Abstract
ROS1 gene‐rearrangement in non‐small‐cell lung cancer (NSCLC) patients has recently been identified as a driver gene and benefited from crizotinib treatment. However, no data are available for ROS1‐positive NSCLC about chemotherapeutic options and prognostic data. We investigated pemetrexed‐based treatment efficacy in ROS1 translocation NSCLC patients and determined the expression of thymidylate synthetase (TS) to provide a rationale for the efficacy results. We determined the ROS1 status of 1750 patients with lung adenocarcinoma. Patients' clinical and therapeutic profiles were assessed. In positive cases, thymidylate synthetase (TS) mRNA level was performed by RT‐PCR. For comparison, we evaluated the TS mRNA status and pemetrexed‐based treatment efficacy from 170 NSCLC patients with anaplastic lymphoma kinase (ALK) translocation (n = 46), EGFR mutation (n = 50), KRAS mutation (n = 32), and wild‐type of EGFR/ALK/ROS1/KRAS (n = 42). Thirty‐four ROS1 translocation patients were identified at two institutions. Among the 34 patients, 12 with advanced stage or recurrence were treated with pemetrexed‐based first‐line chemotherapy. The median progression‐free survivals of pemetrexed‐based first‐line chemotherapy in ROS1 translocation, ALK translocation, EGFR mutation, KRAS mutation, and EGFR/ALK/ROS1/KRAS wild‐type patients were 6.8, 6.7, 5.2, 4.2, and 4.5 months, respectively (P = 0.003). The TS mRNA level was lower in patients with ROS1‐positive than ROS1‐negative patients (264 ± 469 × 10−4 vs. 469 ± 615 × 10−4, P = 0.03), but similar with ALK‐positive patients (264 ± 469 × 10−4 vs. 317 ± 524 × 10−4, P = 0.64). Patients diagnosed with ROS1 translocation lung adenocarcinoma may benefit from pemetrexed‐based chemotherapy. TS mRNA level enables the selection of therapeutic options for ROS1 translocation patients.
Keywords: Efficacy, non‐small‐cell lung cancer, pemetrexed, ROS1, thymidylate synthetase
Introduction
Chromosomal rearrangements of the gene encoding ROS1 proto‐oncogene receptor tyrosine kinase (ROS1) define a distinct molecular subset of non‐small‐cell lung cancer (NSCLC)1. ROS1 rearrangements occur in approximately 1% to 2% of patients with NSCLC2, 3. ROS1 fusion has been identified as a driver gene and fusion partners including CD74, SLC34A2, TPM3, SDC4, EZR, LRIG3, KDELR2, and CCDC6 have been identified4. Due to highly similar tyrosine kinase domains, preclinical and clinical data suggest that ROS1 can be targeted by ALK inhibitors5. Several studies with limited number of patients demonstrated that crizotinib, an ALK inhibitor, was effective against NSCLC in patients harboring ROS1 translocation6, 7. In China, crizotinib is not commonly used for ROS1 translocation NSCLC patients due to lack of efficacy and toxicity data. Chemotherapeutic agents are the standard of care for ROS1 translocation NSCLC patients currently. For patients with ROS1 translocation, platinum‐based chemotherapy is recommended currently in clinical practice.
The efficacy of pemetrexed‐based treatment is superior to that of other regimens in ALK translocation patients8, 9, 10, 11. In addition, low TS level was significantly associated with better clinical efficacy in nonsquamous NSCLC patients who were treated with pemetrexed‐based chemotherapy8. Recent studies demonstrated that TS mRNA levels in ALK translocation tumor specimens were significantly lower compared with ALK translocation tissue, which may partially explain the clinical outcome of pemetrexed‐based chemotherapy in this population 8, 11. However, for lower frequency of ROS1 translocation than ALK translocation NSCLC, the TS mRNA level in ROS1 translocation samples is not well investigated 12. No clinical efficacy data of pemetrexed‐based chemotherapy efficacy exists currently.
In this study, we have carried out an analysis of prevalence and clinical efficacy of pemetrexed‐based chemotherapy of ROS1 translocation NSCLC patients, and further investigated TS mRNA levels in ROS1‐positive and ROS1‐negative patients.
Materials and Methods
Study populations
Consecutive patients who had been diagnosed with NSCLC between January 2010 and December 2014, at Zhejiang Cancer Hospital and Zhangzhou Municipal Hospital were screened for ROS1 gene. For comparison, 170 patients identified as EGFR mutation, ALK translocation, KRAS mutation, and EGFR/ALK/ROS1/KRAS wild‐type during the same period were collected. Tumor histology was classified according to the World Health Organization criteria (2004 version)13. Lung cancer staging was performed in all patients according to the 7th TNM classification. This study was approved by the Institutional Review Board of the two institutions.
Genetic analysis and measurement of TS mRNA
The EGFR/ALK/ROS1/KRAS detection Kit (Amoy Diagnostics, Xiamen, China) is based on the reverse‐transcriptase polymerase chain reaction (RT‐PCR) technology. All positive samples were validated with Sanger sequencing. All experiments were performed following the user manual. Details of the method have been described in previous report14. RNA was extracted for thymidylate synthase (TS) mRNA level from unstained sections according to standard protocols. Relative quantification for the TS genes was done using a quantitative RT‐PCR detection method (LightCycler 2.0; Roche Applied Science, Penzberg, Germany) and an internal reference gene (β‐actin) as a control. The details of the operation were carried out as previously described11.
Statistical analysis
Fisher's exact test and Wilcoxon rank‐sum test were used to assess the baseline characteristics of the different groups. Progression‐free survival (PFS) was estimated by the Kaplan–Meier method, and the log‐rank test was used to compare the differences between the groups. The data analysis was performed using Statistics 17.0 (SPSS Inc, Chicago, IL, USA). The last follow‐up time was March 1, 2015.
Results
Clinicopathologic characteristics of ROS1‐positive patients
Thirty‐four patients including 16 males and 18 females with lung adenocarcinoma were identified as ROS1 translocation with a frequency of 1.9% (34/1750). The clinicopathologic characteristics of these patients are listed in Table 1. Four patients have smoking history. According to the pathologic and clinical stages, 15 patients were diagnosed with stage IIIB/IV, and 19 with stage I to IIIA at diagnosis.
Table 1.
Clinicopathologic features comparison among patients harboring different genes
| ROS1 (n = 34) | ALK (n = 46) | KRAS (n = 32) | EGFR (n = 50) | wild‐type (n = 42) | P (ROS1 vs. ALK) | P (ROS1 vs. KRAS) | P (ROS1 vs. EGFR) | P (ROS1 vs. wild‐type) | |
|---|---|---|---|---|---|---|---|---|---|
| Gender | 0.90 | 0.02 | 0.32 | 0.28 | |||||
| Male | 16 | 21 | 24 | 29 | 25 | ||||
| Female | 18 | 25 | 8 | 21 | 17 | ||||
| Age at diagnosis | 0.89 | 0.13 | 0.67 | 0.85 | |||||
| <60 | 19 | 28 | 13 | 32 | 24 | ||||
| ≥60 | 13 | 18 | 19 | 18 | 18 | ||||
| Smoking history | 0.31 | <0.001 | 0.007 | 0.0044 | |||||
| Yes | 5 | 11 | 22 | 21 | 19 | ||||
| No | 29 | 35 | 10 | 29 | 23 | ||||
| Histology | 0.61 | 0.44 | 0.24 | 0.89 | |||||
| Adenocarcinoma | 34 | 44 | 30 | 46 | 41 | ||||
| Nonadenocarcinoma | 0 | 2 | 2 | 4 | 1 | ||||
| Stage at diagnosis | 0.80 | 0.46 | 0.99 | 0.76 | |||||
| I‐IIIA | 19 | 27 | 15 | 28 | 22 | ||||
| IIIB/IV | 15 | 19 | 17 | 22 | 20 | ||||
| Pemetrexed‐based chemotherapy | 0.41 | 0.42 | 0.48 | 0.57 | |||||
| First‐line | 12 | 24 | 19 | 28 | 23 | ||||
| Second or third‐line | 6 | 7 | 4 | 9 | 8 |
Clinicopathologic comparison
Totally, 46 patients with ALK translocation, 32 with KRAS mutation, 50 with EGFR mutation, and 42 with EGFR/ALK/ROS1/KRAS wild‐type were selected in this study concurrently with ROS1‐positive patients. Clinicopathologic comparison of patients with different gene types is presented in Table 1. There was a difference of smoking history between ROS1‐positive and ROS1‐negative patients. No difference was found in gender, age, stage, and histology among different groups.
Treatment efficacy and comparison
Totally, 23 patients with ROS1 translocation received first‐line palliative chemotherapy (15 with stage IIIB/IV at diagnosis and eight with recurrence). Twelve ROS1 translocation patients received platinum/pemetrexed first‐line treatment, and the other 11 patients received platinum‐based double regimens (seven with gemcitabine and four with docetaxel). Six patients received single‐agent pemetrexed as second or third‐line treatment and 13 cases received another single‐agent. No maintenance therapy was used after first‐line treatment in all of patients. The median PFS with first‐line platinum/pemetrexed was 6.8 months, while the median PFS with other platinum‐based double regimens was 5.0 months (P = 0.040)(Fig. 1). The median PFS of patients receiving single‐agent pemetrexed was 4.7 months. In contrast, the median PFS with other single‐agent was 3.3 months (P = 0.958)(Fig. 2).
Figure 1.
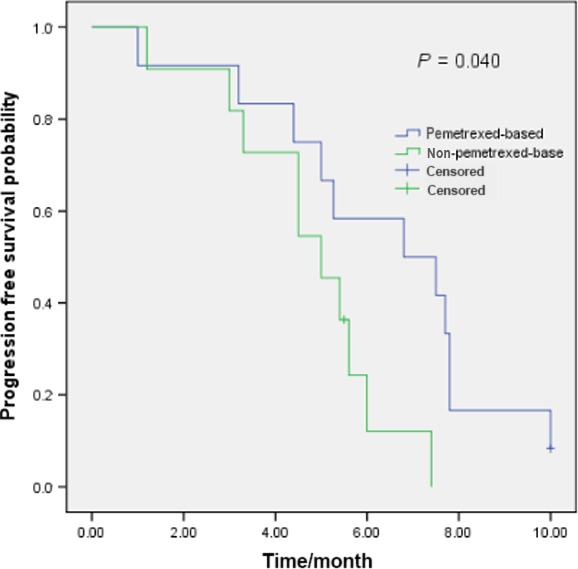
Pemetrexed‐based (n = 12) and non‐pemetrexed‐based(n = 11) first‐line chemotherapy efficacy comparison in ROS1‐positive NSCLC patients.
Figure 2.
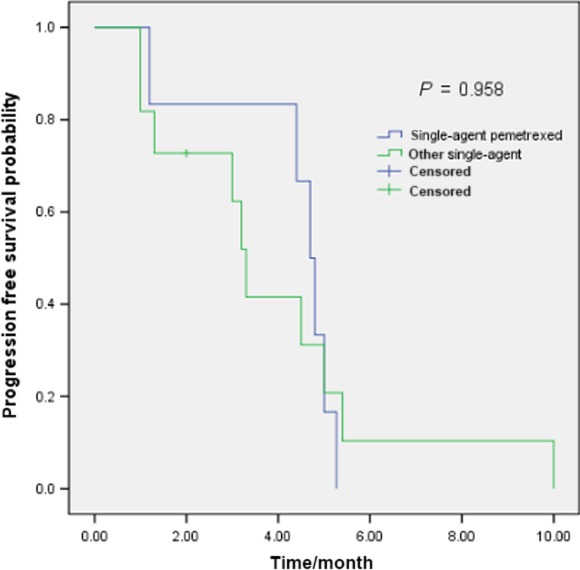
Single‐agent pemetrexed (n = 6) and other single‐agent (n = 13) chemotherapy efficacy comparison in ROS1‐positive NSCLC patients.
Totally, for the 170 patients selected for comparison, there were 27, 22, 34, and 27 patients with ALK translocation, KRAS mutation, EGFR mutation, and EGFR/ALK/ROS1/KRAS wild‐type who received first‐line platinum/pemetrexed treatment. The median PFS of first‐line platinum/pemetrexed chemotherapy for all the patients was 5.2 months. The PFS was 6.8, 6.7, 5.2, 4.2, and 4.5 months in ROS1 translocation, ALK translocation, EGFR mutation, KRAS mutation, and EGFR/ALK/ROS1/KRAS wild‐type patients, respectively (P = 0.003)(Fig. 3).
Figure 3.
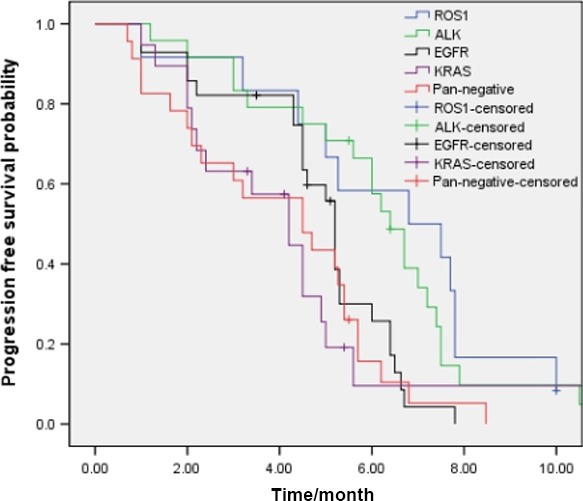
Pemetrexed‐based first‐line chemotherapy efficacy comparison in NSCLC patients with ROS1‐positive(n = 12), ALK‐positive (n = 24), KRAS‐positive(n = 19), EGFR‐positive (n = 28), and pan‐negative patients (n = 23) (P = 0.003).
A difference in PFS of the first‐line platinum/pemetrexed chemotherapy was apparent between ALK/ROS1‐positive and ALK/ROS1‐negative patients (6.7 vs. 4.6 months, P<0.001), while no difference was seen between ALK translocation and ROS1 translocation patients (6.8 vs. 6.7 months, P = 0.498).
For patients with single‐agent pemetrexed treatment, no efficacy difference was found among the ROS1 translocation, ALK translocation, EGFR mutation, KRAS mutation, and EGFR/ALK/ROS1/KRAS wild‐type patients. (P = 0.218)(Fig. 4).
Figure 4.
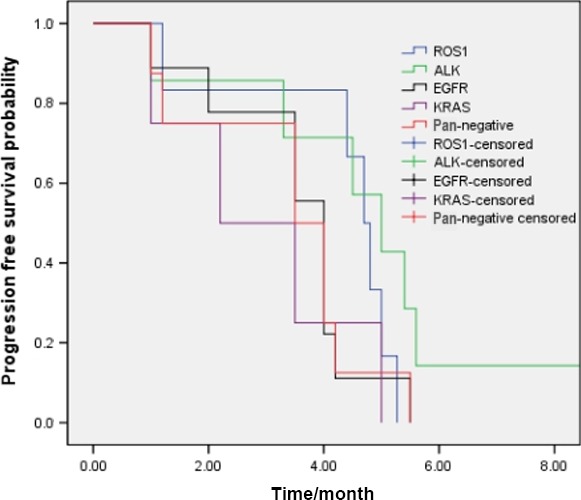
Single‐agent pemetrexed chemotherapy efficacy comparison in NSCLC patients with ROS1‐positive(n = 6), ALK‐positive (n = 7), KRAS‐positive(n = 4), EGFR‐positive (n = 9), and pan‐negative patients (n = 8)(P = 0.218).
Thymidylate synthase (TS) mRNA level comparison
Expression levels of TS mRNA are showed in Figure 5. The TS mRNA levels in ROS1 translocation, ALK translocation, EGFR mutations, KRAS mutations, and EGFR/ALK/ROS1/KRAS wild‐type patients were 264 ± 469 × 10−4, 317 ± 524 × 10−4, 470 ± 422 × 10−4, 770 ± 1085 × 10−4, and 407 ± 251 × 10−4, respectively.
Figure 5.
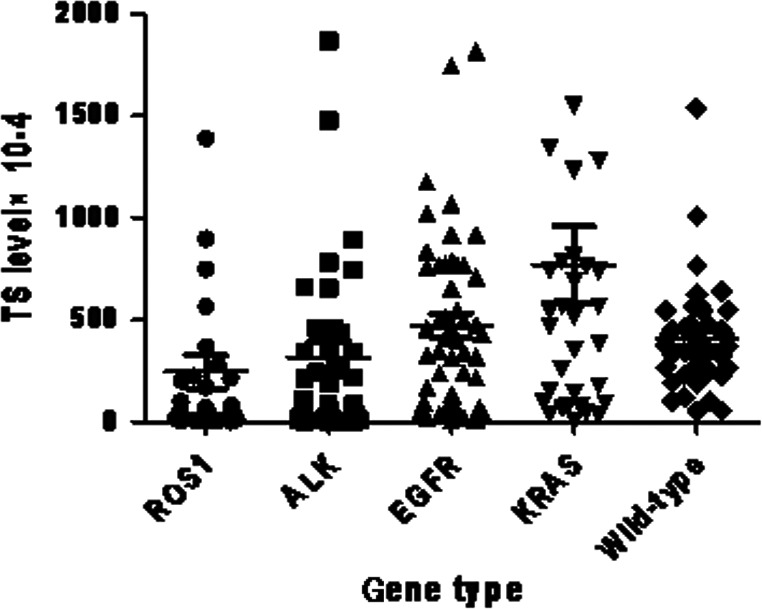
Thymidylate synthase (TS) mRNA levels in NSCLC patients with different driver genes.
TS mRNA levels in ROS1 translocation patients were significantly lower than in patients with KRAS mutation (P = 0.015) and EGFR mutation (P = 0.043), respectively. There was a tendency to lower TS levels in ROS1 translocation than in EGFR/ALK/ROS1/KRAS wild‐type patients (P = 0.11). No difference of TS mRNA level was found between ROS1 translocation and ALK translocation group (P = 0.64)(Fig. 5).
Discussion
We have carried out the study to determine the efficacy of pemetrexed‐based chemotherapy efficacy and the TS mRNA levels in ROS1 translocation lung adenocarcinoma. Our results suggest that ROS1 translocation patients manifest longer PFS with pemetrexed‐based chemotherapy and lower TS mRNA levels compared with ROS1 translocation patients.
To date, no more than one hundred ROS1 translocation patients regardless of retrospective or prospective study treated with crizotinib have been reported. In one study by Shaw, et al., 50 ROS1 translocation patients showed good efficacy with crizotinib treatment with a median PFS of 19.2 months6. Together with EUROS1 study7, ROS1 rearrangement defines another molecular subtype of NSCLC against which crizotinib is highly active. Due to the relative rarity of ROS1 translocation NSCLC, a randomized trial comparing crizotinib with chemotherapy is difficult. Pemetrexed is a multi‐targeted antifolate agent that disrupts the nucleotide syntheses of pyrimidines and purines, resulting in potent antitumor activity 15. Pemetrexed‐based chemotherapy is recommended as first‐line, maintenance and second‐line standard treatment regimens in nonsquamous NSCLC patients 16, 17, 18. Several randomized trials have shown that TS‐negative NSCLC patients benefited from pemetrexed‐based chemotherapy more than TS‐positive patients 19, 20. TS expression was identified as a potential predictive marker of efficacy in pemetrexed‐based regimens of NSCLC treatment.
The efficacy of pemetrexed‐based regimens in ALK translocation NSCLC patients has been identified in previous prospective and retrospective studies 8, 21, 22. ALK translocation patients treated with pemetrexed single‐agent showed a longer PFS than docetaxel in PROFILE 1007 study (4.2 months vs. 2.6 months)21. Similarly, the median PFS reached 7.0 months in ALK translocation patients treated with pemetrexed‐based first‐line chemotherapy in PROFILE 1014 study 22, which was longer than in previous reports of its efficacy 16, 17, 18. Several preclinical and clinical studies demonstrated that TS mRNA levels were significantly lower in patients harboring ALK translocation than in patients without ALK translocation, which may partially explain the outcome in patients amenable to pemetrexed‐based chemotherapy 8, 11. However, due to the rarity of ROS1 translocation patients, few data are available for the chemotherapeutic efficacy of pemetrexed‐based therapy except for two case reports 12, 23, 24 and the TS mRNA levels are unclear currently.
Twelve patients of ROS1 translocation were treated with pemetrexed‐based first‐line chemotherapy in our study. The outcome demonstrated that the PFS of this regimen was similar to that of ALK translocation patients, but longer than in patients without ALK/ROS1 translocation status. Moreover, we detected the TS mRNA levels in patients with different genes and the results showed that TS mRNA levels were lower in ROS1‐positive than in ROS1‐negative patients, which partly explains the efficacy of pemetrexed‐based chemotherapy in this subset.
One of the major limitations of our study is related to the small sample with ROS1 translocation patients. A second major limitation is that treatment regimens varied and not all of the patients were treated with pemetrexed‐based chemotherapy. Third, the retrospective nature of the study may be associated with sampling bias. However, due to lack of study to detect the clinical efficacy and TS mRNA levels in ROS1 translocation patients, our study assumes clinical significance for clinical practice.
In summary, we have shown that ROS1 translocation patients may benefit from pemetrexed‐based chemotherapy and TS mRNA levels are lower in this subset. For ROS1 translocation NSCLC patients, a prospective study with large number of patients is needed to establish the clinical efficacy of different chemotherapy regimens.
Conflicts of interest
None declared.
Acknowledgments
None.
Cancer Medicine 2016; 5(10):2688–2693
References
- 1. Takeuchi, K. , Soda M., Togashi Y., Suzuki R., Sakata S., Hatano S., et al. 2012. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 18:378–381. [DOI] [PubMed] [Google Scholar]
- 2. Pan, Y. , Zhang Y., Li Y., Hu H., Wang L., Li H., et al. 2014. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern‐specific clinicopathologic, histologic and cytologic features. Lung Cancer 84:121–126. [DOI] [PubMed] [Google Scholar]
- 3. Chen, Y. F. , Hsieh M. S., Wu S. G., Chang Y. L., Shih J. Y., Liu Y. N., et al. 2014. Clinical and the prognostic characteristics of lung adenocarcinoma patients with ROS1 fusion in comparison with other driver mutations in East Asian populations. J. Thorac. Oncol. 9:1171–1179. [DOI] [PubMed] [Google Scholar]
- 4. Davies, K. D. , and Doebele R. C.. 2013. Molecular pathways: ROS1 fusion proteins in cancer. Clin. Cancer Res. 19:4040–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kohno, T. , Nakaoku T., Tsuta K., Tsuchihara K., Matsumoto S., Yoh K., et al. 2015. Beyond ALK‐RET, ROS1 and other oncogene fusions in lung cancer. Transl. Lung Cancer Res. 4:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw, A. T. , Ou S. H., Bang Y. J., Camidge D. R., Solomon B. J., Salgia R., et al. 2014. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N. Engl. J. Med. 371:1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mazières, J. , Zalcman G., Crinò L., Biondani P., Barlesi F., Filleron T., et al. 2015. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1rearrangement: results from the EUROS1 cohort. J. Clin. Oncol. 33:992–999. [DOI] [PubMed] [Google Scholar]
- 8. Shaw, A. T. , Varghese A. M., Solomon B. J., Costa D. B., Novello S., Mino‐Kenudson M., et al. 2013. Pemetrexed‐based chemotherapy in patients with advanced, ALK‐positive non‐small cell lung cancer. Ann. Oncol. 24:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee, J. O. , Kim T. M., Lee S. H., Kim D. W., Kim S., Jeon Y. K., et al. 2011. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non‐small cell lung cancer. J. Thorac. Oncol. 6:1474–1480. [DOI] [PubMed] [Google Scholar]
- 10. Camidge, D. R. , Kono S. A., Lu X., Okuyama S., Barón A. E., Oton A. B., et al. 2011. Anaplastic lymphoma kinase gene rearrangements in non‐small cell lung cancer are associated with prolonged progression‐free survival on pemetrexed. J. Thorac. Oncol. 6:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ren, S. , Chen X., Kuang P., Zheng L., Su C., Li J., et al. 2012. Association of EGFR mutation or ALK rearrangement with expression of DNA repair and synthesis genes in never‐smoker women with pulmonary adenocarcinoma. Cancer 118:5588–5594. [DOI] [PubMed] [Google Scholar]
- 12. Chen, Y. F. , Hsieh M. S., Wu S. G., Chang Y. L., Yu C. J., Yang J. C., et al. 2016. Efficacy of pemetrexed‐based chemotherapy in patients with ROS1 fusion‐positive lung adenocarcinoma compared with patients harboring other driver mutations in East Asian populations. J. Thorac. Oncol. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13. Travis, W. D. , Brambilla E., Muller‐Hermelink H. K., and Harris C. C., eds. 2004. WHO Classification of Tumours. Pp. 145–147 in Pathology & Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC Press, Lyon. [Google Scholar]
- 14. Wu, C. , Zhao C., Yang Y., He Y., Hou L., Li X., et al. 2015. High discrepancy of driver mutations in patients with NSCLC and synchronous multiple lung ground‐glass nodules. J. Thorac. Oncol. 10:778–783. [DOI] [PubMed] [Google Scholar]
- 15. Hazarika, M. , White R. M., Johnson J. R., and Pazdur R.. 2004. FDA drug approval summaries: pemetrexed (Alimta). Oncologist 9:482–488. [DOI] [PubMed] [Google Scholar]
- 16. Hanna, N. , Shepherd F. A., Fossella F. V., Pereira J. R., De Marinis F., von Pawel J., et al. 2004. Randomized phase III trial of pemetrexed versus docetaxel in patients with non‐small‐cell lung cancer previously treated with chemotherapy. J. Clin. Oncol. 22:1589–1597. [DOI] [PubMed] [Google Scholar]
- 17. Scagliotti, G. V. , Parikh P., von Pawel J., Biesma B., Vansteenkiste J., Manegold C., et al. 2008. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J. Clin. Oncol. 26:3543–3551. [DOI] [PubMed] [Google Scholar]
- 18. Paz‐Ares, L. , de Marinis F., Dediu M., Thomas M., Pujol J. L., Bidoli P., et al. 2012. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non‐squamous non‐small‐cell lung cancer (PARAMOUNT): a double‐blind, phase 3, randomised controlled trial. Lancet Oncol. 13:247–255. [DOI] [PubMed] [Google Scholar]
- 19. Sun, J. M. , Ahn J. S., Jung S. H., Sun J., Ha S. Y., Han J., et al. 2015. Pemetrexed plus cisplatin versus gemcitabine plus cisplatin according to thymidylate synthase expression in nonsquamous non–small‐Cell lung cancer: a biomarker‐stratified randomized phase II trial. J. Clin. Oncol. 33:2450–2456. [DOI] [PubMed] [Google Scholar]
- 20. Nicolson, M. C. , Fennell D. A., Ferry D., O'Byrne K., Shah R., Potter V., et al. 2013. Thymidylate synthase expression and outcome of patients receiving pemetrexed for advanced nonsquamous non–small‐cell lung cancer in a prospective blinded assessment phase II clinical trial. J. Thorac. Oncol. 8:930–939. [DOI] [PubMed] [Google Scholar]
- 21. Shaw, A. T. , Kim D. W., Nakagawa K., Seto T., Crinó L., Ahn M. J., et al. 2013. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N. Engl. J. Med. 368:2385–2394. [DOI] [PubMed] [Google Scholar]
- 22. Solomon, B. J. , Mok T., Kim D. W., Wu Y. L., Nakagawa K., Mekhail T., et al. 2014. First‐Line Crizotinib versus Chemotherapy in ALK‐Positive Lung Cancer. N. Engl. J. Med. 371:2167–2177. [DOI] [PubMed] [Google Scholar]
- 23. Riess, J. W. , Padda S. K., Bangs C. D., Das M., Neal J. W., Adrouny A. R., et al. 2013. A case series of lengthy progression‐free survival with pemetrexed‐containing therapy in metastatic non–small‐cell lung cancer patients harboring ROS1 gene rearrangements. Clin. Lung Cancer 14:592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang, Y. , Wakelee H. A., and Neal J. W.. 2015. Relationship of driver oncogenes to long‐term pemetrexed response in non‐small‐cell lung cancer. Clin. Lung Cancer 16:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]


