Abstract
Introduction
Acute pain remains highly prevalent in the Emergency Department (ED) setting. This double-blind, randomized, placebo-controlled UK study investigated the efficacy and safety of low-dose methoxyflurane analgesia for the treatment of acute pain in the ED in the adult population of the STOP! trial.
Methods
Patients presenting to the ED requiring analgesia for acute pain (pain score of 4–7 on the Numerical Rating Scale) due to minor trauma were randomized in a 1:1 ratio to receive methoxyflurane (up to 6 mL) or placebo (normal saline), both via a Penthrox® (Medical Developments International Limited, Scoresby, Australia) inhaler. Rescue medication (paracetamol/opioids) was available immediately upon request. Change from baseline in visual analog scale (VAS) pain intensity was the primary endpoint.
Results
300 adult and adolescent patients were randomized; data are presented for the adult subgroup (N = 204). Mean baseline VAS pain score was ~66 mm in both groups. The mean change from baseline to 5, 10, 15 and 20 min was greater for methoxyflurane (−20.7, −27.4, −33.3 and −34.8 mm, respectively) than placebo (−8.0, −11.1, −12.3 and −15.2 mm, respectively). The primary analysis showed a highly significant treatment effect overall across all four time points (−17.4 mm; 95% confidence interval: −22.3 to −12.5 mm; p < 0.0001). Median time to first pain relief was 5 min with methoxyflurane [versus 20 min with placebo; (hazard ratio: 2.32; 95% CI: 1.63, 3.30; p < 0.0001)]; 79.4% of methoxyflurane-treated patients experienced pain relief within 1–10 inhalations. 22.8% of placebo-treated patients requested rescue medication within 20 min compared with 2.0% of methoxyflurane-treated patients (p = 0.0003). Methoxyflurane treatment was rated ‘Excellent’, ‘Very Good’ or ‘Good’ by 77.6% of patients, 74.5% of physicians and 72.5% of nurses. Treatment-related adverse events (mostly dizziness/headache) were reported by 42.2% of patients receiving methoxyflurane and 14.9% of patients receiving placebo; none caused withdrawal and the majority were mild and transient.
Conclusion
The results of this study support the evidence from previous trials that low-dose methoxyflurane administered via the Penthrox inhaler is a well-tolerated, efficacious and rapid-acting analgesic.
Funding
Medical Developments International (MDI) Limited and Mundipharma Research GmbH & Co.KG.
Trial registration
Clinicaltrials.gov identifier: NCT01420159, EudraCT number: 2011-000338-12.
Keywords: Acute pain; Analgesic; Emergency Department; Inhaled analgesic; Methoxyflurane; Pain; Penthrox, pre-hospital; Trauma
Introduction
Despite advances in pain medication and widely accepted guidelines for the treatment of pain such as the World Health Organization’s analgesic ladder [1], inadequate assessment and management of acute pain remains common in both the pre-hospital and Emergency Department (ED) setting, with pain prevalence figures of up to 90% in the ED [2, 3] and many patients undertreated [4–6]. Pain relief regimes work optimally when effective analgesics are supported by formal protocols/guidelines underpinned by staff and patient education [7]. The ‘ideal analgesic’ for acute pain should have rapid onset of action, act over an extended period of time, be well-tolerated and effective across a wide range of pain types in different populations.
Methoxyflurane belongs to the fluorinated hydrocarbon group of volatile anesthetics. It was first introduced as an inhalation anesthetic in the 1960s [8], but its use was generally discontinued by the late 1970s due to availability of newer anesthetic agents and reports of dose-related renal tubular damage at anesthetic doses [9–11]. Among inhalational fluorinated anesthetics, methoxyflurane is unique in having well-documented analgesic properties at low doses [12]. It has been used extensively for over 30 years in Australia and New Zealand (administered via a handheld inhaler; Penthrox®, Medical Developments International Limited, Scoresby, Australia) as a self-administered, rapid-acting analgesic agent for short-term pain relief in emergency medicine, minor surgical and dental procedures. The Penthrox inhaler is a green whistle-shaped single-use device that delivers methoxyflurane in analgesic doses, with a maximum recommended dose in 24 h of two 3 mL vials [13].
The historical concern regarding methoxyflurane has been nephrotoxicity, which was reported following significantly higher doses with deep methoxyflurane anesthesia [9]. Renal damage is most likely due to the metabolism of methoxyflurane in the liver and kidney and release of fluoride ions [14, 15]. Clinical experience suggests that a low but effective analgesic dose is not associated with the risk of renal adverse events [16]. Laboratory evidence also shows a large safety margin for analgesic use of methoxyflurane in the Penthrox inhaler [a dose of 6 mL/day and 15 mL/week results in exposure of 0.59 methoxyflurane minimum alveolar concentration (MAC)-hours, which is well below the reported level of risk of nephrotoxicity of 2.0 MAC-hours [14]]. Therefore, it has been concluded that the use of methoxyflurane in analgesic doses does not carry a risk of nephrotoxicity [17].
Studies of low-dose methoxyflurane as an analgesic agent show decreases in pain scores and indicate that methoxyflurane is an efficacious analgesic in the ED and pre-hospital settings [18–20] and for procedural analgesia [21]. Due to the physiochemical characteristics of methoxyflurane, absorption is rapid, providing fast onset of analgesic action (usually within 6–10 inhalations) to treat acute pain rapidly [13, 17]. The portability of the Penthrox inhaler and self-administration by the patient mean that it has practical advantages over alternatives such as nitrous oxide. Penthrox is a noncontrolled drug making it easier to prescribe and requiring less patient monitoring than opioid analgesics; it does not interfere with other analgesic agents or anesthetic drugs, and therefore does not limit subsequent treatment choices, and its effects are quickly reversible. Its characteristics make it suitable as a bridging analgesic, should more powerful intravenous (IV) analgesia be required, or for patients in whom IV access is difficult or impractical, or patients with contraindications or intolerance to other pain medications including opioids. Penthrox may eliminate the need for opioid analgesia for dislocations or fractures, for example, since the pain relief from Penthrox with or without the addition of simple non-opioid analgesia, may be sufficient for reduction or splinting.
Despite a large volume of published literature supporting the efficacy and safety of methoxyflurane at analgesic concentrations [18], previous studies have been mostly observational and uncontrolled. Furthermore, little data have been generated within an ED setting, or outside Australia and New Zealand. This double-blind, randomized, placebo-controlled, UK-based study evaluated the short-term efficacy and safety of methoxyflurane at low analgesic doses for the treatment of acute pain in patients presenting to the ED with minor trauma. The study included both adult and adolescent patients aged ≥12 years and the results for the full study population have been reported previously [22]. Since Penthrox has recently been approved in Europe for the treatment of adult patients, a subgroup analysis was performed to evaluate the data in patients aged ≥18 years, and the data for this adult subgroup are the focus of this secondary paper.
Methods
Study Design
This was a randomized, double-blind, multicenter, placebo-controlled study (The STOP! Trial, Clinicaltrials.gov identifier: NCT01420159; EudraCT number: 2011-000338-12), undertaken between August 5, 2011 and July 26, 2012, at six EDs in the UK. Patient eligibility for the study was established at time of presentation to the ED. A total of 300 patients presenting with acute pain requiring analgesia were randomized in a 1:1 ratio to receive treatment with either methoxyflurane or placebo via a Penthrox inhaler while in the ED. Study assessments were performed by a blinded research nurse, who remained with the patient in the ED while they were receiving care. Patients attended a post-treatment safety follow-up 14 ± 2 days after discharge from the ED. The randomized study population included 96 adolescent patients (aged 12–17 years) whose data are not presented here, and 204 adult patients whose data have been analyzed separately for this report. The full methodology for this study has been previously described in the primary publication [22].
Study Participants
Eligible patients were those presenting to the ED with minor trauma (where trauma referred to ‘a physical wound or injury’, such as fractures, lacerations, burns, dislocations, contusions or injury due to foreign bodies) and requiring analgesia for acute pain [defined as a pain score ≥4 to ≤7 as measured using the 11-point Numerical Rating Scale (NRS) at the time of admission] who were able to give written informed consent. For this adult subgroup analysis, all patients were aged ≥18 years. The NRS was used for the assessment of patient eligibility only and was not used to evaluate efficacy in this study.
Patients with a life-threatening condition requiring immediate admission to the operating room or intensive care unit, acute intoxication with drugs or alcohol, treatment with any analgesic agent within 5 h before presentation to the ED (except diclofenac sodium, which was prohibited within 8 h before presentation to ED), ongoing use of analgesic agents for chronic pain, use of methoxyflurane within the previous 4 weeks, known personal or familial hypersensitivity to fluorinated anesthetics, or clinically significant respiratory depression, cardiovascular instability, renal or hepatic impairment, were excluded from the participation.
Treatments
Patients were randomized in a 1:1 ratio to receive either methoxyflurane or placebo (sterile normal saline) via a Penthrox inhaler. The handheld Penthrox inhaler is a small, lightweight, disposable, cylindrical polyethylene device, approximately 15 cm long in a distinctive green color comprising a whistle-like mouthpiece on one end and a cap insert at the other end. Internally, the device contains an S-shaped polypropylene wick which absorbs the liquid methoxyflurane/normal saline, and a one-way valve that allows fresh air and methoxyflurane/normal saline vapor to be inhaled through the wick; and prevent expired air and moisture passing back through the wick. An activated carbon unit attached to the outlet of the inhaler minimizes the release of methoxyflurane in the vicinity of the patient.
Treatment randomization (using permuted block randomization), stratified by center and age group (adolescent/adult) was prepared by an independent statistician. At enrollment, each individual patient was allocated the next randomization number in the appropriate stratum. To prevent selection bias and maintain the blind, the assembling and dispensing of study medication was performed by an unblinded research team member, who loaded the inhalers and then placed each inhaler into a plastic bag, which was sealed, labeled with the patient randomization number and weighed. The patient and all other personnel involved with the conduct and interpretation of the study, including the investigators, site personnel and the study team, were blinded to the treatment allocation. The inhalers looked the same, but as methoxyflurane has a characteristic odor, one drop of methoxyflurane was placed on the outside of the primed inhaler before sealing the plastic bag so that the smell between active and placebo treatments was indistinguishable to the patient and treating physician upon opening. The relative density of methoxyflurane (1.42) is greater than that of normal saline (1), therefore to maintain the blind in respect of inhaler weight, a larger volume of saline solution was contained in the placebo inhalers (5 mL) compared with the volume of methoxyflurane in the active inhalers (3 mL).
Patients were supplied with one Penthrox inhaler containing 3 mL of methoxyflurane or 5 mL placebo as soon as possible following enrollment and initial assessments, which was utilized as required. Study medication was self-administered by the patient by inhaling from the device, assisted where required by the research nurse. Each inhaler had a diluter hole at the mouthpiece end, which when covered with the patient’s index finger, allowed the patient to inhale a higher concentration of study medication. A second inhaler containing 3 mL of methoxyflurane or 5 mL placebo was supplied if requested by the patient; no patient received a dose greater than 6 mL methoxyflurane (2 × 3 mL) or 10 mL placebo (2 × 5 mL). It was estimated that each inhaler could provide up to 1 h pain relief when used intermittently. Following use, the inhaler(s) were weighed by the unblinded member of the research team to determine the dose of methoxyflurane or placebo inhaled by the patient.
To ensure that the placebo control study design was ethical and acceptable to patients and investigators, rescue medication was made available immediately upon request for all patients at any time during or after treatment with study medication, as recommended in the Committee for Medicinal Products for Human Use guideline CPMP/EWP/612/00 [23] and guidance from the Declaration of Helsinki on the use of placebo control. Rescue medications permitted while the patient was in the ED included IV, intranasal or oral opioids or paracetamol. At the time of discharge patients received 16 × 500 mg paracetamol tablets as rescue medication for the treatment of pain during the 14 ± 2 day follow-up period.
Efficacy Assessments
Pain intensity was measured using the Painlog™ (Schlenker Enterprises, Ltd., Lombard, IL, USA) 100 mm visual analog scale (VAS) before the first inhalation of study medication, at 5, 10, 15, 20 and 30 min after the start of study medication inhalation and every 30 min thereafter until rescue medication was administered or discharged from the ED, whichever was sooner. The pain VAS is frequently used in pain studies because it is easy to use, requires no verbal or reading skills, and is sufficiently versatile to be employed in a variety of settings [24, 25].
The time point at which the patient first reported pain relief, the number of inhalations administered until pain relief was achieved, and whether the patient covered the hole in the inhaler during inhalation were recorded. It was noted whether or not the patient requested rescue medication, and if applicable, the time of request for rescue medication. Prior to ED discharge, the patient, the treating physician and the research nurse completed an assessment of GMP measured using a 5-point Likert scale (‘Poor’, ‘Fair’, ‘Good’, ‘Very Good’, or ‘Excellent’).
Safety Assessments
Patients were monitored by a research nurse for the duration of the ED visit and any adverse events, not related to the trauma presentation, were recorded from the time of consent until the time of ED discharge. Information on any adverse events occurring during the follow-up period was collected at the 14 ± 2 day follow-up visit. For each adverse event, the investigator provided a ‘Yes/No’ assessment as to whether there was a reasonable possibility that the event may have been caused by methoxyflurane and evaluated its severity according to the National Cancer Institute Common Toxicity Criteria where applicable.
Vital signs (blood pressure, heart rate and rhythm, and respiratory rate) were assessed at enrollment and at 5, 10, 15, 20 and 30 min after the start of study medication inhalation and every 30 min thereafter until rescue medication was administered or discharged from the ED, whichever was sooner. Level of consciousness was measured using the 15-point Glasgow coma score at 10, 20 and 30 min after the start of study medication inhalation and prior to ED discharge. Blood samples were drawn for clinical laboratory tests (complete blood count and clinical chemistry including blood glucose, sodium, potassium, calcium, chloride, serum creatinine, alanine transaminase, aspartate transaminase, gamma-glutamyl transferase, alkaline phosphatase, lactate dehydrogenase, total bilirubin, blood urea nitrogen, albumin and total protein) within −10 to +5 min of the start of study medication inhalation and at the 14 ± 2 day follow-up visit.
Statistical Analyses
The primary efficacy endpoint was the change in pain intensity as measured using the VAS scale from baseline to 5, 10, 15 and 20 min after the start of study medication inhalation, which was analyzed using repeated measures analysis of covariance adjusted for baseline VAS score, and the interaction between time point and treatment. The primary analysis was the overall test for treatment effect considering all four time points. Treatment effects were estimated as least squares mean differences between the methoxyflurane group and the placebo group.
Secondary efficacy endpoints included use of rescue medication within 20 min of the start of treatment (yes/no), time to request for rescue medication, time to first pain relief, number of inhalations taken before first pain relief, and global medication performance (GMP). The time from the start of treatment to first pain relief and first request for rescue medication were each compared between the treatment groups using the Cox proportional hazards model. Time was censored at the soonest of: 2 h from the start of treatment, physician-initiated rescue medication, start of treatment for the injury, or early withdrawal. Use of rescue medication within 20 min of the start of treatment (yes/no) was compared using logistic regression. The assessment of the GMP by the patient, research nurse and treating physician were each compared between the treatment groups using ordinal logistic regression with proportional odds assumption. All analyses were adjusted for baseline VAS scores (patients with no baseline VAS pain score were excluded from the analysis). Other efficacy endpoints were summarized descriptively.
All statistical analyses were performed using SAS® version 9.2 or higher (SAS Institute Inc., Cary, NC, USA). All hypothesis testing was carried out at the 5% (two-sided) significance level unless stated otherwise. There was no imputation of missing data; if a baseline value was missing, no change from baseline was calculated. Baseline was defined as the last recorded value before the first dose. Efficacy analyses were performed using the intention-to-treat population, defined as all randomized patients who received at least one dose of study medication and had at least one post-baseline efficacy assessment.
Safety presentations were descriptive and based on the safety population, which included all randomized patients who received at least one dose of study medication. Adverse events from enrollment to 14 ± 2 days after ED discharge were coded using the Medical Dictionary for Regulatory Activities (MedDRA version 14.0, McLean, VA, USA) coding system. Events were classified as treatment-emergent if they started or increased in severity on or after the first date and time of study medication dosing.
Sample Size
The sample size calculation for the whole study including both adult and adolescent patients, estimated that 150 patients per arm would provide at least 94.5% power to detect a treatment difference of 13 mm [26] in change from baseline of VAS pain score after 20 min using repeated measures analysis of variance of assessments at 5, 10, 15 and 20 min. Given the setting of the study, the dropout rate was expected to be minimal, and a sample size of 150 patients per arm was considered adequate. The planned sample size was achieved in the full study population (300 patients were randomized); however, the study was not intended to be sufficiently powered to demonstrate a statistically significant treatment difference in the adult-only subgroup that we report here (102 patients in the methoxyflurane treatment group and 101 patients in the placebo group).
Compliance with Ethics Guidelines
The study was conducted in accordance with International Council on Harmonization Good Clinical Practice adhering to the ethical principles of the Helsinki Declaration of 1964, as revised in 2013, as well as local guidelines. The protocol was reviewed by and received favorable opinion from a central National Health Service ethics committee. Each participating center’s research and development department reviewed and approved the protocol and all amendments. Written informed consent was obtained from all patients before enrollment.
Results
Study Patients
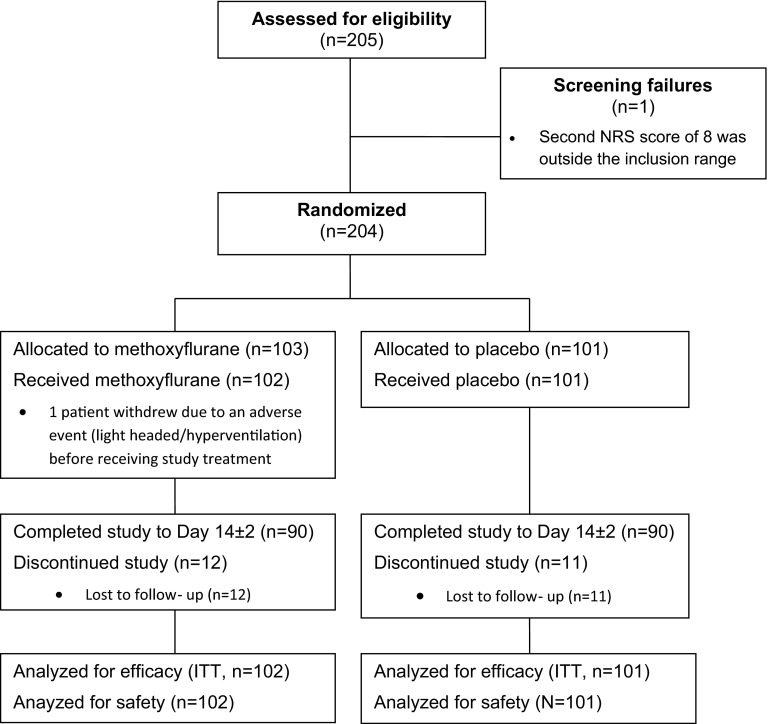
Participant flow is shown in Fig. 1. A total of 205 adult patients were screened; one patient failed screening because her second NRS score of 8 was outside the inclusion range, and the remaining 204 patients were randomized to double-blind treatment (103 patients to methoxyflurane and 101 patients to placebo). One patient in the methoxyflurane group discontinued due to an adverse event (light headed/hyperventilation) before receiving study treatment, therefore 203 patients were treated and analyzed for efficacy (intention-to-treat population) and safety. The majority of patients completed the study to Day 14 ± 2; however, 12 patients (11.7%) in the methoxyflurane group and 11 patients (10.9%) in the placebo group were lost to follow-up.
Fig. 1.
Participant flow. NRS numerical rating scale, ITT intention-to-treat population. The ITT population is defined as those patients in the safety population who have at least one post-baseline efficacy assessment
Demographic and baseline characteristics are presented in Table 1. Most patients (96.1%) were White, with an even gender split (51.2% male: 48.8% female) and a mean age of 36 years (range 18–84 years) overall. First injury type was most commonly classified as ‘other’ (50.2% of patients, largely injuries such as sprains, soft tissue injury and muscular pain); 23.6% of patients had contusions, 17.7% of patients had fractures, while burns, dislocations, lacerations and injuries due to foreign body were each reported for <5% of patients. Eleven patients had >1 injury, including two who had three injuries (second and third injury types included contusions, lacerations and ‘other’). Mean baseline VAS pain intensity score was 66.2 mm in the methoxyflurane group and 65.5 mm in the placebo group, indicating that on average, patients were experiencing pain of at least moderate severity at baseline [27]. Overall, the two treatment groups were evenly matched with regard to patient demographic characteristics, injury type and baseline pain severity.
Table 1.
Demographic and baseline characteristics (intention-to-treat population)
| Variable | Statistic | Methoxyflurane (N = 102) | Placebo (N = 101) | Total (N = 203) |
|---|---|---|---|---|
| Age (years) | n | 102 | 101 | 203 |
| Mean (SD) | 36.7 (13.9) | 35.7 (15.0) | 36.2 (14.4) | |
| Median | 35.0 | 30.0 | 33.0 | |
| Min, Max | 18, 74 | 18, 84 | 18, 84 | |
| Gender [n (%)] | Male | 53 (52.0) | 51 (50.5) | 104 (51.2) |
| Female | 49 (48.0) | 50 (49.5) | 99 (48.8) | |
| Race [n (%)] | White | 99 (97.1) | 96 (95.0) | 195 (96.1) |
| Asian | 1 (1.0) | 2 (2.0) | 3 (1.5) | |
| Black | 2 (2.0) | 2 (2.0) | 4 (2.0) | |
| Other | 0 | 1 (1.0) | 1 (0.5) | |
| Injury type (first injurya) | Burn | 0 | 3 (3.0) | 3 (1.5) |
| Contusion | 26 (25.5) | 22 (21.8) | 48 (23.6) | |
| Dislocation | 1 (1.0) | 2 (2.0) | 3 (1.5) | |
| Fracture | 19 (18.6) | 17 (16.8) | 36 (17.7) | |
| Injury due to foreign body | 2 (2.0) | 1 (1.0) | 3 (1.5) | |
| Laceration | 3 (2.9) | 5 (5.0) | 8 (3.9) | |
| Other | 51 (50.0) | 51 (50.5) | 102 (50.2) | |
| Site | Back | 5 (4.9) | 2 (2.0) | 7 (3.4) |
| Chest | 8 (7.8) | 0 | 8 (3.9) | |
| Face | 1 (1.0) | 0 | 1 (0.5) | |
| Left lower limb | 31 (30.4) | 25 (24.8) | 56 (27.6) | |
| Left upper limb | 11 (10.8) | 14 (13.9) | 25 (12.3) | |
| Other | 7 (6.9) | 5 (5.0) | 12 (5.9) | |
| Right lower limb | 29 (28.4) | 32 (31.7) | 61 (30.0) | |
| Right upper limb | 10 (9.8) | 23 (22.8) | 33 (16.3) | |
| VAS pain intensity (mm) | n | 100 | 99 | – |
| Mean (SD) | 66.2 (16.6) | 65.5 (18.1) | – | |
| Median | 68 | 70 | – | |
| Min, Max | 25, 100 | 10, 100 | – |
SD standard deviation, VAS visual analog scale
a11 patients had >1 injury; in these patients second injuries included contusions (seven patients), laceration (one patient) and ‘other’ (three patients), and third injuries included contusion (one patient) and laceration (one patient)
Efficacy
Methoxyflurane significantly reduced pain intensity compared with placebo. Table 2 shows that for the overall change from baseline in VAS pain (primary analysis), there was a highly significant treatment difference [estimated treatment effect: −17.4 mm; 95% confidence interval (CI): −22.3 to −12.5 mm; p < 0.0001]. The mean change in VAS pain from baseline was also significantly greater for the methoxyflurane group compared with the placebo group at each individual time point (5, 10, 15 and 20 min). The greatest treatment effect was observed at 15 min after the start of dosing (estimated treatment effect: −21.0 mm; 95% CI: −26.8 to −15.3 mm).
Table 2.
Analysis of VAS Pain Intensity Score (intention-to-treat population)
| Time point | Adjusteda change from baseline | Estimated treatment effect (95% confidence interval) | p value | |
|---|---|---|---|---|
| Methoxyflurane (N = 102) | Placebo (N = 101) | |||
| Overall | −29.0 | −11.6 | −17.4 (−22.3,−12.5) | <0.0001 |
| 5 min | −20.7 | −8.0 | −12.6 (−17.0, −8.3) | |
| 10 min | −27.4 | −11.1 | −16.3 (−21.4,−11.1) | |
| 15 min | −33.3 | −12.3 | −21.0 (−26.8,−15.3) | |
| 20 min | −34.8 | −15.2 | −19.7 (−26.0,−13.3) | |
| Time by treatment interaction | 0.0004 | |||
Pain scores recorded following the start of the planned emergency department procedure were excluded from the analysis. Pain scores taken after initiation of rescue medication were included in the analysis
VAS visual analog scale
aLeast squares mean adjusted for baseline VAS pain score and time by treatment interaction
The majority of patients in the methoxyflurane group (82.4%) experienced pain relief. The median time to first pain relief was significantly shorter in the methoxyflurane group (5 min) compared with the placebo group (20 min, Table 3) (hazard ratio for difference between methoxyflurane and placebo: 2.32; 95% CI: 1.63, 3.30; p < 0.0001). In the methoxyflurane group, 45 patients (44.1%) reported pain relief within the first five inhalations, 36 patients (35.3%) reported pain relief within 6–10 inhalations, five patients (4.9%) took >10 inhalations, while 16 patients (15.7%) reported no pain relief prior to taking rescue medication. In contrast, in the placebo group, almost half of the patients [47 (46.5%)] reported no pain relief prior to taking rescue medication, while for those who did report pain relief, overall it was reported after a greater number of inhalations compared with the methoxyflurane group (Table 3).
Table 3.
Analysis of secondary pain relief endpoints (intention-to-treat population)
| Endpoint | Statistic | Methoxyflurane (N = 102) | Placebo (N = 101) |
|---|---|---|---|
| Time to first pain relief | |||
| Kaplan–Meier estimatea (min) | Upper quartile (95% CI) | 10.0 (8.0, 17.0) | NC |
| Median (95% CI) | 5.0 (NC) | 20.0 (10.0, NC) | |
| Lower quartile (95% CI) | 2.0 (2.0, 4.0) | 5.0 (NC) | |
| Number (%) responses | 84 (82.4) | 53 (52.5) | |
| Number (%) censored | 18 (17.6) | 48 (47.5) | |
| Number of inhalations to first pain relief | No relief without rescue medication | 16 (15.7%) | 47 (46.5%) |
| 1 | 1 (1.0%) | 0 | |
| 2 | 6 (5.9%) | 2 (2.0%) | |
| 3 | 11 (10.8%) | 7 (6.9%) | |
| 4 | 19 (18.6%) | 3 (3.0%) | |
| 5 | 8 (7.8%) | 8 (7.9%) | |
| 6 | 9 (8.8%) | 7 (6.9%) | |
| 7 | 4 (3.9%) | 1 (1.0%) | |
| 8 | 10 (9.8%) | 4 (4.0%) | |
| 9 | 4 (3.9%) | 4 (4.0%) | |
| 10 | 9 (8.8%) | 9 (8.9%) | |
| >10 | 5 (4.9%) | 9 (8.9%) |
Times were censored at the soonest of; 2 h from start of treatment, investigator initiated rescue medication, start of treatment for the injury, early withdrawal
CI confidence interval, NC not calculable
aUnadjusted estimates
The proportion of patients who used rescue medication in the first 20 min was significantly lower in the methoxyflurane group (2.0%) than the placebo group (22.8%) (odds ratio: 0.07; 95% CI: 0.02, 0.29; p = 0.0003). When considering requests for rescue medication at any time (prior to censoring), rescue medication use was again significantly lower for the methoxyflurane group (11.8%) compared with the placebo group (38.6%), with a significantly longer time to request for rescue medication (hazard ratio: 0.23; 95% CI: 0.12, 0.44; p < 0.0001). The proportion of patients requesting rescue medication at any time (prior to censoring) did not reach a level where the median time to request could be estimated.
The GMP ratings by the patient, treating physician and research nurse at ED discharge were all significantly better in the methoxyflurane group compared with the placebo group (p < 0.0001, Table 4). Approximately, three-quarters of patients, physicians and research nurses rated methoxyflurane treatment as ‘Excellent’, ‘Very Good’ or ‘Good’ (77.6% of patients, 74.5% of physicians and 72.5% of research nurses).
Table 4.
Global medication performance (intention-to-treat population)
| Methoxyflurane (N = 102) | Placebo (N = 101) | p value for treatment effect | |
|---|---|---|---|
| Patient assessment | |||
| n | 98 | 96 | |
| Excellent | 20 (20.4%) | 4 (4.2%) | |
| Very good | 22 (22.4%) | 6 (6.3%) | |
| Good | 34 (34.7%) | 20 (20.8%) | |
| Fair | 10 (10.2%) | 23 (24.0%) | |
| Poor | 12 (12.2%) | 43 (44.8%) | |
| Ordinal logistic regression | <0.0001 | ||
| Physician assessment | |||
| n | 55 | 54 | |
| Excellent | 6 (10.9%) | 0 | |
| Very good | 10 (18.2%) | 4 (7.4%) | |
| Good | 25 (45.5%) | 10 (18.5%) | |
| Fair | 8 (14.5%) | 20 (37.0%) | |
| Poor | 6 (10.9%) | 20 (37.0%) | |
| Ordinal logistic regression | <0.0001 | ||
| Research nurse assessment | |||
| n | 102 | 101 | |
| Excellent | 19 (18.6%) | 2 (2.0%) | |
| Very good | 20 (19.6%) | 6 (5.9%) | |
| Good | 35 (34.3%) | 18 (17.8%) | |
| Fair | 13 (12.7%) | 22 (21.8%) | |
| Poor | 15 (14.7%) | 53 (52.5%) | |
| Ordinal logistic regression | <0.0001 |
Significance of treatment effect was adjusted for baseline pain score
Inhaler Use
A total of 25 patients (24.5%) in the methoxyflurane group and 15 patients (14.9%) in the placebo group requested a second inhaler. The median time between dispensing the first and second inhalers was 54 min (range 30–120 min) for patients receiving methoxyflurane and 50 min (range 20–72 min) for patients receiving placebo. The number of patients covering the diluter hole on inhalation (allowing the patient to inhale a higher concentration of methoxyflurane/placebo) was slightly higher in the placebo group [43 patients (42.6%)] compared with the methoxyflurane group [37 patients (36.3%)].
Safety
Treatment-emergent adverse events were reported by 64 patients (62.7%) in the methoxyflurane group and 41 patients (40.6%) in the placebo group (Table 5); these were considered to be treatment-related (according to the investigator’s causality assessment) for 43 patients (42.2%) receiving methoxyflurane and 15 patients (14.9%) receiving placebo. The most common adverse events in the methoxyflurane group were dizziness [37 patients (36.3%)] and headache [20 patients (19.6%)], which were both reported more frequently than in the placebo group [dizziness: 11 patients (10.9%); headache: 13 patients (12.9%)]. All other adverse events were reported by <5% of patients in either treatment group. There were no other notable differences between the treatment groups in the incidence of adverse events, except for somnolence, which was reported by five methoxyflurane-treated patients and one placebo-treated patient. No patients discontinued treatment with methoxyflurane due to adverse events and one placebo-treated patient discontinued due to vomiting. The majority of adverse events were mild and transient in nature; no patients experienced a severe adverse event (severity was not recorded for six adverse events). One serious adverse event (lower respiratory tract infection requiring hospitalization) was reported in a methoxyflurane-treated patient 5 days after treatment. The patient had enrolled into the study with blunt trauma (from falling off a chair) and complained of right-sided rib pain and an injury to the right knee. The investigator considered that the event was not related to the study treatment and that the most likely cause was blunt trauma.
Table 5.
Treatment-emergent adverse events (safety population)
| MedDRA system organ class | Methoxyflurane (N = 102) | Placebo (N = 101) | ||||
|---|---|---|---|---|---|---|
| Preferred term | n | N | % | n | N | % |
| Any adverse event | 133 | 64 | (62.7%) | 76 | 41 | (40.6%) |
| Ear and labyrinth disorders | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Ear pain | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Eye disorders | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Diplopia | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Gastrointestinal disorders | 10 | 9 | (8.8%) | 12 | 9 | (8.9%) |
| Abdominal pain upper | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Diarrhea | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Dry mouth | 3 | 3 | (2.9%) | 0 | 0 | (0.0%) |
| Gingivitis | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Nausea | 2 | 2 | (2.0%) | 4 | 4 | (4.0%) |
| Toothache | 1 | 1 | (1.0%) | 2 | 2 | (2.0%) |
| Vomiting | 2 | 2 | (2.0%) | 5 | 4 | (4.0%) |
| General disorders and administration site conditions | 8 | 7 | (6.9%) | 2 | 2 | (2.0%) |
| Chest discomfort | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Chills | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Fatigue | 1 | 1 | (1.0%) | 1 | 1 | (1.0%) |
| Feeling abnormal | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Feeling drunk | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Feeling hot | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Feeling of relaxation | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Hangover | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Hunger | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Infections and infestations | 4 | 4 | (3.9%) | 7 | 6 | (5.9%) |
| Cystitis | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Influenza | 2 | 2 | (2.0%) | 0 | 0 | (0.0%) |
| Lower respiratory tract infection | 1 | 1 | (1.0%) | 1 | 1 | (1.0%) |
| Nasopharyngitis | 1 | 1 | (1.0%) | 4 | 4 | (4.0%) |
| Upper respiratory tract infection | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Injury, poisoning and procedural complications | 3 | 2 | (2.0%) | 1 | 1 | (1.0%) |
| Arthropod bite | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Procedural dizziness | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Procedural nausea | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Procedural pain | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Investigations | 8 | 5 | (4.9%) | 5 | 3 | (3.0%) |
| Alanine aminotransferase increased | 1 | 1 | (1.0%) | 2 | 2 | (2.0%) |
| Aspartate aminotransferase increased | 1 | 1 | (1.0%) | 2 | 2 | (2.0%) |
| Blood alkaline phosphatase increased | 1 | 1 | (1.0%) | 1 | 1 | (1.0%) |
| Blood calcium increased | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Blood lactate dehydrogenase increased | 2 | 2 | (2.0%) | 0 | 0 | (0.0%) |
| Gamma-glutamyl transferase increased | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| White blood cell count increased | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Musculoskeletal and connective tissue disorders | 2 | 1 | (1.0%) | 2 | 2 | (2.0%) |
| Arthralgia | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Back pain | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Musculoskeletal pain | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Neck pain | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Nervous system disorders | 86 | 55 | (53.9%) | 38 | 27 | (26.7%) |
| Amnesia | 2 | 2 | (2.0%) | 0 | 0 | (0.0%) |
| Dizziness | 43 | 37 | (36.3%) | 14 | 11 | (10.9%) |
| Dysarthria | 2 | 2 | (2.0%) | 0 | 0 | (0.0%) |
| Headache | 31 | 20 | (19.6%) | 19 | 13 | (12.9%) |
| Migraine | 2 | 2 | (2.0%) | 1 | 1 | (1.0%) |
| Paraesthesia | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Sinus headache | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Somnolence | 5 | 5 | (4.9%) | 1 | 1 | (1.0%) |
| Syncope | 0 | 0 | (0.0%) | 2 | 1 | (1.0%) |
| Psychiatric disorders | 1 | 1 | (1.0%) | 1 | 1 | (1.0%) |
| Inappropriate affect | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Insomnia | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Reproductive system and breast disorders | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Dysmenorrhoea | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Respiratory, thoracic and mediastinal disorders | 4 | 4 | (3.9%) | 4 | 4 | (4.0%) |
| Cough | 2 | 2 | (2.0%) | 1 | 1 | (1.0%) |
| Dyspnea | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Oropharyngeal pain | 2 | 2 | (2.0%) | 2 | 2 | (2.0%) |
| Skin and subcutaneous tissue disorders | 3 | 3 | (2.9%) | 1 | 1 | (1.0%) |
| Cold sweat | 0 | 0 | (0.0%) | 1 | 1 | (1.0%) |
| Night sweats | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Rash | 2 | 2 | (2.0%) | 0 | 0 | (0.0%) |
| Vascular disorders | 2 | 2 | (2.0%) | 2 | 2 | (2.0%) |
| Hypertension | 1 | 1 | (1.0%) | 0 | 0 | (0.0%) |
| Hypotension | 1 | 1 | (1.0%) | 2 | 2 | (2.0%) |
Includes events reported up to 14 ± 2 days after discharge from the emergency department
Only events not related to the trauma presentation were recorded
MedDRA Medical Dictionary for Regulatory Activities, n number of events, N number of patients, % percentage of patients
The administration of low dose methoxyflurane had no observable effects on cardiovascular or respiratory parameters. Mean changes from baseline in heart rate were within ±5 beats per minute, while mean changes from baseline in systolic and diastolic blood pressure were within ±6 mmHg, and mean respiratory rate remained constant at 14–15 breaths/min. Glasgow coma score was 15 for all patients at all-time points, except two patients who recorded a score of 14 (one at 10, 20 and 30 min and one at 30 min only). There were no renal or liver concerns arising from the results of the clinical laboratory evaluations at baseline and at the follow-up visit, and mean values for all parameters were within normal limits.
Discussion
The results of this study confirm that methoxyflurane is a highly effective analgesic for adult patients in the ED setting. There was a highly significant difference between the methoxyflurane and placebo groups (p < 0.0001) in the analysis of the VAS pain intensity score at all-time points tested, despite a considerable ‘placebo effect’. In a previous hypothesis-generating study, Todd et al. found that a difference of approximately 13 mm on a 100 mm VAS scale represented the minimum change in acute pain that was clinically significant in a cohort of trauma patients [28], which was supported by findings of Gallagher et al. [26]. This was appreciably exceeded in our adult population results, with a treatment difference of −17.4 mm (95% CI: −22.3, −12.5 mm) overall in favor of methoxyflurane. A reduction of approximately two points or 30% in the pain intensity NRS has also been postulated to represent a clinically important difference by Farrar and colleagues [29]. While the primary efficacy results of the current study are based on the VAS pain score and acute pain, rather than the 11-point NRS and chronic pain (as evaluated by Farrar), the overall adjusted change from baseline in VAS pain intensity of −29.0 mm in the methoxyflurane group (from a baseline mean of 66.2 mm) represents a reduction in pain of ~44%, also considerably exceeding this definition of clinical significance. This is important, as small differences in mean VAS score can be declared “statistically significant”, even though they may be of little clinical significance to the patient [30]. In comparison, the overall adjusted change from baseline in VAS pain intensity in the placebo group was −11.6 mm (from a baseline mean of 65.5 mm), representing an 18% reduction. Although the STOP! study was placebo-controlled, our results for methoxyflurane are similar to those observed for IV morphine and intranasal fentanyl in a study by Borland et al. with a similar design and endpoint in pediatric patients presenting to the ED with acute long-bone fractures [31]. This study showed mean changes from baseline in 100 mm VAS pain scores at 5, 10 and 20 min of −25, −26 and −32 mm for morphine and −13, −22 and −31 mm for fentanyl from a baseline of 67 and 68 mm, respectively, compared with our results for methoxyflurane of −21, −27 and −35 mm from a baseline of 66 mm.
The secondary efficacy results supported the findings of the primary efficacy analysis, with significantly fewer methoxyflurane-treated patients requiring rescue medication than placebo-treated patients (11.8% vs. 38.6%), and approximately three-quarters of patients, physicians and research nurses rating methoxyflurane treatment as ‘Excellent’, ‘Very Good’ or ‘Good’. The high patient and treating medical professional satisfaction with methoxyflurane analgesia observed in this study is consistent with results reported by Buntine et al. [20], who reported that 81.9% of paramedics and 72.3% of patients felt satisfied with methoxyflurane pre-hospital analgesia. As expected, the onset of pain relief with methoxyflurane was rapid (median 5 min) with 79.4% of patients experiencing pain relief within 1–10 inhalations. This is consistent with the rapid onset of pain relief reported by Johnston et al. in their study of pre-hospital analgesia for visceral pain, which found a mean reduction in visual/verbal analog scale pain score (0–10 scale) with methoxyflurane of 2.0 (95% CI: 1.7, 2.2) after 5 min and 2.5 (95% CI: 2.1–2.9) on arrival at hospital, compared with 1.6 (95% CI: 1.4, 1.8) and 3.2 (95% CI: 2.9, 3.5), respectively, for intranasal fentanyl [19]. The onset of pain relief with methoxyflurane is also similar to that reported by Tveita et al. for a 10 mg bolus dose of IV morphine [32]. Although methoxyflurane is currently only licensed for emergency relief of pain due to trauma in Europe, several studies in Australia and New Zealand have also demonstrated its effectiveness as a procedural analgesic [21, 33–36].
The rate of treatment-related adverse events was higher with methoxyflurane (42.2%) than placebo (14.9%); this was mostly attributable to a higher incidence of dizziness/lightheadedness and headache in the methoxyflurane group, which are both adverse events already captured in the product label [13]. However, the majority of adverse events were mild and transient in nature, no patients discontinued use of methoxyflurane due to adverse events and patients rated their satisfaction with methoxyflurane treatment highly, (77.6% assessing treatment as ‘Excellent’, ‘Very Good’ or ‘Good’). It should also be taken into account that study treatment exposure was higher in the methoxyflurane group than the placebo group; patients in the placebo group requested rescue medication significantly earlier than patients in the methoxyflurane group, and 24.5% of patients in the methoxyflurane group compared with 14.9% in the placebo group used a second inhaler. While this is unlikely to have affected the key efficacy endpoints, which mainly considered the early effects of study treatment (in the first 20 min), it may have had an impact on safety results. We also observed no effects of methoxyflurane on vital signs, which are consistent with the findings from two retrospective, observational studies of methoxyflurane analgesia in the pre-hospital setting that looked at records of 1217 patients treated by the Australian Ambulance Service [19, 37].
In this study, although clinical laboratory sampling was limited, no evidence of nephro- or hepatotoxicity was observed. Similarly, in patients receiving methoxyflurane as procedural analgesia for bone marrow biopsy, blood analysis of urea and electrolytes was no different between those patients receiving methoxyflurane and those receiving placebo [35]. These observations are consistent with the findings of Dayan [17], who reviewed laboratory and clinical data relevant to nephrotoxicity and methoxyflurane and concluded that low-dose use of methoxyflurane for analgesia has a large safety margin (at least 2.7- to 8-fold based on methoxyflurane MAC-hours or serum fluoride level) and does not carry a risk of causing renal dysfunction or damage. Furthermore, over 5 million doses of Penthrox have been sold with no pharmacovigilance-related trends suggesting nephrotoxicity. In a much larger controlled observational study of patients receiving analgesia during ambulance transport, comparing 17,629 patients receiving methoxyflurane with 118,141 patients not receiving methoxyflurane, no link between methoxyflurane use for emergency analgesia and renal disease (or hepatic disease) was observed [16]. Whilst the literature and post-marketing surveillance in Australia suggest that nephrotoxicity and/or hepatotoxicity of methoxyflurane at analgesic doses is not a risk [16, 17], a study is underway in the UK to understand hepatotoxicity in the pre-hospital and ED settings.
A limitation of the study was the lack of an active comparator, as previously observed by Carley and Body [38]. An active comparator was not considered feasible in this study due to the unique mode of delivery and smell of methoxyflurane, as well as the difficulty in blinding possible inhaled comparators such as nitrous oxide. Due to methoxyflurane’s fast onset of action, if an oral comparator was used, it would have been evident which treatment the patient had been randomized to. Placebo use is considered warranted when the placebo effect is known to be very variable (e.g., pain) and when associated with minimization measures, e.g., rescue treatment [39]. Patients randomized to placebo had immediate access to rescue medication to mitigate the risk of under treatment, therefore the placebo group was considered to be ethically justified and was approved by the National Research Ethics Committee, and the decision to include placebo as the comparator was ratified by regulatory agencies. A double-blind, double-dummy study design would have been required for an active comparator, which would have had implications in terms of the time taken to dispense and administer study medication (when rapid analgesia is required in the ED), and also in terms of affecting the provision of rescue analgesia. Despite the lack of active control, the study provided meaningful and clinically relevant results indicating a beneficial clinical effect of methoxyflurane.
Conclusion
In conclusion, consistent with evidence from previous studies, the results of this study show that low-dose methoxyflurane administered via the Penthrox inhaler is an easy to use, well-tolerated, effective and rapid-acting analgesic in the ED setting. Considering its fast onset of action, ease of use and minimal impact on subsequent treatment choices, methoxyflurane may also lend itself as a bridging agent in the pre-hospital/ED setting until it is possible to administer more powerful analgesia if required, and also as a short-term procedural analgesic.
Acknowledgments
The study was funded by Medical Developments International (MDI) Limited. This article processing charges and open access fee were funded by Mundipharma Research GmbH & Co.KG.
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Editorial assistance in the preparation of this manuscript was provided by Karen Mower of Scientific Editorial and funded by Mundipharma Research GmbH & Co.KG. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Frank Coffey was paid travel and subsistence expenses by MDI for one investigator’s meeting. Frank Coffey’s institution was paid by MDI to run the study. Patrick Dissmann’s institution was paid by MDI to run the study. Kazim Mirza’s institution was paid by MDI to run the study. Mark Lomax is an employee of Mundipharma Research Limited.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Favorable opinion for the study was received from the National Research Ethics Service (REC reference 11/YH/0116). Informed consent was obtained from all patients for being included in the study. ® PENTHROX is a registered trademark of MDI Limited.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D395F0600606EF5E.
References
- 1.Ventafridda V, Saita L, Ripamonti C, De Conno F. WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React. 1985;7:93–96. [PubMed] [Google Scholar]
- 2.Cordell WH, Keene KK, Giles BK, Jones JB, Jones JH, Brizendine EJ. The high prevalence of pain in emergency medical care. Am J Emerg Med. 2002;20:165–169. doi: 10.1053/ajem.2002.32643. [DOI] [PubMed] [Google Scholar]
- 3.Berben SA, Meijs TH, van Dongen RT, et al. Pain prevalence and pain relief in trauma patients in the Accident and Emergency Department. Injury. 2008;39:578–585. doi: 10.1016/j.injury.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Pierik JG, IJzerman MJ, Gaakeer MI, et al. Pain management in the emergency chain: the use and effectiveness of pain management in patients with acute musculoskeletal pain. Pain Med. 2015;16:970–984. doi: 10.1111/pme.12668. [DOI] [PubMed] [Google Scholar]
- 5.Guéant S, Taleb A, Borel-Kühner J, et al. Quality of pain management in the emergency department: results of a multicentre prospective study. Eur J Anaesthesiol. 2011;28:97–105. doi: 10.1097/EJA.0b013e3283418fb0. [DOI] [PubMed] [Google Scholar]
- 6.Dale J, Bjørnsen LP. Assessment of pain in a Norwegian Emergency Department. Scand J Trauma Resusc Emerg Med. 2015;23:86. doi: 10.1186/s13049-015-0166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lord BA, Parsell B. Measurement of pain in the prehospital setting using a visual analogue scale. Prehosp Disaster Med. 2003;18:353–358. doi: 10.1017/S1049023X0000131X. [DOI] [PubMed] [Google Scholar]
- 8.Tomlin PJ. Methoxyflurane. Br J Anaesth. 1965;37:706–709. doi: 10.1093/bja/37.9.706. [DOI] [PubMed] [Google Scholar]
- 9.Crandell WB, Pappas SG, Macdonald A. Nephrotoxicity associated with methoxyflurane anaesthesia. Anesthesiology. 1966;27:591–607. doi: 10.1097/00000542-196609000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Mazze RI, Shue GL, Jackson SH. Renal dysfunction associated with methoxyflurane anaesthesia: a randomised, prospective clinical evaluation. JAMA. 1971;216:278–288. doi: 10.1001/jama.1971.03180280032006. [DOI] [PubMed] [Google Scholar]
- 11.Mazze RI. Methoxyflurane revisited: tale of an anaesthetic from cradle to grave. Anesthesiology. 2006;105:843–846. doi: 10.1097/00000542-200610000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Tomi K, Mashimo T, Tashiro C, et al. Alterations in pain threshold and psychomotor response associated with subanaesthetic concentrations of inhalation anaesthetics in humans. Br J Anaesth. 1993;70:684–686. doi: 10.1093/bja/70.6.684. [DOI] [PubMed] [Google Scholar]
- 13.Penthrox European Summary of Product Characteristics October 2015. http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1460695399101.pdf. Accessed 07 Aug 2016.
- 14.Cousins ML, Mazze RI. Methoxyflurane nephrotoxicity. A study of dose-response in man. JAMA. 1973;225:1611–1616. doi: 10.1001/jama.1973.03220410023005. [DOI] [PubMed] [Google Scholar]
- 15.Mazze RI, Cousins ML. Biotransformation of methoxyflurane. Int Anesthesiol. 1984;2:551–575. doi: 10.1097/00004311-197412020-00011. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs IG. Health effects of patient given methoxyflurane in the pre-hospital setting: a Data Linkage Study. Open Emerg Med J. 2010;3:7–13. doi: 10.2174/1876542401003010007. [DOI] [Google Scholar]
- 17.Dayan AD. Analgesic use of inhaled methoxyflurane: evaluation of its potential nephrotoxicity. Hum Exp Toxicol. 2016;35:91–100. doi: 10.1177/0960327115578743. [DOI] [PubMed] [Google Scholar]
- 18.Grindlay J, Babl FE. Review article: efficacy and safety of methoxyflurane analgesia in the emergency department and prehospital setting. Emerg Med Australas. 2009;21:4–11. doi: 10.1111/j.1742-6723.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnston S, Wilkes GJ, Thompson JA, Ziman M, Brightwell R. Inhaled methoxyflurane and intranasal fentanyl for prehospital management of visceral pain in an Australian ambulance service. Emerg Med J. 2011;28:57–63. doi: 10.1136/emj.2009.078717. [DOI] [PubMed] [Google Scholar]
- 20.Buntine P, Thom O, Babl F, Bailey M, Bernard S. Prehospital analgesia in adults using inhaled methoxyflurane. Emerg Med Australas. 2007;19:509–514. doi: 10.1111/j.1742-6723.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- 21.Gaskell AL, Jephcott CG, Smithells JR, Sleigh JW. Self-administered methoxyflurane for procedural analgesia: experience in a tertiary Australasian centre. Anaesthesia. 2016;71:417–423. doi: 10.1111/anae.13377. [DOI] [PubMed] [Google Scholar]
- 22.Coffey F, Wright J, Hartshorn S, et al. STOP!: a randomised, double-blind, placebo-controlled study of the efficacy and safety of methoxyflurane for the treatment of acute pain. Emerg Med J. 2014;31:613–618. doi: 10.1136/emermed-2013-202909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CHMP guideline CPMP/EWP/612/00. Note for Guidance on Clinical Investigations of Medicinal Products for Treatment of Nociceptive Pain.
- 24.Jensen MP, Karoly P. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 25.Ho K, Spence J, Murphy M. Review of pain measurement tools. Ann Emerg Med. 1996;27:427–431. doi: 10.1016/S0196-0644(96)70223-8. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38:633–638. doi: 10.1067/mem.2001.118863. [DOI] [PubMed] [Google Scholar]
- 27.Collins S, Moore A, McQuay H. The visual analog pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72:95–97. doi: 10.1016/S0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 28.Todd KH, Funk KG, Funk JP, et al. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485–489. doi: 10.1016/S0196-0644(96)70238-X. [DOI] [PubMed] [Google Scholar]
- 29.Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 30.Feinstein AR. Clinical epidemiology: the architecture of clinical research. Philadelphia: WB Saunders; 1985. pp. 396–406. [Google Scholar]
- 31.Borland M, Jacobs I, King B, O’Brien D. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med. 2007;49:335–340. doi: 10.1016/j.annemergmed.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Tvieta T, Thoner J, Klepstad P, Dale O, Jystad A, Borchgrevink PC. A controlled comparison between single doses of intravenous and intramuscular morphine with respect to analgesic effects and patient safety. Acta Anaesthesiol Scand. 2008;52:920–925. doi: 10.1111/j.1399-6576.2008.01608.x. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen NQ, Toscano L, Lawrence M, et al. Patient-controlled analgesia with inhaled methoxyflurane versus conventional endoscopist-provided sedation for colonoscopy: a randomized multicenter trial. Gastrointest Endosc. 2013;78:892–901. doi: 10.1016/j.gie.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen NQ, Toscano L, Lawrence M, et al. Portable inhaled methoxyflurane is feasible and safe for colonoscopy in subjects with morbid obesity and/or obstructive sleep apnea. Endosc Int Open. 2015;3:E487–E493. doi: 10.1055/s-0034-1392366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spruyt O, Westerman D, Milner A, Bressel M, Wein S. A randomised, double-blind, placebo-controlled study to assess the safety and efficacy of methoxyflurane for procedural pain of a bone marrow biopsy. BMJ Support Palliat Care. 2014;4:342–348. doi: 10.1136/bmjspcare-2013-000447. [DOI] [PubMed] [Google Scholar]
- 36.Huang S, Pepdjonovic L, Konstantatos A, Frydenberg M, Grummet J. Penthrox alone versus Penthrox plus periprostatic infiltration of local analgesia for analgesia in trasrectal ultrasound-guided prostate biopsy. ANZ J Surg. 2016;86:139–142. doi: 10.1111/ans.12974. [DOI] [PubMed] [Google Scholar]
- 37.Oxer HF. Effects of Penthrox® (methoxyflurane) as an analgesic on cardiovascular and respiratory functions in the pre-hospital setting. J Military Veterans’ Health. 2016;24(2):14–20. [Google Scholar]
- 38.Carley S, Body R. Methoxyflurane is a better painkiller than placebo: but do we want to know more? Emerg Med J. 2014;31:610. doi: 10.1136/emermed-2014-203690. [DOI] [PubMed] [Google Scholar]
- 39.Ethical Considerations for Clinical Trials on Medicinal Products Conducted with the Paediatric Population. 2008. ftp://ftp.cordis.europa.eu/pub/fp7/docs/ethical-considerations-paediatrics_en.pdf. Accessed 07 Aug 2016. [DOI] [PubMed]