Abstract
Background
This study was conducted to evaluate the utility and effectiveness of Intranasal Dexmedetomidine on Sedation status and pain experience of the patients undergoing surgical extraction of impacted third molar.
Materials and Methods
In this double-blind, split mouth study, in 15 patients, evaluations pertaining to classes of third molar impaction was done. Each patient was randomly assigned to receive either intranasal normal saline (placebo group) or intranasal 1.5 µg/kg atomized Dexmedetomidine during the first session. The other regimen was used during the second session. Study was conducted for over a period of 120 min and data for sedation and pain was collected at an interval of 30 min. The collected data was then compared between the two groups within the same patient. Sedation status was assessed by a blinded observer with a modified Observer’s Assessment of Alertness/Sedation (OAA/S) 13 scale (Annexure- B). Pain experience was evaluated by Visual Analog Scale (VAS) (Annexure-B). Clinical evaluation for sedation and pain was done by a blinded observer at 30, 60, 90, and 120 min after administration of the intranasal solution. The values were then tabulated, and compared between two visits.
Results
The mean values of OAA score of Dexmedetomidine group were significantly higher as compared to Placebo group with a ‘p’ value of 0.000. And the mean values of Pain score of Dexmedetomidine group were significantly lower as compared to Placebo group with a ‘p’ value 0.009.
Conclusion
The results of this study clearly indicated that: Intranasally administered Dexmedetomidine was significantly useful and effective to achieve optimal sedation and analgesia during third molar surgery.
Keywords: Third molar extraction, Dexmedetomidine, Intranasal sedation
Introduction
In Oral & Maxillofacial Surgery, the most common procedure is extraction and surgical removal of impacted teeth. The surgical removal of third molar is always associated with fear of pain and preoperative anxiety. The management of fear and apprehension on the part of the patient undergoing surgery has been an integral part of patient management. Several minor surgical procedures which can be done under local anesthesia subject the patient to a rather unpleasant experience. General anesthesia on other hand demands a more specialized operatory setup, accurate monitoring and increases treatment costs. The possibility of anesthetic complications would be another cardinal drawback of general anesthesia.
Conscious sedation is an important part of pain and anxiety control. The application of conscious sedation techniques for minor oral surgical procedures can greatly reduce the need for general anaesthesia. Recognition of this lead to the use of pharmacological and behavioural therapies to control both the aversiveness of the procedure and patient anxiety. There has been considerable recent advancements in the issue of optimal conscious sedation and the principal of minimum intervention. Monitored anesthesia care has been proposed as one of the suitable techniques for minor oral surgeries which can be taken up in the dental clinic and are not required to be taken up under General Anesthesia. Monitored anesthesia care comprises of sedation combined with use of local anesthesia. The advantage being more quicker and an uneventful recovery [1].
Conscious sedation has been defined as a minimally depressed level of consciousness that retains the patient’s ability to independently and continuously maintain an airway and respond appropriately to physical stimulation and verbal command and that is produced by a pharmacological or non-pharmacological method or combination thereof [2].
The use of intranasal medications for sedation during dental procedures is easy, effective, and associated with few safety issues. The nasal atomization device delivers intranasal medication in a fine mist, which ensures that the exact dose and volume are delivered, enhance absorption, and improves bioavailability (through the nose–brain pathway) for fast and effective drug delivery [3].
Dexmedetomidine is a potent and highly selective α-2 adrenoceptor agonist with sympatholytic, sedative and analgesic properties and is associated with lesser intraoperative respiratory depression and postoperative amnesia [4].
With this background in mind, this study has been designed to evaluate the benefit of sedation technique using intranasal delivery of Dexmedetomidine along with local anesthesia compared to local anesthesia technique alone.
Materials and Methods
In this study, 15 patients having bilateral third molar impacted tooth were selected from Department of Oral and Maxillofacial Surgery, J.S.S Dental College and Hospital, Mysore and Dental Out Patient Department, J.S.S Medical College and Hospital, Mysore.
Inclusion Criteria
Patients willing to give their consent for the procedure.
Patients within the age group of 18–35 years
Patients with bilateral third molar impactions
Patients with ASA physical status of 1.
Exclusion Criteria
Individuals with
(BMI) > 27 kg/m2
Systemically unfit individuals
Pregnant Women
Methodology
After obtaining the consent, each subject had undergone bilateral impaction procedure in two surgical sessions, with extraction of a single third molar during each session. They were randomly assigned to receive either intranasal normal saline (placebo group, Group II) or intranasal 1.5 µg/kg atomized Dexmedetomidine (Group I) at the first session. Subsequently, during the second session, the other regimen was used.
The solution for intranasal administration (drug or placebo) was prepared by an independent investigator (the attending anaesthesiologist in a 2.5 ml syringe. A parenteral preparation of 100 µg/ml dexmedetomidine was used without dilution at a dose of 1.5 µg/kg, and 0.9 % saline was added to make a final volume of 1.5 ml. The volume of placebo was equivalent to 1.5 ml. The syringe containing the drug/placebo was attached via a luer lock connector to a nasal mucosal atomization device (MAD).
At approximately 30 min before the planned procedure, the drug or placebo was sprayed into both nostrils of the subject, while the subject was seated in a semi-reclining position in the dental chair (Fig. 1)
At 30 min after drug administration, local anaesthesiac using 2 % lignocaine hydrochloride with 1:200,000 adrenaline was given in accordance with the standard protocol.
Fig. 1.
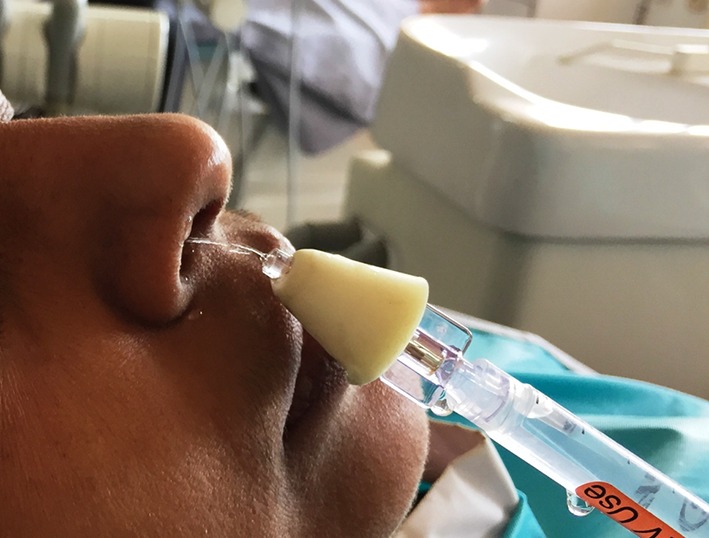
Intranasal delivery of solution
Standard third molar extraction procedure was performed (Fig. 2)
Suitable flap was used for exposure of the tooth
Standard impaction procedure was carried out, if required, bone removal was done.
Verbal contact was maintained with the patient throughout the procedure. Patient was monitored via noninvasive blood pressure cuff, and pulse oximeter throughout the entire study period (from administration of the drug to 30 min into the recovery period).
The relevant data was recorded at regular intervals during the procedure.
Patient was discharged as soon as the general condition was near normal.
The time between the two appointments was one week.
Fig. 2.
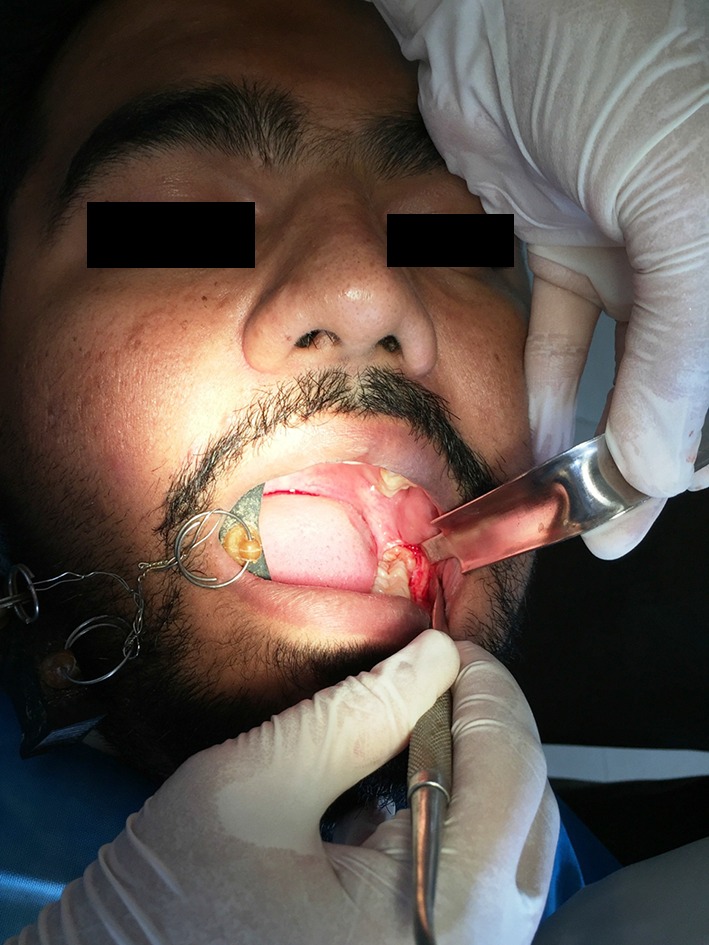
Surgical Extraction of Mandibular third molar
Clinical Evaluation for Sedation
Clinical evaluation for sedation was done by a blinded observer at 30, 60, 90, and 120 min after administration of the drug and assessment was done using modified Observer’s Assessment of Alertness/Sedation (OAA/S)13 scale (Modified Observer’s Assessment of Alertness/Sedation scale).
6: Appears alert and awake, responds readily to name spoken in normal tone
5: Appears asleep but responds readily to name spoken in normal tone
4: Lethargic response to name spoken in normal tone
3: Responds only after name is called loudly or repeatedly
2: Responds only after mild prodding or shaking
1: Does not respond to mild prodding or shaking
0: Does not respond to noxious stimulus
These values were then tabulated, and compared between two visits.
Clinical Evaluation for Pain
Pain experience was evaluated by Visual Analog Scale (VAS).
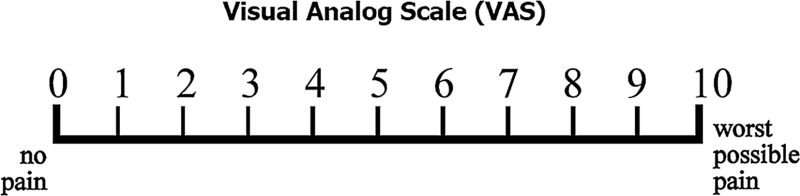
These values were then tabulated, and compared between two visits.
Results
A total of 15 patients were included in this study.
The two groups showed similar surgical durations (i.e. time from injection to the end of the surgery).
All the patients tolerated intranasal administration of dexmedetomidine or normal saline well, except one which complained of local irritation during intranasal administration of dexmedetomidine which subsided immediately after the spray.
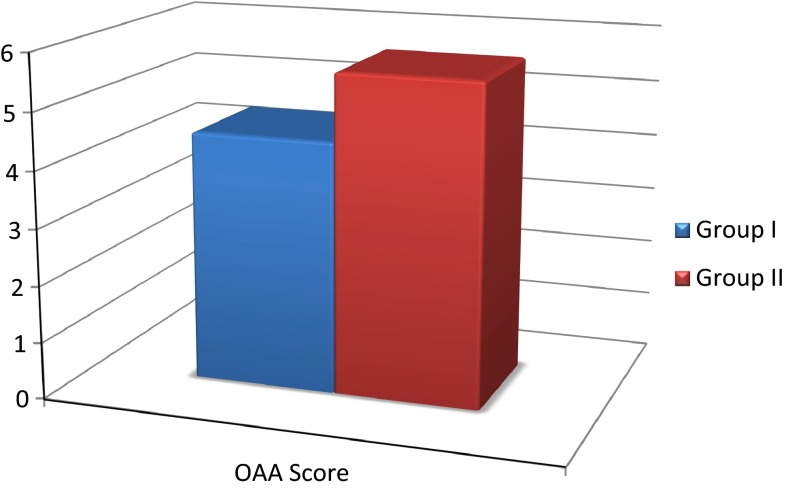
None of the patients were anxious at baseline. No patient experienced severe anxiety at any stage of treatment (Table 1; Graph 1).
Table 1.
Mean values of sedation as analyzed by modified observer’s assessment of alertness/sedation scale and the paired differences of the same
| Paired T test | ||||
|---|---|---|---|---|
| Mean | N | Standard deviation | Standard error mean | |
| Group I | 4.4467 | 15 | 0.38705 | 0.09994 |
| Group II | 5.6400 | 15 | 0.28983 | 0.07483 |
| Paired ‘t’ test | |||||
|---|---|---|---|---|---|
| Paired differences | t | df | Sig. (2-tailed) | ||
| Mean | Std. deviation | ||||
| Group I to Group II | −1.19333 | 0.29873 | −15.471 | 14 | 0.000 |
Graph 1.
Mean values of sedation as analyzed by modified observer’s assessment of alertness/sedation scale
Significant differences were observed in the mean scores of sedation scores of Dexmedetomidine and Placebo group intra and post operatively.
The mean values of OAA score of Dexmedetomidine group were significantly higher as compared to Placebo group.
The ‘t’ value for sedation score is 15.471. ‘p’ value 0.000 is significant (Table 2; Graph 2).
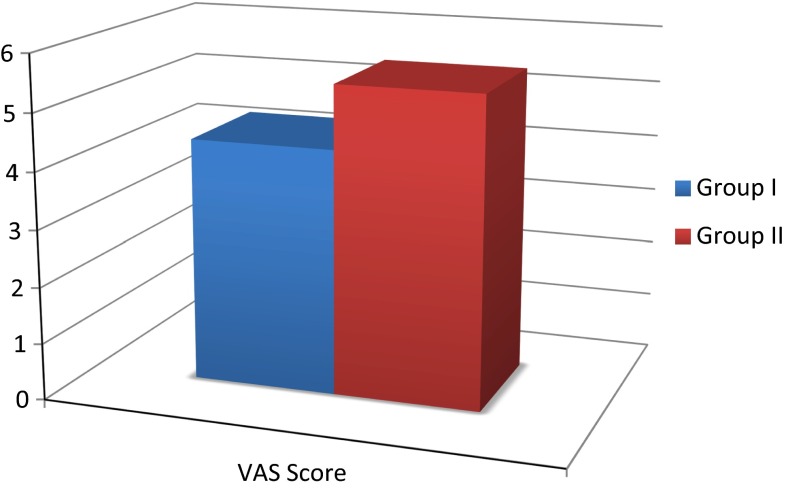
Table 2.
Mean values of pain score as analyzed VAS, showing the values and the paired differences of the same
| Paired T test | ||||
|---|---|---|---|---|
| Mean | N | Standard deviation | Standard error mean | |
| Group I | 4.3200 | 15 | 1.66098 | 0.42886 |
| Group II | 5.4467 | 15 | 1.22758 | 0.31696 |
| Paired ‘t’ test | |||||
|---|---|---|---|---|---|
| Paired differences | t | df | Sig. (2-tailed) | ||
| Mean | Std. deviation | ||||
| Group I to Group II | −1.12667 | 1.44047 | −3.029 | 14 | 0.009 |
Graph 2.
Mean values of pain score as analyzed VAS
Significant differences were observed in the mean scores of pain scores of Dexmedetomidine and Placebo group intra and post operatively.
The mean values of Pain score of Dexmedetomidine group were significantly lower as compared to Placebo group. The ‘t’ value for pain score is 3.029. ‘p’ value 0.009 is significant.
Discussion
Safe and adequate administration of sedative and analgesic medications can make painful and anxiety-provoking situations tolerable. Specifically, conscious sedation is a tool available to oral surgeons in the out patient setting that can improve patient tolerance and acceptability of unpleasant procedures.
Dexmedetomidine is a tasteless, colorless, and odourless agent that acts as a selective alpha-2 adrenergic agonist with both sedative and analgesic effects via actions in the central nervous system.
Dexmedetomidine is used as an adjuvant for premedication because of its sedative, anxiolytic, analgesic, sympatholytic, and stable hemodynamic profile [5]. Dexmedetomidine attenuates hemodynamic stress response to intubation and extubation by sympatholysis [6].
Dexmedetomidine is an effective and safe agent for controlled hypotension mediated by its central and peripheral sympatholytic action. Its easy administration, predictability with anesthetic agents, and lack of toxic side effect while maintaining adequate perfusion of the vital organs makes it a near-ideal hypotensive agent. Spinal fusion surgery for idiopathic scoliosis, septoplasty and tympanoplasty operations, and maxillofacial surgery have been safely done with dexmedetomidine-controlled hypotension [7].
This study evaluated the intranasal administration of atomized dexmedetomidine to sedate adult patients undergoing impacted third molar removal.
The objective and subjective results showed that 1.5 mcg/kg dexmedetomidine produced effective sedation with minimal side effects.
The present study is unique in that healthy adult patients comprised the study population.
The use of the atomized form of intranasal dexmedetomidine, which may improve bioavailability issues, is another novel feature of this study.
Atomized intranasal administration is achieved by using a product known as a Mucosal Atomizer Device (Fig. 3). This latex free device attaches directly to a luer-lock syringe and atomizes medications to a particle size of 30–100 µm.
Fig. 3.
Mucosal atomization device (MAD)
The intranasal route of administration is not only noninvasive and better tolerated by patients, but it is also very convenient to use the Mucosal Atomization Device.
Higher drug serum levels are achieved when a drug is given in its atomized form, due to a more extensive distribution of the medication across the nasal mucosa and increased bioavailability of the drug. In addition, because atomized medication is delivered as a mist, it is less likely to be blown back out of the nose [8].
Talon et al. [9] compared intranasal dexmedetomidine given via atomizer with oral midazolam in children younger than 18 years. Both products had similar effects on preoperative sedation and anxiolysis for induction of general anesthesia with no significant adverse effects, and only modest hemodynamic effects. Yuen et al. [10] investigated time to onset and duration of sedative effects for intranasal dexmedetomidine administered via droplets with similar sedative efficacy and pharmacodynamic results.
In our study, LA injection was performed at 30 min after intranasal administration of dexmedetomidine. Modified OAA/S revealed significant sedation (avg. score = 4.4467) compared to placebo (avg. Score = 5.6400), which commenced at 30 min after dexmedetomidine administration.
The analgesic effect of dexmedetomidine is controversial. In a previous randomized, double-blind study, when used as an i.v. sedative during third molar surgery under LA, dexmedetomidine did not offer additional analgesic benefit compared to midazolam [11].
In our study, when combined average of all the patients was compared, Visual Analog scale (VAS) revealed significant analgesia compared to placebo after dexmedetomidine administration during and after the procedure. However, two patients in our study showed a higher VAS score in Dexmedetomidine group but the difference was insignificant when compared to placebo group.
The site of action of dexmedetomidine is in the locus coeruleus of the central nervous system, where it induces a state similar to natural sleep. Therefore, it is not surprising that external stimulation should facilitate arousal [12].
Compliance with Ethical Standards
Conflict of interest
Dr. Sujeeth K. Shetty and Dr. Garima declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Sujeeth Kumar Shetty, Email: shettymaxfax@gmail.com.
Garima Aggarwal, Email: garimaukl@gmail.com.
References
- 1.Kazim K, Fahrettin Y, Nebahat G, Cemil C, Murat S, Hasan K. Comparision of Dexmedetomidine and Midazolam for monitored anesthesia care with tramadol via patient controlled analgesia in endoscopic nasal surgery: a prospective, randomized, double blind, clinical study. Curr Ther Res. 2007;68(2):69–81. doi: 10.1016/j.curtheres.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malamed SF (2003) Sedation: a guide to patient management, 4th edn, Mosby Inc, Missouri, pp 9–12, 23
- 3.Merkus P, Ebbens FA, Muller B, Fokkens WJ. The ‘best method’ of topical nasal drug delivery: comparison of seven techniques. Rhinology. 2006;44:102–107. [PubMed] [Google Scholar]
- 4.Afonso J, Reis F. Dexmedetomidine: Current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012;62(1):118–133. doi: 10.1016/S0034-7094(12)70110-1. [DOI] [PubMed] [Google Scholar]
- 5.Taittonen MT, Kirvela OA, Aantaa R, Kanto JH. Effect of clonidine and dexmedetomidine premedication on perioperative oxygen consumption and haemodynamic state. Br J Anaesth. 1997;78:400–406. doi: 10.1093/bja/78.4.400. [DOI] [PubMed] [Google Scholar]
- 6.Guler G, Akin A, Tosun E, Eskitafloglu E, Mizrak A, Boyaci A. Single dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand. 2005;49:1088–1091. doi: 10.1111/j.1399-6576.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5:128–133. doi: 10.4103/0259-1162.94750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warrington SE, Kuhn RJ. Use of intranasal medications in pediatric patients. Pharmacol Update Orthop. 2011;34(6):456–459. doi: 10.3928/01477447-20110427-20. [DOI] [PubMed] [Google Scholar]
- 9.Talon MD, Woodson LC, Sherwood ER, Aarsland A, McRae L, Benham T. Intranasal dexmedetomidine premedication is comparable with midazolam in burn children undergoing reconstructive surgery. J Burn Care Res. 2009;30(4):599–605. doi: 10.1097/BCR.0b013e3181abff90. [DOI] [PubMed] [Google Scholar]
- 10.Yuen VM, Hui TW, Irwin MG, Yuen MK. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: a double-blinded randomized controlled trial. Anesth Analg. 2008;106:1715–1721. doi: 10.1213/ane.0b013e31816c8929. [DOI] [PubMed] [Google Scholar]
- 11.Cheung CW, Ying CL, Chiu WK, Wong GT, Ng KF, Irwin MG. A comparison of dexmedetomidine and midazolam for sedation in third molar surgery. Anaesthesia. 2007;62:1132–1138. doi: 10.1111/j.1365-2044.2007.05230.x. [DOI] [PubMed] [Google Scholar]
- 12.Nooh N, Sheta SA, Abdullah WA, Abdelhalim AA. Intranasal atomized dexmedetomidine for sedation during third molar extraction. Int J Oral Maxillofac. 2013;42:857–862. doi: 10.1016/j.ijom.2013.02.003. [DOI] [PubMed] [Google Scholar]