or no loss of soft tissue. The bone graft is placed in a well vascularized bed. The adjacent bone fragments are stripped of periosteum so that adequate bone to bone contact is established. The common donor sites for non-vascularized bone grafts are the rib and the iliac crest. The rib can be used as whole rib or split rib graft. The whole rib graft is less successful because it gets revascularized very slowly owing to the absence of exposed cancellous bone. On the contrary split rib grafts provide large areas of exposed cancellous bone for its rapid revascularization. Two split rib grafts (joined to each other anteriorly and inserted into the rami posteriorly on each side—Frys technique) can be used to reconstruct the entire mandible [45].
The iliac crest is another favoured site for non-vascularized bone grafts as it provides good amount of cortical as well as cancellous bone and is easily accessible. It can be used to reconstruct the medium size defects as well as for reconstruction of entire ramus and posterior portion of the mandibular body. Because of the natural curvature the iliac crest can be sculpted to reconstruct the hemi mandible. Careful preoperative planning, designing of the template and selection of the side is required.
Iliac Bone
The harvested bone is primarily cancellous bone and is an excellent substrate for implantation due to its substantial height and thickness. The iliac bone can be contoured to fit most segmental mandibular defects. Opening osteotomies performed in the iliac bone allow reliable reconstruction of anterior mandibular defects.
Costochondral Rib
The costochondral graft is used predominantly for condylar reconstruction in children and adolescents. Poswillo [46] was the first surgeon to truly establish the physiologic compatibility of costochondral grafting for the TMJ. The rib graft also may be used in reconstructing mandibular segmental defects that do not involve the condyles in infants. The advantage of the costochondral graft over other reconstructive modalities is that it has the potential for continued growth. This advantage makes it a better graft than the fibula flap when used in growing patients. This benefit brings with it some uncertainties. The graft has been shown to grow at a different rate than the contralateral natural condyle, whereas others have shown no growth, excessive growth, and deficient growth [47–49]. In cases in which there has been deficient or no growth, evidence exists to the benefit of distraction osteogenesis of the graft. Although other autogenous donor sites, such as the sternoclavicular and metacarpal joints, have been used, the costochondral graft is the autogenous reconstruction material of choice for TMJ arthroplasty in the pediatric population. The costochondral rib graft is adaptable to the TMJ not only because of its native size and dimensions but also because its hyaline cartilaginous cap (as opposed to fibrocartilage) can withstand the biomechanical stresses of the TMJ and act as a new growth center.
A costochondral rib graft with a cartilaginous cap secured to the native mandible can be expected to grow spontaneously in a growing patient. The rate of reankylosis after costochondral rib grafting is most common in adult patients, especially individuals with multiple previous operations, because this patient population is prone to heterotopic bone formation [50]. One of the most unpredictable factors regarding costochondral bone grafting is the degree of growth. Although lack of growth and potential reankylosis are possible, overgrowth of the costochondral rib graft is the most common scenario after TMJ arthroplasty in growing patients [51].
Bone Graft Substitutes
Additional bone substitutes for mandibular reconstruction include a variety of autologous free-bone grafts, irradiated or cryopreserved mandible, and alloplastic materials. The overall success for these in immediate reconstruction has also been disappointing [52–54].
In 1944, Mowlem demonstrated the superior osteogenic potential of cancellous bone grafts (PBCM) [11]. However, sufficient global application of jawbone reconstruction in the clinical setting has not occurred. In 1964, Burwell [55] reported that the cells capable of bone formation are included in PCBM originated from undifferentiated mesenchymal cells. Rappaport [56] showed that the use of PBCM grafts afforded a higher rate of osteogenesis and lower rate of surgical complications as compared to cortical grafts. In 1969, Boyne [57] demonstrated the success of PBCM grafts using metal cribs. Cribs of titanium, vitallium, tantalum, chrome cobalt and stainless steel have since been used. In 1972, Leake and Rappoport [58] described the use of dacron-coated polyurethane trays in dogs for mandibular reconstruction and good healing was demonstrated. Cheung et al. [59] showed in a clinical study that the bone height at both ends and the middle of the reconstructed segment underwent even resorption, and 80 % of the bone height was retained over a 3-year period. Lawson et al. [25] noted a high failure rate for immediate reconstruction using titanium hollow screw reconstruction plate with particulate cancellous bone. The high failure rate was primarily attributed to the inability of the graft to withstand intraoral contamination. Higher success rates were noted when the reconstruction was delayed.
The advantages of PBCM grafts are the potential to create an anatomic mandibular reconstruction of adequate height, symmetrical arch form and width, and the ability to adequately support dental implants. The graft may be used to bridge mandibular defects of any length, and even the entire mandible, when used together with costochondral grafts for reconstruction of condyles. The disadvantages include remodeling resorption, and wound dehiscence and infection may still occur leading to loss of the graft. The latter is the main reason why PBCM grafts are not frequently used in patients with malignant tumors where the soft tissues are compromised or where radiation therapy has been or will be used. Complications may also arise from the containment system, such as tray exposure or foreign-body reactions to bone cribs, leading to subsequent infection [60].
Complications reported with the use of metal or Dacron trays are wound dehiscence, tray exposure and postoperative infection that can lead to partial or total loss of the graft. Interference of the tray with subsequent prosthetic or implant rehabilitation may require removal of the tray. Poly (l-lactide) (PLLA) or Poly (d, l lactide) (PDLLA) trays have the advantage of being biodegradable. Kinoshita et al. [61] showed good results in an animal study using particulate bone and cancellous marrow (PBCM) grafts with PLLA trays for mandibular reconstruction. Louis et al. [62] reported the use of a PDLLA mesh tray as a containment system for PBCM grafts in three patients for reconstruction of fractured atrophic edentulous mandibles with satisfactory results.
Currently, a variety of implantable materials are available to aid in mandibular reconstruction. One of the most commonly used is Hydro Set (Stryker, Kalamazoo, MI), a calcium phosphate cement which converts in situ to hydroxyapatite, serving as an effective osteoconductive and osteointegrative material [63]. Other implantable options include customized implants (75 % methyl methacrylate styrene copolymer, 15 % poly methyl methacrylate, and 10 % barium sulfate), Delta [poly (l) lactide, 10 % glycolide, and 5 % poly (d) lactide], and MedPor (high density porous polyethylene).
Tideman et al. [64, 65]. described a titanium mesh system for mandible reconstruction. A custom-made titanium tray was designed to match the segment of mandible to be resected and fixed to the residual segments. Autologous cancellous bone blocks were inserted into the tray to reconstruct the mandible in the desired position and shape. Warnke et al. [66] created an individually made titanium-mesh cage filled with xenogenic bone mineral, recombinant human bone morphogenetic protein (BMP) and fresh bone marrow.
The current disadvantages in mandibular reconstruction using PLLA mesh and PCBM include limited indication for certain patients and an increased risk of infection. This method is contraindicated in patients with an extensive bilateral defect, poor regional blood circulation, those of an advanced age with poor bone regenerative capacity or those who have received a full-dose irradiation. Infection can be prevented by: (1) dense closure of the oral wound; (2) strict and immediate initial fixation; (3) avoidance of simultaneous reconstruction of soft tissue and bone; and (4) strict patient selection.
The discovery of bone morphogenic protein (BMP), the key activator of bone induction, has been a crucial step in the development of synthetic bone grafts. BMPs are members of the transforming growth factor β superfamily [67] and induce pluripotent mesenchymal stem cells to differentiate into osteoblasts, stimulating new bone formation. Presently, these methods are still under investigation and have a limited role in the reconstruction following tumor removal. The first reported mandibular reconstruction using rhBMP in a human was reported by Moghadam et al. [68]. Over the 10 years since the first publication, only a few articles have been reported. Herford et al. [69] did clinical applications of rhBMP-2 in maxillofacial surgery. Clokie and Sandor [70] carried out reconstruction of 10 major mandibular defects using bioimplants containing BMP-7. Carter et al. [71], Glied and Kraut [72] and Herford and Cicciu [73] similarly used recombinant human bone morphogenetic protein type 2 for mandibular reconstruction.
Vascularized Free Flaps
Vascularized bone allows for healing independent of a compromised recipient bed. This is in contrast with nonvascularized bone, which heals by resorption of old bone and deposition of new bone, i.e., creeping substitution. Microvascular free flaps allow long-term reliability and stability along with the ability to osseointegrate in one primary stage. In addition, for anterior mandibular defects, no other reconstruction methods have the ability of vascularized bone flaps for providing a solid arch necessary to restore form and function. During the past decade, a variety of donor sites for vascular bone flaps and soft tissue have evolved. Ideally, the bone must provide enough length to bridge the defect with sufficient width and height to accommodate endosteal implants and withstand mastication. In addition, soft-tissue reconstruction is critical to the restoration of function. Thus, when the defect includes soft tissue, the flap must also provide adequate soft tissue to restore function.
Fibular Free Flap
The fibular free flap receives its blood supply from the peroneal artery via endosteal and periosteal branches. Excellent segmental periosteal blood supplies allow the fibula to be osteotomized as many times as necessary. Because it receives both a segmental and intraosseous blood supply, multiple osteotomies can be made without devascularizing the bone [15, 74, 75]. The bone length provided by the fibula is up to 25 cm, which is greater than any other donor site [76]. The location provides for an easy two-team approach. Osseointegration is controversial, although Frodel et al. [76] showed adequate bone stock in a cadaver study. In addition, Hayder has also shown this clinical success of osseointegration [76]. The major concern of this flap in the past has been an unreliable skin paddle with variable septocutaneous perforators. Incorporating a cuff of soleus muscle or dissecting the cutaneous perforators through the soleus muscle has eliminated most of these concerns [77]. In addition, Hayder has shown a cutaneous sensory nerve to the skin paddle, providing a sensate osteocutaneous flap [78]. The known advantages are an abundant supply of bicortical bone that is available for reconstruction of defects across the midline, eligibility for the subsequent insertion of dental implants, the opportunity for simultaneous dissection of the fibula while operating at the head, and little morbidity of the donor site.
Since this long bicortical bone provides excellent quality bone for osseointegration, it can be used for both lateral segment and anterior arch reconstruction [79].
The peroneal vessels, measuring 1.5–3.0 mm in diameter, offer favourable conditions for anastomosis to branches of both the external carotid artery and the jugular vein. The vascular pedicle measures approximately 4 cm in length, and will be short in comparison to other donor areas only if the full length of available bone is used [80].
Secondary morbidity of the donor site was remarkably low, which is one of the main advantages of the fibula as compared to other donor sites. If a distal portion of at least 7 cm of the fibula, including the tibio-fibular syndesmosis, is preserved, instability of the ankle joint does not occur [81]. In contrast to BROUGH, a flexion deficit of the hallux after resection of the flexor hallucis longus muscle has not been observed [82]. The pedicle for the fibular free flap is the peroneal artery, a branch off the tibio peroneal trunk. The peroneal artery courses with paired venae comitantes along the entire distance of the fibula; along its medial aspect. The fibula is nourished by both periosteal and endosteal blood supplies. It is this dual blood supply that permits multiple osteotomies and contouring for restoration of large defects including angle to angle defects. The vascular pedicle can be lengthened by harvesting a more distal segment of bone while performing a sub periosteal dissection of the soft tissue surrounding the proximal bone and discarding it [83].
The fibula is touted as “the most donateable bone in the body,” with up to 25-cm of bone available for harvest and adequate bone stock to support dental implantation. With such lengths of bone available, the entire mandible may be reconstructed with vascularized bone if required. Multiple osteotomies may be performed to shape the fibula to reconstruct the anterior arch, body, angle, or ramus of the mandible, as long as the fibular periosteum is not disrupted. The skin of the lower lateral leg is thin and pliable, with fairly large amounts of skin available, and may be transferred in a sensate fashion. With smaller skin paddles, the donor defect may be closed primarily. With larger defects, a split-thickness skin graft is utilized.
Confirmation of three-vessel flow to the distal lower extremity should be determined preoperatively to avoid vascular compromise of the foot after harvest of the peroneal artery. The primary disadvantage of the fibula flap is the limitations of the skin paddle. The skin is inadequate for larger soft tissue defects and most three-layer defects, requiring a second flap for soft tissue repair. If dental implants are not planned, use of the fibula bone results in a very broad and rounded neomandible, which is quite difficult to fit for a tissue-borne prosthesis. The donor site morbidity of the fibula flap involves prolonged pain on ambulation for some patients.
Radial Forearm Free Flap
The radial forearm fasciocutaneous flap has been widely used because it is thin and pliable and provides an abundant amount of skin. A portion of the underlying radius can be included, creating a composite flap. A total of 10–12 cm in length and up to 40 % of the circumference of the bone can be procured [84]. According to Urken et al., the thickness of the bone graft is limited to 40 % of the cross-sectional area of the radius, which is inadequate to support osseointegrated dental implants or to allow for multiple osteotomies. A cutaneous sensory nerve has been identified providing sensation to the entire skin paddle [79]. The radial forearm free flap (RFFF) has also been described as an osteocutaneous radial forearm free flap (OCRFFF), with harvest of a portion of the radius bone based on perforators in the intermuscular septum passing to the periosteum.
There are some significant disadvantages of this osteofasciocutaneous flap that have limited its use. A major disadvantage is the limited amount of bone length and width. There is not enough bone present for osseointegration or structural strength for mastication [37]. The bone has no natural curve; therefore, it requires multiple osteotomies to shape the mandible. In addition, pathological fractures of the remaining radius have been reported up to one-fourth of the patients. Grip, pinch, and range of motion were significantly reduced in the affected hand when fractures occurred [85].
The osteocutaneous radial forearm free flap (RFFF) is based on the radial artery, which runs a course between the flexor carpi radialis and brachioradialis muscles before it terminates in the deep palmar arch. The artery travels with its two paired venae comitantes and a more superficial cephalic vein [86]. The radius is vascularized because its periosteum is supplied through attachments with the intermuscular septum. The skin paddle is considered reliable, thin, and pliable enough to serve as an ideal replacement for intraoral lining. Additionally, the vascular pedicle is considered of excellent length and diameter for microvascular anastomosis. A superficial branch of the radial nerve lies lateral to the brachioradialis tendon distally. When elevating the radial forearm flap, it is imperative to identify and preserve the integrity of this nerve, which provides sensation to the lateral digits.
Despite some key advantages, the use of the forearm in mandibular reconstructive surgery is limited by poor bone quality and high donor site morbidity. Postharvest pathologic fracture rates of 23–42 % have been reported in the literature. However, this number can be decreased with prophylactic plating of the donor site [86]. The most dreaded complication, an ischemic hand, could be prevented by the use of a diligent preoperative evaluation. The Allen’s test remains the most accurate method of clinically evaluating blood flow to the digits.
The prophylactic internal fixation of the radius bone after OCRFFF harvest has been shown to successfully eliminate this risk. Despite the limitations of bone availability with the OCRFFF, it has been used successfully for oromandibular reconstruction, with fewer complications than those associated with the fasciocutaneous RFFF with plate reconstruction. For limited mandibular defects, the radius bone is quite adequate and can easily bear a tissue borne prosthesis (denture) [87]. This characteristic has proved beneficial, because many patients do not have the financial means for dental implantation, which is frequently not covered by many third-party payers. In this setting, the radius bone provides a better contour for the support of a tissue-borne prosthesis than either the fibula or scapula bone.
Scapula Free Flap
The scapula osteocutaneous free flap is attractive for reconstructing composite defects of the head and neck with large soft-tissue loss. Based on the subscapular artery, this system of flaps can include lateral scapula and overriding skin as well as latissimus dorsi and serratus anterior muscles. The subscapular artery terminates in the circumflex artery and the thoracodorsal artery. The terminal branches of the circumflex artery include the descendent branch and the transverse branch, which supply the scapular and the parascapular fasciocutaneous flaps, respectively. The thoracodorsal artery supplies the latissimus dorsi muscle and terminates in the angular artery, which supplies the tip of the scapula and a branch to the anterior serratus muscle [79, 88]. This vascular system allows one to harvest, in a single flap, a wide amount of muscle, soft tissue, and bone to reconstruct large three-dimensional defects. The pedicle length, which depends on how proximal the dissection is continued and the inclusion of the subscapular artery, ranges from 11 to 14 cm. In addition, when facial reanimation is desired, the latissimus dorsi muscle may be reinnervated. A major disadvantage of the flap is the difficulty in positioning the patient to allow for simultaneous resection and flap procurement. In addition, in some cases there is a limited cross-sectional area of bone, making osseointegration questionable. Finally, sensory reinnervation of this flap has not been described [89]. The free scapular/parascapular flap is based on the circumflex scapular artery (CSA) and paired venae. The CSA is one of two terminal branches of the subscapular system. After passing through a muscular triangular space formed by the teres minor, teres major, and long head of the triceps muscles, it divides into a descending branch and a transverse branch that supply the scapular flap and the parascapular flap, respectively. The CSA has periosteal branches, which supply the lateral aspect of the scapula and allows for the harvest of approximately 10–14 cm of bone as an osteocutaneous scapular flap. A composite flap that incorporates the scapular tip, supplied by the angular artery, can be harvested to reconstruct angle defects. Advantages to the scapular flap include the constant and easily dissected pedicle of good length and calibre, the ability to tolerate multiple osteotomies, and the large quantity of soft tissue that can be harvested. Another favourable characteristic of the subscapular system is the ability to harvest a unique composite flap composed of bone, muscular components, and multiple skin islands.
The scapula remains largely underutilized due to the location of the donor site. Patients require intraoperative repositioning for the harvest and inset, which prolongs operative time. Other drawbacks to the use of the scapular free flap for mandibular reconstruction is the quality of bone stock, which may be unsuitable for dental implants except in larger male patients [15, 89].
For extremely large defects, some surgeons have employed the scapular “megaflap,” which includes not only the scapular bone and extensive skin as described earlier but also the latissimus dorsi muscle or the serratus anterior muscle for additional bulk and coverage. The muscles are based on the thoracodorsal artery and vein that branch off the subscapular vessels, and therefore can be harvested on the same vascular pedicle, requiring only one arterial anastomosis and one venous anastomosis. The megaflap offers significant mobilization of the various tissue components relative to one another as a result of the branching vascular supply, providing great reconstructive versatility for the largest of defects [90].
Anteriolateral Thigh Flap
The ALT flap is a perforator flap that is based on the descending branch of the lateral circumflex femoral artery. It is currently one of the most favoured flaps for head and neck reconstruction in Asia and has recently gained popularity in the United States. The use of the ALT did not reach its current status as “the radial forearm’s big brother” until Chang Gung Memorial Hospital’s (Taipei, Taiwan) experience with 672 ALT flaps [91, 92]. The slow adoption of the ALT in the United States has been attributed to the difficulty of dissection, the variations of the vascular anatomy, and the thick thigh commonly found in Westerners [93, 94]. Despite these concerns, the ALT has continued to gain popularity as more microvascular surgeons have become familiar with the nuances of harvesting this flap. The advantages of the ALT flap are numerous. One of the great appeals of this flap is that it has low donor site morbidity and is at a site where it can be easily hidden. A large amount of skin territory can be harvested with ease of harvest, allowing a two-team approach. Another benefit of the ALT is its ability to withstand thinning. That ability allows the ALT to be “custom” fitted to the defect, not only in size but also in depth.
The main drawback of the ALT flap is the difficulty in elevation when the main perforator is of a musculocutaneous type. The boundaries of reconstructive surgery continue to be pushed. A testament to this is the publication by Wei and colleagues on the concept of “free style perforator flaps,” where Doppler ultrasonography is used to find a perforator in a suitable area, and a flap is outlined and raised down to the axial vessel [95]. This approach will be rendered even more commonplace with the advent of “super-microsurgery” [96]. These concepts make it a very exciting time to be a surgeon involved in the reconstruction of these most difficult head and neck defects.
The Pectoralis Major Myocutaneous Flap
The pectoralis major myocutaneous (PM) flap was first described for head and neck reconstruction in 1979 [97]. It quickly became the cornerstone technique for reconstruction of large defects of the lower third of the face and neck. Despite the increased contemporary use of versatile microvascular free flaps, the PM flap continues to play a useful role in the reconstruction of traumatic and ablative head and neck defects.
The ideal use of the PM flap is for the mandible, floor of mouth, upper neck, and lower one-third of the face. When defects are primarily mucosal or cutaneous, the bulk of the PM muscle and subcutaneous tissues can be problematic. A thinner or more delicate free flap should be considered in these instances. The bulk of muscle and subcutaneous tissue may be advantageous for large vessel coverage when a neck dissection or large resection is to be performed. If an osseous continuity defect is to be restored, a reconstruction bar is placed to maintain the native anatomy and prevent contracture. Definitive osseous reconstruction may be performed at a later date.
The pectoralis major muscle originates from the medial one-third of the clavicle, sternum and cartilage of the upper seven ribs, and the aponeurosis of the external oblique muscle. It inserts on the greater tubercle of the humerus. The blood supply is from the thoracoacromial artery, a branch of the second portion of the axillary artery, which enters the muscle on its deep surface at the junction of the middle and lateral one-third of the clavicle. Once harvested, the pectoralis flap is tunneled beneath the skin and over the clavicle to reconstruct external facial defects and intraoral lining. For oromandibular reconstruction, the pectoralis flap is usually wrapped around a reconstruction plate that has been placed to bridge a segmental defect.
The Metatarsus Osteocutaneous Flap
The metatarsus osteocutaneous flap is supplied by the dorsalis pedis artery and is based on the second metatarsal bone. It has been used to reconstruct anterior floor of mouth mandibular composite defects. The skin is thin and receives sensory input from the superficial peroneal nerve. The pliability of the skin is a major advantage of this flap.
Disadvantages of metatarsus include difficulty in flap elevation and limited amounts of bone and skin. The average length of the second metatarsal bone is 7–8 cm and only approximately 10 cm of skin can be procured. Atherosclerosis can narrow the vessels. The donor-site morbidity rate can be significant, including poor healing of the skin graft over the paratenons, repeated breakdowns from local trauma, and loss of sensation to the dorsum of the foot [98].
Iliac Crest Free Flap
The harvested bone is primarily cancellous bone and is an excellent substrate for implantation due to its substantial height and thickness. The iliac bone can be contoured to fit most segmental mandibular defects. The iliac crest was initially selected for use because of its similarity in shape to the hemimandible. Opening osteotomies performed in the iliac bone allow reliable reconstruction of anterior mandibular defects [99]. The hemi-mandible can be recreated from the ipsilateral ilium using the anterior superior iliac spine to restore the mandibular angle [100]. By including the ascending branch of the DCIA, the internal oblique muscle can be harvested and used for intraoral mucosal defect reconstruction. The internal oblique muscle is thin, pliable and can be maneuvered independent of the bone more easily and more reliably than the overlying skin flap [101]. The donor site morbidity is a primary concern related to the use of the iliac donor site. However, a critical appraisal of this donor site in patients who underwent harvest has not supported such claims [102]. The issues related to the donor site include the challenge of restoring the abdominal wall to prevent hernia formation, as well as the rehabilitation required to achieve normal ambulation. The large amount of bone and natural shape make the ilium a popular replacement for the resected mandible. The osteocutaneous flap is based on the deep circumflex iliac artery (DCIA).
A major advancement of this flap was the identification of the ascending branch of the DCIA as the dominant supply to the internal oblique muscle [103]. Limitations of this flap include the poor pliability of the overlying skin and the overall bulk of the flap that can add difficulty while in setting the flap [79].
Clavipectoral Osteomyocutaneous Free Flap
In 1996, Seikaly et al. [104] introduced the clavipectoral osteomyocutaneous free flap as an additional option for reconstruction of mandibular defects. An average of 16.1 cm (range of 12–20 cm) can be obtained with total clavicular harvest, whereas an average of 10.5 cm (range of 7–15 cm) can be harvested if the distal clavicle is left intact to minimize shoulder morbidity [104–106]. The clavicle has a normal contour that simulates that of the mandible and can be osteotomized because of its periosteal blood supply. It has sufficient width and height to support dental implants, and its biomechanics closely match that of the mandible [78, 107]. The clavicular head of the pectoralis major muscle and the overlying skin are ideal for reconstruction of composite mandibular defects. A mega flap can also be harvested by encompassing the territory of the pectoral artery and adding the sternal head of the pectoralis major muscle, overlying skin, and possibly ribs 4–6. The flap has the potential for sensory and motor reinnervation. The surgical anatomy is very familiar to the head and neck surgeon, and harvesting is amenable to a two-team approach.
There was minimal donor-site morbidity in our case series, even though all patients had full-thickness clavicular harvest. The clavicle serves mainly as a stabilizing force for the shoulder and a place for insertion of opposing muscles. It also transmits the force of the trapezius to the scapula through the thoracoacromial ligament. Clavicular resection has been reported in the literature for numerous conditions, such as thoracic outlet syndrome, tumours, and brachial plexus disorders.
Advantages associated with this flap include the availability of the donor site during tumor resection, surgical anatomy that is familiar to the head and neck surgeon, and minimal functional and cosmetic donor site morbidity. One disadvantage of this flap is the relatively short pedicle which may require the use of interposition grafts for both the arterial and venous anastomosis.
The clavipectoral flap has bone and soft tissue components that are especially suited for composite mandibular defects, but it should be used as a second-line flap owing to the short pedicle and the regular need for vein grafts.
Radiation Therapy and Osteoradionecrosis (ORN)
Radiation therapy can lead to both early- and late onset tissue reactions [108, 109]. The late reactions are typical radiation-induced fibrosis and bone demineralization, in conjunction with a diminished ability to resist infection. The irradiated osseous structure is more liable to infection because of diminished perfusion. Moreover, radiation causes endarteritis, resulting in tissue hypoxia, hypocellularity, and hypovascularity, which cause tissue collapse, resulting in chronic nonhealing wounds (Marx [110]). ORN has been described as bone exposure within a field of radiation that has not healed with conservative therapy after a 3-month period (Alam et al. [111]).
In an effort to improve success rates in patients treated with radiation, various vascularized osseous flaps have been used to improve the vascularity of the recipient bed in preparation for the subsequent bone graft (Dufresne et al. [112]). Hirsch et al. [113] compared the outcome and complications between patients undergoing vascularized osseous flap reconstruction for ORN and similarly reconstructed patients who received radiation therapy but did not develop ORN, as well as with unradiated controls. In their study the overall flap survival was 88 %, and did not differ significantly between ORN (86 %), no ORN (87 %), and controls (90 %); the complication rates also did not differ between the three groups. These results suggest that free flap transfers using the fibula, iliac crest, and scapula are viable options for advanced mandibular ORN.
Recent Advances
Medical modelling is a new tool for the reconstructive surgeon and has many applications for mandibular reconstruction. Although it is not necessarily cost-effective or required for all cases, it is extremely helpful in cases with primary bone malignancies as well as cases with involvement of the outer table of the mandible which makes it impossible to perform direct plate contouring prior to resection. Technological advances in medical imaging and rapid prototyping allows for the production of three-dimensional models. In cases where the mandible has been previously resected or destroyed by osteoradionecrosis, a digitally created “virtual” mandibular arch based on mirroring or a CT dataset with an appropriate occlusal relationship to the maxilla permits the reconstructive surgeon to contour a plate preoperatively or intra-operatively that will provide the patient with optimal post-operative occlusion. Three-dimensional modelling of the bone graft can also produce templates for contouring osteotomies, which saves the surgeon time and maximizes bone to bone contact to promote a strong bony union [114].
Transport Disc Distraction Osteogenesis (TDDO)
For mandibular reconstruction, a technique known as transport disc distraction osteogenesis (TDDO) is used. A segment of bone is cut adjacent to the defect and moved gradually across the defect by a mechanical device. New bone fills in between the two bone segments. The piece of bone being moved or transported is referred to as the transport disc. Over several years, Costantino et al. [115] made a substantial contribution to the clinical application of TDDO for mandibular reconstruction. In 1995, Constantino et al. successfully applied transport distraction to restore the continuity of a mandibular defect formed as a result of cancer resection following radiation therapy in a patient. External devices were employed in early cases but these caused problems of facial scarring along the pin tracks [116]. To overcome this problem, an internal plate-guided distraction device was described by Herford [117].
Gonzalez-Garcıa et al. described its usefulness in treating patients who were unsuitable for more aggressive surgery or undergoing prolonged surgical time because of poor general health or for patients in whom primary treatment using a vascularized free-osseous flap had failed. TDDO has also been reported to provide sufficient bone to allow dental implant placement, an important functional outcome [118].
Transport distraction for reconstruction of continuity defects is most efficient for defects of the mandibular body. When used to reconstruct a defect of the body of the mandible, the transported segment not only achieves bone continuity but also, through histiogenesis, the associated attached tissue is reconstructed achieving a natural ridge with a vestibule. Transport distraction is limited to reconstruction of relatively straight line defects as seen with defects of the mandibular body. This is because the connective tissue stroma which regenerate dictates the shape of the reconstructed tissue. If attempts are made to move a transport disc around a curve, the regenerate forms a straight segment between the point of transport origin and completion. Thus, if it is necessary to reconstruct a defect of the symphysis, the best plan is to create transport discs from the right and left posterior stumps of the mandible and move them toward the symphysis. The residual defect in the symphysis requires a bone graft. An alternative plan is to transport right and left transport discs forward from the body with a vector that lets them consolidate in the midline and then follow that procedure with a second midline osteotomy and application of a midline distracter to widen the symphysis.
Modular Endoprosthesis
Over the past decade, modular endoprosthetic reconstruction has become a routine method in limb sparing surgery (Malawer and Chou [119]). This technique emphasises the removal of all diseased bone, followed by replacement with an artificial device fixed within the remaining bone using bone cement. A modular system combines standardised units to allow flexibility in the reconstruction of various defect sizes. Endoprostheses anchored with bone cement has proven to achieve long term stability with good function in long bones, with 15-year survival rates of 94 % reported [120]. When applying this reconstructive method to the mandible, however, several unique challenges had to be addressed. The mandible is a curved bone with a flat, tear-drop-shaped cross section unlike the long bone which is straight with a concentric cross-section. A mandibular modular endoprosthesis has been described as a novel method for alloplastic mandible reconstruction with replacement of the mandibular body and the ascending ramus/condyle unit [121]. Endoprosthesis is a metallic device fixed into the medullary space of the mandibular stump after resection. There is no need for screw fixation. The variable length of the bone gap can be bridged by using modules that allow for accurate three-dimensional reconstructions. The modules are connected by a locking system. Initial designs had stems cemented into the medullary space with bone cements. Animal studies have been published detailing the results of replacements of the mandibular body and ramus/condyle. Tideman and Lee [122] recently introduced the concept of a modular endoprosthesis for mandibular reconstruction in a study on Macaca monkeys. The principle has been known in the orthopaedic community for almost 10 years. In principle, the mandible would qualify for such an endoprosthesis because of the existing medullary space. Animal studies have already been published detailing the results of mandibular body and also ramus/condyle replacements (Lee et al. [122]; Goh et al. [123]). These studies revealed that replacement of the mandibular body encountered persistent problems with loosening of the module connections, infection and loss of peri-implant bone mineral density whereas uneventful healing was experienced with the ramus/condyle replacement. It was apparent that the endoprosthesis design for mandibular body replacement was unable to withstand the stresses that developed with mastication, leading to failure [124].
Occlusal rehabilitation may be achieved on implants that are screwed into existing holes of the endoprosthesis. Whether this system is applicable to patients with compromised soft tissues remains to be determined, but the principle is worthy of further research.
Tissue-Engineering
Tissue engineering is an interdisciplinary field that combines the principles of engineering, material and biological sciences toward the development of therapeutic strategies and biological substitutes that restore, maintain, replace or improve biological functions. Growth factors and smad proteins, such as bone morphogenic proteins, and platelet rich plasma osteoprogenitor cells produced on a recipient bed can induce adequate regeneration. The technique of tissue engineering makes it possible to regenerate functional tissue by autologous cells from patients. In benign situations there is a choice of therapeutical options including free bone grafts and tissue engineering techniques. However, for malignancies and especially for irradiated patients with large mandibular defects the clinical options are almost exclusively limited to microvascular bone flaps. However, reconstruction of the mandible with free bone flaps has two limitations. (1) The shape of the bone does not match exactly the requirements. (2) Limited availability/morbidity of donor sites.
Tissue engineering can offer solutions. (1) The shape can be controlled by customised scaffolds on the basis of CAD/CAM and radiographic imaging data. (2) Bone can be grown in muscular environments and then be harvested with reduced morbidity. An ideal site for such a bone flap prefabrication is the latissimus dorsi muscle.
Gronthos reported the reconstruction of a human mandible by a bone–muscle-flap in vivo prefabrication technique in a 56-year-old patient [125]. A titanium mesh was chosen for the external scaffold, and loaded with HA blocks coated with rhBMP-7 and BMSC. The patient served as his own bioreactor as the scaffold was implanted into his latissimus dorsi muscle to allow for growth of heterotopic bone and ingrowth of vessels from the thoracodorsal artery. After 7 weeks the mandible replacement was transplanted, along with the adjacent vessel pedicle, into the mandibular defect. The vessel pedicle was anastomosed onto the external carotid artery and the cephalic vein by microsurgery. Within 4 weeks the patient regained the masticatory function, allowing him to enjoy solid meals [126]. In this study, Gronthos used both rhBMP-7 and whole bone marrow to maximize bone induction. Therefore, they could not conclude whether regeneration of bone tissue was attributable mostly to the bone-marrow cells or BMP7 or both. However the same group demonstrated, in a minipig model, the engineering of individual human-size mandible replacements following bone induction by rhBMP-7 [127]. Heliotis et al. [128] described the generation in a patient of a vascularized pedicled-bone flap useful for reconstruction of a hemi-mandible; the flap was obtained after intramuscular implantation of a HA/rhBMP-7 composite without any addition of harvested bone, bone marrow, or stem cells. In tissue engineering principle, engineering a graft at the site of the defect would be more of an advantage to other methods, but would require a period of healing in which the mandible should be immobilized. Apart from the technical problems there is the question of unknown effects on various tissue cells and even plain oncogenic effects. This is one of the main reasons why morphogenetic growth factors are not freely available yet. Autogenous growth factors, like those present in platelets, are mainly mitogenetic and are not known to be oncogenetic, at least not for epithelial cells.
Dental Rehabilitation
Dental implants play an important role in the rehabilitation of masticatory function, allowing the fixation of prosthetics and protecting the existing bone by providing an approximation of physiologic bone healing (Boyne et al. [129]). Blake et al. [130] studied the susceptibility of implants to inflammation following autogenous bone transplantation and evaluated the association between the soft-tissue response and different types of autogenous bone grafting. They reported that the rate of peri-implant inflammation varied between 9 and 38 % depending on the type of reconstruction. Rates of 16.3–24.1 % were seen for mucositis, while 30–70.9 % of sites exhibited no inflammation. There were high rates of soft-tissue inflammation adjacent to implants in autogenously transplanted bone, and it was found that the choice of donor site and the mode of transplantation together appeared to influence the development of periimplant inflammation.
The application of primary implants prior to radiotherapy has been advocated by Schoen et al. and Schepers et al. Advantages include implant healing before radiation, reducing the risk of radiation-induced complications such as osteoradionecrosis and avoidance of adjunctive hyperbaric oxygen therapy. Rohner et al. [131] described a technique using prefabricated osseous free tissue transfer. In the first stage, implants are placed in the donor bone in a preplanned position and lined with a split-thickness skin graft. After 6 weeks, a second-stage procedure is carried out for flap transfer and reconstruction of the mandible using an implant-mounted dental prosthesis as a template for occlusion. Immediate functional loading can be achieved with this technique.
Implant placement is done in two stages: fixture placement followed by exposure of the implant and placement of the trans mucosal attachment. Following placement, the implant is allowed to integrate for 4 months in the mandible and 6 months for maxillary implants. The trans-mucosal attachment is then placed and 2 weeks later the denture is attached and load bearing follows. Vascularized bone flaps for mandibular reconstruction have facilitated the use of primary implant placement. Advantages of implanting at the time of the primary reconstruction include having optimal bone exposure in the primary setting, reduced time to dental rehabilitation and avoidance of hyperbaric oxygen therapy if radiation therapy is planned [132].
Furthermore, the microsurgically reanastomosed fibula seemed to be most resistant to the inflammatory process, followed by reanastomosed iliac crest, free iliac crest, and free fibula. Thus, the microsurgically reanastomosed fibula appears to be the best suited for maxillofacial reconstruction in terms of the long-term incidence of peri-implantitis, followed by the microsurgically reanastomosed iliac crest flap. The scapula bone stock, which relies on the lateral edge of the scapula, is often too thin for use in implants (Mücke et al. [133]). In the case of oral rehabilitation with dental implants in irradiated free transferred tissue, there is no reliable consensus regarding the effects of the level of irradiation (Garrett et al. [134]). However, Raoul et al. [135] suggested that implant placement should be avoided in areas that have received radiotherapy doses of more than 50 Gy. Alam et al. also reported that the risk of developing ORN is significantly higher in patients receiving total radiotherapy doses exceeding 50. Raoul et al. reported that implant loss occurred only in 1 irradiated vascularized fibula flap among a total of 18 implants placed in 6 patients, representing a success rate of 94 %. Salinas et al. [136] reported a 72.5 % osseointegration rate for 51 implants placed in 22 patients. They found that the radiation dose had statistically significant effect on implant success.
Foster et al. [137] reported an implant success rate of 99 % in vascularized bone flaps versus 82 % in non-vascularized bone grafts over a mean follow-up period of 3 years. There is no significant difference in implant loss rates in irradiated versus nonirradiated free bone flaps [138–140]. Implants may be placed either at the time of ablative and reconstructive surgery (primary implants) or at a later date (secondary implants) [141].
Conclusion
Although there are many options for mandibular reconstruction, vascularized bone flaps are unique in that they permit reconstruction of the oromandibular complex even though the recipient bed is often compromised by salivary contamination and prior irradiation. In contrast to nonvascularized bone grafts, vascularized bone grafts remain capable of healing to the adjacent native mandible and eventually withstand the loading forces associated with mastication. The fibular, iliac, and scapular donor sites all provide bone stock sufficient for dental implants in the majority of patients, which has been demonstrated as an essential factor for full oral rehabilitation. Furthermore, the soft tissue components harvested with each of these three composite flap donor sites provide a source of tissue for either intraoral lining or extraoral coverage. Inherent differences with regard to bone stock, soft tissue quality, potential for sensory reinnervation, and pedicle geometry dictate which of the available donor sites will provide the optimal source for reconstruction. While the fibula osteocutaneous flap, is the most often choice of flap, allows for osteotomy and placement of dental implants, there are various modifications to improve the oral sphincter, reduce postoperative wound infection, and reduce donor site morbidity. The improvements include pedicled myoosseous flap with free skin flap, double free flaps with the tensor fascia lata for composite oromandibular defect reconstruction, and the fibula flap with a segmental soleus muscle for augmentation of submandibular dead space.
Finally, in terms of dental rehabilitation associated with mandibular reconstruction, it is important to remember the significance of tongue function in mastication; surgeons may consider using free flaps to replace missing portion of the anterior tongue if indicated.
Contributor Information
Batchu Pavan Kumar, Phone: +918682272255, Email: pavankumarbatchu@yahoo.co.in.
V. Venkatesh, Phone: +918682272255
K. A. Jeevan Kumar, Phone: +918682272255, Email: jeevan1983@yahoo.com.
B. Yashwanth Yadav, Email: dryash2204@gmail.com.
S. Ram Mohan, Email: rammohan.shagore@gmail.com.
References
- 1.Ivy RH. Bone grafting for restoration of defects of the mandible. Plast Reconstr Surg (1946) 1951;7(4):333–341. doi: 10.1097/00006534-195107040-00009. [DOI] [PubMed] [Google Scholar]
- 2.Tessier P, Kawamoto H, Matthews D, Posnick J, Raulo Y, Tulasne JF, Wolfe SA. Taking long rib grafts for facial reconstruction: tools and techniques—III. A 2900-case experience in maxillofacial and craniofacial surgery. Plast Reconstr Surg. 2005;116(5 Suppl):38S–46S. doi: 10.1097/01.prs.0000173906.35257.e0. [DOI] [PubMed] [Google Scholar]
- 3.Testelin S. History of microsurgical reconstruction of the mandible. Ann Chir Plast Esthet. 1992;37(3):241–245. [PubMed] [Google Scholar]
- 4.de Fries HO. Reconstruction of the mandible: use of combined homologous mandible and autologous bone. Otolaryngol Head Neck Surg. 1981;89(4):694–697. doi: 10.1177/019459988108900433. [DOI] [PubMed] [Google Scholar]
- 5.White S. The employment of silver wire to bridge the gap after resection of a portion of the lower jaw. Br Med J. 1909;2(2552):1525. doi: 10.1136/bmj.2.2552.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowlicht RA, Delacure MD, Sasaki CT. Allogeneic (homograft) reconstruction of the mandible. Laryngoscope. 1990;100(8):837–843. doi: 10.1288/00005537-199008000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Attie JN, Catania A, Ripstein CB. Stainless steel mesh prosthesis for immediate replacement of hemimandibulectomy. Surgery. 1953;33:712. [PubMed] [Google Scholar]
- 8.Blocker TC, Stout RA. Mandibular reconstruction. World War II. Plast Reconstr surg. 1949;4:153. doi: 10.1097/00006534-194903000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Castermans A, Van Garse R, Vanwick R. Primary resection of mandible after resection for oral cancer. Acta Chir Belg. 1977;2:205–208. [PubMed] [Google Scholar]
- 10.Bowerman JE. A review of reconstruction of the mandible. Proc R Soc Med. 1974;67(7):610–614. [PMC free article] [PubMed] [Google Scholar]
- 11.Albert TW, Smith JD, Everts E, Cook TA. Dacron mesh tray and cancellous bone in reconstruction of mandibular defects. Arch Otolaryngol Head Neck Surg. 1980;8:78–83. doi: 10.1001/archotol.1986.03780010055010. [DOI] [PubMed] [Google Scholar]
- 12.Wersäll J, Bergstedt H, Körlof B, Lind MG (1984) Split-rib graft for reconstruction of the mandible. Otolaryngol Head Neck Surg 92:270–275 [PubMed]
- 13.Taylor GI, Townsend P, Corlett R. Superiority of the deep circumflex iliac vessels as the supply for free groin flaps. Experimental work. Plast Reconstr Surg. 1979;64(5):595–604. doi: 10.1097/00006534-197964050-00001. [DOI] [PubMed] [Google Scholar]
- 14.Swartz WM, Banis Newton JCED. The osteocutaneous scapular flap for mandibular and maxillary reconstruction. Plast Reconstr Surg. 1986;77(4):530–545. doi: 10.1097/00006534-198604000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84(1):71–79. doi: 10.1097/00006534-198907000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Bradley PF. A two-stage procedure for reimplantation of autogenous freeze-treated mandibular bone. J Oral Maxillofac Surg. 1982;40:278. doi: 10.1016/0278-2391(82)90218-X. [DOI] [PubMed] [Google Scholar]
- 17.Dong YJ, Zhang GZ, Wang SP, Li Z. The use of immediate frozen autogenous mandible, for benign tumour mandibular reconstruction. Br J Oral Maxillofac Surg. 1996;34(1):58–61. doi: 10.1016/S0266-4356(96)90137-0. [DOI] [PubMed] [Google Scholar]
- 18.Kuo YR, Shih HS, Chen CC, et al. Free fibula osteocutaneous flap with soleus muscle as a chimeric flap for reconstructing mandibular segmental defect after oral cancer ablation. Ann Plast Surg. 2010;64(6):738–742. doi: 10.1097/SAP.0b013e3181a72f62. [DOI] [PubMed] [Google Scholar]
- 19.Bak M, Jacobson AS, Buchbinder D, Urken ML. Contemporary reconstruction of the mandible. Oral Oncol. 2010;46(2):71–76. doi: 10.1016/j.oraloncology.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Lin PY, Lin KC, Jeng SF. Oromandibular reconstruction: the history, operative options and strategies, and our experience. ISRN Surg. 2011;2011:824251. doi: 10.5402/2011/824251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misch CE, Qu ZM, Bidez MW. Mechanical properties of trabecular bone in the human mandible: implications for dental implant treatment planning and surgical placement. J Oral Maxillofac Surg. 1999;57:700–706. doi: 10.1016/S0278-2391(99)90437-8. [DOI] [PubMed] [Google Scholar]
- 22.Yi WJ, Heo MS, Lee SS, Choi SC, Huh KH, Lee SP. Direct measurement of trabecular bone anisotropy using directional fractal dimension and principal axes of inertia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:10–116. doi: 10.1016/j.tripleo.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Jewer DD, Boyd JB, Manktelow RT, Zuker RM, Rosen IB, Gullane PJ et al (1989) Orofacial and mandibular reconstruction with the iliac crest free flap: a review of 60 cases and a new method of classification. Plast Reconstr Surg 84:391–405 [PubMed]
- 24.Maurer P, Eckert AW, Kriwalsky MS, Schubert J (2010) Scope and limitations of methods of mandibular reconstruction: a long-term follow-up. Br J Oral Maxillofac Surg 48:100–104 [DOI] [PubMed]
- 25.Schusterman MA, Harris SW, Raymond AK, Goepfert H. Immediate free flap mandibular reconstruction: significance of adequate surgical margins. Head Neck. 1993;15:204. doi: 10.1002/hed.2880150305. [DOI] [PubMed] [Google Scholar]
- 26.Lawson W, Loscalzo LJ, Baek SM, et al. Experience with immediate and delayed mandibular reconstruction. Laryngoscope. 1982;92:5. doi: 10.1288/00005537-198201000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Marx RE, Ames JR. The use of hyperbaric oxygen therapy in bony reconstruction of the irradiated and tissue deficient patient. J Oral Maxillofac Surg. 1982;40:410. doi: 10.1016/0278-2391(82)90076-3. [DOI] [PubMed] [Google Scholar]
- 28.Marx RE. Mandibular reconstruction. J Oral Maxillofac Surg. 1993;51:466. doi: 10.1016/S0278-2391(10)80501-4. [DOI] [PubMed] [Google Scholar]
- 29.Carlson ER, Monteleone K. An analysis of inadvertent perforations of mucosa and skin concurrent with mandibular reconstruction. J Oral Maxillofac Surg. 2004;62:1103. doi: 10.1016/j.joms.2004.05.114. [DOI] [PubMed] [Google Scholar]
- 30.Cordeiro PG, Hidalgo DA. Conceptual considerations in mandibular reconstruction. Clin Plast Surg. 1995;22:61. [PubMed] [Google Scholar]
- 31.Baker A, McMahon J, Parmar S. Immediate reconstruction of continuity defects of the mandible after tumor surgery. J Oral Maxillofac Surg. 2001;59:1333. doi: 10.1053/joms.2001.27825. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Zhu K, Liu F, Li H. Assessment of quality of life in giant ameloblastoma adolescent patients who have had mandible defects reconstructed with a free fibula flap. World J Surg Oncol. 2014;12:201. doi: 10.1186/1477-7819-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netscher DT, Meade RA, Goodman CM, et al. Quality of life and disease specific functional status following microvascular reconstruction for advanced (T3 and T4) oropharyngeal cancers. Plast Reconstr Surg. 2000;105:1628. doi: 10.1097/00006534-200004050-00005. [DOI] [PubMed] [Google Scholar]
- 34.Weymuller EA, Yueh B, Deleyiannis FWB, et al. Quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2000;126:329. doi: 10.1001/archotol.126.3.329. [DOI] [PubMed] [Google Scholar]
- 35.Boyd JB, Mullholland RS, Davidson J, et al. The free flap and plate in oromandibular reconstruction: long-term review and indications. Plast Reconstr Surg. 1995;95:1018. doi: 10.1097/00006534-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Havlik RJ (1996) Reconstruction of the pediatric mandible. Oper Tech Plastic Reconstr Surg 3(4):272–288. https://www.infona.pl/resource/bwmeta1.element.elsevier-22f19fc2-1ab2-3f4b-8a75-4ed01a222baf
- 37.Gullane PJ, Holmes H. Mandibular reconstruction new concept. Arch Otolaryngol Head Neck Surg. 1976;112:714–719. doi: 10.1001/archotol.1986.03780070026006. [DOI] [PubMed] [Google Scholar]
- 38.Urken ML. Composite free flaps in oro-mandibular reconstruction. Arch Otolaryngol Head Neck Surg. 1991;117:724–732. doi: 10.1001/archotol.1991.01870190036009. [DOI] [PubMed] [Google Scholar]
- 39.Komisar A. The functional result of mandibular reconstruction. Laryngoscope. 1990;100:364–374. doi: 10.1288/00005537-199004000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Peled M, El-Naaj IA, Lipin Y, Ardekian L. The use of free fibular flap for functional mandibular reconstruction. J Oral Maxillofac Surg. 2005;63:220–224. doi: 10.1016/j.joms.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 41.Yim KK, Wei FC. Fibula osteoseptocutaneous flap for mandible reconstruction. Microsurgery. 1994;15(4):245–249. doi: 10.1002/micr.1920150405. [DOI] [PubMed] [Google Scholar]
- 42.Haughey BH, Fredrickson JM, Lerrick AJ, et al. Fibular and iliac crest osteomuscular free flap reconstruction of the oral cavity. Laryngoscope. 1994;104:1305. doi: 10.1288/00005537-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Vuillemin T, Raveh J, Sutter F. Mandibular reconstruction with the titanium hollow screw reconstruction plate (THORP) system: evaluation of 62 cases. Plast Reconstr Surg. 1998;82:804. doi: 10.1097/00006534-198811000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Schusterman MA, Reece KP, Kroll SS, et al. Use of the A–O plate for mandibular reconstruction in cancer patients. Plast Reconstr Surg. 1991;88:588–593. doi: 10.1097/00006534-199110000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Chow J, Hill J. Primary mandibular reconstruction using the A–O reconstruction plate. Laryngoscope. 1986;96:768–773. doi: 10.1288/00005537-198607000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Poswillo D. Experimental reconstruction of the mandibular joint. Int J Oral Surg. 1974;3:400. doi: 10.1016/S0300-9785(74)80005-0. [DOI] [PubMed] [Google Scholar]
- 47.Guyuron B, Lasa C. Unpredictable growth pattern of costochondral graft. Plast Reconstr Surg. 1992;90:880–886. doi: 10.1097/00006534-199211000-00024. [DOI] [PubMed] [Google Scholar]
- 48.Medra AM. Follow up of mandibular costochondral grafts after release of ankylosis of the temporomandibular joints. Br J Oral Maxillofac Surg. 2005;43(2):118–122. doi: 10.1016/j.bjoms.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Lata J, Kapila BK. Overgrowth of a costochondral graft in temporomandibular joint reconstructive surgery: an uncommon complication. Quintessence Int. 2000;31(6):412–414. [PubMed] [Google Scholar]
- 50.Fernandes R, Fattahi T, Steinberg B. Costochondral rib grafts in mandibular reconstruction. Atlas Oral Maxillofac Surg Clin North Am. 2006;14(2):179–183. doi: 10.1016/j.cxom.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Caccamese JF, Ruiz RL, Costello BJ. Costochondral rib grafting. Atlas Oral Maxillofac Surg Clin North Am. 2005;13:139–149. doi: 10.1016/j.cxom.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Hamaker RC. Irradiated autogenous mandibular grafts in primary reconstructions. Laryngoscope. 1981;91:1031–1051. doi: 10.1288/00005537-198107000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Leipzig B, Cummings CW. The current status of mandibular reconstruction using autogenous frozen mandibular grafts. Head Neck Surg. 1984;8:992–998. doi: 10.1002/hed.2890060603. [DOI] [PubMed] [Google Scholar]
- 54.Schmoper RR. Mandibular reconstruction using a special plate: animal experiments and clinical appearance. J Maxillofac Surg. 1983;11:99–108. doi: 10.1016/S0301-0503(83)80025-3. [DOI] [PubMed] [Google Scholar]
- 55.Burwell RG. Studies in transplantation of bone. VII. The fresh composite homograft-autograft of cancellous bone; an analysis of factors leading to osteogenesis in marrow transplants and in marrow-containing bone grafts. J Bone Joint Surg Br. 1964;46:110–140. [PubMed] [Google Scholar]
- 56.Rappaport I, Boyne PV, Nethery J. The particulate graft in tumor surgery. Am J Surg. 1971;122(6):748–755. doi: 10.1016/0002-9610(71)90439-9. [DOI] [PubMed] [Google Scholar]
- 57.Boyne PJ. Restoration of osseous defects in maxillofacial casualities. J Am Dent Assoc. 1969;78(4):767–776. doi: 10.1016/S0002-8177(69)84023-7. [DOI] [PubMed] [Google Scholar]
- 58.Leake DL, Rappoport M. Mandibular reconstruction: bone induction in an alloplastic tray. Surgery. 1972;72(2):332–336. [PubMed] [Google Scholar]
- 59.Cheung LK, Samman N, Tong AC, Tideman H. Mandibular reconstruction with the Dacron urethane tray: a radiologic assessment of bone remodeling. J Oral Maxillofac Surg. 1994;52(4):373–380. doi: 10.1016/0278-2391(94)90440-5. [DOI] [PubMed] [Google Scholar]
- 60.Cheung LK, Samman N, Chow TW, Clark RKF, Tideman H. A bone graft condensing syringe system for maxillofacial reconstructive surgery. Br J Oral Maxillofac Surg. 1997;35:267–270. doi: 10.1016/S0266-4356(97)90045-0. [DOI] [PubMed] [Google Scholar]
- 61.Kinoshita Y, Kobayashi M, Hidaka T, Ikada Y. Reconstruction of mandibular continuity defects in dogs using poly (l-lactide) mesh and autogenic particulate cancellous bone and marrow: preliminary report. J Oral Maxillofac Surg. 1997;55(7):718–723. doi: 10.1016/S0278-2391(97)90585-1. [DOI] [PubMed] [Google Scholar]
- 62.Louis P, Holmes J, Fernandes R. Resorbable mesh as a containment system in reconstruction of the atrophic mandible fracture. J Oral Maxillofac Surg. 2004;62(6):719–723. doi: 10.1016/j.joms.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 63.Takagi S, Chow LC, Markovic M, Friedman CD, Costantino PD. Morphological and phase characterizations of retrieved calcium phosphate cement implants. J Biomed Mater Res. 2001;58(1):36–41. doi: 10.1002/1097-4636(2001)58:1<36::AID-JBM50>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 64.Samman N, Cheung LK, Tideman H. Functional reconstruction of the jaws: new concepts. Ann R Australas Coll Dent Surg. 1996;13:184–192. [PubMed] [Google Scholar]
- 65.Tideman H, Samman N, Cheung LK. Functional reconstruction of the mandible: a modified titanium mesh system. Int J Oral Maxillofac Surg. 1998;27(5):339–345. doi: 10.1016/S0901-5027(98)80061-1. [DOI] [PubMed] [Google Scholar]
- 66.Warnke PH, Springer IN, Wiltfang J, Acil Y, Eufinger H, Wehmöller M, Russo PA, Bolte H, Sherry E, Behrens E, Terheyden H. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364(9436):766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 67.Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, Wozney JM. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci USA. 1990;87(24):9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moghadam HG, Urist MR, Sandor GK, Clokie CM. Successful mandibular reconstruction using a BMP bioimplant. J Craniofac Surg. 2001;12(2):119–127. doi: 10.1097/00001665-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Herford AS, Boyne PJ. Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2) J Oral Maxillofac Surg. 2008;66(4):616–624. doi: 10.1016/j.joms.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 70.Clokie CM, Sándor GK. Reconstruction of 10 major mandibular defects using bioimplants containing BMP-7. J Can Dent Assoc. 2008;74(1):67–72. [PubMed] [Google Scholar]
- 71.Carter TG, Brar PS, Tolas A, Beirne OR. Off-label use of recombinant human bone morphogenetic protein-2 (rhBMP-2) for reconstruction of mandibular bone defects in humans. J Oral Maxillofac Surg. 2008;66(7):1417–1425. doi: 10.1016/j.joms.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 72.Glied AN, Kraut RA. Off-label use of rhBMP-2 for reconstruction of critical-sized mandibular defects. N Y State Dent J. 2010;76(4):32–35. [PubMed] [Google Scholar]
- 73.Herford AS, Cicciù M. Recombinant human bone morphogenetic protein type 2 jaw reconstruction in patients affected by giant cell tumor. J Craniofac Surg. 2010;21(6):1970–1975. doi: 10.1097/SCS.0b013e3181f502fa. [DOI] [PubMed] [Google Scholar]
- 74.Hidalgo DA. Aesthetic improvements in free-flap mandible reconstruction. Plast Reconstr Surg. 1991;88(4):574–585. doi: 10.1097/00006534-199110000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Hidalgo DA, Rekow A. Review of 60 consecutive fibula free flap mandible reconstructions. Plast Reconstr Surg. 1995;96:585. doi: 10.1097/00006534-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 76.Frodel JL, Jr, Funk GF, Cappis DT, et al. Osseointegrated implants: a comparative study of bone thickness in four vascularized bone flaps. Plast Reconstr Surg. 1993;92:449–455. doi: 10.1097/00006534-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 77.Kuriloff DB, Sullivan MJ. Mandibular re-construction using vascularized bone graft. Otolaryngol Clin North Am. 1991;24:1391–1418. [PubMed] [Google Scholar]
- 78.O’Leary MJ, Martin PJ, Hayden RE. The neurocutaneous free fibula flap in mandibular reconstruction. Otolaryngol Clin North Am. 1994;27(6):1081–1096. [PubMed] [Google Scholar]
- 79.Chim H, Salgado CJ, Mardini S, Chen HC. Reconstruction of mandibular defects. Semin Plast Surg. 2010;24(2):188–197. doi: 10.1055/s-0030-1255336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilbert A. Free vascularized bone grafts. Int Surg. 1981;66(1):27–31. [PubMed] [Google Scholar]
- 81.Wol KD, Ervens J, Herzog K, Hoefmister B. Experience with the osteocutaneous fibula flap: an analysis of 24 consecutive reconstructions of composite mandibular defects. J Cranio-Maxillofac Surg. 1996;24:330–338. doi: 10.1016/S1010-5182(96)80033-3. [DOI] [PubMed] [Google Scholar]
- 82.Goodacre TE, Walker CJ, Jawad AS, Jackson AM, Brough MD. Donor site morbidity following osteocutaneous free fibula transfer. Br J Plast Surg. 1990;43(4):410–412. doi: 10.1016/0007-1226(90)90004-J. [DOI] [PubMed] [Google Scholar]
- 83.Thoma A, Levis C, Young JE (2005) Oromandibular reconstruction after cancer resection. Clin Plastic Surg 32(3): 361–375 [DOI] [PubMed]
- 84.Soutar DS, Widdowson WP (1986) Immediate reconstruction of the mandible using a re-vascularized segment of radius. Head Neck Surg 8(4):232–246 [DOI] [PubMed]
- 85.Jaquet Y, et al. Radial forearm free flap donor site morbidity ulnar-based transposition flap vs. split-thickness skin graft. Arch Otolaryngol Head Neck Surg. 2012;138(1):38–43. doi: 10.1001/archoto.2011.216. [DOI] [PubMed] [Google Scholar]
- 86.Rockwell GM, Thoma A. Should the donor radius be plated prophylactically after harvest of a radial osteocutaneous flap? A cost-effectiveness analysis. J Reconstr Microsurg. 2004;20:297–306. doi: 10.1055/s-2004-824887. [DOI] [PubMed] [Google Scholar]
- 87.Cummings Discuss the options for mandibular reconstruction in lateral and anterior arch defects (non-vascularized vs. vascularized bone grafts) Sem Surg Oncol. 1995;11:190. doi: 10.1002/ssu.2980110304. [DOI] [Google Scholar]
- 88.Baker SR, Sullivan MJ. Osteocutaneous free scapular flap for one stage mandibular reconstruction. Arch Otolaryngol Head Neck Surg. 1988;114:267–277. doi: 10.1001/archotol.1988.01860150049015. [DOI] [PubMed] [Google Scholar]
- 89.Urken ML, Buchbinder D, Weinberg H, et al. Primary placement of osseointegrated implants in microvascular mandibular reconstruction. Otolaryngol Head Neck Surg. 1989;101:58–73. doi: 10.1177/019459988910100111. [DOI] [PubMed] [Google Scholar]
- 90.Sullivan MJ, et al. The free scapular flap for head and neck reconstruction. Am J Otolaryngol. 1990;11:319–327. doi: 10.1016/0196-0709(90)90062-Z. [DOI] [PubMed] [Google Scholar]
- 91.Lueg E. The anterolateral thigh flap: radial forearm’s “big brother” for extensive soft tissue head and neck defects. Arch Otolaryngol Head Neck Surg. 2004;130:813–818. doi: 10.1001/archotol.130.7.813. [DOI] [PubMed] [Google Scholar]
- 92.Wei FC, Jain V, Celik N, et al. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg. 2002;109:2219–2226. doi: 10.1097/00006534-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 93.Yu P. Characteristics of the anterolateral thigh flap in a Western population and its application in head and neck reconstruction. Head Neck. 2004;26:759–769. doi: 10.1002/hed.20050. [DOI] [PubMed] [Google Scholar]
- 94.Walton RL. Discussion. Free anterolateral thigh flaps for reconstruction of head and neck defects. Plast Reconstr Surg. 1993;92:429–430. doi: 10.1097/00006534-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 95.Wei FC, Jain V, Suominen S, et al. Confusion among perforator flaps: What is a true perforator flap? Plast Reconstr Surg. 1988;81:561. doi: 10.1097/00006534-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 96.Koshima I. Discussion. Free anterolateral thigh flap for reconstruction of head and neck defects following cancer ablation. Plast Reconstr Surg. 2000;105:2358–2360. doi: 10.1097/00006534-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 97.Rajan R. The pectoralis major myocutaneous flap in head and neck reconstruction. Indian J Otolaryngol Head Neck Surg. 1997;49(4):368–373. doi: 10.1007/BF02994653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McLeod AM, Robinson DW. Reconstruction of defects involving the mandible and floor of mouth by free osteocutaneous flaps derived from the foot. Br J Plast Surg. 1982;35:239–248. doi: 10.1016/S0007-1226(82)90108-4. [DOI] [PubMed] [Google Scholar]
- 99.Urken ML, Buchbinder D, Costantino PD, et al. Oromandibular reconstruction using microvascular composite flaps: report of 210 cases. Arch Otolaryngol Head Neck Surg. 1998;124(1):46–55. doi: 10.1001/archotol.124.1.46. [DOI] [PubMed] [Google Scholar]
- 100.Manchester WM. Some technical improvements in the reconstruction of the mandible and temporomandibular joint. Plast Reconstr Surg. 1972;50(3):249–256. doi: 10.1097/00006534-197209000-00009. [DOI] [PubMed] [Google Scholar]
- 101.Urken ML, Vickery C, Weinberg H, Buchbinder D, Lawson W, Biller HF. The internal oblique-iliac crest osteomyocutaneous free flap in oromandibular reconstruction. Report of 20 cases. Arch Otolaryngol Head Neck Surg. 1989;115(3):339–349. doi: 10.1001/archotol.1989.01860270081019. [DOI] [PubMed] [Google Scholar]
- 102.Rogers SN, Lakshmiah SR, Narayan B, et al. A comparison of the long-term morbidity following deep circumflex iliac and fibula free flaps for reconstruction following head and neck cancer. Plast Reconstr Surg. 2003;112(6):1517–1525. doi: 10.1097/01.PRS.0000082817.26407.86. [DOI] [PubMed] [Google Scholar]
- 103.Ramasastry SS, Tucker JB, Swartz WM, et al. The internal oblique muscle flap: an anatomic and clinical study. Plast Reconstr Surg. 1984;73:721–730. doi: 10.1097/00006534-198405000-00001. [DOI] [PubMed] [Google Scholar]
- 104.Seikaly H, Calhoun K, Rassekh C, Slaughter D. The clavipectoral osteomyocutaneous free flap. Otolaryngol Head Neck Surg. 1997;117(5):547–554. doi: 10.1016/S0194-5998(97)70029-9. [DOI] [PubMed] [Google Scholar]
- 105.Milroy BC, Korula P. Vascularized innervated transfer of the clavicular head of the pectoralis major muscle in established facial paralysis. Ann Plast Surg. 1988;20:273–282. doi: 10.1097/00000637-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 106.Reid CD, Taylor GI, Waterhouse N. The clavicular head of pectoralis major musculocutaneous free flap. Br J Plast Surg. 1986;39:57–65. doi: 10.1016/0007-1226(86)90005-6. [DOI] [PubMed] [Google Scholar]
- 107.Seikaly H, Chau J, Li F, Driscoll B, Seikaly D, Calhoun J, Calhoun KH. Bone that best matches the properties of the mandible. J Otolaryngol. 2003;32(4):262–265. doi: 10.2310/7070.2003.41646. [DOI] [PubMed] [Google Scholar]
- 108.Pogrel MA, Podlesh S, Anthony JP, et al. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg. 1997;55:1200–1206. doi: 10.1016/S0278-2391(97)90165-8. [DOI] [PubMed] [Google Scholar]
- 109.Granström G, Jacobsson M, Tjellström A. Titanium implants in irradiated tissue: benefits from hyperbaric oxygen. Int J Oral Maxillofac Implants. 1992;7:15–25. [PubMed] [Google Scholar]
- 110.Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41:351–357. doi: 10.1016/S0278-2391(83)80005-6. [DOI] [PubMed] [Google Scholar]
- 111.AleAlam DS, Nuara M, Christian J. Analysis of outcomes of vascularized flap reconstruction in patients with advanced mandibular osteoradionecrosis. Otolaryngol Head Neck Surg. 2009;141:196–201. doi: 10.1016/j.otohns.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 112.Dufresne C, Cutting C, Valauri F, et al. Reconstruction of mandibular and floor of mouth defects using the trapezius osteomyocutaneous flap. Plast Reconstr Surg. 1987;79:687–696. doi: 10.1097/00006534-198705000-00001. [DOI] [PubMed] [Google Scholar]
- 113.Hirsch DL, Bell RB, Dierks EJ, et al. Analysis of microvascular free flaps for reconstruction of advanced mandibular osteoradionecrosis: a retrospective cohort study. J Oral Maxillofac Surg. 2008;66:2545–2556. doi: 10.1016/j.joms.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 114.Foley BD, et al. Mandibular reconstruction using computer-aided design and computer-aided manufacturing: an analysis of surgical results. J Oral Maxillofac Surg. 2013;71(2):e111–e119. doi: 10.1016/j.joms.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 115.Costantino PD, Shybut G, Friedman CD, Pelzer HL, Masini M, Shindo ML, Sisson GA., Sr Segmental mandibular regeneration by distraction osteogenesis: an experimental study. Arch Otolaryngol Head Neck Surg. 1990;116:535–545. doi: 10.1001/archotol.1990.01870050035003. [DOI] [PubMed] [Google Scholar]
- 116.Costantino PD, Johnson CS, Friedman CD, Sisson GA. Bone regeneration within a human segmental mandible defect: a preliminary report. Am J Otolaryngol. 1995;16:56–65. doi: 10.1016/0196-0709(95)90011-X. [DOI] [PubMed] [Google Scholar]
- 117.Herford AS. Use of a plate-guided distraction device for transport distraction osteogenesis of the mandible. J Oral Maxillofac Surg. 2004;62:412–420. doi: 10.1016/j.joms.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 118.González-García R, Naval-Gías L. Transport osteogenesis in the maxillofacial skeleton: the outcomes of a versatile reconstruction method following tumor ablation. Arch Otolaryngol Head Neck Surg. 2010;136:243–250. doi: 10.1001/archoto.2010.2. [DOI] [PubMed] [Google Scholar]
- 119.Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995;77:1154–1165. doi: 10.2106/00004623-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 120.Riede U, Luem M, Ilchmann T, Eucker M, Ochsner PE. The M.E Müller straight stem prosthesis: 15 year follow-up. Survivorship and clinical results. Arch Orthop Trauma Surg. 2007;127:587–592. doi: 10.1007/s00402-007-0342-7. [DOI] [PubMed] [Google Scholar]
- 121.Tideman H, Lee S. The TL endoprosthesis for mandibular reconstruction—a metallic yet biological approach. Asian J Oral Maxillofac Surg. 2006;18:5. doi: 10.1016/S0915-6992(06)80024-5. [DOI] [Google Scholar]
- 122.Lee S, Goh BT, Tideman H, Stoelinga PJ, Jansen JA. Modular endoprosthesis for mandibular body reconstruction: a clinical, micro-CT and histologic evaluation in eight Macaca fascicularis. Int J Oral Maxillofac Surg. 2009;38:40–47. doi: 10.1016/j.ijom.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 123.Goh BT, Lee S, Tideman H, Stoelinga PJ. Replacement of the condyle and ascending ramus by a modular endoprosthesis in Macaca fascicularis part 1: a clinical and radiographic study. J Oral Maxillofac Surg. 2009;67:1392–1400. doi: 10.1016/j.joms.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 124.Wong RC, Tideman H, Merkx MA, Jansen J, Goh SM. The modular endoprosthesis for mandibular body replacement. Part 2: finite element analysis of endoprosthesis reconstruction of the mandible. J Craniomaxillofac Surg. 2012;40:e487–e497. doi: 10.1016/j.jcms.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 125.Gronthos S. Reconstruction of human mandible by tissue engineering. Lancet. 2004;9436:735–736. doi: 10.1016/S0140-6736(04)16948-1. [DOI] [PubMed] [Google Scholar]
- 126.Warnke PH, Wiltfang J, Springer I, Acil Y, Bolte H, Kosmahl M, et al. Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials. 2006;17:3163–3167. doi: 10.1016/j.biomaterials.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 127.Warnke PH, Springer IN, Acil Y, Julga G, Wiltfang J, Ludwig K, et al. The mechanical integrity of in vivo engineered heterotopic bone. Biomaterials. 2006;7:1081–1087. doi: 10.1016/j.biomaterials.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 128.Heliotis M, Lavery KM, Ripamonti U, Tsiridis E, di Silvio L. Transformation of a prefabricated hydroxyapatite/osteogenic protein-1implant into a vascularised pedicled bone flap in the human chest. Int J Oral Maxillofac Surg. 2006;3:265–269. doi: 10.1016/j.ijom.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 129.Boyne PJ. Transplantation, implantation and grafts. Dent Clin North Am. 1971;15:433–453. [PubMed] [Google Scholar]
- 130.Blake F, Bubenheim M, Heiland M, et al. Retrospective assessment of the peri-implant mucosa of implants inserted in reanastomosed or free bone grafts from the fibula or iliac crest. Int J Oral Maxillofac Implants. 2008;23:1102–1108. [PubMed] [Google Scholar]
- 131.Rohner D, Jaquiery C, Kunz C, Bucher P, Maas H, Hammer B. Maxillofacial reconstruction with prefabricated osseous free flaps: a 3-year experience with 24 patients. Plast Reconstr Surg. 2003;112:748–757. doi: 10.1097/01.PRS.0000069709.89719.79. [DOI] [PubMed] [Google Scholar]
- 132.Marunick MT, Roumanas ED. Functional criteria for mandibular implant placement post resection and reconstruction for cancer. J Prosthet Dent. 1999;82(1):107–113. doi: 10.1016/S0022-3913(99)70136-8. [DOI] [PubMed] [Google Scholar]
- 133.Mücke T, Hölzle F, Loeffelbein DJ, Ljubic A, Kesting M, Wolff KD, Mitchell DA. Maxillary reconstruction using microvascular free flaps. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(1):51–57. doi: 10.1016/j.tripleo.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 134.Garrett N, Roumanas ED, Blackwell KE, Freymiller E, Abemayor E, Wong WK, Gerratt B, Berke G, Beumer J, 3rd, Kapur KK. Efficacy of conventional and implant-supported mandibular resection prostheses: study overview and treatment outcomes. J Prosthet Dent. 2006;96(1):13–24. doi: 10.1016/j.prosdent.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 135.Raoul G, Ruhin B, Briki S, Lauwers L, Haurou Patou G, Capet JP, Maes JM, Ferri J. Microsurgical reconstruction of the jaw with fibular grafts and implants. J Craniofac Surg. 2009;20(6):2105–2117. doi: 10.1097/SCS.0b013e3181bec611. [DOI] [PubMed] [Google Scholar]
- 136.Salinas TJ, Desa VP, Katsnelson A, Miloro M. Clinical evaluation of implants in radiated fibula flaps. J Oral Maxillofac Surg. 2010;68(3):524–529. doi: 10.1016/j.joms.2009.09.104. [DOI] [PubMed] [Google Scholar]
- 137.Foster RD, Anthony JP, Sharma A, Pogrel MA. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck. 1999;21(1):66–71. doi: 10.1002/(SICI)1097-0347(199901)21:1<66::AID-HED9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 138.Weischer T, Mohr C. Ten-year experience in oral implant rehabilitation of cancer patients: treatment concept and proposed criteria for success. Int J Oral Maxillofac Implants. 1999;14(4):521–528. [PubMed] [Google Scholar]
- 139.Werkmeister R, Szulczewski D, Walteros-Benz P, Joos U. Rehabilitation with dental implants of oral cancer patients. J Craniomaxillofac Surg. 1999;27(1):38–41. doi: 10.1016/S1010-5182(99)80008-0. [DOI] [PubMed] [Google Scholar]
- 140.Shaw RJ, Sutton AF, Cawood JI, Howell RA, Lowe D, Brown JS, Rogers SN, Vaughan ED. Oral rehabilitation after treatment for head and neck malignancy. Head Neck. 2005;27(6):459–470. doi: 10.1002/hed.20176. [DOI] [PubMed] [Google Scholar]
- 141.Sclaroff A, Haughey B, Gay WD, Paniello R. Immediate mandibular reconstruction and placement of dental implants at the time of ablative surgery. Oral Surg Oral Med Oral Pathol. 1994;78(6):711–717. doi: 10.1016/0030-4220(94)90085-X. [DOI] [PubMed] [Google Scholar]