Abstract
The homeostatic controls over eating are inextricably linked to the reward aspects of eating. The result is an integrated response that coordinates the internal milieu with the prevailing environment. Thus, appetite, which reflects a complex interaction among the external environment, behavioral profile, and subjective states as well as the storage and metabolism of energy, has an important role in the regulation of energy balance. In the prevailing food environment which offers an abundance of food choices it is likely that the motivation to consume from a wide range of delectable foods plays a greater role in contributing to overeating than in the past when the motivation to eat was largely governed by metabolic need. The response to food-related cues can promote strong desires to eat known as cravings by activating the mesocorticolimbic dopamine neurocircuitry. Cravings are associated with subsequent eating and weight-related outcomes. Being able to control food cravings is a determinant of success at adhering to an energy-restricted diet regimen. Increased understanding of the neurocircuitry of appetite regulation, especially reward-related eating behavior, has provided potential targets for therapeutic anti-obesity agents specifically directed at reward mechanisms. The naltrexone–bupropion combination and lorcaserin, which are both approved by the US Food and Drug Administration (FDA) for long-term weight management, have shown promise in addressing craving-related eating behavior. Phentermine and liraglutide are approved as monotherapies for weight management. Preliminary research suggests that liraglutide, as well as phentermine alone or in combination with lorcaserin, may be effective in targeting food cravings. Food components such as thylakoid membranes have also been shown to influence food cravings. This review explores the concepts related to appetite and reward-induced eating behavior, as well as the pharmacological options and food-derived components that may be used to address food cravings.
Keywords: Appetite, Food cravings, Pharmacology, Reward-related eating
Introduction
Globally, the leading causes of death are cardiovascular disease (CVD), especially coronary heart disease and stroke [1]. Overweight and obesity are important risk factors of CVD [2–4], operating in part through mechanisms such as elevated levels of blood pressure, dyslipidemia, and abnormal glucose metabolism. However, interventions that address these abnormalities resolve approximately half of the excess risk for coronary heart disease that may be attributed to a high body mass index [5]. Thus, maintenance of optimum body weight through strategies that can curb or reverse adiposity is of paramount importance in reducing CVD and type 2 diabetes [5].
Current obesity guidelines suggest that sustained weight loss of just 3–5% achieved through lifestyle interventions can result in clinically significant positive metabolic outcomes [2]. Any obesity treatment involves creating a negative energy balance. Finding the optimal diet composition for achieving a negative energy balance has proven elusive. What appears to be important is not the type of diet, but adherence to a diet regimen that promotes energy restriction [6]. Lifestyle change programs can help many individuals to achieve weight loss but a large percentage will fail to do so and many of those who achieve it will regain body weight. Weight loss induces neuroendocrine changes that impinge on what is called “willpower”, as a result of counter-regulatory mechanisms that provoke a powerful unconscious impulse to eat [7]. There are associated increases in neural activity in areas of the brain involved in processing the rewarding value of food stimuli [8, 9].
Economic growth and technological developments are important for prosperity, but the trade-off to business and trade by way of less regulated global markets has led to the creation of cheap, easily available food sources [10]. The biological drive to eat is inextricably linked not only to the satiating and satiety-enhancing power of food but also to its rewarding value. In an environment that presents a plethora of inviting food choices the consumer is often prodded to disregard dietary recommendations [11]. Obesity results from a chronic energy imbalance; hence, controlling the urge to eat assumes importance. This review explores the concepts related to appetite regulation with special emphasis on reward-induced eating and interventions that target reward mechanisms.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Appetite
Appetite reflects a complex interaction between the external environment, the behavioral profile, and subjective states as well as the storage and metabolism of energy [12]. The expression of appetite manifests the interplay of events and processes that occur at three levels: (1) psychological events such as perceptions of hunger, food cravings, or hedonic sensations, and the corresponding behavioral actions; (2) responses in the peripheral physiologic system and metabolic events which stem from nutrient absorption, metabolism, and storage; and (3) central neural processes that translate the physiologic events [13].
The hypothalamus is the cerebral appetite center, integrating peripheral humoral signals that transmit information about food intake and energy expenditure with neuronal signals from the brainstem and higher cortical center dealing with cognition, pleasure, and emotion [14]. Within the arcuate nucleus of the hypothalamus there are two neuronal populations with opposing effects on food intake. The neurons which co-express neuropeptide Y and agouti-related peptide (AgRP) increase appetite, whereas the neurons that co-express the cocaine- and amphetamine-regulated transcript (CART) and proopiomelanocortin (POMC) decrease appetite, by acting on the melanocortin-4 receptor (MC-4R). Proopiomelanocortin cells produce α-melanocyte stimulating hormone (α-MSH) an MC-4R agonist, whereas AgRP is an MC-4R antagonist [15, 16]. Following the ingestion of food, sensory information is transmitted from the gastrointestinal tract to the central nervous system either through vagal and somatosensory afferent fibers or via bloodstream signals which may be the gut hormones [17]. The presence of an incomplete blood–brain barrier in the regions of the brain such as the area postrema permit many circulating signals, including the gut hormones, direct access to the central nervous system [18]. Thus, complex neuronal pathways with reciprocal connections between the hypothalamus, brainstem, and higher cortical areas [19] are involved in the control of appetite acting through endocrine and neuronal feedback signals from the periphery to synchronize appetite perception, food intake behavior, and energy homeostasis.
The widely accepted homeostatic view of the control of food intake is that adiposity signals such as leptin and insulin influence the sensitivity to meal-derived satiation signals to regulate meal size in response to changes in body weight [20]. Energy homeostasis is coordinated by two sets of signals. Long-term or tonic signals emanate from tissue stores, predominantly adipose, and include chemical signals such as leptin, insulin, adiponectin, and certain cytokines. Short-term or episodic signals are generated periodically as food intake occurs and for the most part arise in the gastrointestinal tract. Some of the chemical components of episodic signals are cholecystokinin (CCK), peptide YY (PYY), glucagon-like peptide-1 (GLP-1), ghrelin, and oxyntomodulin released from cells in and around the gastrointestinal tract. Cerebral integration of tonic and episodic signals reflects the current state of energy stores and the flux of nutrients prompted by an eating episode [21].
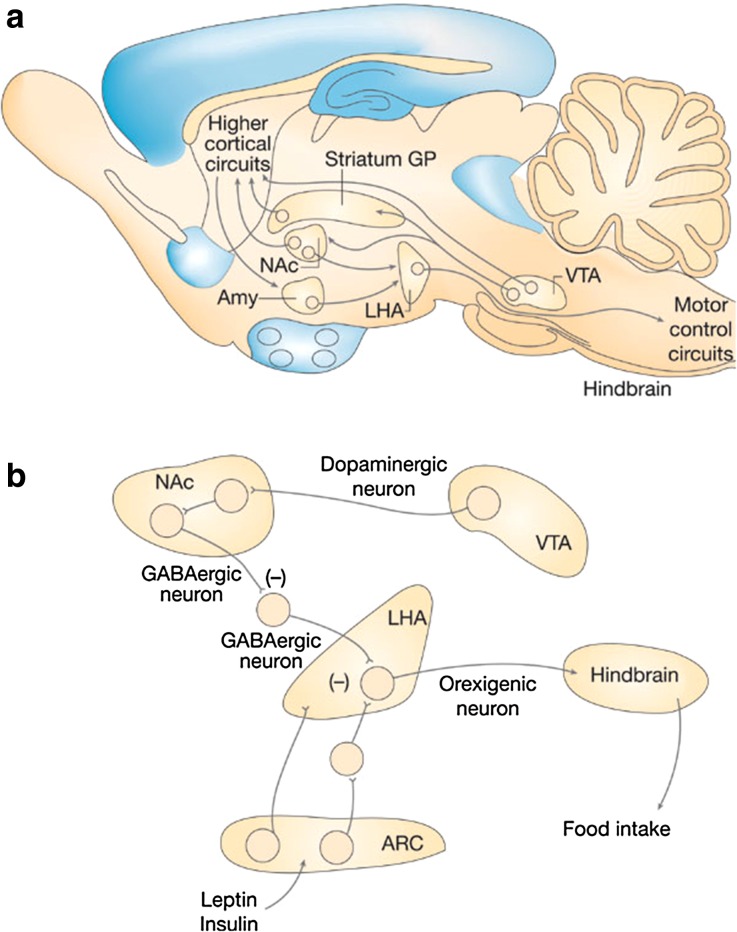
Translation of the metabolic need into behavioral action is closely intertwined with reward and is orchestrated by a cognitive and emotional brain that operates on factors such as past experiences, cost, and availability [22]. The mesocorticolimbic system is involved in reward processing through dopamine release from neurons in the ventral tegmental area and their projections to the nucleus acumbens, amygdala, prefrontal complex, and other forebrain regions [23]. In the reward system, dopamine and opioid systems interact in the determination of eating behavior [24]. Dopamine signaling plays an important role in translating motivation into action [25], and increases the drive to obtain a rewarding stimulus, but it is opioid peptide transmission in the nucleus acumbens rather than dopamine that modulates the hedonic or pleasure impact of food [23, 26] (Fig. 1). Specifically, opioids, particularly the µ-opioids, covey the reward sensation of desirable foods whereas dopamine regulates the reward value of food and the motivation to obtain that food [27]. Thus, appetite reflects (1) physiologic hunger or a conscious sensation reflecting an urge to eat and (2) reward that can be triggered without any physiologic hunger, but is still controlled physiologically.
Fig. 1.
Brain reward circuitry. a Neurons in the VTA of the midbrain project to forebrain areas including the NAc, striatum, and cortex, and assign reward value to palatable food. b Perception of pleasure associated with consumption of a palatable food involves neuronal activation in the NAc and striatum, which through activation of opiate peptide receptors disinhibits the lateral hypothalamic area and thereby stimulates feeding. Amy amygdala, GP globus pallidus, NAc nucleus accumbens, VTA ventral tegmental area. Reprinted with permission from Macmillan Publishers: Morton et al. [26]
Reward-Induced Eating Behavior
In the modern world, humans often eat in the absence of any metabolic feedback indicating a diminution of reserves [22]. This non-homeostatic eating involves perceptions of food reward. The psychological components of reward include (1) learning which includes knowledge resulting from associative and cognitive processes; (2) affect (emotion) or liking which reflects the immediate experience or eagerness to experience pleasure from the hedonic value of consuming a food; and (3) motivation to actually eat, or wanting [28]. Components of affective and motivational processes can exist objectively, without conscious awareness of them. Therefore, they can be implicit which assumes importance because individuals can react to a rewarding stimulus without conscious awareness of either the stimulus or their hedonic response to it [28].
Perceptions of food reward can occur by encountering food-related cues such as the sight, smell, and taste of the food, sensations that often initiate an intense desire to eat known as craving: a conditioned response to food that is often accompanied by increased salivation, physiologic arousal, and neural stimulation. Such craving is a form of food cue reactivity, called cue-induced craving. Another type of craving that arises independently of external cues as may occur when merely imagining a favorite food causes the individual to be consumed with the desire to eat and is known as tonic craving. Other forms of craving include state craving which is tonic or cue-related craving assessed at a particular moment in time and trait craving which is a general tendency to crave with or without the presence of cues [29]. Further, there are gender differences in cravings and the response to them. Men exhibit a yearning for savory foods [8, 30, 31], whereas women prefer high-fat sweet foods such as chocolate [8, 31, 32]. Women have a greater inclination than men to succumb to cue-related food cravings, and more so in the luteal phase of the menstrual cycle [29].
Both cue-related and tonic craving are associated with subsequent eating and weight-related outcomes. Moreover, reactivity to visual cues such as images of food is as related to eating behavior as real food [33]. Individuals unsuccessful at adhering to dietary energy restriction report greater food cravings that are related to loss of control over eating and plans to consume food than those who achieve success at following a diet regimen [34]. Meta-analyses of brain imaging studies suggest that major brain regions involved in cue reactivity to alcohol, drugs of abuse, sex, gambling, smoking, and food for the most part overlap, and consist of a network that processes reward responses [35, 36]. These biological and behavioral parallels suggest that eating behavior is addictive. It appears that food cravings are a mediator between addictive eating behavior and increases in body weight [37].
Hypothalamic signals regulating energy intake can influence the activity of the hedonic or reward systems in the corticolimbic structures [38]. Similarly, signals generated in the corticolimbic structures processing reward information can modulate hypothalamic processes involved in homeostatic control [39]. The result is a complex integration of multiple interacting neuronal circuits and a number of factors that strengthen, attenuate, or override others. For instance, food deprivation exerts a stimulatory effect on reward perception. The proposed mechanism is that leptin and insulin tonically inhibit the reward circuitry in the brain, and energy restriction by lowering circulating levels of these hormones increases the responses to rewarding stimuli as an adaptive mechanism to prevent drastic depletion of body stores [26]. Conversely, circulating levels of the hormone ghrelin are elevated during fasting and fall after eating, suggesting that ghrelin exerts an orexigenic effect to stimulate hunger. Ghrelin also modulates both liking and wanting aspects of reward-driven eating and increases the motivation to obtain and consume foods in part by activating dopaminergic neurons in the ventral tegmental area and stimulating dopamine release within the nucleus acumbens [40].
Obesity trends suggest that overriding of homeostatic control by corticolimbic processes may be the cause of energy imbalance; however, it remains debatable as to whether the short-term regulation of food intake can truly be called a homeostatic response if the correction of imbalances is not critical [20]. Thus, targeting perceptions of food reward can have significant implications for public health initiatives directed at reducing energy intake [41].
Measuring Reward-Driven Appetite
To evaluate the intensity and type of food cravings an individual experiences, the Control of Eating Questionnaire (COEQ) has often been used [42–44]. In a recent validation study, loss of control due to cravings and a positive mood were associated with increased total energy intake at an ad libitum snack service. Further, the craving for sweet was strongly associated with intake and selection of sweet foods. However, the craving for savory foods did not show the same association which may reflect differences between choices at main meals and snacks [42]. The Food Craving Inventory (FCI) has also been shown to be a valid and reliable measure of general and specific food cravings [45]. Both of these questionnaires appear to converge with data from psychometric tests such as the Eating Inventory which measures traits (predispositions exhibited by an individual that are relatively stable across time) related to eating behavior [46]. The State and Trait Food Craving Questionnaires are validated means of measuring state and trait cravings [47, 48]. The Yale Food Addiction scale is a measure of addictive-like eating behaviors that has been validated to various extents across different age groups, races, and among subjects with obesity or eating disorders. However, the concept of food addiction has been criticized because of the lack of evidence for substance-based food addiction [49]. Neuroimaging using functional magnetic resonance imaging to permit measuring and mapping of brain activity that is specific to food stimuli in the reward circuitry is also a possible way to measure motivational signals [50, 51].
Targeting Reward Mechanisms
The term obesity was paired with each of the following terms using the operator AND: food cues, food cravings, and reward-induced eating. The paired terms were used to perform a literature search on PubMed. Six human intervention trials investigating the effects of medications approved by the US Food and Drug Administration (FDA) for weight management on reward-induced eating and three human trials investigating the effect of a food-derived component on reward-related mechanisms were identified for the period ending August 31, 2016. Additionally, one medication-related trial Moldovan et al. was identified through a personal communication. The results of the PETAL study [52, 53], a trial relating to food cravings, were presented at the meeting of the Obesity Society, 2015.
Pharmacologic Interventions
Anti-obesity medications are approved for individuals with a BMI of at least 30 kg/m2 or a BMI of at least 27 kg/m2 having at least one obesity-related co-morbidity. The combination of naltrexone extended release (ER) and bupropion ER (Contrave™) is approved by the FDA for long-term weight management in patients with obesity [54]. Naltrexone and bupropion are approved by the FDA as monotherapies for other conditions. Bupropion, a dopamine reuptake inhibitor, is approved for the treatment of depression and seasonal affective disorder, and to aid in smoking cessation [55, 56]; whereas, naltrexone, an opioid receptor antagonist, is approved for the treatment of alcohol and opioid dependence [57, 58]. Bupropion is thought to stimulate secretion of α-MSH from POMC cells which produces an anorexic effect. Further, the antidepressant effects of bupropion and its effectiveness in aiding smoking cessation suggest that it mediates processes in the reward system [59]. Although bupropion stimulates POMC activity, the anorexic effects of POMC are curtailed by the melanocortin system’s inherent feedback mechanism that limits sustained stimulation of POMC cells by simultaneous secretion of endogenous opioids such as β-endorphins that inhibit α-MSH secretion [27].
Naltrexone monotherapy has little efficacy for weight management. However, consistent with the established role of opiates in the reward aspects of eating and stimulation of consumption of energy dense sugar and fat-laden foods [60], naltrexone antagonism of opioid receptors reduces the subjective pleasantness or liking of certain foods [61, 62]. Blockade of the µ-opioid receptor with naltrexone to counteract the auto-inhibitory actions of bupropion-stimulated release of endogenous opioids forms the basis of the combination treatment of bupropion and naltrexone for obesity treatment. Moreover, naltrexone and bupropion both reduce food intake in mice when injected directly into the reward system; but the effect is synergistic when they are administered together. Results from the phase III trials showed that the combination treatment of naltrexone and bupropion consistently reduced measures of food reward assessed using the COEQ [43, 44, 63]. Subjects reported reduced frequency and strength of food cravings (Table 1).
Table 1.
Therapies for reducing reward-related eating
| Therapy | Effect | References |
|---|---|---|
| Naltrexone + bupropion (Contrave™) | Reduces frequency and strength of food cravings | [43, 44, 63] |
| Phentermine (Adipex-P™, Ionamin™)a | Suppresses appetite | [66] |
| Lorcaserin (Belviq™) | Improves facets of inhibitory control | [79–81] |
| Lorcaserin + phentermine | Reduces frequency and strength of food cravings. Reduces cravings for high-fat foods, sweets, fast foods | [52, 53] |
| Liraglutide (Saxenda™) | Reduces attention to food cues | [82, 88] |
| Thylakoid membranes (Appethyl™) | Reduces desire for sweet foods and chocolate | [93, 94] |
aSome common brand names
Phentermine (common brand names Adipex-P™, Ionamin™) is primarily a noradrenergic and perhaps dopaminergic sympathomimetic amine that acts as an appetite suppressant [64]. It was approved by the FDA for use in conjunction with lifestyle change efforts for short-term (few weeks) weight management [65]. A 12-week randomized controlled trial evaluated the effect of phentermine and a meal replacement system along with nutritional counseling on weight loss and food cravings. A greater proportion of subjects in the phentermine group achieved weight loss of 5% or more, and the craving for fats and sweets (evaluated using the Trait and State Food Cravings Questionnaires) was reduced in the phentermine group, compared to the group receiving the meal replacement and counseling along with a placebo. Further, the reduction in craving positively correlated with weight loss over the 12-week period [66].
Serotonin (5-hydroxytryptamine, 5-HT) is a neurotransmitter that regulates food intake and energy expenditure by acting on the central nervous system, with the key mediators being the 5-HT2C receptors (5-HT2CR) [67]. Agonists of 5-HT2CR activate POMC neurons which results in the release of α-MSH and activation of the anorexigenic central melanocortin pathway through MC-4R [68–70]. Lorcaserin (Belviq™) is a highly specific 5-HT2CR approved by the FDA for the long-term treatment of obesity [71]. Studies showed that lorcaserin produced weight loss without the adverse cardiac outcomes associated with non-specific serotonin receptor agonists [72–74]. The role of 5-HT2CR in the regulation of forebrain dopaminergic systems is well established [75–77], suggesting that agonists of 5-HT2CR should affect behaviors motivated by food [78]. On the basis of research in substance abuse, it appears that agonists of 5-HT2CR including lorcaserin improve facets of inhibitory control, which has the potential to prevent relapse following a period of abstinence [78–81]. Given the neurobiological and behavioral commonalities between obesity and drug addiction, it is not surprising that lorcaserin has been investigated for its effects on food cravings.
In the Pilot Evaluation of Tolerability and Safety of Lorcaserin and phentermine (PETAL) study, conducted to evaluate if administration of the combination of lorcaserin with immediate release phentermine for 12 weeks was associated with adverse events, the effect of the drug administration on food cravings was also evaluated as a secondary endpoint. Subjects were given lorcaserin 10 mg twice daily or lorcaserin 10 mg twice + phentermine 15 mg once daily, or lorcaserin 10 mg twice + phentermine 15 mg twice daily. Weight loss at 12 weeks was 3.3%, 6.7%, and 7.2% in the lorcaserin only and the combination with phentermine once or twice daily, respectively.
Analysis of the food craving data evaluated using the FCI indicated that subjects in all treatment groups showed improvement in the FCI total score from baseline to week 12 as well as in the FCI subscale scores for high-fat foods, sweets, carbohydrates, and fast foods without any one particular category of foods driving the overall effect [52]. Subjects also reported improvements in control of eating in all treatment groups at week 12 compared to baseline, evaluated using the COEQ. The frequency and strength of food cravings reduced including the craving for chocolate, sweets, non-sweets, and starchy foods in a sample that comprised 85% women. The limitation of this study is that there was no placebo-treated group [53].
In the brains of humans GLP-1 receptors have been identified in the hypothalamus, medulla, and parietal cortex [82]. In human subjects, the GLP-1 receptor agonist exenatide reduced activation in response to food cues in brain areas involved in the regulation of reward, the effect of which was greatly attenuated by GLP-1 receptor blockade [83, 84]. The response to GLP-1 receptor blockade was more pronounced in subjects with type 2 diabetes and obesity than in lean healthy subjects [85]. In response to treatment for 17 days with liraglutide (Saxenda™) a GLP-1 agonist approved by the FDA for long-treatment of obesity [86], activity in the parietal cortex decreased when subjects viewed images of highly desirable foods compared to placebo. The parietal cortex is involved in controlling the location of attention [87]; hence, it is likely that there was a reduced appeal of the food cues, especially since liraglutide treatment also increased fullness and reduced food intake [82]. However, gastric inhibitory peptide increased and serum leptin decreased in response to food cues, each of which has opposing effects in the areas of the brain involved with reward mechanisms [88]. Leptin is secreted by fat cells and binds to specific leptin receptors on dopaminergic neurons in the ventral tegmental area to inhibit dopamine signaling in the nucleus acumbens and amygdala. Unlike gastric inhibitory peptide, leptin reduces reward-related eating behavior [89]. The short-term effects of liraglutide on reward mechanisms warrant corroboration in future studies.
Food-Based Interventions
Thylakoids are compartments inside the chloroplasts of green plants such as spinach and are composed of membranes that form the internal photosynthetic membrane system of chloroplasts. By interacting with lipids and delaying fat digestion, thylakoid membranes promote the release of hormones that mediate satiety [90]. Studies investigating the effects of thylakoids (Appethyl™) on eating behavior have demonstrated increases in perceptions of satiety [91, 92], and a reduction in body weight [93]. Additionally, two studies demonstrated a decrease in the craving for sweet foods and chocolate among women in the overweight or obese body mass index range, measured subjectively [93, 94]. In another study, men tended to reduce their intake at a pizza meal; whereas, among women food intake did not change following a 5-g dose of thylakoids [91]. Men and women tend to crave different kinds of foods, have different experiences of craving, and differ in their responses. Men report a craving for savory foods, and a disproportionately large percentage of those who experience a craving for chocolate are women [29]. The gender differences demonstrated by studies investigating the effects of thylakoids suggest that thylakoids may influence the reward system.
Conclusions
Neural systems, located primarily in the brainstem and hypothalamus, represent the homeostatic regulators of food intake; whereas, the neural pathways and functions located in the corticolimbic structures are responsible for reward-related control of eating [38]. Nevertheless, hypothalamic signals regulating energy intake can influence the activity of the hedonic or reward systems in the corticolimbic structures [38]. Similarly, signals generated in the corticolimbic structures processing sensory, cognitive, and reward information can modulate hypothalamic processes involved in homeostatic control [39]. Although the balance between energy intake and expenditure is controlled by a powerful unconscious biological system, the laws of thermodynamics predict that body weight can be controlled by consciously balancing food intake and energy expenditure. If being able to control food cravings is a determinant of success at adhering to a diet regimen then addressing cravings may be particularly relevant to people with obesity. Therefore, anti-obesity medications that reduce reward-induced eating behavior can be especially helpful in overcoming strong desires to eat.
Lorcaserin and the combination of naltrexone and bupropion, which are both approved by the FDA for long-term weight management, have shown promise in addressing craving-related eating behavior. Phentermine and liraglutide are approved as monotherapies for weight management. Preliminary research suggests that liraglutide, as well as phentermine alone or in combination with lorcaserin may be effective in targeting food cravings. Food components such as thylakoid membranes have also been shown to influence food cravings. Strategies that effectively target food cravings can facilitate weight loss and maintenance leading to long-term improvements in obesity-related comorbidities. Thus, it is important that clinicians are aware of options to address the reward components of eating behavior that may be used in conjunction with lifestyle change efforts, when appropriate.
Acknowledgments
This work is based in part on work that was supported by the National Institutes of Health under an award (T32 A T004094) from the National Center for Complementary and Integrative Health. No funding or sponsorship was received for the publication charges of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
FL Greenway is a consultant for Eisai Inc., and Orexigen/Takeda, and is on the Advisory Boards of Novo Nordisk and Zafgen. C.J. Rebello has nothing to disclose
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/53E6F0606CEEE6CA.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh GM, Danaei G, Farzadfar F, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8(7):e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prospective Studies C, Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors C, Wormser D, Kaptoge S, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Hajifathalian K, Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 7.Jequier E, Tappy L. Regulation of body weight in humans. Physiol Rev. 1999;79(2):451–480. doi: 10.1152/physrev.1999.79.2.451. [DOI] [PubMed] [Google Scholar]
- 8.Pelchat ML. Food cravings in young and elderly adults. Appetite. 1997;28:103–113. doi: 10.1006/appe.1996.0063. [DOI] [PubMed] [Google Scholar]
- 9.Hill AJ. The psychology of food craving. Proc Nutr Soc. 2007;66:277–285. doi: 10.1017/S0029665107005502. [DOI] [PubMed] [Google Scholar]
- 10.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 11.Cohen DA. Neurophysiological pathways to obesity: below awareness and beyond individual control. Diabetes. 2008;57:1768–1773. doi: 10.2337/db08-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blundell JE, Lawton CL, Cotton JR, Macdiarmid JI. Control of human appetite: implications for the intake of dietary fat. Annu Rev Nutr. 1996;16:285–319. doi: 10.1146/annurev.nu.16.070196.001441. [DOI] [PubMed] [Google Scholar]
- 13.Blundell J. Pharmacological approaches to appetite suppression. Trends Pharmacol Sci. 1991;12:147–157. doi: 10.1016/0165-6147(91)90532-W. [DOI] [PubMed] [Google Scholar]
- 14.Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 16.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 17.Buhmann H, le Roux CW, Bueter M. The gut-brain axis in obesity. Best Pract Res Clin Gastroenterol. 2014;28:559–571. doi: 10.1016/j.bpg.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57:359–372. doi: 10.1507/endocrj.K10E-077. [DOI] [PubMed] [Google Scholar]
- 20.Woods SC, Langhans W. Inconsistencies in the assessment of food intake. Am J Physiol Endocrinol Metab. 2012;303:E1408–E1418. doi: 10.1152/ajpendo.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blundell JE, Levin F, King NA, et al. Overconsumption and obesity: peptides and susceptibility to weight gain. Regul Pept. 2008;149:32–38. doi: 10.1016/j.regpep.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33(Suppl 2):S8–S13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finlayson G, King N, Blundell JE. Liking vs. wanting food: importance for human appetite control and weight regulation. Neurosci Biobehav Rev. 2007;31:987–1002. doi: 10.1016/j.neubiorev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 26.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 27.Billes SK, Sinnayah P, Cowley MA. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res. 2014;84:1–11. doi: 10.1016/j.phrs.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 29.Hallam J, Boswell RG, DeVito EE, Kober H. Gender-related differences in food craving and obesity. Yale J Biol Med. 2016;89:161–173. [PMC free article] [PubMed] [Google Scholar]
- 30.Weingarten HP, Elston D. Food cravings in a college population. Appetite. 1991;17:167–175. doi: 10.1016/0195-6663(91)90019-O. [DOI] [PubMed] [Google Scholar]
- 31.Zellner DA, Garriga-Trillo A, Rohm E, Centeno S, Parker S. Food liking and craving: a cross-cultural approach. Appetite. 1999;33:61–70. doi: 10.1006/appe.1999.0234. [DOI] [PubMed] [Google Scholar]
- 32.Drewnowski A. Metabolic determinants of binge eating. Addict Behav. 1995;20:733–745. doi: 10.1016/0306-4603(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 33.Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obes Rev. 2016;17:159–177. doi: 10.1111/obr.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meule A, Lutz A, Vogele C, Kubler A. Food cravings discriminate differentially between successful and unsuccessful dieters and non-dieters. Validation of the Food Cravings Questionnaires in German. Appetite. 2012;58:88–97. doi: 10.1016/j.appet.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Noori HR, Cosa Linan A, Spanagel R. Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: a comprehensive meta-analysis. Eur Neuropsychopharmacol. 2016;26:1419–1430. doi: 10.1016/j.euroneuro.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Edwards CA, Johnson IT, Read NW. Do viscous polysaccharides slow absorption by inhibiting diffusion or convection? Eur J Clin Nutr. 1988;42:307–312. [PubMed] [Google Scholar]
- 38.Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.la Fleur SE, van Rozen AJ, Luijendijk MC, Groeneweg F, Adan RA. A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int J Obes (Lond) 2010;34:537–546. doi: 10.1038/ijo.2009.257. [DOI] [PubMed] [Google Scholar]
- 40.Edwards A, Abizaid A. Driving the need to feed: insight into the collaborative interaction between ghrelin and endocannabinoid systems in modulating brain reward systems. Neurosci Biobehav Rev. 2016;66:33–53. doi: 10.1016/j.neubiorev.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 41.Potenza MN, Grilo CM. How relevant is food craving to obesity and its treatment? Front Psychiatry. 2014;5:164. doi: 10.3389/fpsyt.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalton M, Finlayson G, Hill A, Blundell J. Preliminary validation and principal components analysis of the Control of Eating Questionnaire (CoEQ) for the experience of food craving. Eur J Clin Nutr. 2015;69:1313–1317. doi: 10.1038/ejcn.2015.57. [DOI] [PubMed] [Google Scholar]
- 43.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 44.Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21:935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 46.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 47.Cepeda-Benito A, Gleaves GH, Williams TL, Erath SA. Development and validation of the state and trait food cravings questionnaire. Behav Ther. 2000;31:151–173. doi: 10.1016/S0005-7894(00)80009-X. [DOI] [PubMed] [Google Scholar]
- 48.Nijs IM, Franken IH, Muris P. The modified Trait and State Food-Cravings Questionnaires: development and validation of a general index of food craving. Appetite. 2007;49:38–46. doi: 10.1016/j.appet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Long CG, Blundell JE, Finlayson G. A systematic review of the application and correlates of YFAS-diagnosed ‘food addiction’ in humans: are eating-related ‘addictions’ a cause for concern or empty concepts? Obes Facts. 2015;8:386–401. doi: 10.1159/000442403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tricomi E, Lempert KM. Value and probability coding in a feedback-based learning task utilizing food rewards. J Neurophysiol. 2015;113:4–13. doi: 10.1152/jn.00086.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rolls ET, McCabe C. Enhanced affective brain representations of chocolate in cravers vs. non-cravers. Eur J Neurosci. 2007;26:1067–1076. doi: 10.1111/j.1460-9568.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- 52.Greenway FL, PIlson R, Ma T, Fain R. The impact of weight loss therapy on control of eating: an exploratory analysis from a 12-week pilot safety study. Obesity Society (TOS). 2015; Abstract. T-P-3153, Available at http://2015.obesityweek.com/app/uploads/2015/11/1104-Wednesday-ObesityWeek-2015-TOS-Poster-Abstracts.pdf. Accessed 4 Oct 2016.
- 53.Greenway FL, PIlson R, Ma T, Fain R. The impact of weight loss therapy on food cravings: an exploratory analysis from a 12-week pilot safety study with lorcaserin and phentermine. Obesity Week (TOS). 2015; Abstract. T-P-3154, Available at http://2015.obesityweek.com/app/uploads/2015/11/1104-Wednesday-ObesityWeek-2015-TOS-Poster-Abstracts.pdf. Accessed 4 Oct 2016.
- 54.Package Inserts. http://general.takedapharm.com/content/file.aspx?filetypecode=CONTRAVEPI&cacheRandomizer=eedcee06-8dad-4cf0-910b-06e13004a474 Accessed 17 Aug 2016.
- 55.Foley KF, DeSanty KP, Kast RE. Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother. 2006;6:1249–1265. doi: 10.1586/14737175.6.9.1249. [DOI] [PubMed] [Google Scholar]
- 56.Ascher JA, Cole JO, Colin JN, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- 57.Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359:715–721. doi: 10.1056/NEJMct0801733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101:491–503. doi: 10.1111/j.1360-0443.2006.01369.x. [DOI] [PubMed] [Google Scholar]
- 59.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 60.Reece AS. Hypothalamic opioid-melanocortin appetitive balance and addictive craving. Med Hypotheses. 2011;76:132–137. doi: 10.1016/j.mehy.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Yeomans MR, Gray RW. Effects of naltrexone on food intake and changes in subjective appetite during eating: evidence for opioid involvement in the appetizer effect. Physiol Behav. 1997;62:15–21. doi: 10.1016/S0031-9384(97)00101-7. [DOI] [PubMed] [Google Scholar]
- 62.Yeomans MR, Gray RW. Selective effects of naltrexone on food pleasantness and intake. Physiol Behav. 1996;60:439–446. doi: 10.1016/S0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- 63.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19:110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 65.Package Insert. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/085128s065lbl.pdf. Accessed 17 Aug 2016.
- 66.Moldovan CP, Weldon AJ, Daher NS, et al. Effects of a meal replacement system alone or in combination with phentermine on weight loss and food cravings. Obesity (Silver Spring) 2016 doi: 10.1002/oby.21649. [DOI] [PubMed] [Google Scholar]
- 67.Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 68.Heisler LK, Cowley MA, Tecott LH, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 69.Heisler LK, Jobst EE, Sutton GM, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Lam DD, Przydzial MJ, Ridley SH, et al. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Package Insert (Belviq). http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf. Accessed 17 Aug 2016.
- 72.O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 73.Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 74.Aronne L, Shanahan W, Fain R, et al. Safety and efficacy of lorcaserin: a combined analysis of the BLOOM and BLOSSOM trials. Postgrad Med. 2014;126:7–18. doi: 10.3810/pgm.2014.10.2817. [DOI] [PubMed] [Google Scholar]
- 75.Millan MJ, Dekeyne A, Gobert A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology. 1998;37:953–955. doi: 10.1016/S0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 76.Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38:1195–1205. doi: 10.1016/S0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 77.Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. Biochemical and electrophysiological evidence that RO 60-0175 inhibits mesolimbic dopaminergic function through serotonin(2C) receptors. Brain Res. 2000;865:85–90. doi: 10.1016/S0006-8993(00)02246-0. [DOI] [PubMed] [Google Scholar]
- 78.Higgins GA, Fletcher PJ. Therapeutic potential of 5-HT2C receptor agonists for addictive disorders. ACS Chem Neurosci. 2015;6:1071–1088. doi: 10.1021/acschemneuro.5b00025. [DOI] [PubMed] [Google Scholar]
- 79.Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- 80.Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther. 2000;295:1183–1191. [PubMed] [Google Scholar]
- 81.Higgins GA, Silenieks LB, Rossmann A, et al. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology. 2012;37:1177–1191. doi: 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farr OM, Sofopoulos M, Tsoukas MA, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016;59:954–965. doi: 10.1007/s00125-016-3874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Bloemendaal L, IJzerman RG, Ten Kulve JS, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186–4196. doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 84.van Bloemendaal L, Veltman DJ, Ten Kulve JS, et al. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes Metab. 2015;17:878–886. doi: 10.1111/dom.12506. [DOI] [PubMed] [Google Scholar]
- 85.ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia. 2015;58:2688–2698. doi: 10.1007/s00125-015-3754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Package Insert Liraglutide. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206321Orig1s000lbl.pdf. Accessed 17 Aug 2016.
- 87.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 88.Farr OM, Tsoukas MA, Triantafyllou G, et al. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: a randomized, placebo-controlled, crossover study. Metabolism. 2016;65:945–953. doi: 10.1016/j.metabol.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reichelt AC, Westbrook RF, Morris MJ. Integration of reward signalling and appetite regulating peptide systems in the control of food-cue responses. Br J Pharmacol. 2015;172:5225–5238. doi: 10.1111/bph.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albertsson PA, Kohnke R, Emek SC, et al. Chloroplast membranes retard fat digestion and induce satiety: effect of biological membranes on pancreatic lipase/co-lipase. Biochem J. 2007;401:727–733. doi: 10.1042/BJ20061463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rebello CJ, Chu J, Beyl R, Edwall D, Erlanson-Albertsson C, Greenway FL. Acute effects of a Spinach extract rich in thylakoids on satiety: a randomized controlled crossover trial. J Am Coll Nutr. 2015;34:470–477. doi: 10.1080/07315724.2014.1003999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stenblom EL, Egecioglu E, Landin-Olsson M, Erlanson-Albertsson C. Consumption of thylakoid-rich spinach extract reduces hunger, increases satiety and reduces cravings for palatable food in overweight women. Appetite. 2015;91:209–219. doi: 10.1016/j.appet.2015.04.051. [DOI] [PubMed] [Google Scholar]
- 93.Montelius C, Erlandsson D, Vitija E, Stenblom EL, Egecioglu E, Erlanson-Albertsson C. Body weight loss, reduced urge for palatable food and increased release of GLP-1 through daily supplementation with green-plant membranes for three months in overweight women. Appetite. 2014;81:295–304. doi: 10.1016/j.appet.2014.06.101. [DOI] [PubMed] [Google Scholar]
- 94.Stenblom EL, Montelius C, Erlandsson D, et al. Decreased urge for palatable food after a two-month dietary intervention with green-plant membranes in overweight women. Obesity Weight Loss Therapy. 2014;4:238. [Google Scholar]